Uptake of Copper and Zinc Ions by Georgian Natural Heulandite and Resulting Changes in Its Chemical Composition and Structure
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Composition of the Ion-Exchanged Samples
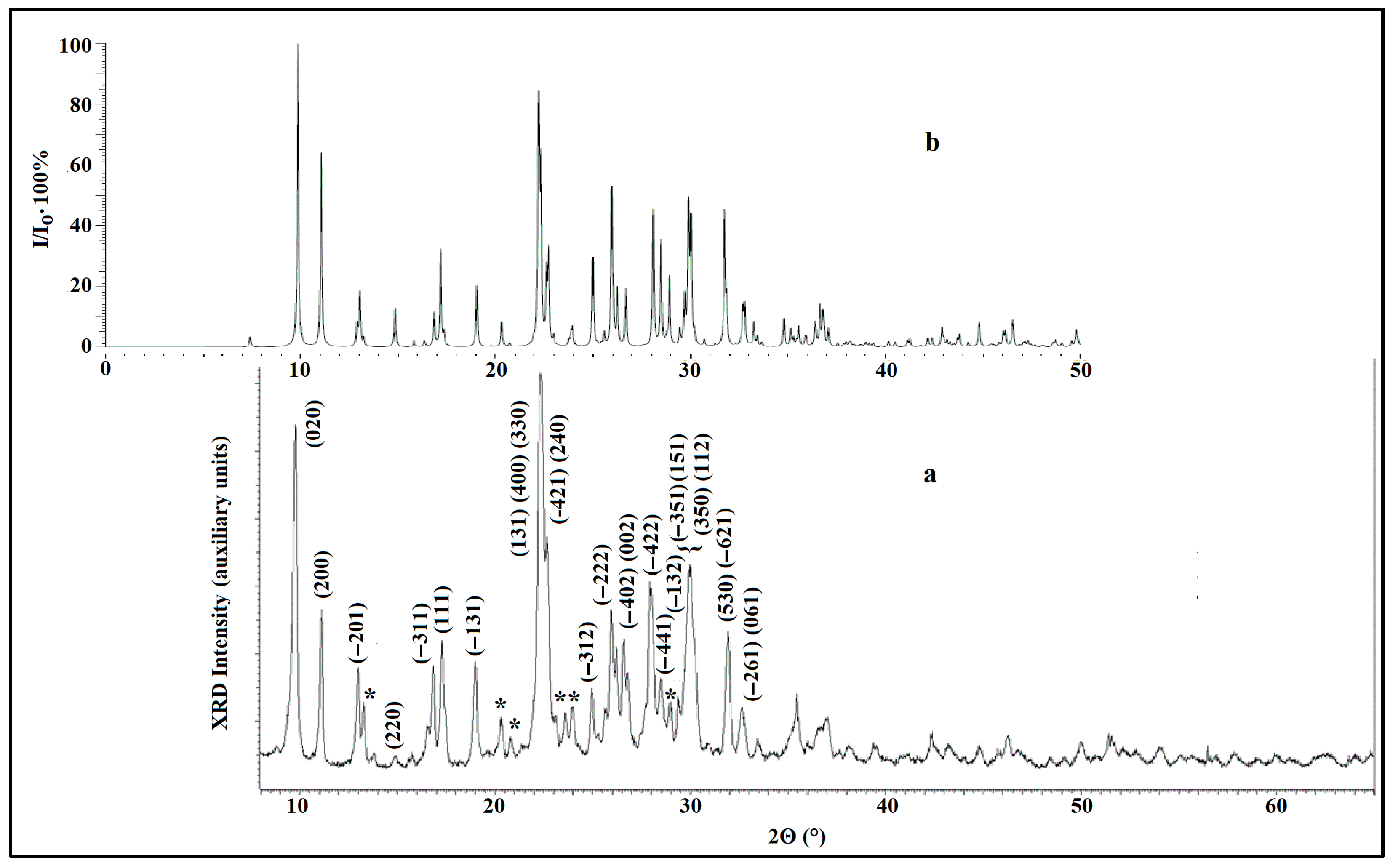
3.2. Crystal Structure of the Ion-Exchanged Samples
3.3. Water Adsorption
3.4. Benzene Adsorption
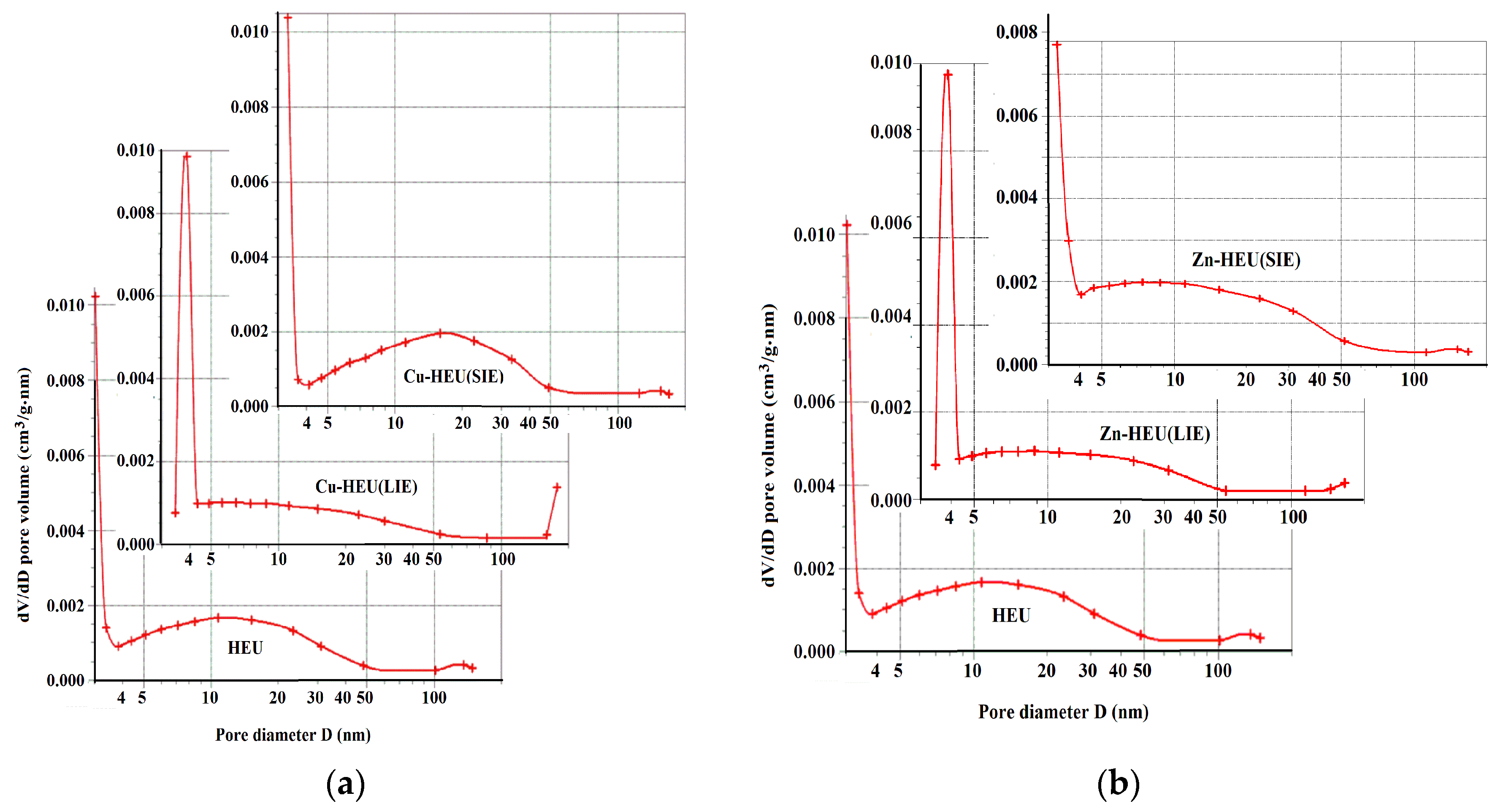
3.5. Nitrogen Adsorption
4. Conclusions
- Ion exchange reactions, regardless of the method of their implementation, cause slight dealumination of the zeolite framework without the formation of “hydroxyl nests”.
