Initial Characterization of Titanium and Vanadium-Rich Magnetite from the Manastir Heights in Southeast Bulgaria Aiming at Future Environmentally Friendly Beneficiation
Abstract
1. Introduction
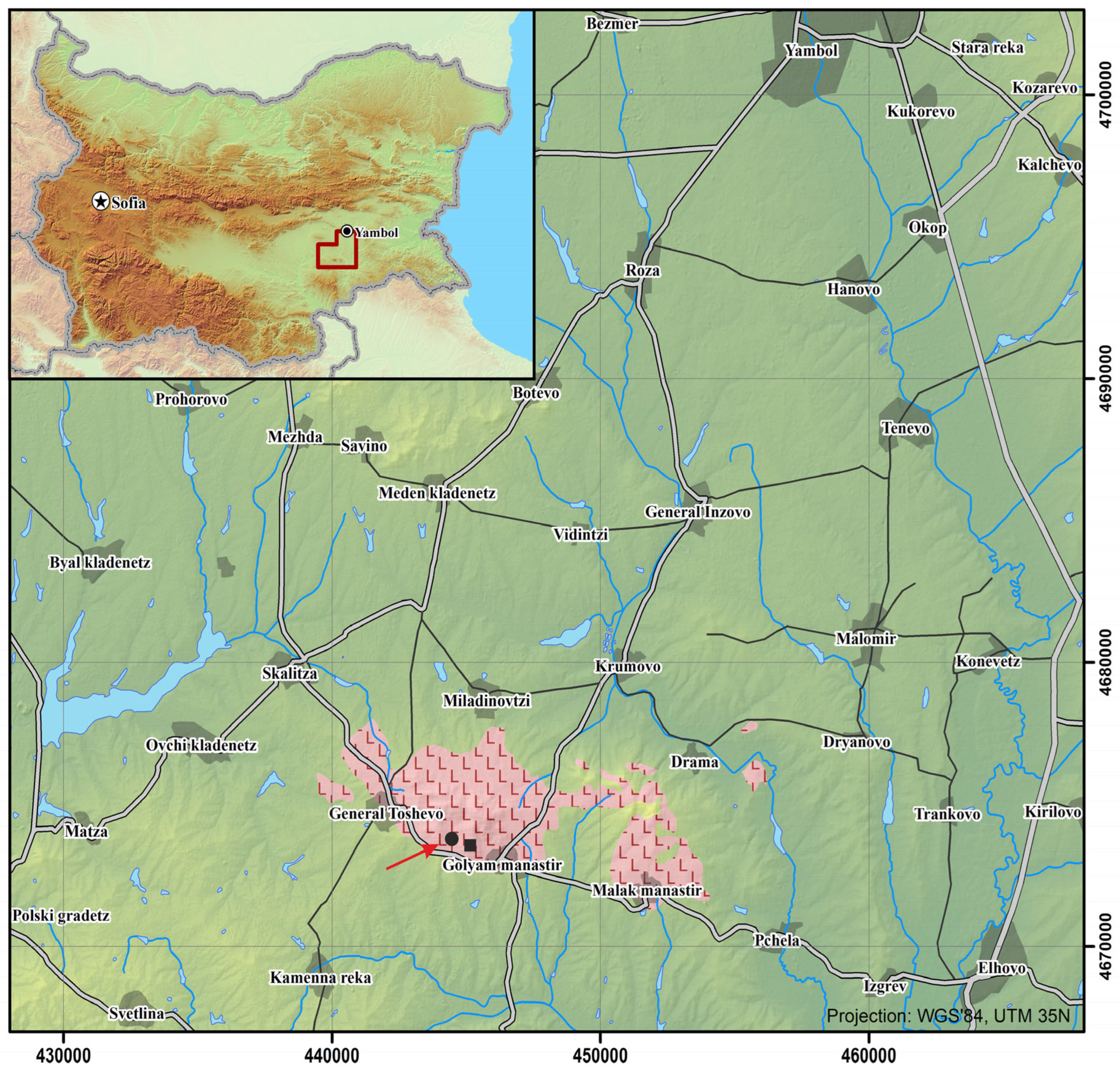
2. Geological Position of the Fe-Ti-V Magnetite Deposit of the Monastir Intrusion
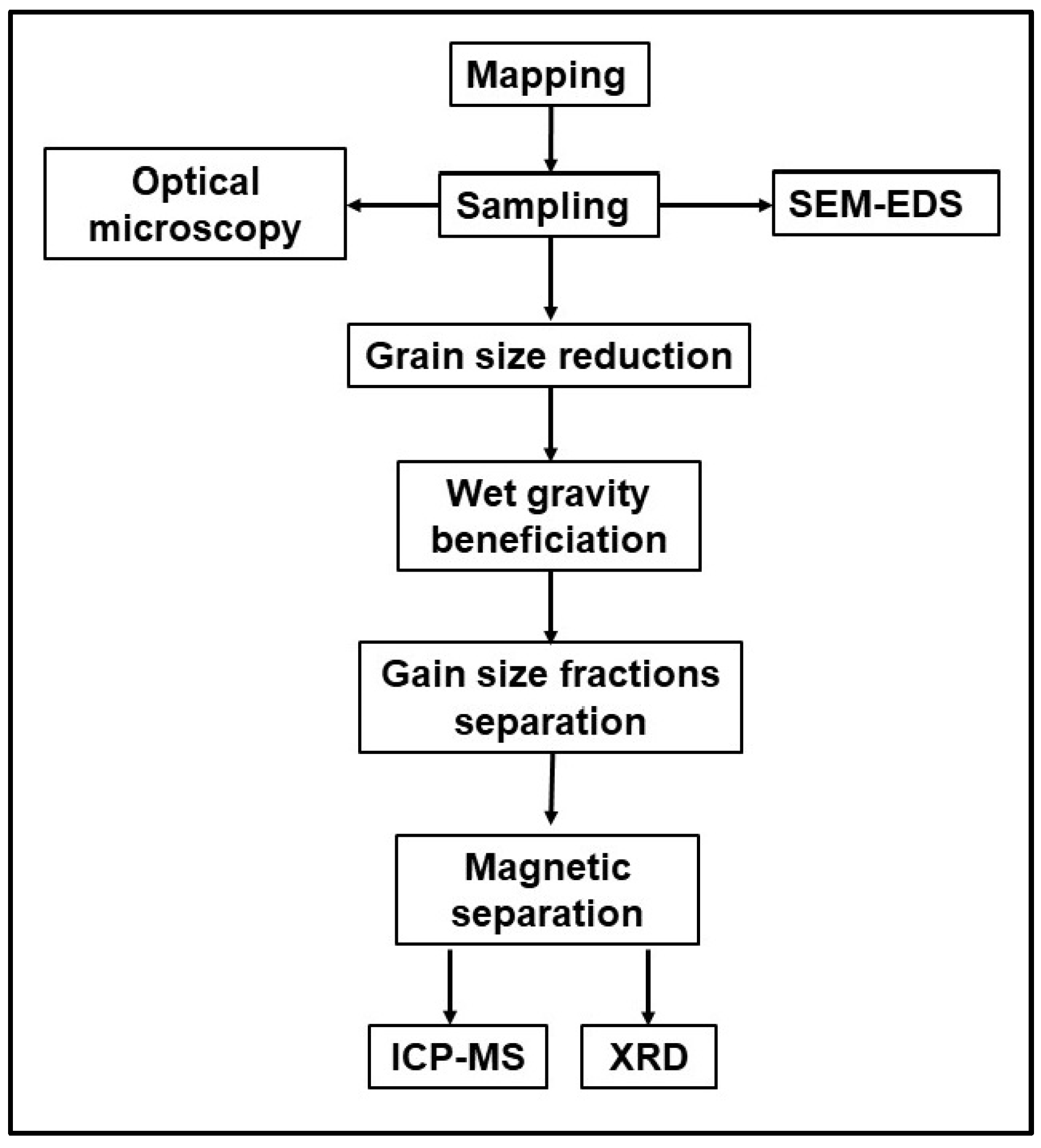
3. Materials and Methods
4. Results
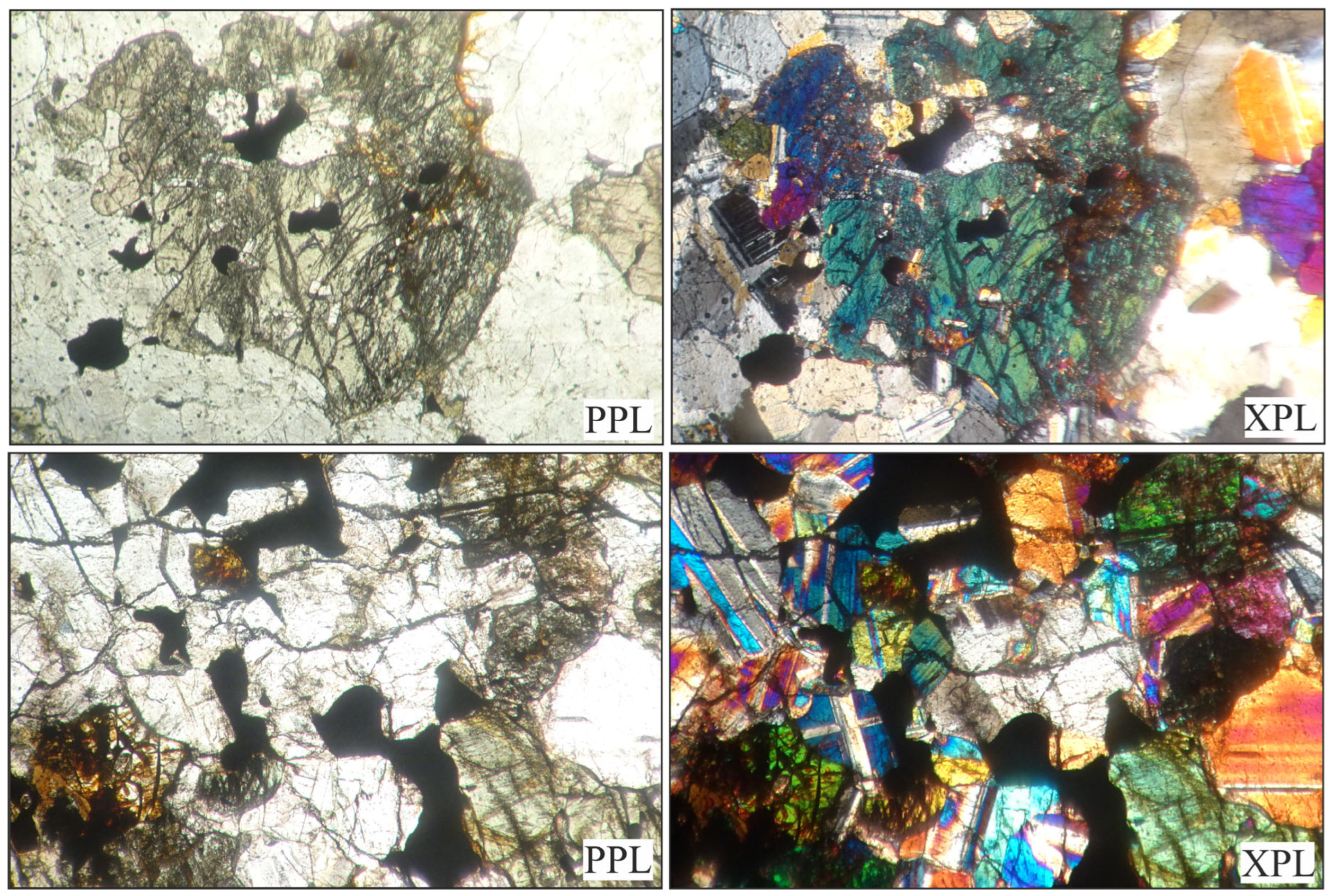
4.1. Observations in Thin Sections
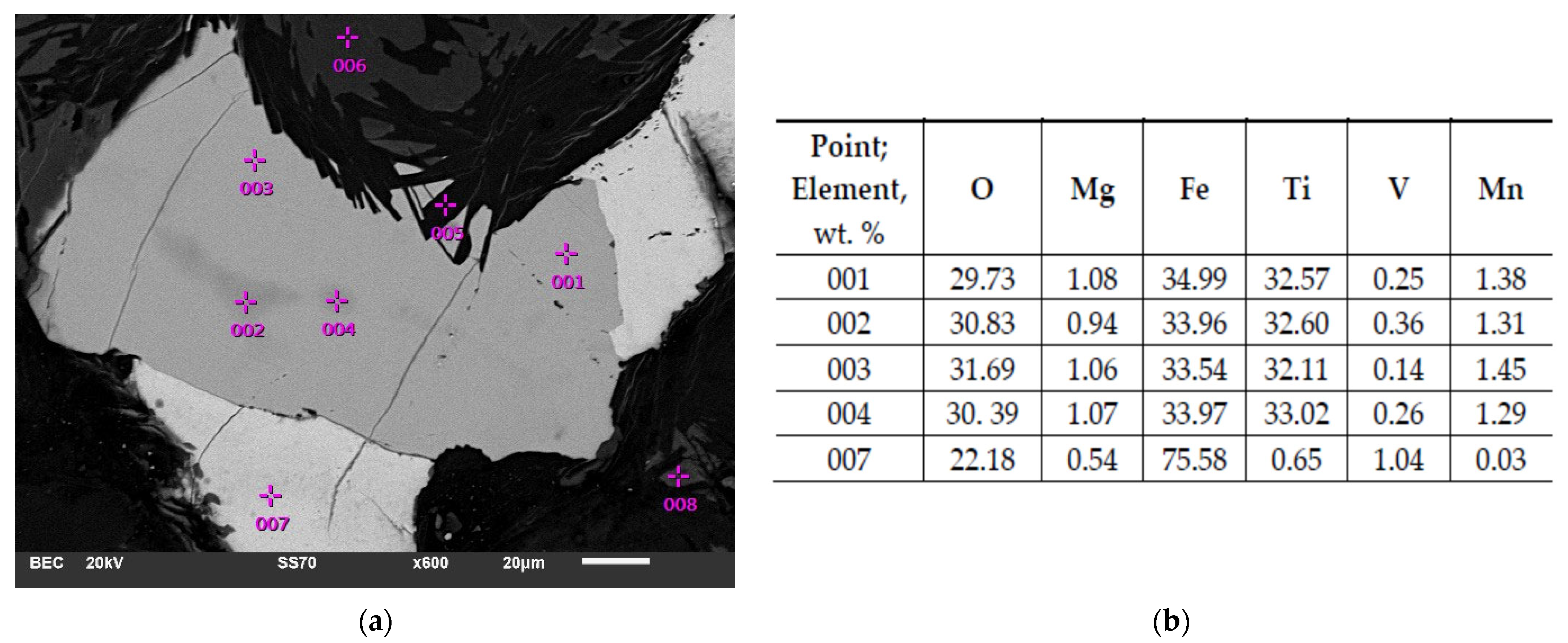
4.2. SEM-EDS Investigation
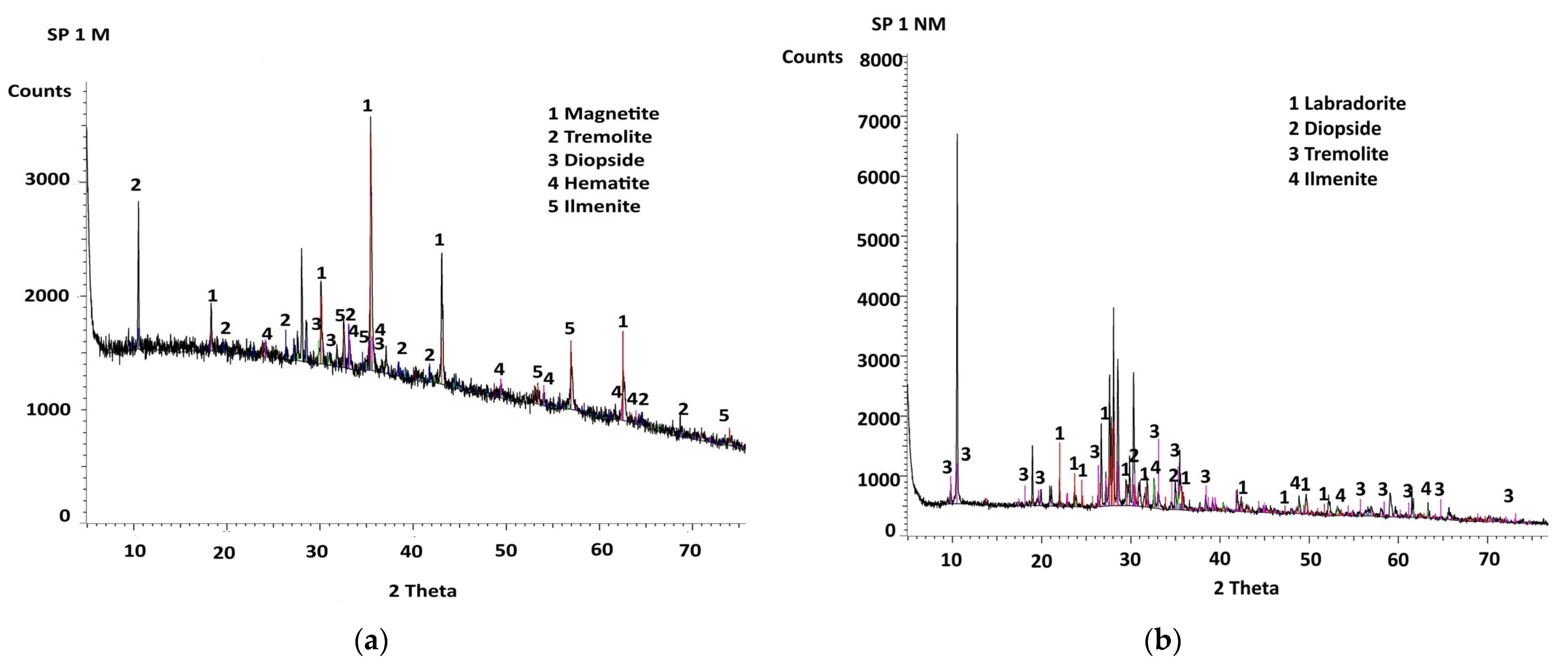
