Fluid Components in Cordierites from Granulite- and Amphibolite-Facies Rocks of the Aldan Shield and Yenisei Ridge, Russia: Evidence from Pyrolysis-Free GC-MS, Raman, and IR Spectroscopy
Abstract
1. Introduction
2. Geology
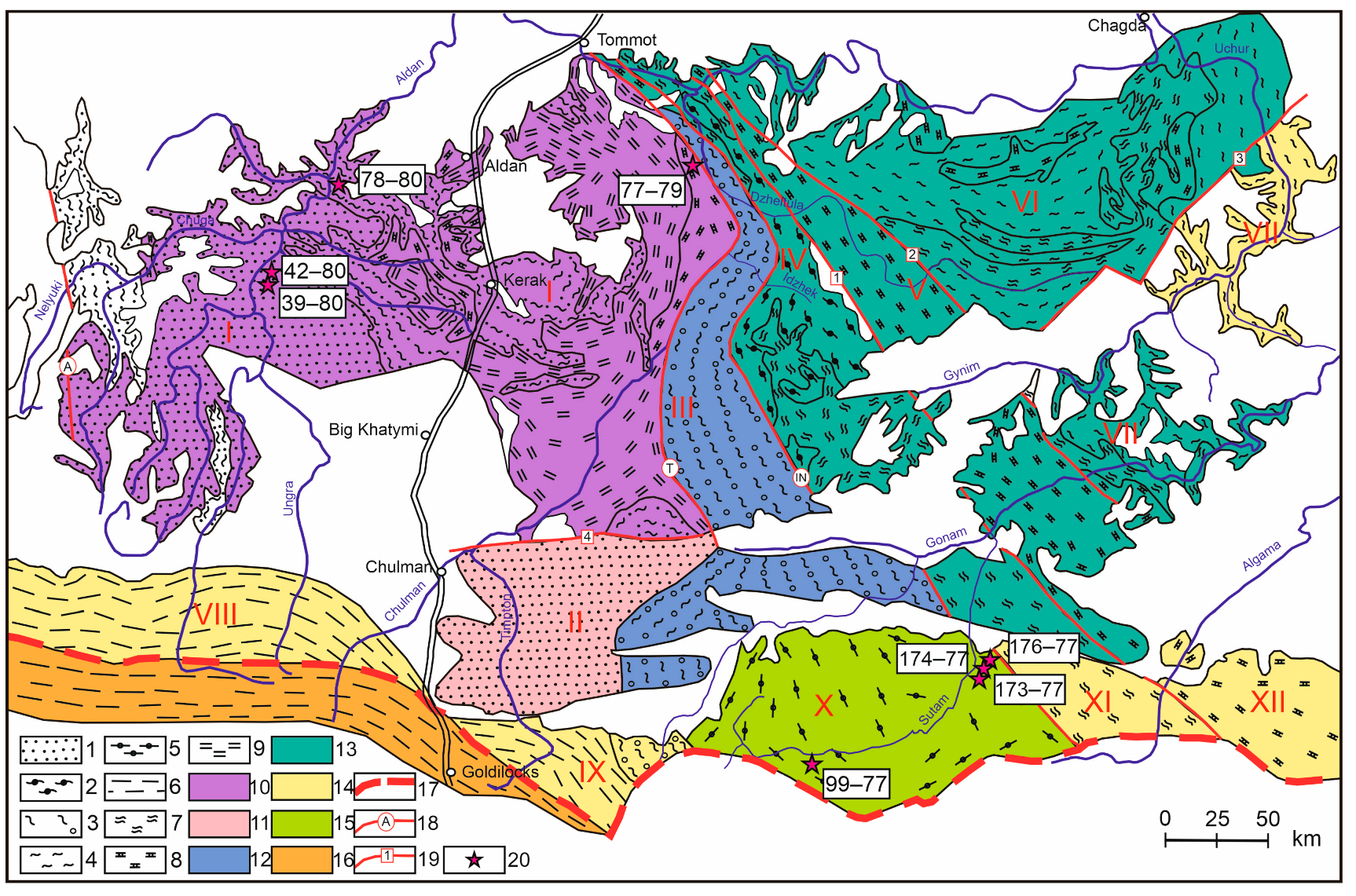
2.1. Aldan–Stanovoy Shield
2.2. South Yenisei Ridge
3. Materials and Methods
3.1. Sampling and Preparation
3.2. Electron Microprobe Method
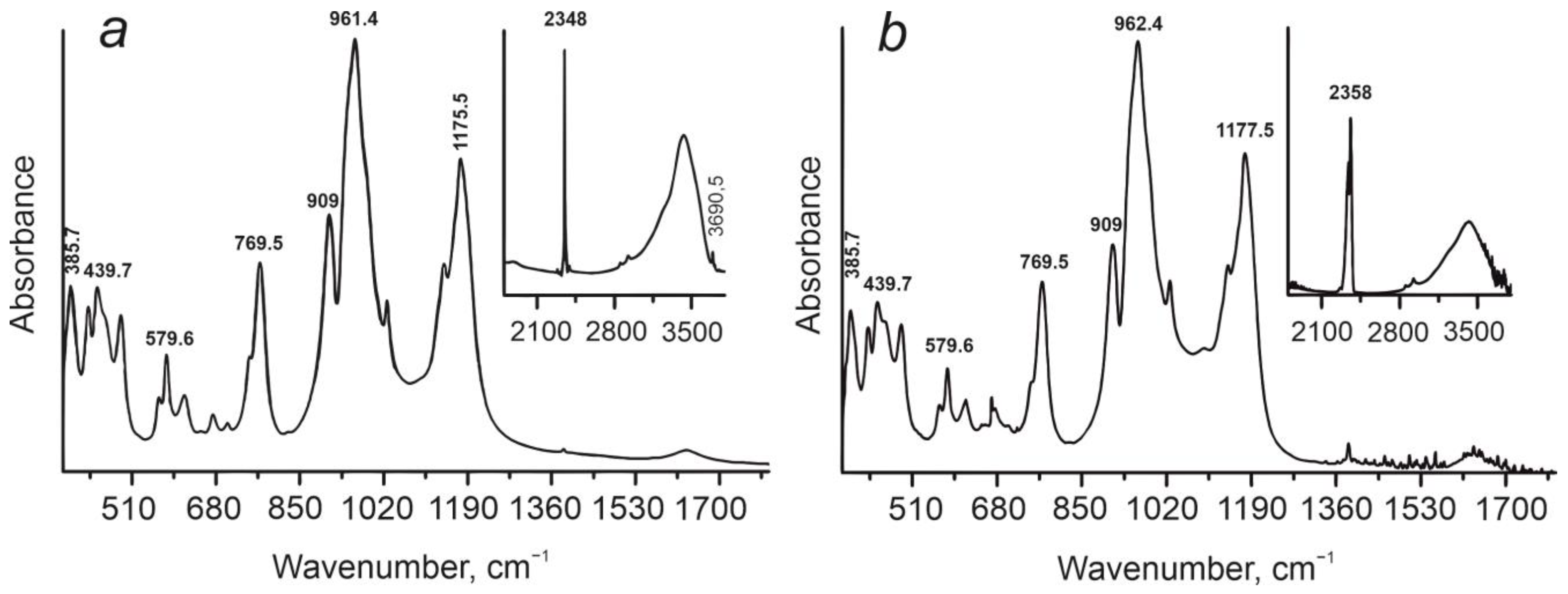
3.3. Infrared Spectroscopy
3.4. Raman Spectroscopy
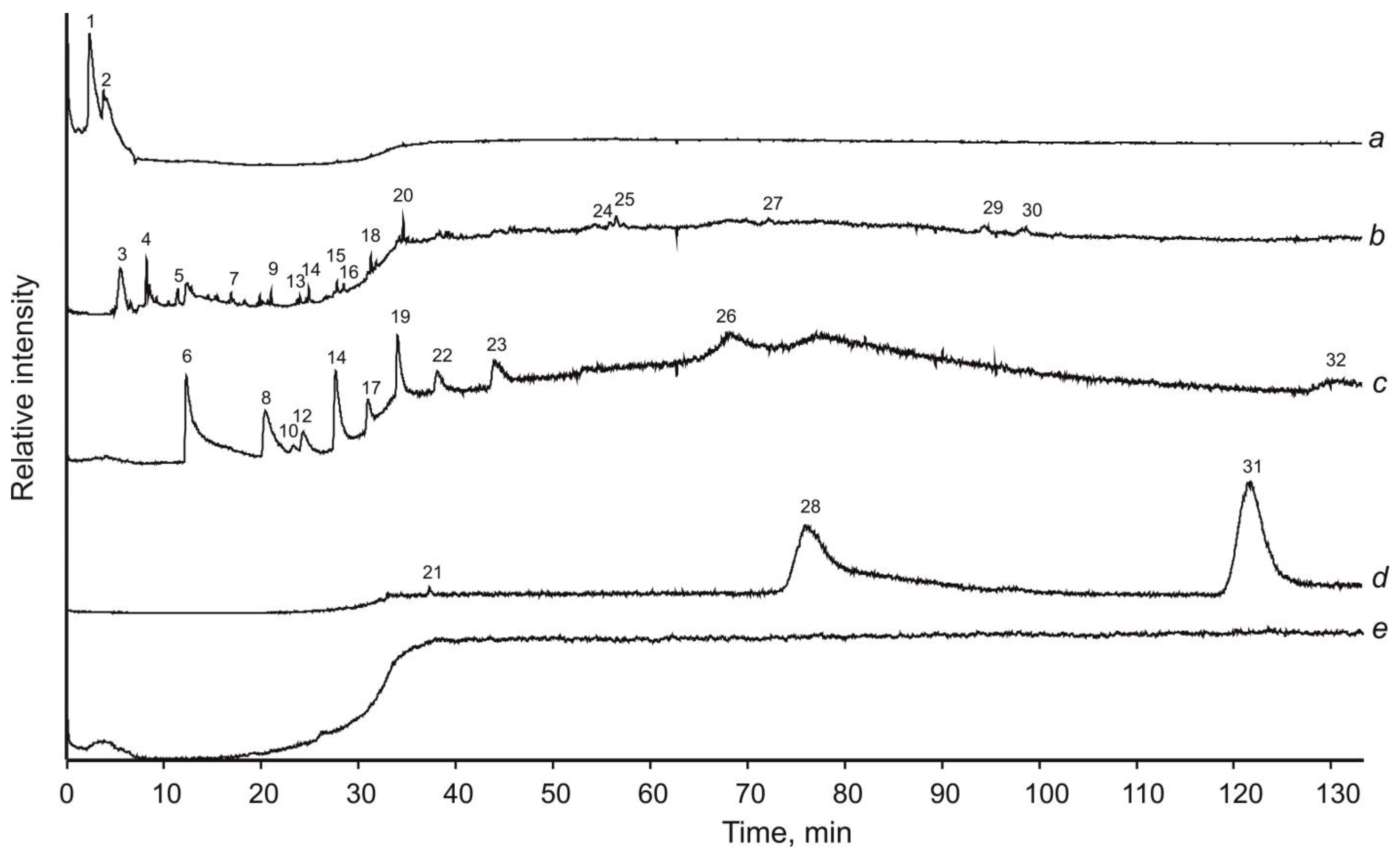
3.5. Pyrolysis-Free Gas Chromatography–Mass Spectrometry with Mechanical Sample Destruction
4. Results
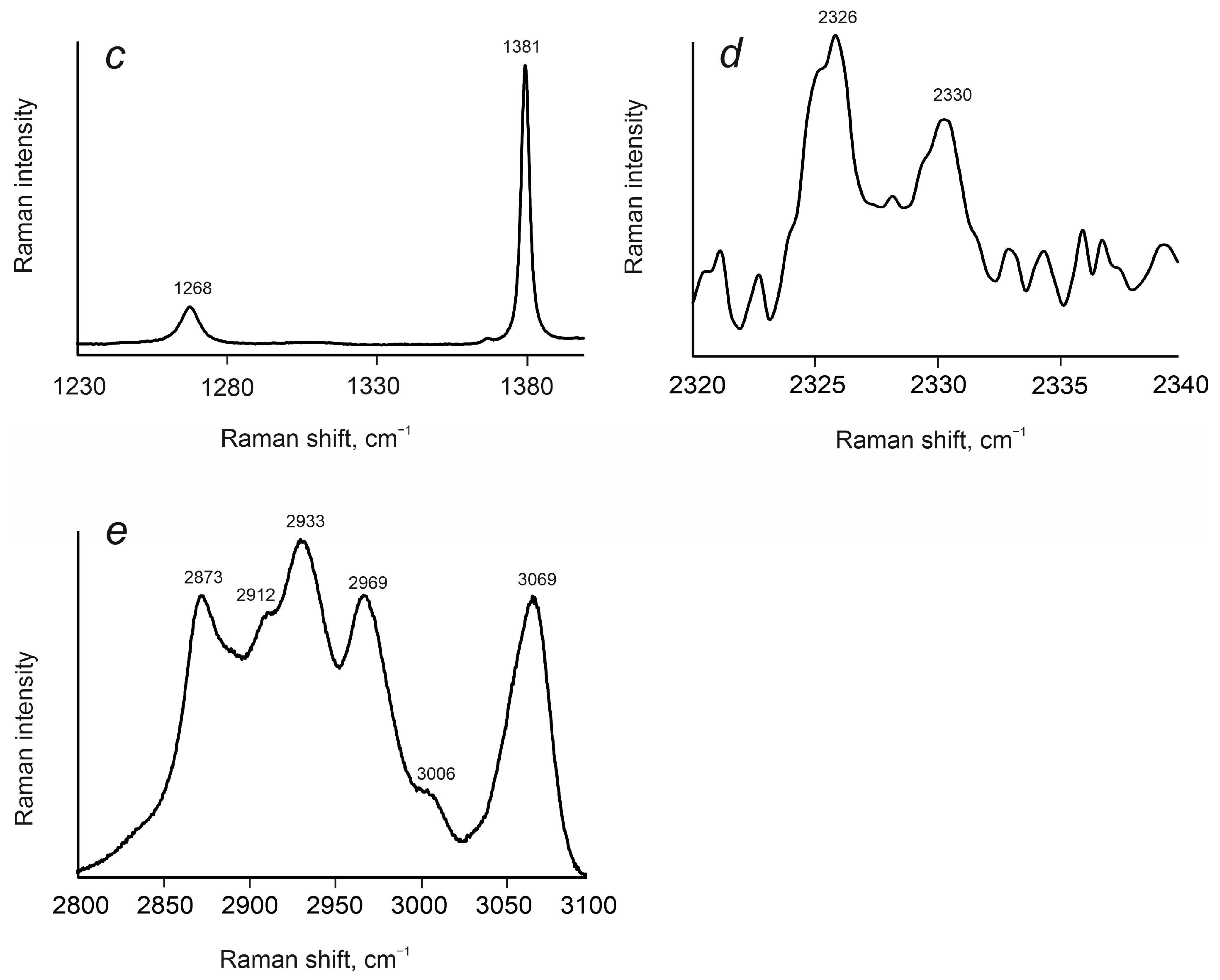
4.1. Cordierites from Granulite-Facies Rocks of Sutam and Nimnyr Blocks of the Aldan Shield
4.2. Cordierites from Granulite-Facies Metamorphism of Kan Series, Yenisei Ridge
4.3. Cordierites from Amphibolite-Facies Metamorphism of Yenisei Series, Yenisei Ridge
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lepezin, G.G.; Bul’bak, T.A.; Sokol, E.V.; Shvedenkov, G.Y. Fluid components in cordierites and their significance for metamorphic petrology. Russ. Geol. Geophys. 1999, 40, 99–116. [Google Scholar]
- Bul’bak, T.A.; Shvedenkov, G.Y.; Lepezin, G.G. On saturation of magnesian cordierite with alkanes at high temperatures and pressures. Phys. Chem. Mineral. 2002, 29, 140–154. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Shvedenkov, G.Y.; Ripinen, O.I. Kinetics of H2O–CO2 Molecular Exchange in the Structural Channels of (Mg, Fe2+)-Cordierite. Geochem. Int. 2005, 43, 386–394. [Google Scholar]
- Vry, J.K.; Brown, P.E.; Valley, J.W. Cordierite volatile content and the role of CO2 in hight–grade metamorphism. Am. Mineral. 1990, 75, 71–88. [Google Scholar]
- Harley, S.L.; Carrington, D.P. The distribution of H2O between cordierite and granitic melt: H2O incorporation in cordierite and its application to high-grade metamorphism and crustal anatexis. J. Petrol. 2001, 42, 1595–1620. [Google Scholar] [CrossRef]
- Harley, S.L.; Thompson, P.; Hensen, B.J.; Buick, I.S. Cordierite as a sensor of fluid conditions in high-grade metamorphism and crustal anatexis. J. Metamor. Geol. 2002, 20, 71–86. [Google Scholar] [CrossRef]
- Zatolokina, K.I.; Tomilenko, A.A.; Bul’bak, T.A. Fluid Components in Cordierite from the Rocks of Epidote-Amphibole Facies of the Muzkol Metamorphic Complex, Tajikistan: Pyrolysis-Free GC-MS Data. Minerals 2023, 13, 323. [Google Scholar] [CrossRef]
- Schreyer, W.; Yoder, H.S. Cordierite—H2O system. In Annual Report of the Director of the Geophysical Laboratory; Carnegie Institution: Washington, DC, USA, 1958; p. 197. [Google Scholar]
- Schreyer, W.; Schairer, I.F. Compositions and structural states of anhydrous Mg-cordierites: A reinvestigation of the central part of the system MgO-Al2O3-SiO2. J. Petrol. 1961, 2, 324–406. [Google Scholar] [CrossRef]
- Schreyer, W.; Seifert, F. Compatibility relations of the aluminium silicates in the systems MgO-Al2O3-SiO2-H2O and K2O-MgO-Al2O3-SiO2-H2O at high pressures. Am. J. Sci. 1969, 267, 371–388. [Google Scholar] [CrossRef]
- Seifert, F.; Schreyer, W. Lower temperature stability limit of Mg cordierite in the range 1–7 kb water pressure: A redetermination. Contr. Mineral. Petrol. 1970, 27, 225–238. [Google Scholar] [CrossRef]
- Seifert, F. Low-temperature compatibility relations of cordierite in haplopelites of the system K2O-MgO-Al2O3-SiO2-H2O. J. Petrol. 1970, 11, 73–99. [Google Scholar] [CrossRef]
- Newton, R.C. An experimental determination of the high-pressure stability limits of magnesian cordierite under wet and dry conditions. J. Geol. 1972, 80, 398–420. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Shvedenkova, S.V. Experimental study of the equilibrium of cordierite with potassium feldspar in water-carbon-dioxide fluid. Russ. Geol. Geophys. 1998, 39, 851–855. [Google Scholar]
- Bul’bak, T.A.; Shvedenkov, G.Y. Experimental study on incorporation of C-H-O-N fluid components in Mg-cordierite. Eur. J. Mineral. 2005, 17, 829–838. [Google Scholar] [CrossRef]
- Likhacheva, A.Y.; Goryainov, S.V.; Krylov, A.S.; Bul’bak, T.A.; Prasad, P.S.R. Raman spectroscopy of natural cordierite at high water pressure up to 5 GPa. J. Raman Spectrosc. 2012, 43, 559–563. [Google Scholar] [CrossRef]
- Likhacheva, A.Y.; Goryainov, S.V.; Bul’bak, T.A. An X-ray diffraction study of the pressure-induced hydration in cordierite at 4-5 GPa. Am. Mineral. 2013, 98, 181–186. [Google Scholar] [CrossRef]
- Miletich, R.; Gatta, G.D.; Willi, T.; Mirwald, P.W.; Lotti, P.; Merlini, M. Cordierite under hydrostatic compression: Anomalous elastic behavior as a precursor for a pressure-induced phase transition. Am. Mineral. 2014, 99, 479–493. [Google Scholar] [CrossRef]
