Enrichment and Fractionation of Rare Earth Elements in High-Altitude Thick Weathered Crust Elution-Deposited Rare Earth Ore
Abstract
1. Introduction
2. Sampling and Analytical Methods
2.1. Sample Characteristics
2.1.1. Mineral Composition Analysis
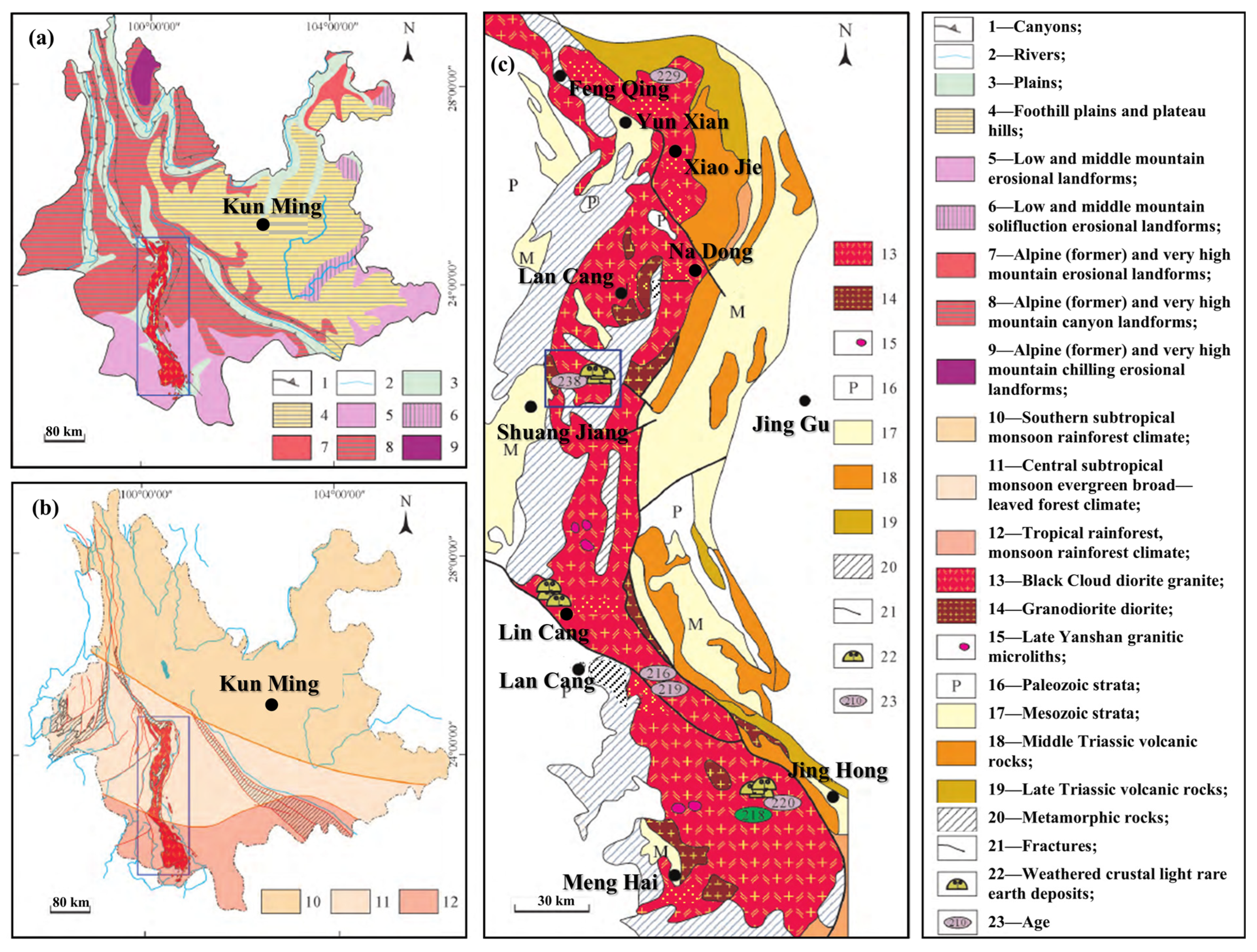
2.1.2. Parent Rock Characterization
2.2. Analysis Method
2.2.1. Mineral Composition
2.2.2. Major and Trace Element Analysis
- (1)
- Dried samples were crushed, ground, and passed through a 200-mesh standard sieve. The major chemical components were analyzed using X-ray fluorescence spectrometry (XRF).
- (2)
- Finely ground samples (<200 mesh) were precisely weighed and subjected to acid digestion and dilution. REEs and other trace elements were then quantified using inductively coupled plasma mass spectrometry (ICP-MS).
2.2.3. Analysis of REE Occurrence States
2.2.4. Analysis of Abrasion pH in Weathering Crust
2.2.5. Mineral Morphology Characterization
3. Results and Discussion
3.1. Mineralogical Analysis of Weathering Profiles
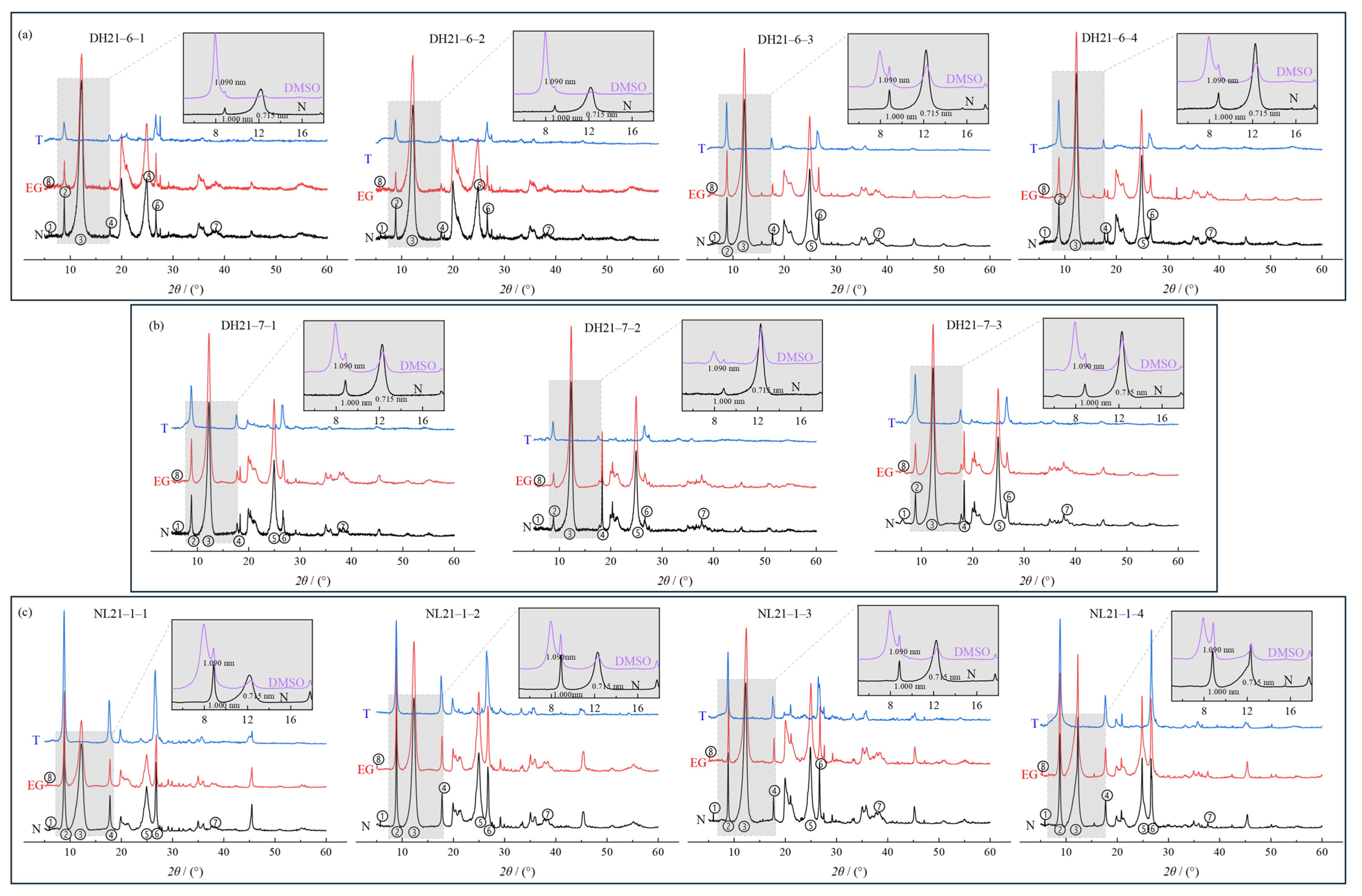
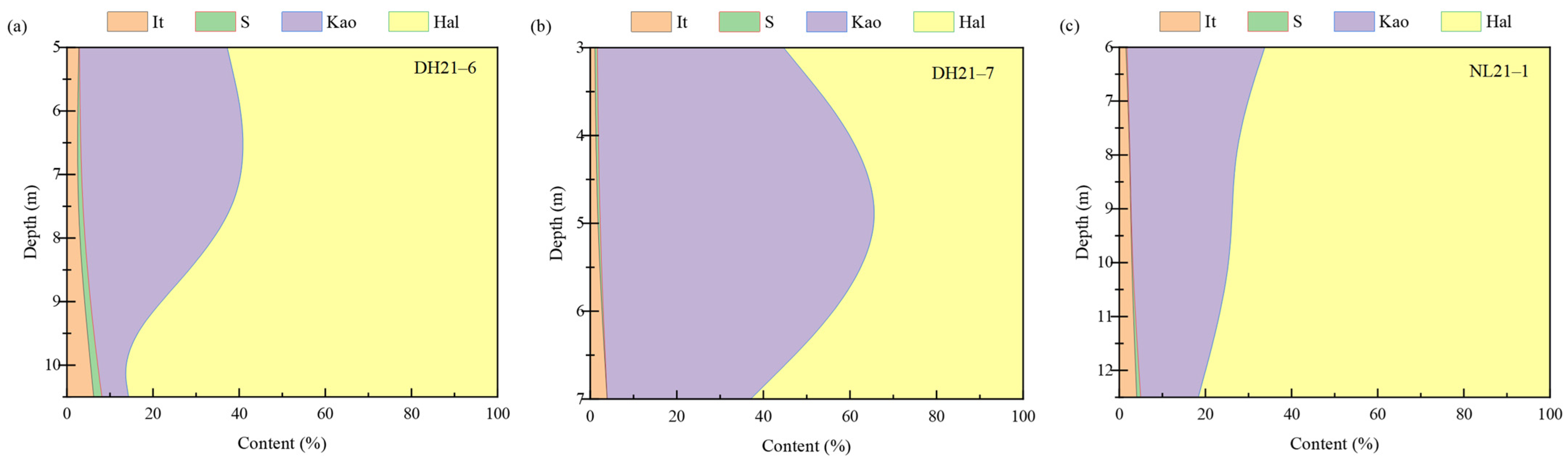
3.1.1. Patterns of Mineral Composition and Content Changes in Weathering Profiles
3.1.2. Weathering Degree of the Weathering Profile and Distribution Patterns of Major Elements
3.1.3. Microscopic Morphological Characteristics of the Fine Particle Fraction in Weathering Profiles
3.2. Distribution Characteristics of REEs in Weathering Profiles
3.2.1. REEs Content Distribution in Weathering Profiles
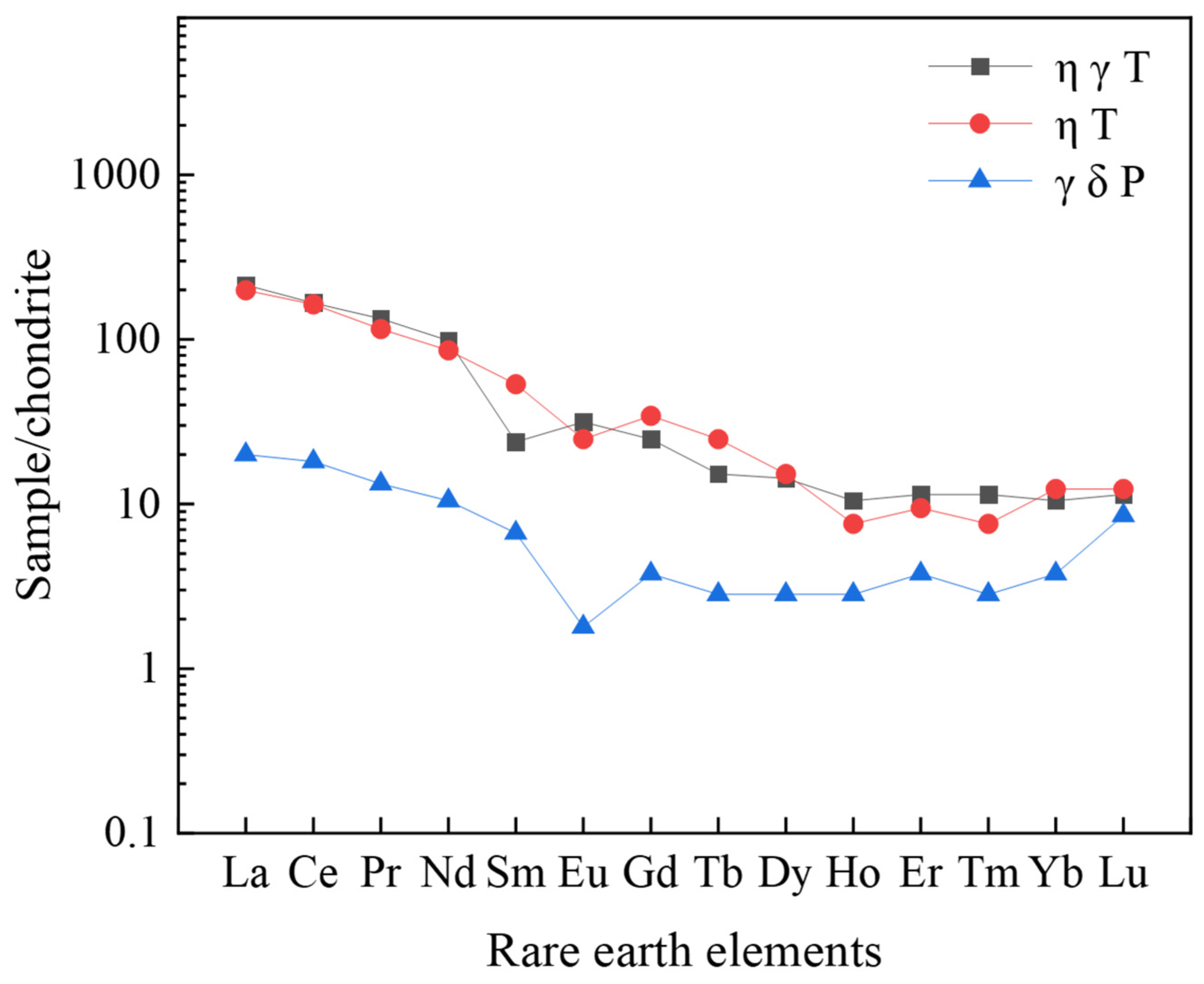
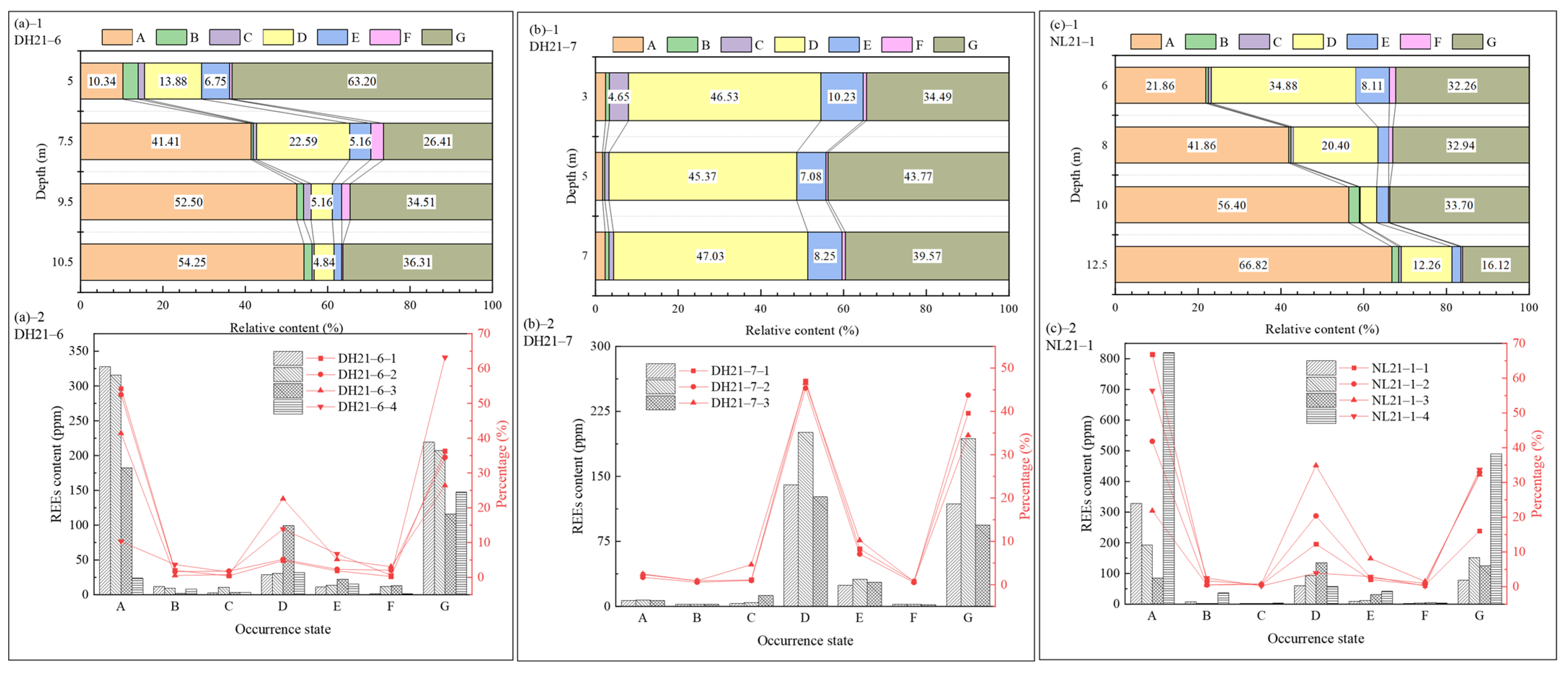
3.2.2. Occurrence States and Fractionation Characteristics of REEs in Weathering Profiles
3.3. Distribution of REEs in Major Occurrence States and Partitioning Characteristics
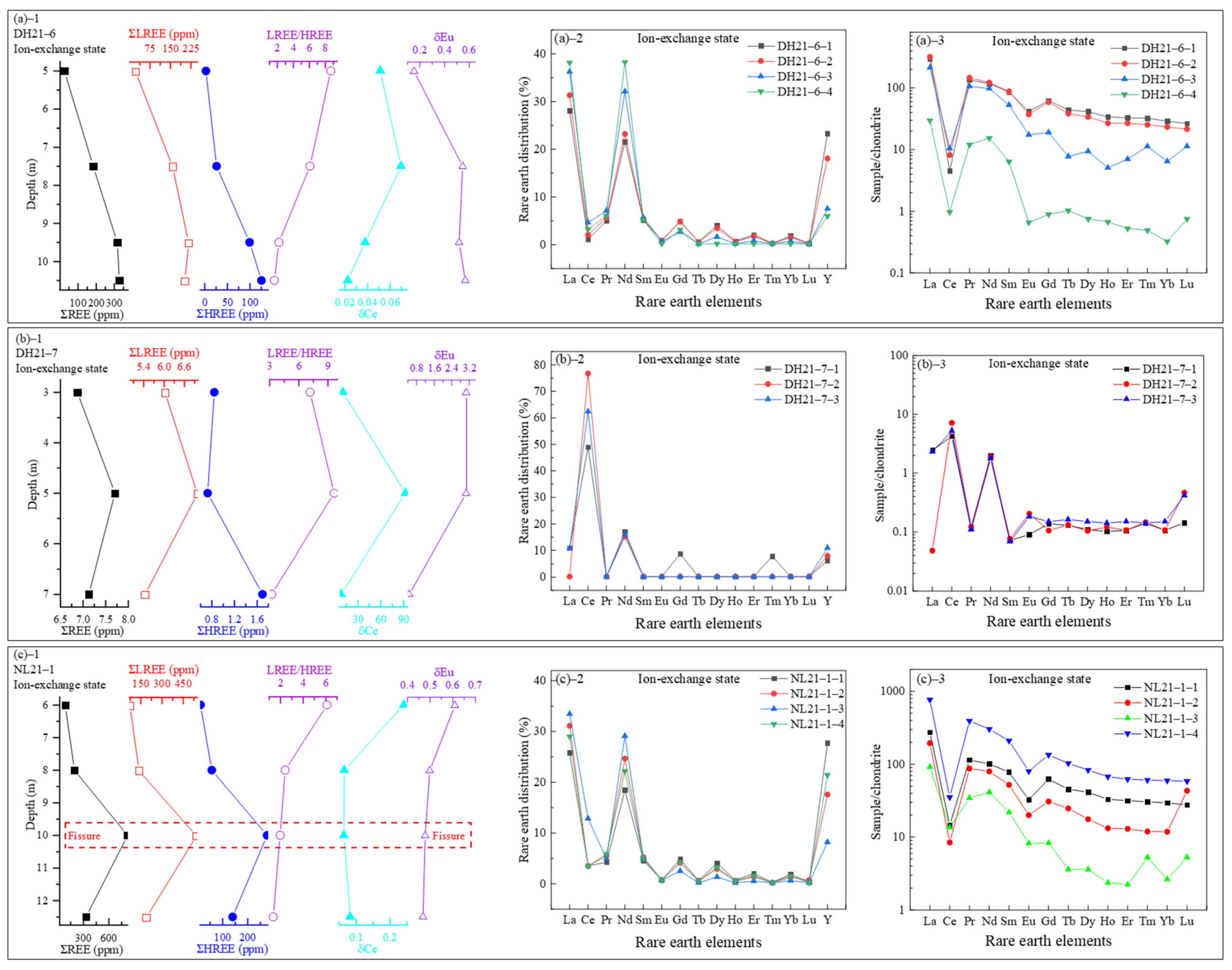
3.3.1. REEs in the Ion-Exchange State
3.3.2. REEs in the Residual State
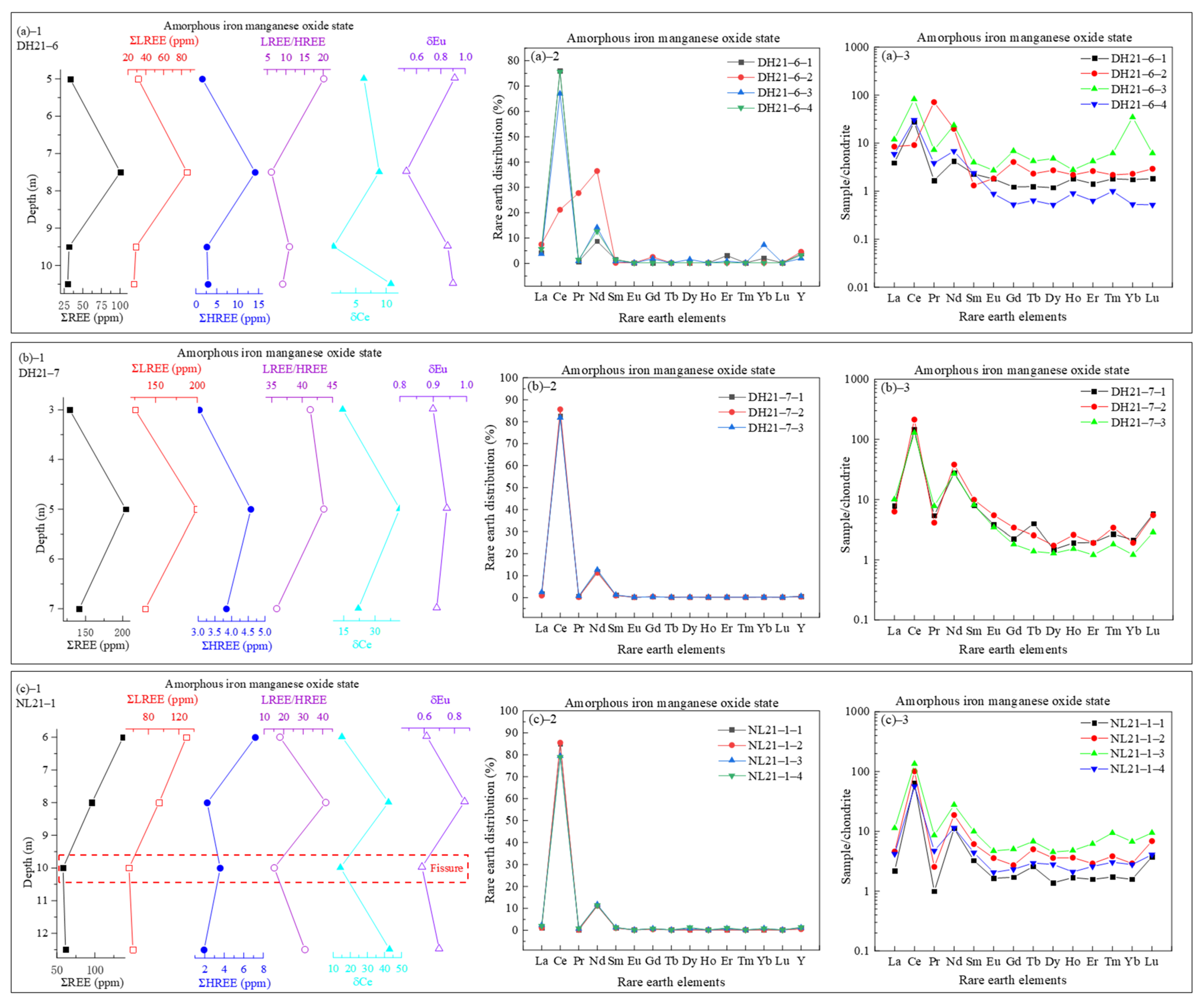
3.3.3. REEs in Amorphous Iron-Manganese Oxide State