- The degree of copper uptake by heulandite does not depend on the ion exchange procedure; however, zinc uptake is more than twice as high as unexchanged zeolite when the zeolite interacts with the liquid phase.
- The uptake of transition metal ions occurs mainly due to the leaching of sodium and magnesium ions. Calcium ions are leached to a lesser extent, and potassium ions barely participate in ion exchange processes; that is, based on the same scheme as during decationization under acidic conditions.
- The crystalline framework of heulandite does not change as a result of ion exchange reactions; changes in the peak intensities in powder XRD patterns are due to changes in cationic composition.
- The change in the adsorption capacity of micropores for water molecules after the uptake of transition metals is insignificant; the adsorption of benzene molecules indicates only minor changes in the hydrophobicity of the outer surface of heulandites.
- The volume of micropores accessible to nitrogen molecules and the BET surface area are increased by all ion exchange procedures, but to a much lesser extent than by acid treatment. The LIE procedure decreases the volume of mesopores, and pores with a diameter of 4 nm become predominant. The SIE procedure increases the volume of nano-sized mesopores, and the distribution of pore sizes depends on the nature of the immobilized metal.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| XR-ED | X-Ray Energy Dispersion |
| XRD | X-Ray Diffraction |
| LIE | Liquid-phase Ion Exchange |
| SIE | Solid-state Ion Exchange |
| BET | Brunauer–Emmett–Teller model |
| BJH | Barrett–Joyner–Halenda model |
| STP | Standard Temperature and Pressure: 273.15 K and 101.325 kPa |
| ZVI | Zero-Valent Iron |
Appendix A
| Simulated Pattern [64] | Experimental Pattern | ||||
|---|---|---|---|---|---|
| Miller Indices hkl | d (Å) | 2Θ (°) | I/Io (%) | 2Θ (°) | I/Io (%) |
| 020 * | 8.979 | 9.85 | 100 | 9.82 | 100 |
| 200 * | 7.989 | 11.07 | 64.0 | 11.04 | 48 |
| −201 | 6.792 | 13.03 | 17.8 | 13.00 | 31 |
| −311 | 5.258 | 16.86 | 11.3 | 16.90 | 32 |
| 111 | 5.157 | 17.29 | 32.0 | 17.24 | 29 |
| −131 | 4.661 | 19.04 | 20.3 | 19.06 | 33 |
| 131 * 400 330 −421 240 | 4.003 3.995 3.979 3.931 3.914 | 22.21 22.25 22.34 22.62 22.72 | 60.2 34.7 53.8 21.9 29.4 | 22.5 | 115 |
| −312 | 3.563 | 24.99 | 29.4 | 25.00 | 25 |
| −222 | 3.433 | 25.96 | 50.8 | 26.00 | 47 |
| −402 | 3.396 | 26.24 | 18.5 | 26.20 | 39 |
| 002 | 3.341 | 26.68 | 19.0 | 26.70 | 29 |
| −422 | 3.176 | 28.09 | 45.1 | 28.06 | 55 |
| −441 | 3.132 | 28.49 | 34.1 | 28.56 | 28 |
| −132 | 3.086 | 28.93 | 22.8 | 28.88 | 21 |
| −351 151 350 112 | 3.007 2.988 2.978 2.975 | 29.71 29.90 30.01 30.03 | 15.0 41.6 27.8 12.7 | 30.0 | 59 |
| 530 −621 | 2.819 2.810 | 31.74 31.85 | 43.6 14.0 | 31.80 | 41 |
| −261 061 | 2.739 2.732 | 32.70 32.79 | 10.6 12.8 | 32.76 | 20 |
| Miller Indices hkl | HEU | Cu-HEU(LIE) | Cu-HEU(SIE) | Zn-HEU(LIE) | Zn-HEU(SIE) |
|---|---|---|---|---|---|
| 020 * | 100 | 100 | 100 | 100 | 100 |
| 200 * | 48 | 38 | 33 | 31 | 37 |
| −201 | 31 | 24 | 23 | 22 | 21 |
| −311 | 32 | 27 | 35 | 33 | 26 |
| 111 | 29 | 32 | 28 | 23 | 28 |
| −131 | 33 | 30 | 30 | 32 | 28 |
| 131 *, 400, 330, −421, 240 | 115 | 104 | 121 | 109 | 102 |
| −312 | 25 | 24 | 26 | 22 | 20 |
| −222 | 47 | 42 | 45 | 40 | 40 |
| −402 | 39 | 38 | 54 | 44 | 38 |
| 002 | 29 | 27 | 35 | 29 | 27 |
| −422 | 55 | 50 | 73 | 53 | 49 |
| −441 | 28 | 24 | 26 | 22 | 23 |
| −132 | 21 | 20 | 20 | 13 | 11 |
| −351, 151, 350, 112 | 59 | 48 | 49 | 44 | 44 |
| 530, −621 | 41 | 36 | 32 | 33 | 33 |
| −261, 061 | 20 | 18 | 22 | 17 | 16 |
Appendix B
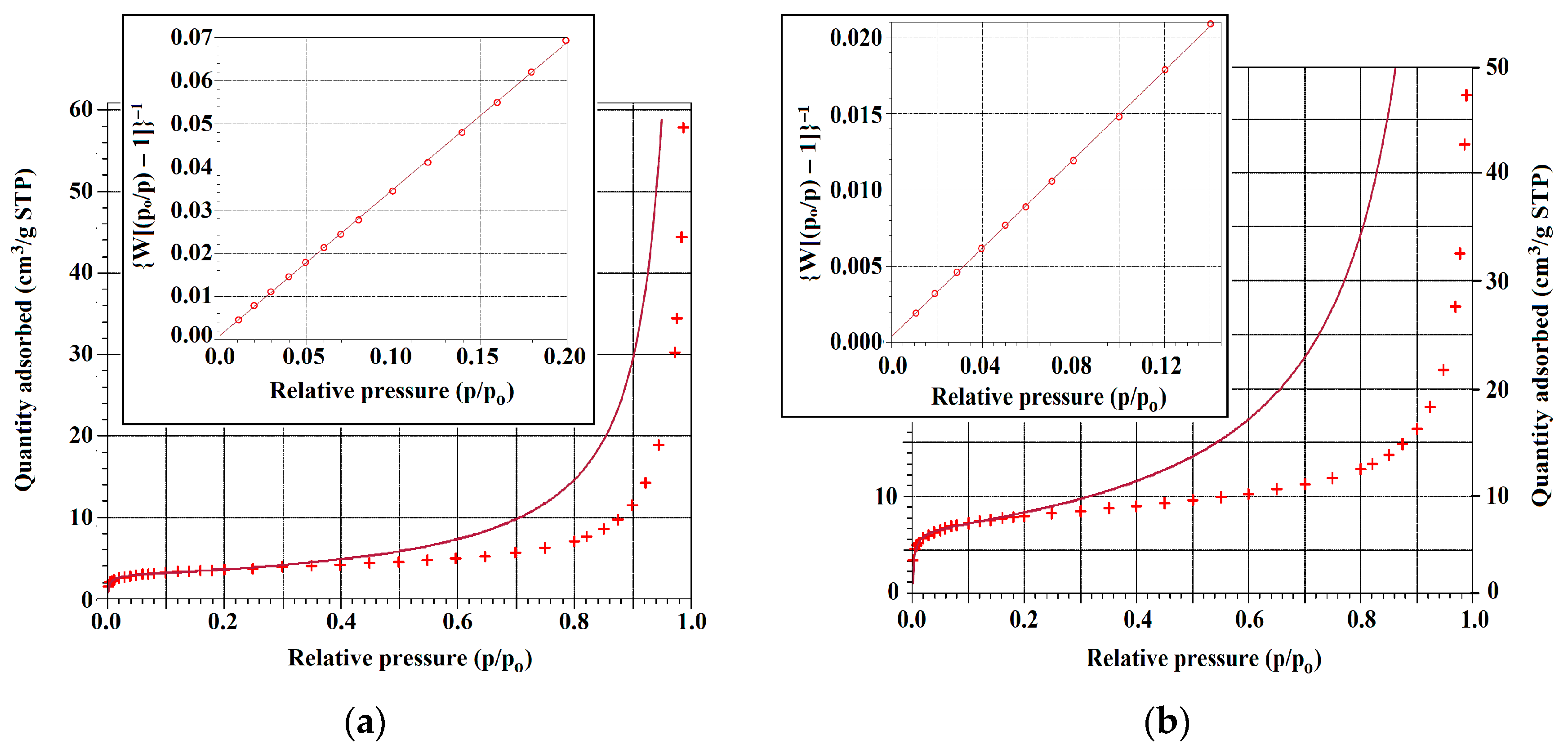
| Sample | Slope (g/cm3 STP) | Y-Intercept (g/cm3 STP) | Correlation Coefficient | Wm (cm3/g STP) |
|---|---|---|---|---|
| HEU | 0.204521 ± 0.000661 | 0.000582 ± 0.000064 | 0.9999426 | 4.8756 ± 0.0158 |
| Cu-HEU(LIE) | 0.145230 ± 0.000546 | 0.000374 ± 0.000042 | 0.9999364 | 6.8680 ± 0.0256 |
| Cu-HEU(SIE) | 0.221737 ± 0.000949 | 0.000379 ± 0.000082 | 0.9999084 | 4.5021 ± 0.0193 |
| Zn-HEU(LIE) | 0.197662 ± 0.000791 | 0.000362 ± 0.000069 | 0.9999199 | 5.0499 ± 0.0202 |
| Zn-HEU(SIE) | 0.220244 ± 0.000650 | 0.000942 ± 0.000070 | 0.9999477 | 4.5211 ± 0.0133 |
