Abstract
The present study investigates post-emplacement zeolitization processes in two widespread pyroclastic units from Central Italy: the Cimina Ignimbrite and the Sorano Ignimbrite. A total of seventy-five samples from ten outcrops were analyzed using optical and environmental scanning electron microscopy, electron probe microanalysis, X-ray powder diffraction, and inductively coupled plasma optical emission spectrometry. Analytical results allow the mineral distribution, zeolite composition, textural relationships, and geochemical features of the zeolite-bearing rocks to be defined. In the Cimina Ignimbrite, zeolitization affects the glassy portion of the groundmass, where the glass transforms into a medium- to high-temperature mineral assemblage dominated by clinoptilolite-Ca and cristobalite. This transformation is restricted to the innermost parts of the deposit. In contrast, zeolitization in the Sorano Ignimbrite involves the entire glassy fraction of pumice clasts, with extensive alteration of the glass into medium- to low-temperature zeolites such as chabazite-K and phillipsite-K. The results reveal a significant correlation between the chemical composition of the juvenile material and that of the newly formed zeolites in both types of ignimbrites, particularly in the Sorano Ignimbrite. Zeolitization in Central Italy ignimbrites likely occurs in a natural autoclave-like setting, where hot fluids remain trapped in the deposit for a long time.
1. Introduction
Zeolites are a large group of minerals that share similar composition, distribution, and genesis. They belong to the subclass of tectosilicates and are distinguished by the presence of large cavities and channels in their structure, which can accommodate large cations or water molecules [1,2]. Structurally, zeolites consist of a framework of interconnected TO4 tetrahedra (where T is primarily Al or Si), with each oxygen atom shared between two T sites. The TO2 unit is electrically neutral when T = Si, but carries a negative charge when T = Al. In the latter case, the charge is balanced by extra-framework cations that occupy the internal cavities, along with H2O molecules [3,4]. In nature, approximately 40 species of zeolites have been identified, with Si/Al ratios typically ranging from 1 to 5, except in rare cases [5,6,7,8].
Natural zeolites typically crystallize within cavities in volcanic rocks through hydrothermal processes [9,10] or form in volcanic to volcaniclastic rocks and saline-lake sediments via diagenesis [11]. In recent years, rocks with high zeolite content (“zeolitized rocks”) have gained strategic importance in environmental and ecological applications. Their unique physical properties, combined with their natural abundance and low extraction costs, underpin their established use in a wide range of sectors, including soil decontamination from heavy metals and radioactive elements, water and wastewater treatment, hydroponic farming and horticulture, adsorption and catalysis, aquaculture and animal feed, pet litter and waste treatment, gas purification and separation, and supplementary cementitious materials [12,13,14,15]. However, despite their potential applications, some zeolites pose environmental and health hazards. Numerous studies on erionite, a zeolite often showing fibrous, asbestos-like morphology, have demonstrated its carcinogenicity, particularly its link to malignant pleural mesothelioma [16,17]. Natural zeolites such as clinoptilolite, phillipsite, and mordenite have been preliminarily evaluated for toxicity and classified by the IARC as Group 3 substances [18,19], whereas information on other strongly fibrous zeolites remains limited [20,21,22,23,24].
Many currently exploited zeolite deposits occur in large pyroclastic formations (ignimbrites) that are naturally rich in alkalis and silica, such as alkali rhyolites, trachytes, and phonolites. In these rocks, the alteration of volcanic glass to zeolites has been the subject of several experimental and field-based studies [11,25,26,27,28,29,30]. These works consistently highlight the strong control exerted by the chemical composition of the parent glass on the type of authigenic zeolites formed during diagenesis. According to experimental investigations [29,30], trachytic glasses typically yield phillipsite and, less commonly, chabazite as the earliest zeolites to crystallize. Phonolitic glasses, in contrast, tend to produce chabazite as the dominant zeolite phase. Alkaline-trachytic compositions may result in assemblages of phillipsite alone or phillipsite–chabazite mixtures, depending on fluid composition and temperature. These experimental works, conducted under hydrothermal conditions (100–270 °C), further show that zeolitization typically starts with phillipsite formation, followed by analcime and potassium feldspar, with chabazite forming last. The likelihood of volcanic glass undergoing zeolitization, as well as the resulting mineral assemblages, depends not only on magma composition but also on emplacement conditions, (e.g., cooling rate and water availability) which are critical for zeolite crystallization.
In Central Italy, several studies documented extensive replacement of the glassy matrix in trachytic and phonolitic ignimbrites by phillipsite and chabazite, in line with experimental predictions [30,31,32]. They also highlighted the role of “geoautoclave” conditions, where thick, impermeable pyroclastic sheets trap heat and fluids for long periods, allowing zeolitization to proceed to completion. However, despite this progress, the understanding of post-emplacement zeolitization in these compositions remains incomplete. Factors such as magma chemistry, cooling rate, deposit permeability, and water composition can all influence the distribution of zeolite species. Additionally, compositional heterogeneity within a single ignimbrite sheet means that different zeolite assemblages may occur laterally or vertically in the same deposit. Consequently, detailed mineralogical and textural analyses remain essential for reconstructing the full alteration history and for predicting the distribution of zeolite types in a given pyroclastic body.
The present study examines two major pyroclastic deposits from Central Italy, the Cimina Ignimbrite and the Sorano Ignimbrite, selected for their wide regional distribution, intensive exploitation, contrasting chemical compositions, and distinctly different field characteristics. Over seventy samples were analyzed using optical (OM) and environmental scanning electron microscopy (ESEM), electron probe microanalysis (EPMA), X-ray powder diffraction (XRPD), and inductively coupled plasma optical emission spectrometry (ICP-OES). The objectives are to determine the mineral distribution, zeolite composition, textural relationships, and geochemical characteristics of these zeolite-bearing rocks, and to explore the factors controlling their zeolitization, with implications for both resource utilization and hazard assessment.
2. Geological Background
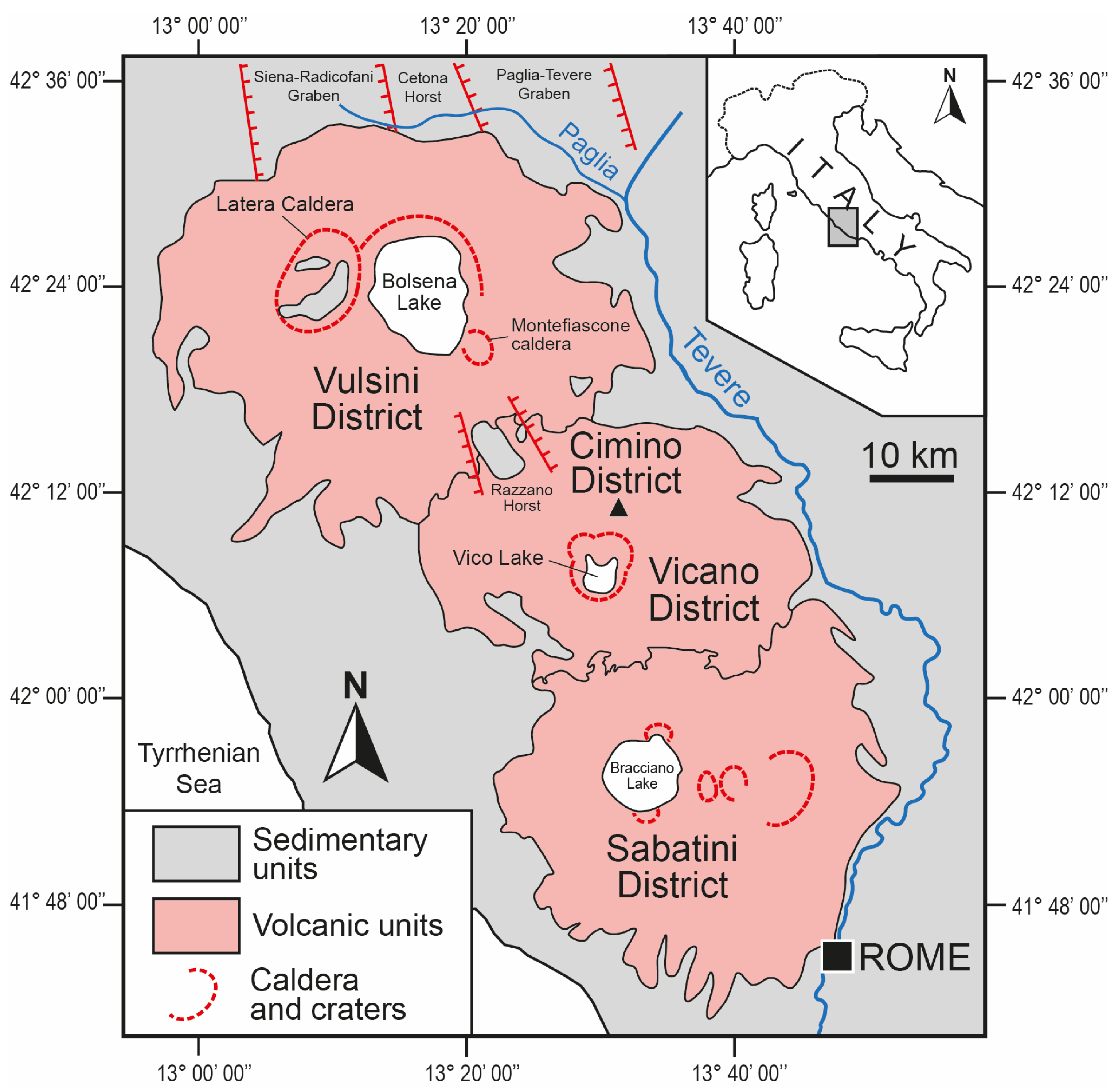
The magmatic rocks outcropping at the Tyrrhenian border of Central Italy belong to four different volcanic districts (Vulsino, Cimino, Vicano, and Sabatino; Figure 1) and are attributable to the volcanism of the Tuscan–Lazio area, which developed in a structurally depressed belt parallel to the Tyrrhenian belt and between the highest sector of the Apennine chain and the coastal areas, at the edge of the Tyrrhenian basin [33,34,35]. The volcanics of the Tuscan–Lazio area are attributable to different magmatic series, including rocks with compositions ranging from acidic to intermediate and rocks characterized by typically potassic and ultrapotassic compositions [36,37,38]. The first group includes the terms with hybrid composition corresponding to the volcanics of the Cimino Volcanic District, while the second group includes the rocks with high K content of the Roman Comagmatic Province, to which the volcanics of the Vulsino, Vicano, and Sabatino volcanic districts are attributable. In particular, post-zeolitization processes concern several ignimbrites resulting from explosive activity of the Vulsino and Cimino districts.
Figure 1.
Geological sketch map of Central Italy showing the locations of the Vulsini, Cimino, Vicano, and Sabatini Volcanic Districts.
The Vulsino Volcanic District is the northernmost and largest of the Roman Province and its products cover an area of about 2200 km2, distributing radially with respect to the volcano-tectonic depression occupied by Lake Bolsena (Figure 1). In the evolution of the Vulsini District, whose activity is between 576 and 127 ka, five volcanic complexes have been distinguished, whose products partially overlap in space and time [39,40,41]: Paleobolsena, Bolsena, Montefiascone, Latera, and Neobolsena. The first phases of activity (Paleobolsena) refer to Plinian pumice horizons and pyroclastic flow deposits (“basal ignimbrites” or “Nenfri” Auct.), which caused the collapse of the caldera in the northern sector of the present Lake Bolsena. Volcanic activity reached its peak in the north-eastern sector, with Plinian eruptions and the emplacement of the Orvieto–Bagnoregio Ignimbrite. Explosive activity was then concentrated first in the south-eastern sectors of the district (Montefiascone complex) and then in the western sectors (Latera complex) where highly explosive activity developed, giving rise to numerous pyroclastic flows, which caused the polygenic collapse of the caldera [39,41,42]. The final activity (Neobolsena) was characterized by small-scale explosive and effusive eruptions from several monogenetic centers (isolated or grouped scoria cones, tuff rings, and tuff cones [39]).
The activity of the Cimino Volcanic District is limited to a very narrow time interval (1.36–1.29 Ma; [43]) and its evolution is very simple, both from a structural and magmatological point of view. The activity first develops along NW-SE feeding fissures and then, in a subsequent phase, along NE-SW feeding fissures. The number of eruptive phases that accompanied the activity of the Cimino Volcanic District is very limited, even if the volume of magma emitted during each eruption is considerable, and can be traced back to three main eruptive cycles [44]. The activity of the first cycle begins with the emplacement of a series of lava domes through cracks oriented mainly NW-SE. The rise of further melt through the same feeding cracks that guided the emplacement of the lava domes determines the emplacement of a welded ignimbrite (Cimina Ignimbrite) with characteristic flame-like structures. A second eruptive cycle develops along new fractures and determines the genesis of a new series of lava domes, followed by the emission of turbulent pyroclastic flows and a second welded ignimbrite. During the third eruptive cycle, a central volcanic apparatus corresponding to the current Monte Cimino is generated.
3. Materials and Methods
3.1. Materials
The rock samples investigated in this study belong to two different volcanic units: the Cimina Ignimbrite (Cimino District) and the Sorano Ignimbrite (Latera Complex, Vulsini District, Figure 1). These ignimbrites were chosen for their broad regional spread, heavy use, contrasting chemistries, and clearly different field features.
The Cimina Ignimbrite [44,45,46] is the most significant product of the activity of the Cimino Volcanic District and is also referred to in the literature as the “Peperino Tipico” [47] or “Quarzolatitic Ignimbrite” (Sheet 137 “Viterbo” of the Geological Map of Italy, scale 1:100,000). It crops out extensively across the eastern sector of Viterbo town, where it is mainly found along major fluvial incisions. The Cimina Ignimbrite is characterized by good workability and excellent compressive and tensile strength, which have made it a widely quarried material, both historically and in present times.
The Sorano Ignimbrite comprises a series of pyroclastic flow deposits originating from the activity of the Latera complex (Vulsini Volcanic District). In the literature, it is variously referred to as “Ignimbrite D” [41], “Lithoid Yellow Tuff” [48], “Sorano Formation” [49,50], or the “TG ignimbrite” [51]. As with the Cimina Ignimbrite, the Sorano Ignimbrite is of considerable importance as a building material. Numerous quarries have been established in these deposits for the extraction of tuff or pumice blocks.
