Navigating the Limits: Unraveling Unidentified Fossil Bone and Tooth Fragments Through Histology, Chemistry, and Multivariate Statistics
Abstract
1. Introduction
2. Materials and Methods
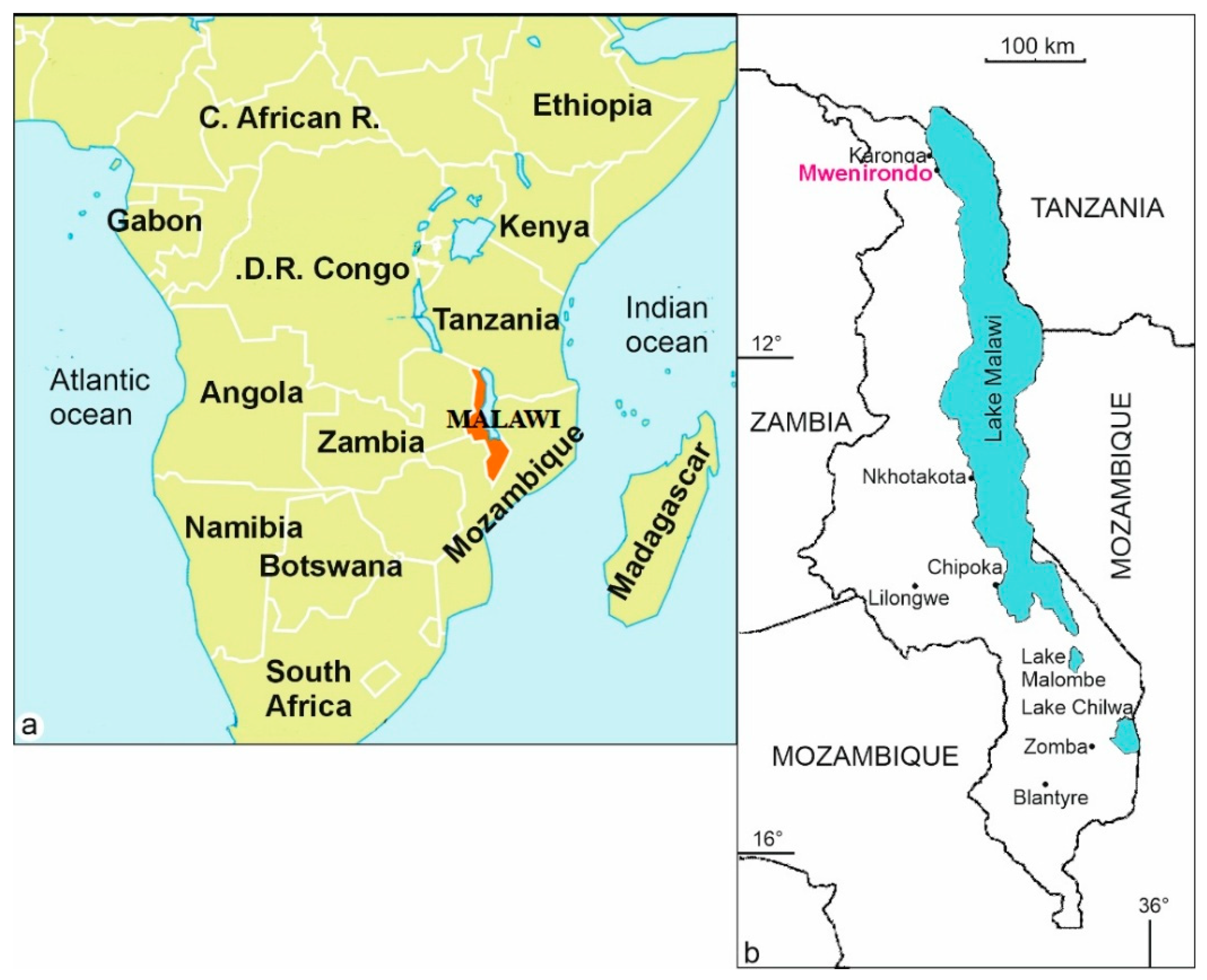
2.1. Materials
2.2. Sample Preparation
2.3. Microstructures
2.4. Chemical Analyses
3. Results
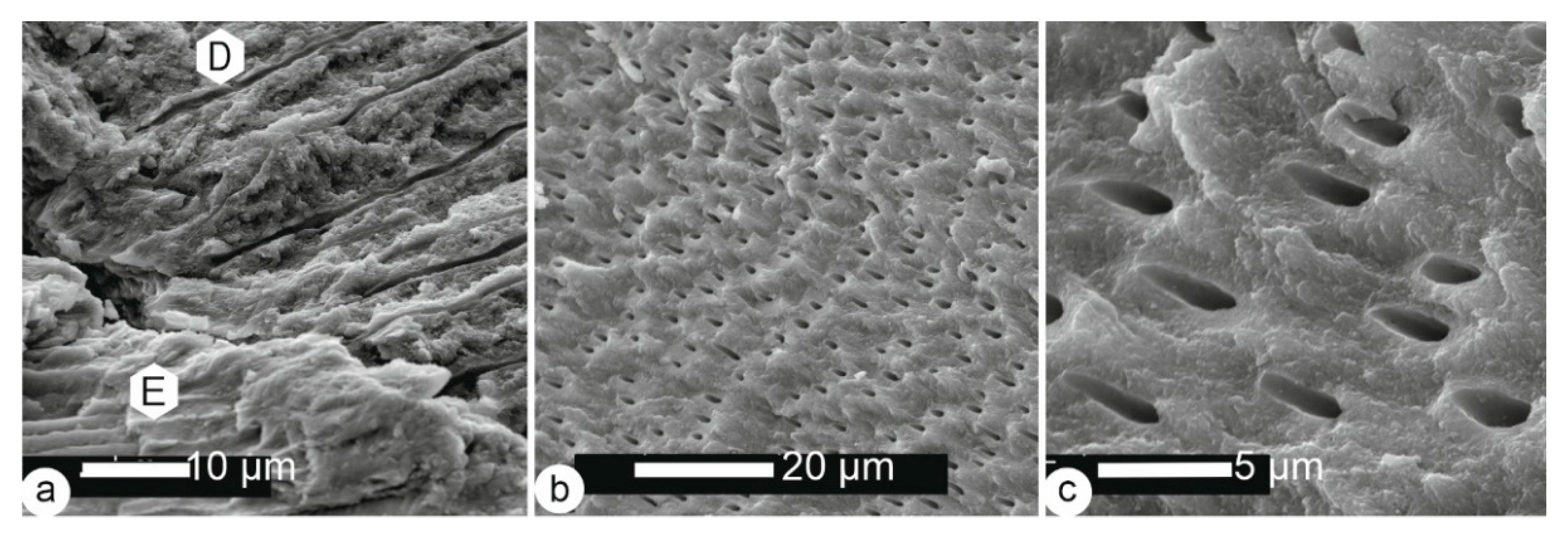
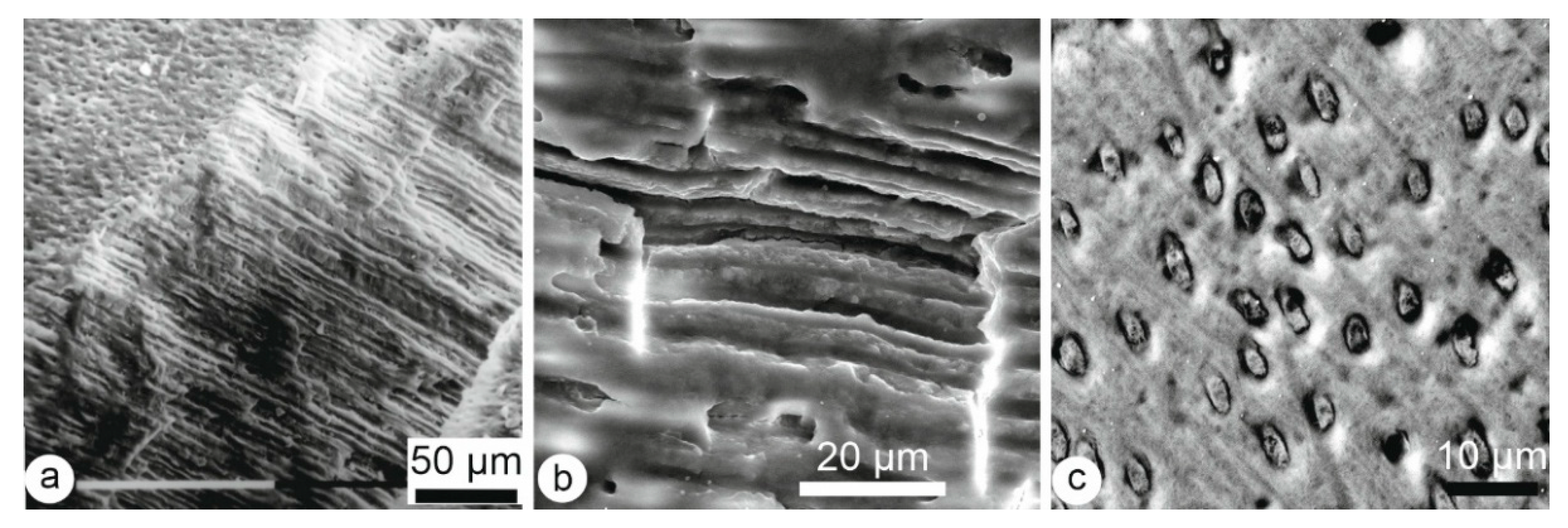
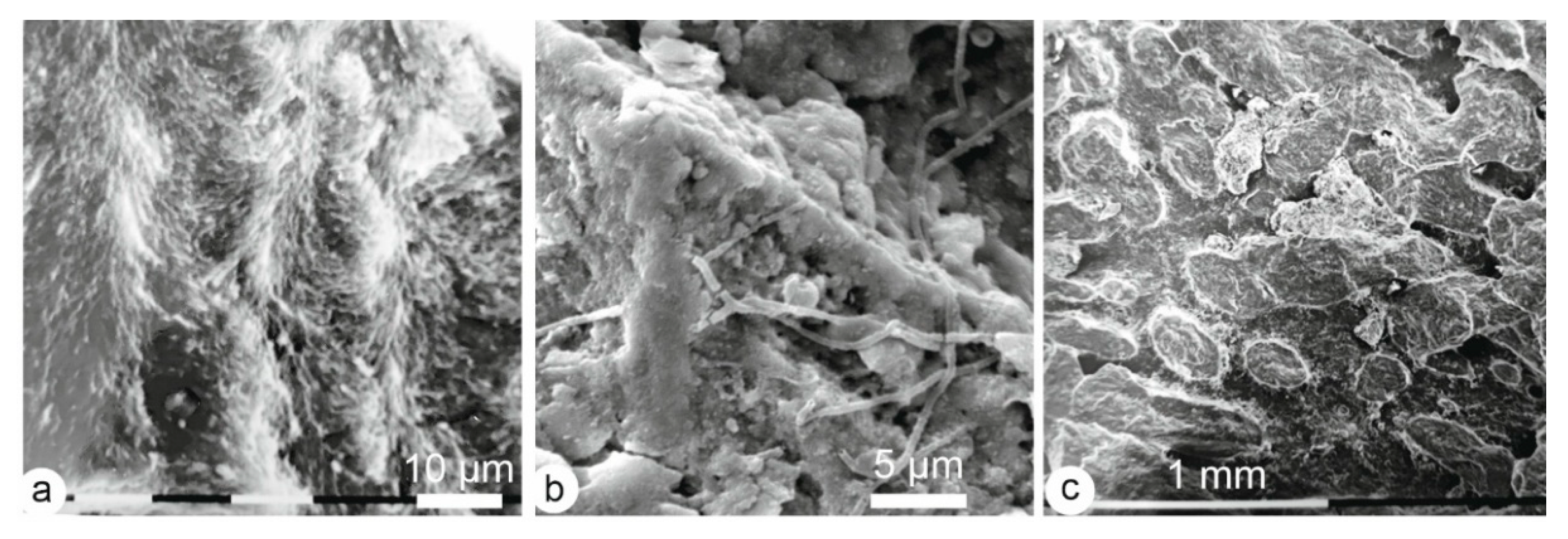
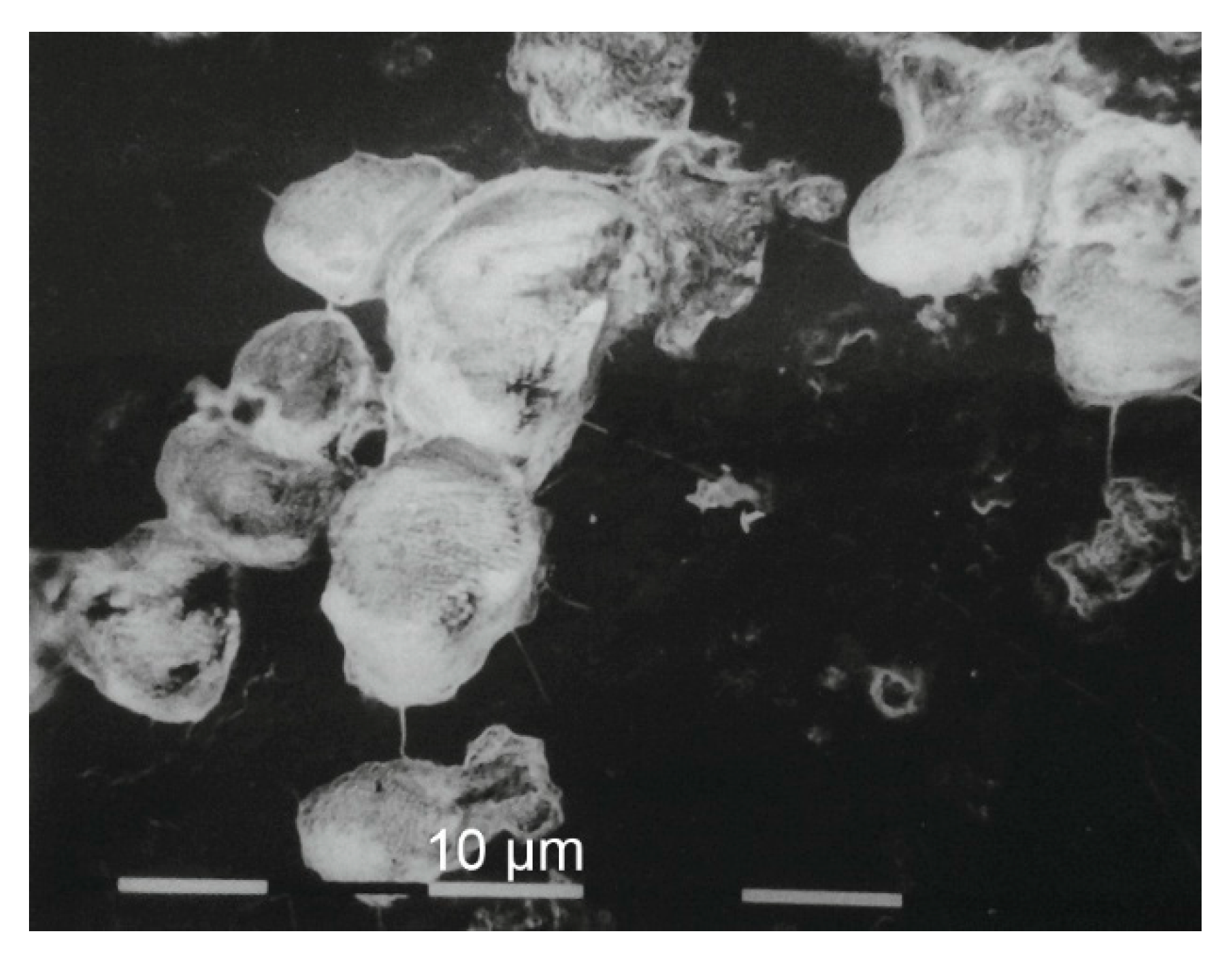
3.1. Microstructures
3.1.1. Enamel
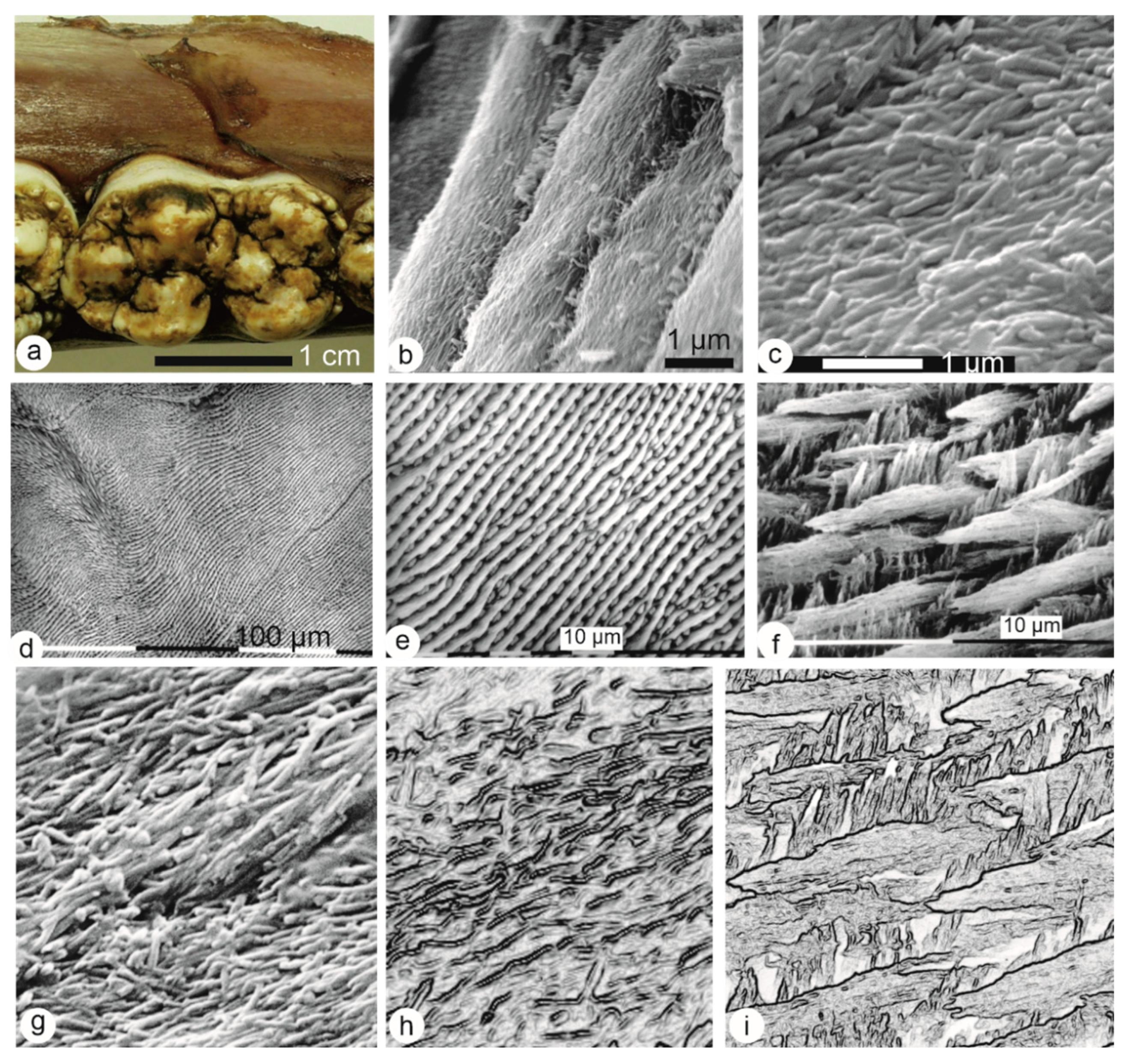
3.1.2. Dentin
3.1.3. Bone
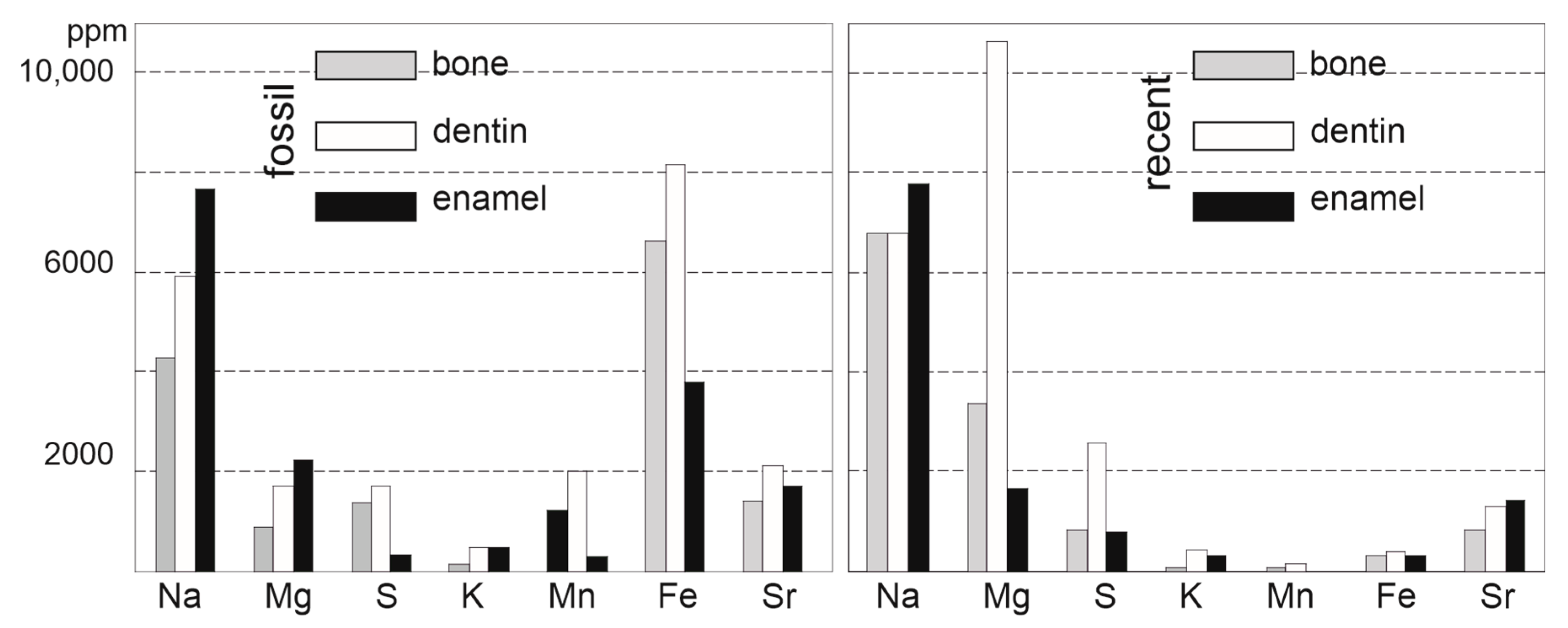
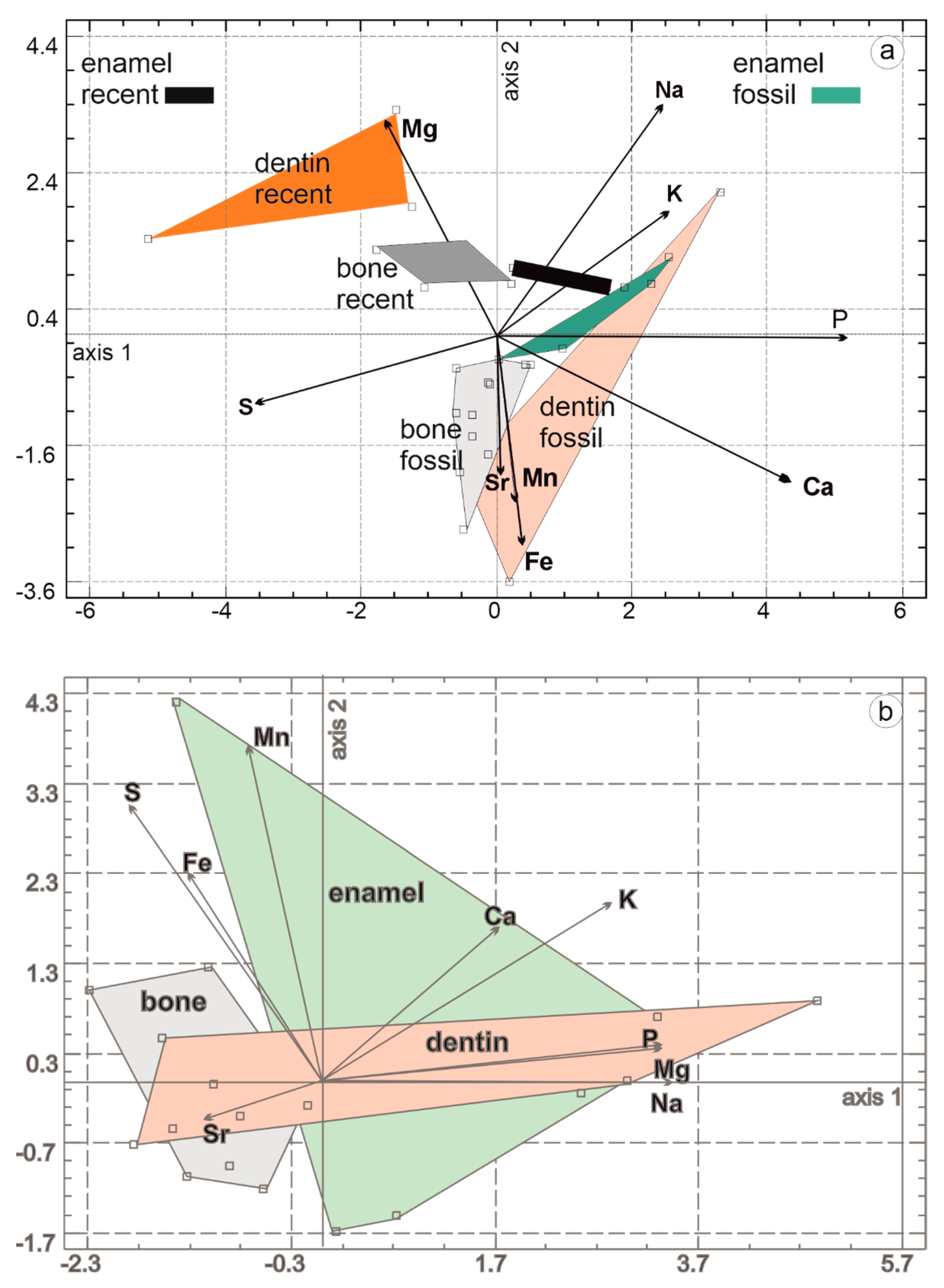
3.2. Composition
4. Discussion
4.1. Comparison Moder—Fossils from Malawi
4.2. Why Has This Been “Successful”?
4.3. Is It Possible to Use These Data to Improve Interpretations of a Fossil Site?
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gümbel, C.W. Beiträge zur Geognosie der bayerischen Alpen. Abh. Bayer. Akad. Wiss. Math.-Phys. Kl. 1868, 10, 567–634. [Google Scholar]
- Efremov, I.A. Taphonomy: New branch of paleontology. Pan-Amer. Geol. 1940, 74, 81–93. [Google Scholar]
- Brain, C.K. Bone weathering and the problem of bone pseudotools. S. Afr. J. Sci. 1967, 63, 97–99. [Google Scholar]
- Tappen, N.C.; Peske, G.R. Weathering cracks and split-line patterns in archeological bone. Am. Antiq. 1970, 35, 383–386. [Google Scholar] [CrossRef]