| Sample | (p/po)max | CBET | (p/po)mono | (p/po)mono-cal | Δ (%) * |
|---|---|---|---|---|---|
| HEU | 0.14 | 369 ± 64 | 0.055 | 0.050 | 9.1 |
| Cu-HEU(LIE) | 0.10 | 389 ± 44 | 0.041 | 0.048 | 17.1 |
| Cu-HEU(SIE) | 0.10 | 585 ± 127 | 0.043 | 0.039 | 9.3 |
| Zn-HEU(LIE) | 0.08 | 547 ± 10 | 0.046 | 0.041 | 10.9 |
| Zn-HEU(SIE) | 0.09 | 234 ± 17 | 0.064 | 0.061 | 4.7 |
References
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.-G.; Huc, Q.; Zhao, F.-J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Das, S.; Sultana, K.W.; Ndhlala, A.R.; Mondal, M.; Chandra, I. Heavy metal pollution in the environment and its impact on health: Exploring green technology for remediation. Environ. Health Insights 2023, 17, 11786302231201259. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Dehmani, Y.; Mohammed, B.B.; Oukhrib, R.; Dehbi, A.; Lamhasni, T.; Brahmi, Y.; El-Kordy, A.; Franco, D.S.P.; Georgin, J.; Lima, E.C.; et al. Adsorption of various inorganic and organic pollutants by natural and synthetic zeolites: A critical review. Arab. J. Chem. 2024, 17, 105474. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. A comprehensive review on novel zeolite-based adsorbents for environmental pollutant. J. Hazard. Mater. Adv. 2025, 17, 100617. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Hakim, M.; Iqbal, R.M.; Adany, F.; Putra, R.; Nitriany, I.; Telaumbanua, I.; Sitorus, R.; Dewi, R. A review on development of porous aluminosilicate-based zeolite adsorbent for heavy metal pollution treatment. J. Sains Mater. Indones. 2024, 25, 85–99. [Google Scholar] [CrossRef]
- Mambetova, M.; Dossumov, K.; Baikhamurova, M.; Yergaziyeva, G. Sorbents based on natural zeolites for carbon dioxide capture and removal of heavy metals from wastewater: Current progress and future opportunities. Processes 2024, 12, 2071. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; Garcia-Sanchez, A.; Querol, X. Purification of metal electroplating waste waters using zeolites. Water Res. 2003, 37, 4855–4862. [Google Scholar] [CrossRef]
- Dal Bosco, S.M.; Jimenez, R.S.; Carvalho, W.A. Removal of toxic metals from wastewater by Brazilian natural scolecite. J. Colloid. Interf. Sci. 2005, 281, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, C.; García, R.; Arriagada, R.; Yánez, J.; Garland, M.T. Cr(III) exchange on zeolites obtained from kaolin and natural mordenite. Micropor. Mesopor. Mater. 2006, 88, 220–231. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, V.; Magro, J.D. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Kalla, E.B.S.; Supriyanto, G.; Suyanto; Puspaningsih, N.N.T. Adsorption of hexavalent chromium from aqueous solutions using acid activated of natural zeolite collected from ende-flores, Indonesia. Rasayan J. Chem. 2017, 10, 606–612. [Google Scholar] [CrossRef]
- Álvarez, A.M.; Guerrón, D.B.; Calderón, C.M. Natural zeolite as a chromium VI removal agent in tannery effluents. Heliyon 2021, 7, e07974. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Adam, M.R.; Liang, X.; Goh, H.; Anouzla, A.; Sillanpää, M.; Mohyuddin, A.; Chew, K.W. Chromium removal from aqueous solution using natural clinoptilolite. Water 2023, 15, 1667. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Selvasembian, R.; Ashiq, A.; Gunarathne, V.; Ekanayake, A.; Perera, V.O.; Wijesekera, H.; Mia, S.; Ahmad, M.; Vithanage, M.; et al. A systematic review on adsorptive removal of hexavalent chromium from aqueous solutions: Recent advances. Sci. Total Environ. 2022, 809, 152055. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Rubio, J. On the removal of Mn2+ ions by adsorption onto natural and activated Chilean zeolites. Miner. Eng. 2009, 22, 336–343. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Rubio, J. Removal of Mn2+ from aqueous solution by manganese oxide coated zeolite. Miner. Eng. 2010, 23, 1131–1138. [Google Scholar] [CrossRef]
- Neag, E.; Török, A.I.; Tanaselia, C.; Aschilean, I.; Senila, M. Kinetics and equilibrium studies for the removal of Mn and Fe from binary metal solution systems using a Romanian thermally activated natural zeolite. Water 2020, 12, 1614. [Google Scholar] [CrossRef]
- Lee, W.S.; Aziz, H.A.; Tajarudin, H.A. Removal of Fe and Mn from the groundwater by using zeolite with Rossellomorea sp. Water Environ. Res. 2023, 95, e10913. [Google Scholar] [CrossRef]
