1. Introduction
For a long time, China’s coal resources, the dominant energy consumption structure system [
1], have inevitably produced a large amount of coal gangue solid waste in its mining, washing, and utilization processes [
2,
3,
4,
5]. On average, the production of a ton of coal will produce 10% to 20% gangue [
4]. In 2024, China’s raw coal production was 4.76 billion tons, accompanied by more than 800 million tons of gangue production. In the Ordos area alone, newly added coal gangue amounts to more than 100 million tons every year, causing serious environmental problems [
5,
6]. Coal gangue is often regarded as a type of low-value industrial waste. At present, coal gangue is mainly disposed of by stockpiling or backfilling. It wastes a lot of land, producing a large number of coal gangue mountains. What is more important is the existing hidden dangers, such as landslides and spontaneous combustion. In addition, long-term stacking releases heavy metal ions, contaminating soil, groundwater, and the atmosphere [
7,
8,
9]. At present, China’s comprehensive utilization of coal gangue technology has not yet achieved a fundamental breakthrough. The traditional use of coal gangue is concentrated in generating electricity, the production of construction and refractory materials, the backfilling of empty areas, and other aspects explored in previous studies [
6,
8,
10]. First of all, the use of coal gangue in power generation requires a relatively high calorific value. Therefore, gangue with a calorific value below 4500 kcal/kg is unsuitable. When blended with high-calorific-value coal, it not only introduces a low level of resource utilization, but produces a large amount of fly ash waste that is more difficult to deal with [
11]. Secondly, the use of coal gangue in the production of construction and refractory materials requires high firing temperatures. This entails a high energy consumption. Moreover, the added value of products is extremely low [
12]. Additionally, when coal gangue is used in molding backfilling, the cost of the backfill technology is much greater than the benefits. The average cost is 80 CNY/ton, yielding almost no added value [
13]. Therefore, backward technology and a high cost are two main challenges in the traditional use of coal gangue, which also brings about difficult decentralized use, low resource utilization, low value-added products, and so on [
14,
15].
In recent years, in the context of the “dual carbon” policy, promoting the comprehensive utilization of solid waste, including coal gangue, has been of great concern to the CPC Central Committee and all walks of life [
16,
17,
18]. The related departments have issued a number of corresponding policies, such as “the Guiding Opinions on the 14th Five-Year Bulk Solid Waste Comprehensive Utilization” and “People’s Republic of China Solid Waste Pollution Prevention and Control Law” to encourage the research and development of new technologies in solid waste comprehensive utilization and promote the comprehensive utilization of solid waste [
19,
20]. From the point of view of elemental value, coal gangue is not only an industrial solid waste but also a resource enriched with valuable elements (carbon, silicon, aluminum, iron, potassium, titanium, etc.), which have potential application value in the preparation of alumina, silica, catalysts, aerogels, and molecular sieves [
21]. The difficulty is that the mineral components of coal gangue are complex (including quartz, kaolinite, feldspar, pyrite, a small amount of mica, etc.). These mineral phases are highly stable and often intergrown, which makes direct utilization difficult. Also, it is often difficult to efficiently separate out the pure Si, Al, and Fe fractions with the traditional direct acid leaching or simple roasting process [
22]. Kong et al. studied the extraction of aluminum and iron from coal gangue. Through simple calcination and hydrochloric acid leaching, the extraction rate can reach 90% and 60%, respectively. Unfortunately, without taking the treatment of silicon elements into consideration, after the acid dissolves a large number of residues, the silicon elements still cannot be dealt with. In addition, coal gangue is not deeply dissociated, which results in the low leaching rate of aluminum [
23]. Xiao et al. investigated the extraction of aluminum and silicon in coal gangue under high-temperature acid leaching conditions. The results showed that heating would promote the extraction of aluminum, aluminum, and iron components. The results showed that warming will promote the dissolution of aluminum and iron, but the quartz component was still very stable. The extraction efficiency of silicon remains low, and considerable unreacted silicon phases persist in the residue [
24]. Yang et al. found that for high-iron gangue, the iron leaching rate could reach 87.64% through roasting and acid leaching, but the extraction rate of aluminum was still low [
25]. Similarly, Xu et al. studied the extraction of Al and Si from coal gangue through high-temperature fusion. Although this method could achieve a certain extraction rate, the high energy consumption and strict requirements on equipment limited its industrial scale-up [
