Abstract
As the most unstable crystalline form of calcium carbonate, vaterite is rarely found in nature due to being highly prone to phase transitions. However, its high specific surface area, excellent biocompatibility, and high solubility properties have led to a research boom and the following breakthroughs in the last two decades: (1) From primitive calculations and spectroscopic analyses to modern multidimensional research methods combining calculations and experiments, the crystal structure of vaterite has turned from early identifications in orthorhombic and hexagonal crystal systems to a complex polymorphic structure within the monoclinic crystal system. (2) The formation process of vaterite not only conforms to the classical crystal growth theory but also encompasses the nanoparticle aggregation theory, which incorporates the concepts of oriented nanoparticle assembly and mesoscale transformation. (3) Regardless of the conditions, the formation of vaterite depends on an excess of CO32− relative to Ca2+, and its stability duration relates to preservation conditions. (4) Vaterite demonstrates significant value in biomedical applications—including bone repair scaffolds, targeted drug carriers, and antibacterial coating materials—leveraging its porous structure, high specific surface area, and exceptional biocompatibility. While it also shows utility in environmental pollutant adsorption and general coating technologies, the current research remains predominantly concentrated on its medical applications. Currently, the rapid transformation of vaterite presents the primary limitation for its industrial application. Future research should prioritize investigating its formation kinetics and stability.
1. Introduction
Vaterite, a metastable polymorph of calcium carbonate (CaCO3), has become a focus of research in mineralogy and materials science due to its unique physicochemical properties, such as high specific surface area and multistage pore structure [1]. Its crystal structure has long been controversial, with early studies proposing a hexagonal crystal system model based on X-ray diffraction (XRD) [2]. Only in 2023 did San et al. [3] establish a polymorphic structure model based on the coexistence of monoclinic modules (space group C12/c1, cell parameters a = 12.17 Å, b = 7.12 Å, and c = 9.47 Å, β = 118.37°) through deep neural network potential function simulations, subsequently validated by Okumura et al. [4], possibly ending the century-long crystallographic debate. In practical applications, vaterite particles characterized by a hollow or porous morphology (pore size: 3–50 nm) and large specific surface area [5] demonstrate significant utility in functional materials. For example, as fillers in coatings, they substantially enhance hardness [6,7]; in the biomedical field, their high solubility and biocompatibility promoted the development of drug carriers [8,9,10]. However, compared with calcite and aragonite, which are widely present in nature, vaterite is relatively rare and is mainly distributed in biological mineralization systems, such as ascidians [11], nacre [12], and fish otoliths [3]. In recent years, vaterite has also been found on plant leaves [13]. However, such a scarcity of vaterite is not sufficient to support its application in daily life and industrial fields. Therefore, laboratory preparation and its potential industrial production have become crucial [14].
Since the first report of the mineral by the German mineralogist Vater in 1897 [15], vaterite research has progressed through three phases: the period of structural exploration (1900s–1960s), the period of mechanistic analysis (1970s–2000s), and the period of expanding applications (2010s–present). Bibliometric analysis (Figure 1) shows that 3108 papers were published in the Web of Science (WoS) database between 1990 and 2024, with 61.84% published in the last decade; research topics are now heavily focused on practical applications. Overall, crystal structure (accounting for 33.62%), formation mechanism (accounting for 15.99%), and application development (accounting for 21.69%) constitute the three core research directions. In this paper, we systematically review the breakthroughs in recent years, focusing on the unified model of monoclinic crystal structure, non-classical crystallization pathways, and interdisciplinary application scenarios, with the aim of providing theoretical frameworks and technological insights for the development of the field.
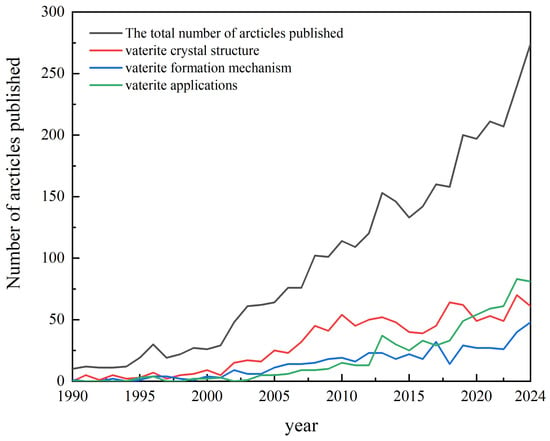
Figure 1.
International research on vaterite.
2. Crystal Structure: From Controversy to Consensus
Since R. E. Gibson first clearly distinguished the crystal structures of vaterite, calcite, and aragonite through X-ray diffraction (XRD) in 1953 [16], the crystallographic study of vaterite has emerged as a central topic within this field. So far, a variety of information regarding the crystal structure of vaterite has been presented (as shown in Table 1). In early theoretical explorations, Olshausen (1925) [17] proposed that vaterite might have hexagonal-symmetry cells by mathematical derivation, but for a long time, this hypothesis was not supported by any experimental evidence. It was not until 1959 that Meyer first proposed the orthorhombic cell model (space group Pbnm, cell parameters a = 4.13 Å, b = 7.15 Å, and c = 8.48 Å) based on XRD data, which laid the experimental foundation for the study of vaterite’s structure [18]. However, this model was soon challenged by Kamhi (1963), who proposed a hexagonal crystal system (space group P63/mmc; a = b = 4.13 Å, c = 8.49 Å) and pointed out that disordered carbonate ion distribution was the source of structural complexity, using the same XRD experiments [2]. In subsequent research, Meyer (1969) [19] further discovered diffuse streaks and satellite reflections in the diffraction patterns, he speculated that vaterite might form a dynamically disordered structure through glide reflections and spiral stacking. Unfortunately, however, Meyer did not provide a detailed atomic-level analysis of this structure. Consequently, the controversy surrounding the crystal structure of vaterite remained unresolved.
