Biogeochemical Interactions and Their Role in European Underground Hydrogen Storage
Abstract
1. Introduction
2. Microbial Risk During UHS
2.1. Physicochemical Properties of H2 Gas
2.2. Key Microbial Groups Involved in UHS
3. Biogeochemical Interactions by Different Microbial Groups
3.1. Sulfate-Reducing Bacteria (SRB)
3.2. Methanogens
3.3. Acetogens
3.4. Iron-Reducing Bacteria (IRB)
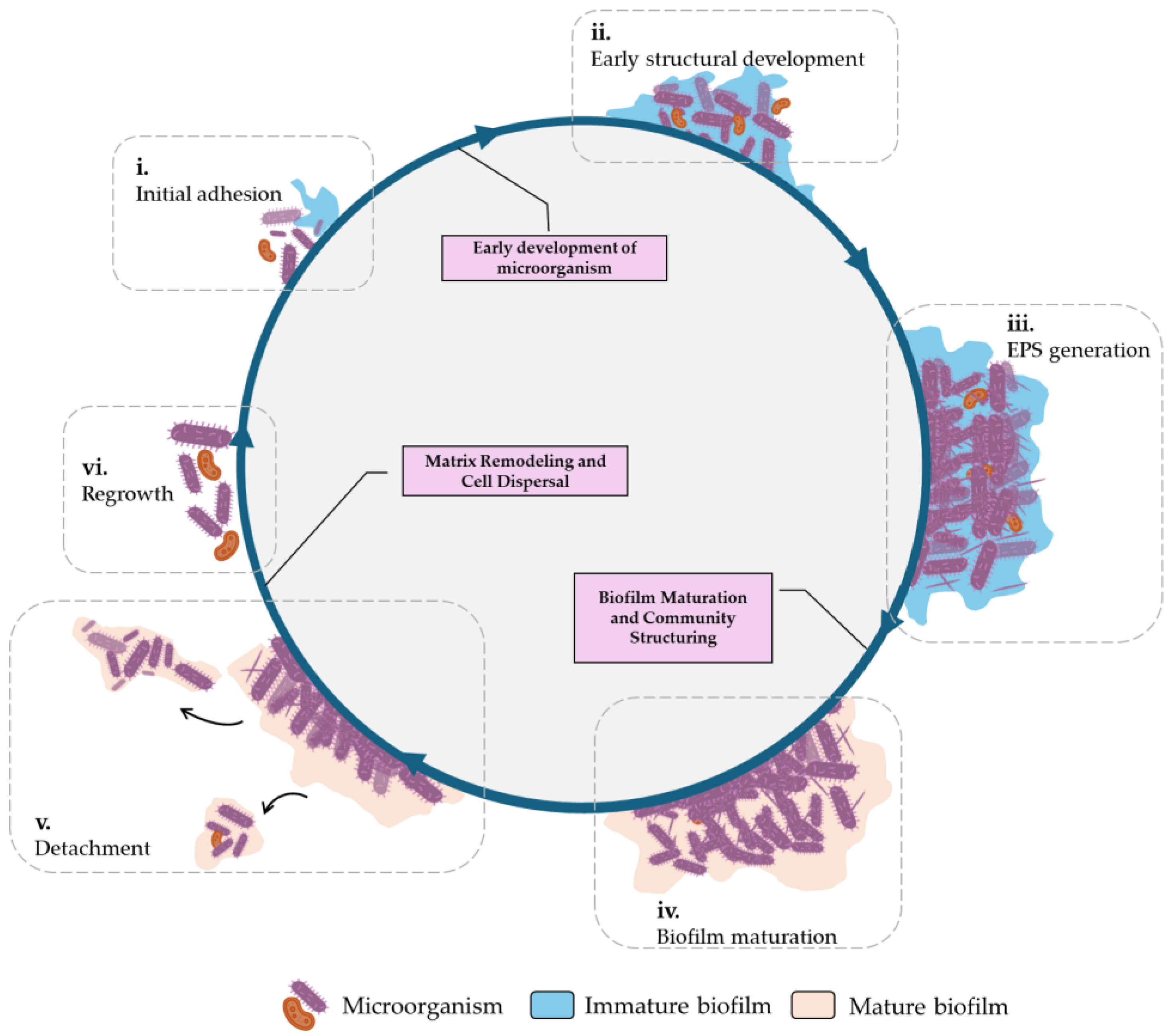
3.5. Biofilms
4. Potential Storage Sites
4.1. Salt Caverns
4.2. Depleted Hydrocarbon Reservoirs (DHR)
4.3. Aquifers
| Aquifer Type | Rock Type | Minerals | Interaction |
|---|---|---|---|
| Unconsolidated sedimentary | Sand and gravel | Qz, Or, Ab, Ill, Mt, Kln | A|M, IRB |
| Consolidated sedimentary | Sandstone | Qz, Or, Ab, Ill, Mt, Kln | IRB, A|M |
| Limestone | Cal, Dol, Py | A|M | |
| Fractured rock | Igneous (granite/gneiss) | Qz, Or, Ab, Py | IRB |
| Metamorphic (schist/gneiss) | Qz, Or, Ab, Py | IRB | |
| Karst | Carbonate (limestone/dolomite) | Cal, Dol | A|M, IRB (indirect) |
5. European Union Projects
6. Methodological Framework Approaches: Experiments to Simulations
6.1. Experimental Methodologies for Investigating Biogeochemical Processes
6.2. Modeling Microbial and Geochemical Interactions: From Laboratory Data to Field-Scale Simulations
7. Knowledge Gaps and Research Priorities
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UHS | Underground hydrogen storage |
| DHR | Depleted hydrocarbon reservoir(s) |
| SRB | Sulfate-reducing bacteria |
| IRB | Iron-reducing bacteria |
| A|M | Acetogens|Methanogens |
| GLRM | Gas–liquid–rock–microorganism |
| AQU | Aquifer |
| Qz | Quartz |
| Or | Orthoclase |
| Ab | Albite |
| Ill | Illite |
| Mt | Montmorillonite |
| Kln | Kaolinite |
| Cal | Calcite |
| Dol | Dolomite |
| Py | Pyrite |
| aw | Water activity |
References
- Epelle, E.I.; Obande, W.; Udourioh, G.A.; Afolabi, I.C.; Desongu, K.S.; Orivri, U.; Gunes, B.; Okolie, J.A. Perspectives and Prospects of Underground Hydrogen Storage and Natural Hydrogen. Sustain. Energy Fuels 2022, 6, 3324–3343. [Google Scholar] [CrossRef]
- Wei, X.; Shi, X.; Li, Y.; Ma, H.; Ban, S.; Liu, X.; Liu, H.; Yang, C. Analysis of the European Energy Crisis and Its Implications for the Development of Strategic Energy Storage in China. J. Energy Storage 2024, 82, 110522. [Google Scholar] [CrossRef]
- Albadi, M.H.; El-Saadany, E.F. Overview of Wind Power Intermittency Impacts on Power Systems. Electr. Power Syst. Res. 2010, 80, 627–632. [Google Scholar] [CrossRef]
- Gomez Mendez, I.; El-Sayed, W.M.M.; Menefee, A.H.; Karpyn, Z.T. Insights into Underground Hydrogen Storage Challenges: A Review on Hydrodynamic and Biogeochemical Experiments in Porous Media. Energy Fuels 2024, 38, 20015–20032. [Google Scholar] [CrossRef]
- Giraldo, L.J.; Medina, O.E.; Ortiz-Pérez, V.; Franco, C.A.; Cortés, F.B. Enhanced Carbon Storage Process from Flue Gas Streams Using Rice Husk Silica Nanoparticles: An Approach in Shallow Coal Bed Methane Reservoirs. Energy Fuels 2023, 37, 2945–2959. [Google Scholar] [CrossRef]
- Cihlar, J.; Mavins, D.; Van der Leun, K. Picturing the Value of Underground Gas Storage to the European Hydrogen System; Guidehouse: Chicago, IL, USA, 2021. [Google Scholar]
- Fuel Cells and Hydrogen 2 Joint Undertaking Hydrogen Roadmap Europe—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/0817d60d-332f-11e9-8d04-01aa75ed71a1/language-en (accessed on 27 June 2025).