- Dufour, M.S.; Popova, V.A. Processes of regional metamorphism in the Muzkol metamorphic complex in the Central Pamirs. Vestn. Leningr. Univ. 1975, 12, 14–20. [Google Scholar]
- Lepezin, G.G.; Melenevsky, V.N. On the problem of water diffusion in the cordierites. Lithos 1977, 10, 49–57. [Google Scholar] [CrossRef]
- Palin, R.M.; Palmer, Z.; Holm-Denoma, C.; Hernández-Uribe, D.; Hernández-Montenegro, J.D. On the occurrence of amphibolite-facies sapphirine, spinel, phlogopite, anorthite, and corundum in the Wet Mountains, Colorado, USA. Lithos 2023, 440–441, 107024. [Google Scholar] [CrossRef]
- Holdaway, M.J.; Lee, S.M. Fe-Mg cordierite stability in high-grade pelitic rocks based on experimental, theoretical, and natural observations. Contrib. Mineral. Petrol. 1977, 63, 175–198. [Google Scholar] [CrossRef]
- Winter, J.D. Principles of Igneous and Metamorphic Petrology, 2nd ed.; Pearson: Harlow, UK, 2014; 739p. [Google Scholar]
- Kalt, A.; Altherr, R.; Ludwig, T. Contact metamorphism in pelitic rocks on the island of Kos (Greece, Eastern Aegean Sea): A test for the Na-in-cordierite thermometer. J. Petrol. 1998, 39, 663–688. [Google Scholar] [CrossRef]
- Phillips, G.N.; Wall, V.J.; Clemens, J.D. Petrology of the Strathbogie Batholith: A Cordierite-Bearing Granite. Can. Mineral 1981, 19, 47–63. [Google Scholar]
- Sokol, E.V.; Maksimova, N.V.; Nigmatulina, E.N.; Sharygin, V.V.; Kalugin, V.M. Combustion Metamorphism; SB RAN Publisher: Novosibirsk, Russia, 2005. [Google Scholar] [CrossRef]
- Grapes, R.; Korzhova, S.; Sokol, E.; Seryotkin, Y. Paragenesis of unusual Fe-cordierite (sekaninaite)-containing paralava and clinker from the Kuznetsk coal basin, Siberia, Russia. Contrib. Mineral. Petrol. 2011, 162, 253–273. [Google Scholar] [CrossRef]
- Heinrich, E.W. Cordierite in pegmatite near Micanite, Colorado. Am. Mineral. 1950, 35, 173–184. [Google Scholar]
- Newton, R.C. BeO in pegmatitic cordierite. Mineral. Mag. 1966, 35, 920–927. [Google Scholar] [CrossRef]
- Černý, P.; Povondra, P. Beryllian cordierite from Vĕžná: (Na,K)+Be→Al. Neues Jahrb. Miner. Monatsh. 1966, 2, 36–44. [Google Scholar]
- Černý, P.; Povondra, P. Cordierite in West-Moravian desilicated pegmatites. Acta Univ. Carol. Geol. 1967, 3, 203–221. [Google Scholar]
- Černý, P.; Chapman, R.; Schreyer, W.; Ottolini, L.; Bottazzi, P.; McCammon, C.A. Lithium in sekaninaite from the type locality, Dolní Bory, Czech Republic. Can. Mineral. 1997, 35, 167–173. [Google Scholar]
- Jobin-Bevans, S.; Černý, P. The beryllian cordierite + beryl + spessartine assemblage, and secondary beryl in altered cordierite, Greer Lake granitic pegmatites, southeastern Manitoba. Can. Mineral. 1998, 36, 447–462. [Google Scholar]
- Grew, E.S.; Yates, M.G.; Barbier, J.; Shearer, C.K.; Sheraton, J.W.; Shiraishi, K.; Motoyoshi, Y. Granulite-facies beryllium pegmatites in the Napier Complex in Khmara and Amundsen Bays, western Enderby Land, East Antarctica. Polar Geosci. 2000, 13, 1–40. [Google Scholar]
- Sokol, E.V.; Seryotkin, Y.V.; Bul’bak, T.A. Na-Li-Be-rich cordierite from the Murzinka pegmatite field, Middle Urals, Russia. Eur. J. Mineral. 2010, 22, 565–575. [Google Scholar] [CrossRef]
- Zatolokina, K.I.; Tomilenko, A.A.; Bul’bak, T.A.; Lepezin, G.G. Volatile components in cordierite and coexisting tourmaline and quartz from pegmatites of the Kuhilal deposit (Pamirs, Tajikistan). Russ. Geol. Geophys. 2021, 62, 1411–1431. [Google Scholar] [CrossRef]
- Dymek, R.F.; Albee, A.L.; Chodos, A.A. Petrology and origin of Boulders #2 and #3, Apollo 17 Station 2. In Proceedings of the Seventh Lunar Science Conference, Houston, TX, USA, 15–19 March 1976; pp. 2335–2378. [Google Scholar]
- Herzberg, C.T.; Baker, M.B. The cordierite-to-spinel-cataclasite transition: Structure of the lunar crust. In Proceedings of the Conference on the Lunar Highlands Crust, Houston, TX, USA, 14–16 November 1979; Pergamon Press: New York, NY, USA, 1980; Volume 1, pp. 113–132. [Google Scholar]
- Marvin, U.B.; Carey, J.W.; Lindstrom, M.M. Cordierite-Spinel Troctolite, a New Magnesium-Rich Lithology from the Lunar Highlands. Science 1989, 243, 925–928. [Google Scholar] [CrossRef]
- Fuchs, L.H. Occurrence of cordierite and aluminous orthoenstatite in the Allende meteorite. Am. Mineral. 1969, 54, 1645–1653. [Google Scholar]
- Sheng, Y.J.; Hutcheon, I.D.; Wasserburg, G.J. Origin of plagioclase-olivine inclusions in carbonaceous chondrites. Geochim. Cosmochim. Acta 1991, 55, 581–599. [Google Scholar] [CrossRef]
- Petaev, M.I.; Zaslavskaya, N.I.; Clarke, R.S., Jr.; Olsen, E.J.; Jarosewich, E.; Kononkova, N.N.; Holmberg, B.B.; Davis, A.M.; Ustinov, V.I.; Wood, J.A. The Chaunskij meteorite: Mineralogical, chemical and isotope data, classification and proposed origin (abstract). Meteoritics 1992, 27, 276–277. [Google Scholar]