In the late 20th century, spectroscopic techniques became a key tool for deciphering the structural controversy, but the experimental results for vaterite were contradictory. The experimental results of infrared spectroscopy by Sato (1969) [20] and Gabrielli (2000) [21] support Kamhi’s hexagonal model and Meyer’s orthogonal model, respectively, but the Raman spectra of Behrens (1995) [22] detect two symmetric scaling modes, which were incompatible with either Kamhi’s or Meyer’s structural models. These disparate experimental results suggest that variations between samples or sample defects may introduce data bias, highlighting the limitations of traditional experimental methods and prompting researchers to combine theoretical calculations with experimental techniques [23,24]. Wang et al. (2009) [25] and Mugnaioli et al. (2012) [15] used the Density Functional Theory (DFT) and Automated electron diffraction tomography (ADT), respectively, to propose different vaterite crystal structures (space groups P6522 and C12/c1). Wang et al. emphasized for the first time the critical role of the carbonate interlayer hydrogen bonding network on structural stability. Mugnaioli et al. (2012) [15] first revealed the monoclinic symmetry of vaterite. However, they did not combine theoretical calculations with experiments, that is, the theoretical calculations and experiments did not yield a consistent vaterite crystal structure.
In 2019, Steciuk et al. [26] identified the modulation and polycrystalline properties of vaterite for the first time using electron diffraction experiments. Based on Steciuk’s ideas, San et al. [3] proposed a “polymorph coexistence” model through molecular dynamics simulations driven by a deep neural network potential function (DNN), achieving an error of only 0.3% compared to experiments and successfully explaining the phase transition behavior of vaterite. It is proposed that the pseudohexagonal symmetry of vaterite is actually caused by the slip stackings with different periodicities along a specific direction, and the slight difference in the orientation of carbonate groups leads to the phenomenon of polymorphism. This result was directly observed by Okumura’s team using scanning transmission electron microscopy (STEM) combined with annular dark-field imaging (ADF) [4], which verifies the accuracy of the San model and may mark the end of the phase of controversy regarding the vaterite structure.
From primitive calculations and spectroscopic analyses to modern multidimensional research methods combining calculations and high-resolution TEM and electron diffraction experiments, the crystal structure of vaterite has turned from early identifications in orthorhombic and hexagonal crystal systems to a complex polymorphic structure within the monoclinic crystal system. Despite the remarkable progresses, the complexity and controversial nature of vaterite’s structure still need further exploration with the aim to provide a more solid theoretical basis for applications [27].

Table 1.
Summary of the crystal structure models proposed for vaterite.
Table 1.
Summary of the crystal structure models proposed for vaterite.
| Space Group | Lattice Parameters | Proposed by | Method |
|---|---|---|---|
| Pbnm | a = 4.13 b = 7.15 c = 8.48 | Meyer H. J. [18] | XRD, Microtwinning Hypothesis |
| P63/mmc | a = 4.13 b = 4.13 c = 8.49 | Kamhi S. R. [2] | XRD, Microtwinning Hypothesis |
| P6522 | a = 7.290 b = 7.290 c = 25.302 | Wang J. [25] | First-principles calculations (Density Functional Theory (DFT), Plane-wave pseudopotential method) |
| Ama2 | a = 8.4721 b = 7.1575 c = 4.1265 | Le Bail A. [23], Demichelis R. [24] | Microtwinning Hypothesis, DFT (all-electron quantum mechanics) |
| P65 | a = 7.1120 b = 7.1120 c = 25.4089 | Demichelis R. [24] | DFT (all-electron quantum mechanics) |
| P3221 | a = 7.1239 b = 7.1239 c = 25.3203 | ||
| P212121 | a = 4.3668 b = 6.5831 c = 8.4282 | ||
| C12/c1 | a = 12.170 b = 7.120 c = 9.470 β = 118.37° | Mugnaioli E. [15] | Electron diffraction (ED) data acquisition based on Automated electron diffraction tomography (ADT) and precession electron diffraction (PED) |
| C1 | a = 12.353 b = 7.102 c = 25.733 β = 99.78° | Demichelis R. [24] | |
| C121 | a = 12.245 b = 7.197 c = 9.305 β = 115.16° | ||
| C1c1 | a = 12.281 b = 7.142 c = 9.371 β = 115.48° |
3. Mechanism of Formation: Integration of Classical and Non-Classical Theories
As one of the metastable crystalline forms of calcium carbonate, vaterite has long been the focus of the research on crystallography, mineralogy, and biomineralization due to its non-equilibrium crystallization behavior and complex morphological formation mechanism [28]. Among the existing theoretical systems, the Classical Crystallization Theory (CCT) and Nanoparticle-mediated Non-Classical Crystallization Theory (NCCT) constitute the two core frameworks to explain its formation mechanism [29]. Although both models have made important progress in describing vaterite’s nucleation, growth pathways, and morphological regulation, their theoretical boundaries and implications for applications still need further clarification.
3.1. Vaterite Formation Mechanism from the Perspective of the Classical Crystal Growth Theory
Based on the traditional Gibbs free energy-driven crystallization model, the CCT suggests that vaterite formation follows a continuous nucleation-growth process. The core idea emphasizes that (1) the nucleation phase is triggered by a single critical nucleus; and (2) the growth phase is characterized by crystal amplification through the layer-by-layer deposition of ions/molecules at lattice sites [29]. The typical spherical morphology of vaterite aggregates can evolve through two patterns: One is the radial fiber growth pattern (Figure 2A), that is, crystal fibers starting from the common crystallization core are symmetrically arranged radially to form polycrystalline aggregates; the second one is the filamentous fiber bundle growth pattern (Figure 2B), characterized by the fibers extending from both ends of the crystallization core forming a spherical configuration through helical entanglement or bifurcation in a three-dimensional space [30]. Although the theory successfully explains the phenomenon of the directional growth of regular spherical crystals under laboratory conditions, its linear growth assumption makes it difficult to elucidate the following issues: (1) the mechanism of formation of internal cavities in hollow vaterite; (2) the genesis of asymmetric, heterogeneous surface structures; and (3) the self-organization behavior of multi-level substructures under environmental perturbations.

Figure 2.
Diagrams of two growth modes of spherical vaterite: (A) radial fiber growth pattern; (B) filamentous fiber bundle growth pattern.