- Khan, T.; Waseem, M.; Tahir, M.; Liu, S.; Yu, M. Autonomous Hydrogen-Based Solar-Powered Energy System for Rural Electrification in Balochistan, Pakistan: An Energy-Economic Feasibility Analysis. Energy Convers. Manag. 2022, 271, 116284. [Google Scholar] [CrossRef]
- CORDIS EU. Assessment of the Potential, the Actors and Relevant Business Cases for Large Scale and Long Term Storage of Renewable Electricity by Hydrogen Underground Storage in Europe; CORDIS: Brussels, Belgium, 2024. [Google Scholar]
- Heinemann, N.; Alcalde, J.; Miocic, J.M.; Hangx, S.J.T.; Kallmeyer, J.; Ostertag-Henning, C.; Hassanpouryouzband, A.; Thaysen, E.M.; Strobel, G.J.; Schmidt-Hattenberger, C.; et al. Enabling Large-Scale Hydrogen Storage in Porous Media—The Scientific Challenges. Energy Environ. Sci. 2021, 14, 853–864. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, J.; Elsworth, D. Phenomenal Study of Microbial Impact on Hydrogen Storage in Aquifers: A Coupled Multiphysics Modelling. Int. J. Hydrogen Energy 2024, 79, 883–900. [Google Scholar] [CrossRef]
- Dopffel, N.; Jansen, S.; Gerritse, J. Microbial Side Effects of Underground Hydrogen Storage—Knowledge Gaps, Risks and Opportunities for Successful Implementation. Int. J. Hydrogen Energy 2021, 46, 8594–8606. [Google Scholar] [CrossRef]
- Liu, N.; Dopffel, N.; Stepec, B.A.A. Impact of Microbial Biofilms on Subsurface Energy Systems: From Oil and Gas to Renewable Energy. In Petroleum Microbiology: The Role of Microorganisms in the Transition to Net Zero Energy; CRC Press: Boca Raton, FL, USA, 2024; pp. 21–36. [Google Scholar] [CrossRef]
- Boon, M.; Buntic, I.; Ahmed, K.; Dopffel, N.; Peters, C.; Hajibeygi, H. Microbial Induced Wettability Alteration with Implications for Underground Hydrogen Storage. Sci. Rep. 2024, 14, 8248. [Google Scholar] [CrossRef]
- Dopffel, N.; Mayers, K.; Kedir, A.; Alagic, E.; An-Stepec, B.A.; Djurhuus, K.; Boldt, D.; Beeder, J.; Hoth, S. Microbial Hydrogen Consumption Leads to a Significant PH Increase under High-Saline-Conditions: Implications for Hydrogen Storage in Salt Caverns. Sci. Rep. 2023, 13, 10564. [Google Scholar] [CrossRef]
- Wu, L.; Hou, Z.; Luo, Z. Impacts of Microbial Competition on Underground Bio-Methanation of Hydrogen and Carbon Dioxide: Insights from Biogeochemical Simulations. Renew. Energy 2025, 251, 123458. [Google Scholar] [CrossRef]
- Vasile, N.S. A Comprehensive Review of Biogeochemical Modeling of Underground Hydrogen Storage: A Step Forward in Achieving a Multi-Scale Approach. Energies 2024, 17, 6094. [Google Scholar] [CrossRef]
- Thaysen, E.M.; McMahon, S.; Strobel, G.J.; Butler, I.B.; Ngwenya, B.T.; Heinemann, N.; Wilkinson, M.; Hassanpouryouzband, A.; McDermott, C.I.; Edlmann, K. Estimating Microbial Growth and Hydrogen Consumption in Hydrogen Storage in Porous Media. Renew. Sustain. Energy Rev. 2021, 151, 111481. [Google Scholar] [CrossRef]
- Liu, N.; Kovscek, A.R.; Fernø, M.A.; Dopffel, N. Pore-Scale Study of Microbial Hydrogen Consumption and Wettability Alteration during Underground Hydrogen Storage. Front. Energy Res. 2023, 11, 1124621. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D. Role of Methane as a Cushion Gas for Hydrogen Storage in Depleted Gas Reservoirs. Int. J. Hydrogen Energy 2023, 48, 29663–29681. [Google Scholar] [CrossRef]
- Ebrahimiyekta, A. Characterization of Geochemical Interactions and Migration of Hydrogen in Sandstone Sedimentary Formations: Application to Geological Storage. Ph.D. Thesis, Université d’Orléans, Orléans, France, 2017. [Google Scholar]
- Hassanpouryouzband, A.; Adie, K.; Cowen, T.; Thaysen, E.M.; Heinemann, N.; Butler, I.B.; Wilkinson, M.; Edlmann, K. Geological Hydrogen Storage: Geochemical Reactivity of Hydrogen with Sandstone Reservoirs. ACS Energy Lett. 2022, 7, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Zeng, L.; Chen, Y.; Xie, Q. Geochemical Reactions-Induced Hydrogen Loss during Underground Hydrogen Storage in Sandstone Reservoirs. Int. J. Hydrogen Energy 2021, 46, 19998–20009. [Google Scholar] [CrossRef]
- Hemme, C.; van Berk, W. Hydrogeochemical Modeling to Identify Potential Risks of Underground Hydrogen Storage in Depleted Gas Fields. Appl. Sci. 2018, 8, 2282. [Google Scholar] [CrossRef]
- Viveros, F.E.; Medina, O.E.; Moncayo-Riascos, I.; Lysyy, M.; Benjumea, P.N.; Cortés, F.B.; Franco, C.A. Hydrogen Storage in Depleted Gas Reservoirs Using Methane Cushion Gas: An Interfacial Tension and Pore Scale Study. J. Energy Storage 2024, 98, 113110. [Google Scholar] [CrossRef]
- Bartolomeu, R.A.C.; Franco, L.F.M. Thermophysical Properties of Supercritical H-2 from Molecular Dynamics Simulations. Int. J. Hydrogen Energy 2020, 45, 16372. [Google Scholar] [CrossRef]
- Jahanbakhsh, A.; Louis Potapov-Crighton, A.; Mosallanezhad, A.; Tohidi Kaloorazi, N.; Maroto-Valer, M.M. Underground Hydrogen Storage: A UK Perspective. Renew. Sustain. Energy Rev. 2024, 189, 114001. [Google Scholar] [CrossRef]
- Arnoldini, M.; Cremer, J.; Hwa, T. Bacterial Growth, Flow, and Mixing Shape Human Gut Microbiota Density and Composition. Gut Microbes 2018, 9, 559–566. [Google Scholar] [CrossRef]
- Lysyy, M.; Liu, N.; Landa-Marbán, D.; Ersland, G.; Fernø, M. Impact of Gas Type on Microfluidic Drainage Experiments and Pore Network Modeling Relevant for Underground Hydrogen Storage. J. Energy Storage 2024, 87, 111439. [Google Scholar] [CrossRef]
- Lysyy, M.; Ersland, G.; Fernø, M. Pore-Scale Dynamics for Underground Porous Media Hydrogen Storage. Adv. Water Resour. 2022, 163, 104167. [Google Scholar] [CrossRef]
- Yu, S.; Hu, M.; Steefel, C.I.; Battiato, I. Unraveling Residual Trapping for Geologic Hydrogen Storage and Production Using Pore-Scale Modeling. Adv. Water Resour. 2024, 185, 104659. [Google Scholar] [CrossRef]
- Lysyy, M.; Fernø, M.; Ersland, G. Seasonal Hydrogen Storage in a Depleted Oil and Gas Field. Int. J. Hydrogen Energy 2021, 46, 25160–25174. [Google Scholar] [CrossRef]
- Anderson, R.T.; Chapelle, F.H.; Lovley, D.R. Evidence against Hydrogen-Based Microbial Ecosystems in Basalt Aquifers. Science (1979) 1998, 281, 976–977. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Sakthivel, S.; Yekeen, N.; Norrman, K.; Nzila, A. The Influence of Microbial Activities on the Capillary Pressure during H2 Injection: Implications for Underground H2 Storage. Energy Fuels 2023, 38, 499–505. [Google Scholar] [CrossRef]
- Conrad, R. Contribution of Hydrogen to Methane Production and Control of Hydrogen Concentrations in Methanogenic Soils and Sediments. FEMS Microbiol. Ecol. 1999, 28, 193–202. [Google Scholar] [CrossRef]
- Pichler, M. Underground Sun Storage Results and Outlook. In Proceedings of the EAGE/DGMK Joint Workshop on Underground Storage of Hydrogen, Celle, Germany, 24 April 2019; Volume 2019, pp. 1–4. [Google Scholar] [CrossRef]
- Panfilov, M. Underground Storage of Hydrogen: In Situ Self-Organisation and Methane Generation. Transp. Porous Media 2010, 85, 841–865. [Google Scholar] [CrossRef]
