Abstract
Municipal sewage treatment plants generate significant amounts of polluting sludge, which demands innovative valorization approaches to support its sustainable recycling. This work aimed to evaluate the valorization potential of sludge from a municipal sewage treatment plant (STP) as an alternative raw material to traditional limestone in red wall tile formulations. For this purpose, four red wall tile formulations were performed with 0%, 5%, 10%, and 15% weight of STP sludge replacing traditional limestone. The tile formulations prepared by the dry process were characterized to determine their chemical and mineral compositions, thermal analysis, and sintering behavior. The red wall tile pieces were manufactured by pressing and firing at temperatures ranging from 1150 °C to 1180 °C. The effects of STP sludge incorporation and firing temperature on the densification behavior and technological properties were investigated. The results indicated that the STP sludge exhibited good chemical compatibility for use in red wall tile formulations. Water absorption values varied between 16.52% and 19.70%, indicating compliance with the red wall tile production (BIII group). These findings demonstrate the valorization potential of STP sludge in red wall tiles, which offers a relevant recycling option for the sanitation sector and the circular economy.
1. Introduction
Nowadays, recycling is a global priority. However, in most countries, several polluting solid wastes with recycling potential require specific research to develop the most suitable approaches for their valorization into new products and/or raw materials in an environmentally sustainable way [1,2,3,4]. Such approaches often present very challenging processes, but they yield relevant benefits, including the conservation of natural resources, improved solid waste management, and reduced environmental degradation. They also play a key role in generating economic value and raising awareness among people, companies, and governments about the urgent need for environmental sustainability on the planet. This scenario includes the sludge generated by municipal sewage treatment plants (STPs).
Large volumes of STP sludge are produced annually worldwide. When disposed of inappropriately, they cause significant damage to the environment and public health. Indeed, STP sludges in their raw form are highly harmful, as they consist of a complex and heterogeneous mixture of inorganic and organic compounds, which can contain large amounts of hazardous substances such as heavy metals and pathogens [5,6,7]. As a result, STP sludges are generally classified as hazardous solid waste by environmental legislation [7]. This scenario makes the eco-friendly disposal of STP sludge very complex and highly challenging from both economic and environmental perspectives. In Brazil, for example, STP sludge is generally subjected to a liming treatment process, transforming it into non-hazardous and non-inert solid waste (class IIA), which can be disposed of in sanitary landfills [8]. However, this method is costly and comes with environmental and health restrictions, making it a limiting factor [9]. Consequently, several approaches for sustainable valorization of the STP sludges have been investigated with promising results, including clay bricks [8,10,11,12,13,14,15,16], cement [13,17], concrete [13,18,19], mortars [20,21], lightweight aggregate [22,23], soil substitute [24], activated carbon [25], and thermal processing [26,27].
Wall tiles are construction materials with high water absorption (>10%) and are widely used in both indoor and outdoor environments. They belong to group BIII of the ISO 13006 standard [28]. The most commonly used technological route for manufacturing dry-pressed wall tiles (group BIII) includes preparing the white- or red-firing ceramic paste, followed by uniaxial pressing and fast single-firing. A highly relevant issue in wall tile manufacture concerns the formulations of the triaxial pastes (clay/carbonate material/filler) used, which are composed of non-renewable natural raw materials, such as kaolin and illite clays, carbonates, quartz, feldspars, and talc, among others, in varying amounts [29,30]. Wall tile formulations contain between 10% and 16% carbonate materials (calcite and dolomite), which are essential for obtaining technological properties, crystalline phases based on calcium aluminosilicate, and a porous microstructure after the firing process. However, the intensive use of non-renewable natural raw materials has become a major concern for the ceramic tile industry in many parts of the world, as it may lead to the total depletion of natural raw material deposits.
A sustainable approach that has been successfully applied to mitigate the depletion of natural raw material deposits used by the ceramic sector involves the partial or total replacement of non-renewable ceramic raw material with renewable solid waste. In this scenario, several types of solid waste were tested in the formulation of wall tile pastes with promising results, including blast furnace slag [31], fine fire clay sanitaryware waste [32], grits waste [33], eggshell waste [34], cement raw mix waste dust [35], and marble waste [36].
STP sludges contain appreciable amounts of oxides such as CaO, Al2O3, Fe2O3, and SiO2 [5,8,16]. Therefore, STP sludges are highly attractive for use in ceramic tile formulations. However, to date, few studies have been carried out aiming at the application of municipal sewage sludges in the manufacture of ceramic tile materials. Jordán et al. [37] studied the effects of adding 0%–10% weight of sewage sludge as a partial replacement for clay in ceramic tile production. The results indicated that the incorporation of sewage sludge tends to increase water absorption and decrease mechanical strength. Zhou et al. [38] produced split tiles fired at 1210 °C using untreated municipal sewage sludge as a replacement for kaolin. An optimal tile formulation was found, consisting of 60% weight crude municipal sewage sludge, 15.2% weight quartz, 20.6% weight feldspar, and 14.2% weight kaolin, with acetic acid as a modifier. The resulting split tiles had a bending strength of 25.5 MPa and a water absorption of 1.14%, meeting the property requirements for fine-grade split tiles according to ISO 13006 [28]. Amin et al. [39] investigated the possibility of using municipal sewage sludge for floor tile production. For this purpose, a commercial floor tile formulation was incorporated with up to 35% weight municipal sewage sludge, pressed at 30 MPa, and fired between 1050 °C and 1150 °C. They found that floor tiles could be produced with a maximum addition of 7% weight sewage sludge fired at 1150 °C (for water absorption < 10%), and 10% weight sewage sludge or 5% weight sewage sludge for floor tiles fired at 1150 °C and 1100 °C, respectively (for water absorption > 10%). Therefore, STP sludges have not yet been effectively examined in the formulation of triaxial wall tile pastes for the manufacture of red-firing wall tiles.
This work aims to investigate the potential for valorization of STP sludge from the southeastern region of Brazil as an alternative raw material in the production of red-firing wall tiles. In particular, this investigation proposes a sustainable approach for transforming STP sludge, which is widely available on a global scale, into a renewable raw material source for the ceramic tile industry, which can contribute positively to the basic sanitation sector, support the circular economy, and advance the sustainable development goals outlined in the UN 2030 Agenda.
2. Materials and Methods
This research is a preliminary experimental work developed on a laboratory scale that analyzed the valorization potential of STP sludge in the manufacture of new red-firing wall tiles. For this purpose, the following raw materials were used: common clay, calcitic limestone, quartz, and STP sludge. Common clay, calcitic limestone, and quartz were acquired from commercial companies. A sewage treatment company located in the southeastern region of Brazil supplied the STP sludge. After collecting, the raw STP sludge used in this work was subjected to an inertization treatment process to eliminate pathogens, following the same procedures described by Areias [8]. Briefly, the inertization treatment consisted of drying of raw STP sludge at 65 °C for 48 h, followed by mixing and homogenizing the dried sludge with 15 wt.% of hydrated lime.
All raw materials were subjected to a beneficiation process that included drying at 110 °C for 24 h, followed by crushing in a porcelain ball mill (model 1A, Gardelin, São Paulo, Brazil) and sieving to a fraction < 200 mesh (<75 µm). Table 1 presents the chemical composition of the raw materials, determined using an energy-dispersive X-ray fluorescence spectrometer (model EDX 700, Shimadzu, Tokyo, Japan). Table 2 summarizes the qualitative mineral composition of the raw materials obtained via X-ray diffraction (XRD) analysis.

Table 1.
Chemical composition of the raw materials (% in weight).

Table 2.
Mineral composition of the raw materials used.
To study the effect of STP sludge incorporation, four ceramic pastes for red-firing wall tiles were developed by replacing conventional limestone with STP sludge, as described in Table 3. The STP sludge-free red wall tile formulation (MIA1 Paste), composed of 70% weight clay, 15% weight limestone, and 15% weight quartz, was used as a reference [33]. The red wall tile formulations described in Table 3 underwent a dry granulation process, homogenization, and moisture control to 7% weight [40].

Table 3.
Compositions of the wall tile formulations used (% in weight).
The chemical composition of the wall tile formulations was obtained using an energy-dispersive X-ray fluorescence spectrometer (model EDX 700, Shimadzu, Tokyo, Japan). Mineralogical analysis of the wall tile formulations was carried out by XRD in a conventional diffractometer (model XRD-7000, Shimadzu, Japan) with Cu-Kα radiation at a scanning speed of 1.5° (2θ)/min and 2θ = 10°–70°. Mineral phases were identified using JCPDS-ICDD cards described in Table 4. Thermal analyses of the ceramic pastes were conducted by differential thermal analysis (DTA) and thermogravimetric analysis (TG) using a simultaneous thermal analyzer (model STA 409E, Netzsch, Selb, Germany) over a temperature range of 25 °C to 1100 °C at a heating rate of 10 °C/min. Dilatometric analysis was performed on a dilatometer (model DIL 402 C, Netzsch, Selb, Germany) in the temperature range of 25 °C to 1200 °C, also at a heating rate of 10 °C/min under an air atmosphere.

Table 4.
JCPDS-ICDD cards used in the identification of the mineral phases of the red wall tile pastes.
Rectangular red wall tile pieces measuring 115.0 mm × 25.4 mm × 7.0 mm were compacted by uniaxial pressing at 35 MPa, dried at 110 °C for 24 h, and finally fired in a laboratory kiln at temperatures of 1150 °C, 1160 °C, 1170 °C, and 1180 °C for 5 min. A heating rate of 30 °C/min was applied, and cooling was performed by thermal inertia, switching off the kiln. Five test pieces were prepared for each wall tile formulation.