- Myers, T.P.; Voorhies, M.R.; Corner, R.G. Spiral fractures and bone pseudotools at paleontological sites. Am. Antiq. 1980, 45, 483–490. [Google Scholar] [CrossRef]
- Behrensmeyer, A.K. Time resolution in fluvial vertebrate assemblages. Paleobiology 1982, 8, 211–227. [Google Scholar] [CrossRef]
- Behrensmeyer, A.K. Vertebrate preservation in fluvial channels. Palaeogeog. Palaeoclim. Palaeoecol. 1988, 63, 183–199. [Google Scholar] [CrossRef]
- Gaudzinski, S. Aspects of faunal exploitation in the middle Palaeolithic: Evidence from Wallertheim (Rheinhessen, Germany). Anthropozoologica 1997, 25, 337–342. [Google Scholar]
- Barker, M.J.; Clarke, J.B.; Martill, D.M. Mesozoic reptile bones as diagenetic windows. Bull. Soc. Géol. Fr. 1997, 168, 485–490. [Google Scholar]
- Hao, B.-Q.; Feng, H.; Zhao, Z.-Q.; Ye, Y.; Wan, D.-X.; Peng, G.-Z. Different types of bone fractures in dinosaur fossils. Hist. Biol. 2020, 33, 1636–1641. [Google Scholar] [CrossRef]
- Haynes, G.; Krazinski, K.; Wojtal, P. A Study of fractured proboscidean bones in recent and fossil assemblages. J. Archaeol. Method Theory 2020, 28, 956–1025. [Google Scholar] [CrossRef]
- Weber, K.; Winkler, D.E.; Schulz-Kornas, E.; Kaiser, Y.M.; Tütken, T. The good, the bad and the ugly—A visual guide for common post-mortem wear patterns in vertebrate teeth. Palaeogeogr. Palaeoclim. Palaeoecol. 2021, 578, 110577. [Google Scholar] [CrossRef]