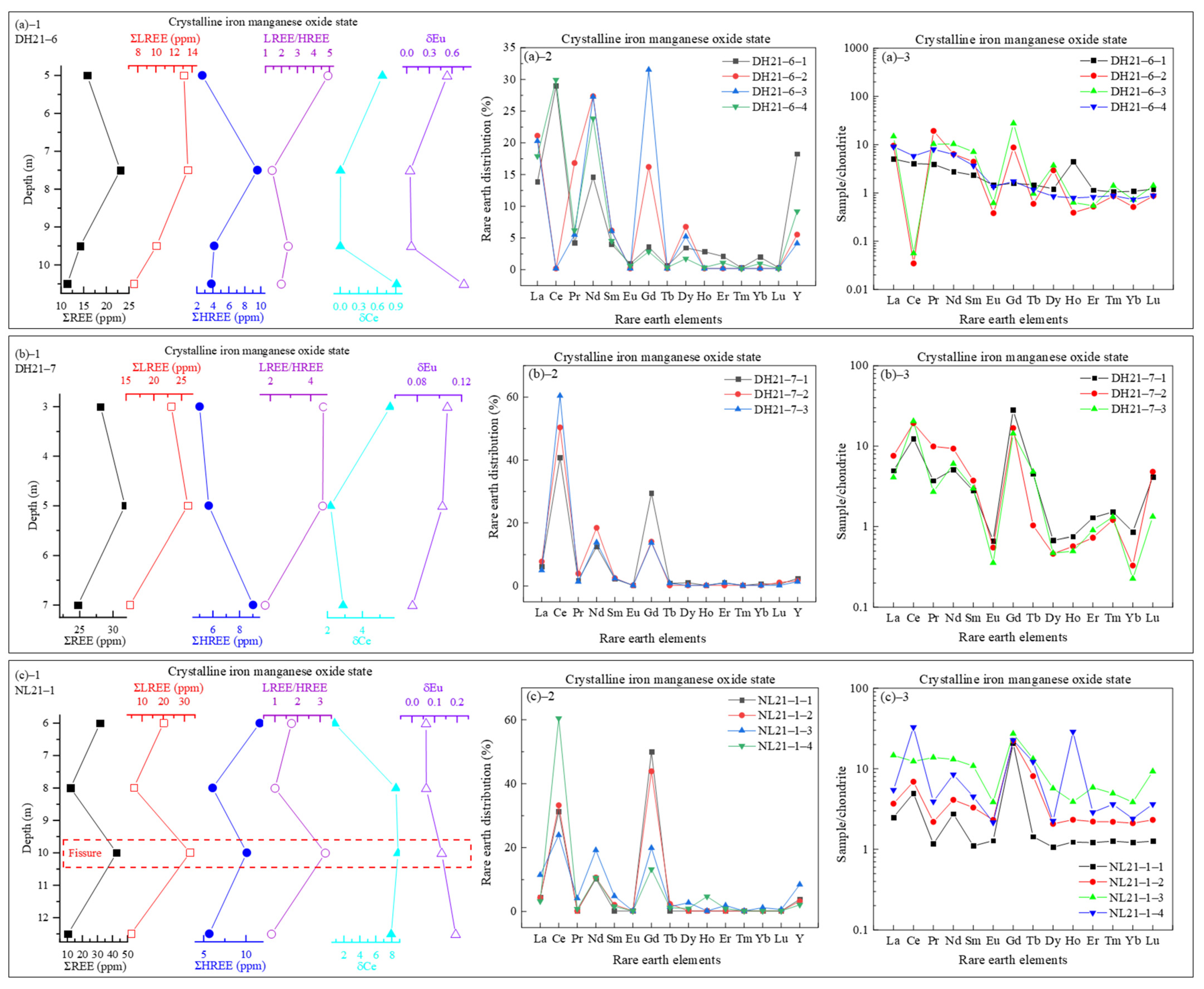
3.3.4. REEs in Crystalline Iron-Manganese Oxide State
3.4. REEs Migration and Fractionation Patterns
3.4.1. Mineral Weathering Processes and REEs Release Mechanisms
3.4.2. Fractionation Characteristics of REE Occurrence States
4. Conclusions
- (1)
- Mineralogical characteristics: The weathering profiles are predominantly composed of clay minerals (kaolinite, halloysite, illite, and minor montmorillonite) and iron oxides, exhibiting typical geochemical signatures of weathered REE deposits—high SiO2 (64.10–74.40 wt.%) and Al2O3 (15.50–20.20 wt.%) and low CaO/MgO. With increasing depth, 2:1-type clay minerals transform into 1:1 types, while iron oxides show increasing crystallinity, transitioning from poorly structured forms in surface layers to well-crystallized hematite at depth;
- (2)
- REE occurrence and fractionation: The total REE contents (ΣREEs: 238.12–1545.53 ppm) display distinct vertical fractionation, with LREEs being enriched in upper layers and HREEs increasing downward. Sequential extraction reveals that REEs primarily occur in ion-exchangeable (peaking at 819.96 ppm), residual, and iron-manganese oxide-bound states (>95% of total). Amorphous iron-manganese oxides show strong Ce affinity (partitioning >80%), while crystalline phases preferentially adsorb HREEs (e.g., Gd partitioning up to 15%);
- (3)
- Structural controls: Fissure systems critically govern REE redistribution, inducing localized enrichment (e.g., ΣREEs reaching 1545.53 ppm in fissure zones). Fluids migrating along fractures concentrate amorphous iron-manganese oxides in overlying strata, facilitating multistage mineralization;
- (4)
- Mineralization mechanisms: Under weakly acidic conditions, weathering-prone minerals release REEs, with LREEs migrating downward and HREEs accumulating in deeper secondary minerals. Residual REEs exhibit increasing Ce partitioning (to 60%) with increasing depth, which indicates the presence of independent REE minerals (e.g., monazite).