1. Introduction
The majority of igneous phosphate ores exhibit diminished P
2O
5 content and require enrichment procedures to generate commercially viable products [
1]. Froth flotation is the most important stage within the mineral beneficiation industry’s concentration workflow. Inefficient performance in flotation results in substantial revenue losses and the unnecessary depletion of mineral reserves [
2]. Phosphate flotation outcomes are deemed economically feasible when the concentrate achieves a P
2O
5 grade exceeding 30% while simultaneously maintaining phosphorus recovery rates above 60% [
3].
Flotation constitutes a complex separation technique involving multiple constituents, phases, and heterogeneous systems wherein suspended solid particles in aqueous medium interact with air bubbles, creating an air–pulp dispersion [
4]. Throughout this enrichment operation, chemical reagents are introduced to selectively alter the surface characteristics of target minerals, rendering the valuable mineral hydrophobic and consequently amenable to flotation, while simultaneously making gangue minerals hydrophilic and therefore non-floatable [
5]. During the flotation procedure, valuable particles are captured by air bubbles within the liquid phase and transported toward the pulp–froth interface, whereas hydrophilic particles remain suspended in the pulp and are discharged as tailings [
6].
Overall, froth flotation effectiveness depends upon three primary sub-processes: collision, adhesion, and detachment [
7]. The probability of particle–bubble contact, the adherence of particles to bubbles subsequent to collision, and the persistence of this attachment throughout the pulp phase are mechanisms strongly dependent on particle size and bubble diameter, which, in turn, are directly influenced by the gas holdup. Thus, the operating variables that change the gas holdup also influence the flotation performance [
8]. Therefore, understanding the relationship between gas holdup and bubble–particle interaction and the forms to enhance it is extremely important for enhancing mineral flotation processes [
9].
Column flotation has been effectively employed in apatite beneficiation owing to its superior performance compared to traditional cells, generating foam with enhanced flow properties [
10]. Gangue entrainment within the froth is minimized while bubble residence time in the pulp is extended, leading to improved selectivity [
11].
Processing fine particles of low-grade apatite ore through flotation remains problematic for phosphate companies, chiefly because of the limited probability of collisions between particles and air bubbles, which reduces attachment potential and subsequently lowers flotation effectiveness [
12]. Thus, the focus in the fine particle’s flotation is the improvement in the conditions that lead to more favorable bubble–particle contact [
13].
Bubble–particle contact is influenced by particle and bubble sizes. The distribution of bubble sizes is affected by gas holdup. Gas holdup is defined as the volumetric fraction of gas (air bubbles) present in the gas–liquid–solid mixture within the flotation column, typically expressed as a percentage. This parameter can be managed via air flow rate and the incorporation of surfactants, in addition to the concentration of flotation chemicals, such as collectors and depressants [
14]. Surfactants within the flotation operation are predominantly employed to boost the likelihood of collisions between particles and air bubbles through the creation of fine bubbles and the provision of foam stability [
15]. The introduction of surfactants minimizes bubble coalescence and drives bubbles from the slurry region toward the foam layer, owing to the extended residence duration of air bubbles. As a result, the likelihood of bubble–particle collision may be improved [
16].
The present research was designed to evaluate how certain process variables and chemical dosages influence gas holdup and its correlation with the efficiency of column flotation for fine particles derived from a low-grade apatite ore.
This research encompasses a comprehensive investigation of gas holdup’s effect on column flotation systems, specifically targeting low-grade apatite ore processing. The scope includes (a) a systematic evaluation of operating variables on gas holdup and flotation performance; (b) the development of predictive statistical models; and (c) implications for industrial applications.
2. Materials and Methods
2.1. Characterization of Phosphate Ore
The sample utilized in this investigation consisted of a low-grade apatite ore obtained from the Mosaic Fertilizantes, situated in Araxá, Minas Gerais State, Brazil. The country rock formations primarily comprise carbonatites and glimmerites [
17].
