Modal mineralogy, elemental assay and deportment, mineral liberation degree, and mineral association and free-surface area analyses, as well as image analyses using both BSE, SEM-EDS and EDS mapping, were conducted to characterize the nepheline syenite rock in detail.
3.1. Modal Mineralogy, Elemental Assay, and Deportment
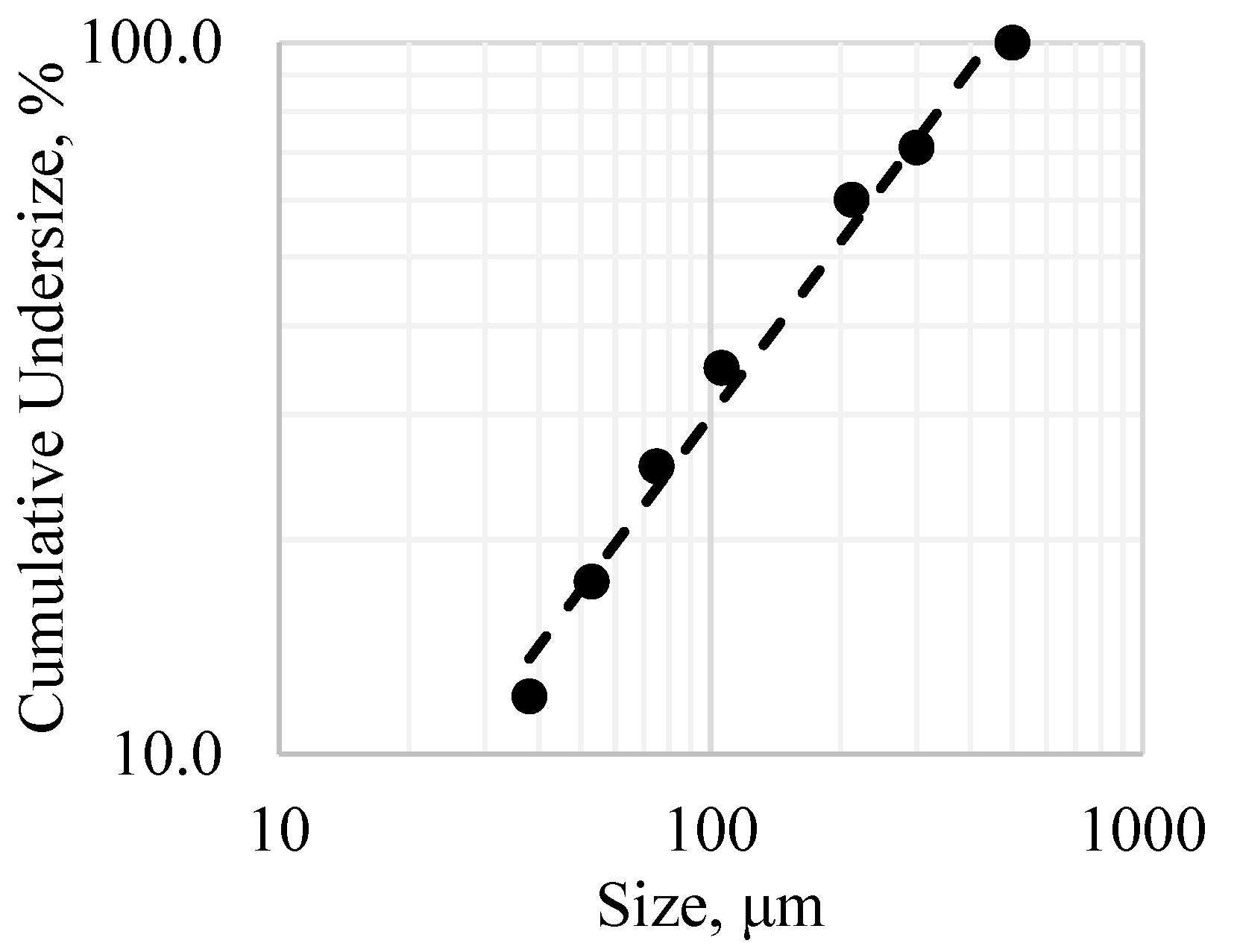