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borst, A.M.; Smith, M.P.; Finch, A.A.; Estrade, G.; Villanova-De-Benavent, C.; Nason, P.; Marquis, E.; Horsburgh, N.J.; Goodenough, K.M.; Cheng, X. Adsorption of rare earth elements in regolith-hosted clay deposits. Nat. Commun. 2020, 11, 4386. [Google Scholar] [CrossRef]
- Kynicky, J.; Smith, M.P.; Xu, C. Diversity of rare earth deposits: The key example of China. Elements 2012, 8, 361–367. [Google Scholar] [CrossRef]
- García, M.V.R.; Krzemień, A.; Del Campo, M.Á.M.; Álvarez, M.M.; Gent, M.R. Rare earth elements mining investment: It is not all about China. Resour. Policy 2017, 53, 66–76. [Google Scholar] [CrossRef]
- Li, M.Y.H.; Zhou, M.F. The role of clay minerals in formation of the regolith-hosted heavy rare earth element deposits. Am. Miner. 2020, 105, 92–108. [Google Scholar] [CrossRef]
- Yan, C.H.; Huang, X.W. Review on research of rare earths field in China in 2023. J. Chin. Soc. Rare Earths 2024, 42, 381–418. [Google Scholar]
- Zhang, L.; Wu, K.X.; Chen, L.K.; Zhu, P.; Ouyang, H. Overview of metallogenic features of ion-adsorption type REE deposits in Southern Jiangxi Province. J. Chin. Soc. Rare Earths 2015, 33, 10–17. [Google Scholar]
- Cheng, J.C. Tectonic surroundings forming west Yunnan granitoids and their rock characters. Yunnan Geol. 1989, 8, 205–212. [Google Scholar]
- Li, X.L. Basic characteristics and formation structural environment of Lincang composite granite batholith. Yunnan Geol. 1996, 15, 1–18. [Google Scholar]
- Dong, G.C.; Mo, X.X.; Zhao, Z.D.; Zhu, D.C.; Goodman, R.C.; Kong, H.L.; Wang, S. Zircon U–Pb dating and the petrological and geochemical constraints on Lincang granite in Western Yunnan, China: Implications for the closure of the Paleo-Tethys Ocean. J. Asian Earth Sci. 2013, 62, 282–294. [Google Scholar] [CrossRef]