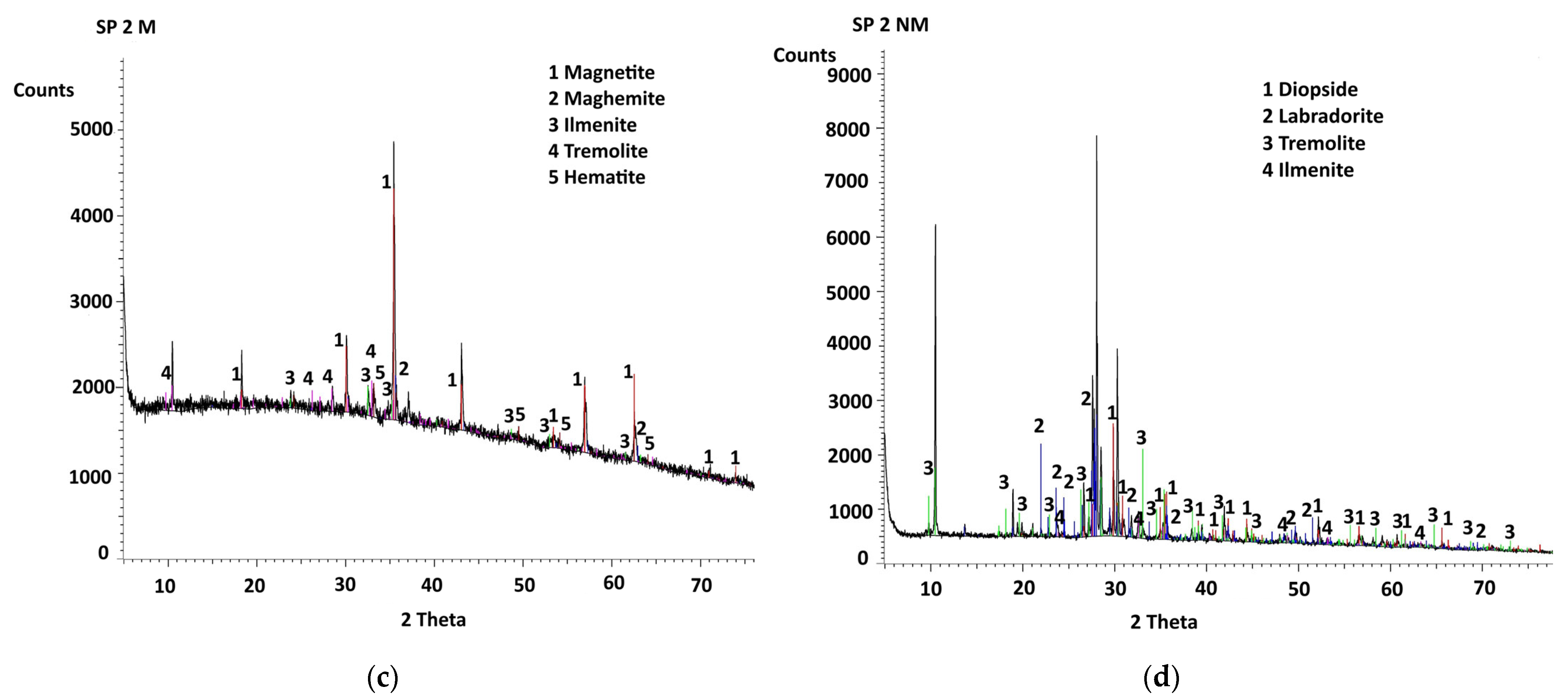
4.3. X-Ray Diffractograms
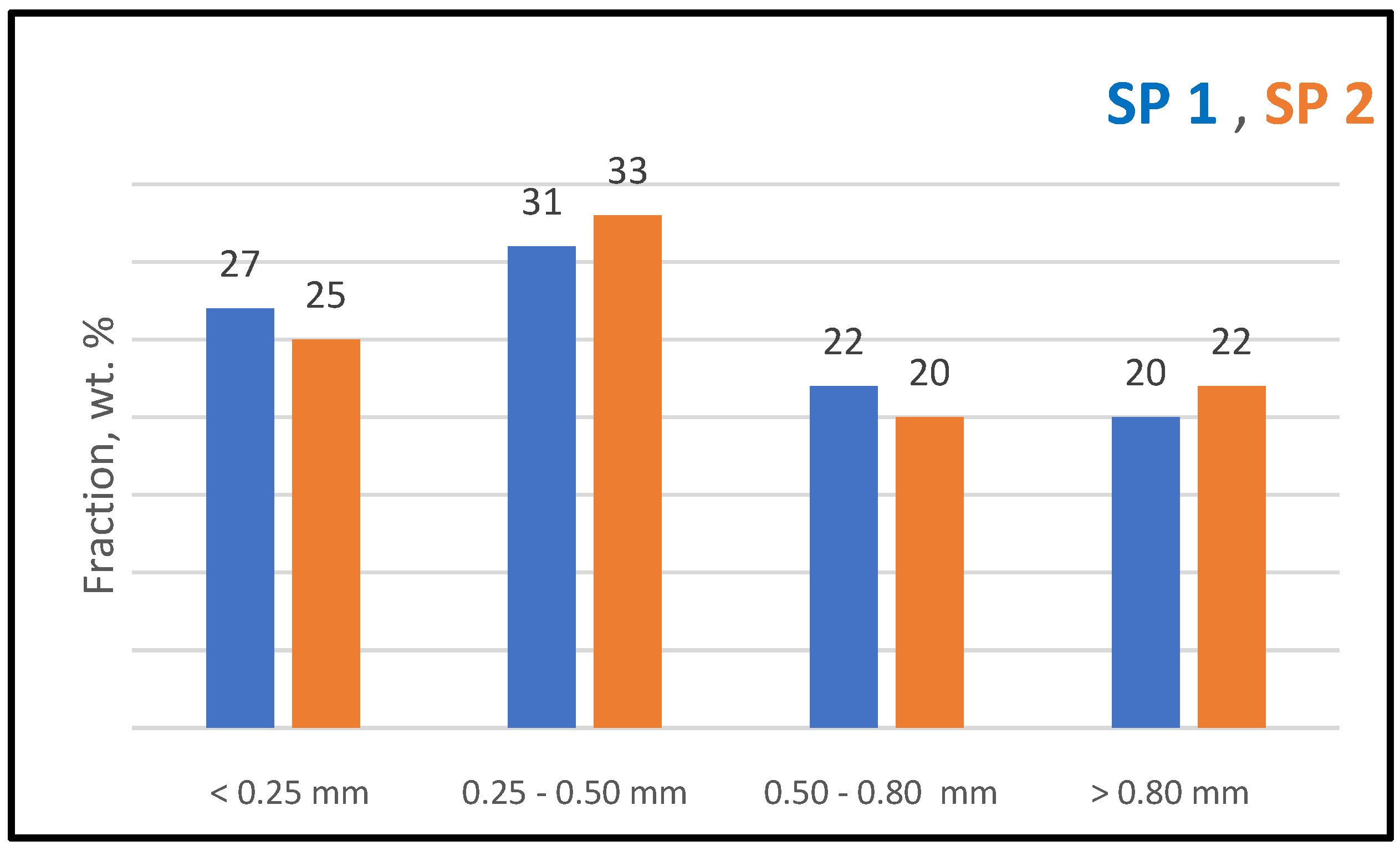
4.4. Gravity Concentration and Particle Size Distribution
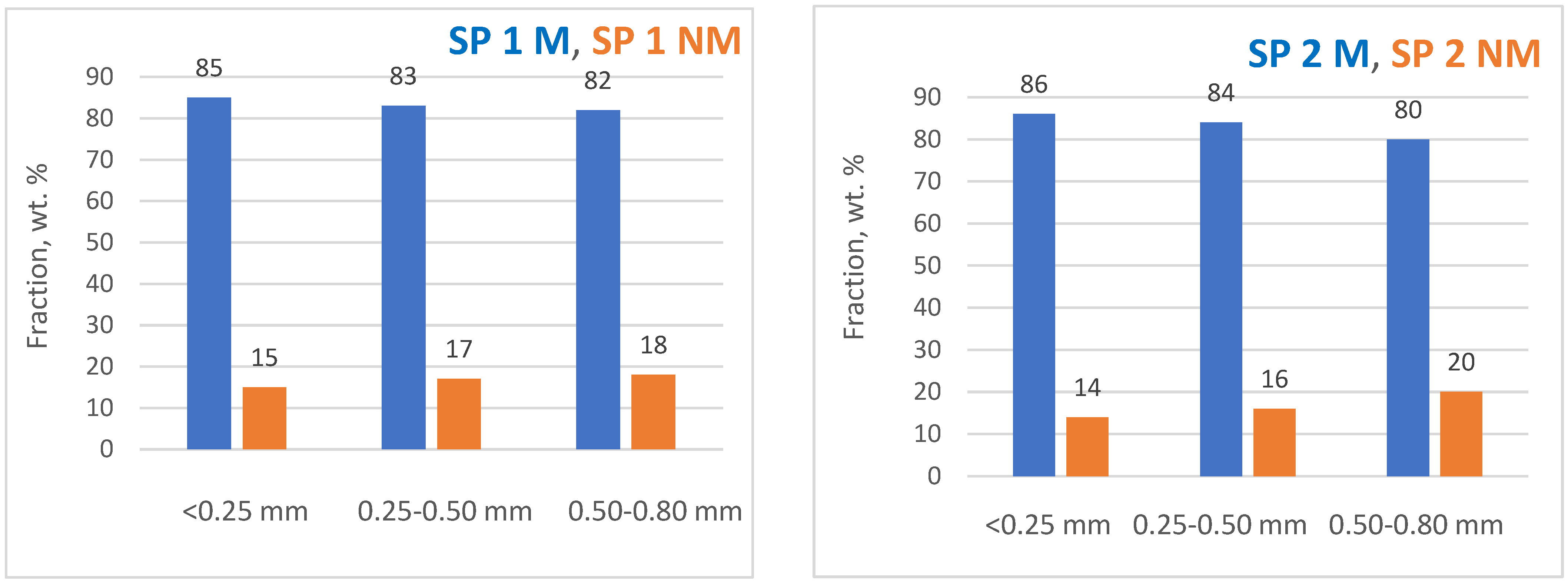
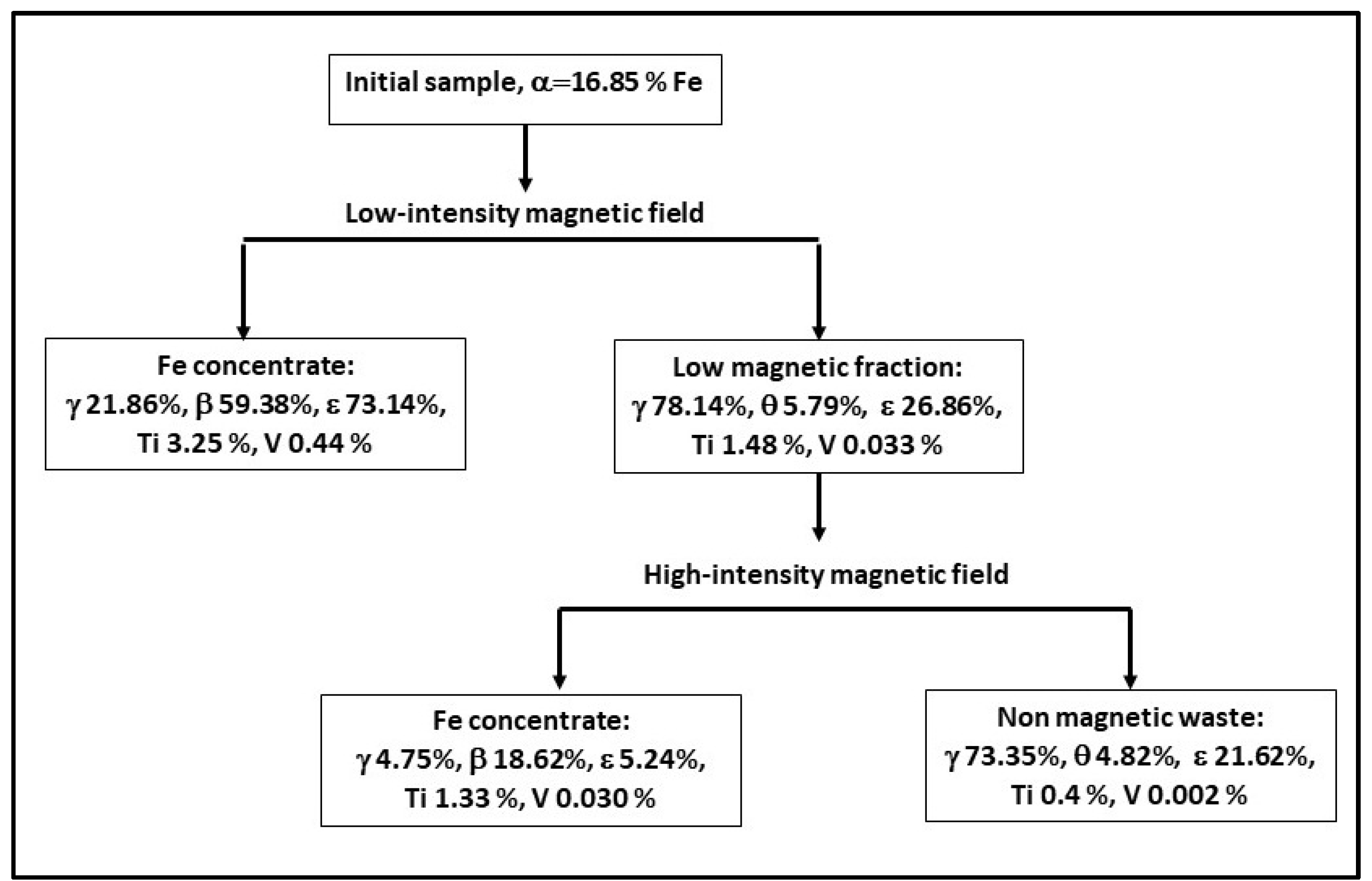
4.5. Magnetic Separation Studies and ICP MS Analyses
4.6. Test on Magnetic Beneficiation Without Predating Gravity Separation
5. Discussion
5.1. The Effect of the Oxy-Exsolution and Martitisation on Magnetic Enrichment
5.2. Economic Validation of the Featured Fe-Ti-V Concentrate
5.3. Opportunity for Rock Waste Material Handling
