Mineral-Based Synthesis of CuFe2O4 Nanoparticles via Co-Precipitation and Microwave Techniques Using Leached Copper Solutions from Mined Minerals
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of CuSO4 Solution from Minerals
2.3. Synthesis of CuFe2O4 Nanoparticles
3. Characterizations
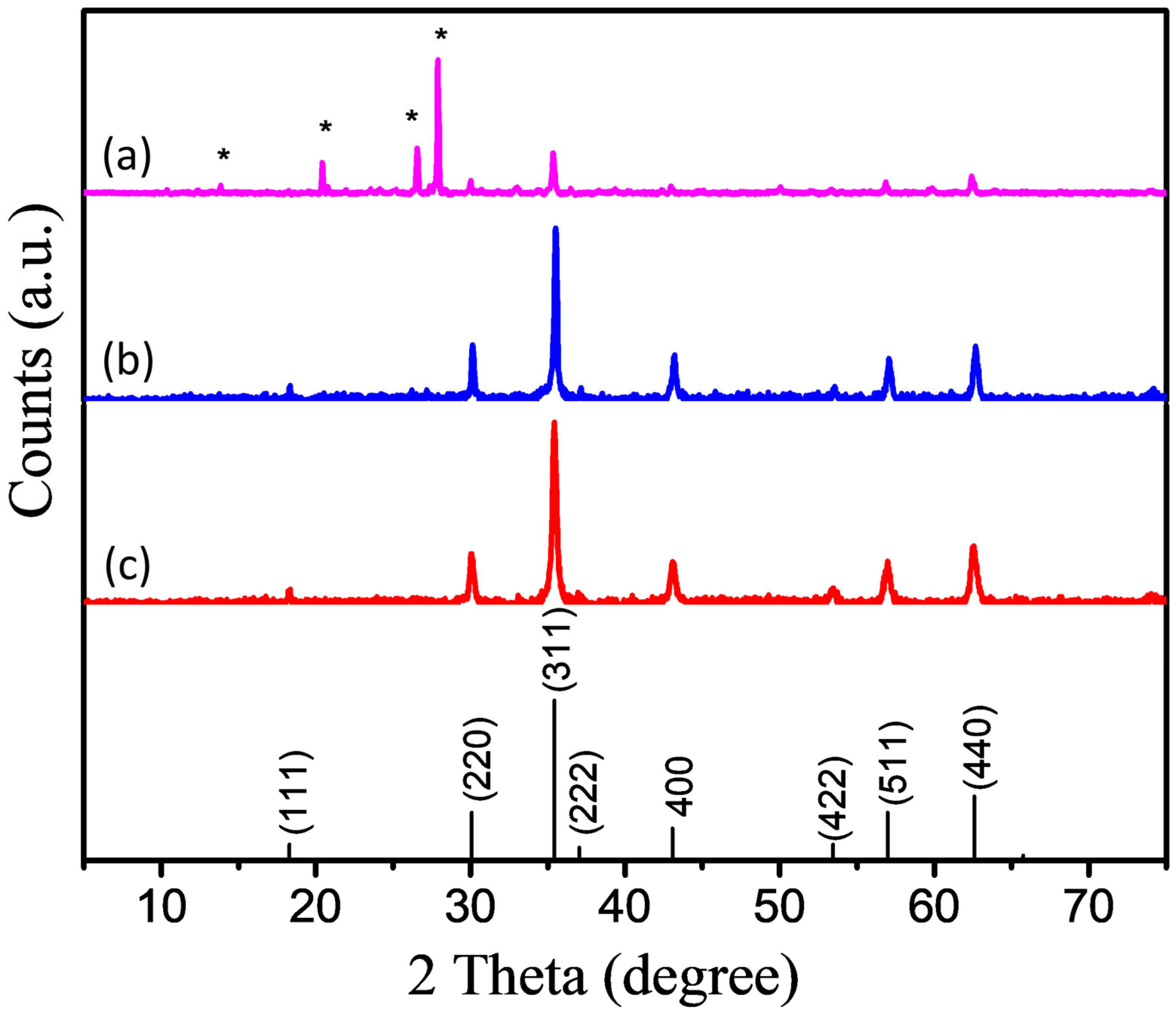
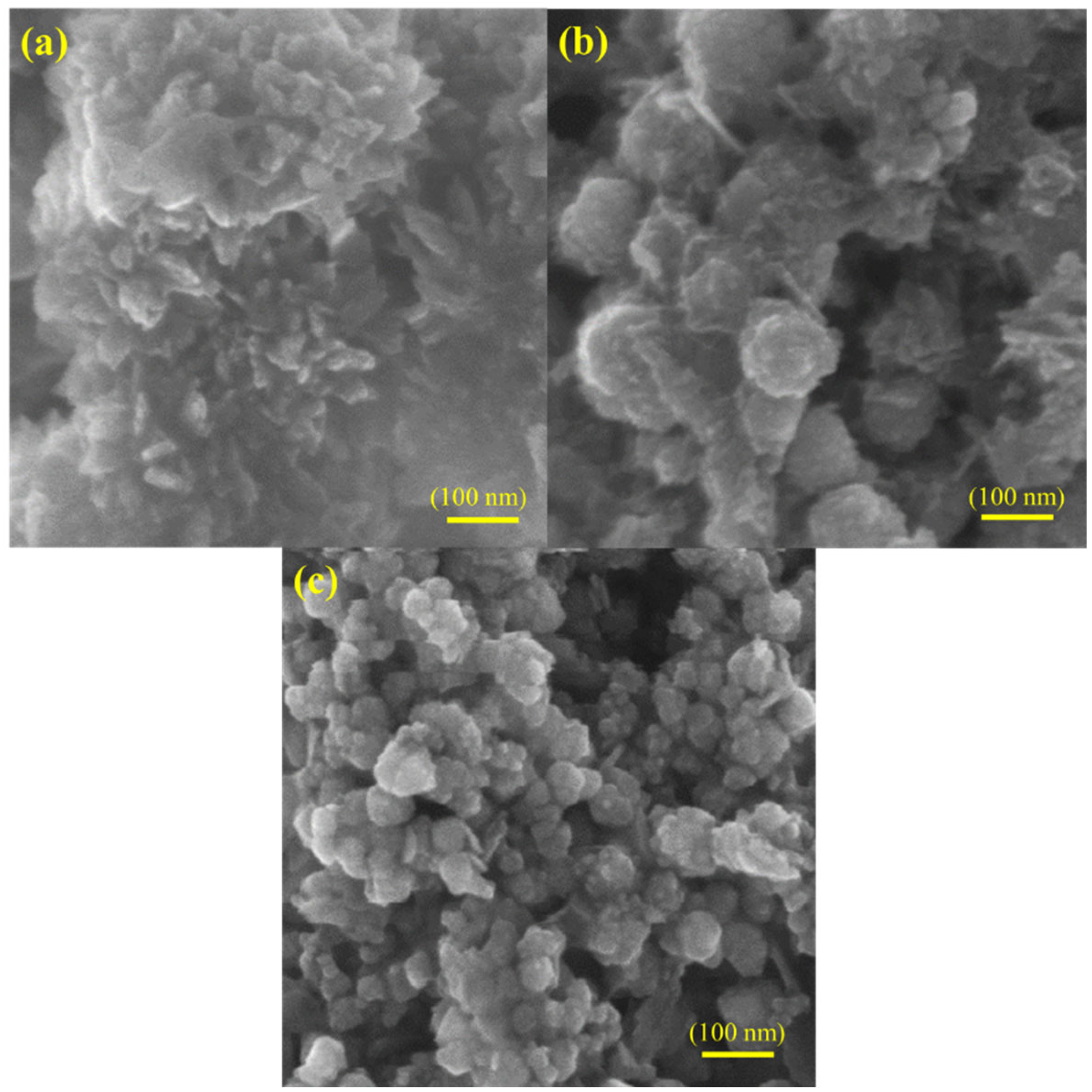
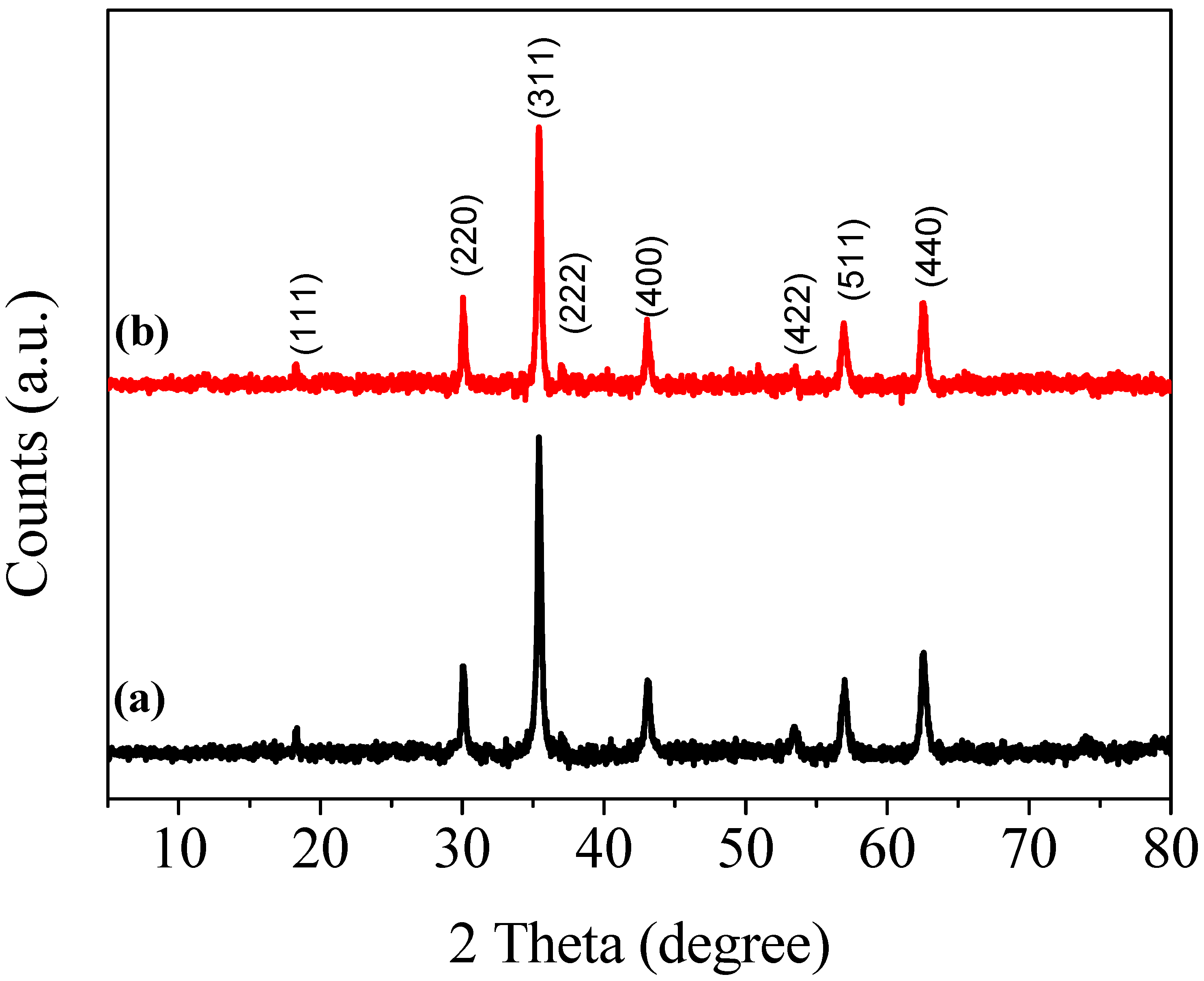
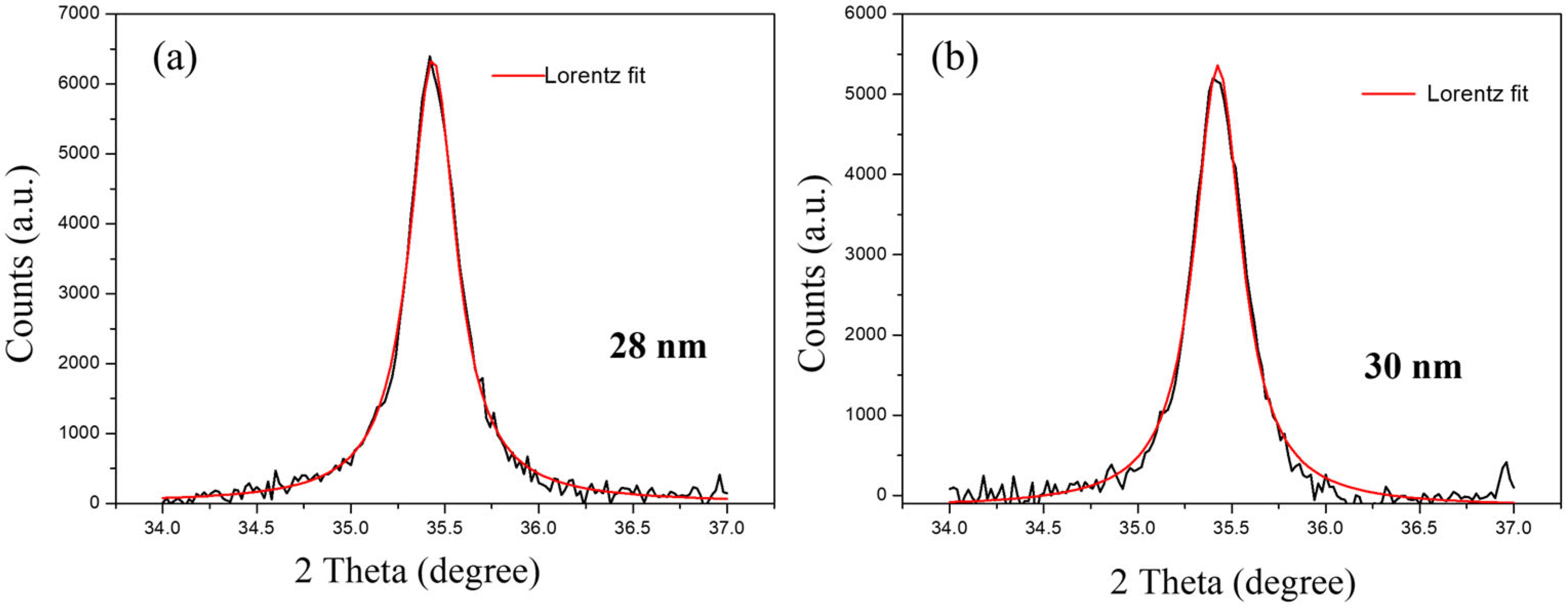
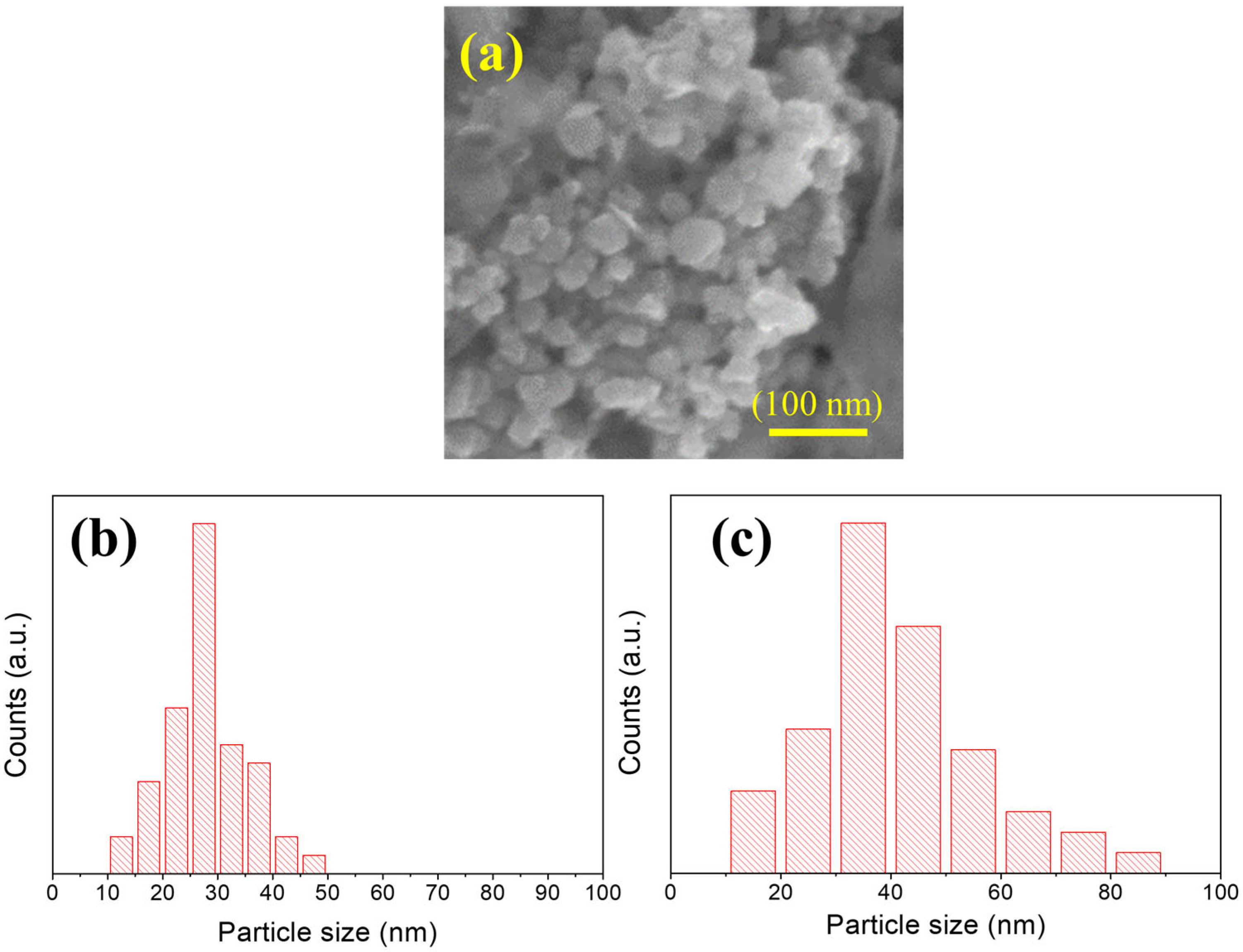
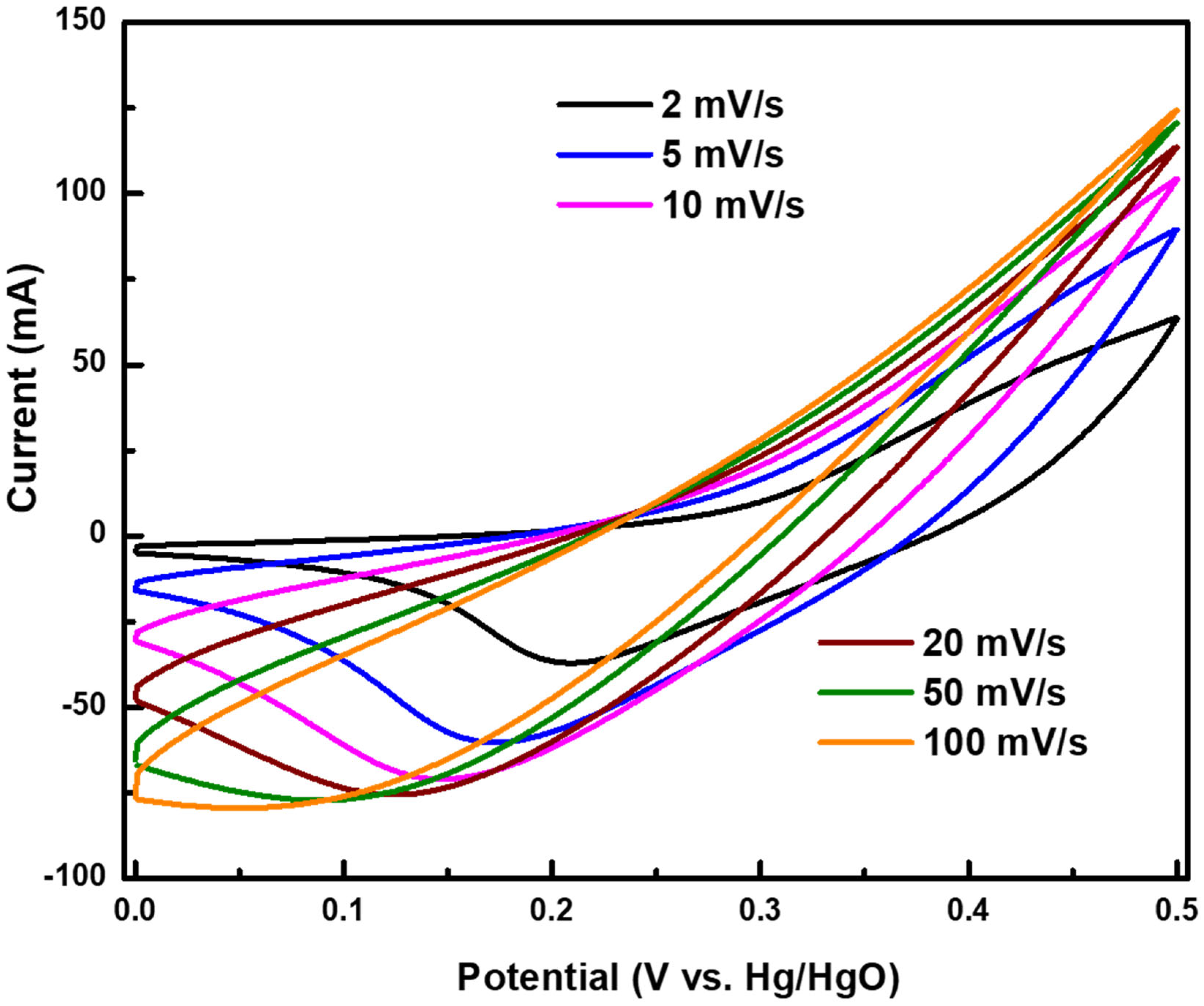
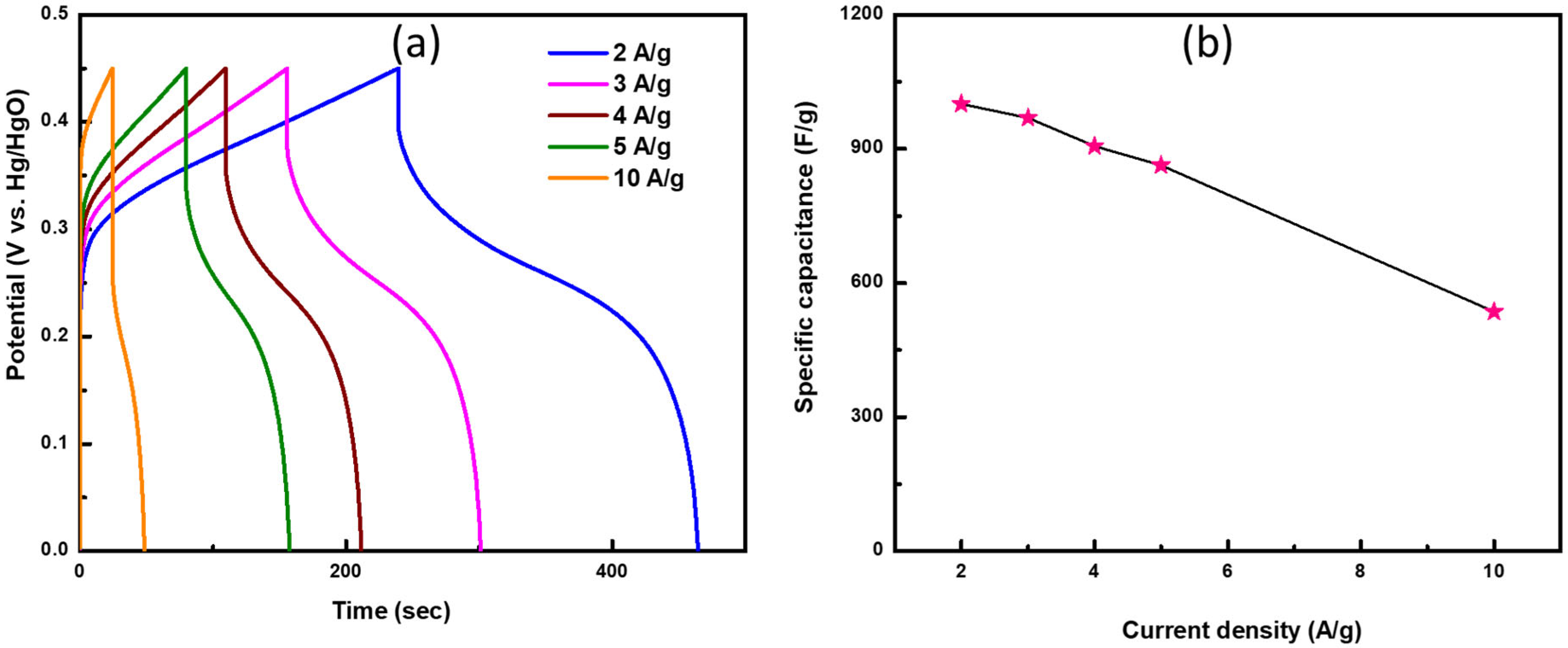
4. Results and Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Backman, C.-M. Global Supply and Demand of Metals in the Future. J. Toxicol. Environ. Health Part A 2008, 71, 1244–1253. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B.K. Copper demand, supply, and associated energy use to 2050. Glob. Environ. Change 2016, 39, 305–315. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Govindaraj, R.; Dhanabalan, S.; Sakthivel, P.; Chidhambaram, N.; Udayabhaskar, R.; Kamaraj, S.K.; Abarzúa, C.V.; Nazer, J.C.; Morel, M.J. Strategic analysis of lithium resources by addressing challenges and opportunities for sustainable electric vehicle battery development in the era of global EV domination. Z. Für Phys. Chem. 2025, 239, 457–465. [Google Scholar] [CrossRef]
- Lagos, G.; Peters, D.; Lima, M.; Jara, J.J. Potential copper production through 2035 in Chile. Miner. Econ. 2020, 33, 43–56. [Google Scholar] [CrossRef]