26]. Some scholars attempted to apply high-temperature alkali roasting to directly turn Si or Al phases into easily leachable materials. However, the product was impure and the rate of recycling was low due to the interaction between Si and Al during the reaction process. The study by Wang et al. proposed a combined process of roasting, alkali leaching, and the Bayer method to extract Si and Al from high-alumina gangue. Although this method improved the extraction efficiency, the complex process, high energy consumption, and strict equipment requirements limited its industrial application [
27]. Some studies have shown that a stepwise or graded extraction process might be more efficient [
28]. By controlling different temperature segments or using different activators, the valuable elements in coal gangue can be selectively extracted in stages, realizing the efficient enrichment of different elements [
29].
In this context, this paper selects the typical high-silica–aluminum coal gangue in the Ordos area as the raw material, and puts forward a step-by-step extraction process of “alkaline activation aids depth activation priority separation of silica acid leaching to extract aluminum”. The process makes use of soda ash that preferentially combines with the silica component in the gangue at a certain temperature and can interrupt the silica–aluminum connection in the coal gangue in depth. Then, priority water leaching activation products are used in stages to obtain high-purity liquid water glass products (sodium silicate). This is followed by the use of hydrochloric acid leaching of the remaining aluminum-rich residue to obtain high-purity aluminum chloride solution, in order to achieve the efficient separation of silica–aluminum elements in the gangue and the high value-added use of the gangue. This process differs from existing research on the direct acid leaching of coal gangue by simple roasting [
30,
31,
32]; the route proposed here shows clear advantages. The Bayer process requires long digestion at a high temperature and pressure, which not only consumes a large amount of energy but also produces by-products that reduce alumina quality [
27]. Acid roasting, in turn, causes serious equipment corrosion and often leads to silica gel formation that hinders leaching [
30]. By contrast, our process only needs 30 min of activation at 800 °C under normal pressure, transforming aluminosilicate into soluble sodium silicate and acid-leachable nepheline. This stepwise strategy avoids strong acid treatment, reduces unwanted by-products, and improves both efficiency and product purity. Therefore, this study highlights these advantages and investigates the mechanisms of Si and Al separation during activation and leaching. Through XRD, SEM, FTIR, and other means of characterization, we analyze the elemental migration paths and the mechanism of mineral phase transformation during the activation process in depth, design orthogonal experiments, and discuss the influencing factors of different experimental parameters on the separation of silica and aluminum and the purification of the elements based on the response surface methodology. Through this systematic study, the efficient extraction route of coal gangue is optimized to provide guidance for industrial application, and to offer new ideas for the green, efficient, high extraction rate, and high value-added application of coal gangue in the whole country.
2. Materials and Methods
2.1. Materials
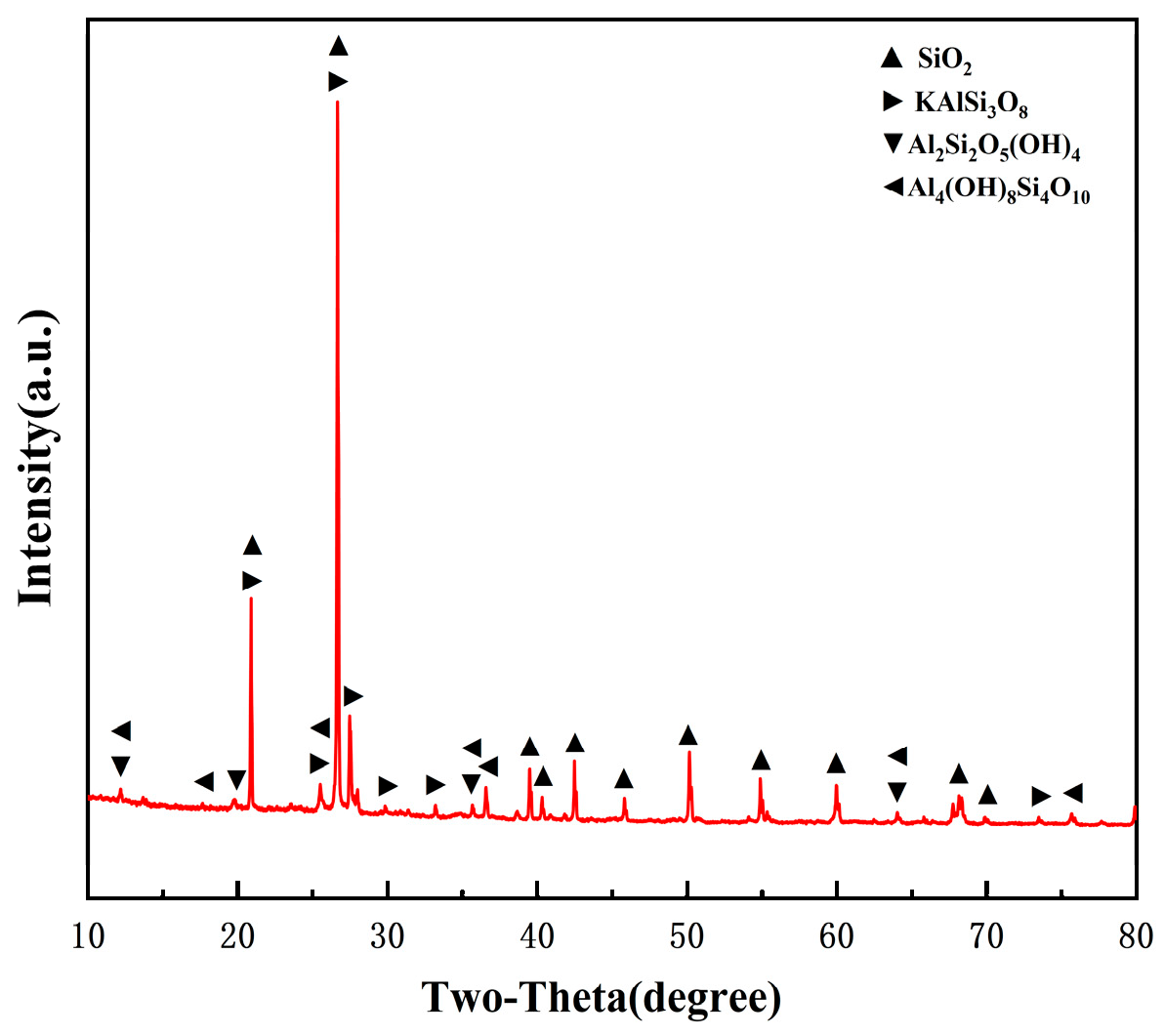
The coal gangue is extracted from several large-scale coal mines in Ordos, and the main mineral composition includes quartz (SiO
2), kaolinite (Al
2Si
2O
5(OH)
4), feldspar (KalSi
3O
8), a small amount of pyrite (FeS
2), and mica (Kal
2(AlSi
3O
10)(OH)
2). Its mineralogical characteristics are identified by XRD, as shown in
Figure 1. The main elemental composition and proximate analysis results are listed in
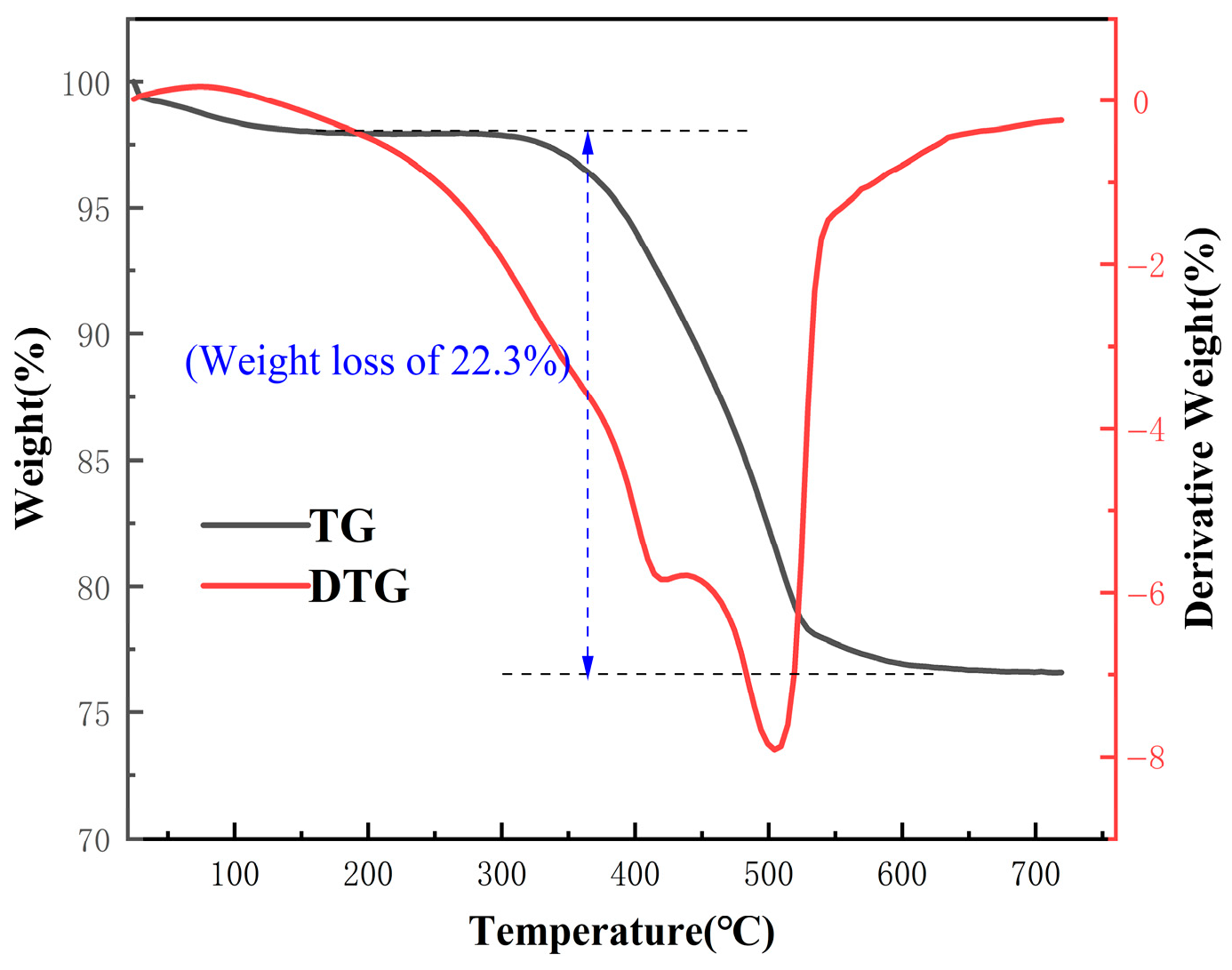
Table 1. In addition, the thermal decomposition behavior of the raw coal gangue is illustrated in
Figure 2. According to the analysis, it can be classified as a typical high-silica–alumina coal gangue.
HCl, NaOH, anhydrous ethanol (C2H6O), and sodium carbonate (Na2CO3) are all Analytical Reagent (AR) grade and purchased from Tianjin Hengxing Chemical Reagent Manufacturing Company Limited (Tianjin, China), and deionized water is made using a laboratory high-purity water machine.
2.2. Experiments
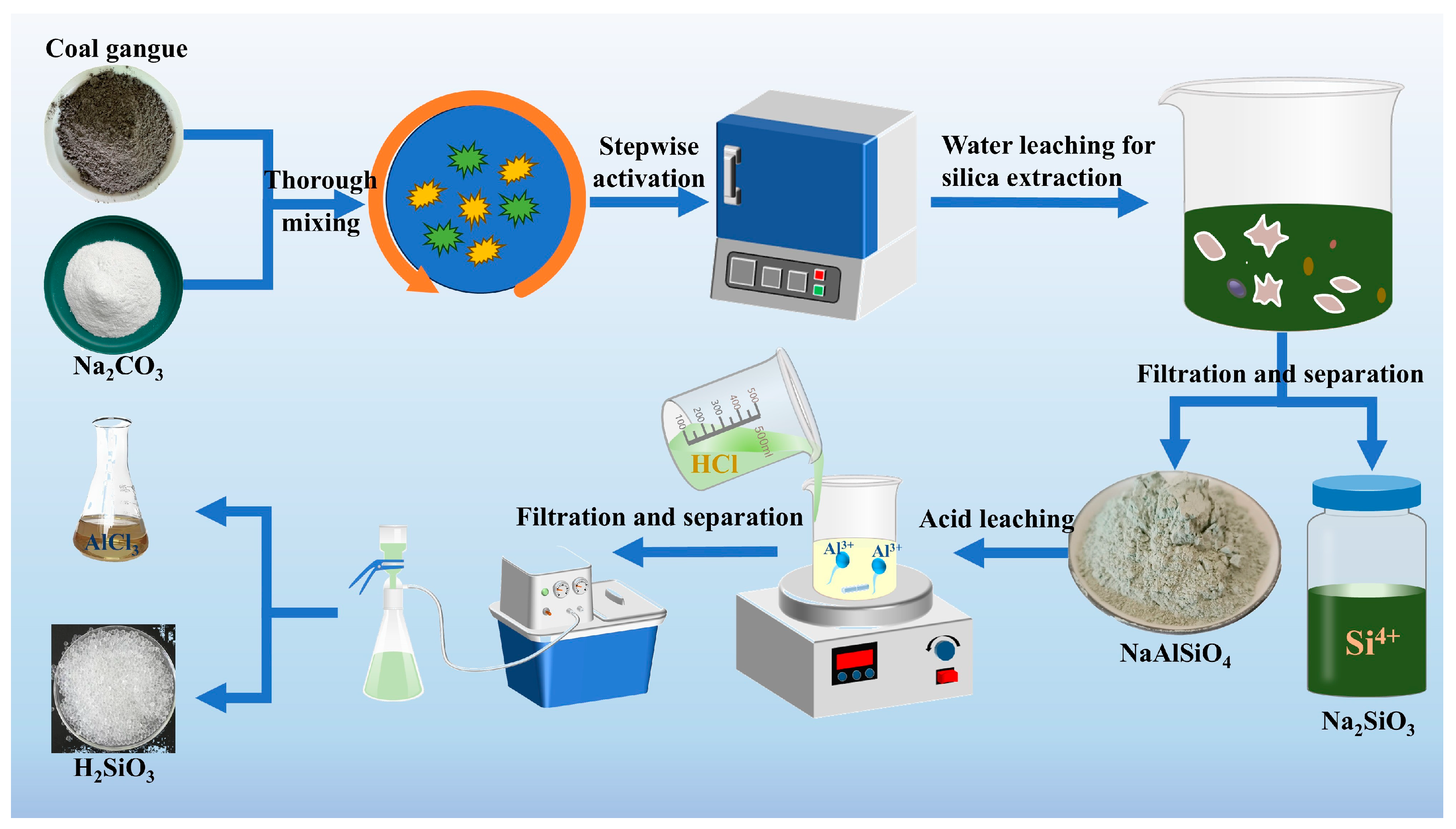
2.2.1. Deep Activation and Silicon Extraction Experiment
Pre-treatment of coal gangue: First of all, the coal gangue is crushed and ground to pass a 120-mesh sieve. The sample was then dried in an oven at 105 °C for 12 h. The coal gangue and sodium carbonate powder were mixed uniformly at mass ratios of 1:0.5, 1:0.8, 1:1, and 1:1.2. The mixtures were placed into covered alumina crucibles to minimize the volatilization of Na2CO3 and then heated in a muffle furnace under ambient air atmosphere. The activation was carried out under different reaction times (40 min, 50 min, 60 min, and 70 min) and temperatures (700 °C, 750 °C, 800 °C, and 850 °C).