- Petaev, M.I.; Clarke, R.S.; Olsen, E.J.; Jarosewich, E.; Davis, A.M.; Steele, I.M.; Lipschutz, M.E.; Wang, M.S.; Clayton, R.N.; Mayeda, T.K.; et al. Chaunskij: The most highly metamorphosed, shock-modified and metal-rich mesosiderite (abstract). Lunar Planet. Sci. 1993, 24, 1131–1132. [Google Scholar]
- Schreyer, W. Experimental studies on cation substitutions and fluid incorporation in cordierite. Bull Mineral. 1985, 108, 273–291. [Google Scholar] [CrossRef]
- Zimmermann, J.L. Application petrogenetique de l’etude de la liberation de l’eau et du gaz carbonique des cordierites. CR Acad. Sci. 1972, 275, 519–522. [Google Scholar]
- Zimmermann, J.L. Etude par spectrometric de masse de la composition des fluides dans quelques cordierites du sud de la Norvege. In Reunion Annuelle des Sciences de la Terre; Societe Geologique de France: Paris, France, 1973. [Google Scholar]
- Zimmermann, J.L. La liberation de l’eau, du gaz casrbonique et des hydrocarbures des cordierites. Cinetiques des mecanismes. Determination des sides. Interet petrogenetique. Bull. Mineral. 1981, 104, 325–338. [Google Scholar]
- Beltrame, R.J.; Norman, D.I.; Alexander, E.C.; Savkins, F.J. Volatiles released by step-heating a cordierite to 1200 °C. Trans. Am. Geophys. Union 1976, 57, 352. [Google Scholar]
- Goldman, D.S.; Rossman, G.R.; Dollase, W.A. Channel constituents in cordierite. Am. Mineral. 1977, 62, 1144–1157. [Google Scholar]
- Johannes, B.; Schreyer, W. Experimental introduction of CO2 and H2O into Mg- cordierite. Am. J. Sci. 1981, 281, 299–317. [Google Scholar] [CrossRef]
- Armbruster, T.; Bloss, F.D. Orientation and effects of channel H2O and CO2 in cordierites. Am. Mineral. 1982, 67, 284–291. [Google Scholar]
- Mottana, A.; Fusi, A.; Bianchi Potenza, B.; Crespi, R.; Liborio, G. Hydrocarbon-containing cordierite from Dervio-Colico road tunnel (Como, Italy). Neues Jahrb. Miner. Abh. 1983, 148, 181–199. [Google Scholar]
- Armbruster, T. Ar, N2, and CO2 in the Structural Cavites of Cordierite, an Optical and X-ray Single-Crystal Study. Phys. Chem. Miner. 1985, 12, 233–245. [Google Scholar] [CrossRef]
- Khomenko, V.M.; Langer, K. Aliphatic hydrocarbons in structural channels of cordierite: A first evidence from polarized single-crystal IR absorption spectroscopy. Am. Mineral. 1999, 84, 1181–1185. [Google Scholar] [CrossRef]
- Kolesov, E.A.; Geiger, C.A. Cordierite II: The role of CO2 and H2O. Am. Mineral. 2000, 85, 1265–1274. [Google Scholar] [CrossRef]
- Khomenko, V.; Langer, K.; Geiger, C.A. Structural locations of the iron ions in cordierite: A spectroscopic study. Contrib. Mineral. Petrol. 2001, 141, 381–396. [Google Scholar] [CrossRef]
- Kolesov, B.A. Raman spectra of single H2O molecules isolated in crystal cavities. Zhurnal Strukturnoi Khimii 2006, 47, 27–40. [Google Scholar] [CrossRef]
- Smith, J.V.; Schreyer, W. Location of argon and water in cordierite. Miner. Mag. 1962, 33, 226–236. [Google Scholar] [CrossRef]
- Farrel, E.E.; Newnham, R.E. Electronic and vibrational absorption spectra. Am. Mineral. 1967, 52, 380–389. [Google Scholar]
- Armbruster, T.; Bloss, F.D. Channel CO2 in cordierites. Nature 1980, 286, 140–141. [Google Scholar] [CrossRef]
- Carson, D.G.; Rossman, G.R.; Vaughan, R.V. Orientation and motion of water molecules in cordierite: A proton nuclear magnetic resonance study. Phys. Chem. Miner. 1982, 8, 14–19. [Google Scholar] [CrossRef]
- Mirwald, P. Crystal chemical effects of sodium on the incorporation of H2O and CO2 in Mg-cordierite. Terra Cogn. 1983, 3, 163. [Google Scholar]
- Aines, R.D.; Rossman, G.R. The high temperature behavior of water and carbon dioxide in cordierite and beryl. Am. Mineral. 1984, 69, 319–327. [Google Scholar]
- Armbruster, T. The role of Na in the structure of low cordierite. A syngle crystal X-ray study. Am. Mineral. 1986, 71, 746–757. [Google Scholar]
- Giampaolo, C.; Putnis, A. The kinetics of dehydration and order-disorder of molecular H2O in Mg-cordierite. Eur. J. Miner. 1989, 1, 193–202. [Google Scholar] [CrossRef]
- Suknev, V.S.; Kitsul, V.I.; Lazebnik, Y.D.; Brovkin, A.A. On the presence and qualitative estimation of CO2 in cordierites based on data of IR spectroscopy and chemical analysis. Doklady Akademii Nauk SSSR 1971, 200, 950–952. [Google Scholar]
- Kitsul, V.I.; Lazebnik, Y.D.; Brovkin, A.A.; Suknev, V.O. Diagram for determination of the iron content of cordierites. Doklady Akademii Nauk SSSR 1971, 200, 1419–1422. [Google Scholar]
- Schreyer, W.; Gordillo, C.E.; Werding, G. A new sodian-berillian cordierite from Soto, Argentina, and the relationship between distortion index, Be content, and state of hydration. Contrib. Miner. Petrol. 1979, 70, 421–428. [Google Scholar] [CrossRef]