- Ming, T.X.; Tang, Z.; Bao, C.F.; Li, R.; Zhan, D.Q.; Yang, Q.B.; Hao, X.F.; Yu, H.J. Prospect, research progress and distribution characteristics of rare earth minerals in Yunnan Province. J. Chin. Soc. Rare Earths 2022, 40, 577–590. [Google Scholar]
- Fan, W.M.; Pang, T.P.; Wang, Y.J. Triassic magmatism in the southern Lancangjiang zone, southwestern China and its constraints on the tectonic evolution of Paleo-Tethys. Earth Sci. Front. 2009, 16, 291–302. [Google Scholar]
- Jian, P.; Liu, D.Y.; Zhang, Q.; Zhang, F.Q.; Shi, Y.R.; Shi, G.H.; Zhang, C.Q.; Tao, H. Shrimp dating of ophiolite and leucocratic rocks within ophiolite. Earth Sci. Front. 2003, 10, 439–456. [Google Scholar]
- Lu, L.; Wang, D.H.; Wang, C.H.; Zhao, Z.; Feng, W.J.; Xu, X.C.; Chen, C.; Zhong, H.R. The metallogenic regularity of ion-adsorption type REE deposit in Yunnan Province. Acta Geol. Sin. 2020, 94, 179–191. [Google Scholar]
- Lin, L. Rock Geochemical Characteristics of Lincang Granodiorite and Its Genesis in Yunnan. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2017. [Google Scholar]
- Li, J.F.; Ma, K.M.; Lu, Y.Y.; Fu, J.M.; Cheng, S.B.; Li, Y.; Li, C.B. Timing and tectonic setting of the Gaoaobei Tungsten-Molybdenum deposit in Nanling Range, south China. J. Earth Sci. 2024, 35, 890. [Google Scholar] [CrossRef]
- Xu, Q.H.; Yang, L.F.; Wang, D.S.; Hou, X.; Sun, Y.Y.; Zhou, X.Z.; Zhou, X.M.; Li, Y.X. Evaluating the fractionation of ion-adsorption rare earths for in-situ leaching and metallogenic mechanism. J. Rare Earths 2018, 36, 1333–1341. [Google Scholar] [CrossRef]
- Bao, Z.W.; Zhao, Z.H. Geochemistry of mineralization with exchangeable REY in the weathering crusts of granitic rocks in south China. Ore Geol. Rev. 2008, 33, 519–535. [Google Scholar] [CrossRef]
- Liu, W.; Tian, G.; Guo, Y.C.; Gu, Z.L.; Yang, Z.F. Study on extraction method of ionic phase rare earth from ionic rare earth ore. J. Chin. Soc. Rare Earths 2023, 43, 173–182. [Google Scholar]