- Barros, K.S.; Vielmo, V.S.; Moreno, B.G.; Riveros, G.; Cifuentes, G.; Bernardes, A.M. Chemical Composition Data of the Main Stages of Copper Production from Sulfide Minerals in Chile: A Review to Assist Circular Economy Studies. Minerals 2022, 12, 250. [Google Scholar] [CrossRef]
- Texeira, L.; Calisaya-Azpilcueta, D.; Cruz, C.; Botero, Y.L.; Cisternas, L.A. Impact of the use of seawater on acid mine drainage from mining wastes. J. Clean. Prod. 2023, 383, 135516. [Google Scholar] [CrossRef]
- Adamovic, D.; Ishiyama, D.; Dordievski, S.; Ogawa, Y.; Stevanovic, Z.; Kawaraya, H.; Sato, H.; Obradovic, L.; Marinkovic, V.; Petrovic, J.; et al. Estimation and comparison of the environmental impacts of acid mine drainage-bearing river water in the Bor and Majdanpek porphyry copper mining areas in Eastern Serbia. Resour. Geol. 2021, 71, 123–143. [Google Scholar] [CrossRef]
- Punia, A. Role of temperature, wind, and precipitation in heavy metal contamination at copper mines: A review. Environ. Sci. Pollut. Res. 2021, 28, 4056–4072. [Google Scholar] [CrossRef]
- Leiva González, J.; Onederra, I. Environmental Management Strategies in the Copper Mining Industry in Chile to Address Water and Energy Challenges—Review. Mining 2022, 2, 197–232. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Z.; Yang, S.; Liu, Z.; Liu, Z.; Liu, Y.; Drewniak, L.; Jiang, C.; Li, Q.; Li, W.; et al. Life cycle assessment and cost analysis for copper hydrometallurgy industry in China. J. Environ. Manag. 2022, 309, 114689. [Google Scholar] [CrossRef]