A total of seventy-five samples were collected from both major extraction sites (quarries) and natural outcrops of the two studied ignimbrites, covering ten investigated locations. At each site, between five and fifteen samples were gathered, depending on the outcrop’s size. Sampling was conducted both vertically, through the full thickness of the exposed deposit, and horizontally to capture possible lateral variations. Generally, samples were taken at 2–3 m intervals; however, in areas showing facies changes or signs of alteration, sampling density was increased. Whenever possible, the entire vertical profile was sampled, including the base, the central portion (with at least three vertically spaced samples), and the roof. Particular attention was given to microscale observations to identify the presence and distribution of cavities and vesicles where zeolites may develop, with a focus on compact facies, as well as evidence of devitrification and/or transformation of the glassy matrix.
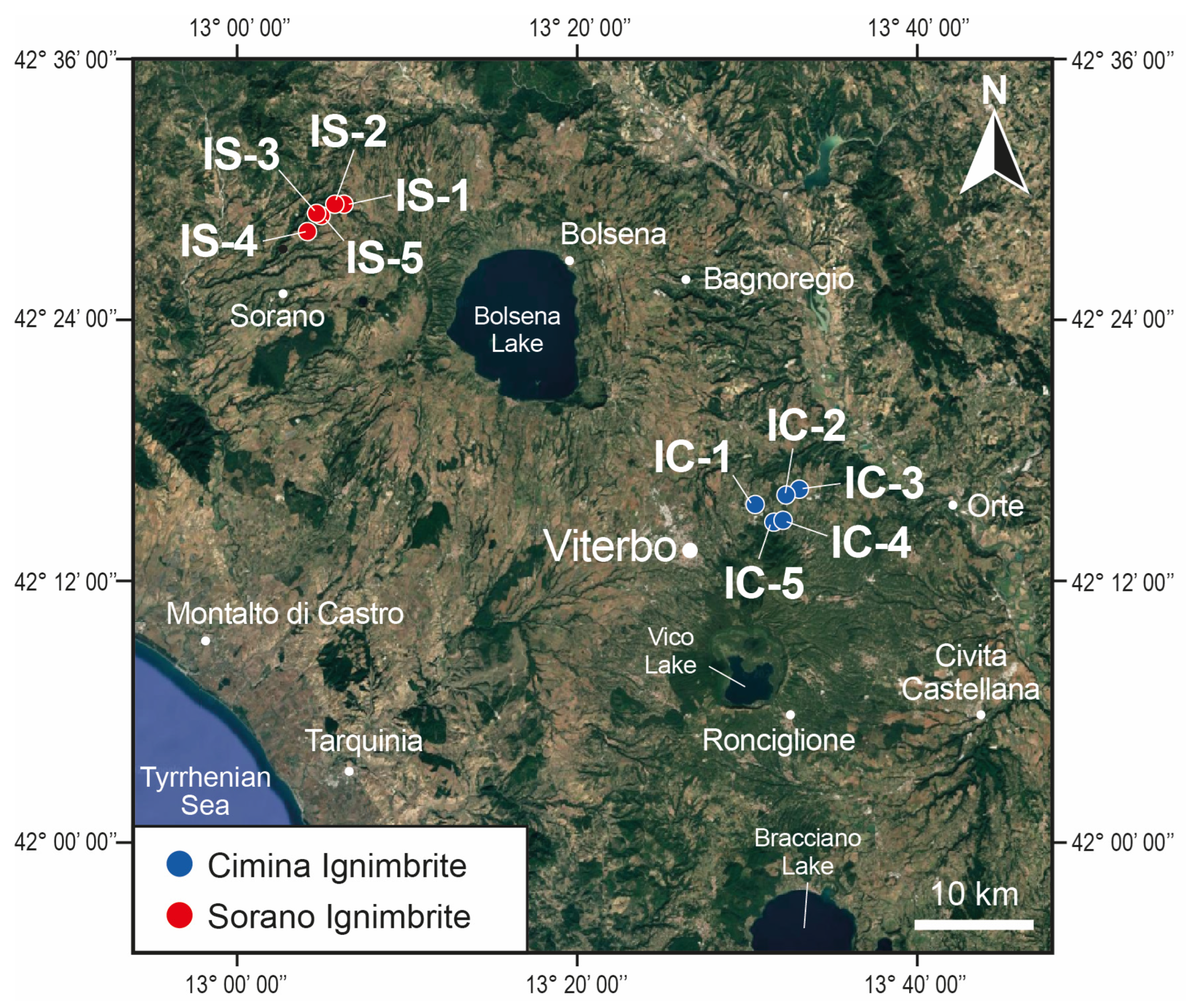
Subsequently, all field-collected samples were carefully examined in the laboratory using both a binocular and a polarizing microscope. This initial phase revealed that, in the Cimina Ignimbrite, the zeolitized facies is confined to the innermost and deepest part of the deposit, spanning approximately 20–30 m in thickness. In contrast, the upper and lateral portions of the ignimbrite contain fresh glass with no signs of zeolitization. Conversely, in the Sorano Ignimbrite, authigenic zeolites form throughout the entire thickness of the deposit. Given this, and considering the notable homogeneity observed under the microscope in samples from the zeolitized zones, we selected two representative areas from each ignimbrite for further analysis. The area from the Cimina Ignimbrite was located near Vitorchiano, east of the town of Viterbo (IC sites, Figure 2), while the area from the Sorano Ignimbrite was located in the San Leonardo locality, northeast of Sorano village (IS sites, Figure 2). Five sites were identified within each area, and a substantial number of samples were collected from each site for subsequent analyses.
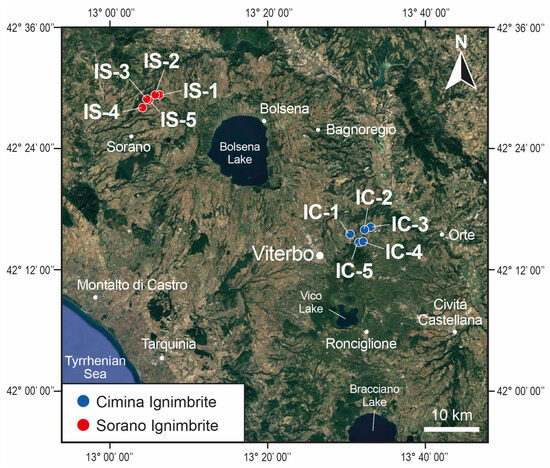
Figure 2.
A Google-based satellite image showing the volcanic material extraction sites examined in this study (IS = Sorano Ignimbrite; IC = Cimina Ignimbrite).
3.2. Sample Preparation
Sample preparation followed two different procedures, depending on the type of ignimbrite and the zeolitization processes involved.
For the Cimina Ignimbrite, the zeolitized portion is very fine-grained and confined to the interstitial glass, which prevented direct identification and separation of the newly formed crystals under the binocular OM. To overcome this limitation, several ~5 mm thick slices of ignimbrite were prepared and gently crushed with an agate mortar. From the resulting grains, zeolitized glassy portions were handpicked, embedded in epoxy resin, thinned to 40–50 µm, and carefully polished. For each fragment used to produce a thin section, corresponding “twin” fragments were prepared for XRPD and ICP-OES analyses, allowing simultaneous acquisition of data from all three analytical techniques. It is important to note that, for the Cimina Ignimbrite, high-purity mineralogical data (99%) could only be obtained through thin sections (for chemical composition via EMPA). Data from XRPD and ICP-OES analyses were slightly affected by the presence of very fine-grained phases within the glass accompanying the zeolites, which could not be separated (resulting in sample purity of ~80%).
For the Sorano Ignimbrite, by contrast, the newly formed crystals were widespread within the cavities or vesicles of the juvenile fraction. Their coarser size and clear visibility under a binocular OM enabled manual extraction and separation (handpicking) of the different mineral phases from the zeolitized portions. Particular care was taken to select the purest possible specimens, free of inclusions or alterations. When crystals were particularly small, entire portions of the mineralized cavities were isolated for analysis. Four parallel populations of crystals were then prepared: polished thin sections for EMPA, crystal stubs for ESEM, and powders for XRPD and ICP-OES analyses. The selection and enrichment procedure applied to these populations yielded a purity greater than 99% for crystals embedded in epoxy resin and approximately 95%–97% for those used for XRPD and ICP-OES analyses.
3.3. Optical Microscope (OM) and Environmental Scanning Electron Microscope (ESEM)
The morphological and physical properties of all collected samples were examined directly within cavities and vesicles using a Leica Wild M10 binocular OM (Wetzlar, Germany) equipped with IntraLux 5000-1 fiber optic illumination (Dietikon, Switzerland) and a DCM-510 digital image acquisition system. Petrographic analysis was carried out on polished thin sections using a Nikon TK-1270E polarizing OM (Tokyo, Japan), also equipped with a digital image acquisition system.
Detailed morphological observations were carried out using an ESEM FEI Quanta 200 FEG (Hillsboro, OR, USA), equipped with an energy-dispersive X-ray spectrometer (EDS) for qualitative microchemical analysis. These analyses were conducted directly on separated pure crystals, as well as on the cavities and inner walls of the vesicles, after gold coating the samples to ensure electrical conductivity. The operating conditions included a 25 kV accelerating voltage, an adjustable beam diameter, a working distance of 10–12 mm, and a tilt angle of 0°. The ESEM operated in low-vacuum mode, with chamber pressure maintained between 0.80 and 0.90 mbar. Images were captured using either a single-shot detector (SSD) or an Everhart–Thornley secondary electron detector (ETD).
3.4. Electron Probe Microanalysis (EPMA)
In situ quantitative chemical EPMA analyses of mineral phases were performed using a CAMECA SX 50 (Gennevilliers, France), equipped with five spectrometers and operating in full wavelength-dispersive system (WDS) mode. The accelerating voltage was set to 15 kV, the beam current to 15 nA, and the beam diameter ranged from ~1 μm to ~5 μm depending on the mineral phase. Counting times were 20 s for the peak and 10 s for the background. Analytical uncertainties are ±2% for major and ±5% for minor elements. The following standards were used: Na2O = jadeite [NaAl(Fe3+)Si2O6]; MgO = periclase; TiO2 = rutile; FeO = magnetite (Fe3O4); SiO2/CaO = wollastonite (CaSiO3); Al2O3 = corundum; MnO = rhodonite (MnSiO3); K2O = orthoclase (KAlSi3O8); BaO = barite (BaSO4); SrO = celestine (SrSO4); NiO = pure nickel; and P2O5 = apatite. The quality of the analyses was evaluated using the balance equation E = [Al + Fe − (2Ca + 2Mg + 2Ba + K + Na)]/[2(Ca + Mg + Ba) + K + Na)] [1]. Only results with E < ±10% were used in this study. Heulandite-group zeolites were characterized based on their Si:Al ratio [5] and confirmed by XRPD analyses.
3.5. X-Ray Powder Diffraction (XRPD)
XRPD data were collected using a Philips X’Change PW1830 diffractometer (Amsterdam, The Netherlands) operating at 35 kV and 30 mA, with CuKα radiation (λ = 1.54506 Å). For this type of analysis, the samples (selected pure crystals or zeolitized matrix) were first finely ground into powder using an agate mortar. Measurements were performed in Bragg–Brentano geometry from 2° to 65° 2θ, using a 0.01° step size and a 2.5 s counting time per step to ensure high-intensity patterns. The instrument was configured with a 1° maximum divergence compensating slit, a 0.2 mm receiving slit, and a graphite monochromator. Quantitative phase analysis was conducted by a reference intensity ratio (RIR) method using X’Pert Quantify and X’Pert HighScore Plus software, version 5.2 release, with PDF-4 as a reference database and quartz as the internal calibration standard. Peak width variation remained within ±2% of the average value. Each sample was measured three times. Comparison of the resulting diffraction patterns demonstrates that the XRPD method produces highly reproducible results, with peak values varying only within a very narrow range. Extended diffraction scans, with acquisition times of up to 24 h, were conducted to determine mineralogical composition, assess crystal quality, and verify the absence of impurities.
3.6. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
Whole-rock chemical analyses were performed at Activation Laboratories in Ancaster (Hamilton, ON, Canada), using the Code 4Litho analytical package. Powered samples weighing 200 mg along with standards were fused using a lithium metaborate/tetraborate flux and then totally dissolved using 5% HNO3. Major elements were measured by ICP-OES using a Varian 735 ICP-OES (Pao Alto, CA, USA). Weight loss on ignition (LOI) was determined by standard gravimetric methods after heating the powdered samples at approximately 1100 °C for 5 h. For classification purposes, the sum of oxide percentages was normalized to 100% on an anhydrous basis. Elemental detection limits, international geochemical reference standards, and duplicate data values are available from the corresponding author on request. Further details of analytical techniques are given in http://www.actlabs.com.