The whole-sample modal mineralogy was calculated by combining the results for individual size fractions, weighted by their relative mass contributions. In
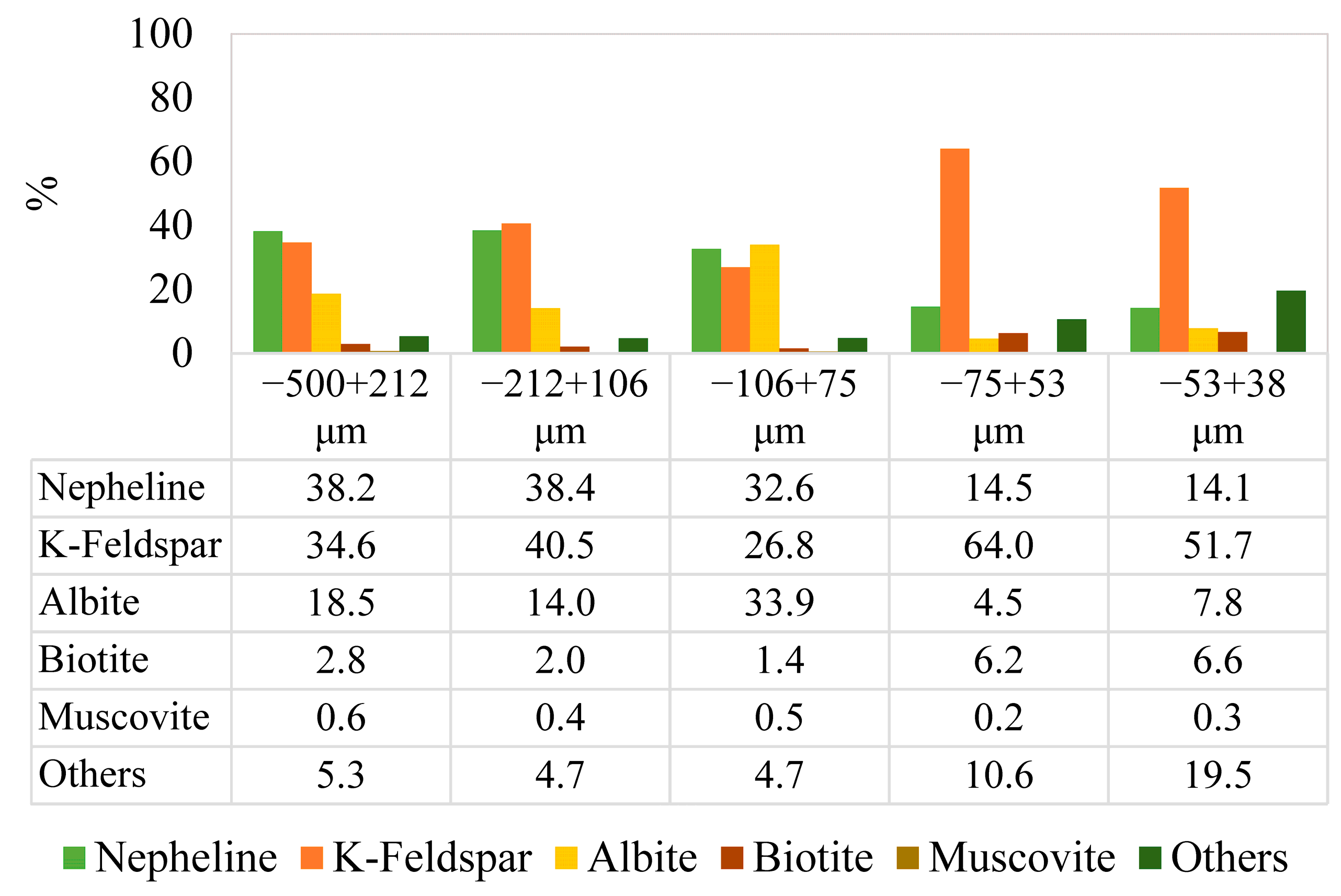
Figure 3, the fractional ratios of the minerals are given, and the fractional bases of the minerals in the different particle size ranges, −500 + 212, −212 + 106, −106 + 75, −75 + 53, and −53 + 38 μm, are illustrated in
Figure 4.