- Seyrankaya, A. Pressure Leaching of Copper Slag Flotation Tailings in Oxygenated Sulfuric Acid Media. ACS Omega 2022, 7, 35562–35574. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, M.; Entezari Zarandi, A.; Pasquier, L.-C.; Azizi, A. Kinetic Investigation on Leaching of Copper from a Low-Grade Copper Oxide Deposit in Sulfuric Acid Solution: A Case Study of the Crushing Circuit Reject of a Copper Heap Leaching Plant. J. Sustain. Metall. 2021, 7, 1154–1168. [Google Scholar] [CrossRef]
- Mohanraj, G.T.; Rahman, M.R.; Arya, S.B.; Barman, R.; Krishnendu, P.; Singh Meena, S. Characterization study and recovery of copper from low grade copper ore through hydrometallurgical route. Adv. Powder Technol. 2022, 33, 103382. [Google Scholar] [CrossRef]
- Calgaro, C.O.; Da Silva, M.D.C.R.; Tanabe, E.H.; Bertuol, D.A. Copper Electrowinning from Supercritical Leachate of Printed Circuit Boards. Metals 2023, 13, 395. [Google Scholar] [CrossRef]
- Fathima, A.; Tang, J.Y.B.; Giannis, A.; Ilankoon, I.M.S.K.; Chong, M.N. Catalysing electrowinning of copper from E-waste: A critical review. Chemosphere 2022, 298, 134340. [Google Scholar] [CrossRef]
- Pizarro Barraza, F.; Thiyagarajan, D.; Ramadoss, A.; Manikandan, V.S.; Dhanabalan, S.S.; Abarzúa, C.V.; Sotomayor Soloaga, P.; Campos Nazer, J.; Morel, M.J.; Thirumurugan, A. Unlocking the potential: Mining tailings as a source of sustainable nanomaterials. Renew. Sustain. Energy Rev. 2024, 202, 114665. [Google Scholar] [CrossRef]