- Sanzana, S.; Abreu, N.J.; Levío-Raimán, M.; Proal-Nájera, J.; Osorio, A.; Maza, S.; Daniele, L.; Castro-Rojas, J.; Soto, V.; González, C.; et al. Enhancing manganese sorption: Batch and fixed-bed column studies on activated zeolite. Environ. Technol. Innov. 2024, 33, 103495. [Google Scholar] [CrossRef]
- Rodríguez, A.; Sáez, P.; Díez, E.; Gómez, J.M.; García, J.; Bernabé, I. Highly efficient low-cost zeolite for cobalt removal from aqueous solutions: Characterization and performance. Environ. Prog. Sustain. Energy 2019, 38, S352–S365. [Google Scholar] [CrossRef]
- Asghar, K.; Ngulimi, M.F.; Kim, S.; Seo, B.K.; Roh, C. Cobalt recovery from industrial and nuclear waste resources: A review. Chem. Eng. J. Adv. 2024, 20, 100668. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Ghoreishian, S.M.; Kwak, C.H.; Han, Y.-K.; Roh, C.; Huh, Y.S. Recent progress in advanced functional materials for adsorption and removal of cobalt from industrial and radioactive effluents. Coord. Chem. Rev. 2025, 527, 216401. [Google Scholar] [CrossRef]
- Al-Abbad, E.A.; Al Dwairi, R.A. Removal of nickel (II) ions from water by Jordan natural zeolite as sorbent material. J. Saudi Chem. Soc. 2021, 25, 101233. [Google Scholar] [CrossRef]
- Mehdi, B.; Belkacemi, H.; Brahmi-Ingrachen, D.; Braham, L.A.; Muhr, L. Study of nickel adsorption on NaCl-modified natural zeolite using response surface methodology and kinetics modeling. Groundw. Sustain. Dev. 2022, 17, 100757. [Google Scholar] [CrossRef]
- Panayotova, M.I. Kinetics and thermodynamics of copper ions removal from wastewater by use of zeolite. Waste Manag. 2001, 21, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Batjargal, T.; Yang, J.-S.; Kim, D.-H.; Baek, K. Removal characteristics of Cd(II), Cu(II), Pb(II), and Zn(II) by natural Mongolian zeolite through batch and column experiments. Separ. Sci. Technol. 2011, 46, 1313–1320. [Google Scholar] [CrossRef]
- Elboughdiri, N. The use of natural zeolite to remove heavy metals Cu (II), Pb (II) and Cd (II), from industrial wastewater. Cogent Eng. 2020, 7, 1782623. [Google Scholar] [CrossRef]
- Logar, N.Z.; Arčon, I.; Kovač, J.; Popova, M. Removal of copper from aqueous solutions with zeolites and possible treatment of exhaust materials. Chem. Ing. Tech. 2021, 93, 941–948. [Google Scholar] [CrossRef]
- Abd-Elaziz, A.; Atress, M.; Haggag, E.A.; Soliman, K.G. Copper removal from aqueous solution of contaminated soil using natural zeolite. Zagazig J. Agric. Res. 2023, 50, 665–684. [Google Scholar] [CrossRef]
- Adinehvand, J.; Shokuhi Rad, A.; Tehrani, A.S. Acid-treated zeolite (clinoptilolite) and its potential to zinc removal from water sample. Int. J. Environ. Sci. Technol. 2016, 13, 2705–2712. [Google Scholar] [CrossRef]
- Kozera-Sucharda, B.; Gworek, B.; Kondzielski, I. The simultaneous removal of zinc and cadmium from multicomponent aqueous solutions by their sorption onto selected natural and synthetic zeolites. Minerals 2020, 10, 343. [Google Scholar] [CrossRef]
- Filatova, E.G.; Pozhidaev, Y.N. Removal of zinc(II) ions from wastewater using natural zeolites. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 666, p. 042034. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/666/4/042034/pdf (accessed on 25 July 2025).
- Onthong, U.; Pungpo, P.; Thongnueakhaeng, W. The applications of natural zeolites for cadmium removal from sample water: Models on laboratory scale. In Renewable and Sustainable Energy, Advanced Materials Research; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2012; Volume 347, pp. 1930–1933. [Google Scholar] [CrossRef]
- Alotaibi, S.; Ibrahim, H.; Alghamdi, A. Application of natural and modified zeolite sediments for the stabilization of cadmium and lead in contaminated mining soil. Appl. Sci. 2024, 14, 10864. [Google Scholar] [CrossRef]
- Budianta, W.; Ardiana, A.; Andriyani, N.D. The removal of lead by natural zeolite. E3S Web Conf. 2020, 200, 06012. [Google Scholar] [CrossRef]
- Hussein, N.S.; Alhadethi, A.A. Remove of lead from contaminated water using zeolite. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2025; Volume 1449, p. 012111. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/1449/1/012111/pdf (accessed on 25 July 2025).
