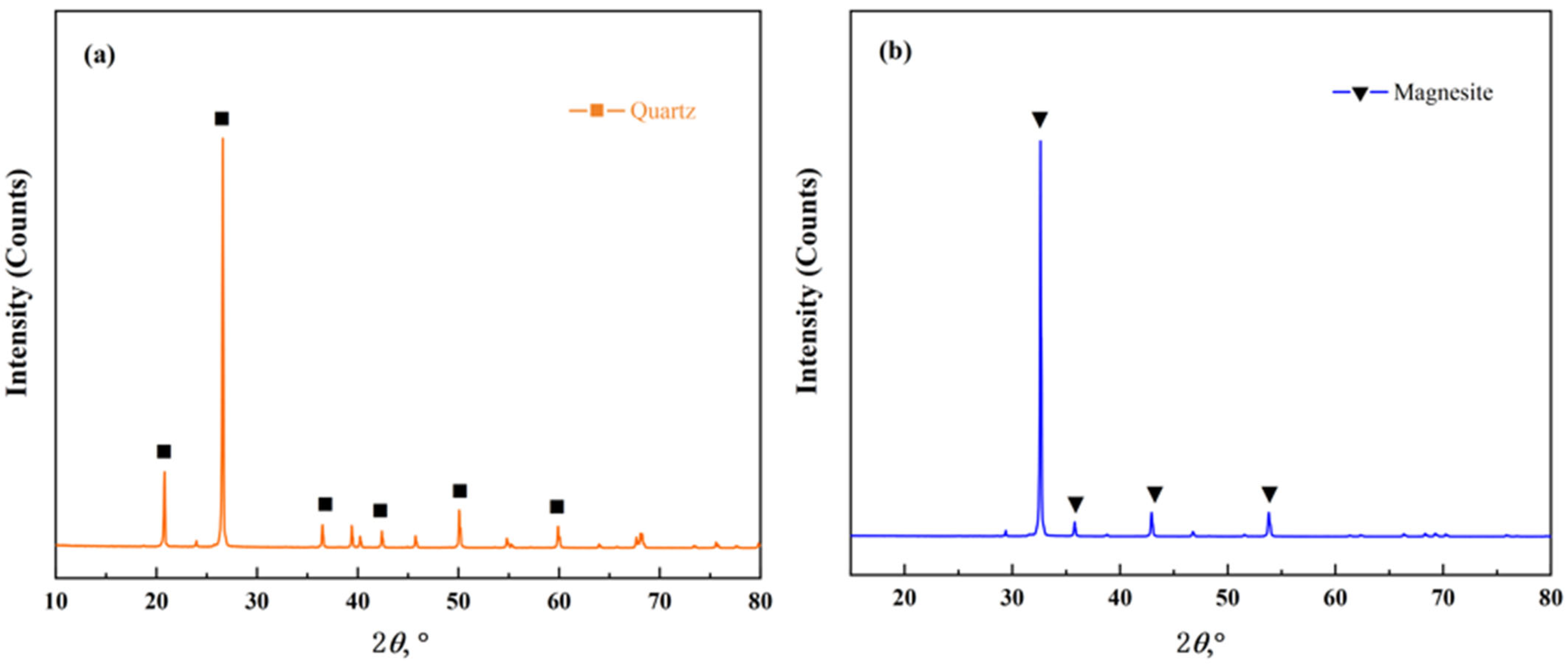
1. Introduction
Magnesium, a critical lightweight metal, plays an indispensable role in modern industries due to its unique physicochemical properties [
1]. It is widely used in applications spanning aviation, electronics, medical devices, and construction materials [
2,
3,
4]. The rising global demand for magnesium products, driven by industrial and technological advancements, has intensified magnesite extraction [
5,
6]. Magnesite, the primary magnesium-bearing mineral, serves as a major source of magnesium and is predominantly mined in northeastern China [
4]. However, decades of unregulated mining practices have depleted high-grade reserves while increasing the prevalence of low-grade ores rich in impurities (e.g., silicon, calcium) [
7]. The traditional strategy of prioritizing high-grade ore extraction while discarding lower-grade deposits is no longer economically viable or environmentally sustainable. Consequently, the development of efficient beneficiation technologies to process low-grade magnesite has become imperative to meet industrial needs and reduce resource waste.
The purification methods for magnesite are closely related to the ore grade [
8]. As the quality of magnesite has declined, beneficiation methods have evolved sequentially from manual sorting to gravity, electrostatic separation, etc. [
9,
10,
11]. Nowadays, as the grade of minerals continues to deteriorate, flotation has become the most effective way to process magnesite [
12,
13]. Flotation takes advantage of the differences in surface properties between ores to achieve separation [
14,
15]. However, the naturally poor floatability of magnesite is insufficient to achieve effective separation. Therefore, various flotation reagents, such as collectors, depressants, and frothers must be introduced to modify the surface hydrophobicity of the particles. At the same time, the diversity of flotation reagents and processes enables the flotation method to be effectively applied to more complex and low-grade magnesite ores [
16,
17].
Siliceous minerals are the most commonly encountered gangue minerals during magnesite mining [
18,
19]. Due to their relatively low content in magnesite ore, reverse flotation—characterized by lower collector consumption—is widely applied in the purification of magnesite [
20,
21]. Meanwhile, cationic collector flotation process has advantages such as sensitively to silicate ores, resistance to low temperatures, and simplicity in reagent systems, which is widely used in the desilication flotation of magnesite. The cationic collector can selectively adsorb on the siliceous minerals surface (e.g., quartz) and then make them float out the foam, resulting in higher quality concentrate product in the pulp [
22,
23]. In this process, the selection of the collector determines whether the flotation process will be successful or not. Fatty amine-based collectors such as dodecylamine (DDA) are often used in cationic reverse flotation processes; however, with the deepening of the ore complexity, conventional collectors have faced problems such as unsatisfactory selectivity and poor foam stability [
24]. In order to solve these shortcomings and improve the efficiency of magnesite purification, a variety of novel cationic collectors have been introduced into the reverse flotation desilication of magnesite. For example, Sun et al. investigated the effect of PTAC on the flotation performance of magnesite and quartz [
25]. Yang et al. introduced PEOLA as a low-cost and highly selective collector to replace DDA [
26]. Li et al. explored the potential of a novel ionic liquid, dodecyl trimethyl ammonium–diethylphosphonic acid (N1211-DEPA), as a flotation reagent for the selective separation of quartz from magnesite [
27]. Liu et al. developed DIPA and DDIPA and applied them to the desilication flotation of magnesite [
28]. Zhao et al. synthesized a novel collector, 14-2G, and demonstrated its application in quartz removal during flotation [
29]. These studies provide good ideas and more choices of agents for the desilication of magnesite in reverse flotation. However, these collectors still have a complicated synthesis process and poor environmental protection, which limit their application in industrial practice. Therefore, it is necessary to find efficient and environmentally friendly magnesite desiliconization collectors.