- Jahanbani Veshareh, M.; Thaysen, E.M.; Nick, H.M. Feasibility of Hydrogen Storage in Depleted Hydrocarbon Chalk Reservoirs: Assessment of Biochemical and Chemical Effects. Appl. Energy 2022, 323, 119575. [Google Scholar] [CrossRef]
- Schwarz, F.M.; Müller, V. Whole-Cell Biocatalysis for Hydrogen Storage and Syngas Conversion to Formate Using a Thermophilic Acetogen. Biotechnol. Biofuels 2020, 13, 32. [Google Scholar] [CrossRef]
- Panfilov, M. Underground and Pipeline Hydrogen Storage. In Compendium of Hydrogen Energy, Volume 2: Hydrogen Storage, Transportation and Infrastructure; Elsevier: Amsterdam, The Netherlands, 2016; pp. 91–115. [Google Scholar] [CrossRef]
- Berta, M.; Dethlefsen, F.; Ebert, M.; Schäfer, D.; Dahmke, A. Geochemical Effects of Millimolar Hydrogen Concentrations in Groundwater: An Experimental Study in the Context of Subsurface Hydrogen Storage. Environ. Sci. Technol. 2018, 52, 4937–4949. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ostertag-Henning, C.; Fernø, M.A.; Dopffel, N. Growth on Hydrogen by the Sulfate-Reducing Oleidesulfovibrio Alaskensis Induces Biofilm Dispersion and Detachment─Implications for Underground Hydrogen Storage. Environ. Sci. Technol. 2025, 59, 7105. [Google Scholar] [CrossRef] [PubMed]
- Vance, I.; Thrasher, D.R. Reservoir Souring: Mechanisms and Prevention. In Petroleum Microbiology; Wiley: Hoboken, NJ, USA, 2014; pp. 123–142. [Google Scholar] [CrossRef]
- Hellerschmied, C.; Schritter, J.; Waldmann, N.; Zaduryan, A.B.; Rachbauer, L.; Scherr, K.E.; Andiappan, A.; Bauer, S.; Pichler, M.; Loibner, A.P. Hydrogen Storage and Geo-Methanation in a Depleted Underground Hydrocarbon Reservoir. Nat. Energy 2024, 9, 333–344. [Google Scholar] [CrossRef]
- Smith, N.W.; Shorten, P.R.; Altermann, E.; Roy, N.C.; McNabb, W.C. A Mathematical Model for the Hydrogenotrophic Metabolism of Sulphate-Reducing Bacteria. Front. Microbiol. 2019, 10, 469258. [Google Scholar] [CrossRef]
- Schwartz, W.; Postgate, J.R. The Sulfate-Reducing Bacteria. J. Basic Microbiol. 1985, 25, 202. [Google Scholar] [CrossRef]
- Ollivier, B.; Hatchikian, C.E.; Prensier, G.; Guezennec, J.; Garcia, J.L. Desulfohalobium retbaense Gen. Nov., Sp. Nov., a Halophilic Sulfate-Reducing Bacterium from Sediments of a Hypersaline Lake in Senegal. Int. J. Syst. Bacteriol. 1991, 41, 74–81. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Isaksen, M.F.; Jannasch, H.W. Bacterial Sulfate Reduction Above 100 °C in Deep-Sea Hydrothermal Vent Sediments. Science 1992, 258, 1756–1757. [Google Scholar] [CrossRef]
- Amina, M.; Lotfi, G. Corrigendum to: An Overview of Extremophile: Microbial Diversity, Adaptive Strategies, and Potential Applications. Microbiol. Biotechnol. Lett. 2024, 52, 520. [Google Scholar] [CrossRef]
- Marzban, G.; Tesei, D. The Extremophiles: Adaptation Mechanisms and Biotechnological Applications. Biology 2025, 14, 412. [Google Scholar] [CrossRef]
- Russell, N.J. Molecular Adaptations in Psychrophilic Bacteria: Potential for Biotechnological Applications. Adv. Biochem. Eng. Biotechnol. 1998, 61, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dopffel, N.; Shaker Shiran, B.; Mayers, K.; An-Stepec, B.A.; Kedir, A.; Heydolph, B.; Hajibeygi, H.; Djurhuus, K. Pressure up to 60 Bar Has No Major Effect on the Overall Hydrogen Consumption of the Sulfate Reducer Oleidesulfovibrio Alaskensis. J. Appl. Microbiol. 2025, 136, 77. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Dupraz, C.; Visscher, P.T. Two Opposing Effects of Sulfate Reduction on Carbonate Precipitation in Normal Marine, Hypersaline, and Alkaline Environments: COMMENT. Geology 2014, 42, e313–e314. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Kading, T.J.; Braissant, O.; Dupraz, C.; Visscher, P.T. Inside the Alkalinity Engine: The Role of Electron Donors in the Organomineralization Potential of Sulfate-Reducing Bacteria. Geobiology 2012, 10, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Schultz, L.; Zhang, W.; Zhu, J.; Meng, F.; Geesey, G.G. Mineral Formation during Bacterial Sulfate Reduction in the Presence of Different Electron Donors and Carbon Sources. Chem. Geol. 2016, 435, 49–59. [Google Scholar] [CrossRef]
- Nogues, J.P.; Fitts, J.P.; Celia, M.A.; Peters, C.A. Permeability Evolution Due to Dissolution and Precipitation of Carbonates Using Reactive Transport Modeling in Pore Networks. Water Resour. Res. 2013, 49, 6006–6021. [Google Scholar] [CrossRef]
- Ganat, T.A.A.O. Fundamentals of Reservoir Rock Properties; Springer: Cham, Switzerland, 2019; 156p. [Google Scholar] [CrossRef]
- Molíková, A.; Vítězová, M.; Vítěz, T.; Buriánková, I.; Huber, H.; Dengler, L.; Hanišáková, N.; Onderka, V.; Urbanová, I. Underground Gas Storage as a Promising Natural Methane Bioreactor and Reservoir? J. Energy Storage 2022, 47, 103631. [Google Scholar] [CrossRef]
- Enzmann, F.; Mayer, F.; Rother, M.; Holtmann, D. Methanogens: Biochemical Background and Biotechnological Applications. AMB Express 2018, 8, 1. [Google Scholar] [CrossRef]
- Underground Sun Storage. Publizierbarer Endbericht. 31 October 2017. Available online: https://www.underground-sun-storage.at/fileadmin/bilder/SUNSTORAGE/Publikationen/UndergroundSunStorage_Publizierbarer_Endbericht_3.1_web.pdf (accessed on 6 June 2025).
- Underground Sun Conversion. Erneuerbares Erdgas Zur Speicherung von Sonne und Wind. Available online: https://www.underground-sun-conversion.at/ (accessed on 6 June 2025).
- Perez, A.; Pérez, E.; Dupraz, S.; Bolcich, J. Patagonia Wind—Hydrogen Project: Underground Storage and Methanation. In Proceedings of the 21st World Hydrogen Energy Conference, Zaragoza, Spain, 13–16 June 2016; p. 255. [Google Scholar] [CrossRef]
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground Bio-Methanation: Concept and Potential. Renew. Sustain. Energy Rev. 2020, 123, 109747. [Google Scholar] [CrossRef]
- Wu, L.; Hou, Z.-M.; Luo, Z.-F.; Fang, Y.-L.; Huang, L.-C.; Wu, X.-N.; Chen, Q.-J.; Wang, Q.-C. Impacts of Microbial Interactions on Underground Hydrogen Storage in Porous Media: A Comprehensive Review of Experimental, Numerical, and Field Studies. Pet. Sci. 2024, 21, 4067–4099. [Google Scholar] [CrossRef]
- Schmitz, R.A.; Peeters, S.H.; Mohammadi, S.S.; Berben, T.; van Erven, T.; Iosif, C.A.; van Alen, T.; Versantvoort, W.; Jetten, M.S.M.; Op den Camp, H.J.M.; et al. Simultaneous Sulfide and Methane Oxidation by an Extremophile. Nat. Commun. 2023, 14, 2974. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.S. Organic Carbon Cycling in Marine Sediments and Seabed Seepage Features in Irish Waters. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2013. [Google Scholar]
- Molenaar, S.D.; Saha, P.; Mol, A.R.; Sleutels, T.H.J.A.; ter Heijne, A.; Buisman, C.J.N. Competition between Methanogens and Acetogens in Biocathodes: A Comparison between Potentiostatic and Galvanostatic Control. Int. J. Mol. Sci. 2017, 18, 204. [Google Scholar] [CrossRef]
- Underground Sun Conversion. Available online: https://www.underground-sun-conversion.at/en/flexstore.html (accessed on 27 June 2025).