4. Results
4.1. Field Description
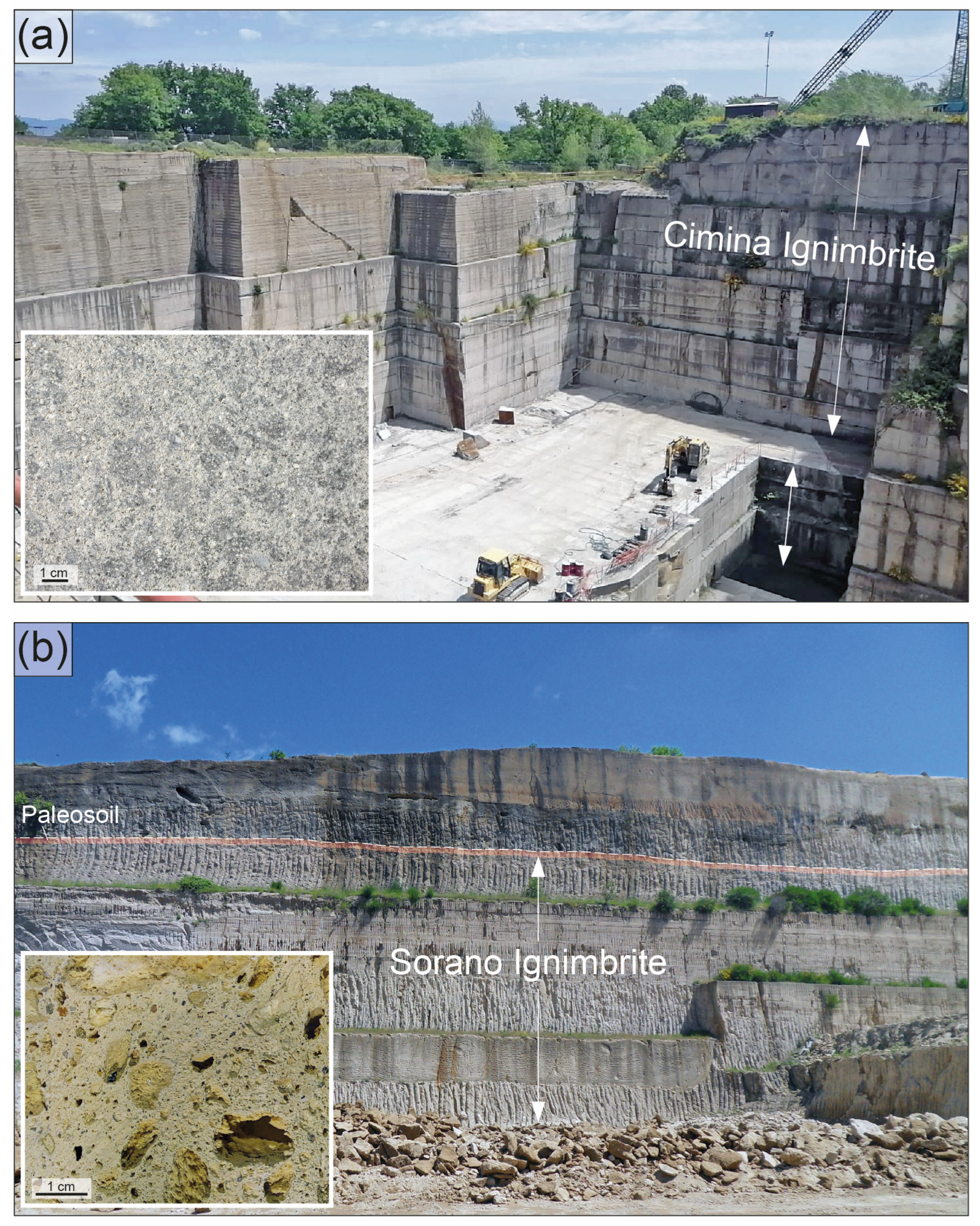
The Cimina Ignimbrite (Figure 3a) appears as a light grey to pinkish pyroclastic flow deposit, fine-grained and variably compacted, composed of at least two superimposed flow units. The deposit is generally massive and lithified by primary welding, transitioning to a non-lithified pumice facies in more distal areas. Pseudostratification features are present, marked by discontinuous lithic-enriched horizons. The lithified facies often displays vertical prismatic jointing. The thickness of the Cimina Ignimbrite varies significantly, depending on the pre-ignimbrite morphology. Field observations show maximum thicknesses ranging from 20 to 40 m in natural outcrops to 60 to 80 m in quarry sites. Survey data from the sampling area [43] indicate a maximum thickness of up to 200 m. The deposit exhibits a pipernoid texture, with characteristic black “flames” composed of stretched vitrophyric fragments. The main mineral constituents include small crystals (up to 0.5 cm) of K-feldspar (sanidine) and biotite. The Cimina Ignimbrite consistently appears compact and lacks cavities or vesicles. No zeolites are observed at either the outcrop or hand-sample scale. In some areas, the deposit is highly altered and appears as a loose, sandy material.
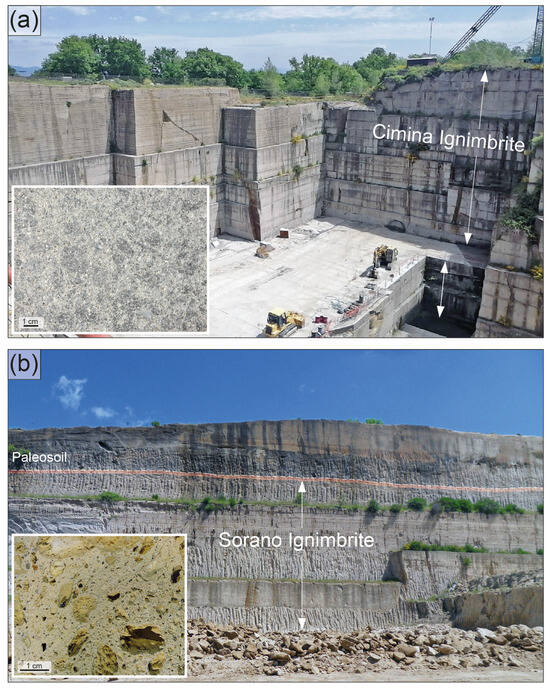
Figure 3.
Field photographs showing the typical outcrop appearance of the two investigated ignimbrites at their respective extraction sites. (a) Cimina Ignimbrite, Vitorchiano quarry. The exposed ignimbrite unit in this quarry reaches a thickness of approximately 40 m. (b) Sorano Ignimbrite, Piandirena quarry. The ignimbrite outcrop here is ~15 m thick, with the top of the deposit clearly marked by a distinct paleosol horizon. Insets on the left side of each image display the macroscopic features of the corresponding ignimbrite samples.
The Sorano Ignimbrite (Figure 3b) consists of multiple ash–-pumice deposits attributed to dilute and turbulent pyroclastic flows, which spread over a wide area surrounding the Latera caldera. As also observed by other studies, the base of the sequence is marked by a yellowish ash layer, overlain by a pumice fall deposit, upon which several pyroclastic flow units are superimposed. These units range in thickness from approximately 1 to 4 m, with a total cumulative thickness that does not exceed 20 m.
The flow units are composed of massive ash-rich deposits, yellowish to light gray or whitish, ranging from incoherent to weakly cemented and zeolitized. They are rich in accretionary lapilli and often contain white pumice lapilli, either scattered or, in places, aligned and concentrated. The pumice clasts are nearly aphyric and are embedded in a matrix of fine- to medium-grained ash. They exhibit a variable degree of vesiculation, with cavities ranging from a few millimeters to several centimeters, often filled with small-scale, secondary mineralizations. At the outcrop scale, the distribution of cavities and vesicles mirrors that of the juvenile fragments containing them, which are regularly distributed without apparent variation either vertically through the deposit or laterally. The uppermost flow unit is distinguished by the presence of dark gray pumice blocks containing millimeter-sized sanidine crystals. The lithic component is consistently very sparse and consists of rare millimeter-sized lava fragments. In the upper part of the ignimbrite deposit, there is a prominent reddish-brown paleosol horizon, about 50 cm thick, with good lateral continuity (Figure 3b).
4.2. Petrography and Geochemistry
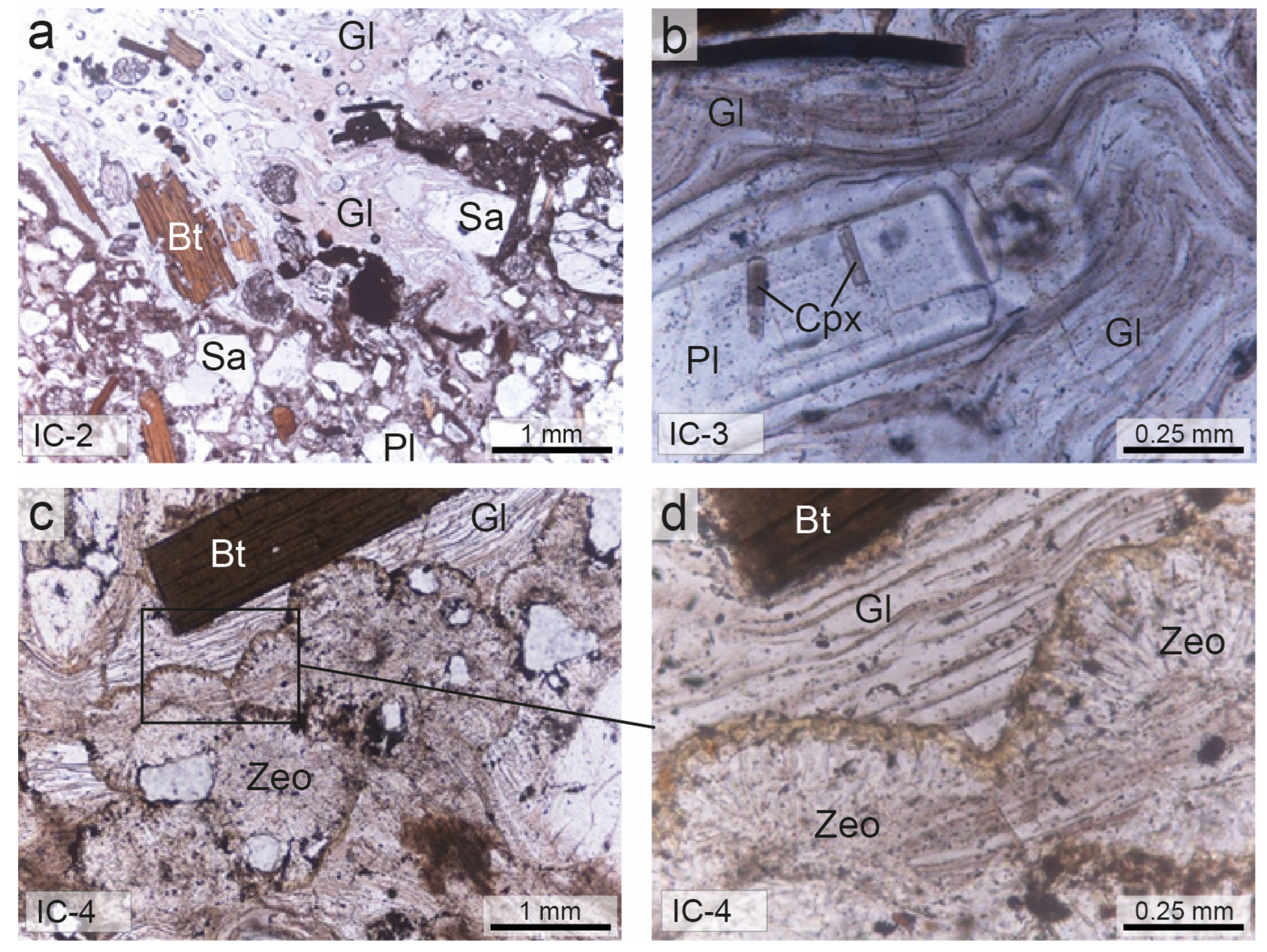
Under the microscope, the Cimina Ignimbrite samples generally display a vitroclastic texture, characterized by a chaotic assemblage of intensely fractured crystals in a glassy groundmass with fluidal features (Figure 4).
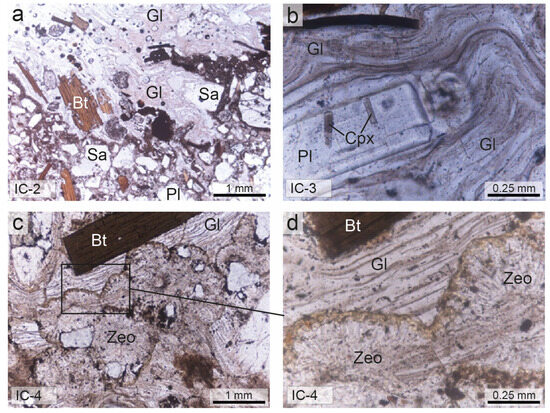
Figure 4.
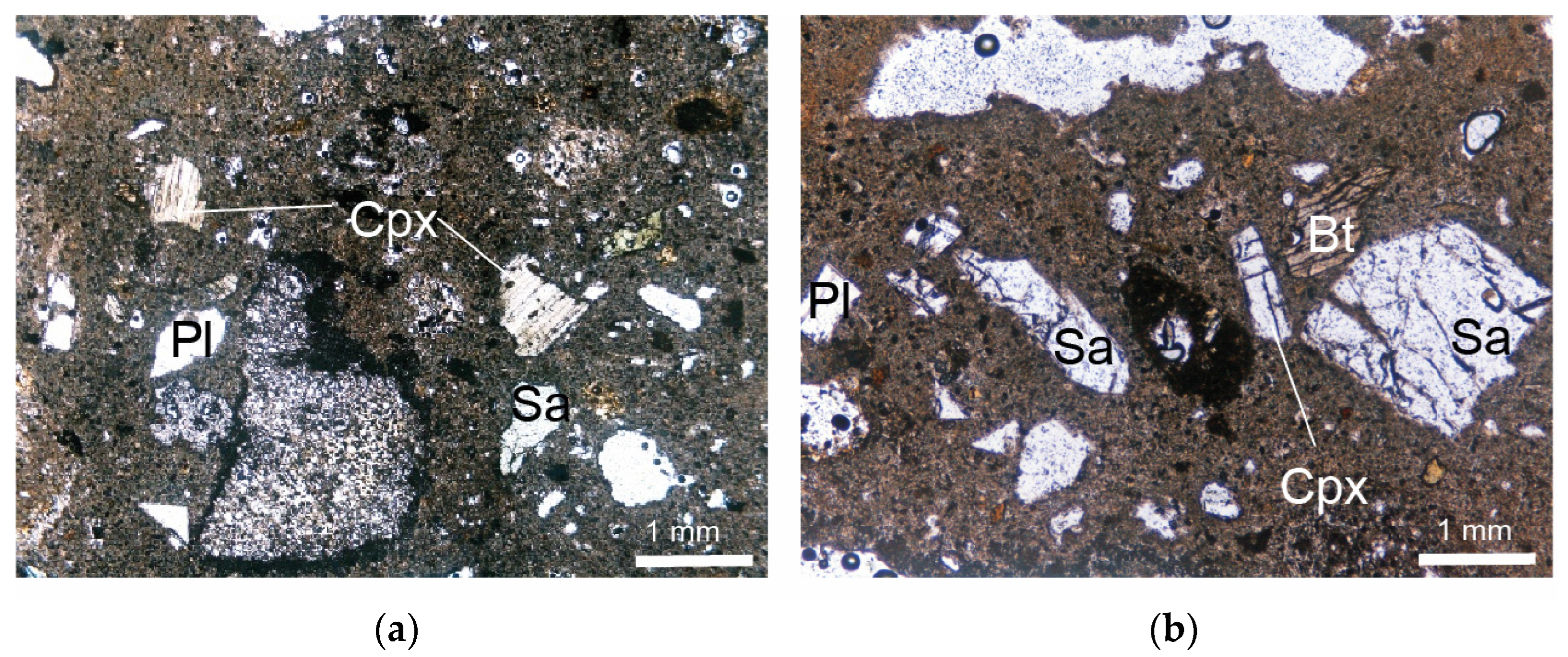
Thin-section micrographs (plane-polarized light) of Cimina Ignimbrite. (a,b) Non-zeolitized facies; (c,d) zeolitized facies. Sa = sanidine; Pl = plagioclase; Bt = biotite; Cpx = clinopyroxene; Zeo = zeolites; Gl = glass. Abbreviations of minerals are according to [52].