3.2. The Interpretation of Complex Morphology by the Non-Classical Crystallization Theory
Compared with the CCT, the NCCT addresses the CCT’s limitations by introducing the concepts of Oriented Attachment (OA) and Mesocrystal Transition (MT) (Mesocrystal Transition: during the crystallization process, particles form highly ordered structures through mesoscopic transitions). This provides a new theoretical framework for understanding mesocrystal formation [31]. The formation of vaterite may go through the multi-stage process of “nanoprecursor → mesocrystal cluster → crystal reorganization”: (1) Primary nanoparticles (approximately 5–20 nm in diameter) undergo directional arrangement through surface charge interactions or molecular templating; (2) At the mesoscopic scale, interfacial fusion or epitaxial growth between nanoparticles forms metastable mesocrystalline structures; and (3) Ostwald maturation or lattice rearrangement occurs within the mesocrystals, eventually creating to crystals with specific morphologies [32].
3.2.1. The Morphology Control Mechanism of Double-Hydrophilic Block Copolymers
Double-hydrophilic block copolymers (DHBCs), as a key factor for vaterite morphology regulation, the structure–activity relationship between their molecular design (such as block length and functional group distribution), and crystal growth have become a research hotspot. The mechanism covers two principles: interfacial energy control (where selective crystallographic adsorption reduces interfacial energy and inhibits growth on dominant crystal faces) and spatial confinement (where micellar templates direct the oriented assembly of nanoparticles) (Figure 3) [33,34]. Taking the poly(ethylene glycol)-block-poly(ethylene imine) (PEG-b-PEI) system as an example [33], the longer PEI block promotes hierarchical growth on specific CaCO3 crystal facets through a high positive-charge density that expands the adsorption area. Conversely, the shorter PEI block combined with high-density PEG stabilizes vaterite and inhibits secondary nucleation via interfacial charge shielding.
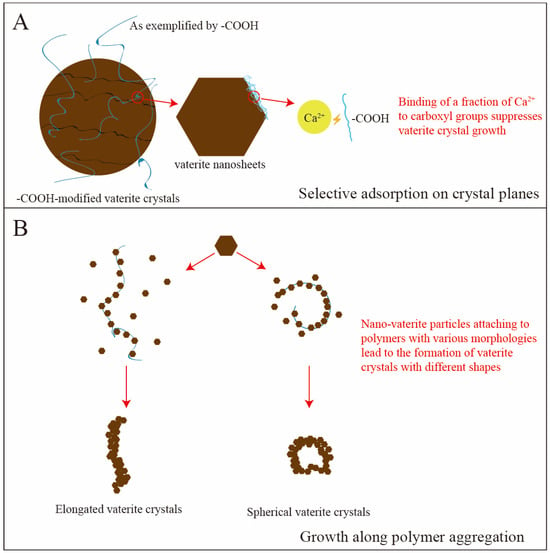
Figure 3.
Schematic diagram of interfacial control and spatial confinement effects by DHBCs on vaterite. (A) -COOH in the solution attaches to the vaterite crystal surface and binds with Ca2+ ions on its specific crystal planes, thereby hindering the transformation of vaterite; (B) Vaterite nanosheets adsorb onto DHBC and grow along them, forming particles with diverse morphologies.
3.2.2. Interfacial Control of Crystallization Pathways
The mechanistic role of solution surface tension (γ) in regulating vaterite morphology has been studied throughout the century-long history of colloid chemistry and crystallography. Seminal early work applied the Gibbs adsorption theory, establishing that γ dominates crystallographic selection by modulating nucleation activation energy (ΔG ∝ γ3). This laid the theoretical foundation for interfacial control research [32]. In low-γ systems (for example, with surfactant addition), reduced interfacial energy causes (Figure 4) (1) enhanced CO2 bubble stability providing more heterogeneous nucleation sites; (2) increased nanoparticle Brownian motion that increases collision–aggregation probability; and (3) a diminished density difference between nascent crystals and solution, slowing sedimentation.

Figure 4.
Effect of low interfacial energy on vaterite particle assembly. (A) Increased CO2 bubbles serve as nucleation templates; (B) enhanced Brownian motion of vaterite nanosheets elevates the probability of collision-induced nucleation; (C) newly formed vaterite nanosheet aggregates in the solution settle to the bottom of the liquid at a reduced rate.
The variation in the surface tension of the solution may contribute to the diverse morphologies observed in vaterite. As illustrated in Figure 5, the initially formed small vaterite particles undergo secondary nucleation driven by local supersaturation at the upper interface. When the surface tension of the solution is elevated, the precipitation rate of vaterite aggregates is relatively slow, allowing for greater aggregation among vaterite particles and facilitating the formation of hollow vaterite structures. Conversely, when the surface tension is reduced, the sedimentation rate of vaterite aggregates increases significantly. Upon settling at the bottom of the solution, it becomes challenging for additional vaterite particles to accumulate on their surfaces, resulting in semi-closed vaterite formations. Additionally, some smaller vaterite particles can form hollow structures by adhering to CO2 bubbles present within the solution.
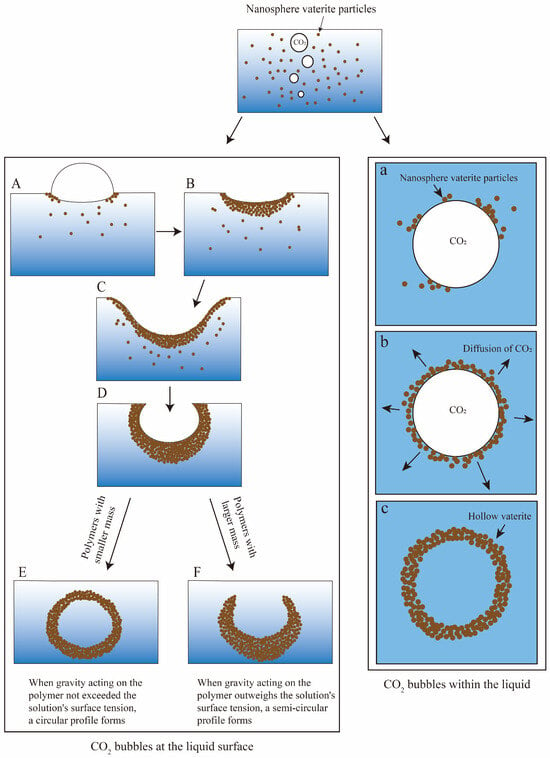
Figure 5.