- Koo, T.H.; Jang, Y.N.; Kogure, T.; Kim, J.H.; Park, B.C.; Sunwoo, D.; Kim, J. wook Structural and Chemical Modification of Nontronite Associated with Microbial Fe(III) Reduction: Indicators of “Illitization”. Chem. Geol. 2014, 377, 87–95. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. Dev. Clay Sci. 2013, 5, 21–81. [Google Scholar] [CrossRef]
- Bhadariya, V.; Kaur, J.; Sapale, P.; Rasane, P.; Singh, J. Hydrogen Storage in Porous Media: Understanding and Mitigating Microbial Risks for a Sustainable Future. Int. J. Hydrogen Energy 2024, 67, 681–693. [Google Scholar] [CrossRef]
- Hoffmann, T.D.; Reeksting, B.J.; Gebhard, S. Bacteria-Induced Mineral Precipitation: A Mechanistic Review. Microbiology 2021, 167, 001049. [Google Scholar] [CrossRef] [PubMed]
- Konhauser, K.O. Diversity of Bacterial Iron Mineralization. Earth Sci. Rev. 1998, 43, 91–121. [Google Scholar] [CrossRef]
- Eshun, L.E.; Coker, V.S.; Shaw, S.; Lloyd, J.R. Strategies for Optimizing Biovivianite Production Using Dissimilatory Fe(III)-Reducing Bacteria. Environ. Res. 2024, 242, 117667. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Al Shehri, D.; Mahmoud, M.; Kamal, M.S.; Alade, O.S. Impact of Iron Minerals in Promoting Wettability Alterations in Reservoir Formations. ACS Omega 2021, 6, 4022–4033. [Google Scholar] [CrossRef]
- Clark, A.; Sharma, S. Relationship between Clay Minerals and Microorganisms in Underground Hydrogen Storage Reservoirs: A Mini Review. Front. Energy Res. 2025, 13, 1515463. [Google Scholar] [CrossRef]
- Ebigbo, A.; Golfier, F.; Quintard, M. A Coupled, Pore-Scale Model for Methanogenic Microbial Activity in Underground Hydrogen Storage. Adv. Water Resour. 2013, 61, 74–85. [Google Scholar] [CrossRef]
- Zhang, T.C.; Bishop, P.L. Density, Porosity, and Pore Structure of Biofilms. Water Res. 1994, 28, 2267–2277. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Alotaibi, G.F. Factors Influencing Bacterial Biofilm Formation and Development. Am. J. Biomed. Sci. Res. 2021, 12, 617–626. [Google Scholar] [CrossRef]
- Liu, N.; Skauge, T.; Landa-Marbán, D.; Hovland, B.; Thorbjørnsen, B.; Radu, F.A.; Vik, B.F.; Baumann, T.; Bødtker, G. Microfluidic Study of Effects of Flow Velocity and Nutrient Concentration on Biofilm Accumulation and Adhesive Strength in the Flowing and No-flowing Microchannels. J. Ind. Microbiol. Biotechnol. 2019, 46, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Eddaoui, N.; Panfilov, M.; Ganzer, L.; Hagemann, B. Impact of Pore Clogging by Bacteria on Underground Hydrogen Storage. Transp. Porous Media 2021, 139, 89–108. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Kannoorpatti, K.; Padovan, A.; Thennadil, S. Sulphate-Reducing Bacteria’s Response to Extreme PH Environments and the Effect of Their Activities on Microbial Corrosion. Appl. Sci. 2021, 11, 2201. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, L.; Fu, Y.; Li, Q.; Liu, Y.; Wang, Z.; Zhu, H. Iron Redox Cycling in Layered Clay Minerals and Its Impact on Contaminant Dynamics: A Review. Sci. Total Environ. 2023, 855, 159003. [Google Scholar] [CrossRef]
- Dong, H.; Huang, L.; Zhao, L.; Zeng, Q.; Liu, X.; Sheng, Y.; Shi, L.; Wu, G.; Jiang, H.; Li, F.; et al. A Critical Review of Mineral–Microbe Interaction and Co-Evolution: Mechanisms and Applications. Natl. Sci. Rev. 2022, 9, nwac128. [Google Scholar] [CrossRef]
- An, B.A.; Shen, Y.; Voordouw, G. Control of Sulfide Production in High Salinity Bakken Shale Oil Reservoirs by Halophilic Bacteria Reducing Nitrate to Nitrite. Front. Microbiol. 2017, 8, 268855. [Google Scholar] [CrossRef]
- Al-Shamari, A.R.; Al-Mithin, A.W.; Olabisi, O.; Mathew, A. Developing a Metric for Microbilogically Influenced Corrosion (MIC) in Oilfield Water Handling Systems. In Proceedings of the CORROSION 2013, Orlando, FL, USA, 17–21 March 2013; pp. 1–15. [Google Scholar] [CrossRef]
- Eckert, R.B. Emphasis on Biofilms Can Improve Mitigation of Microbiologically Influenced Corrosion in Oil and Gas Industry. Corros. Eng. Sci. Technol. 2015, 50, 163–168. [Google Scholar] [CrossRef]
- Al-Jaroudi, S.S.; Ul-Hamid, A.; Al-Gahtani, M.M. Failure of Crude Oil Pipeline Due to Microbiologically Induced Corrosion. Corros. Eng. Sci. Technol. 2011, 46, 568–579. [Google Scholar] [CrossRef]
- Pearce, J.K.; Law, A.C.K.; Dawson, G.K.W.; Golding, S.D. SO2–CO2 and Pure CO2 Reactivity of Ferroan Carbonates at Carbon Storage Conditions. Chem. Geol. 2015, 411, 112–124. [Google Scholar] [CrossRef]
- Tsapekos, P.; Alvarado-Morales, M.; Angelidaki, I. H2 Competition between Homoacetogenic Bacteria and Methanogenic Archaea during Biomethanation from a Combined Experimental-Modelling Approach. J. Environ. Chem. Eng. 2022, 10, 107281. [Google Scholar] [CrossRef]
- Sheikheh, S.; Rabiei, M.; Rasouli, V. A Review of Evaporite Beds Potential for Storage Caverns: Uncovering New Opportunities. Appl. Sci. 2025, 15, 4685. [Google Scholar] [CrossRef]
- Matos, C.R.; Carneiro, J.F.; Silva, P.P. Overview of Large-Scale Underground Energy Storage Technologies for Integration of Renewable Energies and Criteria for Reservoir Identification. J. Energy Storage 2019, 21, 241–258. [Google Scholar] [CrossRef]
- Vandeginste, V.; Ji, Y.; Buysschaert, F.; Anoyatis, G. Mineralogy, Microstructures and Geomechanics of Rock Salt for Underground Gas Storage. Deep. Undergr. Sci. Eng. 2023, 2, 129–147. [Google Scholar] [CrossRef]
- Miocic, J.; Heinemann, N.; Edlmann, K.; Scafidi, J.; Molaei, F.; Alcalde, J. Underground Hydrogen Storage: A Review; Geological Society: London, UK, 2023; Volume 528, pp. 73–86. [Google Scholar] [CrossRef]
- Mouli-Castillo, J.; Heinemann, N.; Edlmann, K. Mapping Geological Hydrogen Storage Capacity and Regional Heating Demands: An Applied UK Case Study. Appl. Energy 2021, 283, 116348. [Google Scholar] [CrossRef]
- Alkan, H.; Bauer, J.F.; Burachok, O.; Kowollik, P.; Olbricht, M.; Amro, M. Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: A Reservoir Engineering Perspective. Appl. Sci. 2024, 14, 6217. [Google Scholar] [CrossRef]
- Francis, W.; Peters, M.C. Petroleum Oils—The Origin and Nature of Crude Petroleum. In Fuels and Fuel Technology: A Summarized Manual; Pergamon Press: Oxford, UK, 1980; pp. 193–197. [Google Scholar] [CrossRef]
- Jia, Y.; Cao, Y.; Lin, C.; Wang, J. Diagenetic Evolution and Its Implication for Reservoir Quality: A Case Study from the Eocene Es4 Interval, Dongying Depression, Bohai Bay Basin, China. Geosci. J. 2018, 22, 91–103. [Google Scholar] [CrossRef]
- Alyafei, N. Fundamentals of Reservoir Rock Properties, 2nd ed.; Qscience: Doha, Qatar, 2021; ISBN 9789927137273. [Google Scholar]
- Hutton, A.C. Petrographic Classification of Oil Shales. Int. J. Coal Geol. 1987, 8, 203–231. [Google Scholar] [CrossRef]
- Uomosul Sandstone. Available online: https://uomosul.edu.iq/public/files/datafolder_2907/_20191217_070616_440.pdf (accessed on 27 June 2025).
- Boggs, S. Petrology of Sedimentary Rocks. In Petrology of Sedimentary Rocks; Cambridge University Press: Cambridge, UK, 2009; 600p. [Google Scholar] [CrossRef]
- Knab, N.J.; Dale, A.W.; Lettmann, K.; Fossing, H.; Jørgensen, B.B. Thermodynamic and Kinetic Control on Anaerobic Oxidation of Methane in Marine Sediments. Geochim. Cosmochim. Acta 2008, 72, 3746–3757. [Google Scholar] [CrossRef]
- Ilin, A.; Yusta, I.; Ilyn, M.; Amesti, A.M.; van der Graaf, C.; Sánchez-Andrea, I.; Scheinost, A.; Prieur, D.; Sorrentino, A.; Li, Z.; et al. Microbial Mediation in Chalcopyrite Formation at Low Temperature. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M.; Bengtson, S.; Heim, C.; Siljeström, S.; Whitehouse, M.J.; Broman, C.; Belivanova, V.; Åström, M.E. Anaerobic Consortia of Fungi and Sulfate Reducing Bacteria in Deep Granite Fractures. Nat. Commun. 2017, 8, 55. [Google Scholar] [CrossRef]
- Gramp, J.P.; Bigham, J.M.; Jones, F.S.; Tuovinen, O.H. Formation of Fe-Sulfides in Cultures of Sulfate-Reducing Bacteria. J. Hazard. Mater. 2010, 175, 1062–1067. [Google Scholar] [CrossRef]
- González-Partida, E.; Camprubí, A.; Pironon, J.; Alfonso, P.; Cienfuegos-Alvarado, E.; Morales-Puente, P.A.; Canet, C.; González-Ruiz, L.E.; Díaz-Carreño, E.H.; González-Partida, E.; et al. Modelo de Formación de Los Yacimientos Estratoligados de Cu En Lechos Rojos de Las Vigas (Chihuahua, México). Boletín Soc. Geológica Mex. 2017, 69, 611–635. [Google Scholar] [CrossRef]
- Subías Pérez, I.; Fernández-Nieto Fernández, C.; González López, J.M. Mineralogía de Las Areniscas Cupríferas de Biel (Zaragoza). Boletín Soc. Española Mineral. 1989, 12, 315–327. [Google Scholar]
- Acceso, Visualización y Consulta de La Información Georreferenciada Del Servicio Geológico Colombiano. Available online: https://www2.sgc.gov.co/sgc/mapas/Paginas/geoportal.aspx (accessed on 27 June 2025).