Chemical composition analysis was conducted using X-ray fluorescence spectrometry with a Bruker spectrometer (S8 Tiger model). The samples’ particle size distribution (PSD) was determined through a laser diffraction methodology employing a Malvern Mastersizer 2000.
Mineralogical identification of the feed material was accomplished by combining X-ray diffraction with scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS). X-ray diffraction analysis was performed using a Rigaku Geigerflex semi-automatic diffractometer. Microstructural investigations were also conducted with SEM/EDS techniques. These measurements were executed using a JSM-5410 SEM microscope and a Noran TN-M3055 EDS spectrometer (JEOL, Peabody, MA, USA).
2.2. Reagents and Ore Conditioning
The conditioning protocol was implemented based on previous investigations [
17,
18], wherein a fatty acid soap derived from crude rice oil at a concentration of 2.5 wt% oil served as the collector, while gelatinized cornstarch at 3.0 wt% concentration functioned as the depressant. A 10% NaOH solution was employed to adjust the pulp pH to approximately pH 11, and Genagen
® surfactant from Clariant was incorporated into the process as a frother.
The Genagen® surfactant plays a crucial role in the flotation process as a frother, acting primarily to control bubble size distribution and foam stability. This non-ionic surfactant belongs to the family of alcohol ethoxylates and functions by reducing surface tension at the gas–liquid interface, thereby preventing bubble coalescence and promoting the formation of smaller, more stable bubbles. The mechanism of action involves the adsorption of surfactant molecules at the bubble surface, creating a protective layer that inhibits bubble merging. This results in increased gas holdup due to the reduced rise velocity of smaller bubbles, which enhances their residence time in the flotation column. The interaction between Genagen® and other flotation reagents is particularly important, as the surfactant can form mixed adsorption layers with the collector molecules at the mineral surface, potentially enhancing the hydrophobicity of apatite particles while maintaining selectivity against gangue minerals.
2.3. Experimental Apparatus and Experimental Measurements
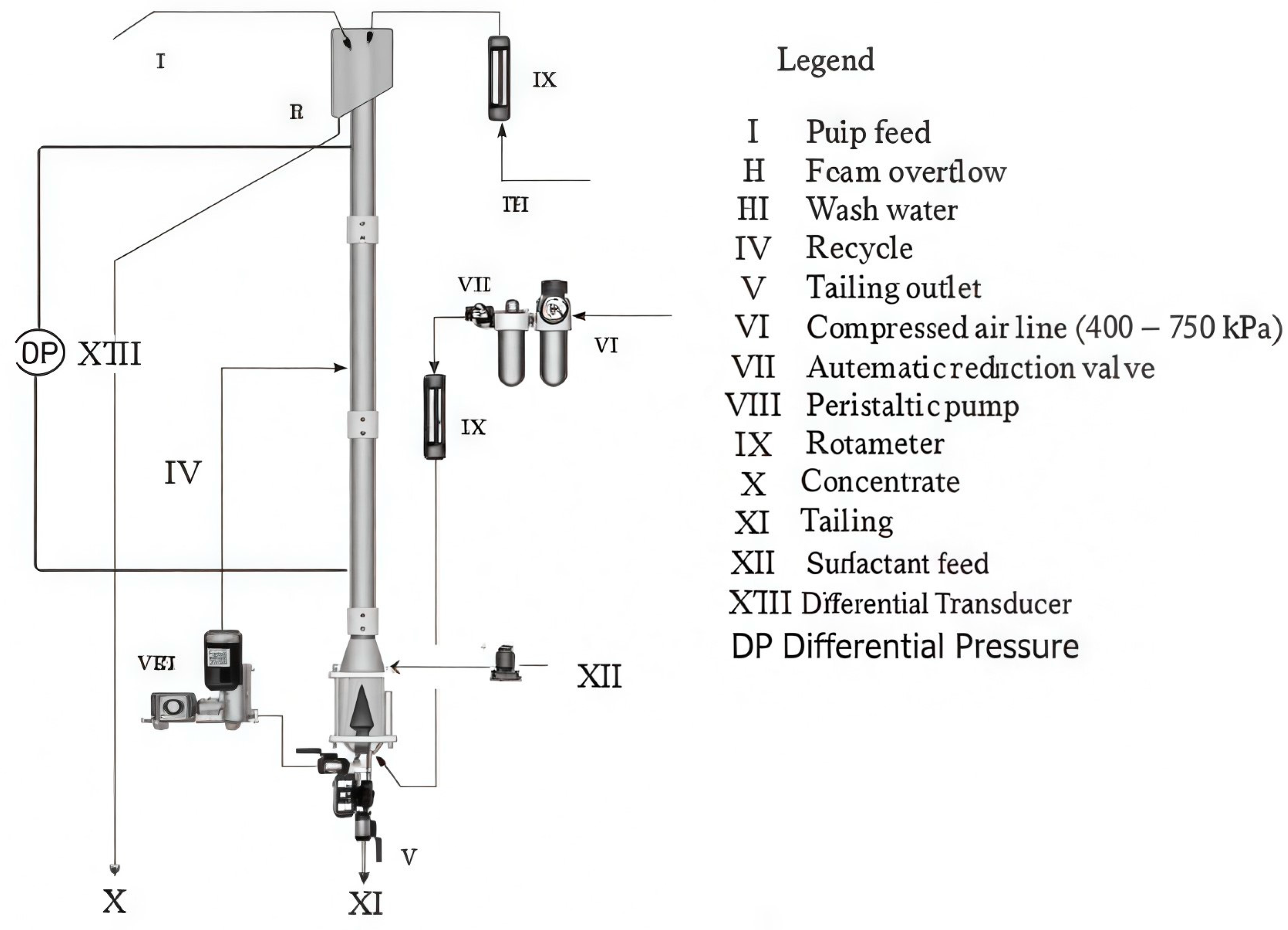
Flotation experiments were carried out in a batch flotation column made of polymethyl methacrylate (PMMA) with a height of 1.50 m and a 40 mm inner diameter, as illustrated in