In this work, the following physical and mechanical properties of the fired wall tile pieces were determined in accordance with the procedures described in the technical standards: linear shrinkage, apparent density, water absorption, apparent porosity, and flexural strength. Linear shrinkage values were obtained by measuring the length of the dried and fired wall tile pieces, following ASTM C326-09 [41]. The apparent density, water absorption, and porosity were measured according to ISO 10545-3 [42]. The flexural strength was determined by a three-point bending test, as specified in ISO 10545-4 [43], using a universal mechanical testing machine (model 5582, Instron, Norwood, MA, USA).
3. Results and Discussion
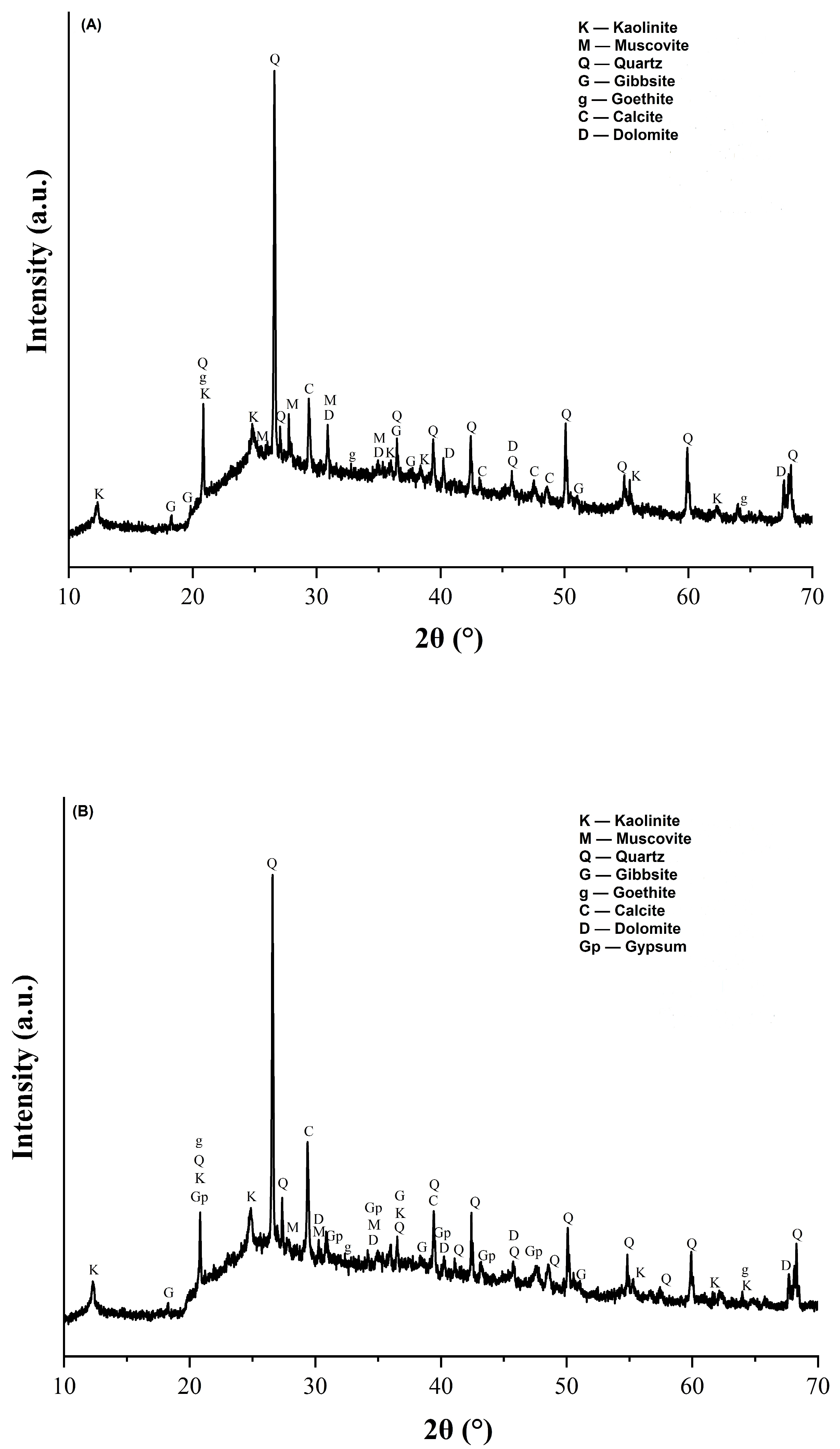
The X-ray diffractograms of the red wall tile pastes are shown in Figure 1. It can be seen in Figure 1A that the mineralogical phases found in the STP sludge-free reference wall tile paste (MIA1 Paste) included kaolinite, muscovite, quartz, gibbsite, goethite, calcite, and dolomite. This mineralogical composition reflects the raw materials used in its paste (Table 2 and Table 3). In the paste containing 10% mass STP sludge (MIA3 Paste), the same mineral phases were identified along with the presence of gypsum, as observed in Figure 1B. Therefore, replacing limestone with STP sludge modifies the mineralogical composition of the red wall tile pastes.
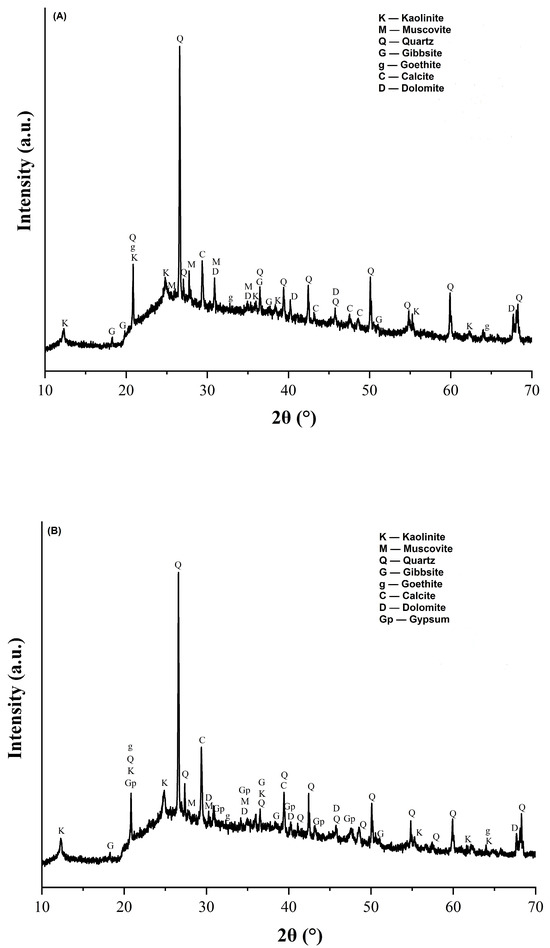
Figure 1.
X-ray diffractograms of the red wall tile pastes: (A) MIA1 and (B) MIA3.
Table 5 presents the chemical analyses and loss on ignition (LoI) of the red wall tile pastes studied. It was found that the incorporation of STP sludge as a replacement for limestone tends to cause small but relevant changes in the chemical composition of the ceramic pastes. The iron oxide content (Fe2O3) in ceramic pastes directly influences the reddish coloration of the produced wall tiles. The effect of incorporating the STP sludge was to provide greater amounts of SiO2, Al2O3, Fe2O3, K2O, and Na2O and a decrease in the amounts of CaO and MgO. Additionally, there was also an increase in the LoI value. This result was consistent with the XRD analysis (Figure 1). Thus, the substitution of limestone with STP sludge is expected to influence the behavior of the technological properties of red wall tiles.

Table 5.
Chemical composition of the red wall tile formulations (% in weight).