The phenocrysts and phenoclasts include sanidine, plagioclase, biotite, orthopyroxene, and clinopyroxene, all embedded in a glassy matrix containing microlites of plagioclase, biotite, pyroxenes, and opaque minerals. Phenocrysts and microphenocrysts are often dotted with minute crystalline and glassy inclusions. Biotite contains fine inclusions of euhedral monazite, magnetite, zircon, apatite, and plagioclase, whereas sanidine may contain biotite crystals. Orthopyroxene is also characterized by abundant inclusions of glass, zircon, and apatite. Accessory monazite and perrierite are rarely observed. The groundmass often shows distinct wavy and sinuous fluidal structures, with glassy shreds plastically adhering to the edges of the crystals.
In samples from the most distal zones of the ignimbrite, where deposit thickness is reduced, or from the outer margins of the thicker deposits, the glass appears clear and the mineral phases are well preserved. In contrast, samples from the innermost parts of the thicker deposits are characterized by a glassy matrix that exhibits clear signs of transformation, including the formation of spherulites with radial and fan-shaped growth microstructures. In these cases, the crystals within spherulites are often extremely fine-grained and are difficult to observe even at high magnification. Locally, a gradual and irregular increase in spherulite volume transforms the matrix into a completely devitrified rock, devoid of any residual volcanic glass. The black flame structures contain intensely fractured crystals of the same crystalline paragenesis previously reported, embedded in a groundmass composed of thin, elongated glassy filaments arranged in a wavy and sub-parallel pattern relative to the base of the deposit.
The juvenile component of the Sorano Ignimbrite consists of whitish to yellow vesiculated pumices ranging from poorly porphyritic to sub-aphyric, containing rare phenocrysts and/or phenoclasts of sanidine, plagioclase, biotite, clinopyroxene, and opaque minerals (Figure 5). Phenocrysts are sometimes characterized by crystalline and glassy inclusions. Sanidine contains very small biotite microcrystals, biotite may include magnetite and plagioclase, while pyroxene is characterized by inclusions of glass.
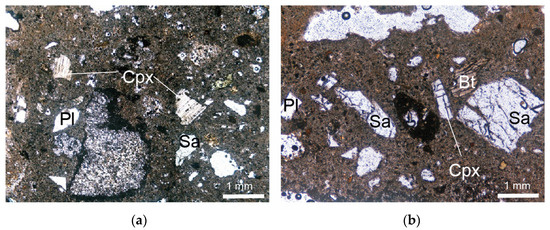
Figure 5.
Thin-section micrographs (plane-polarized light) of Sorano Ignimbrite. (a) IS-1 sample; (b) IS-3 sample. Sa = sanidine; Pl = plagioclase; Bt = biotite; Cpx = clinopyroxene. Abbreviations of minerals are according to [52].
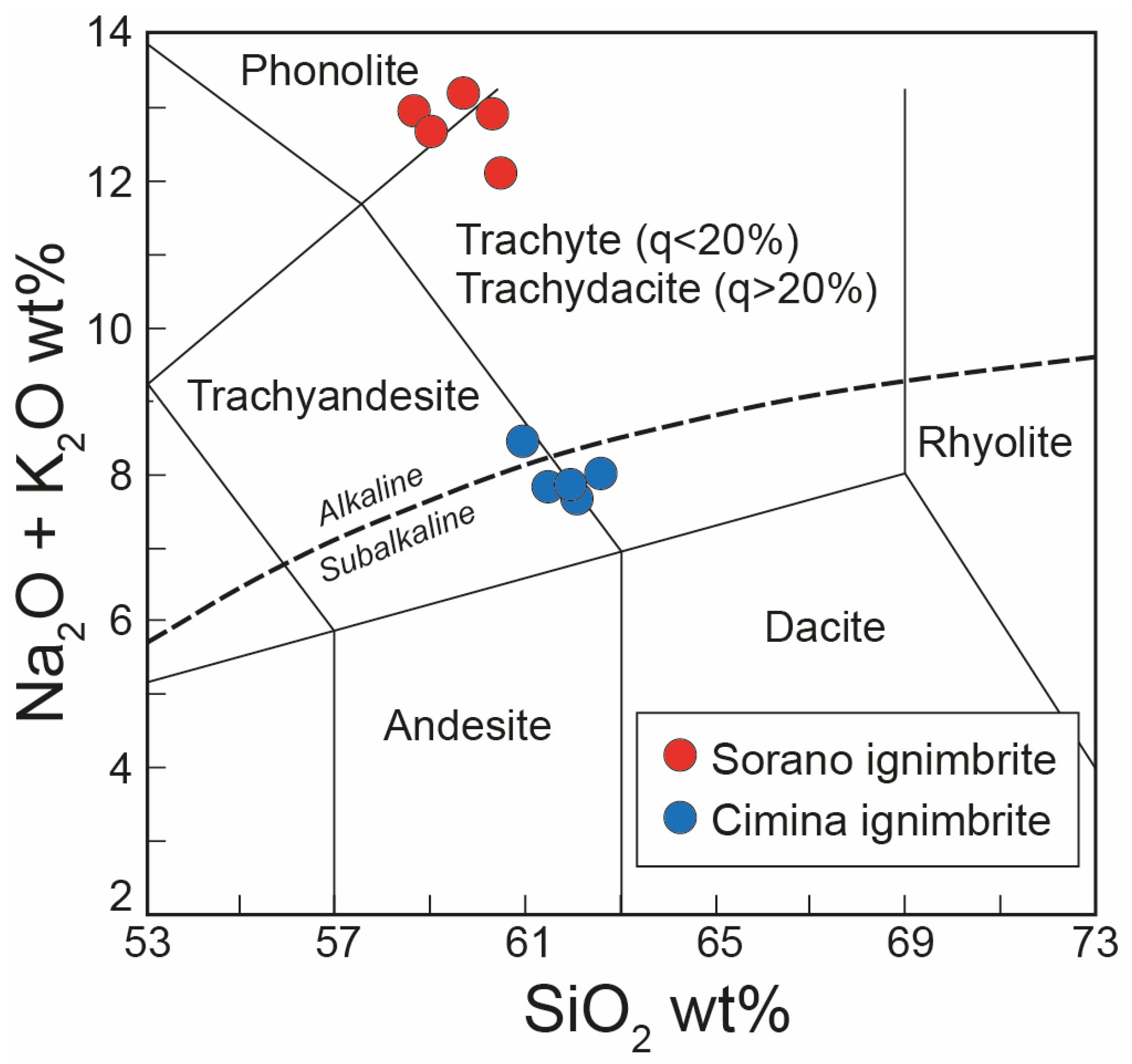
Based on chemical analysis (Table 1), the juvenile fraction of the Cimina Ignimbrite exhibits a LOI-free composition straddling the boundary between trachytic and trachyandesitic fields (Figure 6). This compositional range is characterized by relatively low alkali content (Na2O + K2O between 7.5 and 8.0 wt.%) and silica values ranging from 60.8 to 62.4 wt.%. In terms of cationic content, the Cimina Ignimbrite is characterized by a significant and comparable presence of potassium and calcium, with mean values of 5.32 and 4.30 wt.%, respectively, and a lower concentration of sodium (mean value: 2.39 wt.%).

Table 1.
Whole-rock chemical composition (wt.%) of the juvenile fraction from the Cimina and Sorano Ignimbrites. LOI = loss on ignition.
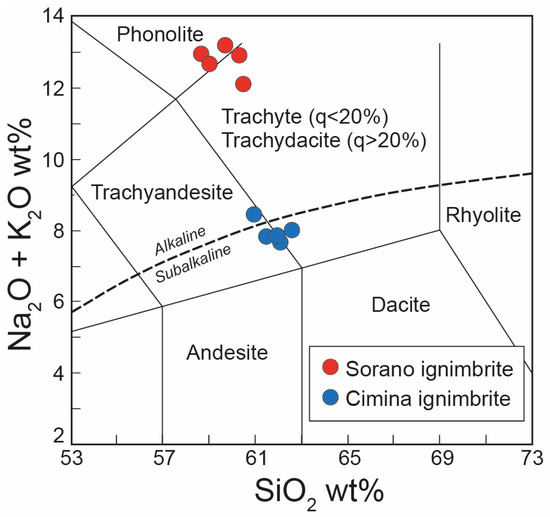
Figure 6.
Total alkali versus silica (TAS) diagram [53] showing the composition of the juvenile fraction from the Cimina and Sorano Ignimbrites. Analyses recalculated to LOI-free basis. q = quartz. Dashed line: alkaline–subalkaline division according to [54].
With regard to the Sorano Ignimbrite, the LOI-free composition of the juvenile fraction plots between the phonolitic and trachytic–trachydacitic fields is shown in Table 1 and Figure 6. The rock exhibits a high alkali content, with Na2O + K2O ranging from 11.5 to 12.9 wt.%. Silica content varies between 58.5 and 60.6 wt.%. In terms of cationic composition, the Sorano Ignimbrite is characterized by high potassium levels (mean: 8.7 wt.%) and comparatively lower sodium and calcium contents, at 3.65 wt.% and 2.59 wt.%, respectively.
4.3. Zeolitization and Zeolite Chemistry
In the Cimina Ignimbrite, the zeolitization process affects the glassy portion of the groundmass, where micro- to cryptocrystalline spherulites of variable size develop. In the early stages of this process, spherulites typically appear as isolated, spherical bodies within the glass. They generally range from <1 to 5 mm in diameter and, in thin sections, exhibit a radial fibrous structure accompanied by concentric zoning, visible through subtle color variations (Figure 7). Spherulites sometimes appear to have nucleated on pre-existing microphenocrysts, particularly feldspar. As zeolitization advances, the number of spherulites increases, and they begin to coalesce into groups, leaving isolated, irregular patches of residual glass. These coalesced spherulites occasionally display polygonal outlines. However, the contacts between spherulites and residual glass are often irregular due to the presence of small spherulites that have nucleated on the surfaces of larger ones.
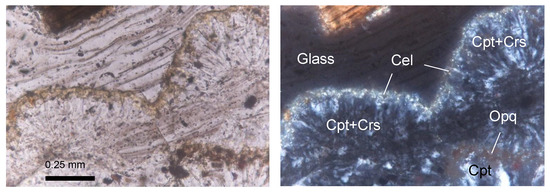
Figure 7.
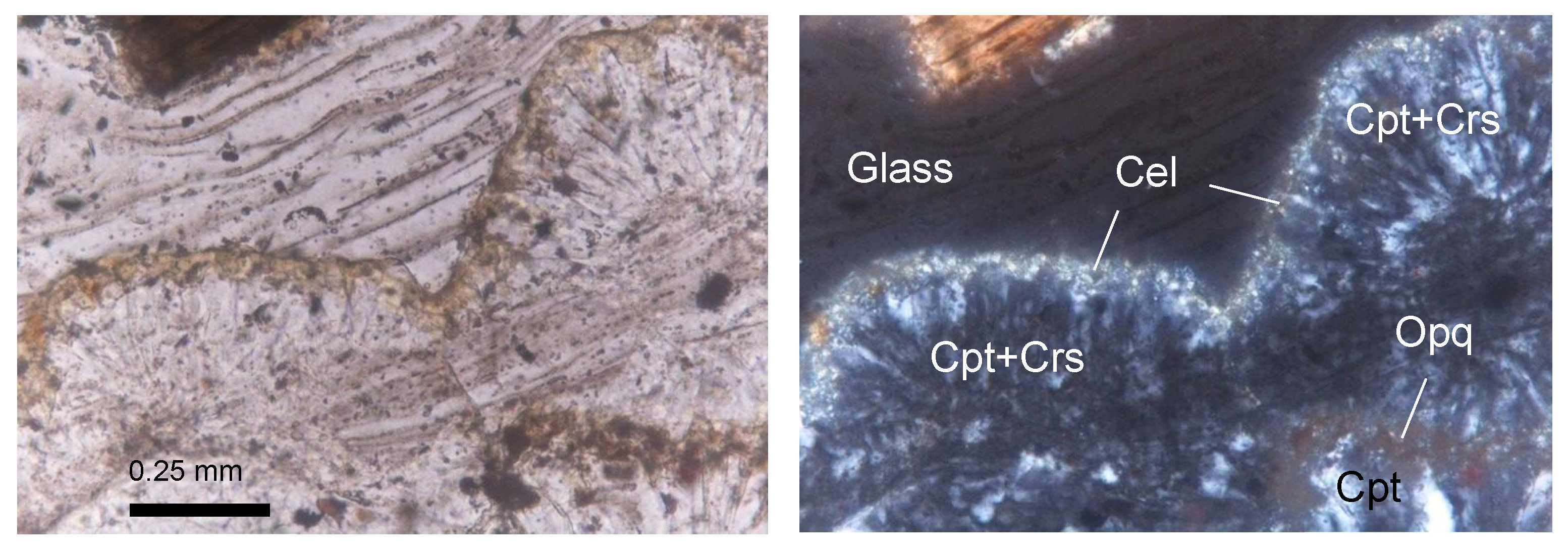
Thin-section micrographs of spherulite development in the Cimino Ignimbrite (IC-4 sample; (left): plane-polarized light; (right): cross-polarized light). Cel = celadonite; Cpt = clinoptilolite; Crs = cristobalite; Opq = opaque minerals. Abbreviations of minerals according to [52].
Quantitatively, the mineral composition was analyzed by the RIR method. The results of the analysis are shown in Table 2.

Table 2.
Quantitative mineral composition (wt.%) of fifteen representative ignimbrite samples based on their XRPD patterns and applying the RIR method for quantification. Av. = average.