Figure 1. The pulp was fed into the top of the column and air was fed and distributed by a sparger (a porous conical device made of sintered bronze) at the bottom of the column. The column functioned using bottom product recirculation, while froth wash water was introduced at the top. Air and wash water flow rates were quantified using rotameters.
The technique used to obtain the air holdup was the same as that developed by Finch and Dobby [
19]. In this approach, the air holdup was calculated from the pressure differential between two points within the column. The pressure differential in the column was measured by employing two pressure transmitters (model VTP-1000, Vectus, Noida, India). The signals were relayed to a data acquisition board (National Instruments, NI USB-6211, NI Austin, TX, USA) and subsequently analyzed and processed using data acquisition software. Equation (1) [
19] was applied to determine gas holdup from the pressure drop information. The solid density was determined using a helium pycnometer (AccuPyc 1340, Micromeritics Instrument Corporation, Norcross, GA, USA) with an accuracy of ±0.02%.
where
Cs is the solid concentration by volume;
εg is the gas holdup, defined as the volumetric fraction occupied by air in the mixture present in the flotation column (dimensionless);
ΔP is the pressure drop (Pa);
is the suspension density (kg/m3);
is the water density (kg/m3);
is the solid density (kg/m3);
L is the distance between the pressure measurement points (m);
g represents the acceleration due to gravity= 9.81 m/s2.
The flotation column operated with a circulating load (0.50 L/min) that suspended the feed particles and guaranteed their transit through the collection zone. The pulp underwent pre-conditioning and was then diluted to supply the batch flotation column from the top (14% solid content in the feed), after air flow rate regulation and pulp recirculation system connection. Wash water (0.15 L/min) was initiated and the floatable material was gathered until the foam layer was exhausted. The surfactant flow rate was maintained at 0.05 L/min. The air supply was provided by an oil-free compressor equipped with a pressure regulator and moisture trap to maintain consistent air quality. The air flow rate was continuously monitored using a rotameter with ±2% accuracy. Additionally, all experiments were conducted under controlled temperature conditions (25 ± 2 °C) to minimize density variations that could affect flow measurements.