After activation, the roasted products were cooled to room temperature, and the composition of the mineral phases of the activated products was analyzed by means of XRD, FTIR, etc., in order to determine the dissociation of silica–aluminum elements in the minerals [
33]. The activated samples were dissolved in water to obtain high-purity sodium silicate solution. Response surface methodology (RSM) was employed to analyze the influence mechanism of each control condition by checking the extraction rate of silicon elements, achieving the optimal reaction conditions.
2.2.2. Aluminum Element Extraction Experiments
After the completion of silica extraction, the aluminum-rich residue was leached using hydrochloric acid. An AlCl
3 solution was obtained through acid leaching under constant stirring in a water bath. The aluminum extraction experiments were conducted at different reaction times (3 h, 4 h, 5 h, 6 h, and 7 h), temperatures (50 °C, 60 °C, 70 °C, 80 °C, and 90 °C), and acid concentrations (10%, 20%, and 30%). After the reaction, solid–liquid separation was performed, and the filtrate was analyzed to determine the Al concentration and leaching rate by ICP-OES. It was also necessary to record the aluminum content in the residue in order to calculate the recovery rate of Al element. Based on these results, the best process for aluminum extraction by acid leaching can be determined [
34], and the overall procedure for additive-assisted activation and subsequent extraction of silicon and aluminum is illustrated in
Figure 3.
2.3. Testing and Characterization
(1) ICP-MS test: An ICP spectrometer (Agilent 7700x, Agilent Technologies, Santa Clara, CA, USA) is used to test the content of Si and Al elements and calculate the leaching rate of Si and Al elements.
(2) SEM characterization: A scanning electron microscope (TESCAN MIRA-3, TESCAN, Brno, Czech Republic) is used to observe the micro-morphological structure of the samples before and after gangue activation.
(3) XRD characterization: X-ray diffraction patterns were recorded using a diffractometer (Rigaku SmartLab SE, Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation (λ = 1.5406 Å, 40 kV, and 40 mA). The scans were collected over a 2θ range of 10–90°, with a step size of 0.02°, and a scanning rate of 5° min−1. The diffraction data were used to identify phase compositions and to analyze the mineral transformation mechanism before and after activation.
(4) FTIR test: The chemical surface structure and composition of the samples before and after activation are tested using a Fourier transform infrared spectrometer (Nicolet iS20, Thermo Fisher Scientific, Waltham, MA, USA).
(5) SiO
2 and Al
2O
3 contents in the alkaline leaching residue are determined using a atomic absorption spectrometer (contrAA-700, Analytik Jena GmbH, Jena, Germany) [
35].
The leaching rate of silicon and aluminum is calculated according to the following equation:
where R is the leaching rate (%); C
1 and C
2 are the mass percentages of silicon or aluminum in the raw material and residue (wt.%); and M
1 and M
2 are the mass of raw material and residue, respectively (g).
3. Results and Discussion
3.1. Using Response Surface Methodology to Explore the Optimal Activation Conditions
In order to study the activation effect of gangue additives and the key parameters affecting the efficiency of silica extraction, this study adopts the response surface methodology (RSM) to optimize the three factors, such as calcination temperature, calcination time, and the mass ratio of gangue to sodium carbonate. The dissolution rate of sodium silicate is used as the response variable to construct a regression model and to elucidate the effects of each key factor [
36].
3.1.1. Experimental Design
A modified Box–Behnken Design (BBD) design table is constructed using a partial factorial combination of three factors and four levels. A total of 15 experimental runs is designed. The experimental factors and levels are summarized in
Table 2, and the Box–Behnken design runs together with the corresponding silicon leaching rate responses are presented in
Table 3.
3.1.2. Regression Model and Analysis of Variance
The RSM takes the dissolution rate of silica Y1 as the response value, and a quadratic polynomial regression model is established as follows:
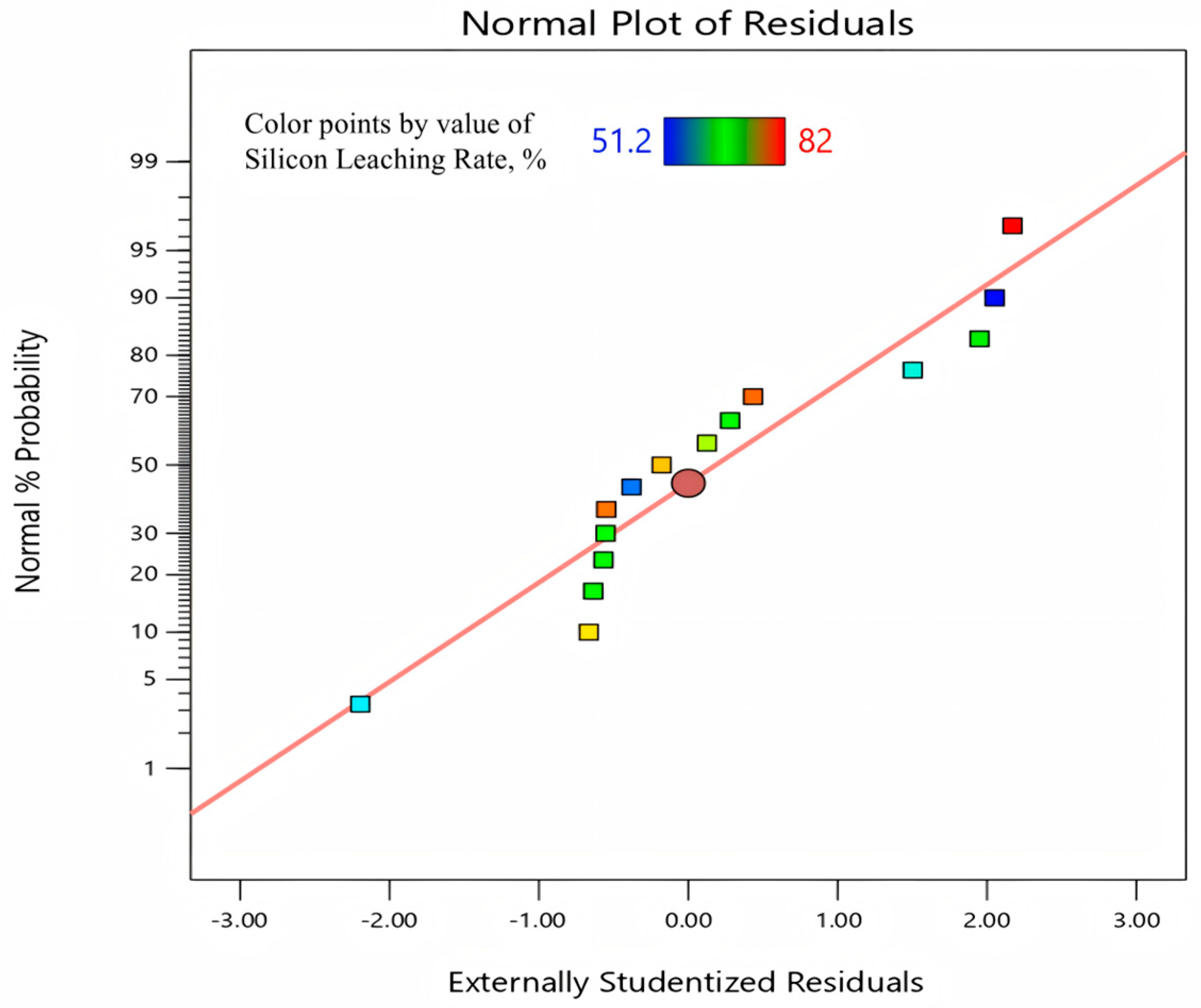
The normal plot of residuals graph (as shown in
Figure 4) shows the fitting degree of the experimental residual values to the standard normal distribution. It is found that most of the data points are distributed along the red fitted line without anomalies deviating from the line. The calculated fitting degree is 99.997%, which indicates that the fitting degree is high and basically conforms to the normal distribution. Therefore, it can be used for optimizing the subsequent prediction. For example, the model predicted a Si extraction efficiency of 82.3% at 800 °C, 30 min, and a gangue-to-Na
2CO
3 ratio of 1:0.8, while the experimental validation under identical conditions yielded 82.1%, with a relative deviation of only 0.2%. This close agreement demonstrates the high reliability of the RSM model.