Cocamidopropyl dimethylamine (PKO-H), a coconut oil-derived amphiphilic surfactant, presents a promising alternative for sustainable mineral processing. PKO-H features a hydrophobic alkyl chain (C12–C18 fatty chains) and a hydrophilic quaternary ammonium group, enabling affinity for mineral surfaces and aqueous phases. Sourced from renewable coconut endosperm via solvent-free mechanical pressing, PKO-H exhibits biodegradability with low ecological toxicity, aligning with green chemistry principles. Initially, PKO-H was adopted in cosmetics and detergents for its emulsifying and foaming properties. Due to its mature production process, easy commercialization, and low price, PKO-H has recently garnered interest in mineral flotation.
This study systematically evaluated the efficacy of PKO-H as a collector in magnesite reverse flotation. Micro-flotation experiments quantified its desilication performance against quartz under varied pH and different dosage conditions, while the FTIR spectroscopy, contact angle measurements, zeta potential analysis, and molecular simulations elucidated adsorption mechanisms at the mineral–water interface. These findings positioned PKO-H as a high-performance and eco-friendly collector capable of replacing traditional amines, advancing the goal of sustainable magnesite resource utilization through reduced reagent consumption and minimized environmental pollution.
3. Results and Discussion
3.1. Single-Mineral Flotation Results
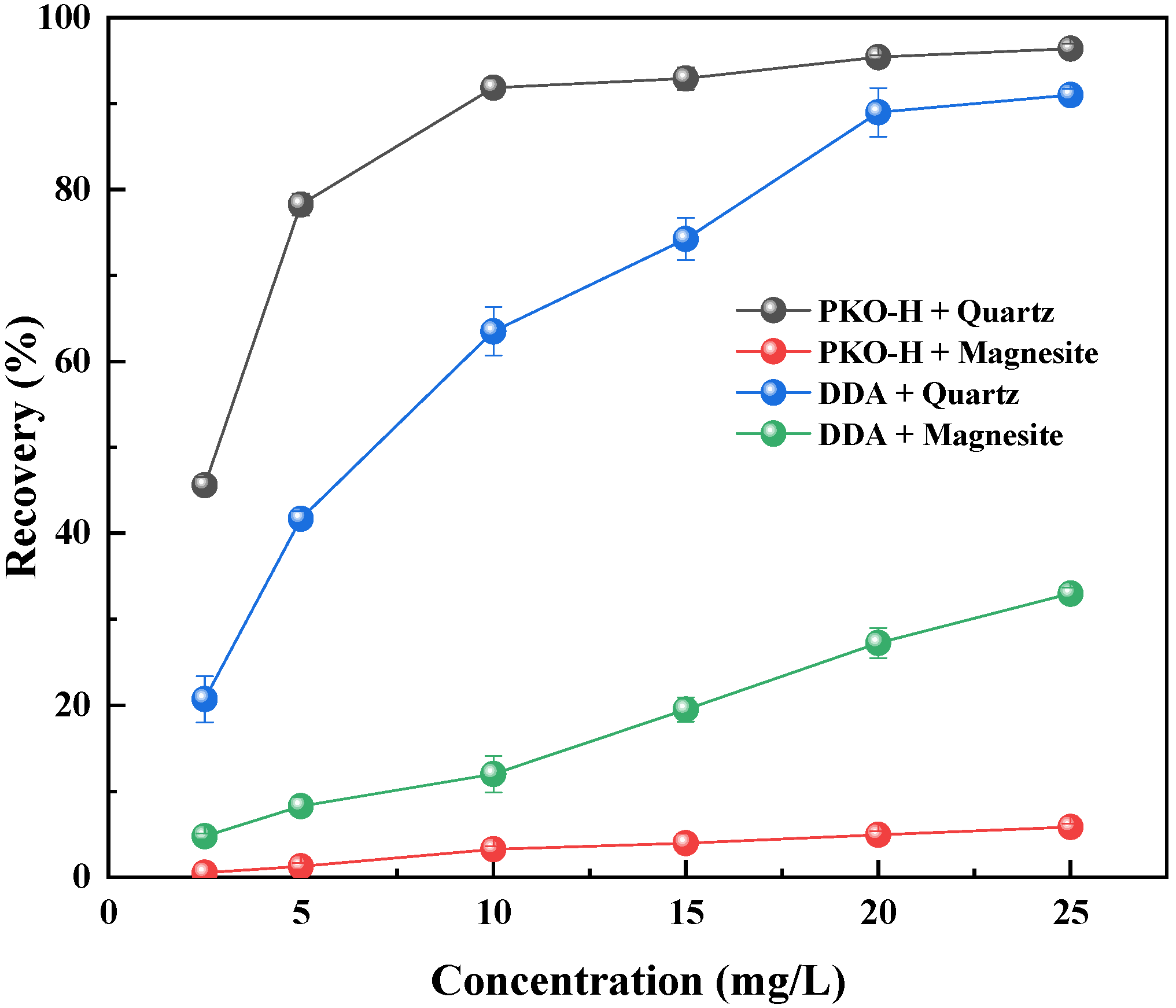
The flotation performance of PKO-H on quartz and magnesite was explored by micro-flotation tests. To evaluate the collection and separation performance of PKO-H, the conventional collector DDA was used as a reference. The relationship between the mineral flotation and collector concentration in the natural pulp pH (approximately 6.5) is presented in
Figure 2.