During each flotation experiment, both flotation and non-flotation products were removed from the equipment and oven-dried at 110 ± 0.5 °C for 24 h, weighed, and analyzed for chemical composition. The chemical composition of the flotation products was determined by X-ray fluorescence spectrometry using a Bruker spectrometer (S8 Tiger, Bruker, Karlsruhe, Germany).
A set of experiments was proposed using a factorial experimental design to evaluate the effects of operating variables on flotation performance. The investigated variables were air flow rate, surfactant concentration, and collector and depressant dosages. The full set of experimental results was evaluated comprehensively using multiple regression analysis, determining the impact of the independent variables on P2O5 grade and recovery.
4. Discussion
4.1. Particle Loading Effects and Bubble Dynamics
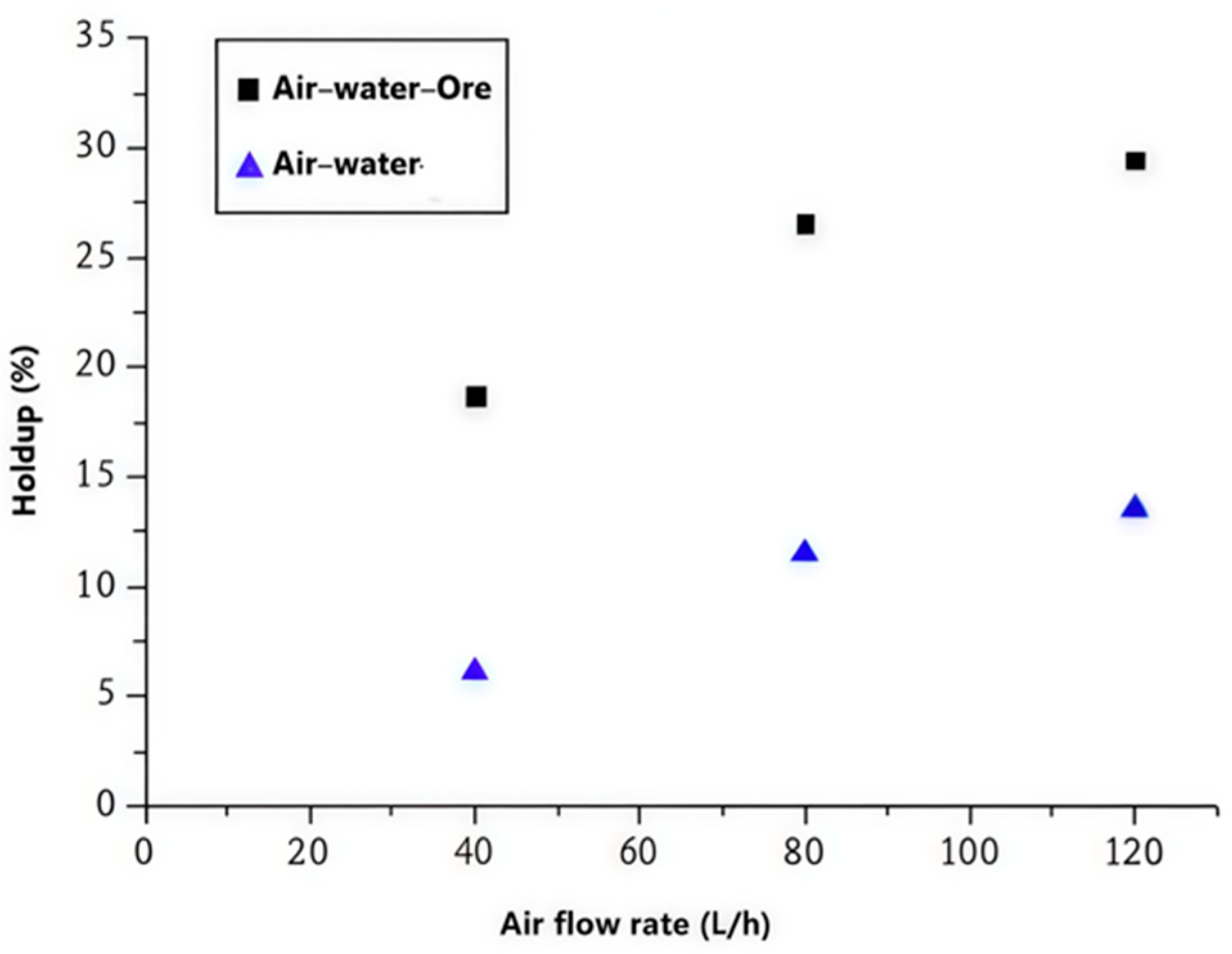
The dramatic increase in gas holdup observed in the presence of mineral particles (2–3 times higher than in air–water systems) can be explained through two complementary mechanisms that operate simultaneously in the flotation system. The first mechanism, known as bubble loading, occurs when particles attach to bubble surfaces, effectively increasing the density of the