Surface tension effects on vaterite’s morphology and schematic of the CO2 bubble templating method for hollow vaterite synthesis. (A) Vaterite nanoparticles aggregate around the CO2 bubble interfaces at the liquid surface; (B) particles accumulate; (C) further particle aggregation proceeds continuously; (D) surface tension drives the formation of quasi-spherical aggregates; (E) when interfacial tension overcomes gravitational force on the aggregate, it develops into a perfect sphere through structural refinement; (F) when gravitational force dominates interfacial tension, hemispherical aggregates settle to the bottom. The diminished probability of particle adhesion on their surfaces results in stabilized hemispheres. (a) Vaterite particles adhere to CO2 bubble surfaces (CO2 bubble serving as mineralization templates); (b) disintegration of CO2 bubbles occurs; (c) hollow vaterite microspheres.
At the beginning of the 21st century, major breakthroughs in surfactant technology significantly reduced the required surface tension (γ) of systems, prompting a shift in the research focus on the dynamic and precise regulation of low-γ systems. In this context, DHBCs with their unique amphiphilic structure and tunable interfacial behaviors rapidly became the core strategy for the controlled synthesis of vaterite, establishing a dominant paradigm in this research field [35,36,37].
4. Stability Regulation: From Molecular Design to Engineering Practice
The structural stability difference between vaterite and calcite is rooted in their crystallographic nature [31]: the carbonate groups of vaterite are arranged parallel to the C-axis (Figure 6B) and compared with the vertically oriented groups in calcite (Figure 6A); it has a lower lattice packing density [3]. This forms an open framework. According to the Born stability criterion (Born stability criterion: the core criterion for judging the mechanical stability of an ideal crystal under static conditions), the low symmetry of the monoclinic crystal system (space group C12/c1) makes its Gibbs free energy higher than that of calcite in the trigonal crystal system, which explains the metastable properties of vaterite. Temperature modulation of stability is nonlinear. Low temperatures (<190 K) trigger secondary-phase transitions and symmetry break-down, reducing the lattice vibrational entropy by 40%, which significantly enhances stability. High temperatures (>320 K), although thermodynamically favorable, accelerate the transition to calcite due to a surge in the rate of dissolution–re-crystallization [3,38,39,40].
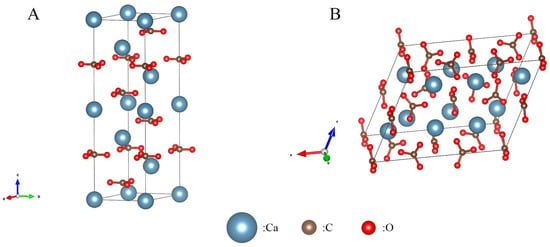
Figure 6.
Crystal structures of calcite and vaterite: (A) calcite crystal structure; (B) vaterite crystal structure.
4.1. Synergistic Regulation of pH and Ionic Effects
At pH 8.5 and Ca2+ concentrations <10 mM, the half-life of vaterite is up to 72 h—only 24 h under neutral conditions. This stabilization is attributed to decreased CO32− activity, resulting in a nucleation free-energy difference of ΔG vaterite–calcite = −2.3 kJ/mol [41]. However, at Ca2+ concentrations >10 mM, the heterogeneous nucleation of calcite dominates (nucleation barrier reduced by 30%), making it difficult to suppress the phase transition even at a low pH. The stabilization mechanisms of metal ions are more diverse: the formation of (Mn,Ca)CO3 solid solution by the lattice substitution of Mn2+ (ionic radius difference Δr = 0.15 Å) increases the solvation activation energy from 45 kJ/mol to 62 kJ/mol [42]; La3+, on the other hand, is formed by surface coordination into a La-O -Ca network (coordination number = 8), which completely blocks the dissolution interface reaction at adsorption densities >2.5 μmol/m2 [41].
4.2. Anisotropic Response of High-Pressure Behavior
The compression of vaterite at pressures in the range of 0–5 GPa shows significant anisotropy, with the a-axis compressing 2.3-times faster than the c-axis. This leads to a transition to the calcite-II phase at lattice mismatches reaching 1.7% [43]. Although Raman spectroscopy of specific carbonate minerals (e.g., calcite and aragonite) under high-pressure, high-temperature conditions has been extensively studied, systematic investigations of vaterite in comparable extreme environments remain significantly lacking [44,45].
4.3. Crystallization Kinetics Regulation Strategy
To address the thermodynamically metastable nature of vaterite, researchers have performed its stabilized preparation through multi-scale strategies (utilizing additives, interface modification, and biomimetic mineralization) [46]. Relevant advances have already transitioned from laboratory exploration to engineering applications.
4.3.1. Inorganic Additive Modulation
- I. Metal ions: dual role of lattice doping and interface modulation
Metal ions (e.g., Mg2+, Mn2+) synergistically induce the formation and enhance the stability of vaterite through lattice doping and interface energy modulation. In the case of Mg2+, for example, its smaller ionic radius (0.72 Å vs. Ca2+ 0.99 Å) allows it to replace Ca2+ sites in the vaterite lattice, forming the [MgO6] octahedral structure. This structure has a longer bond length (2.08 Å) than that of [CaO6] by 13.3%, triggering lattice distortion and increasing the solvation activation energy from 45 kJ/mol to 68 kJ/mol [47,48,49]. Synchrotron X-ray absorption spectroscopy (XAS) showed that Mg2+ preferentially occupies the Ca(1) site, enhances lattice rigidity by shortening the Mg-O bond length (Δ = 0.32 Å), and suppresses phase transition kinetics [48]. Meanwhile, Mg2+ adsorption on the crystal surface reduces the solid–liquid interfacial energy to 25 mN/m, which prioritizes the nucleation of vaterite over calcite by reducing ΔG by 20%.
- II. Non-metal ions: supersaturation drive and kinetic regulation
Non-metal ions (e.g., CO32−, NH4+) induce vaterite nucleation by modulating the solution chemical environment [50]. The urea decomposition system (NH2CONH2 + H2O → 2NH3 + CO2) is exemplified by [51]: when the decomposition rate is controlled at 0.12 mmol/min, the increase in CO32− concentration from 2 to 12 mM and the increase in pH from 7.4 to 9.2 result in a vaterite nucleation rate (1.5 × 1022 m−3s−1) far exceeding that of calcite (3 × 1021 m−3s−1), with a yield of 92%. In situ Raman spectroscopy confirms that NH3 stabilizes ACC precursor clusters with a diameter of ~2 nm through a hydrogen bonding network (bonding energy~15 kJ/mol), thereby retarding Ostwald ripening [52,53].