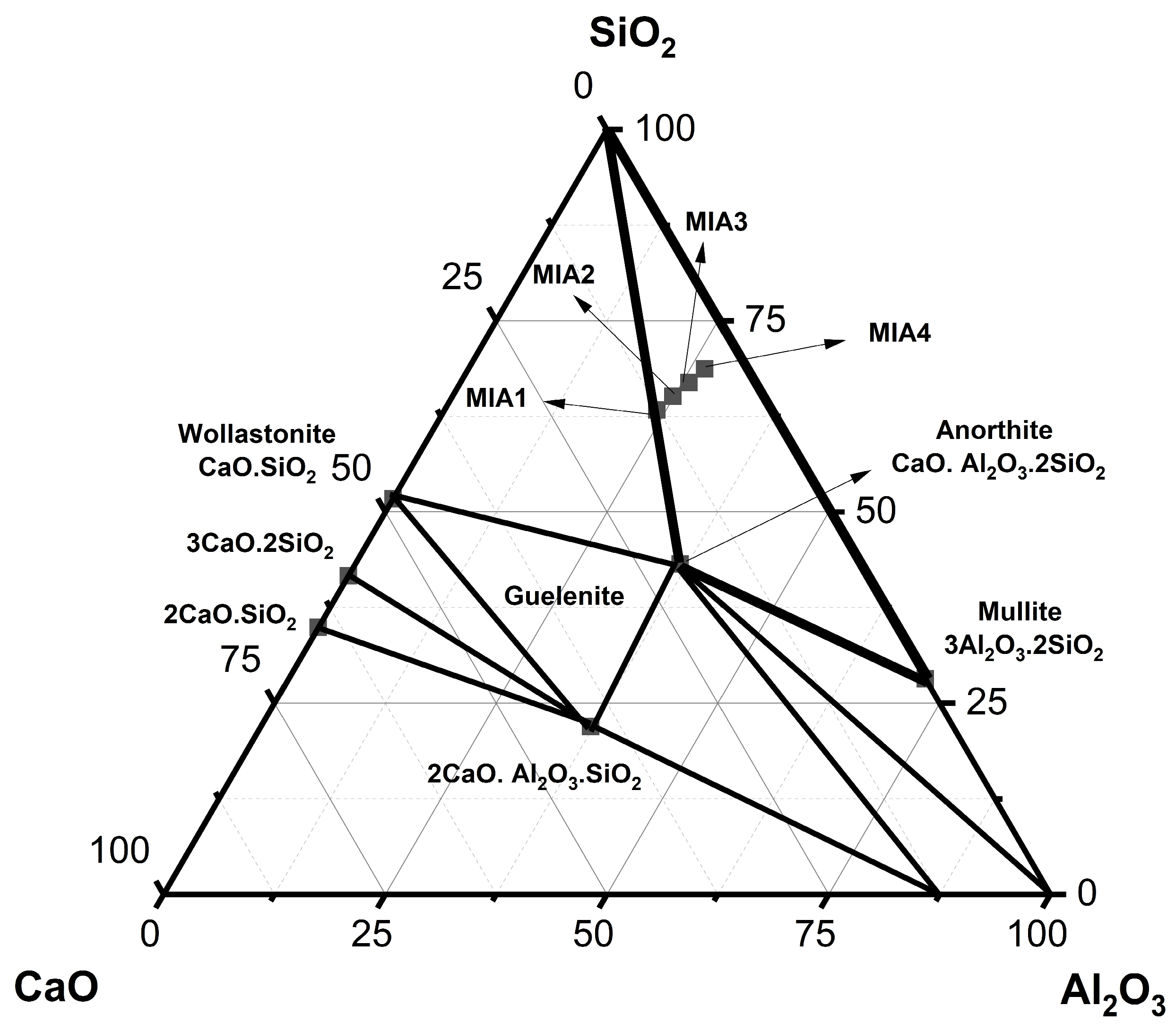
Figure 2 shows the wall tile formulations plotted on the SiO2-Al2O3-CaO phase diagram [44]. For this purpose, only the three main oxides (SiO2, Al2O3, and CaO) described in Table 4 were considered, as they are essential for the formation of the calcium aluminosilicate phases. The MIA1 Paste (STP sludge-free) was positioned at the boundary between two compatibility triangles: quartz–mullite–anorthite and quartz–mullite–wollastonite. It was found that the substitution of limestone with STP sludge moved the MIA2, MIA3, and MIA4 Pastes into the quartz–mullite–anorthite compatibility triangle. This demonstrated good chemical similarity between the wall tile formulations studied. This result is of technological relevance because it indicates the possibility of valorization of STP sludge as a partial or total substitute for natural limestone in wall tile pastes.
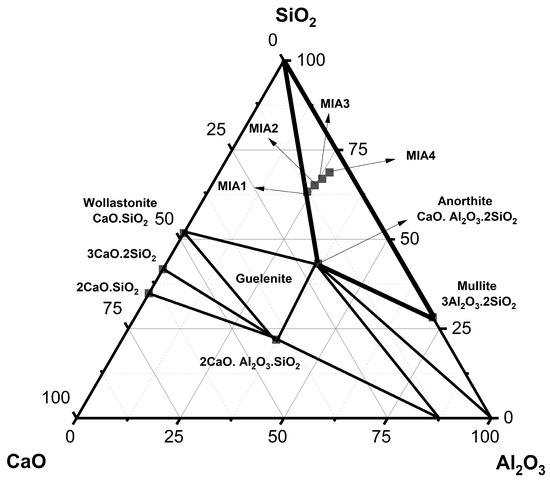
Figure 2.
Representation of the formulation points in the SiO2-Al2O3-CaO phase diagram.
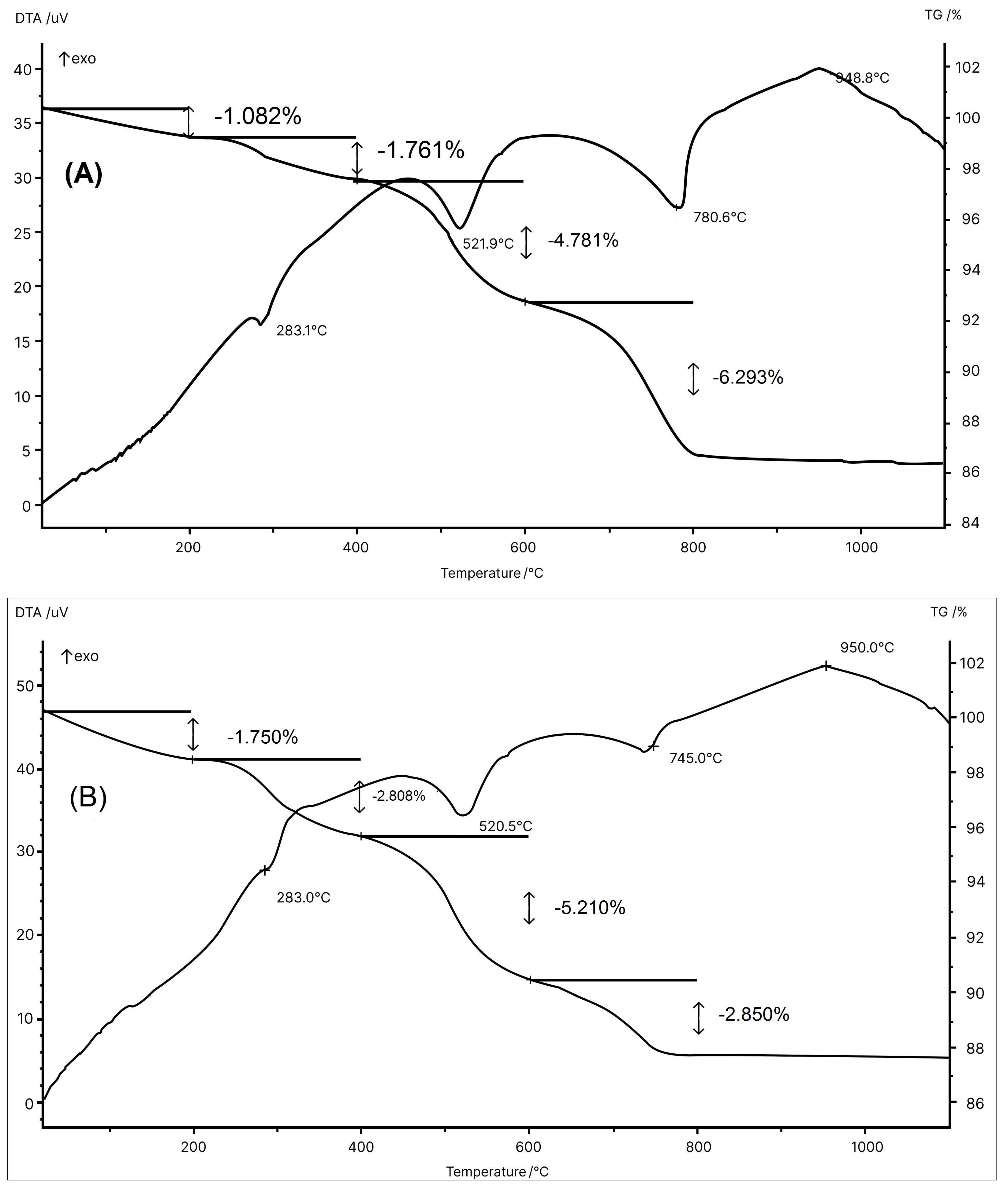
Figure 3 shows the thermal behavior of the MIA1 Paste (STP sludge-free) and the MIA3 Paste (with 10 wt.% STP sludge). Both pastes presented thermal behavior very similar to and typical of ceramic pastes used in wall tile manufacture [45]. The thermal behavior illustrated by the DTA-TG curves in Figure 3 can be described as follows: (a) small endothermic events at ~120 °C with a mass loss of 1.082% (MIA1 Paste) and at ~130 °C with a mass loss of 1.750% (MIA3 Paste) due to the release of physically adsorbed water [33,45]; (b) endothermic events at 283.1 °C with a mass loss of 1.761% (MIA1 Paste) and at 283.0 °C with a mass loss of 2.806% (MIA3 Paste) are related to the dehydration of hydroxides (gibbsite and goethite) [40]; (c) endothermic events at 521.9 °C with mass loss of 4.781% (MIA1 Paste) and at 520.5 °C with mass loss of 5.210% (MIA3 Paste) related to the dehydroxylation of kaolinite to form amorphous metakaolinite [33,40,45]; (d) endothermic events at 780.6 °C with a mass loss of 6.293% (MIA1 Paste) and at 745.0 °C with a mass loss of 2.850% (MIA3 Paste) attributed to the decomposition of carbonates (calcite and dolomite) [46,47,48]; and (e) exothermal events at 948.8 °C (MIA1 Paste) and 950.0 °C (MIA3 Paste) mainly related to the formation of the mullite and calcium aluminosilicate phases [45]. The endothermic event associated with the decomposition of gypsum (CaSO4, calcium sulfate—anhydrous) [49] present in the MIA3 Paste was likely overlapped by the endothermic events of carbonate decomposition (Figure 3B). The total mass losses obtained from the TG curves were 13.917% (MIA1 Paste) and 12.616% (MIA3 Paste). Therefore, these thermal behaviors are in line with the chemical and mineralogical compositions of the wall tile pastes.
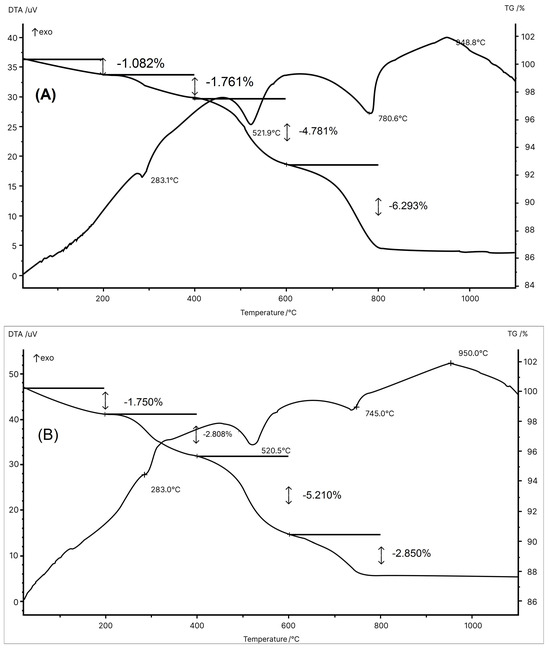
Figure 3.