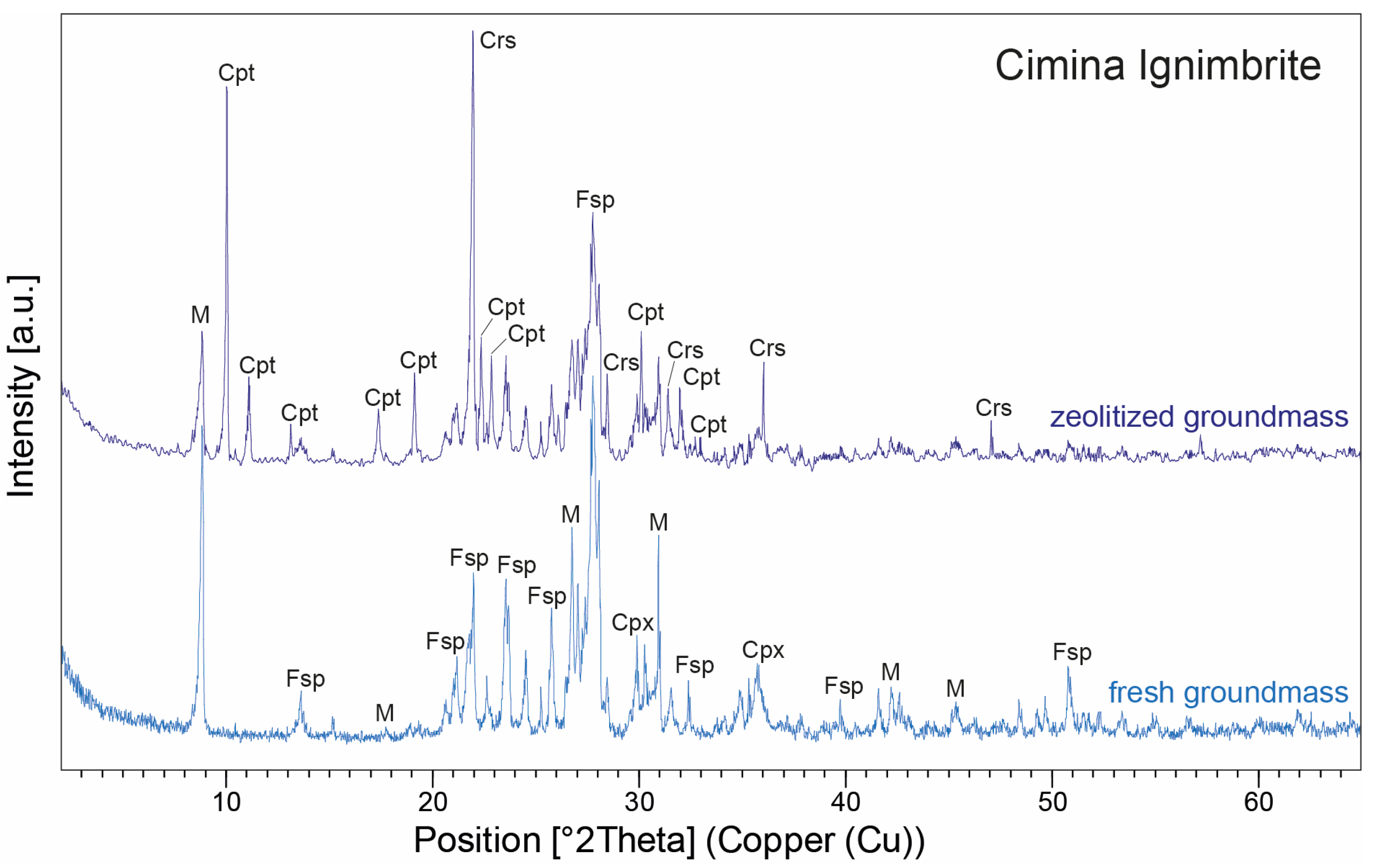
XRPD data in the Cimina Ignimbrite (Figure 8) show that the mineralogical composition of the unaltered (fresh) groundmass closely matches observations from thin sections, with feldspars (both sanidine and plagioclase) and mica minerals being predominant (average 52 wt.% and 34 wt.%, respectively), accompanied by minor amounts of pyroxene (average 14 wt.%). In contrast, during the formation of spherulites, the groundmass undergoes a pronounced mineralogical transformation, marked by the development of authigenic minerals in cryptocrystalline intergrowths at the expense of the original phases, which either disappear or decrease significantly. The interiors of the spherulites are composed primarily of clinoptilolite (average 46 wt.%) and cristobalite (average 34 wt.%), while feldspar and mica decrease markedly (average 9 wt.%), and clinopyroxene is rare (average 3 wt.%). Clinoptilolite was identified by its primary peak (100) at 9.86° 2Θ, together with secondary peaks at 22.45° (90), 30.03° (68), 26.05° (58), and 30.14° (58) 2Θ. Owing to the structural series relationship between heulandite and clinoptilolite, the identification was further confirmed by comparison with ICDD/PDF reference cards for both minerals: the positions and relative intensities of the main reflections matched perfectly with the clinoptilolite phase. Cristobalite, on the other hand, is characterized by a prominent peak (100) at 22° 2Θ, accompanied by smaller peaks at 36.12°, 31.45°, and 28.46° 2Θ. The distribution of clinoptilolite and cristobalite typically follows one of two patterns: (a) one or more layers of finely crystalline clinoptilolite grains aligned perpendicularly to the spherulite walls, alternating with cryptocrystalline zones of cristobalite; or (b) similarly aligned finely crystalline grains with a core partially or entirely filled by anhedral to euhedral grains of either the same zeolite or silica phases. Many spherulites also display a thin film of celadonite on their outer wall, clearly visible in thin sections as a yellowish-green film ~50 µm thick at the spherulites’ margins (Figure 7).
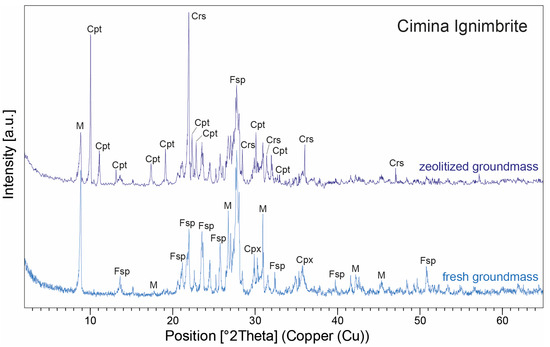
Figure 8.
XRPD patterns of zeolitized and fresh groundmass from two representative samples of the Cimina Ignimbrite, illustrating the mineralogical changes associated with zeolitization. Peak labels: Cpt = clinoptilolite; Crs = cristobalite; Fsp = feldspars; M = mica; Cpx = clinopyroxene. Abbreviations of minerals according to [52].
In contrast to the Cimina Ignimbrites, where zeolitization typically affects the glassy groundmass, the Sorano Ignimbrite exhibits zeolitization predominantly within the vesiculated juvenile fraction. Zeolite crystals occur both within pumice cavities and vesicles, as well as replacing the pumice glass itself. Vesicles and cavities within pumice clasts are mainly filled with chabazite crystals 50–100 µm in size. These crystals are subhedral to euhedral, commonly twinned, and display the characteristic rhombohedral to pseudo-cubic morphology (Figure 9a).
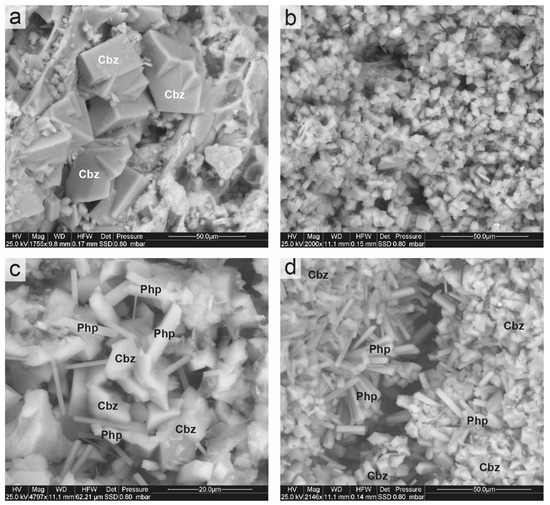
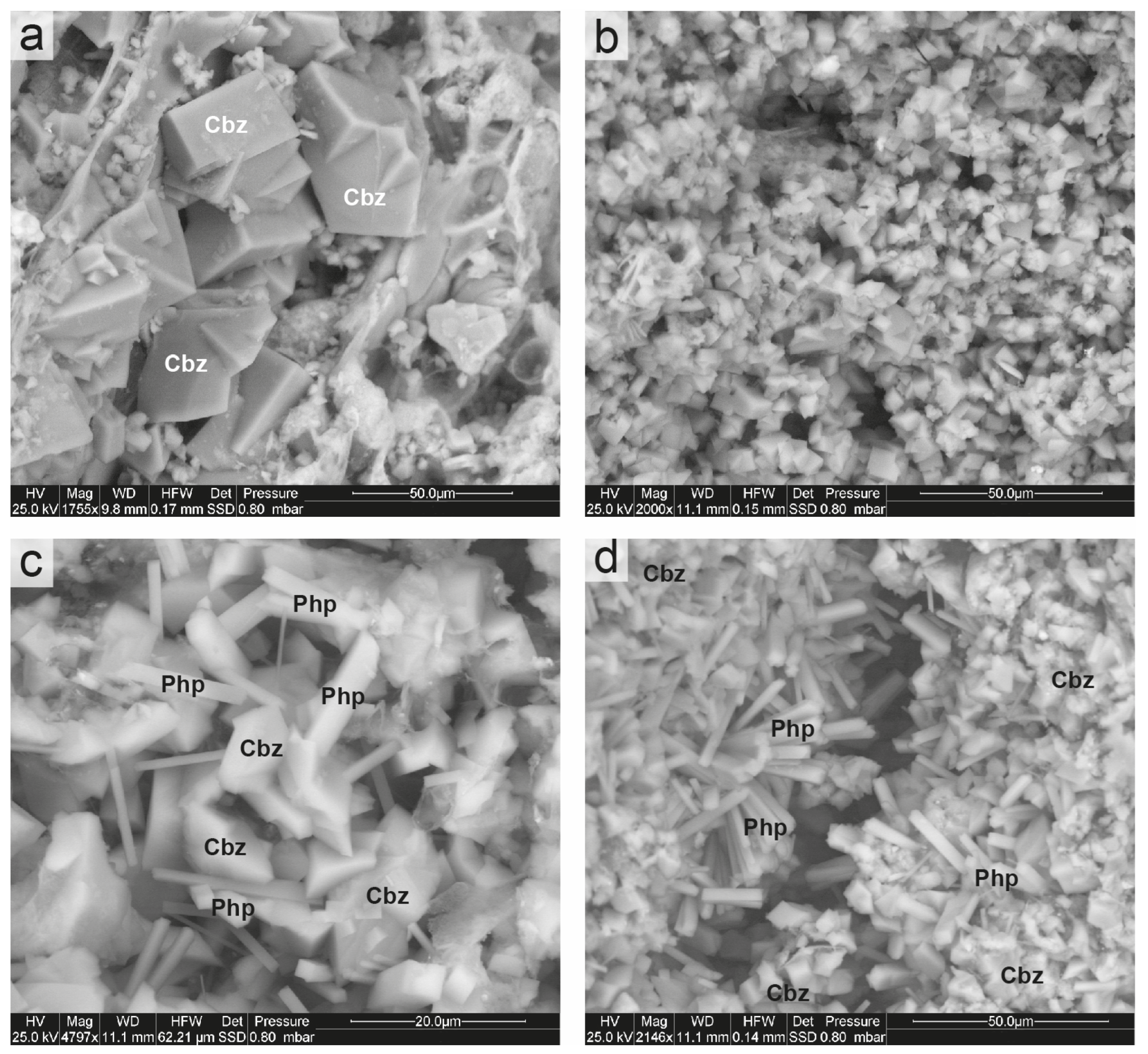
Figure 9.
ESEM images of Sorano Ignimbrite. (a) Euhedral and twinned crystals of chabazite; (b) near-complete replacement of the pumice surface by micrometric chabazite crystals; (c) association of chabazite and phillipsite crystals in a vesicle; (d) radial aggregates of phillipsite, associated with chabazite, within a pumice cavity. Cbz = chabazite; Php = phillipsite. Abbreviations of minerals according to [52].
By contrast, the surfaces of pumice clasts are affected by smaller crystals (1–10 µm), which often completely replace the glassy matrix (Figure 9b). In some cases, elongated prismatic phillipsite crystals (10–20 µm) occur together with chabazite (Figure 9c). Locally, phillipsite forms radial aggregates, acicular clusters of thin prismatic crystals growing within pumice or irregular groups (Figure 9d). Notably, chabazite typically crystallizes by replacing pumice glass and within the ashy matrix, whereas phillipsite preferentially develops inside scoria fragments and along fractures.