From
Figure 2, it can be observed that the flotation recoveries of both minerals increased with the increasing collector concentration. Regardless of the collector type, the quartz recovery was significantly higher than that of magnesite, which was at a relatively low level throughout the entire test. Notably, when PKO-H was employed as the collector, the quartz recovery was significantly higher than that obtained with DDA. Notably, when PKO-H was used as the collector, the flotation recovery of quartz was significantly higher than that obtained with DDA. In contrast, the opposite trend was observed for magnesite: its recovery was markedly higher when DDA was used compared to PKO-H.
Specifically, when the dosage of PKO-H was 20 mg/L, the flotation recovery of quartz reached 95.4%. With further increases in PKO-H concentration, the recovery of quartz tended to plateau, indicating that it had entered a saturation phase. Meanwhile, throughout the entire tested concentration range (5–30 mg/L), the recovery of magnesite remained below 10.0%. In contrast, when DDA was used at a concentration of 20 mg/L, the recovery of quartz stabilized at approximately 90.0%, while the recovery of magnesite reached 27.3% and continued to increase with rising DDA concentration. These flotation results demonstrate that PKO-H possesses a stronger collecting ability for quartz compared to DDA, whereas DDA exhibits superior collecting performance for magnesite. Therefore, PKO-H shows better selectivity in the separation of quartz from magnesite, indicating its greater potential for practical application.
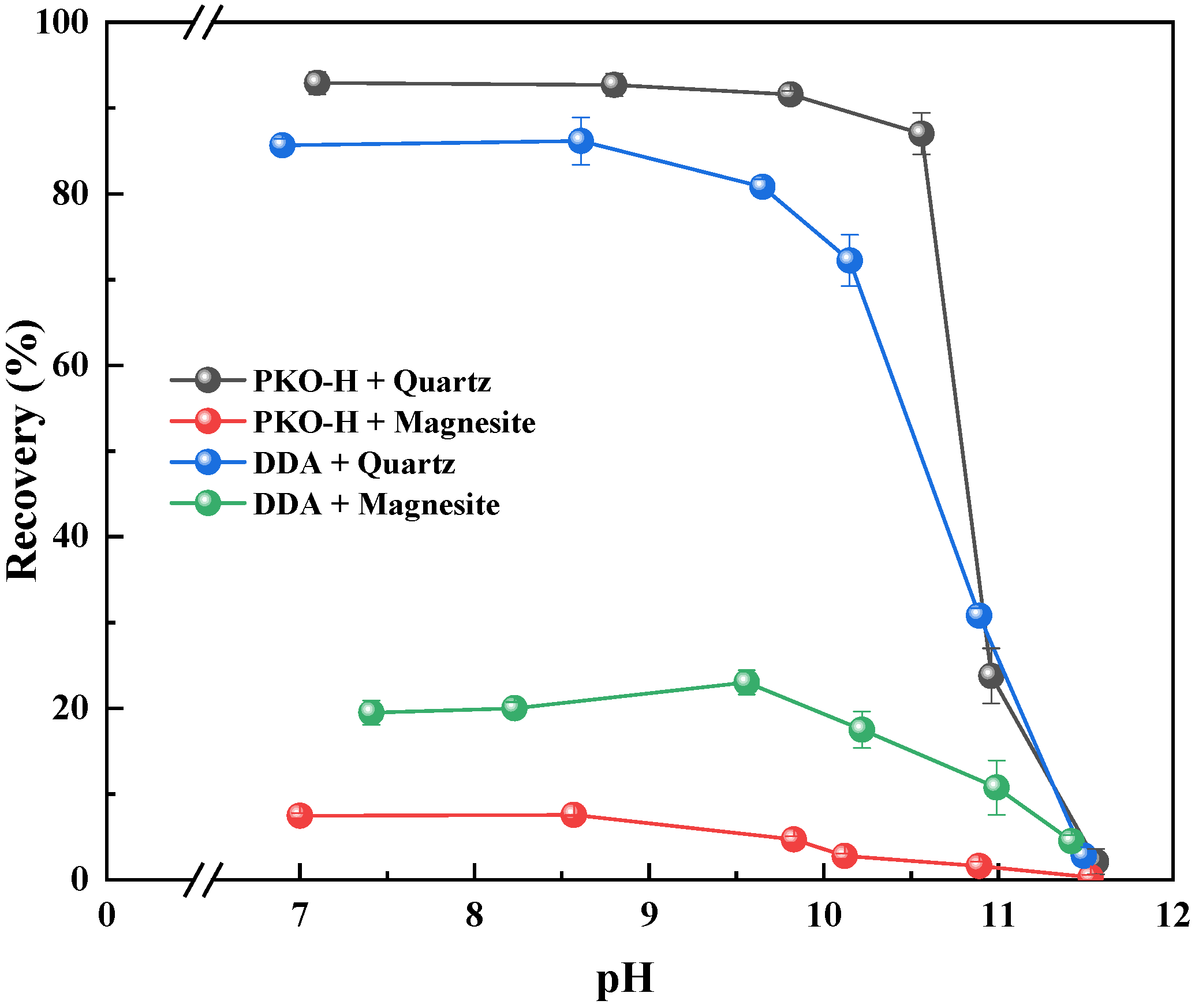
Figure 3 shows the effect of pulp pH on the collection performance of the collectors. Since the carbonate minerals react easily with acids, neutral and alkaline environments are mostly used in the flotation process [
28,
29]. Therefore, the pulp pH range investigated in this study spans from the natural pulp pH (approximately 7) to 11.4. As shown in
Figure 3, the pulp pH has a significant influence on the collection performance of the reagent for both minerals. In the pH range from natural to 10.0, PKO-H exhibits strong collecting ability for quartz, reaching a maximum recovery of 92.9% and 85.7% at around pH 6.8. When the pulp pH exceeds 10.0, the collecting ability for quartz declines sharply, indicating that PKO-H and DDA were highly sensitive to pH. For magnesite, although its recovery decreases with increasing pH, the change is relatively insignificant under neutral-to-mildly alkaline conditions. This variation in flotation results is due to the state of the mineral surface and the form of the agent present in the pulp under different pH conditions. Therefore, to balance flotation performance and reagent cost, the subsequent experiments were conducted under natural pulp pH conditions.