- Mein, C.; Jones, J.R.; Tennick, C.; Williams, A. Recognition of the presence of none fractures through physicochemical changes in diagenetic bone. Appl. Spectr. 2024, 78, 159–174. [Google Scholar] [CrossRef]
- Gallo, G.; Fyhrie, M.; Paine, C.; Ushakov, S.V.; Izuho, M.; Gunchinsuren, B.; Zwyns, N.; Navrotsky, A. Characterization of structural changes in modern and archaeological burnt bone: Implications for differential preservation bias. PLoS ONE 2021, 16, e0254529. [Google Scholar] [CrossRef] [PubMed]
- Brophy, G.P.; Nash, J.T. Compositional, infrared, and X-ray analysis of fossil bone. Am. Miner. J. Earth Planet. Mater. 1968, 53, 445–454. [Google Scholar]
- Sillen, A. Biogenic and diagenetic Sr/Ca in Plio-Pleistocene fossils of the Omo Shungura formation. Paleobiology 1986, 12, 311–323. [Google Scholar] [CrossRef]
- Weiner, S.; Bar-Yosef, O. States of preservation of bones from prehistoric sites in the Near East: A survey. J. Archaeol. Sci. 1990, 17, 187–196. [Google Scholar] [CrossRef]
- Denys, C.; Williams, C.T.; Dauphin, Y.; Andrews, P.; Fernandez-Jalvo, Y. Diagenetical changes in Pleistocene small mammal bones from Olduvai Bed I. Palaeogeogr. Palaeoclim. Palaeoecol. 1996, 126, 121–134. [Google Scholar] [CrossRef]
- Janssens, K.; Vincze, L.; Vekemans, B.; Williams, C.T.; Radtke, M.; Haller, M.; Knöchel, A. The non-destructive determination of REE in fossilized bone using synchrotron radiation induced K-line X-ray microfluorescence analysis. Fresenius J. Anal. Chem. 1999, 363, 413–420. [Google Scholar] [CrossRef]