- Hofmann, J.; Kaiser, G.; Klemm, W.; Paech, H.-J. K/Na Alter von Dolerite und Metamorphiten der Shackleton Range und der Whichaway Nunataks, Ost- und Südostumrandung des Filchner-Eisschelfs (Antarktis). Z. Geol. Wiss. Berl. 1980, 8, 1227–1232. [Google Scholar]
- Armbruster, T.; Schreyer, W.; Hoefs, J. Very high CO2 cordierite from Norwegian Lapland; mineralogy, petrology, and carbon isotopes. Contrib. Mineral. Petrol. 1982, 81, 262–267. [Google Scholar] [CrossRef]
- Lai, K.K.; Ackermand, D.; Raith, P.; Seifert, F. Sapphirine-containing assemblages from Kiranur, Southern India: A study of chemographic relationships in the Na2O-FeO-MgO-Al2O3-SiO2-H2O system. Neues Jahrb. Miner. Abh. 1984, 150, 121–152. [Google Scholar]
- Kurepin, V.A.; Malyuk, G.A.; Kalinichenko, A.M.; Utochkin, D.V. Volatiles in cordierite from Berdichev granites (the Ukrainian Shield). Mineral. Zhurnal 1986, 8, 70–82. [Google Scholar]
- Perreault, S.; Martignole, J. CO2-rich cordierites in high-temperature migmatites, northeastern Grenville province, Quebec (abs). Geol. Assoc. Can. Program Abstr. 1986, 11, 114. [Google Scholar]
- Perreault, S.; Martignole, J. High-temperature cordierite migmatites in the north-eastern Grenville Province. J. Metamorph. Geol. 1988, 6, 673–696. [Google Scholar] [CrossRef]
- Geverk’yan, S.V.; Kuripin, V.A. CO2 in cordierite structure (IR spectroscopic data). Mineral. Zhurnal 1987, 9, 49–53. [Google Scholar]
- Le Breton, N. Infrared investigation of CO2-containing cordierites. Some implications for the study of metapelitic granulites. Contrib. Mineral. Petrol. 1989, 103, 387–396. [Google Scholar] [CrossRef]
- Osorgin, N.Y. The Kinetics of Degassing of (H2O and CO2) Cordierites and Their Significance for Metamorphic Petrology: Manuscript; OIGGM SB RAN: Novosibirsk, Russia, 1991; 16p. [Google Scholar]
- Peck, H.W.; Valley, J.W. Genesis of cordierite—Getrite gneisses, central metasedimentary Belt, boundary thrust zone, Grenville province, Ontario, Canada. Can. Mineral. 2000, 38, 511–524. [Google Scholar] [CrossRef]
- Bertoldi, C.; Proyer, A.; Garbe-Schönberg, D.; Behrens, H.; Dachs, E. Comprehensive chemical analyses of natural cordierites: Implications for exchange mechanisms. Lithos 2004, 78, 389–409. [Google Scholar] [CrossRef]
- Majumdar, A.S.; Mathew, G. Raman-Infrared (IR) Spectroscopy Study of Natural Cordierites from Kalahandi, Odisha. J. Geol. Soc. 2015, 86, 80–92. [Google Scholar] [CrossRef]
- Tropper, P.; Wyhlidal, S.; Haefeker, U.A.; Mirwald, P.W. An experimental investigation of Na incorporation in cordierite in low P/high T metapelites. Miner. Petrol. 2018, 112, 199–217. [Google Scholar] [CrossRef]
- Damon, P.E.; Kulp, J.L. Excess helium and argon in beryl and other minerals. Am. Mineral. 1958, 43, 433–459. [Google Scholar]
- Wood, D.L.; Nassau, K. Infrared spectra of foreign molecules in beryl. J. Chem. Phys. 1967, 47, 2200–2228. [Google Scholar] [CrossRef]
- Saito, K.; Alexander, E.C., Jr.; Dragon, J.C.; Zashu, S. Rare gases in cyclosilicates and cogenetic minerals. J. Geophys. Res. 1984, 89, 7891–7901. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Tomilenko, A.A.; Zatolokina, K.I.; Shaparenko, E.O. Fluid components in cordierites from the Middle Urals (Russia) and Dolní Bory (Czech Republic) pegmatites according to pyrolysis-free gas chromatography-mass spectrometry data. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2025, in press. [Google Scholar]
- Dobretsov, N.L. (Ed.) Early Precambrian of Southern Yakutia; Nauka: Moscow, Russia, 1986; p. 280. [Google Scholar]
- Rundqvist, D.V.; Mitrofanov, F.P. (Eds.) Precambrian Geology of the USSR; Academy of Sciences USSR, The Institute of Precambrian Geology and Geochronology, Nauka: Moscow, Russia, 1988; p. 441. [Google Scholar]
- Popov, N.V.; Smelov, A.P. Metamorphic Formations of the Aldan Shield. Russ. Geol. Geophys. 1996, 37, 148–161. [Google Scholar]
- Smelov, A.P.; Timofeev, V.F. Terrane Analysis and Geodynamic Model of the North Asian Craton in the Early Precambrian. Pac. Geol. 2003, 22, 42–54. [Google Scholar]
- Smelov, A.P.; Timofeev, V.F. The Age of the North Asian Cratonic Basement: An Overview. Gondwana Res. 2007, 12, 279–288. [Google Scholar] [CrossRef]
- Berezkin, V.I.; Smelov, A.P.; Zedgenizov, A.N.; Kravchenko, A.A.; Popov, N.V.; Timofeev, V.F.; Toropova, L.I. Geological Structure of the Central Part of the Aldan-Stanovoy Shield and Chemical Compositions of Early Precambrian Rocks (Southern Yakutia); Smelov, A.P., Ed.; IGABM SO RAN, INGG SO RAN: Novosibirsk, Russia, 2015; p. 459. [Google Scholar]
- Drogova, G.M.; Glebovitsky, V.A.; Duk, V.L.; Kitsul, V.I.; Savelyeva, T.L.; Sedova, I.S.; Semenov, A.P. High-Gradient Metamorphic Regimes in Crustal Evolution; Sokolov, Y.M., Ed.; Nauka: Leningrad, Russia, 1982; p. 229. [Google Scholar]