- Rothe, M.; Kleeberg, A.; Hupfer, M. The Occurrence, Identification and Environmental Relevance of Vivianite in Waterlogged Soils and Aquatic Sediments. Earth Sci. Rev. 2016, 158, 51–64. [Google Scholar] [CrossRef]
- Santos, H.S.; Nguyen, H.; Venâncio, F.; Ramteke, D.; Zevenhoven, R.; Kinnunen, P. Mechanisms of Mg Carbonates Precipitation and Implications for CO2 Capture and Utilization/Storage. Inorg. Chem. Front. 2023, 10, 2507–2546. [Google Scholar] [CrossRef]
- An, S.; Erfani, H.; Godinez-Brizuela, O.E.; Niasar, V. Transition from Viscous Fingering to Capillary Fingering: Application of GPU-Based Fully Implicit Dynamic Pore Network Modeling. Water Resour. Res. 2020, 56, e2020WR028149. [Google Scholar] [CrossRef]
- Zhang, C.; Oostrom, M.; Wietsma, T.W.; Grate, J.W.; Warner, M.G. Influence of Viscous and Capillary Forces on Immiscible Fluid Displacement: Pore-Scale Experimental Study in a Water-Wet Micromodel Demonstrating Viscous and Capillary Fingering. Energy Fuels 2011, 25, 3493–3505. [Google Scholar] [CrossRef]
- Earle, S. 2 Geology of Groundwater. In Introduction to Geology (GEOL 304); Malaspina University-College: Parksville, BC, Canada, 2006. [Google Scholar]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy Clay Nanocomposites—Processing, Properties and Applications: A Review. Compos. B Eng. 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Boggs, S., Jr. Limestones. In Petrology of Sedimentary Rocks; Cambridge University Press: Cambridge, UK, 2009; pp. 313–381. [Google Scholar]
- Pinheiro, A.C.; Mesquita, N.; Trovão, J.; Soares, F.; Tiago, I.; Coelho, C.; de Carvalho, H.P.; Gil, F.; Catarino, L.; Piñar, G.; et al. Limestone Biodeterioration: A Review on the Portuguese Cultural Heritage Scenario. J. Cult. Herit. 2019, 36, 275–285. [Google Scholar] [CrossRef]
- Chidichimo, F.; De Biase, M.; Muto, F.; Straface, S. Modeling a Metamorphic Aquifer through a Hydro-Geophysical Approach: The Gap between Field Data and System Complexity. Hydrology 2023, 10, 80. [Google Scholar] [CrossRef]
- Allan Freeze, R.; Cherry, J.A. Groundwater; Prentice-Hall: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Cohen, A.; Cherry, J. Conceptual and Visual Understanding of Hydraulic Head and Groundwater Flow; The GroundWater Project: Guelph, ON, Canada, 2020. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Swift, G.; Chen, J. Laboratory Investigation of the Effects of Temperature on the Mechanical Properties of Sandstone. Geotech. Geol. Eng. 2013, 31, 809–816. [Google Scholar] [CrossRef]
- Siddall, R. The Composition of Earth: Rocks and Minerals; EOLSS Publishers: Abu Dhabi, United Arab Emirates, 2022. [Google Scholar]
- Jha, N.K.; Al-Yaseri, A.; Ghasemi, M.; Al-Bayati, D.; Lebedev, M.; Sarmadivaleh, M. Pore Scale Investigation of Hydrogen Injection in Sandstone via X-Ray Micro-Tomography. Int. J. Hydrogen Energy 2021, 46, 34822–34829. [Google Scholar] [CrossRef]
- Jangda, Z.; Menke, H.; Busch, A.; Geiger, S.; Bultreys, T.; Lewis, H.; Singh, K. Pore-Scale Visualization of Hydrogen Storage in a Sandstone at Subsurface Pressure and Temperature Conditions: Trapping, Dissolution and Wettability. J. Colloid. Interface Sci. 2023, 629, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, S.; Zhang, Y.; Foroughi, S.; Bijeljic, B.; Blunt, M.J. Trapping, Hysteresis and Ostwald Ripening in Hydrogen Storage: A Pore-Scale Imaging Study. Int. J. Hydrogen Energy 2024, 56, 1139–1151. [Google Scholar] [CrossRef]
- H2 Valley Map|H2Valleys. Available online: https://h2v.eu/hydrogen-valleys (accessed on 27 June 2025).
- HyUSPRe. Available online: https://www.hyuspre.eu/ (accessed on 27 June 2025).
- REPowerEU H2 Infrastructure Map Europe. Available online: https://www.h2inframap.eu/ (accessed on 31 July 2025).
- About Us|BH2C. Available online: https://www.bh2c.org/en/about-us (accessed on 27 June 2025).
- Das Wasserstoffzentrum Nordwest|Clean Hydrogen Coastline. Available online: https://www.clean-hydrogen-coastline.de/ (accessed on 27 June 2025).
- ClusterNortH2. Available online: https://evida.dk/media/04lf5a3w/clusternorth2-engelsk.pdf (accessed on 27 June 2025).
- Grøn Gas. Available online: https://evida.dk/gron-gas/ (accessed on 27 June 2025).
- Heavenn—H2 Energy Applications in Valley Environments for Nothern Netherlands. Available online: https://heavenn.org/ (accessed on 27 June 2025).
- Hydrogen Delta|Smart Delta Resources. Available online: https://www.smartdeltaresources.com/en/hydrogen-delta (accessed on 27 June 2025).
- Hydrogen Valley Estonia. Available online: https://www.hve.ee/ (accessed on 27 June 2025).
- HyNet North West. Available online: https://hynet.co.uk/ (accessed on 27 June 2025).
- Sefe-Storage—Wir Speichern Erdgas. Available online: https://www.sefe-storage.de/en/storage-locations/rehden-storage-facility (accessed on 27 June 2025).
- REN—Redes Energéticas Nacionais. Available online: https://www.ren.pt/en-GB (accessed on 3 August 2025).
- Red de Hidrógeno—Enagás. Available online: https://www.enagas.es/es/transicion-energetica/red-hidrogeno/ (accessed on 3 August 2025).
- HySoW: Developing Low-Carbon and Renewable Hydrogen. Available online: https://www.terega.fr/en/our-activities/hydrogen/hysow-hyrogen-south-west-corridor-of-france-un-projet-dinfrastructures-de/ (accessed on 3 August 2025).
- Corre Energy—Long Duration Energy Storage, CAES, Hydrogen Storage. Available online: https://corre.energy/ (accessed on 3 August 2025).
- HyStock. Available online: https://www.hystock.nl/ (accessed on 3 August 2025).
- GAZ-SYSTEM: Natural Gas, Pipelines. Available online: https://www.gaz-system.pl/en/ (accessed on 3 August 2025).
- Aldbrough Hydrogen Storage. Available online: https://www.aldbroughhydrogen.com/ (accessed on 3 August 2025).
- Home|VGS Gasspeicher. Available online: https://www.vng-gasspeicher.de/home (accessed on 3 August 2025).
- Hydrogen. Available online: https://www.rwe-gasstorage-west.com/en/hydrogen/ (accessed on 3 August 2025).
- SaltHy—Pionierprojekt Wasserstoffspeicher|Storengy. Available online: https://www.salthy.de/de (accessed on 3 August 2025).
- Großtechnischer Wasserstoff-Speicher Huntorf|Clean Hydrogen Coastline|Clean Hydrogen Coastline. Available online: https://www.clean-hydrogen-coastline.de/de/projekte/speicher-huntorf (accessed on 3 August 2025).
- SEFE: Wir Speichern Erdgas. Available online: https://www.sefe-storage.de/ (accessed on 3 August 2025).
- HPC Krummhörn|Uniper. Available online: https://www.uniper.energy/hydrogen-pilot-cavern (accessed on 3 August 2025).
- H2CAST Etzel—Making Energy Transition Work.|H2CAST Etzel. Available online: https://h2cast.com/ (accessed on 3 August 2025).
- NWKG—Start. Available online: https://www.nwkg.de/ (accessed on 3 August 2025).
- Cavanagh, A.; Yousefi, H.; Wilkinson, M.; Groenenberg, R. HyUSPRe Hydrogen Underground Storage in Porous Reservoirs Classifying Hydrogen Storage Potential in Porous Reservoirs as an Aid to European Site Selection the HyUSPRe Consortium; HyUSPRe: Brussels, Belgium, 2023. [Google Scholar]
- Trinity. Available online: https://www.trinity-es.com/es/index.php (accessed on 5 August 2025).
- Startseite|Thüringer Energie Speichergesellschaft. Available online: https://www.speichergesellschaft.de/Default (accessed on 5 August 2025).