4.3.2. Organic Molecular Regulation: From Amino Acids to Proteins
The regulatory efficacy of amino acids is highly correlated with their isoelectric point (pI): when solution pH ≈ pI, glutamic acid (pI = 3.2) selectively adsorbs on the vaterite (001) side via -Ca-OOC-chelating, suppressing its growth rate from 1.5 nm/s to 0.4 nm/s while the (110) side is maintained at 1.2 nm/s, inducing a hexagonal lamellar morphology [54]. Molecular dynamics simulations show that the V interaction energy of the -CH2- group of glycine with the oxygen atoms on the surface of vaterite (−28 kJ/mol) is significantly higher than that of calcite (−15 kJ/mol), which reduces the nuclear energy barrier for vaterite formation by 17% [55]. For protein modulation, silk fibroin protein containing β-fold conformation precisely matches the vaterite Ca2+ lattice (lattice mismatch <2%) by periodic carboxylate arrays (spacing of 4.7 Å), which improves the stability by 3-fold when the β-sheet content exceeds 60% (the half-life is prolonged from 7 days to 21 days) [56,57,58].
4.3.3. Polymer Template Modulation: Space Constraints and Synergistic Adsorption
Hydrophilic block copolymers modulate the crystallization process through space constraints. When the percentage of hydrophobic chain segments is >40%, the dielectric constant of the micellar core is close to that of the organic solvent environment, which reduces the critical radius for CaCO3 nucleation [33,34]. Synergistic adsorption also plays a key role in the stabilization of vaterite. For example, the PAA/SDBS composite system works through the mechanism of “synergistic adsorption”: the sulfonic acid group of SDBS is preferentially adsorbed on the (001) side, while the carboxylic acid group of PAA stabilizes the (110) side through Ca2+ bridging, and this two-site adsorption greatly enhances the activation energy of the phase change in vaterite and prevents the transformation of vaterite to calcite.
5. Microbial Mineralization: Metabolic-Interfacial Synergistic Control Mechanism
In nature, some microorganisms are capable of inducing the precipitation of calcium carbonate minerals. Not only do many microorganisms promote the formation of vaterite, but biogenic vaterite is more stable than abiogenic vaterite [59,60], and the microbial-mediated mineralization of vaterite is not a strain-specific phenomenon, but is a widespread biomineralization process in different microbial groups [59].
5.1. Kinetic Regulation of Vaterite Formation by Microbial Metabolic Activities
Microorganisms alter microenvironmental physicochemical parameters through metabolic pathways, significantly affecting the kinetic processes of calcium carbonate crystallization [60,61,62,63]. First, NH3 released by heterotrophic microorganisms metabolizing nitrogenous organic matter leads to an increase in local microenvironmental pH [59,64]. Typical representatives of urease-producing bacteria bring the environmental pH to an alkaline range of 9.2–9.8 through the following urea decomposition reaction: CO(NH2)2 + H2O → 2NH3 + CO2. This alkaline microenvironment not only significantly enhances the activity of carbonate ions (CO32−) but also reduces the solubility of Ca2+, which together promote the formation of calcium carbonate supersaturation [50].
Second, CO2 produced by microbial metabolism is slowly dissolved into the solution system through a gas–liquid mass transfer process to establish a dynamically balanced carbonate buffer system [65,66]. This process regulates the steady-state concentration of CO2 in solution through the reaction chain CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3− ⇌ 2H+ + CO32−, and this controlled ion release mechanism effectively inhibits the rapid crystallization of calcite, thereby promoting the preferential formation of the vaterite phase.
Microbial extracellular secretions (EPSs) regulate crystallization at the molecular scale [67,68,69]. EPSs rich in ligand groups, such as carboxyl and hydroxyl—for example, acidic amino acids, like glutamic and aspartic acid—form stable complexes with Ca2+ through chelation. This process not only reduces the nucleation activation energy of vaterite, but also inhibits the selective growth of calcite crystal surfaces via spatial site-blocking effects [70,71]. Fourier transform infrared spectroscopy (FTIR) analysis confirms the specific binding of some EPS components to vaterite [59,72,73,74]. For instance, amide groups tightly bind to the vaterite surface through NH-O hydrogen bonding, which can control vaterite’s long-term stability [75,76].
5.2. Nucleation Template Effect Mediated by the Microbial Cell Interface
Microbial cell surfaces act as active interfaces for biomineralization, mediating crystal nucleation processes through chemical interactions. In bacterial secretions, low-molecular-weight amino acids, especially those containing carboxylated glutamic acid and aspartic acid, typically exhibit negative surface Zeta potentials. Abundant anionic groups, such as carboxyl groups (-COOH), on these surfaces adsorb Ca2+ via electrostatic interactions, creating a localized ionic concentration gradient that promotes vaterite formation [77,78,79].
When the system is in a neutral or weakly acidic environment, the carboxyl group on the bacterial surface carries a negative charge due to deprotonation, which effectively attracts Ca2+ in solution, leading to a significant increase in Ca2+ concentration near the interface. At this time, if CO32− is introduced, it combines with the surface-enriched Ca2+ to form structurally stable “bound nuclei”, whereas partially unbound Ca2+ from the bacterial surface combines with CO32− to form energetic “free nuclei” [80]. Both types of nuclei can develop into vaterite crystals. However, free nuclei not affected by bacterial action transform into calcite through rapid growth and phase transformation driven by their high surface energy. Conversely, bound nuclei adsorbed on bacterial surfaces are stabilized by the organic matrix—specifically, organic molecules secreted by microorganisms that reduce vaterite’s surface energy through interfacial adsorption. This effectively slows down further crystal growth and the phase transformation process.
6. Application Frontiers: Environment, Medicine, and Materials
- I. Selective adsorption of vaterite and its application in environmental remediation
With its unique porous structure and large specific surface area, vaterite exhibits a good adsorption capacity, which has led to its reputation as an efficient adsorption material in the field of environmental sciences (Table 2).

Table 2.
Adsorption capacity of vaterite for various pollutants.