- Machiels, L.; Arnout, L.; Yan, P.; Jones, P.T.; Blanpain, B.; Pontikes, Y. Transforming Enhanced Landfill Mining Derived Gasification/Vitrification Glass into Low-Carbon Inorganic Polymer Binders and Building Products. J. Sustain. Metall. 2017, 3, 405–415. [Google Scholar] [CrossRef]
- Krook, J.; Svensson, N.; Eklund, M. Landfill mining: A critical review of two decades of research. Waste Manag. 2012, 32, 513–520. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Méndez, A.; Rodríguez-Pacheco, R.; Paz-Ferreiro, J.; Gascó, G. Recovery of Zinc and Copper from Mine Tailings by Acid Leaching Solutions Combined with Carbon-Based Materials. Appl. Sci. 2021, 11, 5166. [Google Scholar] [CrossRef]
- Nishida, N.; Amagasa, S.; Kobayashi, Y.; Yamada, Y. One-pot production of copper ferrite nanoparticles using a chemical method. Hyperfine Interact. 2016, 237, 111. [Google Scholar] [CrossRef]
- Zaharieva, K.; Rives, V.; Tsvetkov, M.; Cherkezova-Zheleva, Z.; Kunev, B.; Trujillano, R.; Mitov, I.; Milanova, M. Preparation, characterization and application of nanosized copper ferrite photocatalysts for dye degradation under UV irradiation. Mater. Chem. Phys. 2015, 160, 271–278. [Google Scholar] [CrossRef]
- El-Masry, M.M.; El-Shahat, M.; Ramadan, R.; Abdelhameed, R.M. Selective photocatalytic reduction of nitroarenes into amines based on cobalt/copper ferrite and cobalt-doped copper ferrite nano-photocatalyst. J. Mater. Sci. Mater. Electron. 2021, 32, 18408–18424. [Google Scholar] [CrossRef]