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDR | carbon dioxide removal |
| EDS | energy-dispersive X-ray spectroscopy |
| ER | enrichment ratio |
| ICP MS | Inductively Coupled Plasma Mass Spectrometry |
| PPL | plane polarized light |
| SEM | scanning electron microscopy |
| SP | sampling point |
| VTM | vanadium titaniferous magnetite |
| XPL | crossed polarizer |
| XRD | X-ray diffractometry |
References
- Gambogi, J. Titanium and titanium dioxide. In U.S. Geological Survey 2024, Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024; pp. 186–187. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Liu, X.; Zhang, B.; Chi, Q.; Li, D.; Zhou, J.; Liu, H.; Cheng, X.; Wu, H.; et al. Spatial distribution of the critical mineral resource element titanium in China and its influencing factors. J. Geochem. Explor. 2024, 256, 107334. [Google Scholar] [CrossRef]
- Tolcin, A.C. Titanium and titanium dioxide. In U.S. Geological Survey 2025, Mineral Commodity Summaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025; pp. 186–187. [Google Scholar] [CrossRef]
- Maldybayev, G.; Korabayev, A.; Sharipov, R.; Al Azzam, K.M.; Negim, E.-S.; Baigenzhenov, O.; Alimzhanova, A.; Panigrahi, M.; Shayakhmetova, R. Processing of titanium-containing ores for the production of titanium products: A comprehensive review. Heliyon 2024, 10, e24966. [Google Scholar] [CrossRef]
- Department of Energy. Notice of Final Determination on 2023 DOE Critical Materials List. Federal Register 88(149) (Friday, August 4, 2023 Notices), USA. 2023. Available online: https://www.federalregister.gov/documents/2023/08/04/2023-16611/notice-of-final-determination-on-2023-doe-critical-materials-list (accessed on 3 September 2025).