It was detected that K-feldspar was the dominant mineral, accompanied by lower proportions of nepheline, albite, biotite, and other minor minerals.
Due to differences in the breakage characteristics of minerals, the distribution of the mineral phases by weight (%) can differ among particle size fractions. Related to the fractional modal mineralogy analysis, the proportion of biotite increased as the particle size decreased, with values of 2.83%, 1.99%, 1.45%, 6.23%, and 6.62% for progressively finer size fractions. In contrast, the nepheline content showed a declining trend as the particle size decreased, with nepheline contents of 38.15%, 38.43%, 32.61%, 14.46%, and 14.11% through finer size fractions. In
Figure 5, the calculated elemental assay is illustrated. Based on the results, it was observed that the Na% content decreased from 6.06% to 2.57% as the particle size decreased. This trend aligned with the findings from the modal mineralogical analyses conducted for the different size fractions, which revealed that the nepheline content (the primary source of sodium) was higher in coarser size fractions, whereas the biotite content was more prominent in finer sizes. Furthermore, the increase in the Fe% content in finer fractions further supported this observation, as it correlated with the higher concentration of biotite in smaller particle sizes. In
Figure 6a–f, the elemental deportments of K, Al, Fe, Mn, Mg, and Na based on the size fractions and sourced from biotite and other minerals are presented.
Since biotite minerals are composed of biotite (K(Mg,Fe
2+)
3AlSi
3O
10(OH,F)
2), annite (KFe
3+AlSi
3O
10(OH,F)
2), and siderophyllite (KFe
2+Al(Al
2Si
2)O
10(F,OH)
2) [
16], the elemental deportment values for K, Fe, Al, and particularly Mg are presented in the results. The contents of all the elements, except for Mn% and Mg%, are shown in
Figure 5, which illustrates the total elemental contents for each particle size fraction. However, the Mn% contents from the coarsest fraction to the finest fraction were 0.043%, 0.045%, 0.024%, 0.101%, and 0.100%, and the Mg% contents were 0.162%, 0.137%, 0.242%, 0.186%, and 0.237, respectively. Moreover, the elemental assays associated with biotite were calculated using Equation (1), based on the elemental deportment values indicated in
Figure 6a–f and the elemental contents for each particle size fraction demonstrated in
Figure 5. The calculated results are displayed in
Figure 7.
Potassium was primarily sourced from K-feldspar, as confirmed by mineralogical analyses, which revealed the distribution of K within K-feldspar across the particle size fractions. The distributions of potassium from the coarse to fine particle sizes were 66.51%, 70.50%, 64.71%, 86.49%, and 83.51%, respectively. Although biotite inherently contained potassium due to its chemical composition, its contribution to the overall elemental assay and elemental deportment was negligible. Additionally, the distributions of K within nepheline across the particle size fractions from coarse to fine were 29.16%, 26.48%, 31.26%, 7.70%, and 9.08%, respectively.