- Udhaya, P.A.; Ahmad, A.; Meena, M.; Queen, M.A.J.; Aravind, M.; Velusamy, P.; Almutairi, T.M.; Mohammed, A.A.A.; Ali, S. Copper Ferrite nanoparticles synthesised using a novel green synthesis route: Structural development and photocatalytic activity. J. Mol. Struct. 2023, 1277, 134807. [Google Scholar] [CrossRef]
- George, J.; Abraham, K.E. The structural phase change of copper ferrite and its gas-sensing properties. J. Mater. Sci. Mater. Electron. 2021, 32, 13220–13238. [Google Scholar] [CrossRef]
- Mahapatra, P.L.; Das, S.; Keasberry, N.A.; Ibrahim, S.B.; Saha, D. Copper ferrite inverse spinel-based highly sensitive and selective chemiresistive gas sensor for the detection of formalin adulteration in fish. J. Alloys Compd. 2023, 960, 170792. [Google Scholar] [CrossRef]
- Priyadharsini, R.; Manoharan, C.; Bououdina, M.; Sagadevan, S.; Venkateshwarlu, M.; Asath Bahadur, S. Impact of nickel substitution on structural, dielectric, magnetic, and electrochemical properties of copper ferrite nanostructures for energy storage devices. J. Colloid Interface Sci. 2024, 653, 917–929. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.-L.; Das, S.; Pal, U. Catalytic and pseudocapacitive energy storage performance of metal (Co, Ni, Cu and Mn) ferrite nanostructures and nanocomposites. Prog. Mater. Sci. 2022, 130, 100995. [Google Scholar] [CrossRef]
- Mandal, S.; Dasmahapatra, A.K. Hierarchical polyaniline/copper cobalt ferrite nanocomposites for high performance supercapacitor electrode. J. Energy Storage 2023, 74, 109402. [Google Scholar] [CrossRef]