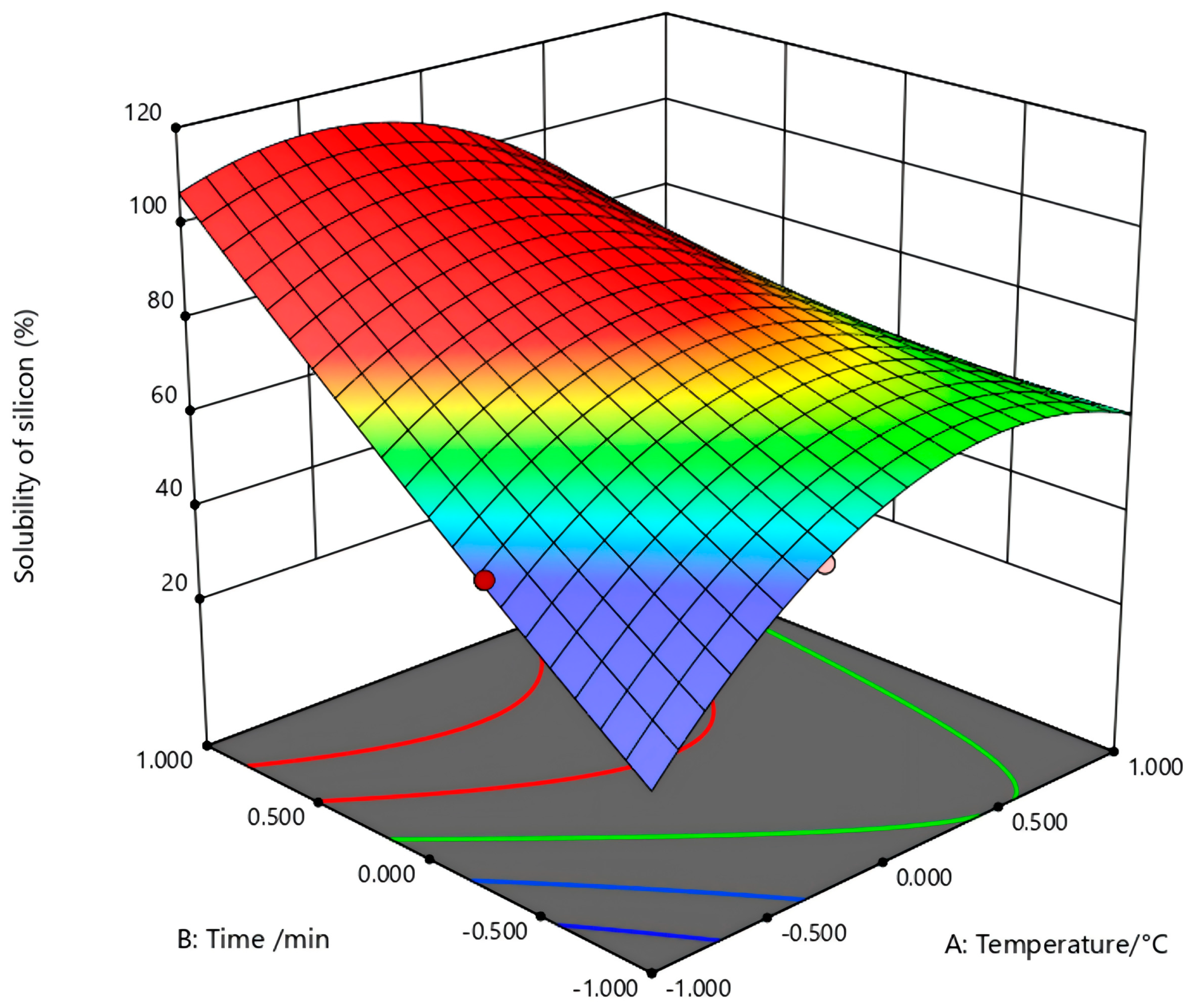
In the figure (
Figure 5) of temperature (A) and activation time (B) to the silicon dissolution rate, the sodium carbonate ratio is 0.8. With the increase in temperature, the dissolution rate increases significantly, reaching a peak at 800 °C, indicating the most effective activation at this temperature. Beyond 800 °C, a further temperature increase led to a decline in the dissolution rate. With the activation time rising from 20 min to 30 min, the dissolution rate is obviously improved. When the time continues to extend, the increase in dissolution efficiency is flat. Compared with this, the temperature has a great influence on the silicon dissolution rate, which is the core factor in the transformation of the mineral phase. Instead, the activation time is a non-dominant factor.
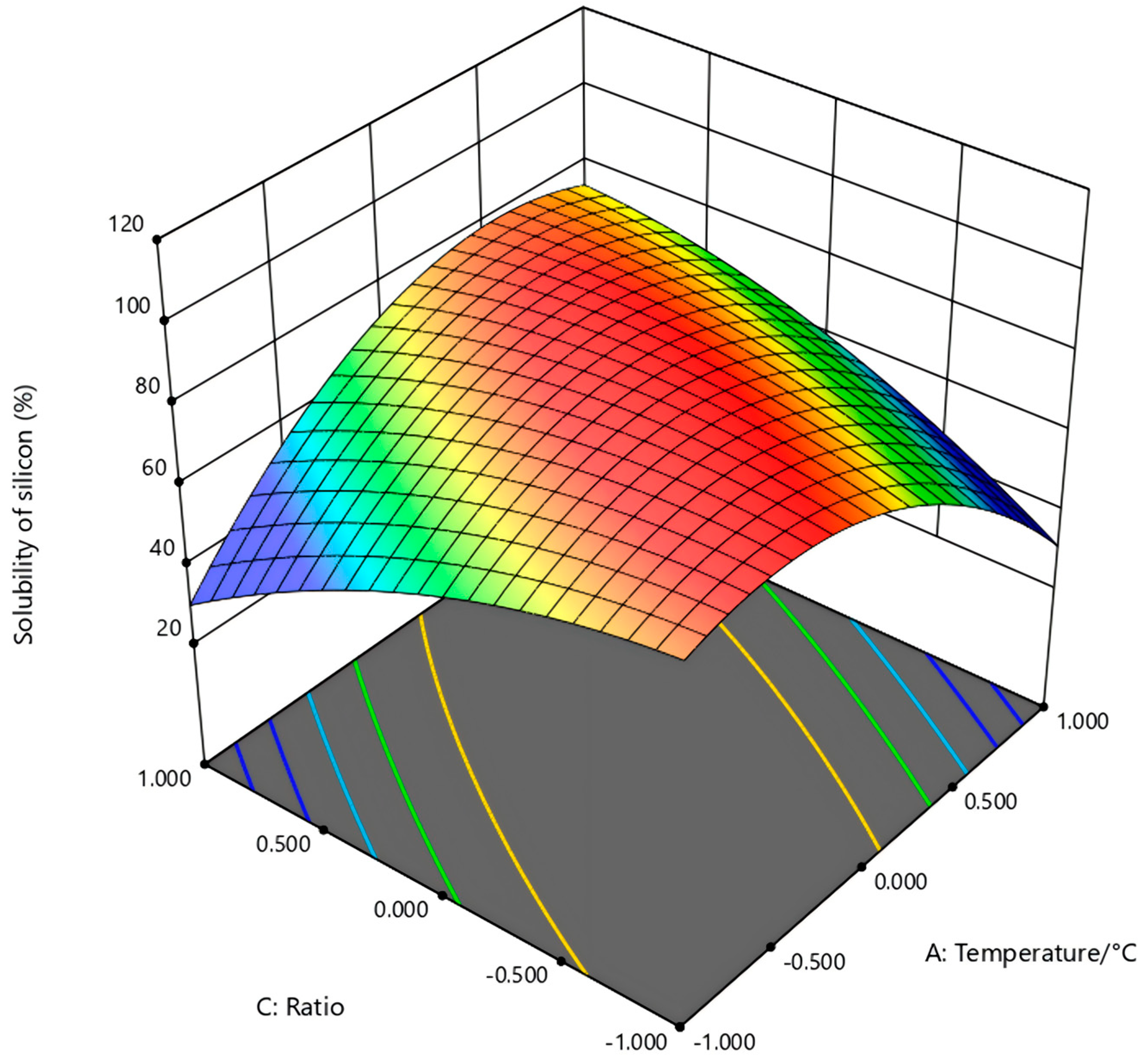
In the figure (
Figure 6) of temperature (A) and sodium carbonate ratio (C) to the silicon dissolution rate, at a fixed activation time of 30 min, the silicon extraction rate increased significantly as the sodium carbonate ratio rose from 0.4 to 0.8. However, further increasing the ratio led to a decline in extraction efficiency. The change process explains that the excess of additives does not enhance the activation effect. It is speculated that the generated product may be wrapped around the surface, hindering ion leaching, thereby leading to a decrease in the silicon dissolution rate. This reflects a typical nonlinear response to the alkali ratio; the optimum is located near 800 °C and a gangue:Na
2CO
3 ratio of 1:0.8.
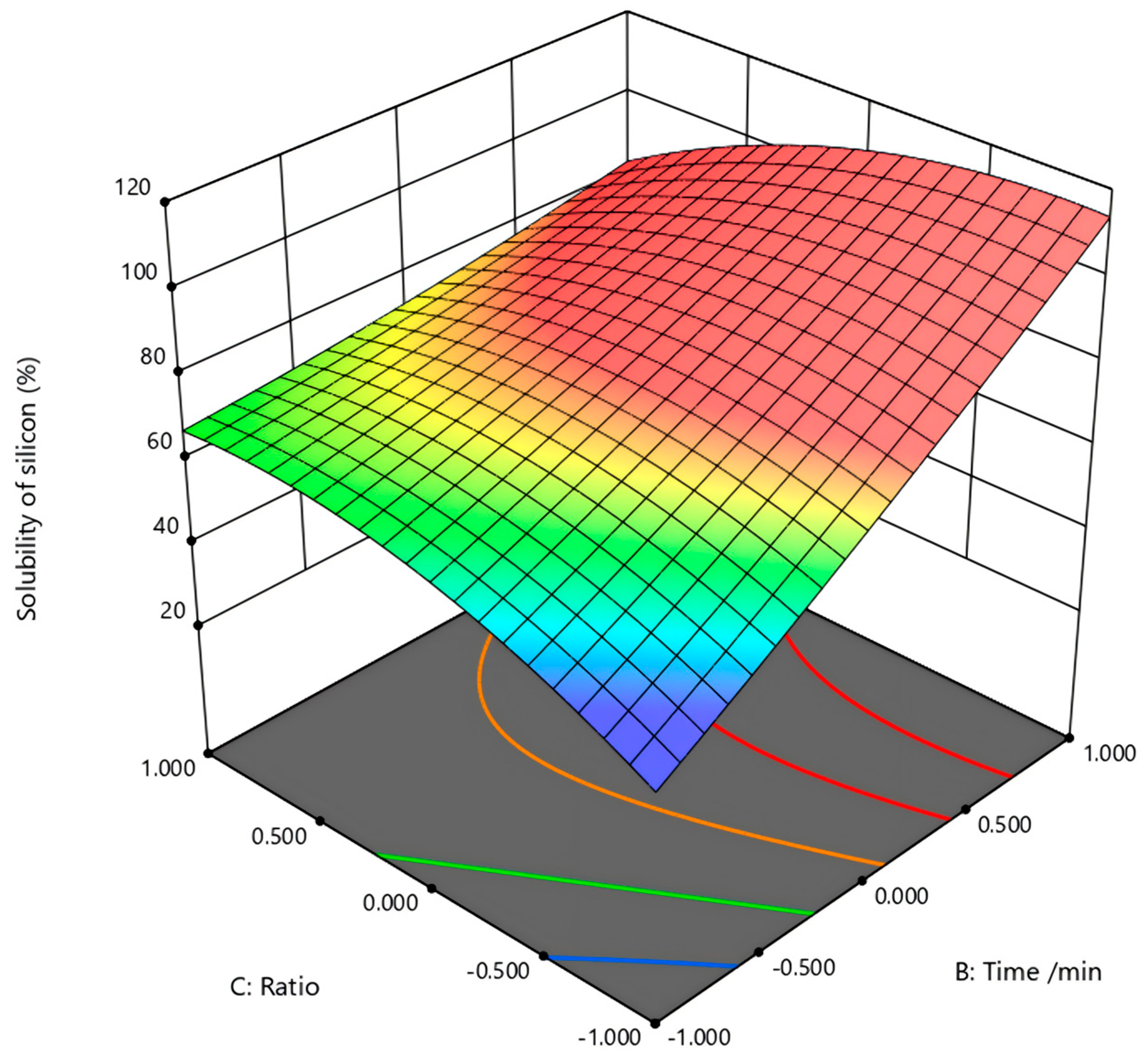
In the figure (
Figure 7) of activation time (B) and sodium carbonate ratio (C) to the silicon dissolution rate, under a fixed activation temperature of 800 °C, the response surface is relatively flat, indicating minor effects of activation time and alkali ratio under 800 °C. The result proves again that the effect of temperature on this experiment is much greater than other factors. The XRD results showed that higher temperatures promoted kaolinite dehydroxylation and enhanced reactivity with Na
2CO
3, leading to nepheline and soluble silicate formation, which improved Si extraction. Increasing Na
2CO
3 dosage further facilitated this process, while extending activation time beyond 30 min had little effect, consistent with the SEM observations of stable particle morphology.