- European Commission. Study on the EU’s List of Critical Raw Materials (2020); Publications Office of the European Union: Luxembourg, 2020; Available online: https://op.europa.eu/en/publication-detail/-/publication/c0d5292a-ee54-11ea-991b-01aa75ed71a1/language-en (accessed on 3 September 2025).
- European Commission. COM (2023) 160 Final ANNEXES to the Proposal for a Regulation of the European Parliament and of the Council Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020; Brussels, 16 March 2023. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:903d35cc-c4a2-11ed-a05c-01aa75ed71a1.0001.02/DOC_2&format=PDF (accessed on 3 September 2025).
- Boni, M.; Bouabdellah, M.; Boukirou, W.; Putzolu, F.; Mondillo, N. Vanadium ore resources of the African continent: State of the Art. Ore Geol. Rev. 2023, 157, 105423. [Google Scholar] [CrossRef]
- Polyak, D.E. Vanadium. In U.S. Geological Survey 2025, Mineral Commodity Summaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025; pp. 192–193. [Google Scholar] [CrossRef]
- Simandl, G.J.; Paradis, S. Vanadium as a critical material: Economic geology with emphasis on market and the main deposit types. Appl. Earth Sci. 2022, 131, 218–236. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, W.; Dai, Z.; Yao, L.; Yang, L.; Zheng, J. Advancements and challenges in vanadium extraction processes from vanadium titano-magnetite and its derivatives. Resour. Conserv. Recycl. 2025, 216, 108178. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Liu, D.; Han, Y. Mineralogical reconstruction of Titanium-Vanadium hematite and magnetic separation mechanism of titanium and iron minerals. Adv. Powder Technol. 2022, 33, 103408. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Zhao, B.; Liu, Z. Extraction of titanium resources from the titanium-containing waste slag: Thermodynamic analysis and experimental verification. Calphad 2020, 71, 102211. [Google Scholar] [CrossRef]
- Hu, T.; Sun, T.; Kou, J.; Geng, C.; Wang, X.; Chen, C. Recovering titanium and iron by co-reduction roasting of seaside titanomagnetite and blast furnace dust. Int. J. Miner. Process. 2017, 165, 28–33. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, J.; Wang, W.Q.; Deng, J.; Chen, B.Y.; Yan, W.; Xiong, S.Q.; Huang, Y.; Liu, J. Flotation behaviors of ilmenite, titanaugite, and forsterite using sodium oleate as the collector. Miner. Eng. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Qu, R.; Zhang, L.; Li, W. Recent technology developments in beneficiation and enrichment of ilmenite: A review. Miner. Eng. 2024, 219, 109084. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Cao, Y.; Sun, W.; Gao, Z.; Han, H.; Chen, P.; Cao, J. Enhanced flotation separation of ilmenite from titanaugite by novel N-hydroxy-3-(4-methoxyphenyl) acrylamide collector. Appl. Surf. Sci. 2025, 707, 163672. [Google Scholar] [CrossRef]
- Gilligan, R.; Nikoloski, A.N. The extraction of vanadium from titanomagnetites and other sources. Miner. Eng. 2020, 146, 106106. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Z.; Jin, J.; Li, Y.; Han, Y. Vanadium extraction from V-bearing shale using oxidation roasting and acid leaching. Miner. Eng. 2023, 192, 107985. [Google Scholar] [CrossRef]
- Liu, G.Z.; Dai, S.J.; Bai, L.M.; Ma, Y.X.; Zhang, Y. Experimental studies on a mineral containing titanium in Baoding area. Adv. Mater. Res. 2013, 826, 34–37. [Google Scholar] [CrossRef]
- Laxmi, T.; Behera, J.R.; Rao, R.B. Beneficiation studies on recovery of ilmenite from red sediments of badlands topography, Andhra Pradesh. Adv. Sci. Lett. 2016, 22, 344–348. [Google Scholar] [CrossRef]
- Lv, J.F.; Zhang, H.P.; Tong, X.; Fan, C.L.; Yang, W.T. Innovative methodology for recovering tita-nium and chromium from a raw ilmenite concentrate by magnetic separation after modifying magnetic properties. J. Hazard. Mater. 2017, 325, 251–260. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Liu, T.; Huang, J. Characterization and Pre-Concentration of Low-Grade Vanadium-Titanium Magnetite Ore. Minerals 2017, 7, 137. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, Z.; Qiu, K.; Peng, W. Separation of Ilmenite from Vanadium Titanomagnetite by combining Magnetic Separation and Flotation Processes. Separations 2023, 10, 95. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Alizadeh, M.; Hosseini, M.R. Mineralogical and physical beneficiation studies for iron extraction from Bardaskan titanomagnetite placer deposit. J. Min. Environ. 2017, 8, 191–201. [Google Scholar] [CrossRef]
- Guo, X.; Dai, S.; Wang, Q. Influence of different comminution flowsheets on the separation of vanadium titano-magnetite. Miner. Eng. 2020, 149, 106268. [Google Scholar] [CrossRef]
- Jena, M.S.; Tripathy, H.K.; Mohanty, J.K.; Mohanty, J.N.; Das, S.K.; Reddy, P.S.R. Roasting Followed by Magnetic Separation: A Process for Beneficiation of Titano-Magnetite Ore. Sep. Sci. Technol. 2015, 50, 1221–1229. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.; Go, B.; Lee, S.; Park, S.; Jeon, H.-S. Optimized magnetic separation for efficient recovery of V and Ti enriched concentrates from vanadium-titanium magnetite ore: Effect of grinding and magnetic intensity. Powder Technol. 2021, 391, 282–291. [Google Scholar] [CrossRef]
- Maldybayev, G.K.; Korabayev, A.S.; Shayakhmetova, R.A.; Khabiyev, A.T.; Baigenzhenov, O.S.; Sharipov, R.H.; Amirkhan, A.A. Separation of iron and titanium from titanium magnetite raw materials by low-temperature treatment and magnetic separation. Case Stud. Chem. Environ. Eng. 2024, 10, 100848. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, X.; Lai, Q.; Yi, F.; Chen, L. First full-scale cHGMS separator for separation of ilmenite. Miner. Eng. 2025, 222, 109151. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Q.; Etsell, T.H. Magnetic properties of ilmenite, hematite and oils and minerals after roasting. Miner. Eng. 2002, 15, 1121–1129. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, K.; Xiang, J.; Huang, P. Upgrading ilmenite by an oxidation-magnetic separation-pressure leaching process. Bulg. Chem. Commun. 2015, 47, 1118–1123. [Google Scholar]
- Li, P.; Yu, J.; Zhu, W.; Li, Y.; Gao, P.; Han, Y.; Bai, L. An innovative process for recovery of ilmenite using mineral phase transformation followed by magnetic separation. Adv. Powder Technol. 2024, 35, 104540. [Google Scholar] [CrossRef]
- European Parliament and Council, Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020, Official Journal of the European Union, EN L Series, 2024/1252 3.5.2024. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202401252 (accessed on 3 September 2025).