- Pavithra, S.I.; Leninraja, D.; Dhevagi, P.; Sathiya Bama, K.; Beulah, A.; Kavitha, M.P. Removal of lead: The synergistic power of clinoptilolite and nano-composite materials—A comprehensive review. Discov. Appl. Sci. 2025, 7, 608. [Google Scholar] [CrossRef]
- Chojnacki, A.; Chojnacka, K.; Hoffmann, J.; Górecki, H. The application of natural zeolites for mercury removal: From laboratory tests to industrial scale. Miner. Eng. 2004, 17, 933–937. [Google Scholar] [CrossRef]
- Andrade, Â.L.; Cavalcante, L.C.D.; Fabris, J.D. Zeolite-magnetite composites to remove Hg2+ from water. Hyperfine Interact. 2019, 240, 83. [Google Scholar] [CrossRef]
- Ugrina, M.; Čeru, T.; Nuić, I.; Trgo, M. Comparative study of mercury(II) removal from aqueous solutions onto natural and iron-modified clinoptilolite rich zeolite. Processes 2020, 8, 1523. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Kudarova, A.; Guney, A.; Kinayat, N.; Tauanov, Z. Efficient mercury removal from water by using modified natural zeolites and comparison to commercial adsorbents. Sustain. Chem. Pharm. 2023, 32, 101017. [Google Scholar] [CrossRef]
- Eremin, O.V.; Epova, E.S.; Filenko, R.; Rusal, O.S.; Bychinsky, V.A. Use of zeolite rocks in metal recovery from mine water. J. Min. Sci. 2017, 53, 915–924. [Google Scholar] [CrossRef]
- Mubarak, M.F.; Mohamed, A.M.G.; Keshawy, M.; elMoghny, T.A.; Shehata, N. Adsorption of heavy metals and hardness ions from groundwater onto modified zeolite: Batch and column studies. Alex. Eng. J. 2022, 61, 4189–4207. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C.Y.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. Adsorption of heavy metals from aqueous solutions on synthetic zeolite. Res. J. Environ. Sci. 2008, 2, 13–22. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Jamil, T.S.; Hegazy, E.Z. Application of zeolite prepared from Egyptian kaolin for the removal of heavy metals: II. Isotherm models. J. Hazard. Mater. 2010, 182, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Yuna, Z. Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Xie, W.-M.; Zhou, F.-P.; Bi, X.-L.; Chen, D.-D.; Li, J.; Sun, S.-Y.; Liu, J.-Y.; Chen, X.-Q. Accelerated crystallization of magnetic 4A-zeolite synthesized from red mud for application in removal of mixed heavy metal ions. J. Hazard. Mater. 2018, 358, 441–449. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Bagherpour, A. Synthesis of zeolite NaY and its nanocomposites with chitosan as adsorbents for lead(II) removal from aqueous solution. Powder Technol. 2018, 338, 744–763. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y.; Hamad, H.; Saad, Z. Synthetic NaX zeolite as a very efficient heavy metals sorbent in batch and dynamic conditions. Colloids Interfaces 2018, 2, 22. [Google Scholar] [CrossRef]
- Bai, S.; Chu, M.; Zhou, L.; Chang, Z.; Zhang, C.; Liu, B. Removal of heavy metals from aqueous solutions by X type zeolite prepared from combination of oil shale ash and coal fly ash. Energy Sources Part A 2019, 2019, 1661549. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Bahaz, H.; Hadj Seyd, A.; Moulai, K.; Aggoun, M.S. Removal of heavy metals from an industrial effluent by synthesized zeolite: Case of Bounoura industrial zone. Leban. Sci. J. 2020, 21, 80–94. Available online: https://applications.emro.who.int/imemrf/322/Lebanese-Sci-J-2020-21-1-80-94-eng.pdf (accessed on 31 July 2025). [CrossRef]
- Lobo-Recio, M.A.; Rodrigues, C.; Jeremias, T.C.; Lapolli, F.R.; Padilla, I.; López-Delgado, A. Highly efficient removal of aluminum, iron, and manganese ions using Linde type-A zeolite obtained from hazardous waste. Chemosphere 2021, 267, 128919. [Google Scholar] [CrossRef] [PubMed]
- Jangkorn, S.; Youngme, S.; Praipipat, P. Comparative lead adsorptions in synthetic wastewater by synthesized zeolite A of recycled industrial wastes from sugar factory and power plant. Heliyon 2022, 8, e09323. [Google Scholar] [CrossRef]
- Kinoti, I.K.; Ogunah, J.; M’Thiruaine, C.M.; Marangu, J.M. Adsorption of heavy metals in contaminated water using zeolite derived from agro-wastes and clays: A review. J. Chem. 2022, 1, 1–25. [Google Scholar] [CrossRef]
- Aloui, L.; Mezghich, S.; Mansour, L.; Hraiech, S.; Ayari, F. Swift removal of the heavy metals cadmium and lead from an aqueous solution by a CAN-zeolite synthesized from natural clay. ChemEngineering 2023, 7, 113. [Google Scholar] [CrossRef]
- Belviso, C.; Lucini, P.; Mancinelli, M.; Abdolrahimi, M.; Martucci, A.; Peddis, D.; Maraschi, F.; Cavalcante, F.; Sturini, M. Lead, zinc, nickel and chromium ions removal from polluted waters using zeolite formed from bauxite, obsidian and their combination with red mud: Behaviour and mechanisms. J. Clean. Prod. 2023, 415, 137814. [Google Scholar] [CrossRef]
- Popaliya, M.; Mishra, A. Modified zeolite as an adsorbent for dyes, drugs, and heavy metal removal: A review. Int. J. Environ. Sci. Technol. 2023, 20, 12919–12936. [Google Scholar] [CrossRef]
- Kuldeyev, E.; Seitzhanova, M.; Tanirbergenova, S.; Tazhu, K.; Doszhanov, E.; Mansurov, Z.; Azat, S.; Nurlybaev, R.; Berndtsson, R. Modifying natural zeolites to improve heavy metal adsorption. Water 2023, 15, 2215. [Google Scholar] [CrossRef]
- Sholikah, L.; Sumari, S.; Yunisari, Y. Modification and application study of activated natural zeolite for the treatment of liquid waste from chemical laboratory. J. Kim. Sains Dan. Apl. 2023, 26, 332–343. [Google Scholar] [CrossRef]
- Bessaha, G.; Bessaha, F.; Bendenia, S.; Khelifa, A. Exchanged zeolite adsorbent for removing Cr(VI): Kinetics, thermodynamics and adsorption mechanism. Int. J. Environ. Anal. Chem. 2022, 105, 1–19. [Google Scholar] [CrossRef]
- Zeng, Y.; Woo, H.; Lee, G.; Park, J. Adsorption of Cr(VI) on hexadecylpyridinium bromide (HDPB) modified natural zeolites. Micropor. Mesopor. Mater. 2010, 130, 83–91. [Google Scholar] [CrossRef]
- Tsitsishvili, V.; Panayotova, M.; Mirdzveli, N.; Dzhakipbekova, N.; Panayotov, V.; Dolaberidze, N.; Nijaradze, M. Acid resistance and ion-exchange capacity of natural mixtures of heulandite and chabazite. Minerals 2023, 13, 364. [Google Scholar] [CrossRef]
- Tasharrofi, S.; Rouzitalab, Z.; Maklavany, D.M.; Esmaeili, A.; Rabieezadeh, M.; Askarieh, M.; Rashidi, A.; Taghdisian, H. Adsorption of cadmium using modified zeolite-supported nanoscale zero-valent iron composites as a reactive material for PRBs. Sci. Total Environ. 2020, 736, 139570. [Google Scholar] [CrossRef]
- Kumara, G.M.P.; Kawamoto, K. Use of natural zeolite and its mixtures to refine high-concentrated heavy metal-contaminated wastewater: An investigation of simultaneous removal of Cd(II) and Pb(II) by batch adsorption method. Water Air Soil Pollut. 2021, 232, 463. [Google Scholar] [CrossRef]