bubble–particle aggregates and reducing their rise velocity. This phenomenon is particularly pronounced with fine particles, where the surface area to volume ratio is high, allowing for multiple particle attachments per bubble.
The second mechanism involves the disruption of bubble coalescence by the presence of solid particles at the gas–liquid interface. Fine mineral particles can act as physical barriers between approaching bubbles, preventing the drainage of the thin liquid film that separates them. This stabilization effect is enhanced by the presence of surfactant molecules, which can form mixed adsorption layers with the particles at the bubble interface. The combination of these effects results in a more stable foam structure with smaller average bubble size and higher gas holdup.
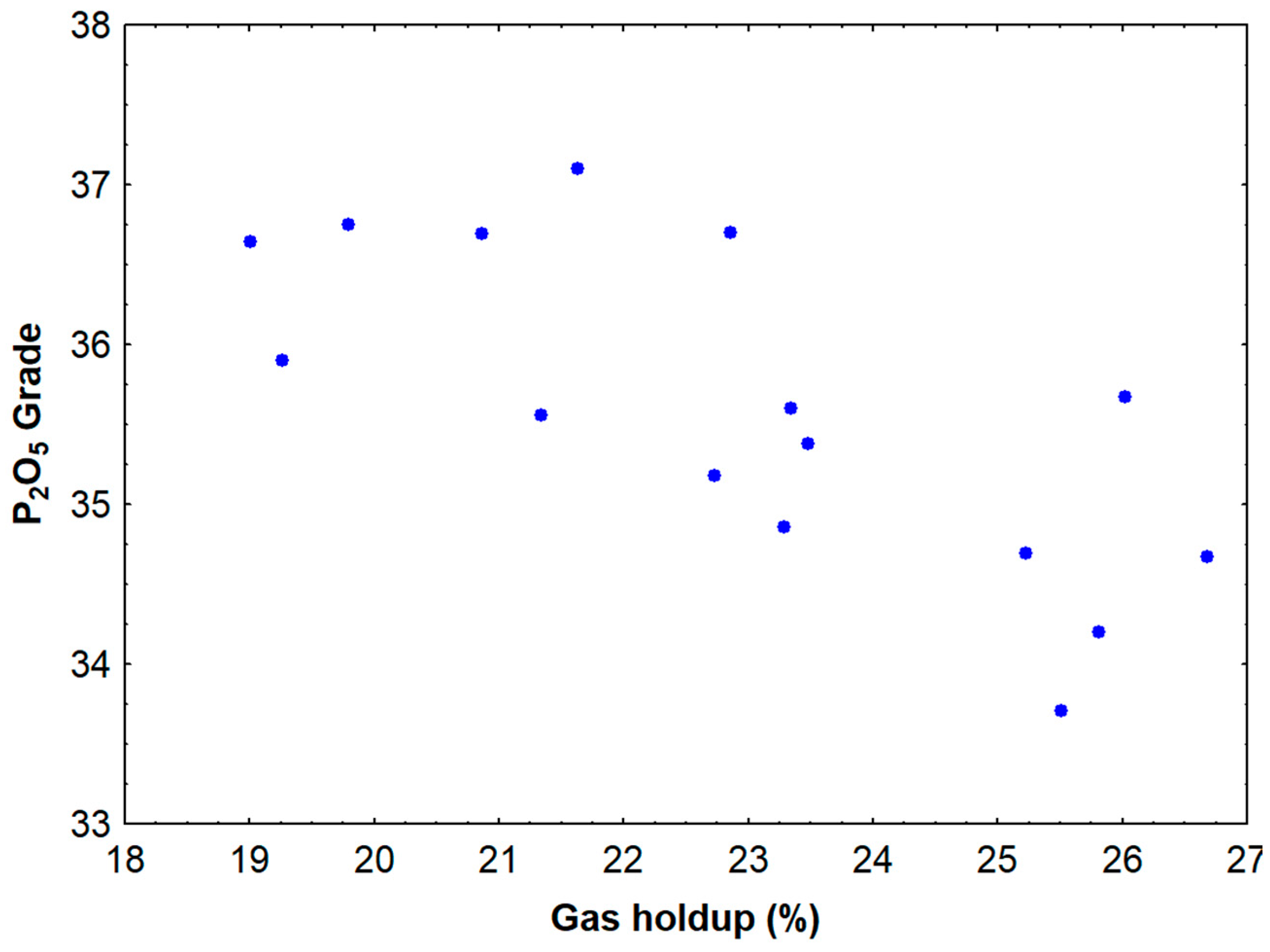
4.2. Trade-Off Between Grade and Recovery
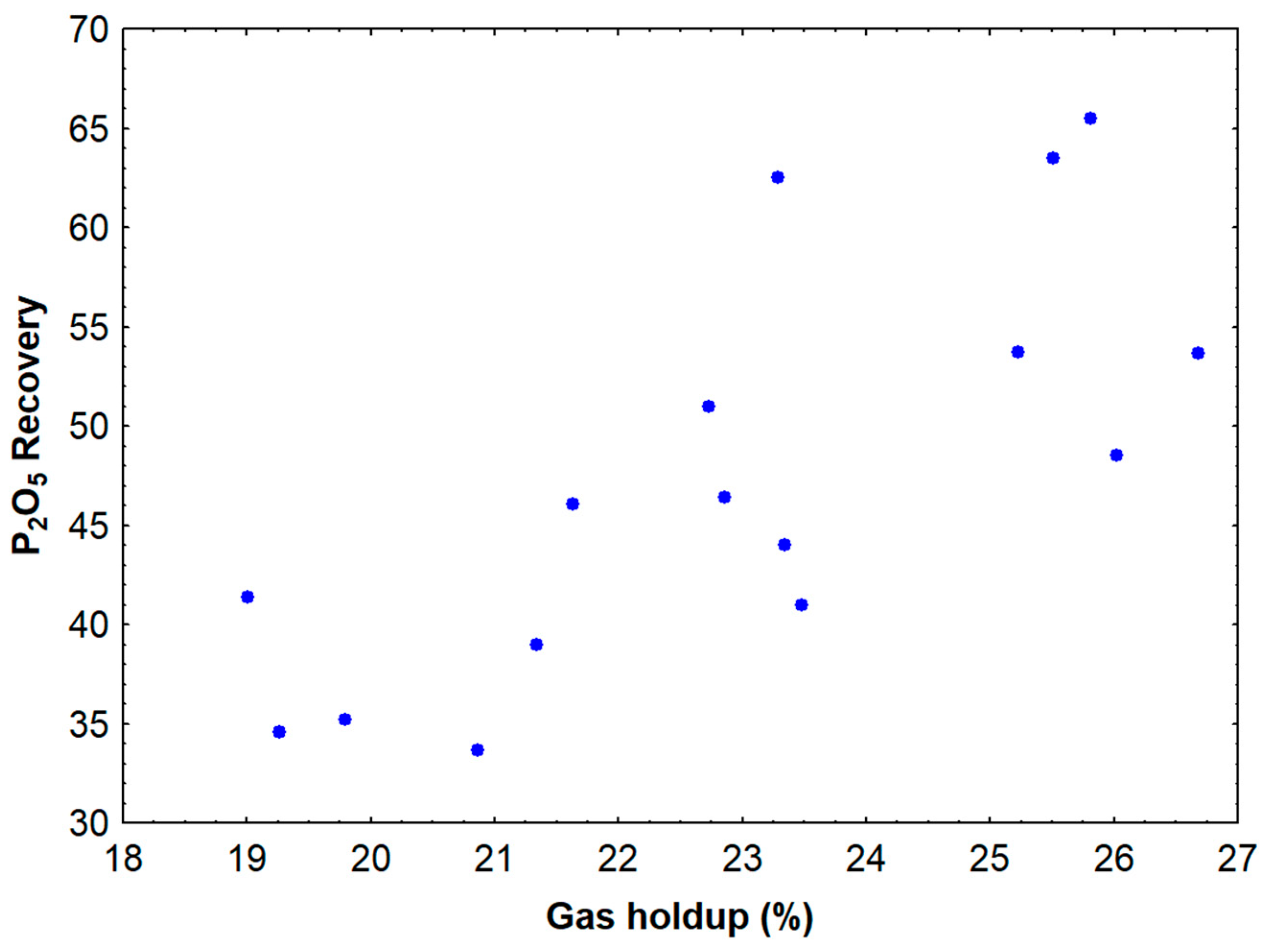
The inverse relationship between P2O5 grade and recovery observed with increasing gas holdup reflects fundamental principles of flotation selectivity and entrainment. At low gas holdup conditions, the system operates with smaller bubbles that provide high selectivity due to their lower carrying capacity and reduced entrainment of gangue particles. However, these conditions