Overall, through the optimization experiments of response surface methodology, it is concluded that the response surface peaks at the activation time of 30 min, 800 °C, and the ratio of 1:0.8. At this time, the water immersion experiments measured the highest Si dissolution rate of 82.1%, which confirmed the reliability of the model prediction. In addition, we learn that the activation temperature is the most critical factor for controlling the activation effect and silica dissolution rate, followed by the sodium carbonate ratio, whereas the activation time shows a relatively smaller influence. To further investigate the mechanism of temperature on the activation path and physical phase transformation, it is necessary to deeply study the evolution rules of the microstructure with the help of XRD, FTIR, and SEM.
3.2. X-Ray Diffraction (XRD) Analysis
Based on the response surface methodology analysis, it is evident that the calcination activation process of the gangue additives is mainly affected by the activation temperature. Therefore, under the optimal coal gangue to sodium carbonate ratio of 1:0.8, the XRD study focused on investigating the intrinsic effects of different activation temperatures (700–850 °C) on mineral phase transformation during activation. The experiment uses the muffle furnace to heat in stages. In the first stage, the samples were heated at 10 °C/min to 500 °C and kept for 20 min to promote the decomposition of the kaolinite structure. In the second stage, the samples were heated at 5 °C/min to the target temperature and activated for 30 min. Subsequently, the samples were collected and subjected to XRD analysis.
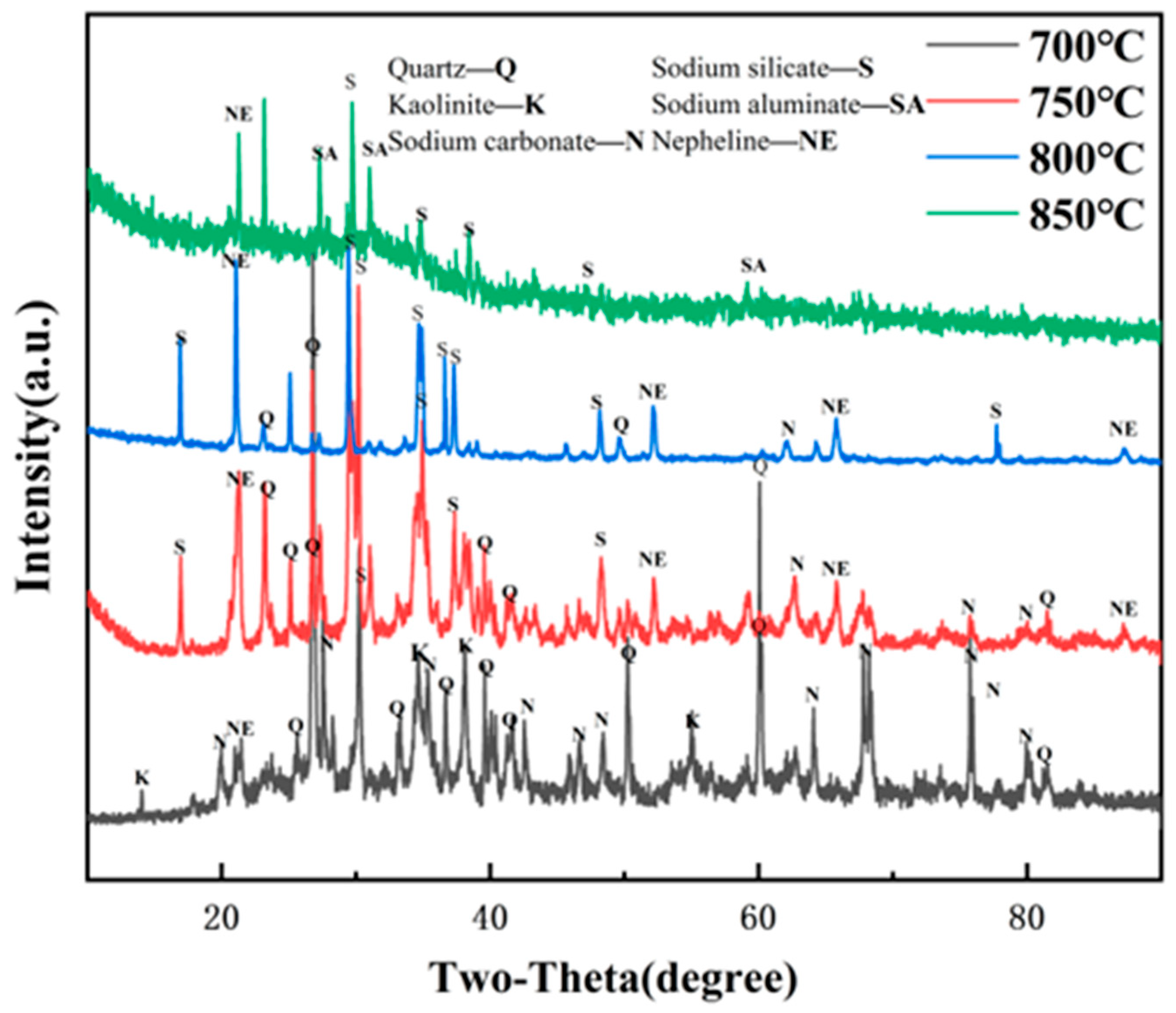
As shown in
Figure 8, after activation at 700 °C, the sample is mainly composed of quartz, sodium carbonate, a very small amount of kaolinite, and nepheline phases. The diffraction peaks are very obvious, indicating that at this temperature quartz minerals have not been involved in the reaction, but kaolinite has been basically decomposed into amorphous metakaolin. When a very small amount of the nepheline phase is generated, the reaction has just begun. With the activation temperature increasing to 750 °C, the main peaks of nepheline and quartz began to weaken. The characteristic peak of kaolinite can no longer be seen, meaning that at this moment, kaolinite has been decomposed completely. At the same time, the peak of sodium carbonate decreases remarkably as well. Sodium silicate peaks also appeared, which may be related to the reaction of part of the quartz and sodium carbonate. When the activation temperature is 800 °C, the main phase is the nepheline phase and sodium silicate. The characteristic peak is obvious and a crystallization effect is great. There exists a very small number of weak quartz peaks. Other peaks can hardly be seen. These results indicate that the aluminum–silicon structure has been completely destroyed and involved in the reaction, and the activation effect is good [
37].
When the activation temperature is 850 °C, the XRD diagram is mainly the amorphous phase diffuse peaks. The crystal structure is obviously destroyed, leaving only a trace of sodium silicate and part of the characteristic peaks of sodium aluminate.
From what has been discussed, we can infer that the activation additives are melted at this temperature, and the melting point of the reaction product (sodium aluminate) is low and begins to transform into the glassy amorphous structure. Therefore, under the influence of the sodium carbonate activation additive, the gangue will experience kaolinite dehydroxylation—nepheline phase generation—sodium silicate enrichment—nepheline phase decomposition—glassy sodium aluminate formation, and the optimal activation temperature is approximately 800 °C, but lower than 850 °C, otherwise the amorphous structure will be stable, making subsequent leaching difficult.