3.2. Artificially Mixed Mineral Flotation Results
The flotation process is carried out in a multiphase system, and different minerals could affect each other [
35]. In order to further investigate the separation performance of PKO-H in the magnesite desilication system, magnesite and quartz were configured into the artificial mixed minerals according to the mass ratio of 7:3. The grades of MgO and SiO
2 in the sample were 32.8%, and 29.9%, respectively. At the natural pulp pH (approximately 6.8) and a collector concentration of 20 mg/L, the concentrate product indices obtained from the separation using DDA and PKO-H are shown in
Figure 4.
The desilication flotation of magnesite is a reverse flotation process in which the concentrate product (magnesite) remains in the flotation cell. When PKO-H was employed as the collector, the recovery of MgO in the concentrate reached 98.8%, with a grade of 45.7%, while the SiO2 grade in the concentrate decreased significantly from 29.9% to 1.4%. In contrast, when DDA was used as the collector, the MgO recovery and grade in the concentrate were only 92.7% and 41.8%, respectively. The SiO2 content remained as high as 4.7% in the magnesite concentrate. These results demonstrate that PKO-H possesses markedly superior separation performance compared to DDA, which is also consistent with the results observed in the single mineral flotation tests. Moreover, PKO-H was able to produce a nearly qualified concentrate without the addition of any depressants, further indicating its strong potential as an effective collector for magnesite desilication flotation.
3.3. Contact Angle Analysis
The contact angles results were tested to investigate the discrepancy of wettability on the magnesite and quartz surface treated with PKO-H.
Figure 5 shows the contact angle results of quartz and magnesite treated with various reagents.
As shown in
Figure 5, the contact angles of untreated quartz and magnesite are 34.61° and 25.82°, respectively, indicating their hydrophilic nature, which is consistent with previous reports [
28,
30]. After treatment with 20 mg/L DDA, the contact angles increased to 55.49° for quartz and 37.92° for magnesite. In comparison, the contact angle of quartz treated with PKO-H increased to 62.33°, while that of magnesite exhibited only a slight change of approximately 2°, suggesting that PKO-H significantly enhances the hydrophobicity of quartz but has a minimal effect on magnesite. Compared with DDA, PKO-H induced a more pronounced difference in the contact angle changes between the two minerals, reflecting its stronger selective adsorption on quartz over magnesite. These findings are in good agreement with the flotation results.
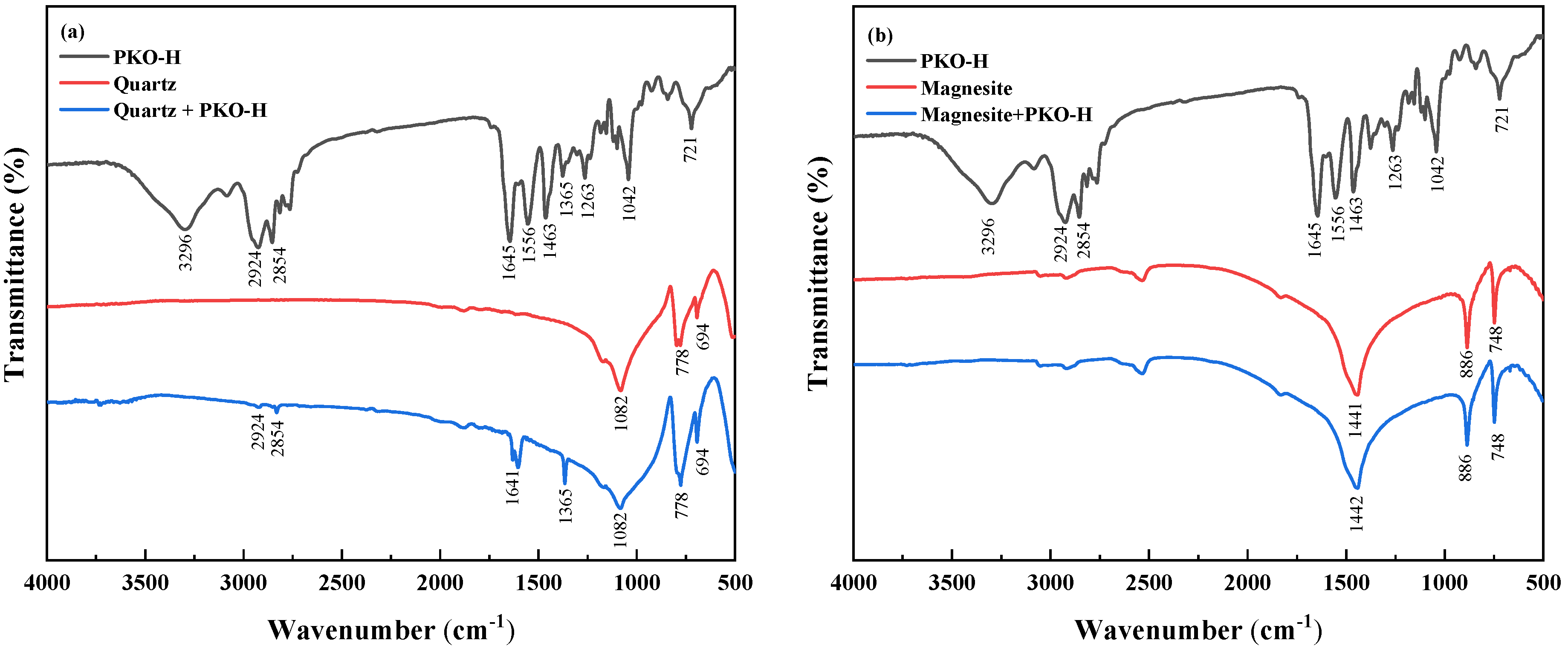
3.4. FTIR Analysis
The FTIR characteristic peaks are usually used to analyze the adsorption behavior of reagents on mineral surfaces [
37]. To further determine the adsorption mechanisms of PKO-H on the quartz and magnesite surface, FTIR measurements were carried out on the treated or untreated minerals, and the results are presented in
Figure 6.