also result in lower recovery because the small bubbles have insufficient buoyancy force to carry particle–bubble aggregates to the froth phase, particularly when dealing with fine particles that may have weak attachment forces.
As gas holdup increases, larger bubbles are formed, which possess greater buoyancy and can more effectively transport attached particles to the froth phase. This explains the observed increase in recovery with higher gas holdup values. However, larger bubbles also have a greater tendency to entrain gangue particles through hydrodynamic forces, leading to the observed decrease in grade. The entrainment mechanism becomes particularly significant when the bubble size approaches or exceeds the particle size, as the hydrodynamic forces around rising bubbles can capture particles regardless of their surface hydrophobicity.
4.3. Role of Reagent Interactions in Performance Optimization
The complex interactions between the flotation reagents used in this study contribute significantly to the observed performance improvements. The gelatinized cornstarch depressant functions through a different mechanism than the collector and frother, acting primarily to increase the hydrophilicity of gangue minerals through hydrogen bonding and physical adsorption. The starch molecules form a protective hydrophilic layer on silicate and iron oxide surfaces, preventing collector adsorption and maintaining these minerals in the pulp phase.
The interaction between the cornstarch depressant and the Genagen® frother is particularly important for maintaining selectivity at higher gas holdup conditions. The presence of starch in the system can influence bubble surface properties by competing with the surfactant for adsorption sites. However, the non-ionic nature of Genagen® reduces this competition compared to ionic surfactants, allowing for more stable foam formation while maintaining the depressant action on gangue minerals.
The best reagent dosages identified in this study (fatty acid soap collector at 175 g/t, gelatinized cornstarch depressant at 450 g/t, and Genagen® surfactant at 11 ppm), associated with an air flow rate of 159.9 L/h, represent a carefully balanced system where each reagent performs its intended function without interfering with the others. The relatively low surfactant concentration required demonstrates the efficiency of Genagen® in modifying bubble characteristics, while the higher depressant dosage ensures adequate gangue suppression even under the enhanced flotation conditions created by optimal gas holdup.