- Wei, X.; Shi, X.; Li, Y.; Liu, H.; Li, P.; Ban, S.; Liang, X.; Zhu, S.; Zhao, K.; Yang, K.; et al. Advances in Research on Gas Storage in Sediment Void of Salt Cavern in China. Energy 2023, 284, 129243. [Google Scholar] [CrossRef]
- Caglayan, D.G.; Weber, N.; Heinrichs, H.U.; Linßen, J.; Robinius, M.; Kukla, P.A.; Stolten, D. Technical Potential of Salt Caverns for Hydrogen Storage in Europe. Int. J. Hydrogen Energy 2020, 45, 6793–6805. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B.; Lewandowska-Śmierzchalska, J.; Matuła, R.; Tarkowski, R. Prospects for the Implementation of Underground Hydrogen Storage in the EU. Energies 2022, 15, 9535. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Youn, H. Effect of Temperature, PH, and Reaction Duration on Microbially Induced Calcite Precipitation. Appl. Sci. 2018, 8, 1277. [Google Scholar] [CrossRef]
- Hydrogen Underground Storage in Porous Reservoirs. Available online: https://storymaps.arcgis.com/stories/2349ba3eb36d4473861b7701a08985e1 (accessed on 27 June 2025).
- Final Report Programmsteuerung Programmabwicklung. 2017. Available online: https://www.underground-sun-storage.at/fileadmin/bilder/03_NEU_SUNSTORAGE/Downloads/Underground_Sun.Storage_Publizierbarer_Endbericht_English.pdf (accessed on 4 August 2025).
- Polgári, M.; Gyollai, I. Geochemical Constraints on the Element Enrichments of Microbially Mediated Manganese and Iron Ores—An Overview. Ore Geol. Rev. 2021, 136, 104203. [Google Scholar] [CrossRef]
- Moon, D.H.; Park, S.S.; Kang, S.P.; Lee, W.; Park, K.T.; Chun, D.H.; Rhim, G.B.; Hwang, S.M.; Youn, M.H.; Jeong, S.K. Determination of Kinetic Factors of CO2 Mineralization Reaction for Reducing CO2 Emissions in Cement Industry and Verification Using CFD Modeling. Chem. Eng. J. 2021, 420, 129420. [Google Scholar] [CrossRef]
- Vasile, N.S.; Suriano, A.; Bellini, R.; Bassani, I.; Vizzarro, A.; Coti, C.; Barbieri, D.; Scapolo, M.; Viberti, D.; Verga, F.; et al. Biogeochemical Modelling of HP-HT Bioreactor Systems for Enhanced Microbial Risk Assessment in Underground Hydrogen Storage. In Proceedings of the Society of Petroleum Engineers—SPE Europe Energy Conference and Exhibition, EURO 2024, Turin, Italy, 26–28 July 2024. [Google Scholar] [CrossRef]
- Gelencsér, O.; Árvai, C.; Mika, L.T.; Breitner, D.; LeClair, D.; Szabó, C.; Falus, G.; Szabó-Krausz, Z. Effect of Hydrogen on Calcite Reactivity in Sandstone Reservoirs: Experimental Results Compared to Geochemical Modeling Predictions. J. Energy Storage 2023, 61, 106737. [Google Scholar] [CrossRef]
- Vasile, N.S.; Bellini, R.; Bassani, I.; Vizzarro, A.; Abdel Azim, A.; Coti, C.; Barbieri, D.; Scapolo, M.; Viberti, D.; Verga, F.; et al. Innovative High Pressure/High Temperature, Multi-Sensing Bioreactors System for Microbial Risk Assessment in Underground Hydrogen Storage. Int. J. Hydrogen Energy 2024, 51, 41–50. [Google Scholar] [CrossRef]
- Thaysen, E.M.; Soler, J.M.; Boone, M.; Cnudde, V.; Cama, J. Effect of Dissolved H2SO4 on the Interaction between CO2-Rich Brine Solutions and Limestone, Sandstone and Marl. Chem. Geol. 2017, 450, 31–43. [Google Scholar] [CrossRef]
- Dohrmann, A.B.; Krüger, M. Microbial H2 Consumption by a Formation Fluid from a Natural Gas Field at High-Pressure Conditions Relevant for Underground H2 Storage. Environ. Sci. Technol. 2023, 57, 1092–1102. [Google Scholar] [CrossRef]
- Thaysen, E.M.; Butler, I.B.; Hassanpouryouzband, A.; Freitas, D.; Alvarez-Borges, F.; Krevor, S.; Heinemann, N.; Atwood, R.; Edlmann, K. Pore-Scale Imaging of Hydrogen Displacement and Trapping in Porous Media. Int. J. Hydrogen Energy 2023, 48, 3091–3106. [Google Scholar] [CrossRef]
- Mushabe, R.; Liu, N.; Dopffel, N.; Ersland, G.; Fernø, M.A. Impact of Specific Surface Area on Anaerobic Microbial Hydrogen Consumption by a Sulfate Reducer: A Sand Pack Study. Int. J. Hydrogen Energy 2025, 166, 150861. [Google Scholar] [CrossRef]
- Liu, N.; Fernø, M.A. Calcite-Functionalized Microfluidic Chip for Pore Scale Investigation of Biogeochemical Interactions in Porous Media. Lab. Chip 2025, 25, 2320–2324. [Google Scholar] [CrossRef]
- Cheng, C.; Busch, B.; Von Dollen, M.; Dohrmann, A.B.; Krüger, M.; Hilgers, C. Natural-Rock Micromodels for Investigation of Micro-Processes and Interactions within Real Pores of Geological Materials. In Proceedings of the 85th EAGE Annual Conference and Exhibition 2024, Oslo, Norway, 10–13 June 2024; Volume 2, pp. 976–980. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Fatah, A.; Zeng, L.; Al-Ramadhan, A.; Sarmadivaleh, M.; Xie, Q. On Hydrogen-Cement Reaction: Investigation on Well Integrity during Underground Hydrogen Storage. Int. J. Hydrogen Energy 2023, 48, 35610–35623. [Google Scholar] [CrossRef]
- Dilshan, R.A.D.P.; Perera, M.S.A. The Impact of Acetic Acid Reaction on Microstructural and Mineralogical Changes in Shale Caprock: A Preliminary Study for Underground Hydrogen Storage Integrity. Int. J. Hydrogen Energy 2025, 166, 150900. [Google Scholar] [CrossRef]
- Bellini, R.; Vasile, N.S.; Bassani, I.; Vizzarro, A.; Coti, C.; Barbieri, D.; Scapolo, M.; Pirri, C.F.; Verga, F.; Menin, B. Investigating the Activity of Indigenous Microbial Communities from Italian Depleted Gas Reservoirs and Their Possible Impact on Underground Hydrogen Storage. Front. Microbiol. 2024, 15, 1392410. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Fatah, A.; Alsaif, B.; Sakthivel, S.; Amao, A.; Al-Qasim, A.S.; Yousef, A.A. Subsurface Hydrogen Storage in Limestone Rocks: Evaluation of Geochemical Reactions and Gas Generation Potential. Energy Fuels 2024, 38, 9923–9932. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Al-Mukainah, H.; Yekeen, N. Experimental Insights into Limestone-Hydrogen Interactions and the Resultant Effects on Underground Hydrogen Storage. Fuel 2023, 344, 128000. [Google Scholar] [CrossRef]
- Grgic, D.; Al Sahyouni, F.; Golfier, F.; Moumni, M.; Schoumacker, L. Evolution of Gas Permeability of Rock Salt Under Different Loading Conditions and Implications on the Underground Hydrogen Storage in Salt Caverns. Rock. Mech. Rock. Eng. 2022, 55, 691–714. [Google Scholar] [CrossRef]
- Zou, S.; Zhang, Y.; Ma, L. Revealing Subsurface Dynamics: Imaging Techniques for Optimizing Underground Energy Storage. Adv. Geo-Energy Res. 2024, 12, 1–7. [Google Scholar] [CrossRef]
- Eric, E.; Hiatt, P.K.P. Cathodoluminescence Petrography of Carbonate Rocks: A Review of Applications for Understanding Diagenesis, Reservoir Quality, and Pore System Evolution. Available online: https://www.researchgate.net/publication/264557734_Cathodoluminescence_petrography_of_carbonate_rocks_A_review_of_applications_for_understanding_diagenesis_reservoir_quality_and_pore_system_evolution (accessed on 18 August 2025).