The primary sources of magnesium in the ore were albite and biotite, with pyroxene and a small amount of muscovite also presented at relatively consistent proportions across all size fractions. The magnesium (Mg) content within albite varied across the particle size fractions, showing values of 0.087%, 0.065%, 0.159%, 0.0212%, and 0.037% from the coarse to fine size fractions, respectively. The presence of magnesium (Mg) within the crystal structure of albite (NaAlSi
3O
8) is typically not attributed to its direct incorporation into the albite lattice but is rather associated with the presence of other coexisting minerals. The identification of minerals that could serve as sources for elements such as iron, titanium, calcium, and magnesium is critical in the characterization of ore deposits containing albite. However, the occurrence of these elements does not necessarily imply their inclusion within the albite crystal structure itself; instead, they can often present in the composition of other minerals coexisting with albite [
17]. However, albite grains generally exhibit negligible levels of magnesium (Mg), consistent with the intrinsic chemical composition of albite, which naturally lacks Mg. The crystal structure of albite is specific to Na and Al ions, and the incorporation of Mg is chemically unfavorable due to ionic radius disparities and charge balance constraints, which induce structural instability. Furthermore, Mg can preferentially be stabilized in coexisting minerals such as pyroxenes rather than within the albite structure. It was recorded that magnesium (with Mg contents typically not exceeding 1%) was found not within albite grains but in pyroxenes associated with albite [
18]. As previously mentioned, minerals such as annite and siderophyllite were closely associated with biotite, and their distinction was directly linked to the magnesium content. Similarly, since the biotite contained trace amounts of manganese (Mn), elemental assay and elemental deportment data for Mn were also obtained. These data played a crucial role in the identification of biotite within the ore. However, as previously highlighted, it was essential to separate the three distinct biotite minerals. The mineral types presented in the nepheline syenite rocks were primarily biotite and annite, with siderophyllite typically not present [
16]. Additionally, although there were no significant differences in the Mg and Mn contents between annite and biotite, the Ti content served as the key differentiating elemental assay. The total elemental assays of titanium were 0.036%, 0.037%, 0.022%, 0.09%, and 0.094% as the particle size decreased. Furthermore, the Ti elemental deportments from biotite were 99.17%, 64.64%, 79.65%, 80.95%, and 87.37%, respectively.
Regardless of the particle size, the primary sources of iron in the ore were iron–spinel and biotite, with minor contributions from Mg-Fe spinel. The characterization of this ore aimed to provide a basis for the accurate design of future beneficiation processes. In this context, the iron content served as a key indicator in chemical analyses for the recovery of biotite, underscoring its critical importance. Furthermore, it also indicated that the biotite was a paramagnetic mineral, which had significant implications for the selection of the appropriate separation methods.
Based on the Al deportment (%) and assay (%), it was evident that aluminum was not a selective elemental component for biotite. The primary sources of Al in the ore were nepheline and K-feldspar. Regarding sodium (Na), regardless of the particle size, its main source was nepheline, followed by albite and, finally, K-feldspar.
For fluorine (F), which was present in the chemical composition of the biotite, the elemental deportment revealed that its distribution was 0% across all size fractions, while the majority (approximately 99–100%) of the fluorine content originated from fluorite. Modal mineralogical analysis revealed the presence of fluorine-containing minerals across all size fractions, including bastnäsite, parisite-Th, apatite, and fluorite. On average, the elemental fluorine distribution in bastnäsite ranged from 0.01% to 0.07%, in parisite-Th it ranged from 0.01% to 0.16%, and in apatite it ranged from 0.19% to 0.65%. In contrast, the fluorine distribution in fluorite was significantly higher, ranging from 99.27% to 99.72% across all size fractions.