- Dauphin, Y.; Williams, C.T.; Andrews, P.; Denys, C.; Fernandez-Jalvo, Y. Diagenetic alterations of micromammal fossil bones from Olduvai Bed I of the Lower Pleistocene sequence at Olduvai Gorge, Tanzania. J. Sedim. Res. 1999, 69, 612–621. [Google Scholar] [CrossRef]
- Hedges, R.E.M. Bone diagenesis: An overview of processes. Archaeometry 2002, 44, 319–328. [Google Scholar] [CrossRef]
- Turner-Walker, G. The chemical and microbial degradation of bones and teeth. In Advances in Human Palaeopathology; Pinhasi, R., Mays, S., Eds.; Wiley & Sons: Chichester, UK, 2008; pp. 3–30. [Google Scholar]
- Weiner, S. Microarchaeology: Beyond the Visible Archaeological Record; Cambridge University Press: Cambridge, UK, 2010; 396p. [Google Scholar]
- Keenan, S.W.; Engel, A.S. Early diagenesis and recrystallization of bone. Geochim. Cosmochim. Acta 2017, 196, 209–233. [Google Scholar] [CrossRef]
- Dauphin, Y.; Denys, C. Diagenèse différentielle chez les rongeurs fossiles—Validité des paramètres géochimiques pour les reconstitutions des régimes alimentaires. Palaeogeog. Palaeoclim. Palaeoecol. 1992, 99, 213–223. [Google Scholar] [CrossRef]
- Dauphin, Y.; Williams, C.T. Diagenetic trends of dental tissues. Comptes Rendus Palevol 2004, 3, 583–590. [Google Scholar] [CrossRef]