DTA/TG curves for the pastes: (A) MIA1 and (B) MIA3.
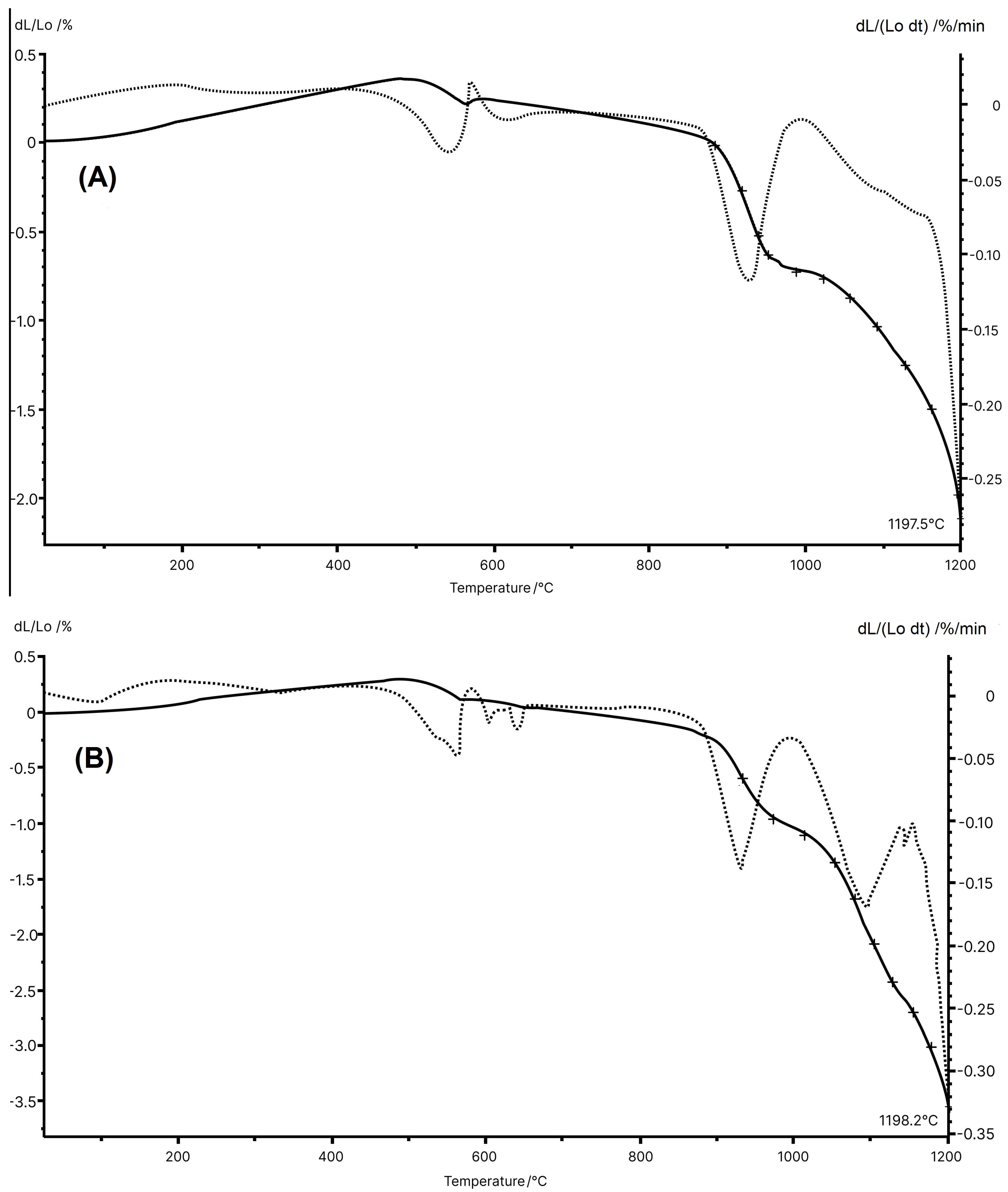
Figure 4 displays the dilatometric curves for the MIA1 Paste (STP sludge-free) and MIA3 Paste (with 10 wt.% STP sludge). The ceramic pastes presented small differences in dilatometric behavior, reflecting their chemical and mineralogical compositions and thermal analysis. However, the incorporation of STP sludge tends to cause greater total firing shrinkage of the red wall tile pieces. It should be noted that both pastes showed slight expansion up to approximately 500 °C, likely due to the thermal expansion of the minerals present in the starting raw materials. Between 500 °C and 580 °C, a small shrinkage was observed due to dehydroxylation of kaolinite. The wall tile pastes showed the beginning of shrinkage at approximately 880 °C (MIA1 Paste) and 890 °C (MIA3 Paste). It was also noted that, from 1055 °C (MIA1 Paste) and 1050 °C (MAI3 Paste), the shrinkage became more pronounced, indicating that sintering was in progress. Finally, it was observed that the temperature of the maximum sintering rate was 1197.5 °C (MIA1 Paste) and 1198.2 °C (MIA3 Paste), respectively.
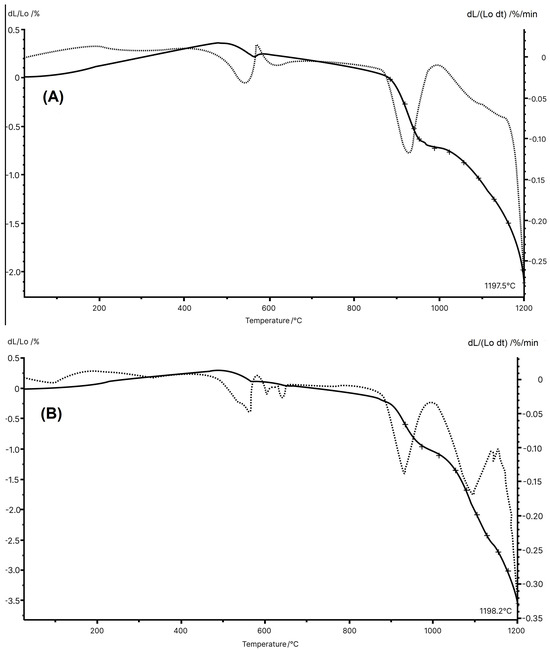
Figure 4.
Dilatometric curves for the pastes: (A) MIA1 and (B) MIA3.
Figure 5, Figure 6, Figure 7 and Figure 8 display the results of the physical properties of the red wall tile pieces as a function of the amount of STP sludge and the firing temperature, whose values are summarized in Table 6.
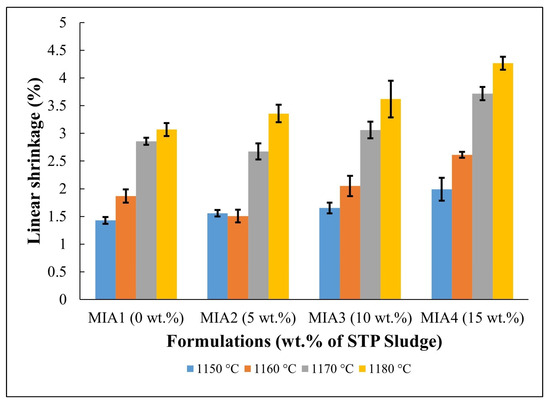
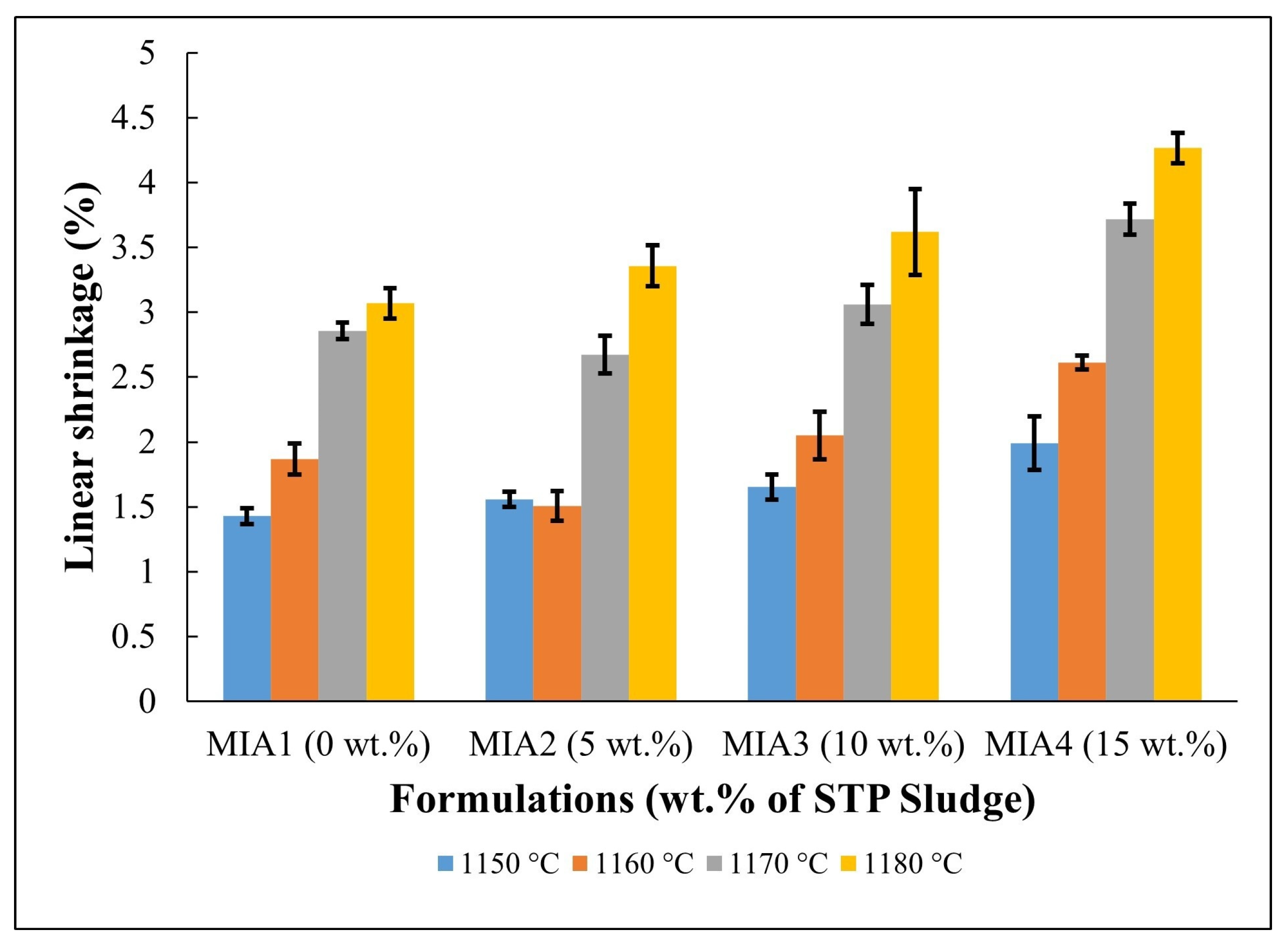
Figure 5.