3.2. Mineral Liberation Degree, Mineral Associations, and Free-Surface Area
The mineral locking and mineral association data obtained through MLA analysis provided critical insights into the distribution and occurrence of associated minerals (e.g., gangue), facilitating a more accurate assessment of their grades. This information plays a pivotal role in optimizing mineral beneficiation processes by enabling more effective separation processes and enhancing the overall process efficiency [
12]. The particle size fractions examined in the liberation study were −500 + 212, −212 + 106, −106 + 75, −75 + 53, and −53 + 38 μm. Within these size fractions, the proportions of biotite mineral particles that presented as liberated and binary-, ternary-, and complex-associated were analyzed and were depicted in
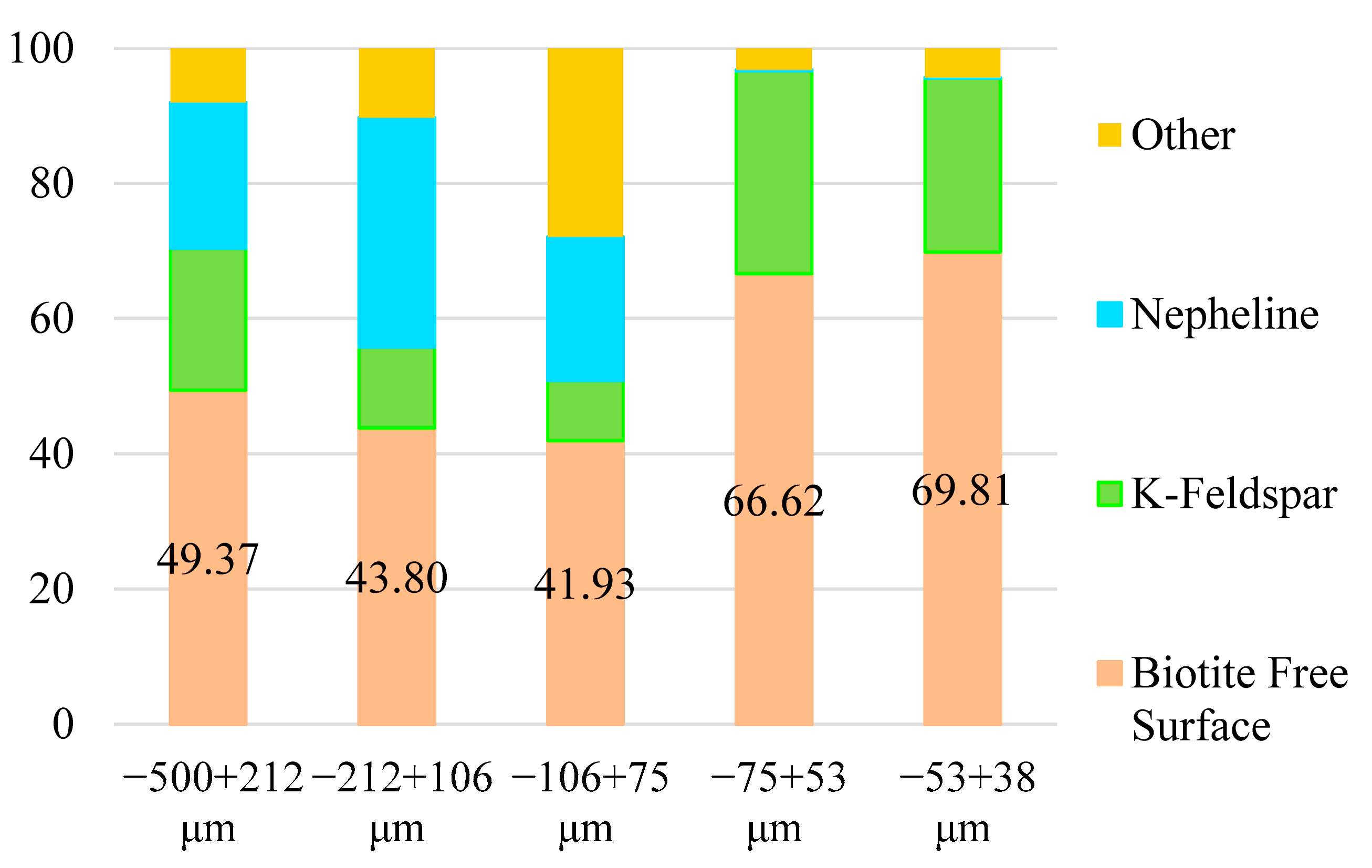
Figure 8. Moreover, the mineral associations and free surface of the biotite were illustrated in
Figure 9.