- Dimitrov, Z.; Kamenov, B. Der Titanomagnetite in den Manastirhöhen, S.O. Bulgariens. Annu Directorate Natural Wealth 1941, A–1, 105–120. Available online: http://bgd.bg/REVIEW_BGS/REVIEW_BGD_2018_1-2/PDF/08_Tchoumatchenco_REV_BGS_2018_1-2.pdf (accessed on 7 September 2025). (In Bulgarian with a German Abstract).
- Kamenov, B. Petrochemical characteristics of the rocks from the Manastir heights. Annu. Sofia Univ. 1969, 61, 1–31. [Google Scholar]
- Kanurkov, G. The Iron-Ore Deposits of Bulgaria; Technika: Sofia, Bulgaria, 1988; (In Bulgarian with an Abstract in French). [Google Scholar]
- Conceição, L.T.; Silva, G.N.; Holsback, H.M.S.; Oliveira, C.F.; Marcante, N.C.; Martins, E.S.; Santos, F.L.S.; Santos, E.F. Potential of basalt dust to improve soil fertility and crop nutrition. J. Agric. Food Res. 2022, 10, 100443. [Google Scholar] [CrossRef]
- Dabovski, C. Fissure intrusions in the Srednogorie. In Structural Analysis, Mathematical and Laboratory Models; BAS Press: Sofia, Bulgaria, 1988. (In Bulgarian) [Google Scholar]
- Kamenov, B.; Tarassova, E.; Nedialkov, R.; Amov, B.; Monchev, P.; Mavroudchiev, B. New radiometric data from Late Cretaceous plutons in Eastern Srednogorie area, Bulgaria. Geochem. Miner. Pet. 2000, 37, 13–24. [Google Scholar]
- Dabovski, C.; Kamenov, B. Upper Cretaceous Magmatism in Bulgaria. In Geology of Bulgaria, 1st ed.; Zagorchev, I., Dabovski, C., Nicolov, T., Eds.; “Prof. Marin Drinov” Academic Publishing House, Bulgarian Academy of Sciences: Sofia, Bulgaria, 2009; Volume II, pp. 632–636. (In Bulgarian) [Google Scholar]
- Strahilov, D. Stress analysis and structural settings in the area of the skarn deposits Krumovo, Southeastern Bulgaria. Rev. Bulg. Geol. Soc. 2020, 81, 178–180. [Google Scholar]
- Dimitrov, I. Tectonic position of the skarn deposits from the Krumovo ore field. In Proceedings of the International Conference “80 Anniversary of the BGS”, Sofia, Bulgaria, 17–18 November 2005; pp. 39–42, (In Bulgarian with English Abstract). [Google Scholar]
- Schwellnns, C.M.; Willemse, J. Titanium and vanadium in the magnetic iron ores of the Bushveld Complex. S. Afr. J. Geol. 1943, 46, 23–38. [Google Scholar]
- Willemse, J. The geology of the Bushveld Igneous Complex, the largest repository of magmatic ore deposits in the world. In Magmatic Ore Deposits—Economic Geology Monograph Series; Wilson, H.D.B., Ed.; Society of Economic Geologists: McLean, VA, USA, 1969; Volume 4, pp. 1–22. [Google Scholar] [CrossRef]
- Harne, D.M.W.; Gerhard, V.G. Ore-forming processes in the upper part of the Bushveld complex, South Africa. J. Afr. Earth Sci. 1995, 20, 77–89. [Google Scholar] [CrossRef]
- Orberger, B.; Tudryn, A.; Wagner, C.; Wirth, R.; Fabris, J.D.; Greneche, J.M.; Morgan, R. Martitisation: Magnetite-hematite transformation and its origin and implications for industrial beneficiation through magnetic separation. In Proceedings of the 12th Biennial Society for Geology Applied to Mineral Deposits (SGA) Meeting, Uppsala, Sweden, 12–15 August 2013; pp. 283–286. [Google Scholar]
- Buddington, A.F.; Lindsey, D.H. Iron-titanium oxide minerals and synthetic equivalents. J. Pet. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Tan, W.; Liu, P.; He, H.; Wang, C.Y.; Liang, X. Mineralogy and origin of exsolution in Ti-rich magnetite from different magmatic Fe-Ti oxide-bearing intrusions. Can. Miner. 2016, 54, 539–553. [Google Scholar] [CrossRef]
- Chen, L.; Deng, G.; Wang, Z.; Zhang, K.; Cao, L.; Luo, H.; Guo, A.; Pu, F.; Luo, D.; Liang, B. Hydrogen-based direct reduction of vanadium-titanium magnetite raw ore: Process optimization and mechanism insights. Chem. Eng. J. 2025, 505, 159152. [Google Scholar] [CrossRef]
- Yan, Z.; Zheng, S.; Zhang, Y.; Zhang, Y.; Zhou, Z.; Qiao, S. Sodium carbonate roasting and mild acid leaching of vanadium titanomagnetite concentrates: Vanadium extraction and residue sodium decrease. Process. Saf. Environ. 2024, 185, 1132–1144. [Google Scholar] [CrossRef]
- Rahman, M.A.; Zaman, M.N.; Uddin, M.N.; Biswas, P.K.; Sultana, M.S. Beneficiation of Ilmenite from Ultramafic Lamprophyres of Mithapukur, Rangpur District of Bangladesh. Int. J. Min. Eng. Miner. Process 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Vametco Mine, Reference Materials. Available online: https://amis.co.za/product/fe-21-98-ti-2-98-v-3763-ppm-vanadium-bushveld-south-africa/ (accessed on 15 July 2025).