- Wahono, S.; Prasetyo, D.J.; Jatmiko, T.; Suwanto, A.; Pratiwi, D.; Hernawan, H.; Vasilev, K. Transformation of mordenite-clinoptilolite natural zeolite at different calcination temperatures. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 251, p. 012009. [Google Scholar] [CrossRef]
- Nguyen, T.B.H.; Van, H.T. Application of modified zeolite in the remediation of heavy metal in contaminated soil: A short review. Technol. Agron. 2024, 4, e002. [Google Scholar] [CrossRef]
- Kumari, S.; Chowdhry, J.; Kumar, M.; Garg, M.C. Zeolites in wastewater treatment: A comprehensive review on scientometric analysis, adsorption mechanisms, and future prospects. Environ. Res. 2024, 260, 119782. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Cadar, O. Modification of natural zeolites and their applications for heavy metal removal from polluted environments: Challenges, recent advances, and perspectives. Heliyon 2024, 10, e25303. [Google Scholar] [CrossRef]
- Jha, V.K.; Matsuda, M.; Miyake, M. Sorption properties of the activated carbon-zeolite composite prepared from coal fly ash for Ni(2+), Cu(2+), Cd(2+) and Pb(2+). J. Hazard. Mater. 2008, 160, 148–153. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.-J.; Wang, H.-G.; Cui, X.-Y.; Yu, F.; Cheng, W.-P.; Ma, J.-H. Removal of Cr3+ in aqueous solutions by zeolite A/activated carbon composite synthesized from elutrilithe. In 2nd 2016 International Conference on Sustainable Development; Atlantis Press: Dordrecht, The Netherlands, 2016; pp. 125–129. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.; Huang, X.; Chen, L.; Hu, P.; Gao, J.-M.; Zhen, Q.; Bashir, S.; et al. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 2020, 390, 124513. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Balaji, G.L.; Rico, I.L.R.; Hamad, H. Removal of Pb2+ ions by ZSM-5/AC composite in a fixed-bed bench scale system. Adsorpt. Sci. Technol. 2021, 2021, 2013259. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M.; Jumah, M.N.B.; Al-Khalawi, N.; Alruhaimi, R.S.; Salama, Y.F.; Allam, A.A. Insight into the adsorption properties of chitosan/ zeolite-A hybrid structure for effective decontamination of toxic Cd (II) and As (V) ions from the aqueous environments. J. Polym. Environ. 2022, 30, 295–307. [Google Scholar] [CrossRef]
- Angaru, G.K.R.; Choi, Y.L.; Lingamdinne, L.P.; Choi, J.S.; Kim, D.S.; Koduru, J.R.; Yang, J.K.; Chang, Y.Y. Facile synthesis of economical feasible fly ash-based zeolite-supported nano zerovalent iron and nickel bimetallic composite for the potential removal of heavy metals from industrial effluents. Chemosphere 2021, 267, 128889. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Lawa, Y.; Naat, J.; Riwu, A.A.P.; Mango, A.W.; Darmokoesoemo, H.; Widyaningrum, B.A.; Iqbal, M.; Kusuma, H.S. Efficiency of activated natural zeolite-based magnetic composite (ANZ-Fe3O4) as a novel adsorbent for removal of Cr(VI) from wastewater. J. Mater. Res. Technol. 2022, 18, 2896–2909. [Google Scholar] [CrossRef]
- Nguyen, T.B.H.; Van, H.-T.; Dang, V.M.; Hoang, V.H.; Nguyen, T.H.; Hoang, T.K. Immobilization of Pb, Cd, and Cr in contaminated soil around mining areas using Mg/Al LDH-zeolite and evaluation of maize growth. Environ. Res. Commun. 2024, 6, 105001. [Google Scholar] [CrossRef]
- Afzal, S.; Alghanem, S.M.S.; Alsudays, I.M.; Malik, Z.; Abbasi, G.H.; Ali, A.; Noreen, S.; Ali, M.; Irfan, M.; Rizwan, M. Effect of biochar, zeolite and bentonite on physiological and biochemical parameters and lead and zinc uptake by maize (Zea mays L.) plants grown in contaminated soil. J. Hazard. Mater. 2024, 469, 133927. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.d.S.R.; Chagas, J.K.M.; Paz-Ferreiro, J.; de Figueiredo, C.C. Enhanced remediation of heavy metal-contaminated soils using biochar and zeolite combinations with additives: A meta-analysis. Environ. Pollut. 2025, 367, 125617. [Google Scholar] [CrossRef]
- de Magalhães, L.F.; da Silva, G.R.; Peres, A.E.C. Zeolite application in wastewater treatment. Adsorpt. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- Laghlimi, M.; Baghdad, B.; Hadi, H.; Bouabdli, A. Phytoremediation mechanisms of heavy metal contaminated soils: A review. Open J. Ecol. 2015, 5, 375–388. [Google Scholar] [CrossRef]
- Wen, J.; Yi, Y.; Zeng, G. Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J. Environ. Manag. 2016, 178, 63–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Miao, J.; Yue, L.; Cheng, M.; Li, Y.; Jing, Z. Self-regulated immobilization behavior of multiple heavy metals via zeolitization towards a novel hydrothermal technology for soil remediation. Environ. Res. 2023, 216, 114726. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef] [PubMed]
- Cadar, O.; Stupar, Z.; Senila, M.; Levei, L.; Moldovan, A.; Becze, A.; Ozunu, A.; Levei, E.A. Zeolites reduce the transfer of potentially toxic elements from soil to leafy vegetables. Materials 2022, 15, 5657. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Lu, P.; Yang, X.; Valtchev, V. Zeolites for the environment. Green Carbon 2024, 2, 12–32. [Google Scholar] [CrossRef]
- Oheix, E.; Reicher, C.; Nouali, H.; Michelin, L.; Josien, L.; Daou, T.J.; Pieuchot, L. Rational design and characterization of novel mono- and bimetallic antibacterial Linde Type A zeolite materials. J. Funct. Biomat. 2022, 13, 73. [Google Scholar] [CrossRef]
- Tsitsishvili, V.; Mirdzveli, N.; Miyamoto, M.; Wajima, T.; Dolaberidze, N.; Nijaradze, M. Antimicrobial and antibacterial activity of metal-containing modified heulandite type natural zeolites. Process. Petrochem. Oil Refin. 2024, 25, 1053–1063. [Google Scholar] [CrossRef]
- Oheix, E.; Daou, T.J.; Pieuchot, L. Antimicrobial zeolites and metal–organic frameworks. Mater. Horiz. 2024, 11, 6222–6256. [Google Scholar] [CrossRef]
- Julien, P.A.; Užarević, K.; Katsenis, A.D.; Kimber, S.A.J.; Wang, T.; Farha, O.K.; Zhang, Y.; Casabanm, J.; Germann, L.S.; Etter, M.; et al. In situ monitoring and mechanism of the mechanochemical formation of a microporous MOF-74 framework. J. Am. Chem. Soc. 2016, 138, 2929–2932. [Google Scholar] [CrossRef]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry. Angew. Chem. Int. Ed. 2010, 49, 9640–9643. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; p. 156. [Google Scholar] [CrossRef]
- Tsitsishvili, G.V.; Andronikashvili, T.G.; Kirov, G.N.; Filizova, L.D. Natural Zeolites; Ellis Horwood Ltd.: Chichester, UK, 1992; pp. 43–47. [Google Scholar]
- Commission Implementing Regulation (EU) No 651/2013 of 9 July 2013 concerning the authorisation of clinoptilolite of sedimentary origin as a feed additive for all animal species and amending Regulation (EC) No 1810/2005. Off. J. Eur. Union 2013, 189, 1–3. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013R0651 (accessed on 31 July 2025).