Figure 6 presents the FTIR of quartz and magnesite before and after the treatment with PKO-H. In
Figure 6a, the broad peak observed at 3296 cm
−1 in the PKO-H spectrum is attributed to the N-H stretching vibration. The peaks located at 2924 cm
−1 and 2854 cm
−1 correspond to the asymmetric and symmetric stretching vibrations of methylene (CH
2) groups, respectively. In addition, the peak at 1654 cm
−1 corresponds to the stretching vibration of the C=O group in the amide moiety, while the peak at 1556 cm
−1 is attributed to a combination of N–H bending and C–N stretching vibrations. The peak at 1365 cm
−1 arises from methyl (CH
3) bending, while the band at 1263 cm
−1 is also associated with C-N stretching. For untreated quartz, characteristic peaks at 1082 cm
−1, 778 cm
−1, and 694 cm
−1 are indicative of Si-O-Si and Si-O stretching vibrations. After reaction with PKO-H, new absorption bands appear at 2924 cm
−1, 1603 cm
−1, and 1365 cm
−1, corresponding to the C-H stretching vibrations of PKO-H. These spectral changes confirm the adsorption of PKO-H onto the quartz surface.
In contrast, the FTIR spectrum of pure magnesite (
Figure 6b) exhibits a prominent peak at 1442 cm
−1, attributed to the asymmetric stretching vibration of the carbonate group (CO
32−), along with bands at 886 cm
−1 and 748 cm
−1 corresponding to its in-plane and out-of-plane bending vibrations, respectively. Notably, after treatment with PKO-H, no additional absorption bands are observed in the magnesite spectrum, suggesting that the interaction between PKO-H and the magnesite surface is limited, indicating that PKO-H may not form effective adsorption on the surface of magnesite.
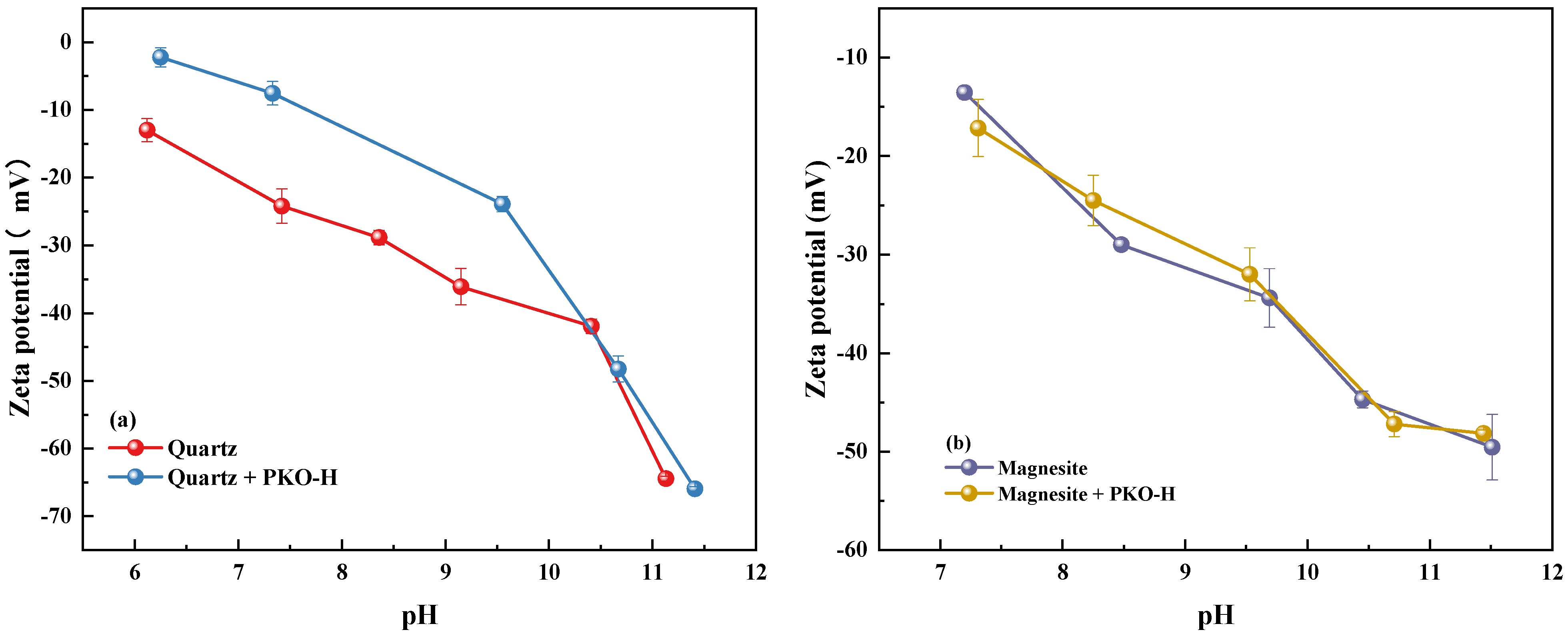
3.5. Zeta Potential Analysis
The adsorption of the collector on the mineral surface leads to changes in the surface properties of the minerals [
38]. To investigate the adsorption mechanism of PKO-H, zeta potential measurements were conducted. The zeta potentials of quartz and magnesite, with and without the addition of 20 mg/L PKO-H, are shown in
Figure 7.
As shown in
Figure 7, the zeta potentials of both quartz and magnesite remain negative throughout the tested pH range, which is consistent with previous studies [
24,
35]. Under natural pulp conditions (pH 6.2 for quartz and pH 7.2 for magnesite), the zeta potentials of quartz and magnesite were −13.00 mV and −13.55 mV, respectively. This is primarily attributed to the breaking of internal bonds during crushing and grinding processes, which generates fresh mineral surfaces that readily undergo hydration, leading to negatively charged surfaces. With increasing pH, the zeta potential measurements of both minerals decrease gradually, which can be ascribed to enhanced surface hydration under alkaline conditions.
Upon the addition of PKO-H, a significant increase in the zeta potential of quartz is observed within the pH range of natural conditions to pH 10, indicating a strong adsorption of PKO-H on the quartz surface. This can be explained by the protonation of PKO-H in an aqueous solution, where the nitrogen atoms accept H+ ions to form positively charged N+ centers. These cationic species are electrostatically attracted to the negatively charged quartz surface. Additionally, hydrogen bonding may occur between hydrogen atoms in PKO-H and surface oxygen atoms on quartz, further enhancing the adsorption strength.
However, as the pH increases beyond 10, the zeta potential of quartz drops sharply, and the difference between treated and untreated samples narrows, suggesting a weakened adsorption of PKO-H under high pH conditions. This is likely due to an increase in OH− concentration, which suppresses the protonation of PKO-H, causing the collector to exist predominantly in its molecular (neutral) form rather than as a cation, thereby reducing its electrostatic attraction to the mineral surface and, consequently, the flotation efficiency.