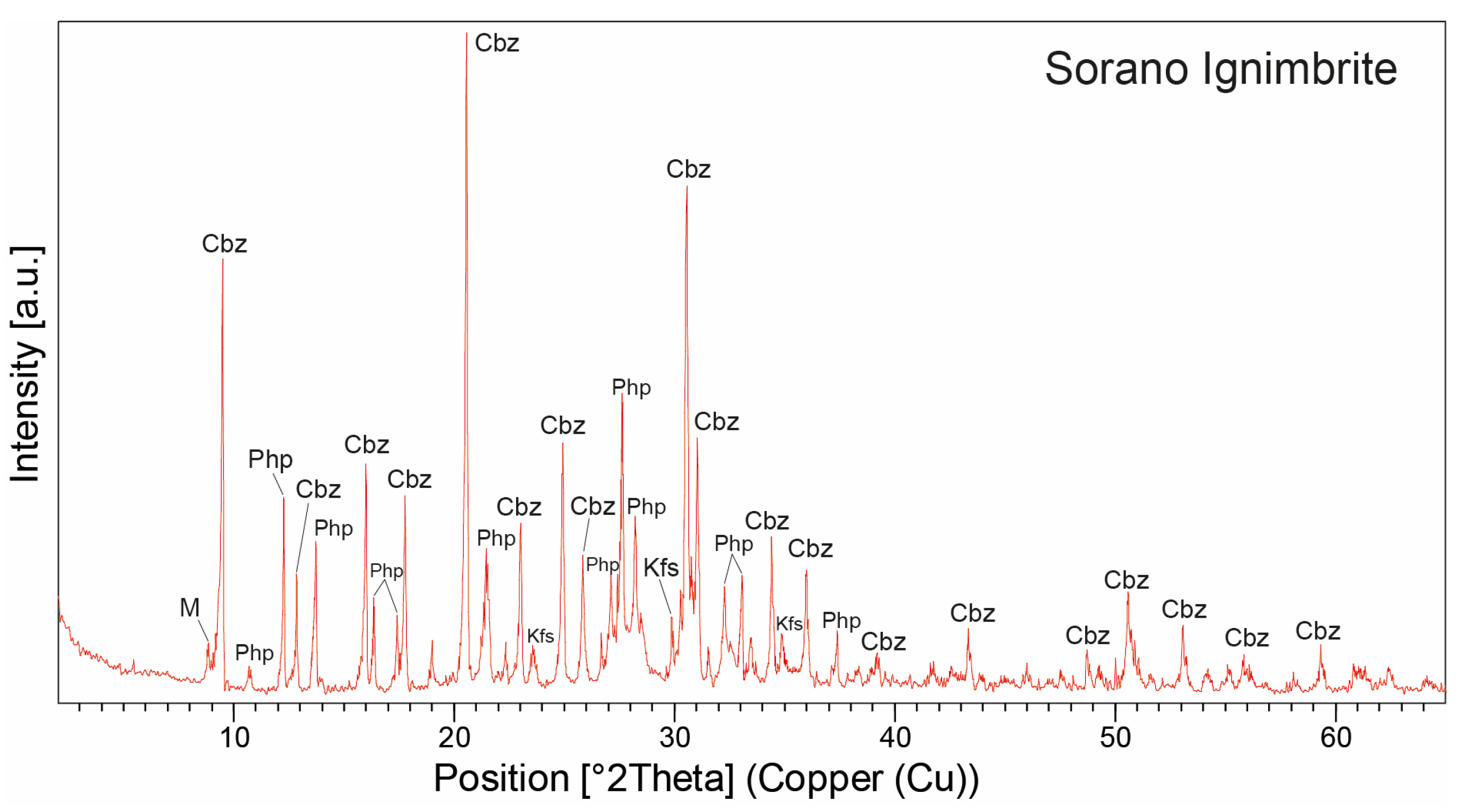
The mineralogical composition determined through ESEM observations and EDS analysis was confirmed by XRPD data (Table 2 and Figure 10). XRPD analyses, performed on pure crystals manually separated under the microscope from vesicles and cavities, show that the Sorano Ignimbrite is predominantly composed of chabazite and minor phillipsite (average 77 wt.% and 20 wt.%, respectively), with traces of K-feldspar (sanidine, average 3 wt.%) and mica (average 3 wt.%), likely occurring as microcrystals associated or included with the zeolite phases. Chabazite is the major mineral phase, as indicated by characteristic peaks at 30.48°, 20.51°, 9.44°, 30.92°, and 24.81° 2Θ), while phillipsite is subordinate, with peaks at 27.6°, 27.74°, 13.73°, 12.32°, 16.41°, 28.26°, and 28.22° 2Θ.
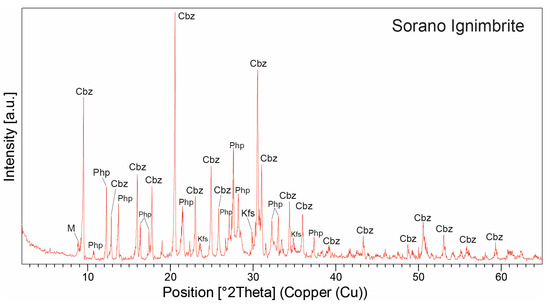
Figure 10.
XRPD patterns of the zeolitized juvenile fraction from one representative sample of the Sorano Ignimbrite. Peak labels: Cbz = chabazite; Php = phillipsite; Kfs = K-feldspar; M = mica. Abbreviations of minerals according to [52].
The microchemical compositions of zeolites from the Cimina Ignimbrite (clinoptilolite) and the Sorano Ignimbrite (chabazite and phillipsite), determined by WDS/EPMA, are reported in Table 3.

Table 3.
Chemical composition of zeolites from the Cimina (clinoptilolite) and Sorano (chabazite and phillipsite) Ignimbrites, based on WDS/EMPA data. Structural formulas are based on 36 oxygens (clinoptilolite), 24 oxygens (chabazite), and 32 oxygens (phillipsite), and are calculated on an anhydrous basis. ΣT represents the sum of the tetrahedral site, while ΣET denotes the sum of the extra-tetrahedral site. R = Si/(Si + Al).
The clinoptilolite in the Cimina Ignimbrite exhibits a chemical composition dominated by calcium as the main extra-framework cation (DEC, average 0.84 atoms per formula unit, apfu), with sodium (Na) and potassium (K) present in subordinate amounts (average 0.69 and 0.47 apfu, respectively). The resulting average chemical composition of clinoptilolite-Ca is Ca0.84Na0.69K0.47[Al3.41Si14.58O36]·12H2O. The R-value, defined as R = Si/(Si + Al), has a mean of 0.81, corresponding to a Si/Al ratio of 4.27. The subcommittee on zeolites of the International Mineralogical Association [5] suggested that minerals in the clinoptilolite series have Si/Al ≥ 4 (clinoptilolite-K, -Na, -Ca), whereas those in the heulandite series have Si/Al < 4 (heulandite-Ca, -Sr, -Na, -K). Accordingly, the analyzed zeolite in the Cimina Ignimbrite (Si/Al > 4) can be classified as clinoptilolite, as also confirmed by the XRPD results.
Microchemical analyses of chabazite crystals from the Sorano Ignimbrite (Table 3) indicate that potassium (K) is consistently the DEC, with an average of 1.55 apfu, while calcium (Ca) and sodium (Na) are subordinate, averaging 0.62 and 0.24 apfu, respectively. The average chemical composition of chabazite-K is K1.55Ca0.62Na0.24[Al3.49Si8.54O24]·11H2O. The DEC in all analyzed crystals comprises K, Ca, or Na; magnesium (Mg) and iron (Fe) are either absent or occur only as secondary extra-framework cations. The R-value, defined as R = Si/(Si + Al), has a mean of 0.71, corresponding to a Si/Al ratio of 2.45.
Analyses of the phillipsite crystals from the Sorano Ignimbrite (Table 3) show that they have a narrow compositional range with K as DEC (3.32 apfu average), and minor amounts of Na (0.54 apfu) and Ca (0.38 apfu). The average chemical composition of phillipsite-K is K3.3Na0.54Ca0.38[Al4.47Si11.53O32]·12H2O. The DEC in phillipsite-K includes K, Na, and Ca, while rare crystals contain trace amounts of magnesium (Mg) and iron (Fe) as secondary cations. The R-value averages 0.72, with an average Si/Al ratio of 2.58.
5. Discussion
The transformation of volcanic glass into zeolites has been extensively studied, and several diagenetic settings for zeolite formation in sedimentary environments have been identified [28,55,56,57,58,59,60]. This process is widespread in pyroclastic flow deposits (i.e., ignimbrites). Zeolitized ignimbrites occur worldwide, including the Niigata oil field (Japan [61]), the Esmeralda Formation (Nevada [62]), the Chambers and Capote Mountains Formations (Texas [63]), the Waitemata Group (New Zealand [64]), and Samos and Santorini (Greece, [65,66]). In Central and Southern Italy, zeolitized ignimbrites are especially widespread. Notable examples include the Tufo Rosso a Scorie Nere (Vico, [26,28]), Tufo Lionato (Colli Albani Volcanic Complex, [67]), Tufo Giallo Napoletano (Campi Flegrei, [68,69]), Logudoro and Asuni (Sardinia, [70,71]), and Cimino and Sorano Ignimbrites (this work).
The mineralogical assemblages, paragenetic relationships, and formation modes of zeolites and other authigenic minerals in the Central Italy ignimbrites closely resemble those found in zeolitized pyroclastic deposits worldwide. These similarities suggest that the key factors controlling zeolitization are broadly consistent across diverse geological settings. Most researchers agree that zeolite formation in ignimbrites is mainly controlled by volcanic glass composition, temperature, and water availability [29,57,69,70,71,72,73].
The zeolite formation through the hydration and dissolution of volcanic glass is driven by cation exchange involving K+, Na+, and Ca2+ [30,56,74,75]. This process is mainly influenced by three factors: (1) the silica content of the host glass; (2) the pH of fluids permeating the deposit, which promotes cation exchange; and (3) the cation content of both the glass and infiltrating fluids [57]. As the glass dissolves, alkali ions (Na+, K+) are released, increasing the solution’s salinity and the alkali-to-H+ activity ratio. These chemical changes favor zeolite crystallization. The specific types of zeolites and other aluminosilicates that form depend largely on the levels of Na, K, Ca, and Si present. The highly alkaline nature of volcanic glass supports zeolite formation, while silica content helps determine which zeolite species develop. Regarding the relationship between the chemical composition of the juvenile fractions in the parent ignimbrites and that of the authigenic mineral assemblages, two distinct behaviors emerge in the ignimbrites studied here.
In the Cimina Ignimbrite, the juvenile fraction has a low-alkali trachytic to trachyandesitic composition (Table 1), with high silica content (61–62 wt.%) and relevant concentrations of Ca (4.30 wt.%) and K (5.32 wt.%). The authigenic mineral assemblage includes clinoptilolite-Ca ± cristobalite (Table 2). The calcium-rich composition of clinoptilolite reflects the high Ca content of the corresponding juvenile fraction. However, none of the studied samples contained K-bearing zeolites, despite the abundance and high mobility of K in fluids. In trachytic ignimbrites with similar compositions, zeolites are generally represented by the phillipsite–chabazite association, as observed in the Neapolitan Yellow Tuff [31], the Campanian Ignimbrite [11,60], and ignimbrite deposits of the Sandıklı–Afyonkarahisar region, Turkey [76]. Clinoptilolite, often associated with mordenite, is more typical of rhyolitic ignimbrites, such as those from Logudoro and Asuni (Sardinia) [70,71] and Samos, Greece [65]. In these rhyolitic cases, zeolite formation is attributed to high-temperature metasomatism involving K-rich alkaline hydrothermal fluids, which increase pH and promote silica supersaturation. In the Cimina Ignimbrite, the presence of clinoptilolite, unusual for a trachytic juvenile fraction, may be related to a higher formation temperature than that typical for chabazite and phillipsite. Another noteworthy aspect is the Si/Al ratio. In the Cimina Ignimbrite, the bulk juvenile fraction has a ratio of ~3.73, whereas in the zeolitized facies, clinoptilolite shows a higher value of 4.27. This difference likely reflects that the juvenile fraction composition does not represent a pure parental glass but rather a glass containing microlites of mineral phases, as indicated by XRD analyses. These microlites influence the bulk Si/Al ratio but do not contribute to the same extent in the ratio measured by EPMA for the newly formed clinoptilolite.
In contrast, the Sorano Ignimbrite has a slightly lower silica content (59–61 wt.%) but is significantly richer in K (8.7 wt.%). When comparing chemical analyses of juvenile bulk rocks (Table 1) with those of zeolites (Table 2), the zeolite crystallization system seems to follow the strongly potassic nature of the whole rock, leading to the crystallization of phillipsite-K and chabazite-K members. Chabazite and phillipsite typically form in potassium- and sodium-rich environments [59]. The Sorano Ignimbrite is characterized by a highly alkaline phonolitic and trachytic–trachydacitic composition, and our results (Table 3) are consistent with this observation. The authigenic mineral assemblages in the Sorano Ignimbrite closely match those found in other ignimbrites of similar composition, both in phase types and relative abundances, such as the Red Tuff with Black Scoria [28], Orvieto Ignimbrite [77], and the tuffs from the Laacher See area in Germany [78]. The relatively high calcium content of the zeolites, particularly chabazite (up to 3.7 wt.% vs. 2.6 wt.% in the juvenile composition), points to an overall higher Ca availability in the deposit. This may in part reflect contributions from bicarbonate-rich waters circulating during the hydrothermal stage, supporting the relationship between permeability and authigenic mineralogy. Additionally, the Si/Al ratio of the zeolites (averaging around 2.45–2.58) is only slightly lower than that of the juvenile fraction (approximately 3.1), further reinforcing the genetic link between the zeolites and the original volcanic material.
Temperature is another key factor influencing zeolite crystallization. Zeolites typically form within a specific temperature range (15–300 °C), with each species indicative of a distinct thermal window [72]. The initial emplacement temperatures of ignimbrite deposits are generally too high for zeolite stability, often exceeding 550 °C [30,75]. Zeolites typically form later, once the deposit has cooled into the appropriate temperature range. According to Hall [75], most ignimbrite deposits cool to around 100 °C within a few years. However, several factors can influence this cooling process, including residual heat flux, circulation of hot fluids, deposition of overlying materials, and geothermal activity [59,74,79,80]. Additionally, water trapped within the ash during cooling may redistribute heat, creating localized hot spots within an otherwise cooler deposit [75]. Clinoptilolite is the only zeolite species identified in the Cimina Ignimbrite. This mineral typically forms at temperatures between 75 and 175 °C [72] and is considered indicative of medium to high temperatures. Clinoptilolite is also found in association with cristobalite, a silica phase that forms under similarly high-temperature conditions. Notably, zeolitization of the volcanic glass in the Cimina Ignimbrite is primarily observed in the most internal parts of the deposit. This suggests that the inner portion of the ignimbrite remained within the stability range for clinoptilolite formation (~150–170 °C) for a relatively extended period. In contrast, the zeolite species found in the juvenile fraction of the Sorano Ignimbrite are chabazite and phillipsite. These minerals crystallize at significantly lower temperatures, generally between 20 and 75 °C, with chabazite stable below 50 °C [72]. Their widespread presence throughout the matrix and within both the rims and cores of pumice clasts suggests that the deposit remained within the optimal temperature range for chabazite and phillipsite crystallization (~50–70 °C). Zeolitization in the Sorano Ignimbrite appears to be homogeneous across the deposit, except for a thin, non-zeolitized layer at its base. The absence of zeolite in this lower portion, also reported in other ignimbrites (e.g., TRSN [28]), may suggest lower emplacement temperatures at the base during deposition.