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Potential environmental pollution from copper metallurgy and methods of management. Environ. Res. 2021, 197, 111050. [Google Scholar] [CrossRef] [PubMed]
- Frausto da Silva, J.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed.; Oxford University Press: Oxford, UK, 2001; pp. 315–339. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 146–158. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin, Germany, 2007; pp. 283–318. Available online: https://link.springer.com/book/10.1007/978-3-540-32714-1 (accessed on 31 July 2025).
- Skhirtladze, N. Genetic Groups of Georgian Zeolites, Their Main Deposits and Manifestations; Tbilisi State University: Tbilisi, Georgia, 1997; pp. 7–9. [Google Scholar]
- Tsitsishvili, G.V.; Skhirtladze, N.S.; Andronikashvili, T.G.; Tsitsishvili, V.G.; Dolidze, A.V. Natural zeolites of Georgia: Occurrences, properties, and application. In Studies in Surface Science and Catalysis; Kiricsi, I., Pál-Borbély, G., Nagy, J.B., Karge, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 125, pp. 715–722. [Google Scholar] [CrossRef]
- Szostak, R. Secondary synthesis methods. In Introduction to Zeolite Science and Practice; van Bekkum, H., Flanigen, E.M., Jacobs, P.A., Jansen, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 261–297. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 184–185. [Google Scholar] [CrossRef]
- Galli, E.; Gottardi, G.; Mayer, H.; Preisinger, A.; Passaglia, E. The structure of potassium-exchanged heulandite at 293, 373 and 593 K. Acta Cryst. 1983, 39, 189–197. [Google Scholar] [CrossRef]
- Olson, D.H.; Haag, W.O.; Borghard, W.S. Use of water as a probe of zeolitic properties: Interaction of water with HZSM-5. Micropor. Mesopor. Mater. 2000, 35, 435–446. [Google Scholar] [CrossRef]
- Yamaka, S.; Malla, P.B.; Komarnani, S. Water sorption and desorption isotherms of some naturally occurring zeolites. Zeolites 1989, 9, 18–22. [Google Scholar] [CrossRef]
- Su, B.L. Adsorption sites for benzene in the 12R window zeolites: A molecular recognition effect. In Studies in Surface Science and Catalysis; Bonneviot, L., Kaliaguine, S., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 97, pp. 303–310. [Google Scholar] [CrossRef]
- Coughlan, B.; Keane, M.A. Adsorption of benzene on a range of activated Y zeolites. J. Chem. Soc. Faraday Trans. 1990, 86, 3961–3966. [Google Scholar] [CrossRef]
- Kukulska-Zając, E.; Kozyra, P.; Datka, J. The interaction of benzene with Cu+ sites in zeolites: IR studies and DFT quantum chemical calculations. Appl. Catal. A 2006, 307, 46–50. [Google Scholar] [CrossRef]
- Archipov, T.; Santra, S.; Ene, A.B.; Stoll, H.; Rauhut, G.; Roduner, E. Adsorption of benzene to copper in CuHY zeolite. J. Phys. Chem. C 2009, 10, 4107–4116. [Google Scholar] [CrossRef]