The particle liberation tests revealed that as the particle size decreased, the proportion of liberated biotite increased, peaking in the −75 + 53 µm size fraction. Consistent with the results of the XRPD and modal mineralogy analyses, the biotite was primarily associated with K-feldspar and nepheline. Typically, biotite occurred in binary locked with K-feldspar, as well as in ternary and complex locked structures with both K-feldspar and nepheline. Although the degree of liberation increased with the decreasing particle size, the mineral association and free-surface ratio analyses indicated that biotite was predominantly associated with K-feldspar rather than with nepheline. While the proportion of ternary and complex structures decreased as the particle size diminished, the fraction of binary associations involving biotite and K-feldspar increased, except for the −75 + 53 µm size range, where biotite liberation reached its maximum. Additionally, the free-surface area of biotite increased with finer particle sizes, while its association with nepheline decreased, confirming that biotite was mostly associated with K-feldspar. The free-surface areas of K-feldspar and nepheline in the different size fractions progressed as follows: 21.35%, 44.88%, 55.24%, 90.42%, and 97.51% for K-feldspar, and 47.95%, 79.43%, 67.04%, 95.23%, and 99.37% for nepheline, as the particle size decreased from coarser to finer size fractions. In addition, the liberation rates of K-feldspar progressively increased from coarse to fine particle sizes, with values of 46.75%, 69.20%, 65.88%, 98.66%, and 98.64%, respectively. Similarly, the liberation rates for nepheline were 68.03%, 89.33%, 81.12%, 94.81%, and 98.37% across the same size fractions. This trend demonstrated a significant enhancement in the liberation of K-feldspar and nepheline as the particle size decreased, indicating reduced mineral interlocking with biotite. Notably, for the −75 + 53 µm and −53 + 38 µm size fractions, the liberation of nepheline was of minimal relevance, as biotite exhibited negligible mineral associations with nepheline in these fractions. The level of liberation achieved during comminution processes plays a critical role in determining the efficiency of the subsequent mineral processing operations. Assessing the liberation properties of zinnwaldite is vital for optimizing comminution methods. As highlighted, while its liberation was essential and dependent on the particle size, the magnetic separation stage served as the primary limitation in its beneficiation. Their findings emphasized that prioritizing the optimization of the separation process was the key to enhancing zinnwaldite recovery, with additional potential for improving the liberation of coarser particles [
13]. However, Sandmann and Gutzmer [
12] asserted that, although the study did not provide a specific target for the degree of liberation, optimization efforts should have been primarily concentrated on refining the separation process rather than on further enhancing the liberation degree.
3.3. SEM-BSE and Energy-Dispersive X-Ray Spectroscopy (EDS)
Imaging was conducted using both the BSE and MLA techniques, focusing on coarser particles (−500 + 212 µm and −212 + 106 µm), where the imaging quality was higher. EDS mapping was made for a more representative assessment of the elemental composition. In
Figure 10, the SEM-BSE images of the representative samples and the EDS spectra corresponding to the particles in these images are presented. Moreover, individual EDS sum spectra of albite, nepheline, K-feldspar, and biotite are given in
Figure 11. EDS spectra were not obtained from a single point; instead, analyses were performed on all particles within the size fractions. Moreover, EDS mapping was conducted for each particle size fraction and is illustrated in
Figure 12, along with SEM-BSE images of all particles in each size fraction.
In
Figure 10, the images illustrate the presence of liberated biotite particles (
Figure 10a) and binary associated particles with nepheline within the −500 + 212 µm size fraction (
Figure 10b). Similarly, within the −212 + 106 µm size fraction, a binary association between albite and biotite was revealed (
Figure 10c). However,
Figure 10d–f also present the corresponding EDS spectra of the particles visualized in the SEM-BSE images.
In
Figure 10a–c, the analysis confirms that the attached particle comprised free biotite, nepheline–biotite, and albite–biotite. The EDS spectrum presented in
Figure 10d further demonstrates that the particle illustrated in
Figure 10c consisted entirely of biotite, with a possible modal composition of 89.86% annite and 10.14% siderophyllite. This finding substantiated the identification of the particle as biotite, with a dominant annite component, attributed to its closer structural and compositional affinity with biotite compared to siderophyllite. Furthermore, the presence of nepheline and albite attached to biotite was corroborated by the EDS spectra provided in
Figure 10e–f.