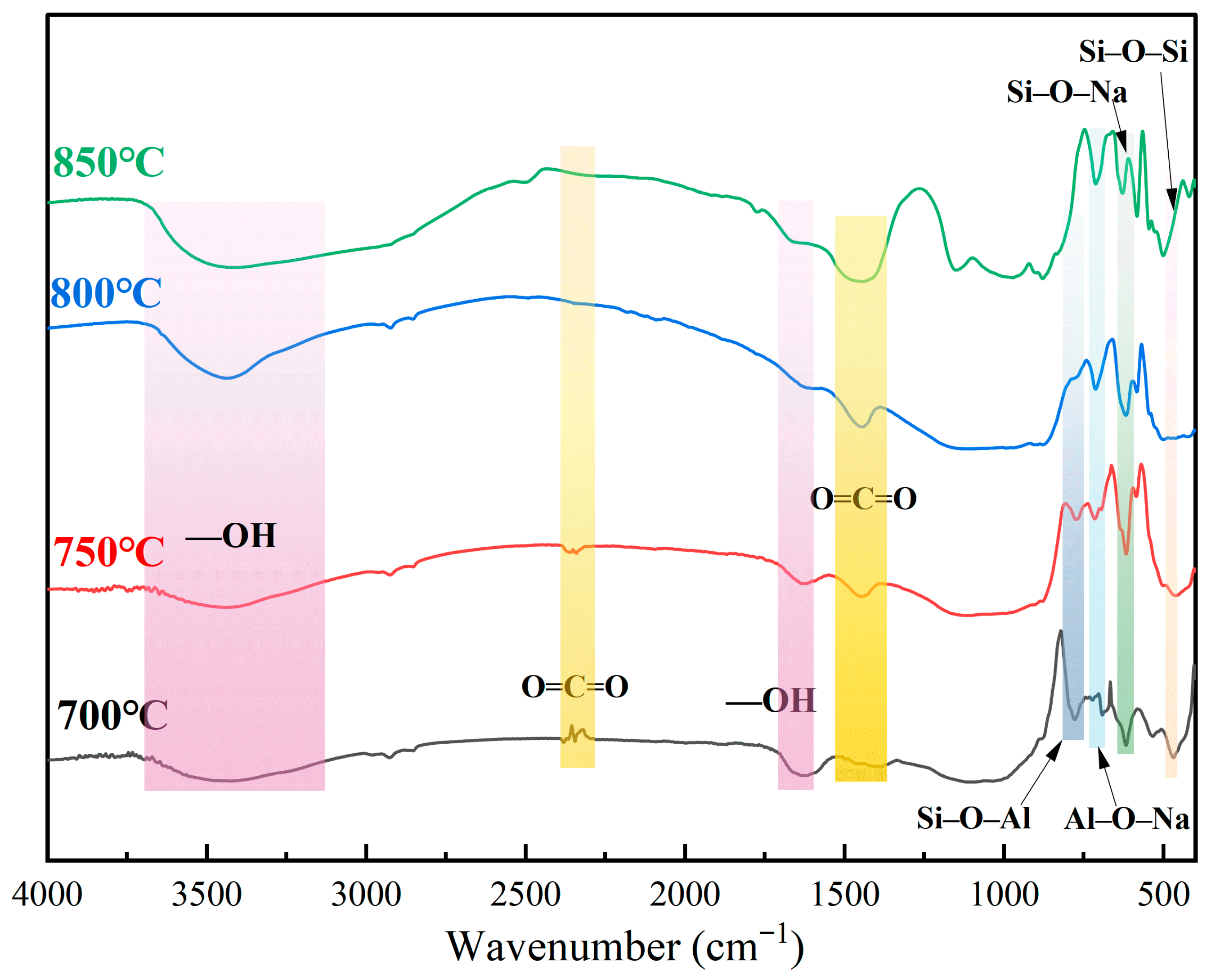
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
In order to further verify the structure transformation process of XRD analysis, FTIR tests are carried out on the products at different calcination temperatures (700–850 °C) and the results are shown in
Figure 9. With the increase in the activation temperature, the stretching vibration peaks (structural water) at 3690–3200 cm
−1 and the H-O-H bending vibration peaks at 1630 cm
−1 gradually weaken. These peaks have almost disappeared at 800 °C. These characteristics indicate that the structural hydroxyl group is removed: Starting from 750 °C, the typical Si-O-Na bond and Al-O-Na bond absorption peaks appear in the sample, indicating that at this time the nepheline phase and sodium silicate begin to form, and the higher the temperature rises, the stronger the vibration peaks become. The typical quartz Si-O-Si vibration peaks weaken at 800 °C, meaning that with the temperature rising, quartz and sodium carbonate react and generate sodium silicate. At the same time, when the temperature increases, the O=C=O vibration peaks weaken, but also verify that sodium carbonate gradually decomposes and becomes involved in the reaction. When the temperature reaches 850 °C, the absorption peaks of the low-wavenumber bands become broader and even merge, which verifies the formation of disorder and the diversity of the amorphous structure and reflects the reconstruction of the crystal structure and the migration of the components [
37].
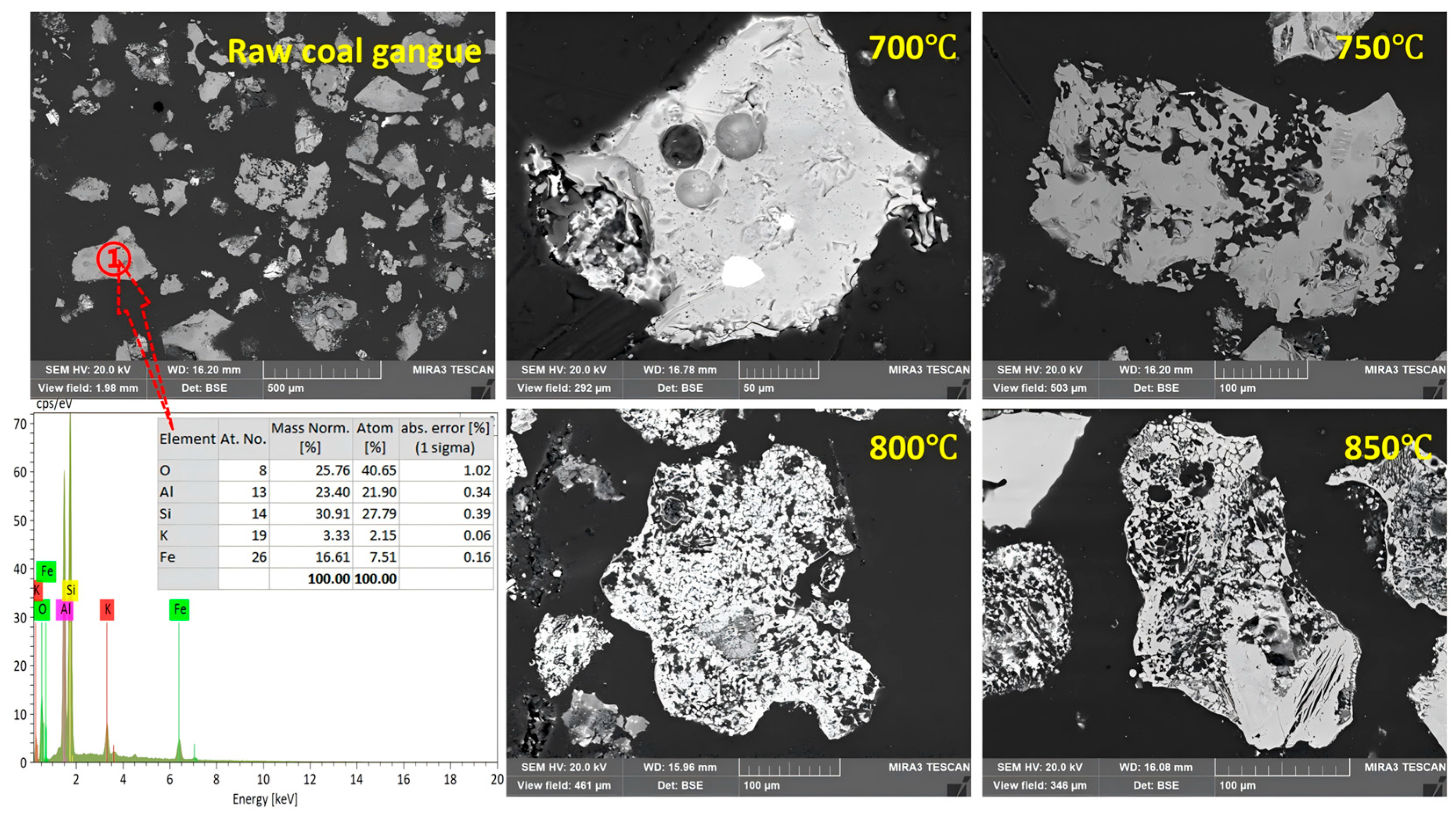
3.4. Field Emission Scanning Electron Microscopy (SEM) Microstructure and Morphological Features Analysis
Field emission scanning electron microscopy analysis is carried out on the raw coal gangue and activation products at different temperatures (700–850 °C) to observe the microstructural characteristics of the sample surface during the activation process and further activation mechanism. Its micro-morphology is shown in
Figure 10. First of all, the morphological features of coal gangue raw ore are mainly irregular limp ore and a dense surface. A variety of minerals are consecutively embedded, and the monomer’s degree of dissociation is low. From the Energy Dispersive Spectroscopy (EDS) analysis, it can be seen that coal gangue is mainly composed of O, Si, Al, and Fe. It is apparent that its main minerals are kaolinite, quartz, and a small trace of iron pyrite. When the activation temperature reaches 700 °C, the gangue sample’s surfaces expand and attach sodium ions and there is deposition on part of the sample’s surface, suggesting that, at this time, kaolinite has been close to the completion of dihydroxylation, and sodium ions begin to combine with the unsaturated defect site’s aluminum atoms as well as silicon atoms in the defective metakaolinite. When the temperature reaches 750 °C, the surfaces of the particles show significant collapse and form a porous structure. At this moment, sodium ions combine with aluminum and silicon, which form new crystals of the nepheline phase. Its structure is relatively loose. At 800 °C, with sodium ions being further eroded, in turn, the internal structure of the mineral is seriously damaged. In addition, the quartz crystals begin to connect with Na ions, generating sodium silicate. After the completion of erosion, the morphology is similar to honeycomb. The surface is porous and loose, and the porosity is obviously enlarged. It shows that at this time Na
2CO
3 is completely reacted, generating the main crystalline phases Na
2SiO
3 and NaAlSiO
4. At the same time, it releases a large amount of CO
2, promoting the formation of a pore structure. When the temperature rises to 850 °C, sodium ions diffuse into the interior of the particles, leading to the breakage and recombination of the Si-O-Al bond. The crystalline phase of NaAlSiO
4 breaks down, and the scaffold begins to disintegrate. It can be noticed that the surfaces of partial particles are melted, which ultimately leads to the reorganization of the structure and transformation into an amorphous glassy phase. At this stage, molten glassy phases precipitate on the particle surfaces, leading to densification and pore blockage. Such microstructural changes decrease the accessible surface area and hinder the penetration of the leaching solution, thereby accounting for the reduced dissolution efficiency observed at temperatures above 850 °C. It turns out that the reaction is most active at an activation temperature of 800 C or so, and the generated structure of the crystalline phase is porous and loose, which is conducive to the following leaching and separation of Al and Si.