- Nachev, G.; Dimitrov, I. Relationship between the soil types the topography and the rocks undersoil in Southeast Bulgaria. Rev. Bulg. Geol. Soc. 2015, 76, 51–68. [Google Scholar]
- Koleva-Rekalova, E.; Dimitrov, I.; Valchev, B.; Iliev, T. Microfabrics and mineral composition of calcretes and dolocretes from Southeast Bulgaria. In Proceedings of the National Conference with International Participation “GEOSCIENCES 2012”, Sofia, Bulgaria, 13–14 December 2012; pp. 87–88. [Google Scholar]
- Chakalov, K.; Popova, T.; Popov, N.; Savov, V.; Bratkova, S.; Angelova, G.; Metodieva, C. Bioremediation of mine waste with chemically extracted or biosolobilized humic substances and zeolites. In Proceedings of the 4th National Conference of BHSS with International Participation, Sofia, Bulgaria, 8–10 September 2016; pp. 385–398. [Google Scholar]
- Buss, W.; Hasemer, H.; Ferguson, S.; Borevitz, J. Stabilisation of soil organic matter with rock dust partially counteracted by plants. Glob. Change Biol. 2024, 30, e17052. [Google Scholar] [CrossRef]
- Vanderkloot, E.; Ryan, P. Quantifying the effect of grain size on weathering of basaltic powders: Implications for negative emission technologies via soil carbon sequestration. Appl. Geochem. 2023, 155, 105728. [Google Scholar] [CrossRef]
- Dimitrov, I.; Sachkov, D.; Valchev, B.; Vasileva, K. Geochemical features of calcretized areas from the Tundzha Depression, Southeast Bulgaria. Rev. Bulg. Geol. Soc. 2010, 71, 25–39, (In Bulgarian with an English Abstract). [Google Scholar]
- Reershemius, T.; Kelland, M.E.; Jordan, J.S.; Davis, I.R.; D’Ascanio, R.; Kalderon-Asael, B.; Asael, D.; Suhrhoff, T.J.; Epihov, D.Z.; Beerling, D.J.; et al. Initial validation of a soil-based mass-balance approach for empirical monitoring of enhanced rock weathering rates. Environ. Sci. Technol. 2023, 57, 19497–19507. [Google Scholar] [CrossRef] [PubMed]

| Sampling Point | Particle Size, mm | Designation |
|---|---|---|
| Kopanica sampling point SP 1 | 0–0.25 | SP1-1 |
| 0.25–0.50 | SP1-2 | |
| 0.50–0.80 | SP1-3 | |
| >0.80 | SP1-4 | |
| Rock Chapel sampling point SP 2 | 0–0.25 | SP2-1 |
| 0.25–0.50 | SP2-2 | |
| 0.50–0.80 | SP2-3 | |
| >0.80 | SP2-4 |
| Point; Element, wt. % | O | Mg | Fe | Al | Ti | V | Mn | Nb | Ta |
|---|---|---|---|---|---|---|---|---|---|
| 001 | 31.52 | 1.17 | 34.51 | 31.12 | 0.31 | 1.38 | |||
| 002 | 31.06 | 1.25 | 34.31 | 32.03 | 0.21 | 1.14 | |||
| 003 | 40.50 | 8.54 | 19.16 | 30.98 | 0.09 | 0.73 | |||
| 004 | 25.93 | 70.09 | 0.91 | 2.21 | 0.86 | ||||
| 005 | 24.94 | 71.36 | 0.60 | 1.30 | 0.81 |
| Metal; Sample | SP1-1 M | SP1-2 M | SP1-3 M | SP1-1 NM | SP1-2 NM | SP1-3 NM |
|---|---|---|---|---|---|---|
| Al, % | 1.95 | 1.73 | 2.19 | 4.12 | 3.50 | 3.32 |
| Ca, % | 2.68 | 2.29 | 6.85 | 9.00 | 11.4 | 10.9 |
| Fe, % | 40.2 | 42.7 | 20.5 | 7.71 | 6.78 | 5.92 |
| K, % | 0.07 | 0.07 | 0.09 | 0.12 | 0.12 | 0.12 |
| Mg, % | 1.97 | 2.18 | 5.54 | 5.11 | 6.57 | 6.10 |
| Mn, ppm | 2863 | 2534 | 2261 | 2909 | 2297 | 2049 |
| Na, % | 0.30 | 0.32 | 0.46 | 0.74 | 0.65 | 0.61 |
| Ti, % | 3.31 | 2.97 | 1.39 | 2.68 | 0.87 | 0.57 |
| V, ppm | 4021 | 4390 | 1960 | 311 | 357 | 310 |
| Zn, ppm | 320.9 | 318.0 | 168.0 | 180.0 | 128.5 | 93.6 |
| Metal; Sample | SP2-1 M | SP2-2 M | SP2-3 M | SP2-1 NM | SP2-2 NM | SP2-3 NM |
| Al, % | 1.39 | 2.42 | 2.31 | 3.87 | 3.23 | 3.07 |
| Ca, % | 0.36 | 1.40 | 3.44 | 8.35 | 10.2 | 11.2 |
| Fe, % | 51.6 | 65.2 | 34.7 | 10.3 | 7.35 | 7.43 |
| K, % | 0.03 | 0.07 | 0.09 | 0.10 | 0.13 | 0.14 |
| Mg, % | 0.65 | 1.89 | 3.21 | 5.07 | 6.49 | 6.75 |
| Mn, ppm | 2012 | 2998 | 2096 | 2552 | 2019 | 1956 |
| Na, % | 0.06 | 0.21 | 0.24 | 0.58 | 0.52 | 0.50 |
| Ti, % | 3.31 | 4.98 | 2.76 | 1.70 | 0.75 | 0.43 |
| V, ppm | 4653 | 5843 | 3031 | 360 | 359 | 381 |
| Zn, ppm | 371.6 | 527.8 | 283.5 | 105.0 | 66.3 | 58.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panayotova, M.; Dimitrov, I.; Sofronieva, A. Initial Characterization of Titanium and Vanadium-Rich Magnetite from the Manastir Heights in Southeast Bulgaria Aiming at Future Environmentally Friendly Beneficiation. Minerals 2025, 15, 964. https://doi.org/10.3390/min15090964
Panayotova M, Dimitrov I, Sofronieva A. Initial Characterization of Titanium and Vanadium-Rich Magnetite from the Manastir Heights in Southeast Bulgaria Aiming at Future Environmentally Friendly Beneficiation. Minerals. 2025; 15(9):964. https://doi.org/10.3390/min15090964
Chicago/Turabian StylePanayotova, Marinela, Ivan Dimitrov, and Angelika Sofronieva. 2025. "Initial Characterization of Titanium and Vanadium-Rich Magnetite from the Manastir Heights in Southeast Bulgaria Aiming at Future Environmentally Friendly Beneficiation" Minerals 15, no. 9: 964. https://doi.org/10.3390/min15090964
APA StylePanayotova, M., Dimitrov, I., & Sofronieva, A. (2025). Initial Characterization of Titanium and Vanadium-Rich Magnetite from the Manastir Heights in Southeast Bulgaria Aiming at Future Environmentally Friendly Beneficiation. Minerals, 15(9), 964. https://doi.org/10.3390/min15090964