- Betzler, C.; Ring, U. Sedimentology of the Malawi Rift: Facies and stratigraphy of the Chiwondo Beds, northern Malawi. J. Hum. Evol. 1995, 28, 23–35. [Google Scholar] [CrossRef]
- Schrenk, F.; Bromage, T.G.; Gorthner, A.; Sandrock, O. Paleoecology of the Malawi Rift: Vertebrate and invertebrate faunal context of the Chiwondo Beds, northern Malawi. J. Hum. Evol. 1995, 29, 59–70. [Google Scholar] [CrossRef]
- Bromage, T.G.; Schrenk, F.; Juwayeyi, Y.M. Palaeobiogeography of the Malawi Rift: Age and vertebrate paleontology of the Chiwondo Beds, northern Malawi. J. Hum. Evol. 1995, 28, 37–57. [Google Scholar] [CrossRef]
- Sandrock, O.; Kullmer, O.; Schrenk, F.; Juwayeyi, Y.M.; Bromage, T.G. Fauna, taphonomy, and ecology of the Plio-Pleistocene Chiwondo Beds, Northern Malawi. Hominin Environments. In The East African Pliocene: An Assessment of the Faunal Evidence; Bobe, R., Alemseged, Z., Behrensmeyer, A.K., Eds.; Wiley: Dordrecht, The Netherlands, 2007; pp. 315–332. [Google Scholar]
- Sandrock, O.; Dauphin, Y.; Kullmer, O.; Abel, R.; Schrenk, F.; Denys, C. Malema: Preliminary taphonomic analysis of an African hominid locality. C. R. Acad. Sci. 1999, 328, 133–139. [Google Scholar] [CrossRef]
- Sandrock, O. The Taphonomy and Paleoecology of the Malema Hominid Site, Northern Malawi. Ph.D. Thesis, Johannes Gutenberg Universität Mainz, Mainz, Germany, 1999; 276p. [Google Scholar]
- Kullmer, O. The fossil suidae from the Plio-Pleistocene Chiwondo Beds of northern Malawi, Africa. J. Vert. Paleontol. 2008, 208, 208–216. [Google Scholar] [CrossRef]