- Masunga, N.; Mamba, B.B.; Getahun, Y.W.; El-Gendy, A.A.; Kefeni, K.K. Synthesis of single-phase superparamagnetic copper ferrite nanoparticles using an optimized coprecipitation method. Mater. Sci. Eng. B 2021, 272, 115368. [Google Scholar] [CrossRef]
- Subha, A.; Shalini, M.G.; Sahu, B.N.; Rout, S.; Sahoo, S.C. Role of surface defects and anisotropy variation on magnetic properties of copper ferrite nanoparticles prepared by co-precipitation method. Mater. Chem. Phys. 2022, 286, 126212. [Google Scholar] [CrossRef]
- Arshad, M.I.; Hasan, M.S.; Rehman, A.U.; Akhtar, M.; Tung, L.D.; Amin, N.; Mahmood, K.; Ali, A.; Trakoolwilaiwan, T.; Thanh, N.T.K. Structural, optical, electrical, dielectric, molecular vibrational and magnetic properties of La3+ doped Mg–Cd–Cu ferrites prepared by Co-precipitation technique. Ceram. Int. 2022, 48, 14246–14260. [Google Scholar] [CrossRef]
- Kumar, A.; Gangawane, K.M. Effect of precipitating agents on the magnetic and structural properties of the synthesized ferrimagnetic nanoparticles by co-precipitation method. Powder Technol. 2022, 401, 117298. [Google Scholar] [CrossRef]
- Kushwaha, P.; Chauhan, P. Influence of different surfactants on morphological, structural, optical, and magnetic properties of α-Fe2O3 nanoparticles synthesized via co-precipitation method. Appl. Phys. A 2022, 128, 18. [Google Scholar] [CrossRef]
- Yi, X.; Peng, Y.; Yao, Z.; Xia, C.; Zhu, S. Microstructure and magnetic properties of FeSiAl soft magnetic composites prepared by chemical in-situ coprecipitation with NaOH. Mater. Chem. Phys. 2021, 267, 124626. [Google Scholar] [CrossRef]