In contrast, as shown in
Figure 7b, the zeta potential of magnesite shows minimal change before and after PKO-H addition, indicating negligible or very weak adsorption of the collector. This may result from the simultaneous presence of electrostatic attraction and repulsion between the PKO-H molecules and the Mg and O atoms exposed on the magnesite surface, preventing the formation of stable adsorption. Consequently, magnesite particles fail to report to the froth and remain in the pulp, achieving reverse flotation desilication.
In conclusion, the zeta potential measurements are in good agreement with the flotation results, further confirming the selective adsorption behavior of PKO-H on different mineral surfaces.
3.6. Molecular Simulations
Since the hydrophobic group of PKO-H generally consists of an alkyl carbon chain with a length of C
12–C
18, a C
14 fatty carbon chain was used as the hydrophobic group in this study. The adsorption process of DDA and PKO-H on magnesite and quartz surfaces was calculated, and the results are shown in
Figure 8 and
Figure 9.
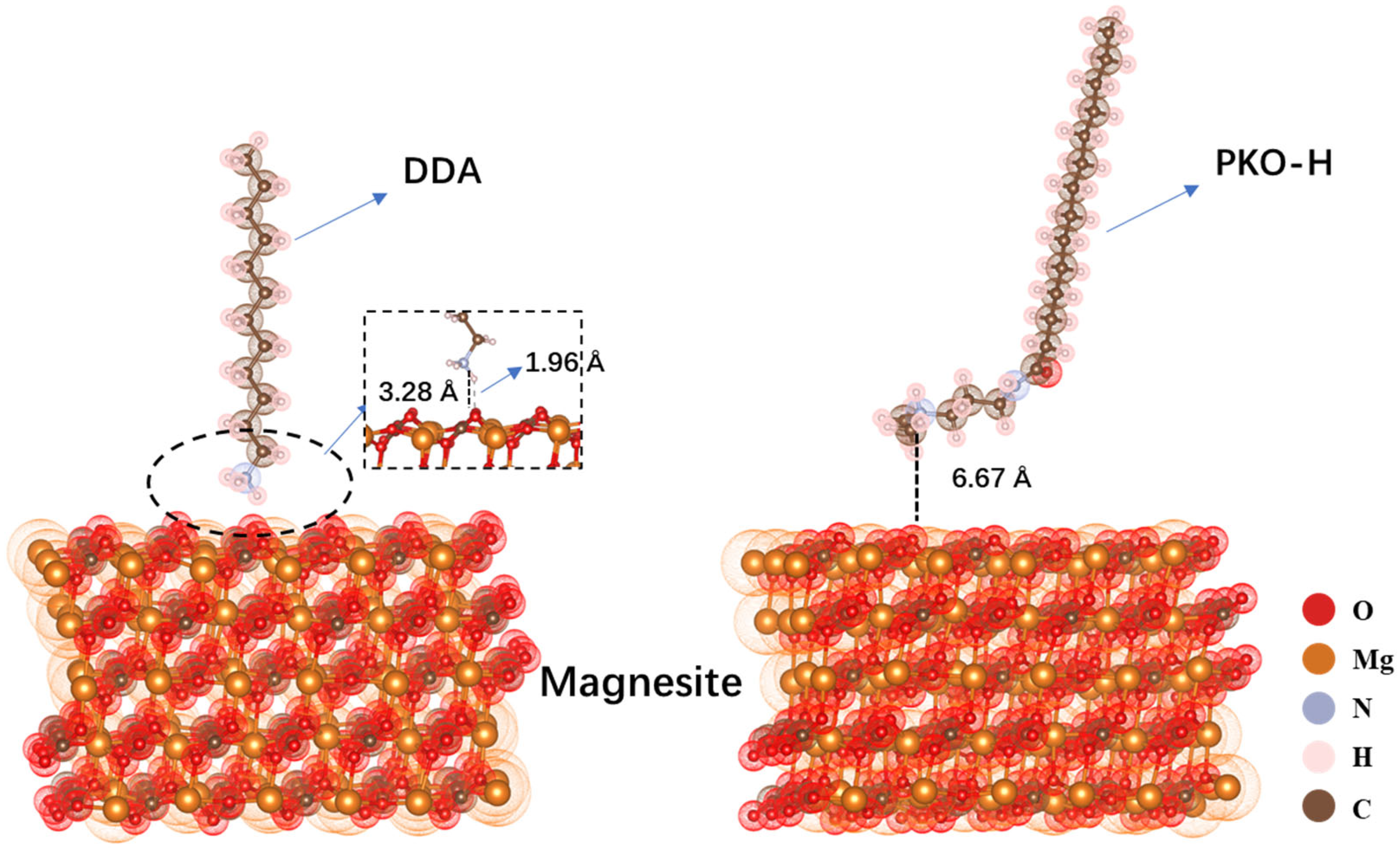
As shown in
Figure 8, after structural optimization, the polar group of the DDA approached the surface of magnesite, with the N atom located at distance of 3.28 Å from the surface—within the effective range of electrostatic interaction. The H atom related to the N atom was found to be 1.96 Å from the surface, a distance greater than that typically required for the formation of a chemical bond with oxygen atoms. From the distance analysis, we found that no new chemical bonds were formed between DDA and the magnesite surface. In contrast, after optimization, the PKO-H molecule remained relatively far from the magnesite surface, with the N atom in its polar group located 6.67 Å away. This large distance suggests that electrostatic interactions between the collector and the mineral surface are unlikely to occur. This finding accounts for the observed flotation activity of DDA toward magnesite in contrast to the poor flotation performance exhibited by PKO-H.