- Kullmer, O.; Sandrock, O.; Abel, R.; Schrenk, F.; Bromage, T.G.; Juwayeyi, M. The first Paranthropus from the Malawi rift. J. Hum. Evol. 1999, 37, 121–127. [Google Scholar] [CrossRef]
- Denys, C.; Otero, O.; Kullmer, O.; Sandrock, O.; Bromage, T.C.; Schrenk, F.; Dauphin, Y. Biominerals fossilisation: Fish bone diagenesis in Plio-Pleitocene hominid sites of Malawi. Minerals 2020, 10, 1049. [Google Scholar] [CrossRef]
- Korvenkontio, V.A. Mikroskopische Untersuchungen an Nagerincisiven unter Hinweis auf die Schmelzstruktur der Backenzähne. Ann. Zool. Soc. Zool. Bot. Fenn. Vanamo 1934, 2, 1–274. [Google Scholar]
- Dauphin, Y.; Massard, P.; Quantin, C. In vitro diagenesis of teeth of Sus scrofa (Mammalia, Suidae): Micro- and nanostructural alterations and experimental dissolution. Palaeog. Palaeocl. Palaeoecol. 2008, 266, 134–141. [Google Scholar] [CrossRef]
- Cuif, J.P.; Dauphin, Y.; Sorauf, J.E. Biominerals and Fossils Through Time; Cambridge University Press: Cambridge, UK, 2011; 490p. [Google Scholar]
- Dauphin, Y.; Denis, A.; Denys, C. Effets de la prédation sur la microstructure et la composition des tissus minéralisés. In TaphonomieS; Brugal, J.P., Ed.; Archives Contemporaines: Paris, France, 2017; Chapter 10; pp. 331–346. [Google Scholar]