In practice, when spherical vaterite was put into a mixed solution containing a variety of pollutants, it showed different adsorption preferences for different types of pollutants. Its adsorption capacity for organic pollutants is significantly better than that of metal ions, which may be due to the stronger interaction of organic pollutant molecules with the surface of vaterite. For metal ions, the adsorption capacity of vaterite showed a certain pattern, with the adsorption capacity decreasing in the order of Pb2+ > Cu2+ > Ni2+ > Cd2+. Such differentiated adsorption characteristics enable vaterite to preferentially adsorb specific pollutants when treating complex polluted water bodies, thus improving the efficiency and effectiveness of water purification. In addition, the adsorption process of vaterite is usually accompanied by complex surface reactions, such as electrostatic adsorption and chemical bonding; the synergy of these mechanisms further enhances its ability to capture pollutants. In actual water pollution control, vaterite is not only used alone but also compounded with other materials to achieve synergistic effects in addressing different types of pollution challenges, providing an effective solution for the remediation and protection of the water environment [92,93].
- II. Triple advantages of vaterite and its application in biomedicine
As a metastable crystalline phase of calcium carbonate, vaterite has attracted widespread attention in biomedical fields in recent years due to its unique physicochemical properties [94,95,96]. This material system exhibits three key advantages: (1) excellent biocompatibility, which effectively reduces host immune rejection; (2) a multi-level pore structure and surface charge properties that synergistically enhance drug loading capacity; and (3) thermodynamic instability, providing a design basis for material functionalization and modulation. These properties demonstrate dual application potential in drug delivery systems and bone tissue regeneration [10].
In the construction of drug-controlled release systems, researchers have established three typical functionalization strategies [10]. Using a surfactant-mediated crystallization-kinetics modulation strategy, vaterite microspheres with a gradient porous structure were successfully prepared; their specific surface area increased by more than 40% compared to conventional structures, significantly enhancing the physical adsorption loading efficiency for hydrophobic drugs [97]. Molecular-level complexation of drug molecules with a calcium carbonate matrix was realized during crystal growth by the in situ co-precipitation loading technique, which can effectively maintain the conformational integrity of bioactive molecules [98]. The chitosan/calcium alginate composite coating system constructed by layer self-assembly technology not only reduces the drug sudden release rate from 65% to 12%, but also endows the carrier with pH-responsive release characteristics, demonstrating the advantages of the design of a intelligent drug delivery system [97].
In bone tissue engineering applications, vaterite achieves multidimensional biological modulation through a dynamic phase transition mechanism. Vaterite can be spontaneously converted to hydroxycarbonate apatite (HCA) in a physiological environment (pH 7.4, 37 °C), the conversion pathway is highly similar to the mineralization process of natural bone matrix, and the formation of an osteoapatite/collagen-like composite structure provides an ideal microenvironment for bone regeneration [99]. From the perspective of ion release, continuously released Ca2+ promotes osteoblast differentiation by activating the calcium-sensing receptor (CaSR), while CO32− enhances osteoclast activity by regulating the local microenvironmental pH. This bionic regulatory mechanism creates a dynamic equilibrium of bone metabolism [100]. At the cellular interaction level, the micro-nanotopography on the material surface upregulates integrin α5β1 expression in mesenchymal stem cells (MSCs) and induces osteogenic differentiation via the Wnt/β-catenin signaling pathway [101,102]. Animal experimental data showed that 8 weeks after the implantation of BMP-2-loaded vaterite scaffolds into rat cranial defects, the newly formed bone volume fraction reached 68.3 ± 5.1%—significantly higher than that in the hydroxyapatite control group (42.7 ± 4.8%) [103,104].
- III. Surface modification of vaterite coating and its application in biomaterials
In the field of biomaterial surface modification, substantial progress has been made in researching vaterite coatings [7,105,106,107]. Chemical deposition of vaterite coatings onto biologically inert materials can systematically modulate surface physicochemical properties [108]. These coatings significantly improve material hydrophilicity, for example, on polypropylene (PP) substrates, the contact angle decreased from 127° to 107° (depending on deposition parameters), while surface energy increased substantially. These altered surface properties enhance cell–material interface interactions, and the elevated surface roughness of modified materials provides additional anchoring sites for cell adhesion. In shell-less hen’s egg models, vaterite-coated PP triggered no observable toxic reactions, confirming the strategy’s biosafety [99]. The synergistic effects of these properties establish vaterite coating technology as an effective means to enhance osseointegration of orthopedic implants and endothelialization efficiency of cardiovascular stents.
7. Challenges and Prospects
- Research Status on Formation and Transformation Mechanisms
As a metastable polymorph of calcium carbonate, the formation pathway and phase transition mechanisms of vaterite remain critical scientific questions. Current research indicates that its crystallization process may follow a non-classical nucleation pathway. For instance, in high-concentration solutions of calcium nitrate and sodium carbonate, the initially formed amorphous calcium carbonate (ACC) precursor undergoes structural reorganization over several minutes to yield vaterite [28]. This discovery challenges the traditional theory of direct ion-by-ion nucleation and suggests that the solution’s chemical environment plays a definitive role in polymorph selection.
Regarding the mechanism of vaterite-to-calcite transformation, the solution-mediated dissolution–recrystallization model is widely accepted. However, systematic studies on phase transition behavior at solid–gas interfaces under atmospheric exposure remain limited.
- 2.
- Technical bottlenecks for controlled preparation and large-scale production
Although the short-term stable preparation of vaterite has been realized in the laboratory, their industrial application is still limited by two challenges: long-term stability control and morphology regulation [109]. Temperature experiments show that vaterite can be formed preferentially in the 20–40 °C range, while calcite phase transition occurs above 40 °C, suggesting that temperature gradient control can be an effective means of crystal shape regulation [110].
In terms of stability enhancement, surface modification strategies show remarkable effects: the introduction of metal ions (e.g., Mg2+, Mn2+) can reduce the solubility of vaterite by forming surface solid solutions or occupying active sites. However, the current research is mostly limited to small batch preparation, and the problems of mass and heat transfer control and the uniform dispersion of additives during continuous production have not been solved yet, which requires the development of a new reactor design and on-line monitoring technology to realize large-scale preparation.
- 3.
- The contradictory relationship between stability and the synergistic optimization of function
The functional application of vaterite needs to solve the contradiction between stability enhancement and surface activity maintenance. Taking the biomedical field as an example, the multistage pore structure of vaterite can significantly increase the drug loading rate, but if crystallinity is too high, it will lead to a decrease in the drug release rate [111]. This calls for the development of smart-responsive carriers, e.g., by surface grafting of environmentally sensitive moieties for controlled release.