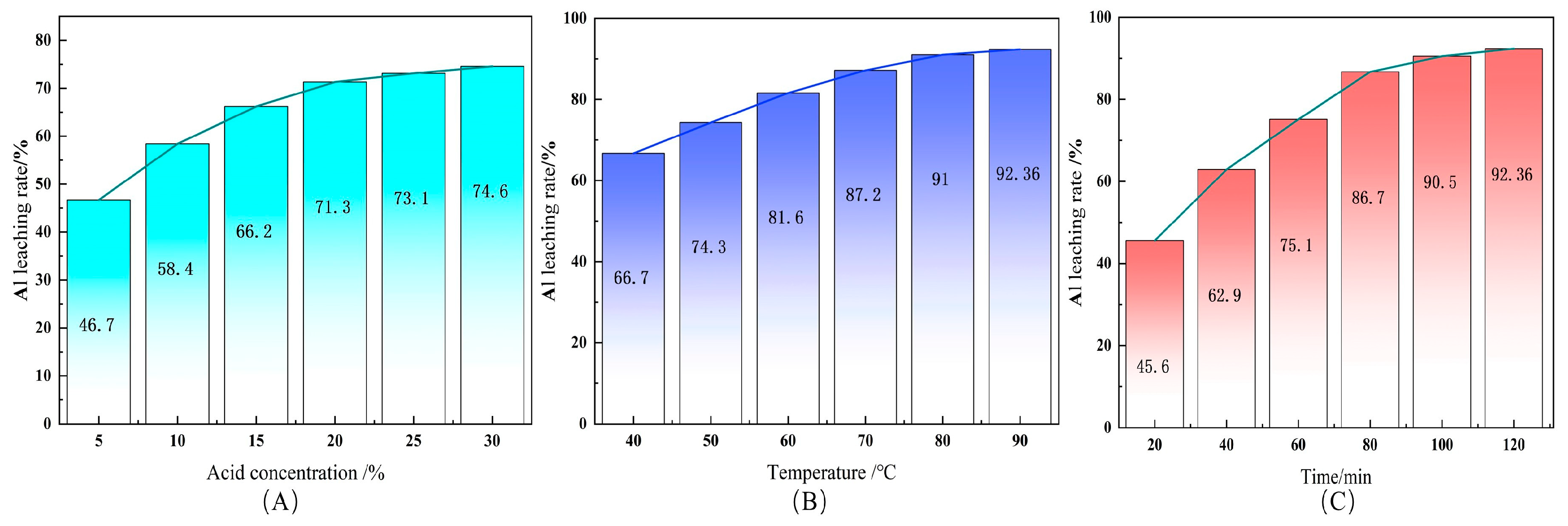
3.5. Experimental Study of the Acid Leaching of Aluminum
In order to systematically reveal the dissolution of aluminum components in the roasting products of sodium carbonate, a one-factor experiment is conducted, which studies how acid concentration, leaching temperature, and leaching time cause the effect and patterns to the dissolution rate of aluminum. After finishing dissolving silica, the solid phase (mainly nepheline powder) is removed and hydrochloric acid is used for leaching. Next, the liquid phase is obtained and the concentration of aluminum ions and tailing residues is tested using ICP to calculate the dissolution rate of aluminum ions [
38]. The results are shown in
Figure 11.
(1) The effect of acid concentration on the aluminum dissolution rate
As shown in
Figure 11A, the higher the acid concentration is, the higher the dissolution rate of aluminum ions will be. When the acid concentration rises from 5% to 25%, the extraction rate increases significantly. Then, when the acid concentration continues to rise, the dissolution rate tends to be flat. The changes indicate that a higher acid concentration is helpful for dissolving the nepheline phase of NaAlSiO
4. However, when the solution is close to being saturated, continuing to increase the acid concentration does not enhance the leaching efficiency.
(2) The effect of leaching temperature on the aluminum dissolution rate
As shown in
Figure 11B, with the temperature rising from 40 °C to 90 °C, the dissolution rate of aluminum, in turn, increases from 66.7% to 92.36%. The higher the temperature, the faster the diffusion rate will be. The dissolution rate increases clearly, in particular, between 60 °Cand 80 °C. Afterwards, continuing to raise the temperature has a smaller influence on the dissolution rate, indicating that the temperature is crucial to the leaching.
(3) The effect of leaching time on the aluminum dissolution rate
As shown in
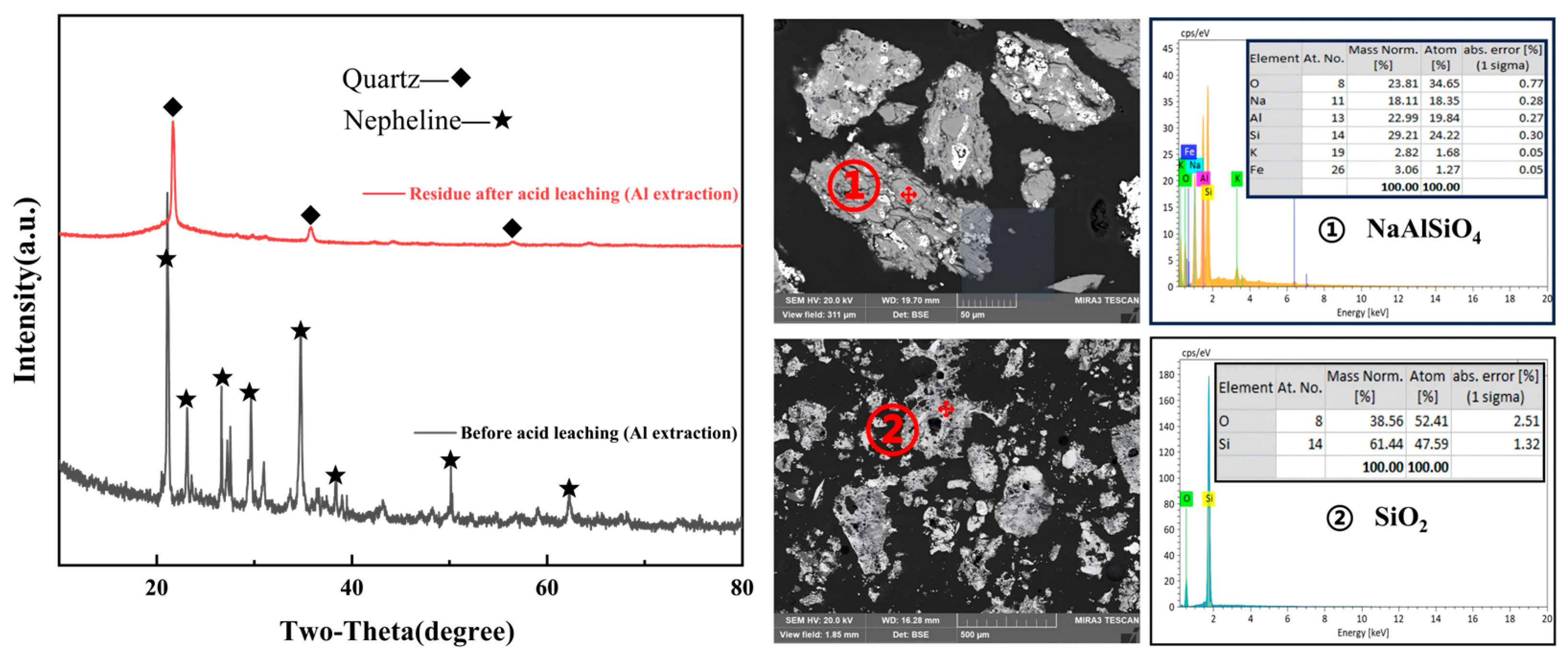
Figure 11C, with the time extending from 10 min to 120 min, the dissolution rate of aluminum gradually increases. In the first 60 min, it increases rapidly and the reaction is relatively violent. After 60 min, it increases slightly slowly, meaning that the reaction is near the equilibrium point, which belongs to the typical diffusion-controlled leaching. As shown in
Figure 12, the phase and morphological evolution of the samples before and after acid leaching clearly confirm the selective dissolution of alumina-bearing minerals under the optimized conditions. The XRD results reveal that nepheline (NaAlSiO
4), the primary alumina-containing phase, is almost completely decomposed during leaching, while quartz remains largely unchanged, owing to its chemical stability, indicating that alumina is preferentially extracted and a silica-rich residue is left behind. The SEM observations further support this conclusion: the particle surfaces before leaching are relatively dense and compact, whereas after leaching they exhibit rough, porous morphologies, which can be attributed to the removal of aluminum phases. The corresponding EDS spectra verify a significant reduction in Al content in the leached residue with Si becoming the dominant element, which is in agreement with the formation of SiO
2. These findings collectively demonstrate that acid leaching effectively dissolves nepheline and enables efficient aluminum release into solution, while simultaneously enriching the residue with stable silica phases.
According to what is mentioned above, the acid leaching of aluminum is simultaneously controlled by the acid concentration, leaching temperature, and leaching time. The temperature increase is the main means of accelerating the extraction of aluminum and the most influential factor, while the acid concentration and the reaction time are of secondary importance. The leaching rate of aluminum will reach 92.36% under the conditions of reaction temperature 90 °C and leaching time ≥ 60 min, which can be further used to prepare a series of high value-added, high-purity aluminum-based fine chemicals, including aluminum hydroxide (Al(OH)3), aluminum oxide (Al2O3), aluminum chloride (AlCl3), aluminum sulfate (Al2(SO4)3), and so on. Based on these results, it is evident that the sodium-assisted activation route decisively influences subsequent leaching. Unlike conventional Bayer digestion or acid roasting, where incomplete conversion and amorphous silica residues restrict Al recovery, this method enabled a rapid and selective transformation, achieving complete activation within 30 min at 800 °C, with direct conversion to nepheline. This unique pathway not only facilitated higher Al leaching ratios (>80%) and minimized secondary phases but also enhanced energy efficiency and ensured higher product purity compared with traditional methods. To further elucidate the origin of these advantages, a mechanistic study of the activation reaction was subsequently conducted.
3.6. Mechanistic Study of the Activation Reaction
The main chemical pathway of the roasting reaction can be simplified as follows.