- Kitsul, V.I.; Zedgenizov, A.N. On the Binary Division of the Aldan Complex. In Stratigraphy of the Archean and Lower Proterozoic of the USSR; Nauka: Leningrad, Russia, 1979; pp. 125–130. [Google Scholar]
- Parfenov, L.M.; Kuzmin, M.I. (Eds.) Tectonics, Geodynamics and Metallogeny of the Territory of the Sakha Republic (Yakutia); MAIK “Nauka/Interperiodica”: Moscow, Russia, 2001; p. 572. [Google Scholar]
- Perchuk, L.L.; Kitsul, V.I.; Podlesskii, K.K.; Gerasimov, V.Y.; Aranovich, L.Y.; Fed’kin, V.V. Evolution of Metamorphism of the Aldan Shield. Preprint, Report. In Proceedings of the the VI All-Union Petrographic Meeting, Leningrad, Russia, 28–29 May 1981; Yakutsk Branch of the Siberian Branch of the USSR Academy of Sciences: Yakutsk, Russia, 1981; p. 63. [Google Scholar]
- Perchuk, L.L.; Aranovich, L.Y.; Podlesskii, K.K.; Lavrant’eva, I.V.; Gerasimov, V.Y.; Fed’kin, V.V.; Kitsul, V.I.; Karsakov, L.P.; Berdnikov, N.V. Precambrian granulites of the Aldan shield, eastern Siberia, USSR. J. Metamorph. Geol. 1985, 3, 265–310. [Google Scholar] [CrossRef]
- Perchuk, L.L.; Kitsul, V.I.; Aranovich, L.Y.; Podlessky, K.K.; Lavrentieva, I.V.; Gerasimov, V.Y.; Fedkin, V.V.; Berdnikov, N.V.; Karsakov, L.P. Mineralogy and Petrology of Granulites of the Aldan-Stanovoy Region; Yakutsk Branch of the USSR Academy of Sciences: Yakutsk, Russia, 1986; p. 40. [Google Scholar]
- Perchuk, L.L.; Kitsul, V.I.; Aranovich, L.Y. Petrology of Granulites of the Aldan Shield; Yakutsk Branch of the USSR Academy of Sciences: Yakutsk, Russia, 1987; p. 80. [Google Scholar]
- Tomilenko, A.A.; Chupin, V.P. Thermobarogeochemistry of Metamorphic Complexes; Nauka: Novosibirsk, Russia, 1983; p. 201. [Google Scholar]
- Tomilenko, A.A. Fluid Regime of Mineral Formation in the Continental Lithosphere at High–Moderate Pressures Based on the Study of Fluid and Melt Inclusions in Minerals. Ph.D. Thesis, Institute of Geology and Mineralogy SB RAS, Novosibirsk, Russia, 2006; p. 31. [Google Scholar]
- Kosygin, Y.A.; Luchitsky, I.V. Structures of the Siberian Platform Margins. In Tectonics of Siberia; SB USSR Academy of Sciences: Novosibirsk, Russia, 1963; Volume 2, pp. 9–12. [Google Scholar]
- Kirichenko, G.I. Tectonics of the Yenisei Ridge. In Tectonics of Siberia; SB USSR Academy of Sciences: Novosibirsk, Russia, 1963; Volume 2, pp. 65–82. [Google Scholar]
- Kuznetsov, Y.A. Petrology of the Precambrian of the South Yenisei Ridge (Materials on the Geology of Western Siberia, Issue 15(57)); West Siberian Geological Survey: Tomsk, Russia, 1941; p. 250. [Google Scholar]
- Kuznetsov, Y.A. Selected Works, Volume 1; Nauka: Novosibirsk, Russia, 1988; p. 217. [Google Scholar]
- Kovrigina, E.K. Experience in Formation Analysis of Metamorphic Sequences Using the Example of the Angara-Kan Part of the Yenisei Ridge. In Proceedings of Annual and Anniversary Sessions of the Scientific Council of VSEGEI; VSEGEI: Leningrad, Russia, 1971; pp. 116–127. [Google Scholar]
- Nozhkin, A.D. Early Precambrian Gneiss Complexes of the Yenisei Ridge and Their Geochemical Features. Geol. Geophys. 1983, 9, 3–10. [Google Scholar]
- Nozhkin, A.D.; Turkina, O.M. Geochemistry of Granulites from the Kan and Sharyzhalgay Complexes; OIGGM SO RAN: Novosibirsk, Russia, 1993; p. 223. [Google Scholar]
- IGG SB USSR Academy of Sciences. Precambrian Crystalline Complexes of the Yenisei Ridge: Guidebook for the Yenisei Excursion; IGG SB USSR Academy of Sciences: Novosibirsk, Russia, 1986; p. 117. [Google Scholar]
- Lepezin, G.G.; Nozhkin, A.D.; Gerya, T.V. Thermodynamic Parameters of Metamorphism of the Kan Series (Yenisei Ridge). Russ. Geol. Geophys. 1986, 9, 11–19. [Google Scholar]
- Perchuk, L.L.; Gerya, T.V.; Nozhkin, A.D. Petrology and retrograde P-T path in granulites of the Kan formation, Yenisei range, Eastern Siberia. J. Metamorphic Geol. 1989, 7, 599–617. [Google Scholar] [CrossRef]
- Popov, N.V. Tectonic Model of the Early Precambrian Evolution of the South Yenisei Ridge. Russ. Geol. Geophys. 2001, 42, 1028–1041. [Google Scholar]
- Nozhkin, A.D.; Turkina, O.M.; Likhanov, I.I.; Dmitrieva, N.V. Late Paleoproterozoic Volcanic Associations in the Southwest of the Siberian Craton (Angara-Kan block). Russ. Geol. Geophys. 2016, 57, 312–332. [Google Scholar] [CrossRef]
- Nozhkin, A.D.; Turkina, O.M.; Likhanov, I.I.; Savko, K.A. Paleoproterozoic Metavolcanosedimentary Sequences of the Yenisei Metamorphic Complex, Southwestern Siberian Craton (Angara–Kan Block): Subdivision, Composition, And U–Pb Zircon Age. Russ. Geol. Geophys. 2019, 60, 1384–1406. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Tomilenko, A.A.; Gibsher, N.A.; Sazonov, A.M.; Shaparenko, E.O.; Ryabukha, M.A.; Khomenko, M.O.; Sil’yanov, S.A.; Nekrasova, N.A. Hydrocarbons in fluid inclusions from native gold, pyrite and quartz of the Sovetskoe deposit (Yenisei Ridge, Russia) according to pyrolysis-free gas chromatography-mass spectrometry data. Russ. Geol. Geophys. 2020, 61, 1535–1560. [Google Scholar] [CrossRef]