Hydration and dissolution of volcanic glass [31] require an adequate water supply. Hall [75] estimated that transforming a pumice fragment into a zeolite aggregate needs water equal to about 10% of its mass. Magmatic water provides only part of this amount, so most of the water must come from external sources. In phreatomagmatic eruptions, magma interacts extensively with groundwater or surface water, supplying sufficient water for zeolitization, which explains the frequent association with zeolitized ignimbrites. Other potential sources include meteoric water, groundwater, and geothermal fluids circulating through the deposit [31,69]. As previously noted, in the Sorano Ignimbrite, chabazite typically replaces pumice glassy surfaces and occurs within the ashy matrix, whereas phillipsite is mainly found inside scoria fragments and along fractures. Similar to observations in the Tufo Rosso a Scorie Nere ignimbrite [28], this distribution reflects the initial permeability contrasts among pumice, scoria, and the matrix. The higher permeability of pumice surfaces and the ashy matrix facilitates more extensive fluid circulation, enhancing glass dissolution and promoting the crystallization of chabazite, whose structure incorporates more water. In contrast, the lower permeability of scoria restricts fluid flow, creating conditions that favor the nucleation and growth of phillipsite, a zeolite with lower water content. This observation supports the conclusion that the composition of zeolite assemblages in volcanic deposits is largely controlled by water availability. Several active high-enthalpy geothermal fields occur along the volcanic belt of Central Italy. The Latera geothermal field lies within the Sorano Ignimbrite emission area, while the Vico system remains active beneath Mount Cimino [80,81,82]. Both fields share similar deep and shallow crustal structures, hydrogeological settings, and subvolcanic systems, with reservoirs likely located at depths of 500–1500 m. Hydrogeochemical data [83] indicate salinities of 8–12 g/L, steam-dominated fluids with 3.5% gas (98% CO2, 1% H2S, 1% N2), reservoir temperatures of 190–230 °C, and pressures near 100 bar at the reservoir top, with free CO2 present. These conditions suggest that, for the studied ignimbrites, the water volume required for zeolitization, together with excess alkalis and favorable pH, could have been supplied by high-enthalpy geothermal fluids mobilized during caldera-forming eruptions and associated collapse events.
Regarding zeolite formation in the ignimbrites of Central Italy, the most widely accepted model is that proposed by Lenzi and Passaglia [84]. In this model, zeolitization occurs in a natural, autoclave-like environment, facilitated by the presence of fluids heated by thermal energy and retained within the deposit for an extended period. In the Cimina Ignimbrite, this environment was characterized by relatively high temperatures, likely between 150 and 200 °C, which favored zeolitization through the interaction of fresh, highly reactive trachytic–trachyandesitic glass with still-hot circulating fluids. By contrast, the Sorano Ignimbrite, owing to eruptive conditions and the smaller volume of magma involved, developed under lower-temperature conditions (100–150 °C), though still sufficient to promote zeolitization. In this case, the fluids induced hydrolysis and dissolution of the more alkaline (phonolitic) glassy fraction, leading to extensive crystallization of chabazite-K and phillipsite-K. Moreover, beyond the physicochemical parameters required for zeolite formation, such as temperature, glass chemistry, and water content, volcanological factors, including eruptive style, transport mechanisms, and emplacement processes, also play a significant role in controlling post-emplacement zeolitization in ignimbrites [26].
This study highlights that in the volcanoes of Central Italy, where both magma chemistry and eruptive mechanisms are highly variable, diverse zeolitization processes and variable authigenic mineral phases can occur. This marked variability reflects the eruption of magmas from different magmatic series within the same or adjacent areas, ranging from acidic to intermediate compositions and including rocks with distinctly shoshonitic, alkaline potassic, and ultrapotassic chemistry. Such diversity warrants special attention, as in some cases zeolitization can produce fibrous zeolites that are hazardous to human health (e.g., erionite, offretite), as recently documented in some pyroclastic deposits of the Vico Volcano in Central Italy [85].
6. Conclusions
Combined field observations, whole-rock chemical compositions, and mineral chemistry of authigenic zeolites from two pyroclastic units of Central Italy (Cimino and Sorano Ignimbrites) allow us to summarize the following points:
- In Central Italy’s volcanoes, where magma chemistry and eruptive mechanisms vary greatly, different types of zeolitization processes and authigenic mineral phases can be recognized.
- In the Cimina Ignimbrite, composition of the juvenile fraction is low-alkali trachytic–trachyandesitic, high silica (61%–62%), and K- and Ca-rich. Zeolitization affects only the inner glassy groundmass, forming clinoptilolite-Ca and cristobalite. These minerals are unusual for trachytic glass compositions but are likely linked to higher formation temperature (150–170 °C).
- In the Sorano Ignimbrite, the juvenile fraction is phonolitic/trachytic–trachydacitic, and has low silica (59%–61%) and high K content. Zeolitization affects the entire glassy fraction of pumice clasts, producing medium- to low-temperature zeolites such as phillipsite-K and chabazite-K, matching strongly the potassic nature of the juvenile fraction.
- In the Cimina Ignimbrite, zeolitization is confined to the innermost and deepest part of the deposit, spanning approximately 20–30 m in thickness. In contrast, the upper and lateral portions of the ignimbrite contain fresh glass with no signs of zeolitization. Conversely, in the Sorano Ignimbrite, authigenic zeolites form throughout the entire thickness of the deposit.
- In the ignimbrites from Central Italy, zeolitization probably occurs in a natural autoclave-like environment, which involves fluids heated by thermal energy and retained in the deposit for a long time. Cimina Ignimbrite experienced high temperatures (150–200 °C), favoring zeolitization of trachytic–trachyandesitic glass with hot circulating fluids, while Sorano Ignimbrite formed at lower temperatures (100–150 °C) due to different eruptive conditions and smaller magma volume, leading to zeolitization of more alkaline phonolitic glass.
- In addition to temperature, glass chemistry, and water content, volcanological factors like eruptive style and emplacement processes also influence zeolitization. Nearby high-enthalpy geothermal systems (Latera, Vico) could have supplied the necessary water, alkalis, and pH conditions during caldera-forming eruptions and collapse.
Author Contributions
M.M.: supervision, conceptualization, methodology, investigation, data curation, writing—original draft preparation, writing—review and editing, and funding acquisition. M.G.: formal analysis, data curation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This project is funded by the INAIL-BRIC 2019 project (grant number ID 60; CUP: B24I20000070006). Part of the research was conducted under the framework of the 2022 research programs of the Department of Pure and Applied Sciences of the University of Urbino Carlo Bo (PROG2022 “New asbestiform natural fibers: mineralogical and physical–chemical characterization of fibrous zeolites”, responsible author, M. Mattioli).
Data Availability Statement
The main data are contained within the article.
Acknowledgments
We warmly thank Laura Valentini for her kind assistance during the acquisition of ESEM images. The authors sincerely thank Lily Zhao and Keely Luo for their kind editorial assistance. We are also grateful to the three anonymous peer reviewers for their valuable suggestions and constructive criticisms, which greatly improved the original version of this paper. During the preparation of this manuscript, the authors used ChatGPT 5.0 (developed by OpenAI) to improve the clarity and style of the scientific English and to identify and correct typographical and grammatical errors. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Gottardi, G.; Galli, E. Natural Zeolites; Springer: Berlin/Heidelberg, Germany, 1985; p. 409. [Google Scholar]
- Bish, D.L.; Ming, D.W. Natural Zeolites: Occurrence, Properties, Applications; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America: Chantilly, VA, USA, 2001; Volume 45, 662p. [Google Scholar]
- Passaglia, E.; Sheppard, R.A. The crystal chemistry of zeolites. In Natural Zeolites: Occurrence, Properties, Applications; Bish, D.L., Ming, D.W., Eds.; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America and Geochemical Society: Washington, DC, USA, 2001; Volume 45, pp. 69–116. [Google Scholar]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 5th ed.; Elsevier: London, UK, 2001; p. 398. [Google Scholar]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended nomenclature for zeolite minerals; report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral. 1997, 35, 1571–1606. [Google Scholar]
- Armbruster, T.; Gunter, M.E. Crystal structures of natural zeolites. In Natural Zeolites: Occurrence, Properties, Applications; Bish, D.L., Ming, D.W., Eds.; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America and Geochemical Society: Washington, DC, USA, 2001; Volume 45, pp. 1–68. [Google Scholar]
- Rhodes, C.J. Properties and applications of zeolites. Sci. Prog. 2010, 93, 223–284. [Google Scholar] [CrossRef]
- Möller, K.; Bein, B. Mesoporosity—A new dimension for zeolites. Chem. Soc. Rev. 2013, 42, 3689–3707. [Google Scholar] [CrossRef]
- Utada, M. Zeolites in burial diagenesis and low-grade metamorphic rocks. In Natural Zeolites: Occurrence, Properties, Applications; Bish, D.L., Ming, D.W., Eds.; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America and Geochemical Society: Washington, DC, USA, 2001; Volume 45, pp. 277–304. [Google Scholar]
- Weisenberger, T.B.; Spürgin, S.; Lahaye, Y. Hydrothermal alteration of the Fohberg phonolite, Kaiserstuhl Volcanic Complex, Germany. Int. J. Earth Sci. 2014, 103, 2273–2300. [Google Scholar] [CrossRef]
- Langella, A.; Bish, D.L.; Cappelletti, P.; Cerri, G.; Colella, A.; de’Gennaro, R.; Graziano, S.F.; Perrotta, A.; Scarpati, C.; de’Gennaro, M. New insights into the mineralogical facies distribution of Campanian Ignimbrite, a relevant Italian industrial material. Appl. Clay Sci. 2013, 72, 55–73. [Google Scholar] [CrossRef]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural Zeolites as Effective Adsorbents in Water and Wastewater Treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- de’Gennaro, B.; Aprea, P.; Colella, C.; Buondonno, A. Comparative ion-exchange characterization of zeolitic and clayey materials for pedotechnical applications-Part 2: Interaction with nutrient cations. J. Porous Mater. 2009, 16, 667–673. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary cementitious materials. Rev. Mineral. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Carbone, M.; Baris, Y.I.; Bertino, P.; Brass, B.; Comertpay, S.; Dogan, A.U.; Gaudino, G.; Jube, S.; Kanodia, S.; Partridge, C.R.; et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc. Natl. Acad. Sci. USA 2011, 108, 13618–13623. [Google Scholar] [CrossRef] [PubMed]
- Giordani, M.; Mattioli, M.; Dogan, M.; Dogan, A.U. Potential carcinogenic erionite from Lessini Mounts, NE Italy: Morphological, mineralogical and chemical characterization. J. Toxicol. Environ. Health A 2016, 79, 808–824. [Google Scholar] [CrossRef]
- Di Giuseppe, D. Characterization of Fibrous Mordenite: A First Step for the Evaluation of Its Potential Toxicity. Crystals 2020, 10, 769. [Google Scholar] [CrossRef]
- Giordani, M.; Ballirano, P.; Pacella, A.; Meli, M.A.; Roselli, C.; Di Lorenzo, F.; Fagiolini, I.; Mattioli, M. Fibrous mordenite from Northern Italy: Another potentially hazardous zeolite. Minerals 2022, 121, 627. [Google Scholar] [CrossRef]
- Cangiotti, M.; Salucci, S.; Battistelli, M.; Falcieri, E.; Mattioli, M.; Giordani, M.; Ottaviani, M.F. EPR, TEM and cell viability study of asbestiform zeolite fibers in cell media. Colloids Surf. B Biointerfaces 2018, 161, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Gandolfi, N.B.; Passaglia, E.; Pollastri, S.; Mattioli, M.; Giordani, M.; Ottaviani, M.F.; Cangiotti, M.; Bloise, A.; Barca, D.; et al. Is fibrous ferrierite a potential health hazard? Characterization and comparison with fibrous erionite. Am. Mineral. 2018, 103, 1044–1055. [Google Scholar] [CrossRef]
- Giordani, M.; Mattioli, M.; Cangiotti, M.; Fattori, A.; Ottaviani, M.F.; Betti, M.; Ballirano, P.; Pacella, A.; Di Giuseppe, D.; Scognamiglio, V.; et al. Characterisation of potentially toxic natural fibrous zeolites by means of electron paramagnetic resonance spectroscopy and morphological-mineralogical studies. Chemosphere 2022, 291, 133067. [Google Scholar] [CrossRef]
- Mattioli, M.; Giordani, M.; Arcangeli, P.; Valentini, L.; Boscardin, M.; Pacella, A.; Ballirano, P. Prismatic to asbestiform offretite from Northern Italy: Occurrence, morphology and crystal-chemistry of a new potentially hazardous zeolite. Minerals 2018, 8, 69. [Google Scholar] [CrossRef]