- Luo, F.; Yan, J.Y.; Liang, J.; Zhang, S.; Wu, D.H.; Rao, G.W.; Hu, W.Q.; Fu, G.M. Application status and prospect of geophysical technology in rare-earth resources field. J. Chin. Soc. Rare Earths 2024, 42, 1088–1101. [Google Scholar]
- Zhang, Z.Y.; Jiang, L.; Guo, W.D.; Yang, J.; Liu, D.F. Flotation behavior and adsorption mechanism of 2-hydroxy-3-naphthyl hydroxamic acid on bastnaesite surface. J. Rare Earths 2024, 43, 1084–1090. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Sun, N.J.; He, Z.Y.; Chi, R.A. Local concentration of middle and meavy rare earth elements in the col on the weathered crust elution-deposited rare earth ores. J. Rare Earths 2018, 36, 552–558. [Google Scholar] [CrossRef]
- Bai, Y.; Deng, Z.X.; Bi, X.L.; Chuan, W.T.; Yin, W.; Xia, J.F. New discovery of rare-earth mineralization of the weathering crust type and ore-search prospect in the Lancang area, Yunnan province. Geol. Explor. 2021, 57, 762–783. [Google Scholar]
- Lu, L.; Wang, D.H.; Wang, C.H.; Zhao, Z.; Feng, W.J.; Xu, X.H.; Yu, S. Mineralization regularity of ion-adsorption type REE deposits on Lincang granite in Yunnan Province. Acta Geol. Sin. 2019, 93, 1466–1478. [Google Scholar]
- Zhang, M.; Li, Y.; He, X.C.; Feng, J.L.; Zheng, Y.; Wang, H.; Du, J.G.; Wang, S.S. Mineralization of the ion adsorption-type REE deposits in the central part of the Lincang granites in western Yunnan. Sediment. Geol. Tethyan Geol. 2018, 38, 37–47. [Google Scholar]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-Ray Diffraction techniques for mineral characterization: A review for engineers of the fundamentals, applications, and research directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Lin, Z.X. Distinguishing Halloysite from Kaolinite by the use of Dimethyl Sulfoxide treatment. Rock Miner. Anal. 1988, 1, 58–61. [Google Scholar]
- GB/T 25282-2010; Sequential Extraction Program for 13 Trace Element Forms in Soil and Sediment: General Administration of Quality Supervision. Standardization Administration of the People’s Republic of China: Beijing, China, 2010; p. 12.