- Khomenko, V.M.; Langer, K. Carbon oxides in cordierite channels: Determination of CO2 isotopic species and CO by single crystal IR spectroscopy. Am. Mineral. 2005, 90, 1913–1917. [Google Scholar] [CrossRef]
- Herms, P.; Schenk, V. Fluid inclusions in granulite-facies metapelites of the Hercynian ancient lower crust of the Serre, Calabria, Southern Italy. Contrib. Mineral. Petrol. 1992, 112, 393–404. [Google Scholar] [CrossRef]
- Santosh, M.; Jackson, D.H.; Harris, N.B.W. The Significance of Channel and Fluid-Inclusion CO2 in Cordierite: Evidence from Carbon Isotopes. J. Petrol. 1993, 34, 233–258. [Google Scholar] [CrossRef]
- Cesare, B.; Maineri, C.; Baron Toaldo, A.; Pedron, D.; Acosta Vigil, A. Immiscibility between carbonic fluids and granitic melts during crustal anatexis: A fluid and melt inclusion study in the enclaves of the Neogene Volcanic Province of SE Spain. Chem. Geol. 2007, 237, 433–449. [Google Scholar] [CrossRef]
- Mirwald, P.W.; Jochum, C.; Maresch, W.V. Rate Studies on Hydration and Dehydration of Synthetic Mg-Cordierite. Mater. Sci. Forum 1986, 7, 113–122. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Tomilenko, A.A.; Wirth, R.; Bul’bak, T.A.; Luk’yanova, L.I.; Fedorova, E.N.; Reutsky, V.N.; Efimova, E.S. Mineral and fluid inclusions in diamonds from the Urals placers, Russia: Evidence for solid molecular N2 and hydrocarbons in fluid inclusions. Geochim. Cosmochim. Acta 2019, 266, 197–219. [Google Scholar] [CrossRef]







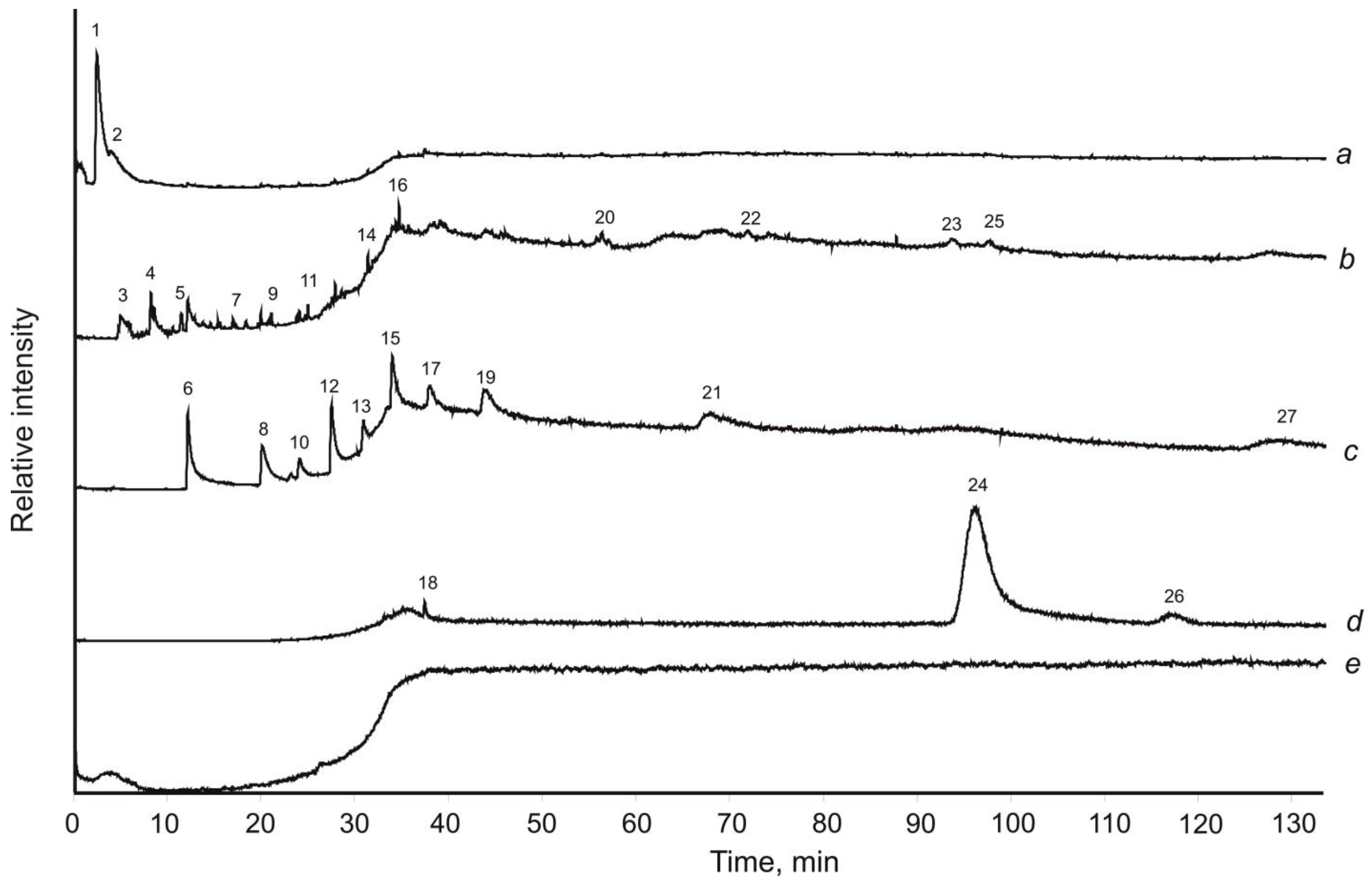
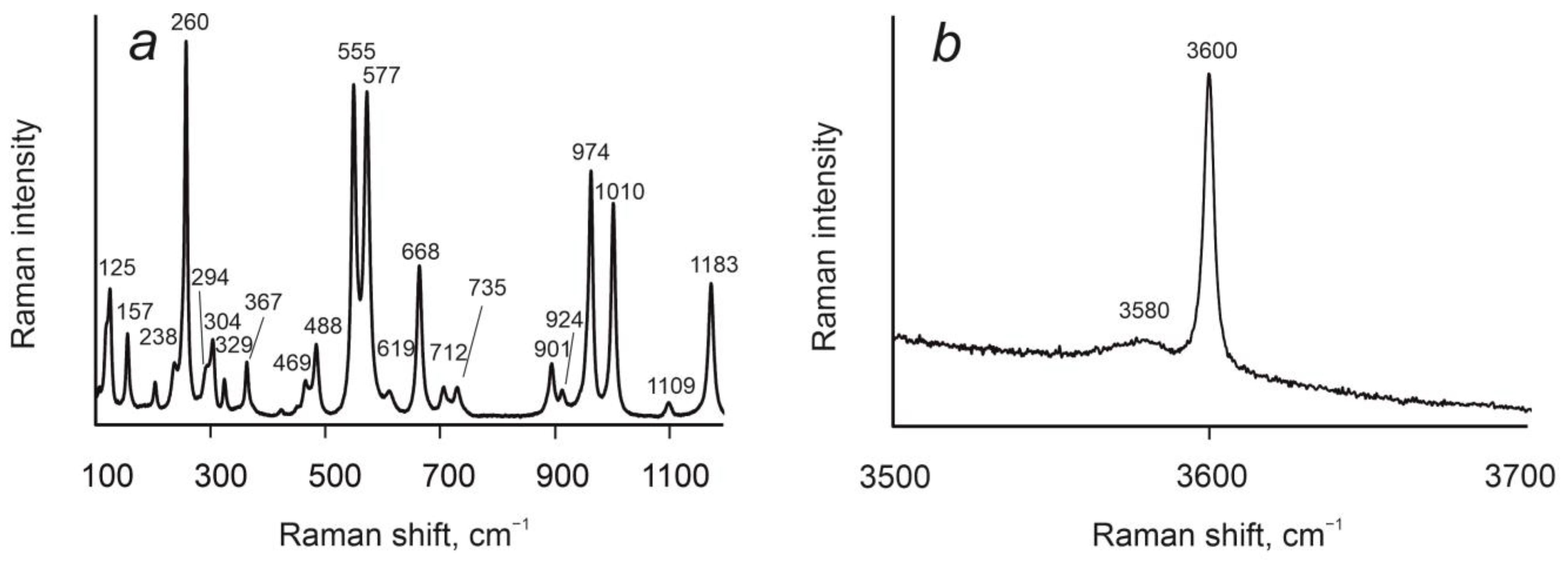
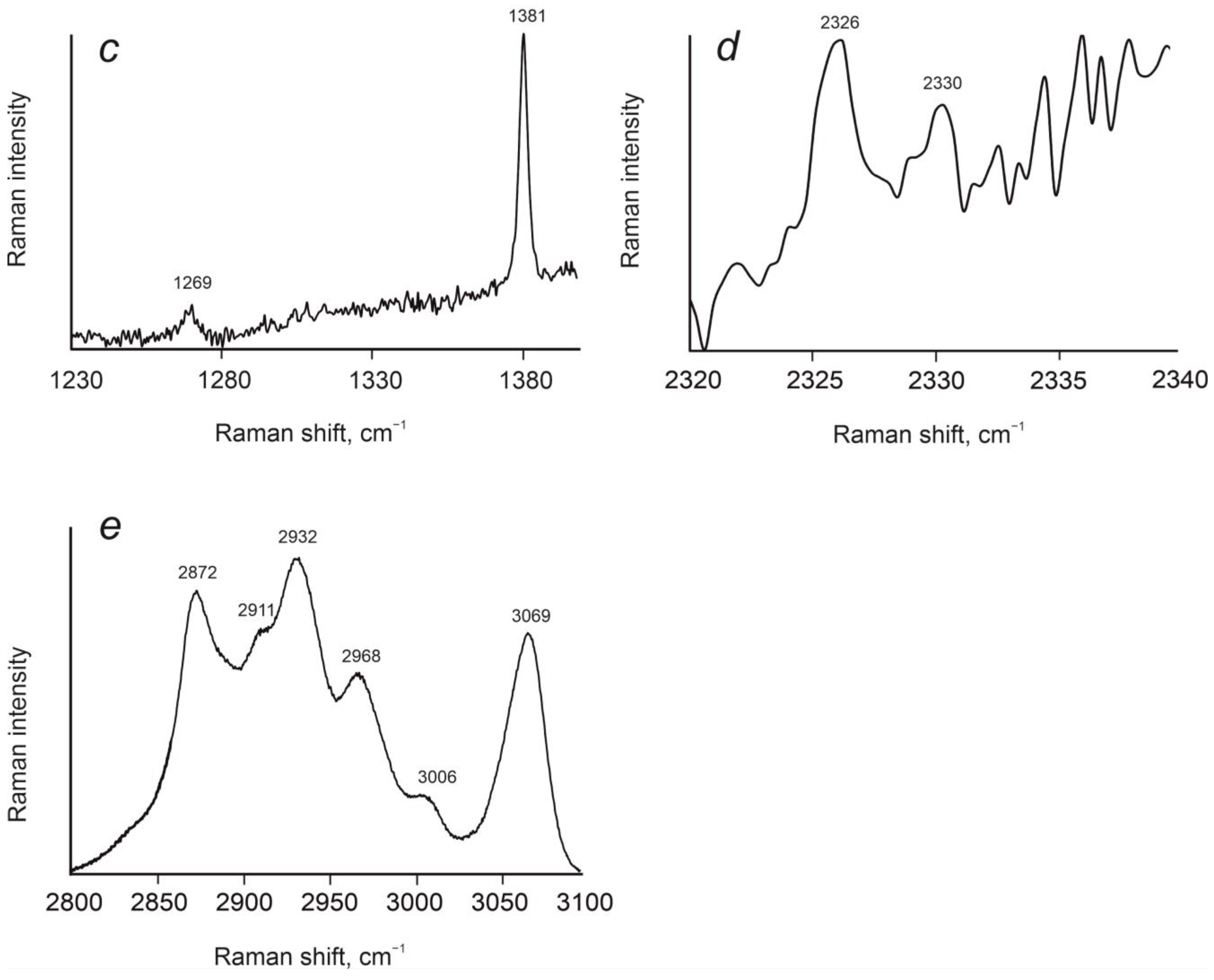
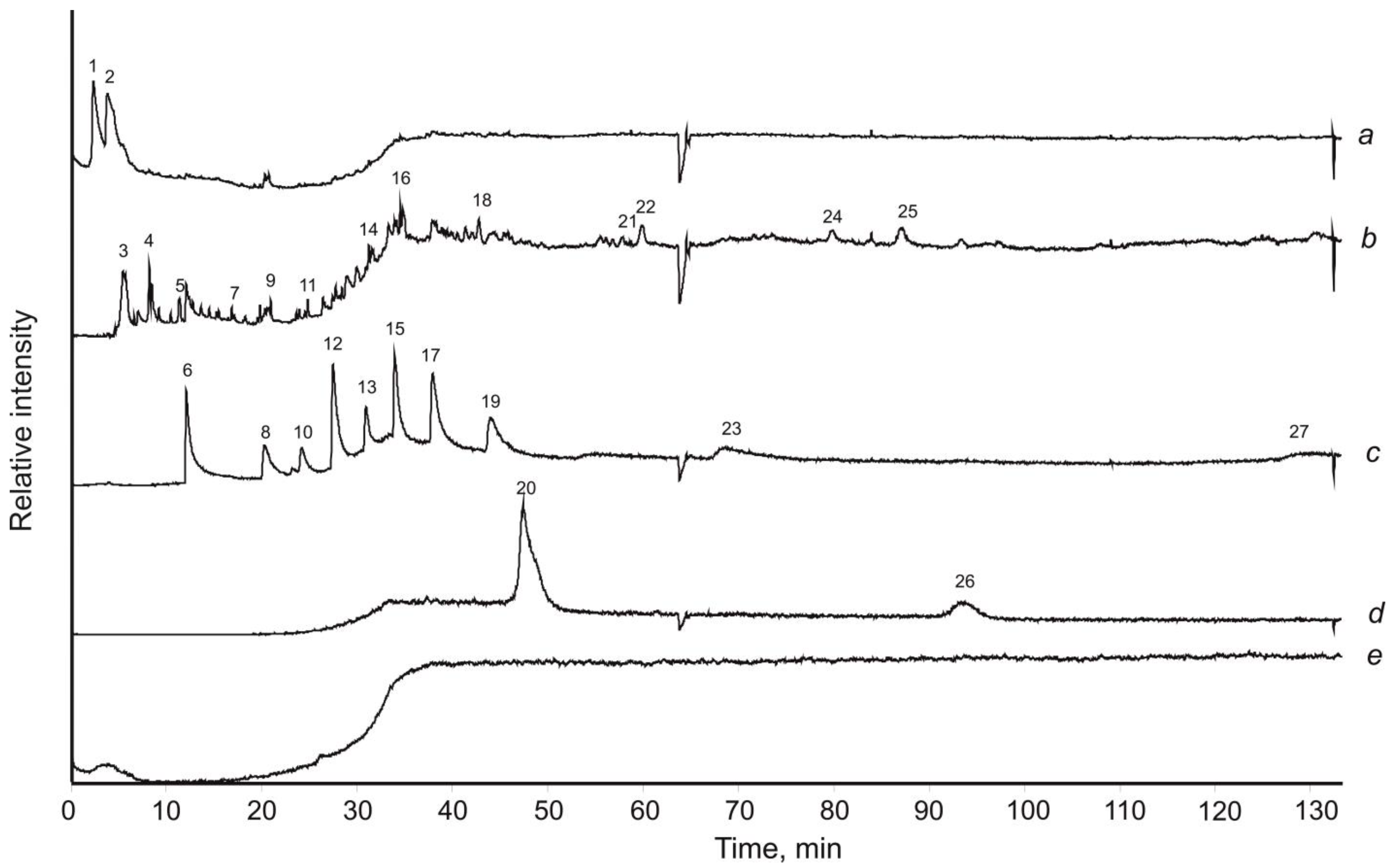


| Component | Sutam Block | Nimnyr Block | ||||||
|---|---|---|---|---|---|---|---|---|
| 99–77 | 173–77 | 174–77 | 176–77 | 77–79 | 39–80 | 42–80 | 78–80 | |
| SiO2 | 48.64 | 49.32 | 49.49 | 48.97 | 48.86 | 48.44 | 48.40 | 48.43 |
| TiO2 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 |
| Al2O3 | 32.93 | 33.23 | 33.34 | 33.02 | 33.04 | 32.87 | 32.71 | 32.75 |
| FeO | 5.23 | 3.71 | 3.38 | 4.16 | 6.55 | 9.93 | 6.64 | 8.09 |
| MnO | 0.02 | 0.05 | 0.03 | 0.04 | 0.20 | 0.08 | 0.17 | 0.03 |
| MgO | 10.69 | 11.51 | 11.72 | 11.26 | 9.77 | 8.10 | 9.92 | 9.09 |
| CaO | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 |
| Na2O | 0.04 | 0.03 | 0.05 | 0.02 | 0.12 | 0.08 | 0.09 | 0.05 |
| K2O | 0.01 | 0.00 | 0.01 | 0.01 | 0.02 | 0.00 | 0.02 | 0.02 |
| Cr2O3 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 |
| Σ | 97.58 | 97.86 | 98.03 | 97.50 | 98.58 | 99.53 | 97.97 | 98.48 |
| Formula for 18 O atoms | ||||||||
| Si | 4.98 | 4.99 | 5.00 | 4.99 | 4.98 | 4.97 | 4.97 | 4.98 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al | 3.97 | 3.97 | 3.97 | 3.97 | 3.97 | 3.97 | 3.96 | 3.97 |
| Fe(II) | 0.45 | 0.31 | 0.29 | 0.35 | 0.56 | 0.85 | 0.57 | 0.69 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.01 | 0.00 |
| Mg | 1.63 | 1.74 | 1.76 | 1.71 | 1.49 | 1.24 | 1.52 | 1.39 |
| Ca | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Na | 0.01 | 0.01 | 0.01 | 0.00 | 0.02 | 0.02 | 0.02 | 0.01 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Σ | 11.04 | 11.02 | 11.03 | 11.03 | 11.04 | 11.05 | 11.06 | 11.05 |
| XMg | 0.78 | 0.85 | 0.86 | 0.83 | 0.72 | 0.59 | 0.72 | 0.67 |
| Band System | Sutam Block | Nimnyr Block | Attribution | |
|---|---|---|---|---|
| A | 385.7 | 385.7 | 385.7 | Lattice, Valent Mg—O(MgO6) + O—Si—O Deformational (SiO4) |
| 422 | 421.4 | 420.5 | ||
| 440.7 | 438.8 | 439.7 | ||
| 488 | 487 | 486 | ||
| B | 564 | 564 | 564 | Valent Al—O (AlO6) + O—Al—O Deformational (AlO4) |
| 579.6 | 579.6 | 579.6 | ||
| C | 618 | 616 | 617 | Deformational (Si,Al)—O—(Si, Al) + (Si,Al) —OH |
| 675 | 674 | 674 | ||
| 704 | 704 | 704 | ||
| D | 750 sh | 748 sh | 750 sh | Valent Al—O (AlO6) |
| 770.5 | 768.6 | 768.6 | ||
| E | 909 | 909 | 909 | Valent Al—O (AlO6) Si—O (SiO6) |
| 961 | 962.4 | 961.4 | ||
| 1027 | 1027 | 1026 | ||
| F | 1143 | 1142 | 1142 | Valent Si—O (SiO6) |
| 1176.5 | 1175.5 | 1175.5 | ||
| 1636 | 1636 | 1636 | ν2 H2O-II | |
| 2348 | 2348 | 2348 | CO2 | |
| Name | MW | Sutam Block | |||
|---|---|---|---|---|---|
| 99–77 | 173–77 | 174–77 | 176–77 | ||
| Aliphatic hydrocarbons | 0.19 | 0.78 | 1.24 | 0.43 | |
| Paraffins (CH4-C17H36) | 16–240 | 0.09 | 0.22 | 0.44 | 0.20 |
| Olefins (C2H2-C17H34) | 26–238 | 0.10 | 0.56 | 0.80 | 0.23 |
| Cyclic hydrocarbons | 0.10 | 0.24 | 0.30 | 0.22 | |
| Cycloalkanes (naphthenes) and cycloalkenes (C3H6-C10H16) | 42–136 | 0.01 | 0.01 | 0.08 | 0.01 |