Linear shrinkage of the fired red wall tile pieces.
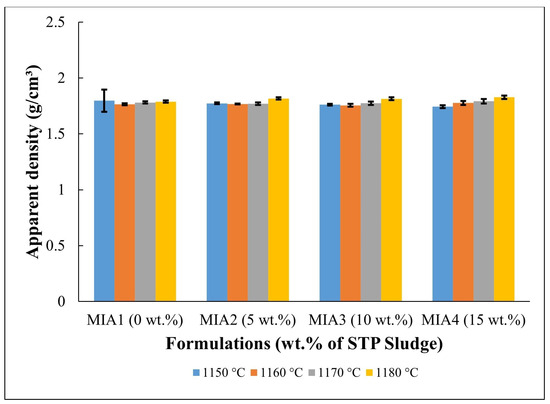
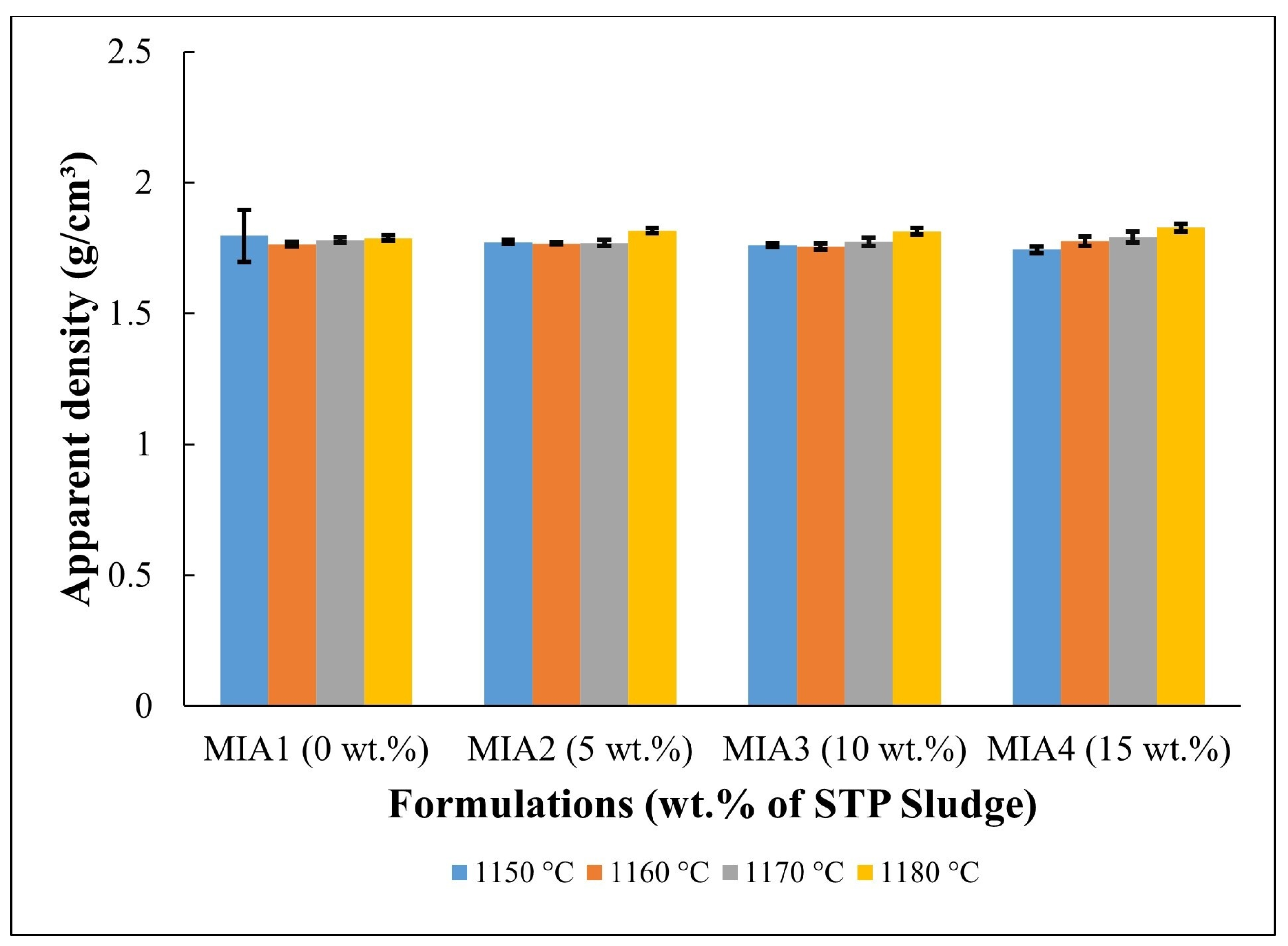
Figure 6.
Apparent density of the fired red wall tile pieces.
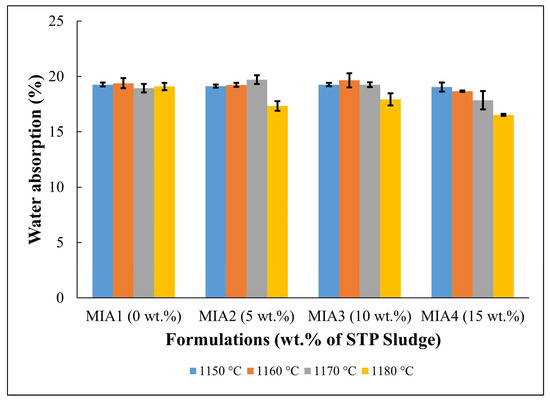
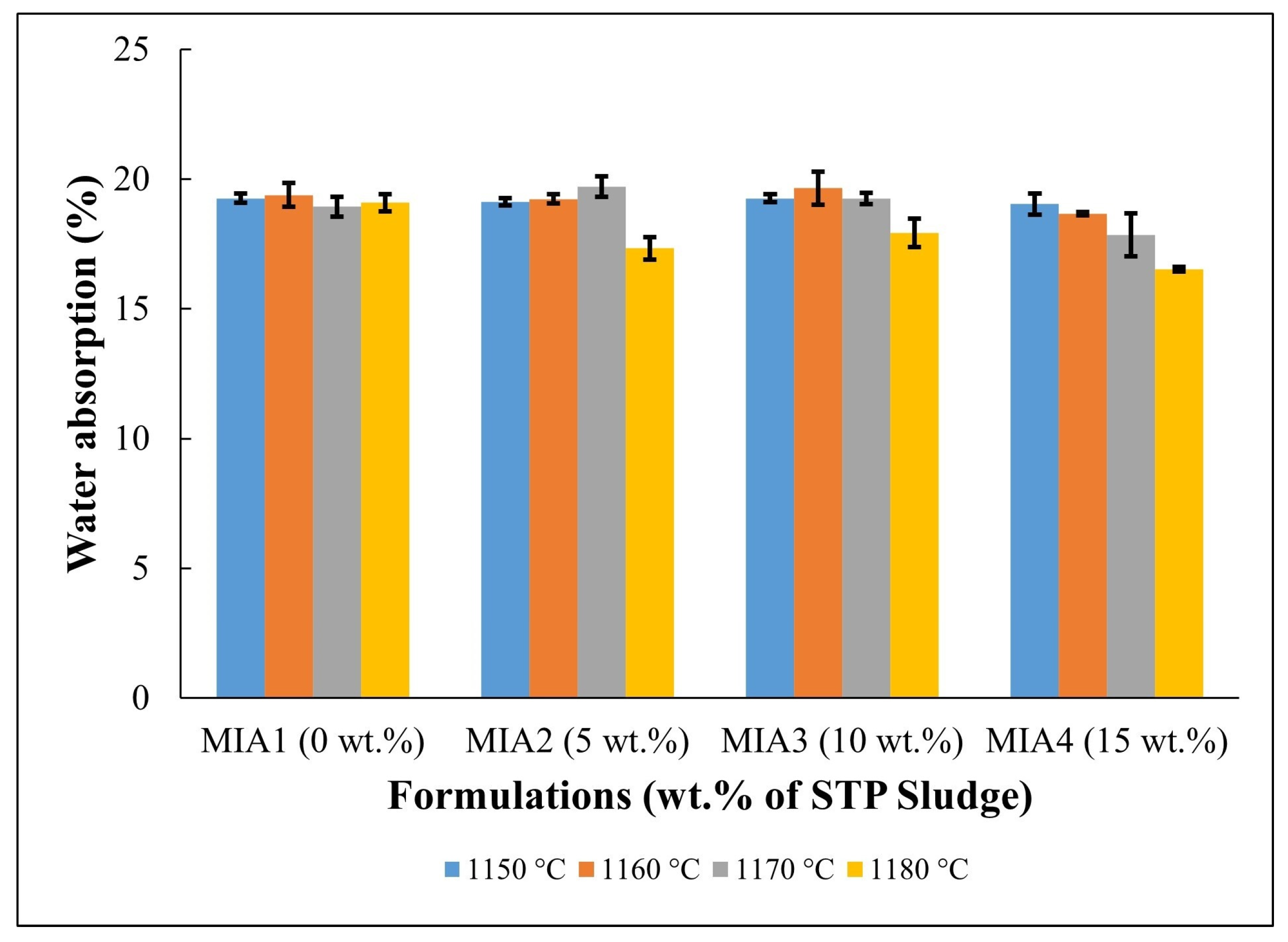
Figure 7.