- Xu, Y.J.; Zuo, W.Z. The preliminary research on the cause of the clay minerals. J. North China Univ. Sci. Technol. (Nat. Sci. Ed.) 2013, 35, 68–72. [Google Scholar]
- Chen, T.; Wang, H.J.; Zhang, Z.Q.; Wang, H. An approach to paleoclimate- reconstruction by clay minerals. Acta Sci. Nat. Univ. Pekin. 2005, 2, 309–316. [Google Scholar]
- Huang, J. REE Enrichment and Fractionation Mechanism of the Renju Ion Adsorption Type REE Deposit in Guangdong Province. Ph.D. Thesis, University of Chinese Academy of Sciences (Guangzhou Institute of Geochemistry, Chinese Academy of Sciences), Beijing, China, 2021. [Google Scholar]
- Rickwood, P.C. Boundary lines within petrologic diagrams which use oxides of major and minor elements. Lithos 1989, 22, 247–263. [Google Scholar] [CrossRef]
- Nesbitt, H.; Young, G. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.W.; Zhou, M.F. Nature of parent rocks, mineralization styles and ore genesis of regolith-hosted REE deposits in South China: An integrated genetic model. J. Asian Earth Sci. 2017, 148, 65–95. [Google Scholar] [CrossRef]
- Deng, Y.F. An attempt to locate hidden leaching adsorption-type rare earth deposits in granite weathering crusts using soil pH values. Earth Environ. 1985, 2, 74–78. [Google Scholar]
- Bern, C.R.; Yesavage, T.; Foley, N.K. Ion-adsorption REEs in regolith of the Liberty Hill pluton, South Carolina, USA: An effect of hydrothermal alteration. J. Geochem. Explor. 2017, 172, 29–40. [Google Scholar] [CrossRef]
- Li, G.L.; Long, W.K.; Luo, W.P.; Yang, Y.L.; Liu, X.D.; Wu, B.; Wei, X.L.; Li, C.X. A detailed research on allanite in weathered sphere from the Xiajialing REE deposit in Xiangshan, Jiangxi, and its metallogenic significance. Acta Petrol. Sin. 2022, 38, 2067–2079. [Google Scholar]
- Peng, L.L.; Chen, B.F.; Zou, X.Y.; Zou, Z.Q.; Xiao, W.G.; Que, X.H. Rare earth partitioning characteristics and new scheme for classification of ion-adsorption type rare earth ore. J. Chin. Soc. Rare Earths 2021, 39, 624–632. [Google Scholar]
- Boynton, W.V. Cosmochemistry of the rare earth elements: Meteorite studies. In Developments in Geochemistry; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Pan, J.X.; Zhao, L.S.; Li, Z.; Huang, X.W.; Feng, Z.Y.; Chen, J. Occurrence and vertical distribution of aluminum and rare earths in weathered crust elution-deposited rare earth ore: Effect of soil solution pH and clay minerals. J. Rare Earths 2024, 42, 1394–1402. [Google Scholar] [CrossRef]
- Jian, D.; Xing, D. Complexation of REE in hydrothermal fluids and its significance on REE mineralization. Minerals 2024, 14, 531. [Google Scholar] [CrossRef]
- Pan, Z.W.; Lu, Y.X.; Luo, J.H.; Tang, Z.; Yu, H.J.; Su, X.Y.; Yang, Q.B.; Fu, H. REE distribution characteristics of the Yingpanshan ion adsorption type rare-earth deposit in the Longchuan area of western Yunnan. Geol. Explor. 2021, 57, 784–795. [Google Scholar]
- He, Y.; Cheng, L.; Li, Y.; Ran, D.J.; Wei, Q.S. The mineralization mechanism of the ion adsorption type rare earths ore and prospecting marks. Chin. Rare Earths 2015, 36, 98–103. [Google Scholar]