| Arenes (C6H6-C16H26) | 78–218 | 0.09 | 0.21 | 0.19 | 0.18 |
| PAH (C10H8-C13H18) | 128–174 | 0.01 | 0.01 | 0.03 | 0.03 |
| Oxygenatedhydrocarbons | 0.69 | 1.48 | 3.06 | 1.45 | |
| Alcohols (CH4O-C12H22O2) | 32–198 | 0.05 | 0.08 | 0.34 | 0.11 |
| Esters and ethers (C4H4O2-C16H19FO2) | 84–262 | 0.09 | 0.13 | 0.16 | 0.10 |
| Aldehydes (CH2O-C16H32O) | 30–240 | 0.14 | 0.35 | 1.37 | 0.33 |
| Ketones (C3H6O-C16H32O) | 58–240 | 0.14 | 0.24 | 0.44 | 0.31 |
| Carboxylic acids (C2H4O2-C14H28O2) | 60–228 | 0.27 | 0.68 | 0.75 | 0.60 |
| Heterocyclic compounds | 0.01 | 0.02 | 0.03 | 0.03 | |
| Dioxanes (C4H8O2-C5H10O2) | 88–102 | <0.01 | <0.01 | <0.01 | <0.01 |
| Furans (C4H4O-C15H26O) | 68–222 | 0.01 | 0.02 | 0.03 | 0.03 |
| Nitrogenated compounds (N2-C13H16F3NO) | 28–259 | 0.14 | 0.35 | 0.88 | 0.26 |
| Sulfonated compounds (H2S-C13H22S) | 34–210 | 0.04 | 0.09 | 0.14 | 0.08 |
| CO2 | 44 | 97.55 | 92.97 | 88.59 | 86.71 |
| H2O | 18 | 1.28 | 4.08 | 5.75 | 10.83 |
| Total number of components | 226 | 237 | 261 | 254 | |
| CO2/(H2O + CO2) | 0.99 | 0.96 | 0.94 | 0.89 | |
| H/(H + O) | 0.05 | 0.14 | 0.21 | 0.24 | |
| Name | MW | Nimnyr Block | |||
|---|---|---|---|---|---|
| 39–80 | 42–80 | 78–80 | 77–79 | ||
| Aliphatic hydrocarbons | 4.39 | 2.24 | 1.05 | 1.19 | |
| Paraffins (CH4-C17H36) | 16–240 | 3.33 | 0.72 | 0.18 | 0.58 |
| Olefins (C2H2-C17H34) | 26–238 | 1.06 | 1.52 | 0.87 | 0.61 |
| Cyclic hydrocarbons | 0.59 | 0.66 | 0.56 | 0.81 | |
| Cycloalkanes (naphthenes) and cycloalkenes (C3H6-C10H16) | 42–136 | 0.13 | 0.01 | 0.07 | 0.06 |
| Arenes (C6H6-C16H26) | 78–218 | 0.35 | 0.55 | 0.45 | 0.70 |
| PAH (C10H8-C13H18) | 128–174 | 0.11 | 0.10 | 0.05 | 0.05 |
| Oxygenated hydrocarbons | 3.22 | 7.91 | 2.35 | 5.86 | |
| Alcohols (CH4O-C12H22O2) | 32–198 | 0.71 | 2.47 | 0.30 | 0.73 |
| Esters and ethers (C4H4O2-C16H19FO2) | 84–262 | 0.88 | 0.53 | 0.68 | 0.83 |
| Aldehydes (CH2O-C16H32O) | 30–240 | 0.77 | 2.37 | 0.68 | 1.73 |
| Ketones (C3H6O-C16H32O) | 58–240 | 0.32 | 0.74 | 0.33 | 1.03 |
| Carboxylic acids (C2H4O2-C14H28O2) | 60–228 | 0.55 | 1.80 | 0.37 | 1.54 |
| Heterocyclic compounds | 0.05 | 0.11 | 0.04 | 0.08 | |
| Dioxanes (C4H8O2-C5H10O2) | 88–102 | 0.01 | 0.01 | <0.01 | <0.01 |
| Furans (C4H4O-C15H26O) | 68–222 | 0.04 | 0.10 | 0.03 | 0.07 |
| Nitrogenated compounds (N2-C13H16F3NO) | 28–259 | 1.32 | 1.76 | 1.03 | 1.13 |
| Sulfonated compounds (H2S-C13H22S) | 34–210 | 0.13 | 0.32 | 0.13 | 0.22 |
| CO2 | 44 | 72.40 | 69.63 | 80.04 | 66.86 |
| H2O | 18 | 17.89 | 17.37 | 14.80 | 23.85 |
| Total number of components | 258 | 246 | 261 | 245 | |
| CO2/(H2O + CO2) | 0.80 | 0.80 | 0.84 | 0.74 | |
| H/(H + O) | 0.41 | 0.43 | 0.32 | 0.44 | |
| Component | Yenisei Ridge Kan Series, Granulite Facies | ||||||
|---|---|---|---|---|---|---|---|
| 16–21 a | 16–21 b | 16–21 c | 16–21 d | 16–21 e | 16–21 f | 16–21 g | |
| SiO2 | 48.68 | 48.49 | 48.57 | 48.58 | 48.62 | 48.34 | 48.50 |
| TiO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 |
| Al2O3 | 32.62 | 32.76 | 32.99 | 32.96 | 32.75 | 32.91 | 32.77 |
| FeO | 6.76 | 6.62 | 6.71 | 6.72 | 7.15 | 7.02 | 7.24 |
| MnO | 0.18 | 0.18 | 0.19 | 0.19 | 0.16 | 0.18 | 0.18 |
| MgO | 9.65 | 9.77 | 9.80 | 9.71 | 9.72 | 9.61 | 9.53 |
| CaO | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 |
| Na2O | 0.04 | 0.06 | 0.10 | 0.05 | 0.07 | 0.04 | 0.04 |
| K2O | 0.00 | 0.02 | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 |
| Cr2O3 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 |
| Σ | 97.95 | 97.91 | 98.40 | 98.22 | 98.51 | 98.11 | 98.30 |
| Formula for 18 O atoms | |||||||
| Si | 5.00 | 4.98 | 4.97 | 4.98 | 4.98 | 4.97 | 4.98 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al | 3.95 | 3.97 | 3.98 | 3.98 | 3.95 | 3.98 | 3.96 |
| Fe(II) | 0.58 | 0.57 | 0.57 | 0.58 | 0.61 | 0.60 | 0.62 |
| Mn | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 |
| Mg | 1.48 | 1.50 | 1.49 | 1.48 | 1.48 | 1.47 | 1.46 |
| Ca | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Na | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Σ | 11.03 | 11.04 | 11.05 | 11.04 | 11.06 | 11.05 | 11.05 |
| XMg | 0.71 | 0.72 | 0.72 | 0.71 | 0.70 | 0.70 | 0.70 |
| Band System | Yenisei Ridge, Kan Series, Granulite Facies | Yenisei Ridge, Yenisei Series, Amphibolite Facies | Attribution |
|---|---|---|---|
| 16–21 (a) | 02–06 (a) | ||
| A | 385.7 | 385.7 | Lattice, Valent Mg—O(MgO6) + O—Si—O Deformational (SiO4) |
| 422 | 421.4 | ||
| 440.7 | 438.8 | ||
| 488 | 487 | ||
| B | 564 | 564 | Valent Al—O (AlO6) + O—Al—O Deformational (AlO4) |
| 579.6 | 579.6 | ||
| C | 618 | 616 | Deformational (Si,Al)—O—(Si, Al) + (Si,Al)—OH |
| 675 | 674 | ||
| 704 | 704 | ||
| D | 750 sh | 748 sh | Valent Al—O (AlO6) |
| 770.5 | 768.6 | ||