Water absorption of the fired red wall tile pieces.
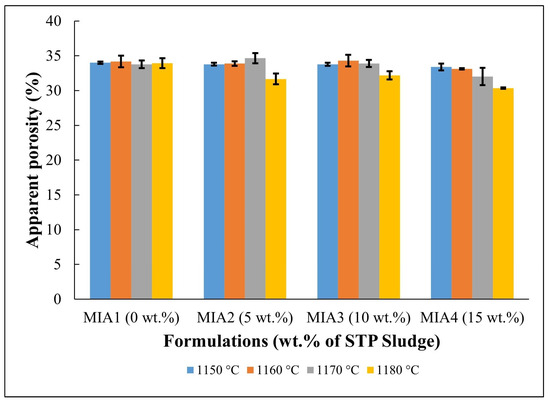
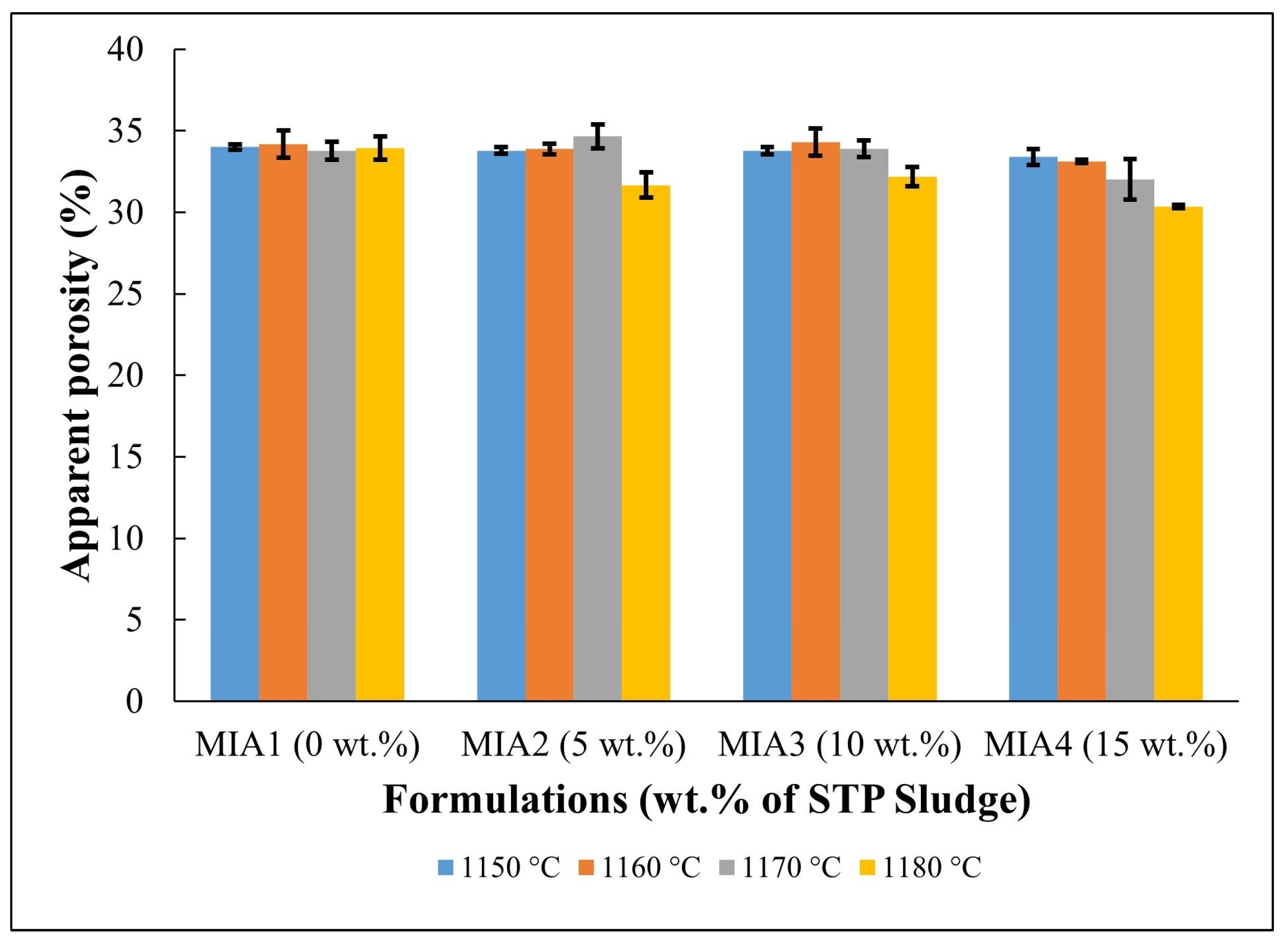
Figure 8.
Apparent porosity of the fired red wall tile pieces.

Table 6.
Values of physical properties of the fired red wall tile pieces.
The linear shrinkage results of the produced red wall tile pieces are shown in Figure 5. Linear shrinkage values ranged from 1.40 to 4.27%, depending on the firing temperature and the amount of STP sludge added. It was found that both the firing temperature and STP sludge contributed to the increase in linear shrinkage. The increase in firing temperature enhances the sintering degree of the red wall tile pieces, particularly above 1160 °C. On the other hand, the incorporation of STP sludge as a substitute for limestone tends to increase the linear shrinkage value due to its chemical composition (Table 1). In fact, as shown in Table 5, the incorporation of STP sludge increases the amount of fluxing oxides (K2O, Na2O, and Fe2O3), which influences sintering behavior. Therefore, the linear shrinkage behavior aligns with the dilatometric curves (Figure 4).
Figure 6 shows the apparent density of the red wall tile pieces. It can be observed that the apparent density of the pieces fired at temperatures between 1150 and 1170 °C presented only a small variation within the dispersion limits (1.74–1.80 g/cm3). At 1180 °C, however, higher apparent density values were achieved (1.79–1.83 g/cm3). This apparent density behavior can be attributed to several simultaneous and competing complex effects occurring during the firing process, including mass loss, mineral decomposition, crystallization of new phases, and sintering, which is typical of wall tile pastes [32,33,34,35,36,45].
Figure 7 displays the water absorption behavior of the red wall tile pieces. The following water absorption values were found: MIA1 Paste (18.93%–19.38%), MIA2 Paste (17.33%–19.70%), MIA3 Paste (17.92%–19.66%), and MIA4 Paste (16.52%–19.04%). The high water absorption values are mainly associated with the decomposition of carbonates and dehydroxylation of kaolinite, as observed in the TG curves in Figure 3, which generate open pores in the fired structure of the red wall tiles. The apparent porosity observed in Figure 8 had a behavior very similar to that of water absorption. Figure 7 also indicates that the red wall tile pieces incorporating STP sludge tend to exhibit lower water absorption values, particularly at higher temperatures. This fact can be explained by the composition of the STP sludge, which provides melting oxides to the ceramic pastes. These oxides influence the sintering behavior of the red wall tile pieces, as seen in the dilatometric curves (Figure 4) and the linear shrinkage (Figure 5).