- Mattioli, M.; Ballirano, P.; Pacella, A.; Cangiotti, M.; Di Lorenzo, F.; Valentini, L.; Meli, M.A.; Roselli, C.; Fagiolino, I.; Giordani, M. Fibrous Ferrierite from Northern Italy: Mineralogical Characterization, Surface Properties, and Assessment of Potential Toxicity. Minerals 2022, 12, 626. [Google Scholar] [CrossRef]
- Marantos, I.; Markopoulos, T.; Christidis, G.E. Zeolitic alteration in the Tertiary Feres volcano-sedimentary basin, Thrace, NE Greece. Mineral. Mag. 2007, 71, 327–345. [Google Scholar] [CrossRef]
- Bear, A.N.; Cas, R.A.F.; Giordano, G. The implications of spatter, pumice and lithic clast rich proximal co-ignimbrite lag breccias on the dynamics of caldera forming eruptions: The 151 ka Sutri eruption, Vico Volcano, Central Italy. J. Volcanol. Geotherm. Res. 2009, 181, 1–24. [Google Scholar] [CrossRef]
- Colella, A.; Di Benedetto, C.; Calcaterra, D.; Cappelletti, P.; D’Amore, M.; Di Martire, D.; Graziano, S.F.; Papa, L.; Langella, A. The Neapolitan Yellow Tuff: An outstanding example of heterogeneity. Constr. Build. Mater. 2017, 136, 361–373. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Cappelletti, P.; Graziano, S.F. A case study of zeolitization process Tufo Rosso a Scorie Nere (Vico volcano, Italy): Inferences for a general model. Eur. J. Mineral. 2021, 33, 315–328. [Google Scholar] [CrossRef]
- de’Gennaro, M.; Franco, E.; Rossi, M.; Langella, A.; Ronca, A. Epigenetic minerals in the volcaniclastic deposits from central southern Italy: A contribution to zeolite genesis. Rend. Lincei Sci. Fis. Nat. 1987, 107–131. [Google Scholar]
- de’Gennaro, M.; Colella, C. The critical role of temperature in the natural zeolitization of volcanic glass. Neues Jahrb. Für Mineral. Monatshefte 1991, 8, 355–362. [Google Scholar]
- de’Gennaro, M.; Cappelletti, P.; Langella, A.; Perrotta, A.; Scarpati, C. Genesis of zeolites in the Neapolitan Yellow Tuff: Geological, volcanological and mineralogical evidence. Contrib. Mineral. Petrol. 2000, 139, 17–35. [Google Scholar] [CrossRef]
- de’Gennaro, R.; Cappelletti, P.; Cerri, G.; De’ Gennaro, M.; Dondi, M.; Langella, A. Neapolitan Yellow Tuff as raw material for lightweight aggregates in lightweight structural concrete production. Appl. Clay Sci. 2005, 28, 309–319. [Google Scholar] [CrossRef]
- Peccerillo, A.; Conticelli, S.; Manetti, P. Petrological characteristics and the genesis of the recent magmatism of the Southern Tuscany and Northern Latium. Per. Mineral. 1987, 56, 157–172. [Google Scholar]
- Peccerillo, A. Plio-Quaternary Volcanism in Italy: Petrology, Geochemistry, Geodynamics; Springer: New York, NY, USA, 2005; 365p. [Google Scholar]
- Carminati, E.; Lustrino, M.; Doglioni, C. Geodynamic evolution of the central and western Mediterranean: Tectonics vs. igneous petrology constraints. Tectonophysics 2012, 579, 173–192. [Google Scholar] [CrossRef]
- Perini, G.; Francalanci, L.; Davidson, J.P.; Conticelli, S. Evolution and genesis of magmas from Vico volcano, Central Italy: Multiple differentiation pathways and variable parental magmas. J. Petrol. 2004, 45, 139–182. [Google Scholar] [CrossRef]
- Conticelli, S.; Melluso, L.; Perini, G.; Avanzinelli, R.; Boari, E. Petrologic, geochemical and isotopic characteristics of potassic and ultrapotassic magmatism in Central-Southern Italy: Inferences on its genesis and on the nature of mantle sources. Per. Mineral. 2004, 73, 135–164. [Google Scholar]
- Conticelli, S.; Avanzinelli, R.; Poli, G.; Braschi, E.; Giordano, G. Shift from lamproite-like to leucititic rocks: Sr-Nd-Pb isotope data from the Monte Cimino volcanic complex vs. the Vico stratovolcano, Central Italy. Chem. Geol. 2013, 353, 246–266. [Google Scholar] [CrossRef]
- Nappi, G.; Renzulli, A.; Santi, P.; Gillot, P.Y. Geological evolution and geochronology of the Vulsinian Volcanic District (Central Italy). Boll. Soc. Geol. Ital. 1995, 114, 599–613. [Google Scholar]
- Palladino, D.M.; Simei, S.; Sottili, G.; Trigila, R. Integrated approach for the reconstruction of stratigraphy and geology of Quaternary volcanic terrains: An application to the Vulsini Volcanoes (central Italy). In Stratigraphy and Geology in Volcanic Areas; Groppelli, G., Viereck-Goette, L., Eds.; The Geological Society of America: Boulder, CO, USA, 2010; Volume 464, pp. 66–84. [Google Scholar]
- Sparks, R.S.J. Stratigraphy and geology of the ignimbrites of the Vulsini Volcano, Central Italy. Geol. Rundsch. 1975, 64, 497–523. [Google Scholar] [CrossRef]
- Palladino, D.M.; Agosta, E. Pumice fall deposits of the Western Vulsini Volcanoes (Central Italy). J. Volcanol. Geotherm. Res. 1997, 78, 77–102. [Google Scholar] [CrossRef]
- Nappi, G.; Mattioli, M.; Valentini, L.; Chiocchini, U.; Madonna, S. Note Illustrative della Carta Geologica d’Italia alla Scala 1:50.000. F. 355 Ronciglione; ISPRA: Rome, Italy, 2016. [Google Scholar]
- Lardini, D.; Nappi, G. I cicli del Complesso Vulcanico Cimino. Rend. Soc. Ital. Mineral. Petrol. 1987, 42, 141–153. [Google Scholar]
- Micheluccini, M.; Puxeddu, M.; Toro, B. Rilevamento e studio geo-vulcanologico della regione del Monte Cimino (Viterbo, Italia). Atti Soc. Tosc. Sc. Nat. Mem. Ser. A 1971, 78, 301–327. [Google Scholar]
- Nappi, G. Evoluzione del Complesso Vulcanico Cimino. Boll. GNV 1985, 128–139. [Google Scholar]
- Sabatini, V. I Vulcani dell’Italia Centrale e i Loro Prodotti: Parte II. Vulcani Cimini; Memorie Descrittive della Carta geologica d’Italia; 1912; Volume 15, pp. 1–636.
- Nappi, G. Stratigrafia e petrografia dei Vulsini sud-occidentali (caldera di Latera). Boll. Soc. Geol. Ital. 1969, 88, 171–181. [Google Scholar]
- Vezzoli, L.; Conticelli, S.; Innocenti, F.; Landi, P.; Manetti, P.; Trigila, R. Stratigraphy of the Latera Volcanic Complex: Proposals for a new nomenclature. Per. Mineral. 1987, 56, 89–110. [Google Scholar]
- Valentine, G.A.; Palladino, D.M.; DiemKaye, K.; Fletcher, C. Lithic-rich and lithic-poor ignimbrites and their basal deposits: Sovana and Sorano formations (Latera caldera, Italy). Bull. Volcanol. 2019, 81, 29. [Google Scholar] [CrossRef]
- Palladino, D.M.; Taddeucci, J. The basal ash deposit of the Sovana eruption (Vulsini volcanoes, central Italy): The product of a dilute pyroclastic density current. J. Volcanol. Geoth. Res. 1998, 87, 233–254. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.D.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Le Maitre, R.W.; Streckeisen, A.; Zanettin, B. A chemical classification of volcanic rocks based on the total alkali–silica diagram. J. Petrol. 1986, 27, 745–750. [Google Scholar] [CrossRef]
- Irvine, T.N.; Baragar, W.R.A. A guide to chemical classification of common volcanic rocks. Can. J. Earth Sci. 1971, 8, 523–548. [Google Scholar] [CrossRef]
- Passaglia, E.; Vezzalini, G. Crystal chemistry of diagenetic zeolites in volcaniclastic deposits of Italy. Contrib. Mineral. Petrol. 1985, 90, 190–198. [Google Scholar] [CrossRef]
- Gottardi, G. The genesis of zeolites. Eur. J. Mineral. 1989, 1, 479–487. [Google Scholar] [CrossRef]
- Passaglia, E.; Vezzalini, G.; Carnevali, R. Diagenetic chabazites and phillipsites in Italy: Crystal chemistry and genesis. Eur. J. Mineral. 1990, 2, 827–839. [Google Scholar] [CrossRef]
- Scherillo, A.; Scherillo, M. Campi Flegri e la stratigrafia Napoletana. Quad. Accad. Pont. 1990, 11, 138. [Google Scholar]
- de’Gennaro, M.; Langella, A.; Cappelletti, P.; Colella, C. Hydrothermal conversion of trachytic glass to zeolite. 3. Monocationic model glasses. Clays Clay Miner. 1999, 47, 348–357. [Google Scholar] [CrossRef]
- Cappelletti, P.; Cerri, G.; Colella, A.; de’Gennaro, M.; Langella, A.; Perrotta, A.; Scarpati, C. Post-eruptive processes in the Campanian ignimbrite. Mineral. Petrol. 2003, 79, 79–97. [Google Scholar] [CrossRef]
- Iijima, A.; Utada, M. Present-day zeolite diagenesis of the Neogene géosynclinal deposits of the Niigata Oil Field, Japan. In Advances in Chemistry; Gould, R.F., Ed.; American Chemical Society: Washington, DC, USA, 1971; pp. 342–349. [Google Scholar]
- Moiola, R.J. Authigenic zeolite and K-feldspar in the Esmeralda Formation, Nevada. Am. Mineral. 1970, 55, 1681–1691. [Google Scholar]
- Walton, A.W. Zeolite diagenesis in Oligocene volcanic sediments, Trans-Pecos Texas. Geol. Soc. Am. Bull. 1975, 86, 615–624. [Google Scholar] [CrossRef]
- Sameshima, T. Zeolites in tuff beds of the Miocene Waitemata Group, Auckland Volcanic Province, New Zealand. In Natural Zeolites: Occurrences, Properties, Use; Sand, L.B., Mumpton, F.A., Eds.; Pergamon Press: New York, NY, USA, 1978; pp. 309–317. [Google Scholar]
- Pe-Piper, G.; Tsolis-Katagas, P. K-rich mordenite from late-Miocene rhyolitic tuffs, Island of Samos, Greece. Clays Clay Miner. 1991, 39, 239–247. [Google Scholar] [CrossRef]
- Tsolis-Katagas, P.; Katagas, C. Zeolites in pre-caldera pyroclastic rocks of the Santorini volcano, Aegean Sea, Greece. Clays Clay Miner. 1989, 37, 497–510. [Google Scholar] [CrossRef]
- Giampaolo, C.; Lo Mastro, S.; De Rita, D.; Giordano, G. Lateral and vertical zeolite grade variations in the Tufo Lionato ignimbrite unit (Colli Albani, Roma, central Italy). In Proceedings of the Zeolite ‘06’-7th International Conference on the Occurrence, Properties and Utilisation of Natural Zeolites, Socorro, NM, USA, 16–21 July 2006. [Google Scholar]
- de’Gennaro, M.; Franco, E.; Langella, A.; Mirra, P.; Morra, V. Le phillipsiti dei Tufi Gialli del Napoletano. Per. Mineral. 1982, 51, 287–310. [Google Scholar]
- de’Gennaro, M.; Langella, A. Italian zeolitized rocks of technological interest. Mineral. Depos. 1996, 31, 452–472. [Google Scholar] [CrossRef]
- Cerri, G.; Cappelletti, P.; Langella, A.; de’Gennaro, M. Zeolitization of Ologo-Miocene volcaniclastic rocks from Logudoro (northern Sardinia, Italy). Contrib. Mineral. Petrol. 2001, 140, 404–421. [Google Scholar] [CrossRef]
- Mormone, A.; Piochi, M. Mineralogy, Geochemistry and Genesis of Zeolites in Cenozoic Pyroclastic Flows from the Asuni Area (Central Sardinia, Italy). Minerals 2020, 10, 268. [Google Scholar] [CrossRef]
- Chipera, S.J.; Apps, J.A. Geochemical stability of natural zeolites. In Natural Zeolites: Occurrence, Properties, Applications; Bish, D.L., Ming, D.W., Eds.; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America and Geochemical Society: Washington, DC, USA, 2001; Volume 45, pp. 117–161. [Google Scholar]
- Langella, A.; De Simone, P.; Calcaterra, P.; de’Gennaro, M. Evidence of the relationship occurring between zeolitisation and lithification in the yellow facies of the Campanian ignimbrite (southern Italy). In Studies in Surface Science and Catalysts; Aiello, R., Giordano, G., Testa, F., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2002; pp. 1775–1782. [Google Scholar]
- de’Gennaro, M.; Colella, A.; Franco, E.; Stanzione, D. Hydrothermal conversion of trachytic glass into zeolite I: Reactions with deionised water. Neues Jahrb. Mineral. 1988, 4, 149–158. [Google Scholar]
- Hall, A. Zeolitisation of volcaniclastic sediments: The role of temperature and pH. J. Sediment. Res. 1998, 68, 739–745. [Google Scholar] [CrossRef]
- Ozpinar, Y.; Semiz, B.; Schroeder, P.A. Zeolites in mafic pyroclastic rocks from the Sandikli-Afyonkarahisar region, Turkey. Clays Clay Miner. 2013, 3, 177–192. [Google Scholar] [CrossRef]
- Gentili, S.; Comodi, P.; Nazzareni, S.; Zucchin, A. The Orvieto-Bagnoregio Ignimbrite: Pyroxene crystal-chemistry and bulk phase composition of pyroclastic deposits, a tool to identify syn- and post-depositional processes. Eur. J. Mineral. 2014, 26, 743–756. [Google Scholar] [CrossRef]
- Bernhard, F.; Barth-Wirsching, U. Zeolitization of a Phonolitic Ash Flow by Groundwater in the Laach Volcanic Area, Eifel. Clays Clay Miner. 2002, 50, 710–725. [Google Scholar] [CrossRef]
- Sersale, R. Occurrences and uses of zeolites in Italy. In Natural Zeolites: Occurrences, Properties, Use; Sand, L.B., Mumpton, F.A., Eds.; Pergamon Press: New York, NY, USA, 1978; pp. 285–302. [Google Scholar]
- Sollevanti, F. Geologic, volcanologic and tectonic setting of the Vico-Cimini area, Italy. J. Volcanol. Geotherm. Res. 1983, 17, 203–217. [Google Scholar] [CrossRef]
- Barberi, F.; Buonasorte, G.; Cioni, R.; Fiordelisi, A.; Foresi, L.; Iaccarino, S.; Laurenzi, M.A.; Sbrana, A.; Vernia, L.; Villa, I.M. Plio-Pleistocene geological evolution of the geothermal area of Tuscany and Latium. Mem. Descr. Carta Geol. d’It. 1994, 49, 77–134. [Google Scholar]
- Buonasorte, G.; Carmelli, C.M.; Ceron, A.; Cioni, R.; Pensieri, R.; Sbrana, A. Results of geothermal exploration in central Italy (Latium–Campania). Proc. World Geoth. Congr. 1995, 2, 1293–1298. [Google Scholar]
- Baldi, P.; Ferrara, G.C.; Masselli, L.; Pieretti, G. Hydrogeochemistry of the region between Monte Amiata and Rome. Geothermics 1973, 2, 124–128. [Google Scholar] [CrossRef]
- Lenzi, G.; Passaglia, E. Fenomeni di zeolitizzazione nelle formazioni vulcaniche della regione sabatina. Boll. Soc. Geol. Ital. 1974, 93, 623–645. [Google Scholar]
- Mattioli, M.; Giordani, M.; Ballirano, P.; Salvioli-Mariani, E.; Bernardini, S.; Della Ventura, G. First occurrence, crystal-chemistry and structure of erionite, a carcinogenic fibrous zeolite, from the volcanic rocks of Latium (Italy). Per. Mineral. 2023, 92, 159–178. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).