- Xia, A.; Jin, C.; Du, D.; Zhu, G. Comparative study of structural and magnetic properties of NiZnCu ferrite powders prepared via chemical coprecipitation method with different coprecipitators. J. Magn. Magn. Mater. 2011, 323, 1682–1685. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, N.; Sun, K.; Yang, T. High rate performance of Li[Ni1/3Co1/3Mn1/3]O2 synthesized via co-precipitation method by different precipitators. J. Phys. Chem. Solids 2009, 70, 727–731. [Google Scholar] [CrossRef]
- Ermakova, Y.A.; Pominova, D.V.; Voronov, V.V.; Yapryntsev, A.D.; Ivanov, V.K.; Tabachkova, N.Y.; Fedorov, P.P.; Kuznetsov, S.V. Synthesis of SrF2:Yb:Er ceramic precursor powder by co-precipitation from aqueous solution with different fluorinating media: NaF, KF and NH4F. Dalton Trans. 2022, 51, 5448–5456. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Han, G.; Zhang, L.; Huang, Y. Facile microwave-assisted synthesis of magnetic ferrite: Rapid interfacial reaction underlying intensifying mechanism. J. Clean. Prod. 2022, 361, 132181. [Google Scholar] [CrossRef]
- Mayakkannan, M.; Siva, V.; Murugan, A.; Shameem, A.; Thangarasu, S.; Asath Bahadur, S. Microwave-assisted synthesis of ternary transition metal ferrite: Structural, morphological, optical, magnetic and electrochemical properties. Phys. E Low-Dimens. Syst. Nanostructures 2023, 147, 115573. [Google Scholar] [CrossRef]