- Ichimura, K.; Sanematsu, K.; Kon, Y.; Takagi, T.; Murakami, T. REE redistributions during granite weathering: Implications for Ce anomaly as a proxy for paleoredox states. Am. Mineral. 2020, 105, 848–859. [Google Scholar] [CrossRef]
- Janots, E.; Bernier, F.; Brunet, F.; Muñoz, M.; Trcera, N.; Berger, A.; Lanson, M. Ce(III) and Ce(IV) (re)distribution and fractionation in a laterite profile from Madagascar: Insights from in situ XANES spectroscopy at the Ce LIII-edge. Geochim. Cosmochim. Acta 2015, 153, 134–148. [Google Scholar] [CrossRef]
- Ohta, A.; Kagi, H.; Nomura, M.; Tsuno, H.; Kawabe, I. Coordination study of rare earth elements on Fe oxyhydroxide and Mn dioxides: Part I. Influence of a multi-electron excitation on EXAFS analyses of La, Pr, Nd, and Sm. Am. Mineral. 2009, 94, 467–475. [Google Scholar] [CrossRef]
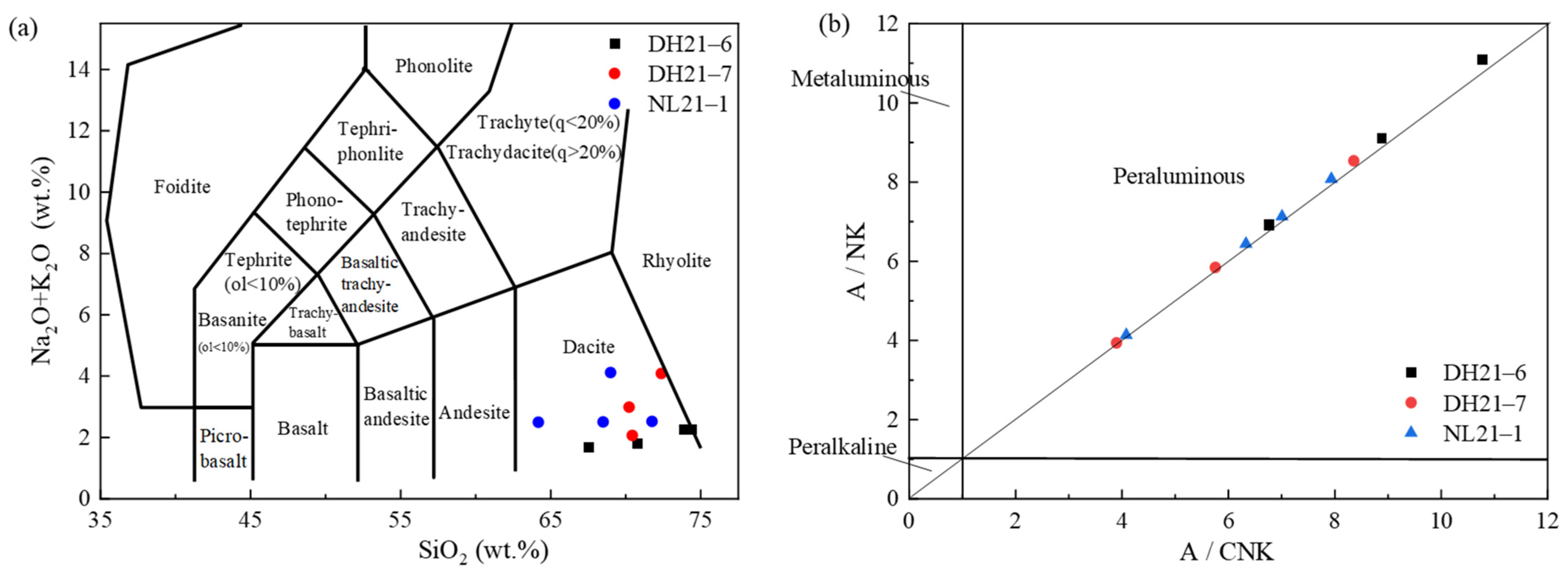
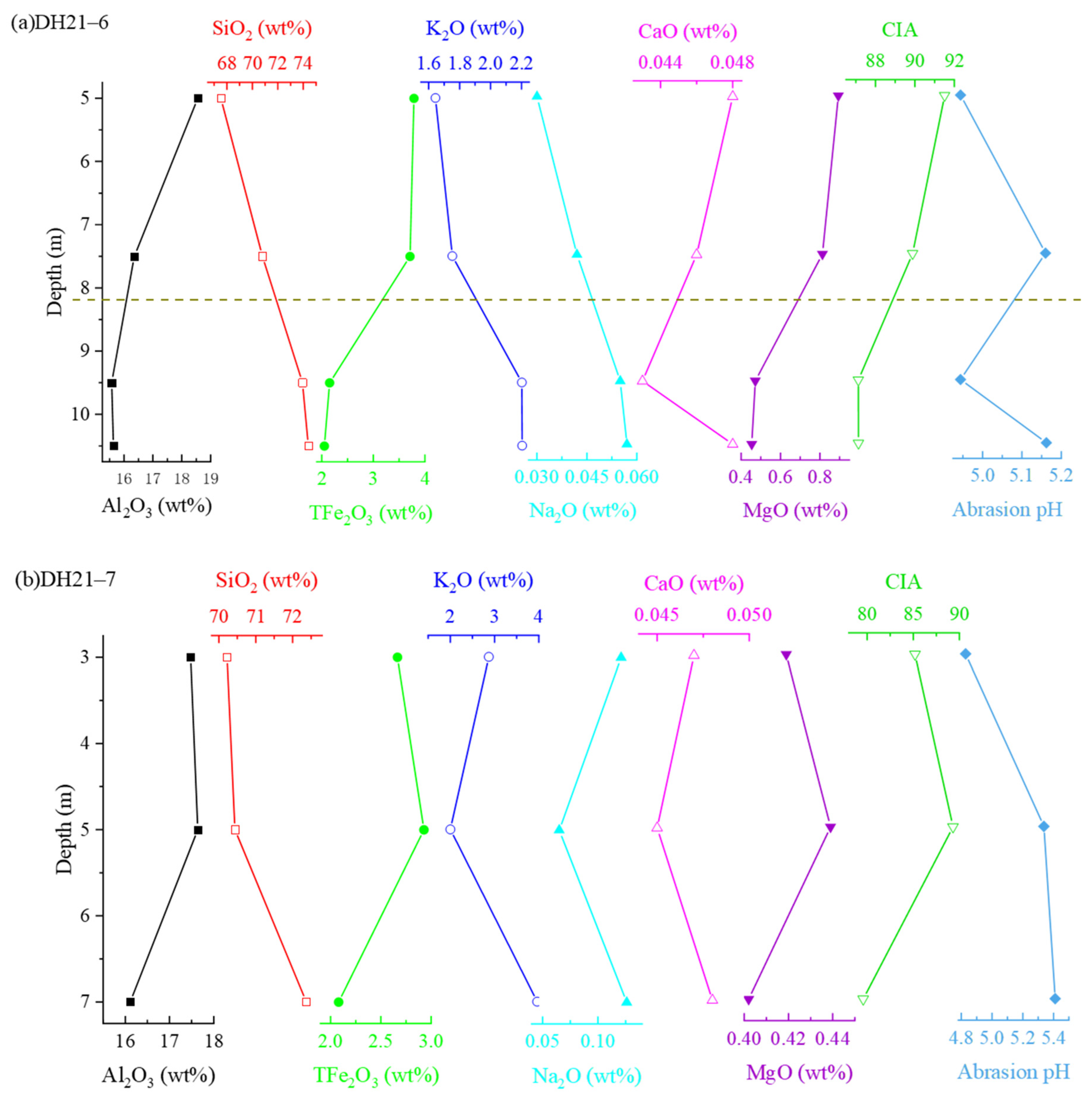

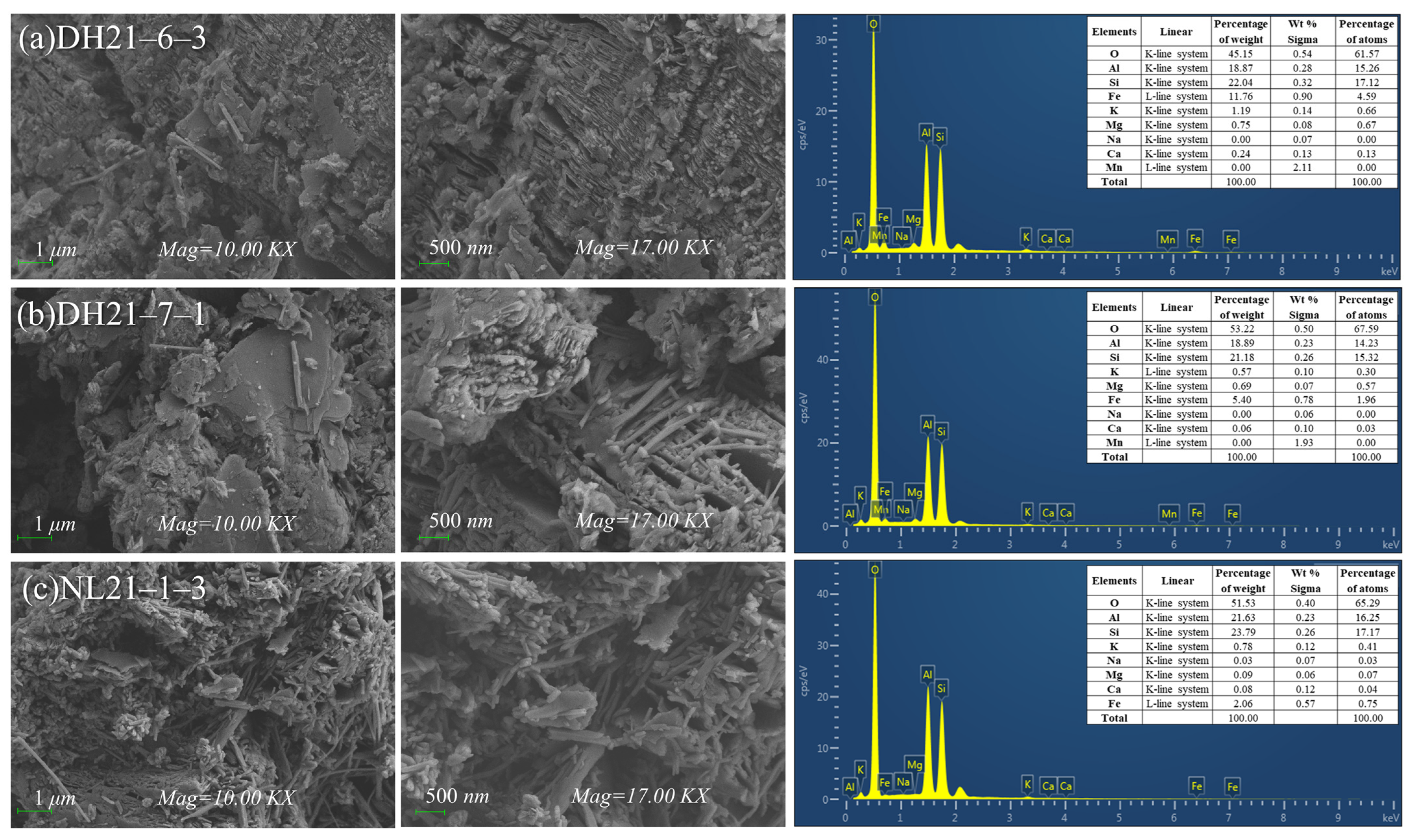
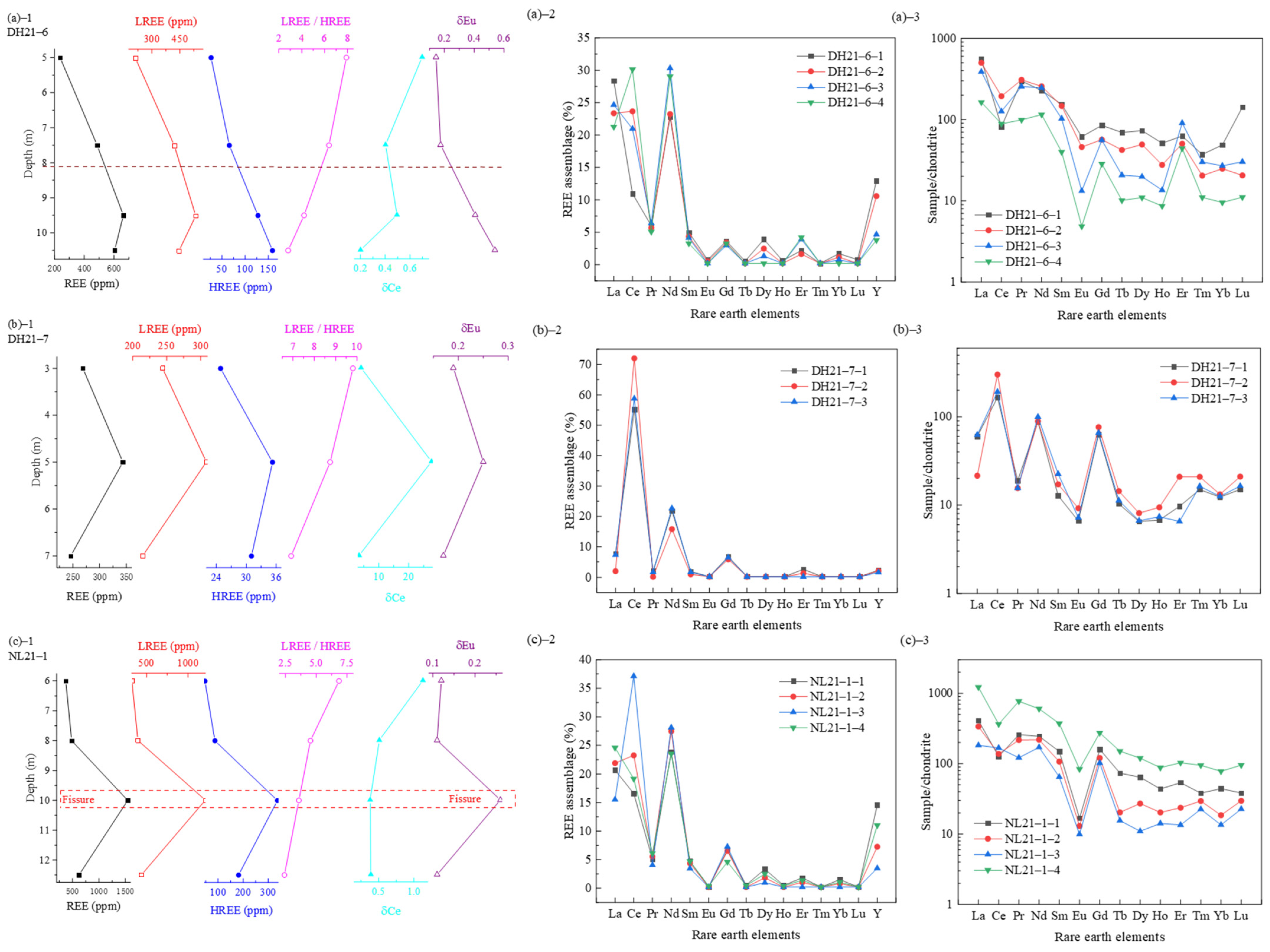

| Profile Number | Altitude (m) | Sampling Number | Depth (m) |
|---|---|---|---|
| DH21–6 Donghe Township | 2070 | DH21–6–1 | 10.5 |
| DH21–6–2 | 9.5 | ||
| DH21–6–3 | 7.5 | ||
| DH21–6–4 | 5 | ||
| DH21–7 Donghe Township | 2120 | DH21–7–1 | 7 |
| DH21–7–2 | 5 | ||
| DH21–7–3 | 3 | ||
| NL21–1 Nanling Township | 1880 | NL21–1–1 | 12.5 |
| NL21–1–2 | 10 | ||
| NL21–1–3 | 8 | ||
| NL21–1–4 | Fissure |
| Rock Mass | Syntexis-Type Intermediate-Acid Hypabyssal Rocks and Porphyry | Lincang and Fengqing Western Rock Bodies | Anatectic Granite | REE Characteristics of Different Lithologies in Lincang Batholith | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | Dongqing Biotite Monzogranite | Biotite Monzogranite | Medium-Grained Biotite Granite | |||
| ΣREE (ppm) | 148 | 164 | 213 | 226 | 212.19 | 221.1 | 215.81 |
| ΣCe/ΣY | 4.48 | 6.30 | 3.91 | 2.33 | 5.16 | 3.87 | 2.86 |
| δEu | 0.94 | 1.03 | 0.52 | 0.32 | 0.59 | 0.53 | 0.14 |
| La/Sm | 7.96 | 8.1 | 6.9 | 6.05 | 6.73 | 5.98 | 5.11 |
| La/Yb | 19.67 | 30.7 | 18.0 | 9.68 | 9.93 | 12.69 | 7.25 |
| (Ce/Yb)n | 7.06 | 11.4 | 6.1 | 3.32 | 5.89 | 7.54 | 4.31 |
| Sampling Number | It (%) | S (%) | Hal/Kao | Kao (%) | Hal (%) |
|---|---|---|---|---|---|
| DH21–6–1 | 6.22 | 1.90 | 13.81 | 6.20 | 85.68 |
| DH21–6–2 | 4.75 | 1.42 | 15.01 | 5.86 | 87.97 |
| DH21–6–3 | 2.11 | 1.14 | 1.28 | 42.44 | 54.31 |
| DH21–6–4 | 2.76 | 0.14 | 1.83 | 34.33 | 62.77 |
| DH21–7–1 | 3.90 | 0.01 | 1.90 | 33.15 | 62.93 |
| DH21–7–2 | 1.47 | 0.60 | 0.29 | 75.72 | 22.20 |
| DH21–7–3 | 1.09 | 0.56 | 1.29 | 43.03 | 55.32 |
| NL21–1–1 | 4.04 | 0.92 | 6.10 | 13.38 | 81.66 |
| NL21–1–2 | 2.78 | 0.14 | 3.06 | 23.89 | 73.19 |
| NL21–1–3 | 2.47 | 0.06 | 3.21 | 23.15 | 74.32 |
| NL21–1–4 | 1.53 | 0.25 | 2.07 | 32.01 | 66.21 |
| Sample | DH21–6 | DH21–7 | NL21–1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | 4 |
| Major Elements (wt.%) | |||||||||||
| SiO2 | 74.394 | 73.929 | 70.794 | 67.521 | 72.367 | 70.433 | 70.219 | 68.990 | 71.750 | 68.490 | 64.175 |