Figure 11 illustrates the total EDS spectra of the major minerals present in the ore along with the weight percentages of the elements constituting these minerals. For example,
Figure 11a indicates that the nepheline mineral consisted of 44.96% oxygen, 11.30% sodium, 17.99% aluminum, 21.14% silicon, and 54.61% potassium by weight. Similar characterizations were conducted for all minerals and are presented in
Figure 11a–d.
In
Figure 11d, the elemental composition of biotite is detailed, which includes 0.19% hydrogen, 42.51% oxygen, 0.55% sodium, 2.26% magnesium, 8.67% aluminum, 19.03% silicon, 7.64% potassium, 1.27% titanium, 1.51% manganese, and 16.35% iron. Despite the lithium (Li) content being extremely low and not visible in the EDS spectra, biotite was characterized as the only lithium source in the ore. Thus, it was inferred that the lithium-bearing mineral in the nepheline syenite was likely to be zinnwaldite, based on the presence of lithium in the biotite. Both the degree of liberation presented in
Figure 8 and the EDS spectra associated with biotite shown in
Figure 11d serve as valuable key factors for the design of future beneficiation processes. These data provide critical insights that can guide the development and optimization of processing strategies in subsequent studies. Geological studies have indicated that both zinnwaldite and biotite are phyllosilicate minerals, which can coexist in certain geological formations. The structure of phyllosilicates allows for cation exchanges within their tetrahedral and octahedral layers, which contain varying levels of hydroxyl groups. Furthermore, these cation exchanges also take place within the interlayer sites of mica minerals, contributing to their unique chemical properties [
19,
20,
21,
22,
23]. However, lepidolite, one of the lithium minerals, is a mica-type mineral, and its geological structure is similar to that of zinnwaldite. The key distinguishing factor, however, lies in the impurities present in zinnwaldite, primarily iron oxide and manganese oxide. These impurities differentiate zinnwaldite from lepidolite despite the structural similarities between the two minerals [
24].
In coarser size fractions, particularly in the −500 + 212 µm and −212 + 106 µm size ranges, nepheline appeared more concentrated. As the particle size decreased, the particle liberation increased, with albite becoming more dominant. Further reduction in the size revealed a stronger concentration of K-feldspar compared to nepheline. Additionally, the biotite mineral, initially less frequent in coarser fractions, exhibited a progressive increase in concentration as the particle size decreased. This trend was consistently confirmed through fractional modal mineralogy analysis, with EDS mapping highlighting biotite’s intensification across finer fractions due to the color contrast observed.
To conclude, in this study, the chemical, mineralogical, and physical characteristics of nepheline syenite rocks were investigated in detail. The behavior of the constituent minerals, including albite, K-feldspar, nepheline, and biotite, was comprehensively characterized. Biotite was identified as the target mineral, either due to its role as a host mineral for lithium or as a result of its partial geological transformation into zinnwaldite. Since biotite, a mica mineral, is currently discarded as waste during nepheline syenite production, its beneficiation presents a viable opportunity for lithium recovery. This approach would not only reduce the amount of industrial waste but also enable the recovery of a valuable byproduct, thereby contributing to the supply of raw materials for electric vehicle battery production from a potential domestic source.
Due to the fact that biotite is the only paramagnetic mineral in the ore, magnetic separation can be applied to produce a pre-concentrate. In a related study carried out with the same rock type, both the dry and wet high-intensity magnetic separation methods were tested with different devices, and even the effects of the particle size fractions were thoroughly examined [
25]. It was found that dry magnetic separation played a critical role in achieving the highest lithium content in the concentrate. However, physical beneficiation processes alone are generally insufficient to achieve high process efficiency. Therefore, combining magnetic separation with flotation as a physicochemical process can significantly improve lithium recovery. For this reason, the magnetic product can be subjected to conventional flotation to produce lithium-enriched concentrates with higher grades in further studies.