Bio-mineralization studies may provide new ideas for synergizing the stability and functionality of vaterite [112]. Biomimetic synthesis with organic matrix modulation can significantly enhance vaterite stability, but requires the precise control of the coverage of the active sites on the surface. Future research should focus on the establishment of a quantitative structure–stability–functionality model to meet the customized needs of different applications.
- 4.
- From Isotope Tracing to Agricultural Innovation: Vaterite Phase-Transition-Inspired Controlled-Release Fertilizer
In nature, vaterite and aragonite spontaneously transform into calcite. It is widely accepted that this transformation occurs in solution via a dissolution–reprecipitation process. However, it remains unclear whether isotope fractionation occurs during the dissolution–reprecipitation process of the vaterite-to-calcite transformation. Furthermore, synthetic vaterite is primarily produced through two main pathways: chemical precipitation and microbially induced mineralization. Due to their distinct formation mechanisms, it is also currently unknown whether vaterite produced by these two methods exhibits differences in isotopic composition. Based on this, we hypothesize that both the synthesis method of vaterite and its transformation process to calcite may influence its isotopic composition.
Presently, environmental pollution issues are of significant concern. In agricultural production, although fertilizer application is unavoidable, its misuse or overuse can lead to varying degrees of environmental pollution. While its environmental impact is generally less severe than industrial pollution, it still warrants serious attention. Notably, plants secrete acidic substances during growth that are sufficient to dissolve calcium carbonate. Based on this, we propose the following concept: utilize vaterite’s porous structure to adsorb fertilizer, then embed it into the soil, where acids secreted by plant roots induce the slow dissolution of vaterite, thereby achieving a controlled release of the fertilizer. Theoretically, this approach offers the following multiple potential advantages: reducing excessive fertilizer application, lowering environmental pollution risks, and potentially preventing seedling death caused by localized high fertilizer concentrations.
Author Contributions
Conceptualization, G.S. and X.L.; methodology, B.L.; software, G.S.; validation, G.S., X.L. and B.L.; formal analysis, S.W.; investigation, G.S.; resources, X.L. and B.L.; data curation, G.S.; writing—original draft preparation, G.S.; writing—review and editing, X.L. and B.L.; visualization, G.S.; supervision, X.L. and B.L.; project administration, S.W.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China: U24A20579.
Data Availability Statement
All crystallographic data of vaterite mentioned in this article were sourced from the American Mineralogist Crystal Structure Database. https://rruff.geo.arizona.edu/AMS/amcsd.php (accessed on 1 June 2025).
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Luca, R.; Cau Ontiveros, M.A.; Miriello, D.; Pecci, A.; Le Pera, E.; Bloise, A.; Crisci, G.M. Archaeometric study of mortars and plasters from the Roman City of Pollentia (Mallorca-Balearic Islands). Period. Mineral. 2013, 82, 353–379. [Google Scholar]
- Kamhi, S.R. On the structure of vaterite CaCO3. Acta Crystallogr. 1963, 16, 770–772. [Google Scholar] [CrossRef]
- San, X.; Hu, J.; Chen, M.; Niu, H.; Smeets, P.J.; Malliakas, C.D.; Deng, J.; Koo, K.; Dos Reis, R.; Dravid, V.P.; et al. Unlocking the mysterious polytypic features within vaterite CaCO3. Nat. Commun. 2023, 14, 7858. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Takahashi, G.; Suzuki, M.; Kogure, T. Stacking Structure of Vaterite Revealed by Atomic Imaging and Diffraction Analysis. Chemistry 2024, 30, e202401557. [Google Scholar] [CrossRef]
- Febrida, R.; Cahyanto, A.; Herda, E.; Muthukanan, V.; Djustiana, N.; Faizal, F.; Panatarani, C.; Joni, I.M. Synthesis and Characterization of Porous CaCO3 Vaterite Particles by Simple Solution Method. Materials 2021, 14, 4425. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Zhao, L.; Zhao, Y.; Ruan, H.; Gong, F. Research Progress of Scale Preparation of Aragonite Calcium Carbonate. Technol. Dev. Chem. Ind. 2020, 49, 25–28+50. [Google Scholar]
- Zafar, B.; Campbell, J.; Cooke, J.; Skirtach, A.G.; Volodkin, D. Modification of Surfaces with Vaterite CaCO3 Particles. Micromachines 2022, 13, 473. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Haase, A.; Antolini, R. Sub-Micrometer Vaterite Containers: Synthesis, Substance Loading, and Release. Chem. Int. Ed. 2012, 51, 1195–1197. [Google Scholar] [CrossRef]
- Jariya, S.I.; Babu, A.A.; Narayanan, T.S.; Vellaichamy, E.; Ravichandran, K. Development of a novel smart carrier for drug delivery: Ciprofloxacin loaded vaterite/reduced graphene oxide/PCL composite coating on TiO2 nanotube coated titanium. Ceram. Int. 2022, 48, 9579–9594. [Google Scholar] [CrossRef]
- Choukrani, G.; Álvarez Freile, J.; Avtenyuk, N.U.; Wan, W.; Zimmermann, K.; Bremer, E.; Dähne, L. High Loading Efficiency and Controlled Release of Bioactive Immunotherapeutic Proteins Using Vaterite Nanoparticles. Part. Part. Syst. Charact. 2021, 38, 7. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Abbott, D.P. Vaterite-Mineralization Product of Hard Tissues of A Marine Organism (Ascidiacea). Science 1975, 188, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Feng, Q.L.; Li, Z. Special vaterite found in freshwater lackluster pearls. Cryst. Growth Des. 2007, 7, 275–279. [Google Scholar] [CrossRef]