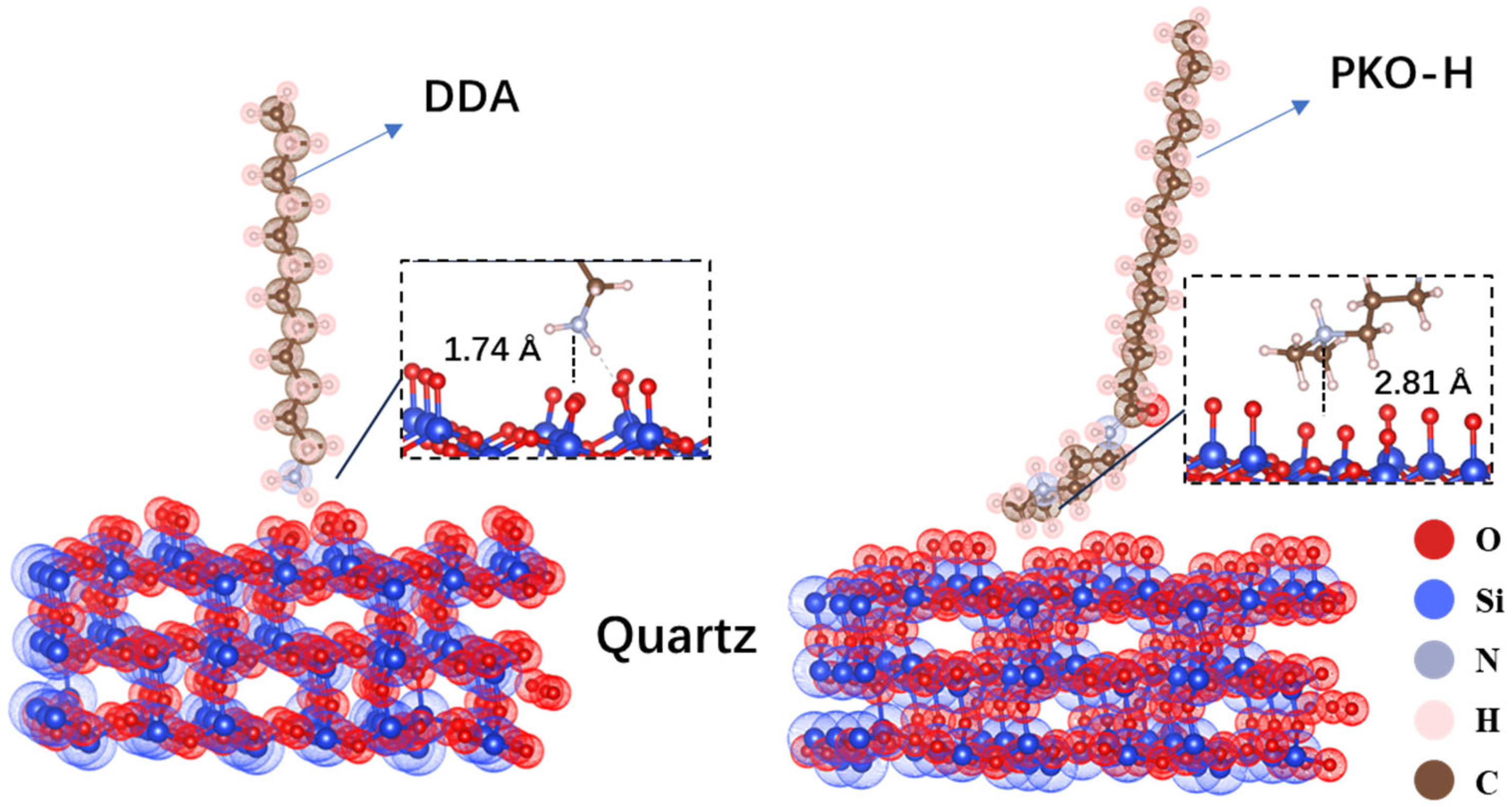
Figure 9 presents the optimized adsorption configurations of DDA and PKO-H on the quartz surface. As shown, the distance between the nitrogen atom in DDA and the quartz surface is 1.74 Å, while that for PKO-H is 2.81 Å, indicating that DDA may exhibit stronger adsorption on the quartz surface. However, PKO-H displays a larger contact area with the mineral surface, which may compensate for its weaker adsorption strength. The absence of bond lengths indicative of covalent interactions further confirms that no new chemical bonds are formed between either the collector and the quartz surface, suggesting that adsorption is primarily driven by electrostatic interactions. Moreover PKO-H possesses a longer hydrophobic chain, contributing to enhanced hydrophobicity and, consequently, a higher flotation recovery for quartz during flotation processes.
Compared with their adsorption behavior on the magnesite surface, both collectors exhibit significantly shorter distances between their polar groups and the quartz surface, indicating stronger interactions with quartz. This adsorption difference helps explain why both DDA and PKO-H achieve much higher flotation recoveries for quartz than for magnesite.
3.7. Molecular Structure Analysis
The structure of a molecule fundamentally determines its performance [
39]. By analyzing the molecular structure of collectors, it is possible to gain deeper insight into the differences in their adsorption behaviors on mineral surfaces. Therefore, the molecular properties of both collectors were analyzed based on DFT calculation.
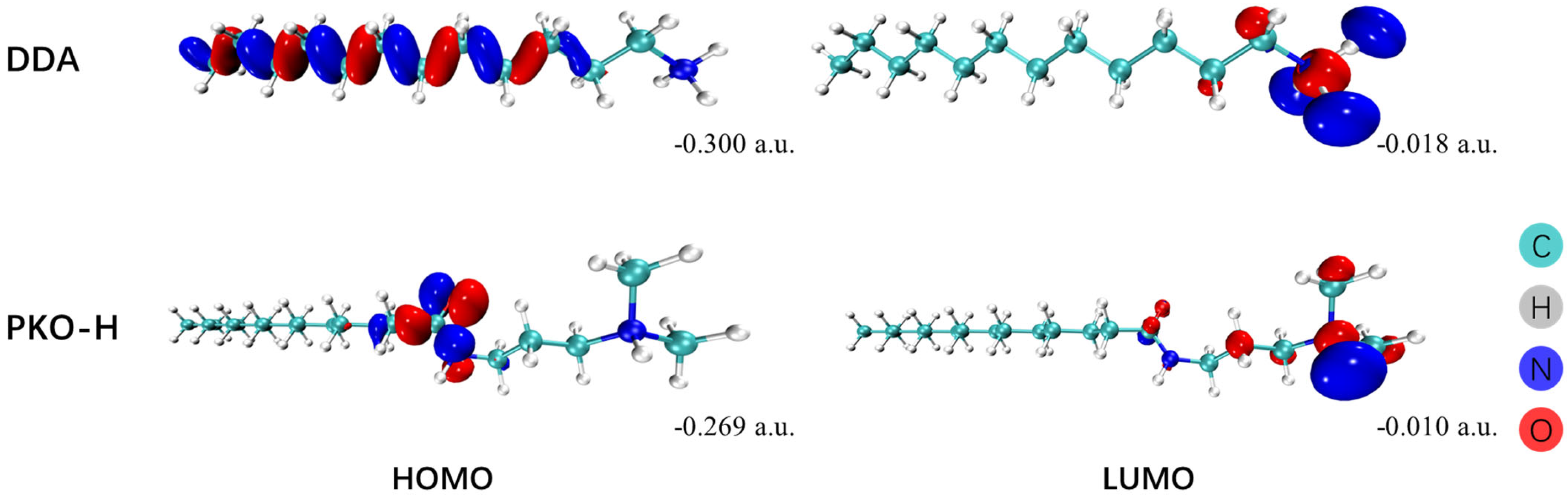
Figure 10 and
Figure 11 present the frontier molecular orbitals (the highest occupied molecular orbital, HOMO, and the lowest unoccupied molecular orbital, LUMO) and electrostatic potential maps (ESP) of the two collectors, respectively.