- Muloiwa, M.; Nyende-Byakika, S.; Dinka, M. Comparison of Unstructured Kinetic Bacterial Growth Models. S. Afr. J. Chem. Eng. 2020, 33, 141–150. [Google Scholar] [CrossRef]
- Shojaee, A.; Ghanbari, S.; Wang, G.; Mackay, E. Interplay between Microbial Activity and Geochemical Reactions during Underground Hydrogen Storage in a Seawater-Rich Formation. Int. J. Hydrogen Energy 2024, 50, 1529–1541. [Google Scholar] [CrossRef]
- Bonk, F.; Popp, D.; Weinrich, S.; Sträuber, H.; Becker, D.; Kleinsteuber, S.; Harms, H.; Centler, F. Determination of Microbial Maintenance in Acetogenesis and Methanogenesis by Experimental and Modeling Techniques. Front. Microbiol. 2019, 10, 414267. [Google Scholar] [CrossRef]
- Wegner, L.; Pohlmeier, A.; Wang, Y.; Klinkenberg, M.; Bosbach, D.; Poonoosamy, J. In Situ Study of Coupled Mineral Dissolution and Precipitation Processes with Gas Production in Porous Media Using Magnetic Resonance Imaging. Water Resour. Res. 2025, 61, e2025WR040035. [Google Scholar] [CrossRef]
- Hendiani, S.; Carbajo, C.; Caicedo, P.N.A.; Verma, T.; Hansen, M.F.; Agbaje, O.B.A.; Mulec, I.M.; Burmølle, M.; Sand, K.K. Reconciling the Role of Mineral Surfaces for Bacterial Evolution: Importance of Minerals in the Dissemination of Antibiotic Resistance. Sci. Total Environ. 2025, 962, 178301. [Google Scholar] [CrossRef]
- Aufrecht, J.A.; Fowlkes, J.D.; Bible, A.N.; Morrell-Falvey, J.; Doktycz, M.J.; Retterer, S.T. Pore-Scale Hydrodynamics Influence the Spatial Evolution of Bacterial Biofilms in a Microfluidic Porous Network. PLoS ONE 2019, 14, e0218316. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Sengupta, A. Microbes in Porous Environments: From Active Interactions to Emergent Feedback. Biophys. Rev. 2024, 16, 173–188. [Google Scholar] [CrossRef]

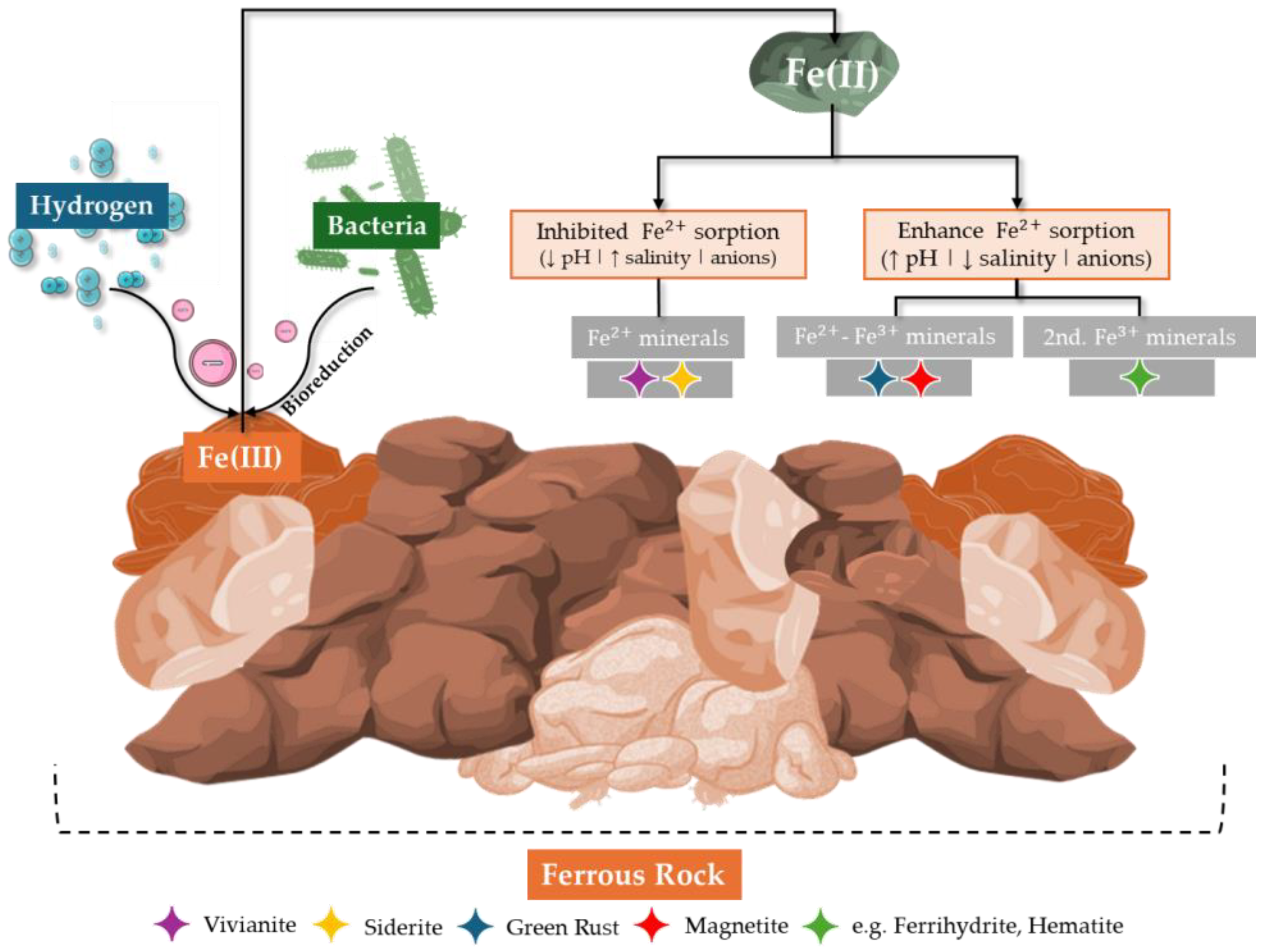


| Hydrogenotrophic Microorganisms | Reaction | Byproduct(s) | Impact |
|---|---|---|---|
| Sulfate-reducing bacteria (SRB) (e.g., Oleidesulfovibrio alaskensis) | H2S | Gas loss [19]; H2S production [12]; wettability alternation [19,34]; pH changes [15]; sulfide precipitation and biofilm formation [19]. | |
| Methanogens (e.g., Methanocalculus halotolerans) | CH4 | Gas contamination [18,35,36]; reducing reservoir pressure [37]; carbonate dissolution and precipitation [38]. | |
| Acetogenic bacteria (e.g., Clostridium scatologenes) | Acetate | Mineral dissolution [39]; pH changes [18]. | |
| Iron-reducing bacteria (IRB) (e.g., Geobacter metallireducens) | Fe2+ | Gas loss [40]; production of Fe2+ [41]; clogging [10]. |
| Mineral | Chemical Formula | Classification | Interaction |
|---|---|---|---|
| Halite | NaCl | Primary component | - |
| Anhydrite | CaSO4 | Common impurity | SRB and A|M |
| Gypsum | CaSO4·2H2O | Common impurity | SRB and A|M |
| Sylvite | KCl | Secondary mineral | - |
| Carnallite | MgCl2 KCl·6H2O | Secondary mineral | A|M |
| Polyhalite | K2Ca2Mg(SO4)4·2H2O | Secondary mineral | SRB and A|M |
| Quartz | SiO2 | Trace impurity | - |
| Calcite | CaCO3 | Trace impurity | A|M |
| Rock Type | Minerals | Chemical Formula | Quantity in Rock (%) | Interaction |
|---|---|---|---|---|
| Sandstone | Quartz | SiO2 | ~45–50 | - |
| Feldspar | (K, Ca, Na, Ba)AlSi2O8 | ~35–40 | - ° | |
| Clay | (Fe, Mg, Ca, K, N)Al2O3 2SiO2·2H2O | ~10–20 | - °° | |
| Shale | Clay | (Fe, Mg, Ca, K, N)Al2O3 2SiO2·2H2O | ~58 | - °° |
| Quartz | SiO2 | ~28 | - | |
| Feldspar | (K, Ca, Na, Ba)AlSi2O8 | ~6 | - ° | |
| Carbonates | CO32− | ~5 | A|M | |
| Iron Oxides | Fe2O3 | ~2 | SRB and IRB | |
| Carbonate | Calcite | CaCO3 | ~50–60 | A|M |
| Dolomite | CaMg(CO3)2 | A|M | ||
| Quartz | SiO2 | ~5–40 | - | |
| Clay | (Fe, Mg, Ca, K, N)Al2O3 2SiO2 2H2O | - °° |
| Project Name | Lead Developer | Location | Type Storage |
|---|---|---|---|
| Amber Hydrogen Valley | Orlen S.A. | Poland | CAV [127] |
| Basque Hydrogen Corridor (BH2C) | Petronor (Repsol Group) | Spain | CAV [130] |
| Clean Hydrogen Coastline | EWE AG | Germany | CAV [131] |
| Cluster NortH2 | Evida and Gas Storage Denmark | Denmark | CAV [132,133] |
| HEAVENN | New Energy Coalition | The Netherlands | CAV [134] |
| Hydrogen Delta | Smart Delta Resources | The Netherlands | CAV [135] |
| Hydrogen Valley Estonia | Participating in Hansa Hydrogen Hubs | Estonia | CAV [136] |
| HyNet North West | Progressive Energy | The UK | CAV [137] |
| Ulster Hydrogen Valley | B9 Energy Storage Ltd. | The UK | CAV [127] |
| ZEV–Zero Emission Valley | Auvergne-Rhône-Alpes Regional Council | France | CAV [127] |
| Rehden Storage Facility | Astora|SEFE Storage GmbH | Germany | CAV [138] |
| H2RENGRID–Carrico UGS | REN | Portugal | CAV [139] |
| H2Burgos | Hidrogeno de Burgos | Spain | CAV [129] |
| H2 storage North-2 | Enagás Infraestructuras de Hidrógeno | Spain | CAV [140] |
| HySoW storage | Terega SA | France | CAV [141] |
| GEOGAZ H2 | Geogaz Lavera | France | CAV [129] |
| Masshylia | ENGIE | France | CAV [129] |
| GeoH2 | Storengy SAS, Geomethane | France | CAV [129] |
| HyManosque | GEOSEL Manosque | France | CAV [129] |
| Extension Aura | Storengy SAS | France | CAV [129] |
| HyPSTER (1st, 2nd, and 3rd phase) | Storengy SAS | France | CAV [129] |
| StorgrHYn (1st, 2nd, and 3rd phase) | Storengy SAS | France | CAV [129] |
| Green Hydrogen Hub Zuidwending | Corre Energy BV | The Netherlands | CAV [142] |
| Hystock Opslag H2 | N.V. Nederlandse Gasunie | The Netherlands | CAV [143] |
| Green Hydrogen Hub Drenthe | Corre Energy BV | The Netherlands | CAV [142] |
| HyCAVmobil | EWE AG et al. | Czechia | CAV [129] |
| Damaslawek Hydrogen Storage | GAZ-SYSTEM S.A. | Poland | CAV [144] |
| Aldbrough Hydrogen Storage | Equinor, SSE Thermal | The UK | CAV [145] |
| H2 storage@Kish | ESB et al. | Ireland | CAV [129] |
| Green Octopus Mitteldeutschland | VNG Gasspeicher | Germany | CAV [146] |
| RWE H2 Storage Staßfurt | RWE Gas Storage West GmbH | Germany | CAV [147] |