| TiO2 | 0.235 | 0.252 | 0.556 | 0.568 | 0.265 | 0.298 | 0.348 | 0.447 | 0.458 | 0.502 | 0.462 |
| Al2O3 | 15.635 | 15.571 | 16.354 | 18.555 | 16.112 | 17.638 | 17.480 | 17.048 | 16.271 | 17.895 | 20.168 |
| TFe2O3 | 2.049 | 2.153 | 3.710 | 3.788 | 2.080 | 2.928 | 2.665 | 3.436 | 3.227 | 3.518 | 4.114 |
| FeO | 0.400 | 0.400 | 0.140 | 0.200 | 0.200 | 0.240 | 0.400 | 0.240 | 0.120 | 0.240 | 0.680 |
| MnO | 0.055 | 0.036 | 0.051 | 0.028 | 0.046 | 0.042 | 0.026 | 0.022 | 0.034 | 0.088 | 0.053 |
| MgO | 0.454 | 0.472 | 0.813 | 0.894 | 0.402 | 0.439 | 0.419 | 0.625 | 0.415 | 0.693 | 0.816 |
| CaO | 0.048 | 0.043 | 0.046 | 0.048 | 0.048 | 0.045 | 0.047 | 0.059 | 0.045 | 0.044 | 0.045 |
| Na2O | 0.057 | 0.055 | 0.042 | 0.030 | 0.126 | 0.065 | 0.121 | 0.154 | 0.079 | 0.109 | 0.053 |
| K2O | 2.204 | 2.202 | 1.753 | 1.644 | 3.962 | 2.001 | 2.870 | 3.966 | 2.447 | 2.401 | 2.444 |
| P2O5 | 0.028 | 0.031 | 0.042 | 0.040 | 0.026 | 0.027 | 0.032 | 0.049 | 0.047 | 0.047 | 0.063 |
| ZrO2 | 0.018 | 0.022 | 0.033 | 0.038 | 0.021 | 0.026 | 0.027 | 0.028 | 0.029 | – | – |
| REO | 0.060 | 0.066 | 0.049 | 0.024 | 0.025 | 0.034 | 0.026 | 0.062 | 0.048 | 0.037 | 0.155 |
| LOI | 4.363 | 4.768 | 5.617 | 6.622 | 4.320 | 5.784 | 5.320 | 4.874 | 5.030 | 5.936 | 6.772 |
| REE (ppm) | |||||||||||
| REEs | 603.750 | 662.500 | 486.880 | 238.120 | 245.870 | 343.570 | 268.610 | 620.510 | 482.950 | 370.180 | 1545.530 |
| LREEs | 445.330 | 535.100 | 420.810 | 211.360 | 214.830 | 308.300 | 243.750 | 438.910 | 395.650 | 322.990 | 1208.720 |
| HREEs | 158.480 | 127.400 | 66.070 | 26.790 | 31.040 | 35.270 | 24.850 | 181.610 | 87.310 | 47.190 | 336.820 |
| LREEs/HREEs | 2.810 | 4.200 | 6.370 | 7.890 | 6.920 | 8.740 | 9.810 | 2.420 | 4.530 | 6.840 | 3.590 |
| δCe | 0.200 | 0.490 | 0.400 | 0.700 | 3.430 | 27.620 | 4.080 | 0.390 | 0.510 | 1.130 | 0.380 |
| δEu | 0.540 | 0.410 | 0.180 | 0.140 | 0.170 | 0.250 | 0.190 | 0.110 | 0.110 | 0.120 | 0.260 |
| La/Yb | 16.780 | 21.240 | 33.770 | 53.150 | 38.350 | 9.850 | 36.600 | 13.790 | 27.040 | 77.600 | 23.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, D.; Long, F.; Chi, R.; Chen, Z. Enrichment and Fractionation of Rare Earth Elements in High-Altitude Thick Weathered Crust Elution-Deposited Rare Earth Ore. Minerals 2025, 15, 932. https://doi.org/10.3390/min15090932
Zhang Z, Li D, Long F, Chi R, Chen Z. Enrichment and Fractionation of Rare Earth Elements in High-Altitude Thick Weathered Crust Elution-Deposited Rare Earth Ore. Minerals. 2025; 15(9):932. https://doi.org/10.3390/min15090932
Chicago/Turabian StyleZhang, Zhenyue, Dan Li, Fei Long, Ruan Chi, and Zhuo Chen. 2025. "Enrichment and Fractionation of Rare Earth Elements in High-Altitude Thick Weathered Crust Elution-Deposited Rare Earth Ore" Minerals 15, no. 9: 932. https://doi.org/10.3390/min15090932
APA StyleZhang, Z., Li, D., Long, F., Chi, R., & Chen, Z. (2025). Enrichment and Fractionation of Rare Earth Elements in High-Altitude Thick Weathered Crust Elution-Deposited Rare Earth Ore. Minerals, 15(9), 932. https://doi.org/10.3390/min15090932