As shown in
Figure 10, the HOMO of DDA is primarily distributed along the alkyl chain, whereas that of PKO-H is concentrated around the amide group. Both exhibited relatively low energy levels, indicating that these regions have limited electron-donating ability. Additionally, the LUMO orbitals of the four reagents are mainly localized around the nitrogen atoms and are predominantly composed of the s orbitals of C, H, and N atoms. This suggested that these orbitals have a low capacity to accept electrons and form new chemical bonds. As a result, both DDA and PKO-H are unlikely to form strong chemisorption on mineral surfaces, which is consistent with the results obtained from FTIR spectroscopy and zeta potential measurements.
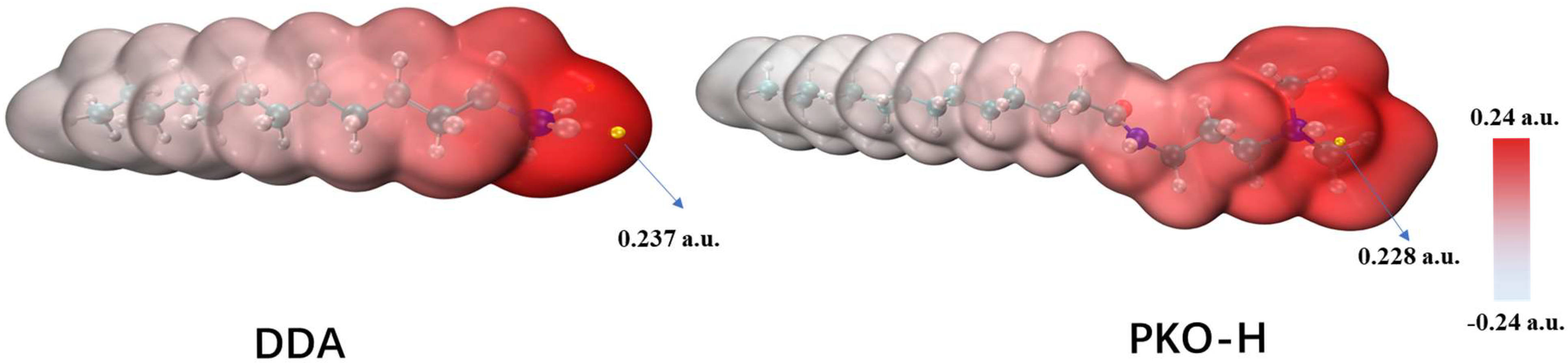
From
Figure 11, the red regions represent areas of positive potential. Comparing the ESP of the two reagents, DDA exhibited a maximum electrostatic potential of 0.237 a.u., while PKO-H has a slightly lower maximum value of 0.228 a.u., suggesting that DDA may possess a stronger overall adsorption capability. However, PKO-H displays a larger positively charged region compared to DDA, which implies that it may have a greater contact area with the negatively charged mineral surfaces. Therefore, it can be inferred that both reagents can achieve effective adsorption on the quartz surface. Nonetheless, PKO-H contains a longer hydrophobic chain, which corresponds to enhanced hydrophobicity, resulting in superior flotation performance during quartz flotation.
In contrast, for minerals such as magnesite, whose surface exposes both O and Mg atoms, the smaller positively charged region of DDA enables more precise electrostatic adsorption with the negatively charged oxygen atoms. Meanwhile, the larger positively charged area of PKO-H may experience repulsion from the positively charged magnesium ions on the magnesite surface during adsorption onto the oxygen atoms. Consequently, due to significant steric hindrance and electrostatic repulsion, PKO-H cannot form stable adsorption on the magnesite surface, which explains why PKO-H exhibits better selectivity than DDA in magnesite flotation.
4. Conclusions
This study systematically investigated the flotation performance and selective adsorption mechanism of the environmentally friendly surfactant PKO-H as a collector in the magnesite desilication system. A comprehensive analysis was conducted using flotation tests, contact angle measurements, Fourier transform infrared spectroscopy (FTIR), and DFT calculations. The results showed that, compared to the conventional cationic collector DDA, PKO-H exhibited superior selectivity and weaker collecting ability toward magnesite. At natural pH and a dosage of 20 mg/L, PKO-H achieved excellent flotation performance with an MgO recovery of 98.8% and a grade of 45.7% for magnesite in artificial mixed minerals. Contact angle measurements indicated that PKO-H significantly enhanced the hydrophobicity of the quartz surface, while its effect on the hydrophobicity of the magnesite surface was limited. Zeta potential and FTIR analyses further confirmed that the strong interaction between PKO-H and the quartz surface was mainly attributed to electrostatic adsorption. DFT calculations revealed that PKO-H, owing to its longer hydrophobic chain and favorable spatial configuration, exhibited different adsorption behaviors on quartz and magnesite surfaces, resulting in improved flotation selectivity.
In summary, this fundamental study demonstrates that PKO-H exhibits excellent selectivity in the desilication flotation of magnesite, indicating strong potential for industrial application. However, it must be acknowledged that PKO-H is still far from industrial application. Therefore, the next step will be to conduct experiments on real ore samples and to explore the influence of plant water recycling in order to provide scientific evidence and technical guidance for potential future applications.