| EWE Hydrogen Storage Rüdersdorf | EWE GASSPEICHER | Germany | CAV [129] |
| UHS Peckensen I and II | Storengy Deutschland GmbH | Germany | CAV [129] |
| Green Hydrogen Hub Moeckow | Corre Energy BV | Germany | CAV [142] |
| UST Hydrogen Storage Epe | Uniper Energy Storage GmbH | Germany | CAV [129] |
| GET H2 IPCEI | RWE Gas Storage West GmbH | Germany | CAV [147] |
| Green Hydrogen Hub Ahaus-Epe | Corre Energy BV | Germany | CAV [142] |
| RWE Gronau (1st, 2nd, 3rd expansion) | RWE Gas Storage West GmbH | Germany | CAV [147] |
| Green Hydrogen Hub Harsefeld | Corre Energy BV | Germany | CAV [142] |
| SaltHy Harsefeld (1st, IIA, IIB phase) | Storengy Deutschland GmbH | Germany | CAV [148] |
| UHS Bremen-Lesum | Storengy Deutschland GmbH | Germany | CAV [129] |
| CHC Hydrogen Storage Huntorf ICPEI | EWE GASSPEICHER | Germany | CAV [149] |
| Green Hydrogen Hub Leer | Corre Energy BV | Germany | CAV [142] |
| CHC Hydrogen Storage Jemgum | EWE GASSPEICHER | Germany | CAV [129] |
| JemgumH2 | SEFE Storage GmbH | Germany | CAV [150] |
| CHC Hydrogen Storage Nüttermoor | EWE GASSPEICHER | Germany | CAV [129] |
| Green Hydrogen Hub Drenthe | Corre Energy BV | Germany | CAV [142] |
| Hystock Opslag H2 | N.V. Nederlandse Gasunie | Germany | CAV [143] |
| Green Hydrogen Hub Zuidwending | Corre Energy BV | Germany | CAV [142] |
| UST Hydrogen Storage Krummhörn | Uniper Energy Storage GmbH | Germany | CAV [151] |
| Green Hydrogen Hub Etzel | Corre Energy BV | Germany | CAV [142] |
| SpHyGer (GSE) | Gasunie Energy Development GmbH | Germany | CAV [129] |
| H2CAST | Storag Etzel | Germany | CAV [152] |
| NWKG H2 Storage | NWKG | Germany | CAV [153] |
| US Conversion | RAG | Austria | DHR [154] |
| USS 2030 | RAG | Austria | DHR [154] |
| USS Scale-Up | RAG | Austria | DHR [154] |
| Aquamarine | HGS | Hungary | DHR [154] |
| Green Hydrogen | dCarbonX | Ireland | DHR [154] |
| UGS Velke Kapusany | Nafta | Slovakia | DHR [154] |
| H21-S&D | Nafta | Slovakia | DHR [154] |
| Aljarafe Project | Trinity Energy Storage SL | Spain | DHR [155] |
| Fiume Trieste UHS pilot test | SNAM/STOGIT | Italy | DHR [129] |
| Sergnano H2 storage | SNAM/STOGIT | Italy | DHR [129] |
| HyUS-Pre | HyUs-Pre | Hungary | DHR [128,155] |
| South Kavala UGS facility | HRADf | Greece | DHR [129] |
| Cretan H2SF–Development of Green H2 | EUNICE | Greece | DHR [129] |
| UGS Lab-H2 | NAFTA a.s. | Slovakia | DHR [129] |
| Project Kestrel | ESB, dCarbonX, Snam partnership | Ireland | DHR [129] |
| HyStorage | Uniper Energy Storage GmbH | Germany | DHR [151] |
| H2 Umstellung UGS Kirchheilingen | Thüringer Energie AG et al. | Germany | DHR [156] |
| H2 Readiness | RWE | Czechia | AQU [154] |
| Lacq Hydrogen | Terega | France | AQU [154] |
| HyPSTER | Storengy | France | AQU [154] |
| H2SS Latvia | Conexus | Latvia | AQU [154] |
| Yela H2 storage | Enagás Infraestructuras de Hidrógeno | Spain | AQU [140] |
| Software | MNS | RPR | PT | SP | Key Input Variables | Applicable Scale | Dimension |
|---|---|---|---|---|---|---|---|
| PHREEQC (v3.8.6) | ✓ | ✓ | ✓ | ✓ | Initial water chemistry, temperature, pressure, mineral solubility constants (Ksp), ion-activity product (IAP), and user-scripted microbial rate laws | Batch reactor, 1-D column | 0-D/1-D |
| DuMuX (v3) | ✓ | ✓ | ✓ | ✓ | Porosity, permeability, reaction kinetics (mineral and microbial), and boundary conditions for flow | Pore to reservoir scale | 1-D/2-D/3-D |
| Flownex (v9.0.1) | × | ✓ | × | × | Fluid properties (density and viscosity), network topology (nodes and conduits), and boundary conditions | System/network scale | 0-D/1-D |
| CoFlow (2024.2) | ✓ | ✓ | × | × | Phase properties, user-defined reaction rates, and pore geometry | Reservoir domain scale | 2-D/3-D |
| Lattice Boltzmann Model | ✓ | ✓ | × | × | Porous medium geometry (mesh), fluid properties, and kinetic parameters | Pore-scale | 2-D/3-D |
| CMG GEM (v2024.10) | ✓ | ✓ | ✓ | ✓ | Reservoir properties (porosity and permeability), PVT data, component balances, and reaction definitions | Field scale | 3-D |
| MATLAB (R2023b) | ✓ | ✓ | ✓ | ✓ | Kinetic equations, growth parameters, and initial conditions | Batch reactor/plug-flow | 0-D/1-D |
| MRST (2024a) | ✓ | ✓ | × | × | Reservoir grids, rock/fluid properties, and user-defined flow and reaction laws | Reservoir scale | 2-D/3-D |
| TOUGHREACT (v3.0) | ✓ | ✓ | ✓ | ✓ | Porosity, permeability, thermal properties, and reaction kinetic parameters | Pore to reservoir scale | 1-D/2-D/3-D |
| COMSOL Multiphysics (v6.1) | ✓ | ✓ | ✓ | ✓ | Fluid/solid properties, user-defined PDEs for kinetics, and boundary and initial conditions | Lab to field scale | 1-D/2-D/3-D |
| OpenFOAM (v11) | ✓ | ✓ | × | × | Geometry and mesh, fluid properties, user-defined and reaction mechanisms | Pore-scale | 2-D/3-D |
| ANSYS Fluent (2024 R2) | ✓ | ✓ | × | × | Geometry, mesh, fluid properties, and user-defined reaction kinetics | Pore-scale | 2-D/3-D |
| OpenGeoSys-Eclipse (e300) | × | ✓ | × | × | Reservoir data, PVT tables, component balances, and user-defined reaction rates | Field scale | 3-D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viveros, F.E.; Liu, N.; Fernø, M.A. Biogeochemical Interactions and Their Role in European Underground Hydrogen Storage. Minerals 2025, 15, 929. https://doi.org/10.3390/min15090929
Viveros FE, Liu N, Fernø MA. Biogeochemical Interactions and Their Role in European Underground Hydrogen Storage. Minerals. 2025; 15(9):929. https://doi.org/10.3390/min15090929
Chicago/Turabian StyleViveros, Frank E., Na Liu, and Martin A. Fernø. 2025. "Biogeochemical Interactions and Their Role in European Underground Hydrogen Storage" Minerals 15, no. 9: 929. https://doi.org/10.3390/min15090929
APA StyleViveros, F. E., Liu, N., & Fernø, M. A. (2025). Biogeochemical Interactions and Their Role in European Underground Hydrogen Storage. Minerals, 15(9), 929. https://doi.org/10.3390/min15090929