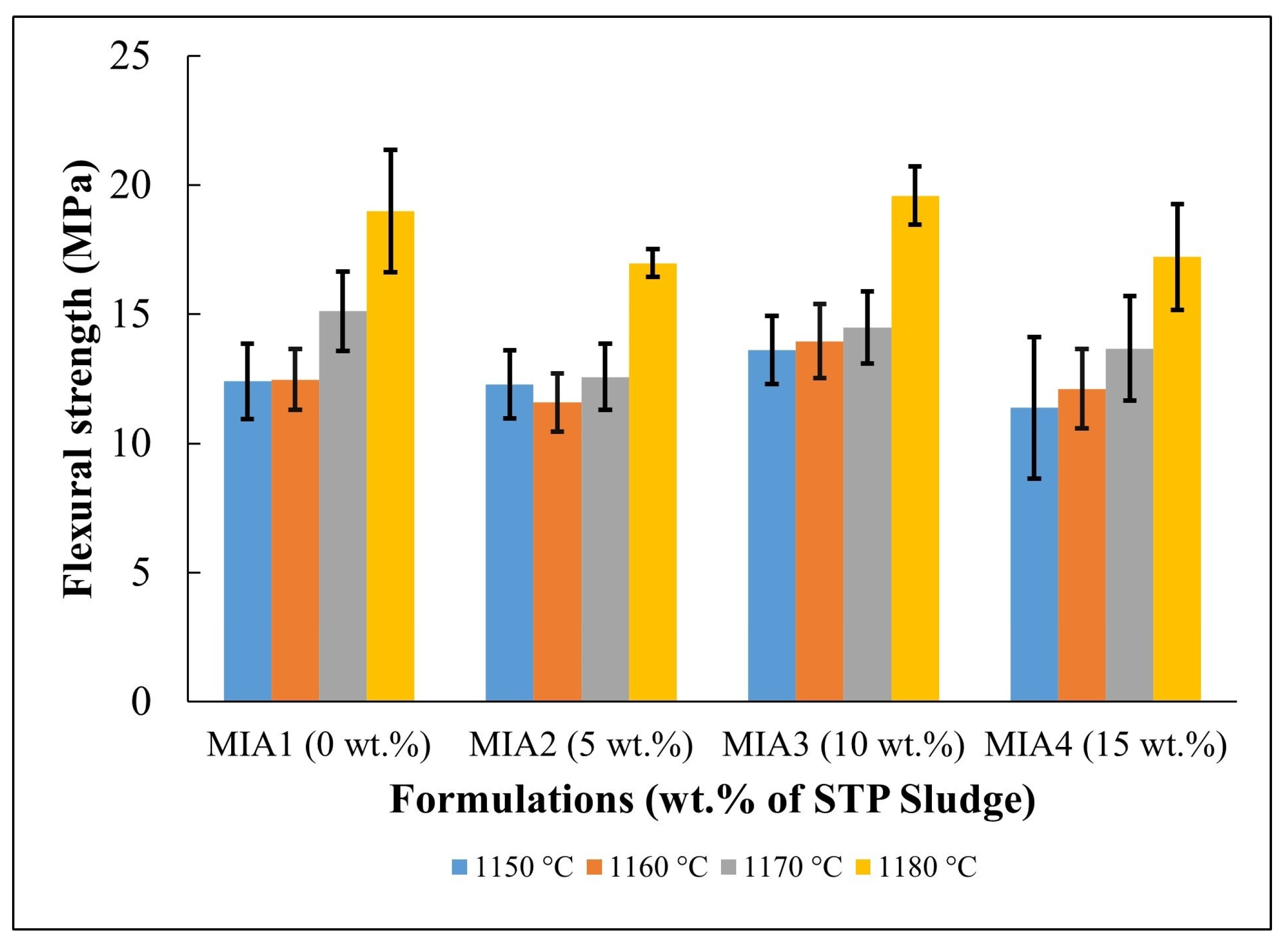
Figure 9 presents the flexural strength behavior of the fired red wall tile pieces. For better visualization, the flexural strength data are summarized in Table 7. Flexural strength values were established in the range of 11.37 to 19.59 MPa, depending on the firing temperature and the amount of STP sludge added. More specifically, it was noted that the flexural strength tended to increase with increasing firing temperature. On the other hand, it was found that at all firing temperatures, the effect of STP sludge incorporation on the flexural strength caused only a small variation within the dispersion limits. In addition, it is suggested that the possibility of formation of the anorthite phase, with greater mechanical strength [50], as described in the phase diagram in Figure 2, may also contribute to increasing the flexural strength of the red wall tiles produced. These findings are well correlated with water absorption (Figure 7) and apparent porosity (Figure 8).
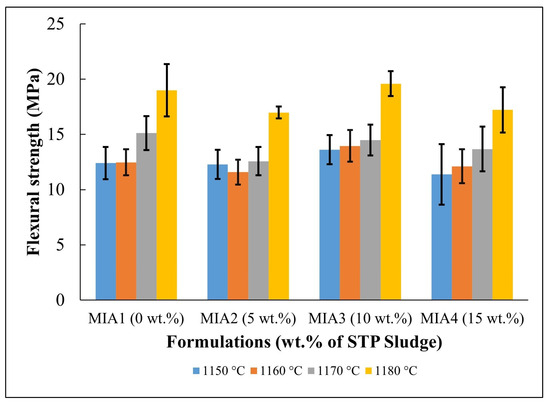
Figure 9.
Flexural strength of the fired red wall tile pieces.

Table 7.
Values of flexural strength of the fired red wall tile pieces (MPa).
In this work, the potential for valorization and practical application of STP sludge in the manufacture of red wall tiles was evaluated based on water absorption values (i.e., the open porosity level of the fired red wall tiles), which correspond to the key physical property for classifying the quality of ceramic tile materials. For wall tiles, the ISO 13006 standard [28] specifies a water absorption value (WA) > 10%, corresponding to classification within the BIII group. According to the water absorption values described in Figure 7, all red wall tile pieces produced fall within the BIII group of the ISO 13006 standard, regardless of firing temperature and the amount of STP sludge incorporated. On the other hand, the ISO 13006 standard [28] also recommends a flexural strength value > 15 MPa for wall tiles with a thickness < 7.5 mm. In this context, based on the flexural strength values described in Figure 9, pieces fired at 1170 °C and 1180 °C are the most favorable for red wall tile manufacture. This result is very relevant, as it indicates that STP sludge can be valorized as a partial or total substitute for conventional limestone in ceramic pastes, enabling the production of red wall tiles with good technical quality.
4. Conclusions
The experimental results indicate that STP sludge can be successfully valorized as a sustainable alternative source of raw material in the development of red wall tiles (BIII group of the ISO 13006 standard). This approach offers a promising sustainable pathway aligned with the principles of the circular economy, contributing to cost reduction and environmental pollution mitigation associated with STP sludge management. It was found that the STP sludge has a complex composition, which influences the chemical and mineralogical compositions, densification behavior, and technical properties of the red wall tiles. The results also show that firing temperatures of 1170 °C and 1180 °C were the most suitable in terms of water absorption and flexural strength for the red wall tile manufacture. Based on these findings, STP sludge can partially or fully replace conventional limestone material in red wall tile pastes (BIII group of the ISO 13006 standard). Therefore, the application of STP sludge for this purpose may be attractive due to its economic and environmental sustainability benefits. Finally, for future research, it is recommended to evaluate the degree of homogeneity of the STP sludge and its quantity, the environmental characterization of inertized STP sludge as secondary raw material, the firing color, the development of the crystalline phases, the microstructural evolution, and the moisture expansion of the red wall tile pieces produced.
Author Contributions
Conceptualization, J.N.F.H.; methodology, I.O.R.A. and J.N.F.H.; validation, I.O.R.A. and J.N.F.H.; investigation, I.O.R.A., F.S.M. and J.N.F.H.; writing—original draft preparation, I.O.R.A. and F.S.M.; writing—review and editing, J.N.F.H.; supervision, J.N.F.H.; funding acquisition, J.N.F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (Grant No. 306147/2023-8) and Foundation for Research Support of the State of Rio de Janeiro (Grant No. E-26/201.137/2022).
Data Availability Statement
The data presented in this research are available in this manuscript.