- Ansari, M.R.; Kem, A.; Agrohi, P.; Mallick, P.K.; Rao, P.; Peta, K.R. Structural, optical, magnetic and anti-bacterial properties of green synthesized spinel zinc ferrite by microwave-assisted method. Mater. Chem. Phys. 2023, 301, 127641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abarzúa, C.V.; Morel, M.J.; Sandoval-Hevia, G.; Kavinkumar, T.; Chidhambaram, N.; Kamaraj, S.K.; Dineshbabu, N.; Thirumurugan, A. Mineral-Based Synthesis of CuFe2O4 Nanoparticles via Co-Precipitation and Microwave Techniques Using Leached Copper Solutions from Mined Minerals. Minerals 2025, 15, 819. https://doi.org/10.3390/min15080819
Abarzúa CV, Morel MJ, Sandoval-Hevia G, Kavinkumar T, Chidhambaram N, Kamaraj SK, Dineshbabu N, Thirumurugan A. Mineral-Based Synthesis of CuFe2O4 Nanoparticles via Co-Precipitation and Microwave Techniques Using Leached Copper Solutions from Mined Minerals. Minerals. 2025; 15(8):819. https://doi.org/10.3390/min15080819
Chicago/Turabian StyleAbarzúa, Carolina Venegas, Mauricio J. Morel, Gabriela Sandoval-Hevia, Thangavel Kavinkumar, Natarajan Chidhambaram, Sathish Kumar Kamaraj, Nagarajan Dineshbabu, and Arun Thirumurugan. 2025. "Mineral-Based Synthesis of CuFe2O4 Nanoparticles via Co-Precipitation and Microwave Techniques Using Leached Copper Solutions from Mined Minerals" Minerals 15, no. 8: 819. https://doi.org/10.3390/min15080819
APA StyleAbarzúa, C. V., Morel, M. J., Sandoval-Hevia, G., Kavinkumar, T., Chidhambaram, N., Kamaraj, S. K., Dineshbabu, N., & Thirumurugan, A. (2025). Mineral-Based Synthesis of CuFe2O4 Nanoparticles via Co-Precipitation and Microwave Techniques Using Leached Copper Solutions from Mined Minerals. Minerals, 15(8), 819. https://doi.org/10.3390/min15080819