| E | 909 | 909 | Valent Al—O (AlO6) Si—O (SiO6) |
| 961 | 962.4 | ||
| 1027 | 1027 | ||
| F | 1143 | 1142 | Valent Si—O (SiO6) |
| 1176.5 | 1175.5 | ||
| 1636 | 1636 | ν2 H2O-II | |
| 2348 | 2358 | CO2 |
| Name | MW | Granulite Facies | Amphibolite Facies | |
|---|---|---|---|---|
| 16–21 | PN-1 | 02–06 | ||
| Aliphatic hydrocarbons | 1.21 | 3.68 | 5.04 | |
| Paraffins (CH4-C18H38) | 16–254 | 0.37 | 1.11 | 1.81 |
| Olefins (C2H2-C18H36) | 26–252 | 0.84 | 2.57 | 3.23 |
| Cyclic hydrocarbons | 0.81 | 1.34 | 1.28 | |
| Cycloalkanes (naphthenes) and cycloalkenes (C10H16) | 82 | 0.01 | 0.03 | 0.03 |
| Arenes (C6H6-C14H22) | 78–190 | 0.74 | 1.20 | 1.08 |
| PAH (C10H8-C13H18) | 128–174 | 0.06 | 0.12 | 0.18 |
| Oxygenated hydrocarbons | 7.21 | 7.39 | 10.35 | |
| Alcohols (CH4O-C8H10O2) | 32–138 | 0.77 | 0.89 | 0.89 |
| Esters and ethers (C4H6O2-C13H8ClFO2) | 84–250 | 1.48 | 0.58 | 0.91 |
| Aldehydes (CH2O-C15H30O) | 30–226 | 1.83 | 3.30 | 3.65 |
| Ketones (C3H6O-C15H30O) | 58–226 | 1.49 | 1.15 | 1.85 |
| Carboxylic acids (C2H4O2-C14H28O2) | 60–228 | 1.64 | 1.47 | 3.05 |
| Heterocyclic compounds | 0.05 | 0.19 | 0.26 | |
| Dioxanes (C4H4O-C6H12O2) | 88–116 | 0.01 | 0.04 | 0.06 |
| Furans (C4H4O-C14H24O) | 68–208 | 0.05 | 0.15 | 0.20 |
| Nitrogenated compounds (N2-C16H24FNO) | 28–265 | 1.06 | 0.35 | 3.72 |
| Sulfonated compounds (H2S-C12H20S) | 34–196 | 0.34 | 1.09 | 0.36 |
| CO2 | 44 | 84.25 | 43.55 | 45.07 |
| H2O | 18 | 5.07 | 42.33 | 33.79 |
| Total number of components | 257 | 263 | 273 | |
| CO2/(H2O + CO2) | 0.94 | 0.51 | 0.57 | |
| H/(H + O) | 0.26 | 0.58 | 0.59 | |
| Component | Yenisei Ridge, Yenisei Series, Amphibolite Facies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PN-1 a | PN-1 b | PN-1 c | PN-1 d | PN-1 e | 02–06 a | 02–06 b | 02–06 c | 02–06 d | 02–06 e | |
| SiO2 | 48.89 | 48.77 | 48.73 | 48.75 | 48.41 | 48.65 | 48.65 | 48.78 | 49.04 | 48.81 |
| TiO2 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al2O3 | 32.83 | 33.08 | 32.89 | 32.91 | 32.53 | 33.15 | 32.90 | 33.17 | 33.11 | 33.22 |
| FeO | 6.66 | 6.68 | 6.72 | 6.63 | 6.63 | 6.62 | 6.56 | 6.71 | 6.70 | 6.69 |
| MnO | 0.29 | 0.30 | 0.32 | 0.30 | 0.28 | 0.31 | 0.33 | 0.28 | 0.30 | 0.32 |
| MgO | 9.89 | 9.90 | 9.81 | 9.75 | 9.74 | 9.81 | 9.66 | 9.68 | 9.86 | 9.84 |
| CaO | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| Na2O | 0.11 | 0.12 | 0.13 | 0.12 | 0.10 | 0.10 | 0.14 | 0.08 | 0.10 | 0.12 |
| K2O | 0.02 | 0.00 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 |
| Cr2O3 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Σ | 98.72 | 98.86 | 98.61 | 98.48 | 97.71 | 98.65 | 98.27 | 98.71 | 99.11 | 99.02 |
| Formula for 18 O atoms | ||||||||||
| Si | 4.99 | 4.97 | 4.98 | 4.98 | 4.99 | 4.96 | 4.98 | 4.97 | 4.98 | 4.96 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al | 3.94 | 3.97 | 3.96 | 3.96 | 3.95 | 3.99 | 3.97 | 3.99 | 3.96 | 3.98 |
| Fe(II) | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 | 0.56 | 0.56 | 0.57 | 0.57 | 0.57 |
| Mn | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 |
| Mg | 1.50 | 1.50 | 1.49 | 1.48 | 1.50 | 1.49 | 1.47 | 1.47 | 1.49 | 1.49 |
| Ca | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Na | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 | 0.02 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Σ | 11.05 | 11.06 | 11.06 | 11.05 | 11.05 | 11.05 | 11.05 | 11.04 | 11.05 | 11.06 |
| XMg | 0.72 | 0.72 | 0.71 | 0.71 | 0.72 | 0.72 | 0.71 | 0.71 | 0.72 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zatolokina, K.; Tomilenko, A.; Bul’bak, T.; Popov, N. Fluid Components in Cordierites from Granulite- and Amphibolite-Facies Rocks of the Aldan Shield and Yenisei Ridge, Russia: Evidence from Pyrolysis-Free GC-MS, Raman, and IR Spectroscopy. Minerals 2025, 15, 890. https://doi.org/10.3390/min15090890
Zatolokina K, Tomilenko A, Bul’bak T, Popov N. Fluid Components in Cordierites from Granulite- and Amphibolite-Facies Rocks of the Aldan Shield and Yenisei Ridge, Russia: Evidence from Pyrolysis-Free GC-MS, Raman, and IR Spectroscopy. Minerals. 2025; 15(9):890. https://doi.org/10.3390/min15090890
Chicago/Turabian StyleZatolokina, Ksenia, Anatoly Tomilenko, Taras Bul’bak, and Nikolay Popov. 2025. "Fluid Components in Cordierites from Granulite- and Amphibolite-Facies Rocks of the Aldan Shield and Yenisei Ridge, Russia: Evidence from Pyrolysis-Free GC-MS, Raman, and IR Spectroscopy" Minerals 15, no. 9: 890. https://doi.org/10.3390/min15090890
APA StyleZatolokina, K., Tomilenko, A., Bul’bak, T., & Popov, N. (2025). Fluid Components in Cordierites from Granulite- and Amphibolite-Facies Rocks of the Aldan Shield and Yenisei Ridge, Russia: Evidence from Pyrolysis-Free GC-MS, Raman, and IR Spectroscopy. Minerals, 15(9), 890. https://doi.org/10.3390/min15090890