- Dorvee, J.R.; Deymier-Black, A.; Gerkowicz, L.; Veis, A. Peritubular dentin, a highly mineralized, non-collagenous, component of dentin: Isolation and capture by laser microdissection. Conn. Tissue Res. 2014, 55 (Suppl. S1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. Mineralization in organic matrix frameworks. Rev. Mineral. Geochem. 2003, 54, 249–289. [Google Scholar] [CrossRef]
- Dauphin, Y. Os et dents. In Message d’os—Archéométrie du Squelette Humain et Animal; Archives Contemporaines: Paris, France, 2015; Chapter 2; pp. 5–21. [Google Scholar]
- Dauphin, Y.; Jaeger, J.J. Géochimie des oeufs de dinosaures du Bassin d’Aix en Provence (Crétacé): Influence de la méthode d’analyse. Neues Jahrb. Geol. Paläontol. Monatshefte 1990, 8, 479–492. [Google Scholar] [CrossRef]
- Koeningswald, W.V.; Sander, P.M. (Eds.) Tooth Enamel Microstructure; A.A. Balkema, Rotterdam; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]



| Na | Mg | S | K | Mn | Fe | Sr | P | Ca | Ca/P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | B | 4260 | 891 | 1363 | 132 | 1214 | 6622 | 1395 | 153,784 | 349,965 | 2.27 |
| O | Sd | 479 | 133 | 343 | 94 | 1579 | 5580 | 238 | 2959 | 9165 | ||
| S | M | D | 5933 | 1701 | 1711 | 485 | 2018 | 8139 | 2101 | 164,818 | 358,038 | 2.17 |
| S | Sd | 2762 | 835 | 607 | 252 | 2195 | 3676 | 666 | 11,094 | 23,716 | ||
| I | M | E | 7665 | 2228 | 315 | 75 | 292 | 3777 | 1713 | 171,239 | 371,246 | 2.17 |
| L | Sd | 1111 | 365 | 245 | 57 | 200 | 489 | 321 | 4496 | 23,503 | ||