The kaolinite transformation is described as follows:
The quartz transformation is described as follows:
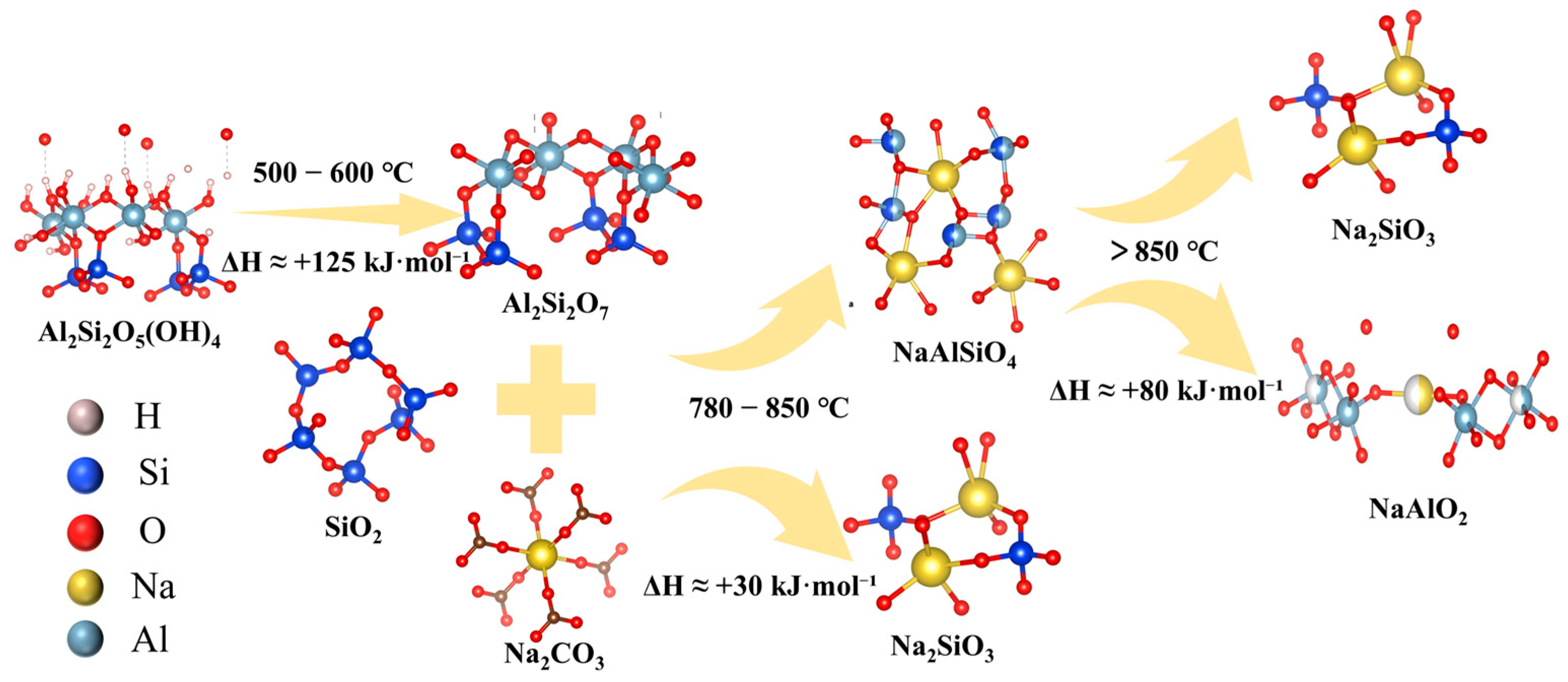
According to the XRD physical phase analysis and SEM morphological characteristics and mineral phase transition analysis results, we constructed
Figure 13 to show the reaction mechanism diagram. During the process of heating activation, the layered aluminosilicate scaffold’s kaolinite first undergoes dehydroxylation (500–600 °C), leading to the destruction of its crystalline structure, and the hydroxyl bond in the aluminum–oxygen octahedron connected to the aluminum atoms is broken, forming metakaolinite (Al
2Si
2O
7) with a crystalline and short-range disordered structure. Therefore, this process generates many defects in the scaffold, exposing that unsaturated Si-O and Al-O active sites easily combine with sodium ions [
39]. With the temperature rising to 780 °C, sodium carbonate crystals begin to decompose. A portion of the released sodium ions reacts with metakaolinite, forming a loose and powdered nepheline phase (NaAlSiO
4), while the remaining sodium ions combine with quartz minerals in coal gangue, forming sodium silicate crystals (Na
2SiO
3). If the temperature continues to rise to 850 °C, this will cause sodium carbonate to further erode the cross-linked Al-O-Si structure in the nepheline scaffold. Consequently, the aluminosilicate scaffold is recombined, generating sodium silicate and sodium aluminate. At the same time, with its low melting point, sodium aluminate progressively melts and encapsulates sodium silicate, forming a complex inclusion system. These phenomena are further supported by microstructural characterization: the SEM images show that the particle surfaces become smooth and compact with blocked pores, while the XRD patterns reveal reduced crystallinity and the emergence of amorphous phases. Such densification and pore blockage hinder the contact between the solid particles and the leaching solution, thereby reducing dissolution efficiency at temperatures above 850 °C. After cooling, it produces a dense glass-phase microcrystalline encapsulation layer, which makes it difficult for the separation of water leaching or acid leaching.
To quantify the feasibility of the above reaction pathways, we calculated the standard Gibbs free energies ΔG°(T) for the key steps using HSC Chemistry 9.5. The results are summarized in
Table 4 and are consistent with the mechanism in
Figure 13. In the roasting window of 700–850 °C, the reactions of kaolinite and quartz with Na
2CO
3 are strongly favorable, corroborating the formation of nepheline (NaAlSiO
4) and sodium silicate (Na
2SiO
3) when Na
2CO
3 starts to decompose near ~800 °C. In contrast, the subsequent conversion of nepheline with Na
2CO
3 is endergonic at 700–800 °C, but becomes slightly exergonic at 850 °C, which explains why this step occurs only near the upper temperature limit and why molten NaAlO
2 can form and encapsulate Na
2SiO
3, leading to densification and reduced leachability above ~850 °C. These thermodynamic results quantitatively support the proposed mechanism and justify the selected activation temperature window.
Therefore, the activation temperature is controlled below 850 °C, which can generate loose sodium silicate powder and nepheline phase powder particles. Since sodium silicate is soluble in water, water leaching results in a clear and transparent sodium silicate solution, which can be used to produce high value-added silicon-based products, such as silica, silicon dioxide, and aerogel. Meanwhile, the generated nepheline powder is difficult to dissolve in water. After the water leaching, it still exists in the form of solid-phase slag. By controlling the conditions of acid leaching, aluminum ions are extracted with hydrochloric acid, which can be used to produce high value-added chemical products, like aluminum hydroxide and alumina. The final residual small amount of tailings is mainly solid-phase silica, with a purity of more than 95%.
Besides the phase transformation and product recovery, the environmental impact of this process also needs attention. Firstly, no strong acid roasting is involved. Acid is only used in the leaching step, and the spent HCl solution can be neutralized with the alkaline liquor generated from the dissolved sodium silicate (“water glass”). After neutralization and clarification, the liquid can be reused, while the solid residue is mainly high-purity silica (>95%), which can serve as a by-product. Secondly, some CO2 is released when Na2CO3 reacts with silicates. Under the chosen conditions (800 °C, 30 min, and gangue:Na2CO3 = 1:0.8), both the carbonate dosage and holding time are reduced, so the CO2 emission is lower than that of conventional alkali roasting. The off-gas can be handled with common kiln treatments such as dust removal and alkaline scrubbing, and it can also be connected to CO2 capture systems at scale. Thirdly, the aluminum-rich filtrate (Al3+/Cl−) can be further concentrated to produce valuable Al salts such as AlCl3 solution, which reduces acidic wastewater. Taken together, by avoiding prolonged high-temperature digestion and strong acid roasting, and by allowing the neutralization and reuse of process streams, the proposed stepwise route improves resource efficiency while lessening typical environmental impacts.
4. Conclusions
This thesis systematically studies the solid-phase reaction mechanism of coal gangue with sodium carbonate additives activated in stages and ion migration behavior. It puts forward a graded leaching and step-by-step extraction process. For example, the process is applied to extract silicon and aluminum in gangue. To some extent, the phase stability and refractory nature of typical high-silicon aluminum coal gangue are effectively addressed. The main research conclusions are as follows:
(1) The optimal activation conditions are determined through RSM optimization experiments: activation time 30 min, temperature 800 °C, and coal gangue and sodium carbonate ratio of 1:0.8. At this time, the highest dissolution rate of silicon is 82.1%. The activation temperature is the most critical control factor, followed by sodium carbonate ratio, while the effect of activation time is relatively small.
(2) XRD and FTIR analyses show that with the activation temperature rising, the coal gangue is converted into nepheline (NaAlSiO4) and sodium silicate (Na2SiO3). At 800 °C or so, the reaction is the fullest and the activation effect is the best, which promote the transformation of the crystalline phase and generates soluble silicon- and aluminum- based materials. As the activation temperature exceeds 850 °C, nepheline is gradually eroded by sodium carbonate and then transformed into amorphous-phase glassy sodium aluminate, which wraps sodium silicate, resulting in highly densified products that are hard to leach.
(3) SEM morphology analysis reveals that the activated products present a loose and porous surface structure. Highly rich porosity is helpful for the leaching and diffusion of the products.
(4) The acid leaching of aluminum is simultaneously controlled by the acid concentration, leaching temperature, and leaching time. The temperature increase is the main means of accelerating the extraction of aluminum and the most influential factor, while the acid concentration and the reaction time are of secondary importance. The leaching rate of aluminum will reach 92.36% under the conditions of reaction temperature 90 °C and leaching time ≥ 60 min, which can be further used to prepare a series of high value-added, high-purity aluminum-based fine chemicals.
(5) The activation reaction mechanism analysis displays the phase transformation paths and element migration rules, validating the scientific basis of this process. It provides both a theoretical foundation and practical path for developing a low-energy-consumption, low-emission, high value-added comprehensive extraction technology which can be applied to multi-element extraction from coal gangue. Together with the low-energy and low-emission features of this route, these results also demonstrate its environmental compatibility and suggest a more sustainable pathway for the comprehensive utilization of coal gangue. Future studies may focus on process scale-up and the recovery of additional valuable elements (e.g., Fe, Ti) to realize multi-element resource utilization from coal gangue.