4.4. Mechanistic Understanding of the Results
The appropriate results reported in this study can be attributed to the synergistic effects of the optimized reagent system and controlled gas holdup conditions. The combination of Genagen® frother with the fatty acid soap collector creates an interfacial environment that enhances both collision probability and attachment strength between bubbles and apatite particles.
The mechanism behind this enhancement involves the formation of mixed adsorption layers at the bubble–particle interface. The collector molecules provide the primary hydrophobic interaction with the apatite surface, while the surfactant molecules stabilize the bubble structure and modify the local hydrodynamic environment. This combination results in stronger and more stable bubble–particle aggregates that are less susceptible to detachment during transport to the froth phase.
Furthermore, the controlled gas holdup conditions ensure that the bubble size distribution remains within the optimal range for fine particle flotation. The balance between collision probability (favored by smaller bubbles) and transport efficiency (favored by larger bubbles) is achieved through careful control of the gas holdup, which, in turn, is influenced by the air flow rate and surfactant concentration.
4.5. Implications for Industrial Applications
The results of this study have significant implications for the industrial processing of low-grade phosphate ores. The ability to achieve economically attractive results (P2O5 grade > 30% and recovery > 60%) through gas holdup optimization represents a cost-effective approach to improving flotation performance. Unlike capital-intensive solutions such as equipment replacement or circuit redesign, gas holdup optimization can be implemented through relatively simple adjustments to operating conditions and reagent dosages.
When compared to conventional flotation cells operating under similar conditions [
32,
33], the optimized column flotation process demonstrated superior performance metrics. The enhanced performance can be attributed to the controlled gas holdup environment and the inherent design limitations of the conventional cells [
34].
The statistical models developed in this study (Equations (4) and (5)) provide valuable tools for process control and optimization in industrial settings. These models can be used to predict flotation performance under various operating conditions, allowing operators to make informed decisions about process adjustments. The high correlation coefficients (R2 > 0.98) indicate that the models accurately capture the relationships between operating variables and flotation responses, making them suitable for implementation in process control systems.
The conditions currently used in processing plants for low-grade phosphate ores vary significantly depending on ore characteristics, plant design, and operational constraints. Considering the variability in industrial conditions and the limitations of conventional flotation technology, this research was specifically designed to evaluate how process variables and chemical dosages influence gas holdup and its correlation with column flotation performance. The systematic approach allows for the development of appropriate conditions that can be adapted to different industrial scenarios.
5. Conclusions
This study successfully investigated the influence of gas holdup on column flotation performance for fine particles of low-grade apatite ore, quantifying the effects of operating variables and reagent dosages on P2O5 grade and recovery. Gas holdup values greater than 23.5% were identified as the critical threshold for simultaneously achieving the desired P2O5 grade (>30%) and recovery (>60%), providing a clear operational target for industrial implementation. Surfactant addition substantially enhanced gas holdup by reducing bubble coalescence, with mineral particles increasing gas holdup by 2–3 times compared to air–water systems. The best performance was achieved with fatty acid soap collector at 175 g/t, gelatinized cornstarch depressant at 450 g/t, Genagen® surfactant at 11 ppm, and an air flow rate of 159.9 L/h. These conditions demonstrated the importance of balanced reagent interactions, with excessive collector or surfactant dosages reducing selectivity through non-selective gangue flotation. Empirical models with high correlation coefficients (R2 > 0.98) were developed to predict flotation performance under various operating conditions, offering a low-cost optimization strategy through equipment adjustment rather than capital investment. The comprehensive understanding of gas holdup mechanisms and reagent synergistic effects provides a foundation for optimizing flotation circuits processing low-grade phosphate ores, with future research focusing on scaling up these findings to industrial conditions and investigating their applicability to other mineral systems.