| R | M | B | 6783 | 3374 | 822 | 75 | 55 | 326 | 843 | 146,686 | 281,269 | 1.92 |
| E | Sd | 446 | 492 | 297 | 57 | 21 | 102 | 586 | 10,011 | 25,148 | ||
| C | M | D | 6788 | 10,621 | 2572 | 438 | 147 | 396 | 1319 | 127,343 | 222,162 | 1.74 |
| E | Sd | 178 | 2390 | 1691 | 204 | 69 | 68 | 87 | 16,653 | 25,972 | ||
| N | M | E | 7774 | 1676 | 796 | 309 | 0 | 300 | 1403 | 172,375 | 327,005 | 1.90 |
| T | Sd | 557 | 101 | 596 | 116 | 0 | 108 | 36 | 10,425 | 21,487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauphin, Y. Navigating the Limits: Unraveling Unidentified Fossil Bone and Tooth Fragments Through Histology, Chemistry, and Multivariate Statistics. Minerals 2025, 15, 807. https://doi.org/10.3390/min15080807
Dauphin Y. Navigating the Limits: Unraveling Unidentified Fossil Bone and Tooth Fragments Through Histology, Chemistry, and Multivariate Statistics. Minerals. 2025; 15(8):807. https://doi.org/10.3390/min15080807
Chicago/Turabian StyleDauphin, Yannicke. 2025. "Navigating the Limits: Unraveling Unidentified Fossil Bone and Tooth Fragments Through Histology, Chemistry, and Multivariate Statistics" Minerals 15, no. 8: 807. https://doi.org/10.3390/min15080807
APA StyleDauphin, Y. (2025). Navigating the Limits: Unraveling Unidentified Fossil Bone and Tooth Fragments Through Histology, Chemistry, and Multivariate Statistics. Minerals, 15(8), 807. https://doi.org/10.3390/min15080807