Acknowledgments
The support provided by the UENF (Recent Doctor Scholarship Program) is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abubakar, I.R.; Maniruzzaman, K.M.; Dano, U.L.; AlShihri, F.S.; AlShammari, M.S.; Ahmed, S.M.S.; Al-Gehlani, W.A.G.; Alrawaf, T.I. Environmental sustainability impacts of solid waste management practices in the global south. Int. J. Environ. Res. Public Health 2022, 19, 12717. [Google Scholar] [CrossRef]
- Peng, X.; Jiang, Y.; Chen, Z.; Osman, A.I.; Farghali, M.; Rooney, D.W.; Yap, P.S. Recycling municipal, agricultural and industrial waste into energy, fertilizers, food and construction materials, and economic feasibility: A review. Environ. Chem. Lett. 2023, 21, 765–801. [Google Scholar] [CrossRef]
- Hamada, H.M.; Shi, J.; Abed, F.; Al Jawahery, M.S.; Majdi, A.; Yousif, S.T. Recycling solid waste to produce eco-friendly ultra-high performance concrete: A review of durability, microstructure and environment characteristics. Sci. Total Environ. 2023, 876, 162804. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Tam, V.W.Y.; Xue, C. Advanced progress in recycling municipal and construction solid wastes for manufacturing sustainable construction materials. Resour. Conserv. Recycl. X 2020, 6, 100036. [Google Scholar] [CrossRef]
- Aadraoui, M.; Elbaghdadi, M.; Rais, J.; Barakat, A.; Ennaji, W.; Karroum, L.A.; Oumenskou, H.; Ouigmane, A.; Mechadi, M.; Didi, S. Characteristics of sewage sludge produced from wastewater treatment plant in the Moroccan city Khouribga. Desalination Water Treat. 2018, 112, 179–185. [Google Scholar] [CrossRef]
- Lin, X.; Chen, C.; Li, H.; Hei, L.; Zeng, L.; Wei, Z.; Chen, Y.; Wu, Q.T. Comprehensive recycling of fresh municipal sewage sludge to fertilize garden plants and achieve low carbon emission: A pilot study. Front. Environ. Sci. 2022, 10, 1023356. [Google Scholar] [CrossRef]
- Silva, J.A. Wastewater treatment and reuse for sustainable water resources management: A systematic literature review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Areias, I.O.R. Environmental and Microstructural Evaluation of Red Ceramic Incorporated with Sludge from a Sewage Treatment Plant (STP). Ph.D. Thesis, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, Brazil, 2019. [Google Scholar]
- Bringhenti, J.R.; Boscov, M.E.G.; Piveli, R.P.; Günther, W.M.R. Co-disposal of sewage sludge in Brazilian sanitary landfills: Technical considerations and minimal criteria for application. Eng. Sanit. Ambient. 2018, 23, 891–899. [Google Scholar] [CrossRef]
- Liew, A.G.; Idris, A.; Wong, C.H.K.; Samad, A.A.; Noor, M.J.M.M.; Baki, A.M. Incorporation of sewage sludge in clay brick and its characterization. Waste Manag. Res. 2004, 22, 226–233. [Google Scholar] [CrossRef]
- Silva, C.R.L.; Chinelatto, A.L.; Chinelatto, A.S.A. Viability of use of sludge from sewage treatment plant in the ceramic mass production of ceramic bricks. Cerâmica. 2015, 61, 31–40. [Google Scholar] [CrossRef]
- Kadir, A.A.; Salim, N.S.A.; Sarani, N.A.; Rahmat, N.A.I.; Abdullah, M.M.A.B. Properties of fired clay brick incorporating with sewage sludge waste. AIP Conf. Proc. 2017, 1885, 020150. [Google Scholar] [CrossRef]
- Chang, Z.; Long, G.; Zhou, J.L.; Ma, C. Valorization of sewage sludge in the fabrication of construction and building materials: A review. Resour. Conserv. Recycl. 2020, 154, 104606. [Google Scholar] [CrossRef]
- Zat, T.; Bandieira, M.; Sattler, N.; Segadães, A.M.; Cruz, R.C.D.; Mohamad, G.; Rodríguez, E.D. Potential re-use of sewage sludge as a raw material in the production of eco-friendly bricks. J. Environ. Manag. 2021, 297, 113238. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, Y.; Yu, L.; Liu, L.; Wang, X.; Zhang, Y. Effect of sewage sludge addition on microstructure and mechanical properties of kaolin-sewage sludge ceramic bricks. Coatings 2022, 12, 944. [Google Scholar] [CrossRef]
- Silva, J.D.S.S.; Lopes, R.L.; Torres, D.M.; Silva, R.D.R. Use of WWTP sludge in ceramic brick production: A systematic literature review. Res. Soc. Dev. 2021, 10, e22010817200. [Google Scholar] [CrossRef]
- Świerczek, L.; Cieślik, B.M.; Konieczka, P. Challenges and opportunities related to the use of sewage sludge ash in cement-based building materials—A review. J. Clean. Prod. 2021, 287, 125054. [Google Scholar] [CrossRef]
- Zegardło, B.; Brzyski, P.; Rymuza, K.; Bombik, A. Analysis of the effects of aggressive environments simulating municipal sewage on recycled concretes based on selected ceramic waste. Materials 2018, 11, 2565. [Google Scholar] [CrossRef]
- Singh, J.; Chaudhary, A.; Dhiman, V.K.; Kumar, A.; Kanoungo, A.; Goyal, A. Impact of dry sewage sludge on characteristics of concrete. Mater. Today Proc. 2022, 52 Pt 3, 818–824. [Google Scholar] [CrossRef]
- Chen, Z.; Chi Sun Poon, C.S. Comparing the use of sewage sludge ash and glass powder in cement mortars. Environ. Technol. 2017, 38, 1390–1398. [Google Scholar] [CrossRef]
- Liang, C.; Le, X.; Fang, W.; Zhao, J.; Fang, L.; Hou, S. The utilization of recycled sewage sludge ash as a supplementary cementitious material in mortar: A review. Sustainability 2022, 14, 4432. [Google Scholar] [CrossRef]
- Franus, M.; Barnat-Hunek, D.; Wdowin, M. Utilization of sewage sludge in the manufacture of lightweight aggregate. Environ. Monit. Assess. 2016, 188, 10. [Google Scholar] [CrossRef]
- Souza, M.M.D.; Anjos, M.A.S.D.; Araújo, A.L.C.; Soares, A.V.D.O.; Souza, P.C.A.D. Use of sewage sludge in the production of light aggregates: A systematic review of literature. Matéria 2020, 25, e-12596. [Google Scholar]
- Artico, M.; Firpo, B.A.; Artico, L.L.; Tubino, R.M.C. Integrated use of sewage sludge and basalt mine waste as soil substitute for environmental restoration. Int. Eng. J. 2020, 73, 225–232. [Google Scholar] [CrossRef]
- Al-mahbashi, N.; Kutty, S.R.M.; Jagaba, A.H.; Al-Nini, A.; Ali, M.; Saeed, A.A.H.; Ghaleb, A.A.S.; Rathnayake, U. Column study for adsorption of copper and cadmium using activated carbon derived from sewage sludge. Adv. Civ. Eng. 2022, 2022, 3590462. [Google Scholar] [CrossRef]
- Rosa, A.P.; Chernicharo, C.A.L.; Melo, G.C.B. Contribution to the energetic recovery of sludge from WWTP in thermal processes. Rev. DAE 2015, 55–62. [Google Scholar] [CrossRef]
- Đurđević, D.; Blecich, P.; Jurić, Z. Energy recovery from sewage sludge: The case study of Croatia. Energies 2019, 12, 1927. [Google Scholar] [CrossRef]
- ISO 13006; Ceramics Tiles—Definitions, Classification, Characteristics and Marking. ISO: Geneva, Switzerland, 2018.
- Barba, A.; Beltran, V.; Felíu, C.; García, J.; Ginés, F.; Sánchez, E.; Sanz, V. Materias Primas Para la Fabricación of Soportes de Baldosas Cerâmicas, 2nd ed.; ITC: Castellón, Spain, 2002.
- Oliveira, A.P.N.; Hotza, D. Tecnologia Para Fabricação de Revestimentos Cerâmicos, 2nd ed.; Editora da UFSC: Florianópoli, Brazil, 2011. [Google Scholar]
- Ozturk, Z.B.; Gultekin, E.E. Preparation of ceramic wall tiling derived from blast furnace slag. Ceram. Int. 2015, 41, 12020–12026. [Google Scholar] [CrossRef]
- Tarhan, M.; Tarhan, B.; Aydin, T. The effects of fine fire clay sanitaryware wastes on ceramic wall tiles. Ceram. Int. 2016, 42, 17110–17115. [Google Scholar] [CrossRef]
- Siqueira, F.B.; Holanda, J.N.F. Application of grits waste as a renewable carbonate material in manufacturing wall tiles. Ceram. Int. 2018, 44, 19576–19582. [Google Scholar] [CrossRef]
- Vilarinho, I.S.; Filippi, E.; Seabra, M.P. Development of eco-ceramic wall tiles with bio-CaCO3 from eggshells waste. Open Ceram. 2022, 9, e100220. [Google Scholar] [CrossRef]
- Aydin, T. The effects of cement raw mix waste dust on thermal properties of ceramic wall tile bodies. J. Mater. Cycles Waste Manag. 2012, 23, 1189–1200. [Google Scholar] [CrossRef]
- Ramos, J.C.R.; Passalini, P.G.S.; Holanda, J.N.F. Utilization of marble waste as a sustainable replacement for calcareous in the manufacture of red-firing wall tiles. Constr. Build. Mater. 2023, 377, 131115. [Google Scholar] [CrossRef]
- Jordán, M.M.; Almendro-Candel, M.B.; Romero, M.; Rincón, J.M. Application of sewage sludge in the manufacturing of ceramic tile bodies. Appl. Clay Sci. 2005, 30, 219–224. [Google Scholar] [CrossRef]
- Zhou, J.; Li, T.; Zhang, Q.; Wang, Y.; Shu, Z. Direct-utilization of sewage sludge to prepare split tiles. Ceram. Int. 2013, 39, 9179–9186. [Google Scholar] [CrossRef]
- Amin, S.K.; Hamid, E.M.A.; El-Sherbiny, S.A.; Sibak, H.A.; Abadir, M.F. The use of sewage sludge in the production of ceramic floor tiles. HBRC J. 2018, 14, 309–315. [Google Scholar] [CrossRef]
- Sousa, S.J.G.; Holanda, J.N.F. Development of red wall tiles by the dry process using Brazilian raw materials. Ceram. Int. 2005, 31, 215–222. [Google Scholar] [CrossRef]
- ASTM C326-09; Standard Test Method for Drying and Firing Shrinkages of Ceramic Whiteware Clays. ASTM International: West Conshohocken, PA, USA, 2018.
- ISO 10545-3; Ceramic tiles—Part 3: Determination of Water Absorption, Apparent Porosity, Apparent Relative Density and Bulk Density. ISO: Geneva, Switzerland, 2018.
- ISO 10545-4; Ceramic Tiles—Part 4: Determination of Modulus of Rupture and Breaking Strength. ISO: Geneva, Switzerland, 2019.
- Jordán, M.M.; Sanfeliu, T.; Fuente, C. Firing transformations of tertiary clays used in the manufacturing of ceramic tile bodies. Appl. Clay Sci. 2001, 20, 87–95. [Google Scholar] [CrossRef]
- Faria, K.C.P.; Holanda, J.N.F. Thermal behavior of ceramic wall tile pastes bearing solid wastes. J. Therm. Anal. Calorim. 2016, 123, 1119–1127. [Google Scholar] [CrossRef]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef]
- Resio, L.C. Dolomite thermal behaviour: A proposal to establish a definitive decomposition mechanism in a convective air atmosphere. Open Ceram. 2023, 15, 100405. [Google Scholar] [CrossRef]
- Zhuang, D.; Chen, Z.; Sun, B. Thermal decomposition of calcium carbonate at multiple heating rates in different atmospheres using the techniques of TG, DTG, and DSC. Crystals 2025, 15, 108. [Google Scholar] [CrossRef]
- Almeida, K.S.; Soares, R.A.L.; Matos, J.M.E.; Almeida, C.S.M.; Almeida, J.S. Incorporation of gypsum residue into a formulation for ceramic adoquim. Cerâmica Ind. 2020, 25, 1–11. [Google Scholar]
- Vodová, L.; Sokovar, R.; Hroudova, J. The effect of CaO addition on mechanical properties of ceramic tiles. Int. Sch. Sci. Res. Innov. 2014, 8, 717–720. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).