- Tsitsishvili, V.G.; Dolaberidze, N.M.; Nijaradze, M.O.; Mirdzveli, N.A.; Amiridze, Z.S.; Khutsishvili, B.T. Acid and thermal treatment of natural heulandite. Chem. Phys. Technol. Surf. 2023, 14, 519–533. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.; Deming, W.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents? In Studies in Surface Science and Catalysis; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouquerol, J., Seaton, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 49–56. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Chen, S.; Popovich, J.; Zhang, W.; Ganser, C.; Haydel, S.E.; Seo, D.-K. Superior ion release properties and antibacterial efficacy of nanostructured zeolites ion-exchanged with zinc, copper, and iron. RSC Adv. 2018, 8, 37949–37957. [Google Scholar] [CrossRef]
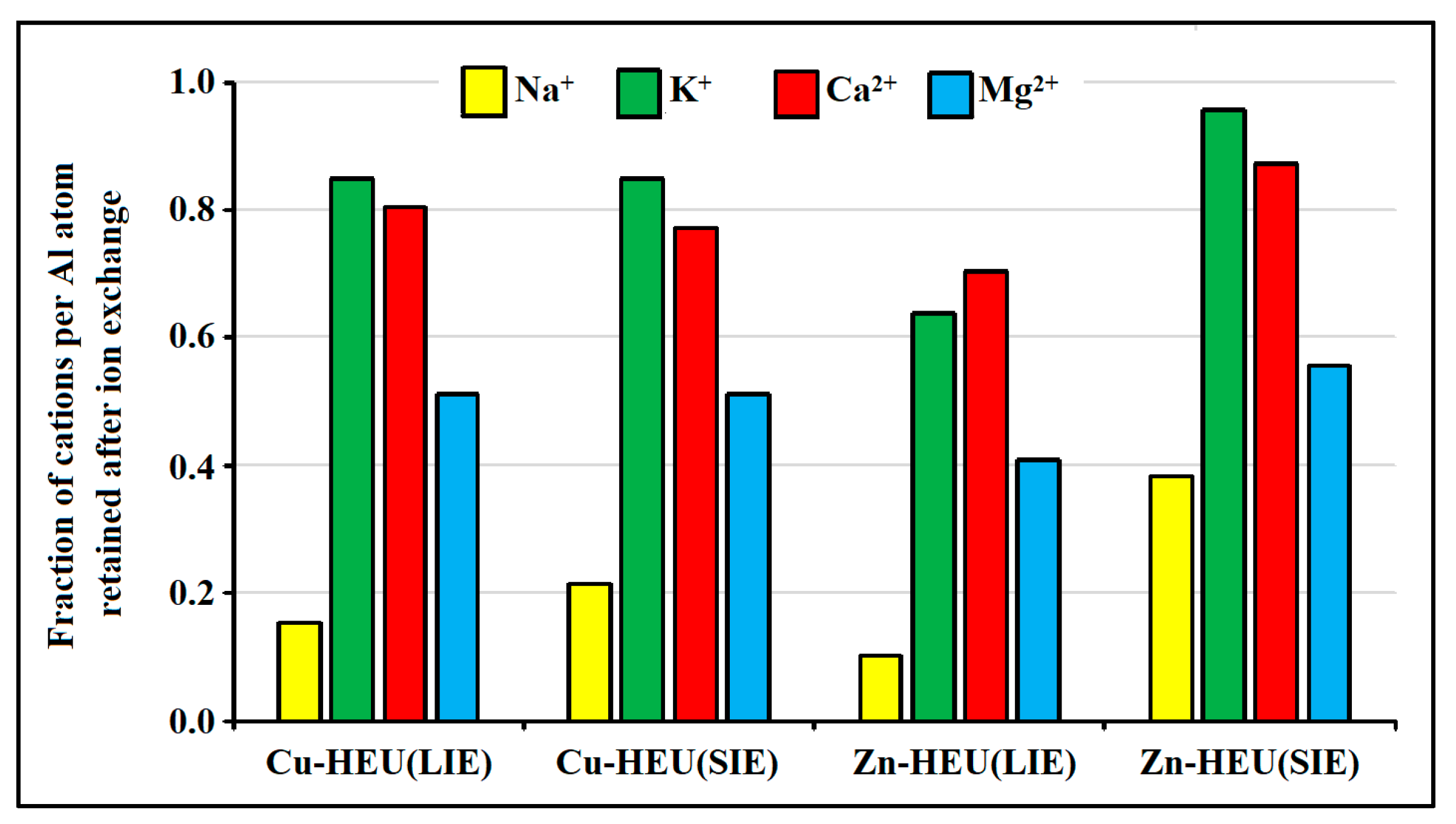
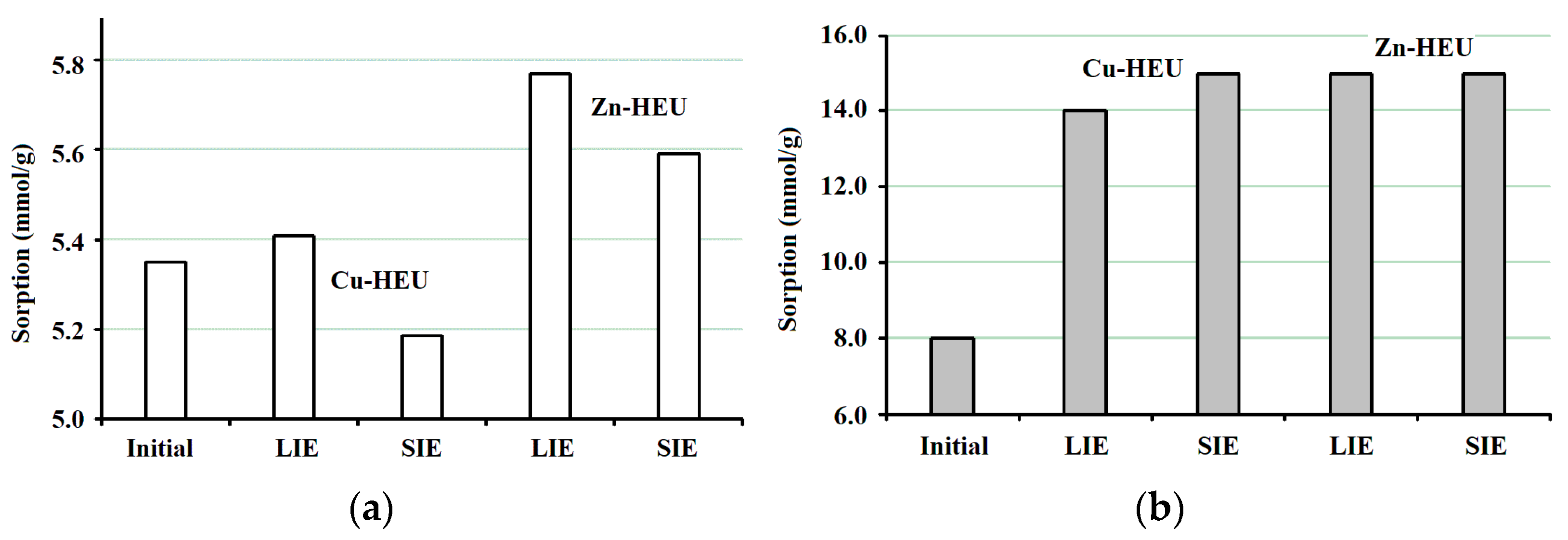


| Sample | Empirical Formula | Si/Al | M+/Al |
|---|---|---|---|
| HEU | (Na1.96K0.47Ca1.49Mg1.17)[Al7.8Si28.2O72] | 3.62 ± 0.12 | 1.00 ± 0.03 |
| Cu-HEU(LIE) | Cu1.1(Na0.30K0.40Ca1.2Mg0.60)[Al6.7Si29.3O72] | 4.37 ± 0.15 | 0.97 ± 0.04 |
| Cu-HEU(SIE) | Cu0.95(Na0.42K0.40Ca1.15Mg0.60)[Al6.3Si29.7O72] | 4.71 ± 0.16 | 0.98 ± 0.04 |
| Zn-HEU(LIE) | Zn1.4(Na0.20K0.30Ca1.05Mg0.48)[Al6.4Si29.6O72] | 4.62 ± 0.16 | 0.99 ± 0.05 |
| Zn-HEU(SIE) | Zn0.67(Na0.75K0.45Ca1.3Mg0.65)[Al6.7Si29.3O72] | 4.37 ± 0.15 | 0.96 ± 0.05 |
| Porosity Parameter | HEU | Cu-HEU(LIE) | Cu-HEU(SIE) | Zn-HEU(LIE) | Zn-HEU(SIE) |
|---|---|---|---|---|---|
| Specific volume of micropores Vm (cm3/g) | 0.00673 | 0.0141 | 0.00977 | 0.0106 | 0.0103 |
| Surface area SBET (m2/g) | 12.8 | 29.9 | 19.6 | 22.0 | 19.7 |
| Specific total pore volume Vp (cm3/g) * | 0.0895 | 0.0730 | 0.1188 | 0.0720 | 0.120 |
| Average pore diameter DBJH (nm) | 17.2 | 18.1 | 21.5 | 17.9 | 19.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsitsishvili, V.; Panayotova, M.; Mirdzveli, N.; Panayotov, V.; Dolaberidze, N.; Nijaradze, M.; Amiridze, Z.; Khutsishvili, B. Uptake of Copper and Zinc Ions by Georgian Natural Heulandite and Resulting Changes in Its Chemical Composition and Structure. Minerals 2025, 15, 902. https://doi.org/10.3390/min15090902
Tsitsishvili V, Panayotova M, Mirdzveli N, Panayotov V, Dolaberidze N, Nijaradze M, Amiridze Z, Khutsishvili B. Uptake of Copper and Zinc Ions by Georgian Natural Heulandite and Resulting Changes in Its Chemical Composition and Structure. Minerals. 2025; 15(9):902. https://doi.org/10.3390/min15090902
Chicago/Turabian StyleTsitsishvili, Vladimer, Marinela Panayotova, Nato Mirdzveli, Vladko Panayotov, Nanuli Dolaberidze, Manana Nijaradze, Zurab Amiridze, and Bela Khutsishvili. 2025. "Uptake of Copper and Zinc Ions by Georgian Natural Heulandite and Resulting Changes in Its Chemical Composition and Structure" Minerals 15, no. 9: 902. https://doi.org/10.3390/min15090902
APA StyleTsitsishvili, V., Panayotova, M., Mirdzveli, N., Panayotov, V., Dolaberidze, N., Nijaradze, M., Amiridze, Z., & Khutsishvili, B. (2025). Uptake of Copper and Zinc Ions by Georgian Natural Heulandite and Resulting Changes in Its Chemical Composition and Structure. Minerals, 15(9), 902. https://doi.org/10.3390/min15090902