- Wightman, R.; Wallis, S.; Aston, P. Leaf margin organisation and the existence of vaterite-producing hydathodes in the alpine plant Saxifraga scardica. Flora 2018, 241, 27–34. [Google Scholar] [CrossRef]
- Konopacka-Lyskawa, D. Synthesis Methods and Favorable Conditions for Spherical Vaterite Precipitation: A Review. Crystals 2019, 9, 223. [Google Scholar] [CrossRef]
- Mugnaioli, E.; Andrusenko, I.; Schüler, T.; Loges, N.; Dinnebier, R.E.; Panthöfer, M.; Tremel, W.; Kolb, U. Ab Initio structure determination of vaterite by automated electron diffraction. Angew. Chem. Int. Ed. Engl 2012, 51, 7041–7045. [Google Scholar] [CrossRef]
- Gibson, R.E.; Wyckoff, R.W.G.; Merwin, H.E. Vaterite and mu-calcium carbonate. Am. J. Sci. 1925, 10, 325–333. [Google Scholar] [CrossRef]
- von Olshausen, S. Strukturunterschungen nach der Debye-Scherrer-Methode. Zeitschr. Kristallographie 1925, 61, 463–514. [Google Scholar]
- Meyer, H.J. Uber Vaterit Und Seine Struktur. Angew. Chem. Int. Ed. 1959, 71, 678. [Google Scholar]
- Meyer, H.J. Struktur und Fehlordnung des Vaterits. Z. Für Krist. Cryst. Mater. 1969, 128, 183–212. [Google Scholar] [CrossRef]
- Sato, M.; Matsuda, S. Structure of vaterite and infrared spectra. Z. Für Krist. 1969, 129, 405–410. [Google Scholar] [CrossRef]
- Gabrielli, C.; Jaouhari, R.; Joiret, S.; Maurin, G. In situ Raman spectroscopy applied to electrochemical scaling. Determination of the structure of vaterite. J. Raman Spectrosc. 2000, 31, 497–501. [Google Scholar] [CrossRef]
- Behrens, G.; Kuhn, T.L.; Ubic, R.; Heuer, H.A. Raman spectra of vateritic calcium carbonate. Spectrosc. Lett. 1995, 28, 983–995. [Google Scholar] [CrossRef]
- Le Bail, A.; Ouhenia, S.; Chateigner, D. Microtwinning hypothesis for a more ordered vaterite model. Powder Diffr. 2012, 26, 16–21. [Google Scholar] [CrossRef]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Dovesi, R. A new structural model for disorder in vaterite from first-principles calculations. Crystengcomm 2012, 14, 44–47. [Google Scholar] [CrossRef]
- Wang, J.W.; Becker, U. Structure and carbonate orientation of vaterite (CaCO3). Am. Mineral. 2009, 94, 380–386. [Google Scholar] [CrossRef]
- Steciuk, G.; Palatinus, L.; Rohlíček, J.; Ouhenia, S.; Chateigner, D. Stacking sequence variations in vaterite resolved by precession electron diffraction tomography using a unified superspace model. Sci. Rep. 2019, 9, 9156. [Google Scholar] [CrossRef]
- Christy, A.G. A Review of the Structures of Vaterite: The Impossible, the Possible, and the Likely. Cryst. Growth Des. 2017, 17, 3567–3578. [Google Scholar] [CrossRef]
- Andreassen, J.P. Formation mechanism and morphology in precipitation of vaterite-nano aggregation or crystal growth? J. Cryst. Growth 2005, 274, 256–264. [Google Scholar] [CrossRef]
- Gránásy, L.; Pusztai, T.; Tegze, G.; Warren, J.A.; Douglas, J.F. Growth and form of spherulites. Phys. Rev. E 2005, 72, 011605. [Google Scholar] [CrossRef]
- Ma, M.G.; Su, R.C. Biomineralization and Biomimetic Synthesis of Biomineral and Nanomaterials. In Advances in Biomimetics; Anne, G., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapther 2. [Google Scholar]
- Dietzsch, M.; Andrusenko, I.; Branscheid, R.; Emmerling, F.; Kolb, U.; Tremel, W. Snapshots of calcium carbonate formation-A step by step analysis. Z. Für Krist. Cryst. Mater. 2017, 232, 255–265. [Google Scholar] [CrossRef]
- Rudloff, J.; Cölfen, H. Superstructures of temporarily stabilized nanocrystalline CaCO3 particles: Morphological control via water surface tension variation. Langmuir 2004, 20, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Yu, S.H.; Guo, X.H. Double hydrophilic block copolymer controlled growth and self-assembly of CaCO3 multilayered structures at the air/water interface. Langmuir 2006, 22, 6125–6129. [Google Scholar] [CrossRef]
- Guo, X.H.; Yu, S.H.; Cai, G.B. Crystallization in a mixture of solvents by using a crystal modifier: Morphology control in the synthesis of highly monodisperse CaCO3 microspheres. Angew. Chem. Int. Ed. Engl. 2006, 45, 3977–3981. [Google Scholar] [CrossRef]
- Qi, L.M.; Li, J.; Ma, J.M. Biomimetic morphogenesis of calcium carbonate in mixed solutions of surfactants and double-hydrophilic block copolymers. Adv. Mater. 2002, 14, 300–303. [Google Scholar] [CrossRef]
- Meng, Q.W.; Wang, X.; Chen, D.Z.; Yu, X.H. Influence of hyperbranched DHBC with carboxyl terminal groups on the crystallization of CaCO3. Chin. J. Inorg. Chem. 2006, 22, 447–450. [Google Scholar]
- Qun, Z.; Chuanbao, C.; Juan, F.; Liang, F.; Liying, R. Influence of A Double-Hydrophilic Block Copolymer With Sulfonic Groups on The Crystallization of CaCO3. Acta Polym. Sin. 2008, 10, 1010–1014. [Google Scholar] [CrossRef]
- Borisov, S.V.; Magarill, S.A.; Pervukhina, N.V. Crystallographic Analysis of Symmetry-Stability Relations in Atomic Structures. J. Struct. Chem. 2019, 60, 1191–1218. [Google Scholar] [CrossRef]
- Borisov, S.V.; Magarill, S.A.; Pervukhina, N.V. Crystallographic Analysis of Three Modifications of Caco3: Calcite, Aragonite, Vaterite. J. Struct. Chem. 2021, 62, 1027–1037. [Google Scholar] [CrossRef]
- Maciejewski, M.; Oswald, H.R.; Reller, A. Thermal Transformations of Vaterite and Calcite. Thermochim. Acta 1994, 234, 315–328. [Google Scholar] [CrossRef]
- Tsuno, H.; Kagi, H.; Akagi, T. Effects of trace lanthanum ion on the stability of vaterite and transformation from vaterite to calcite in an aquatic system. Bull. Chem. Soc. Jpn. 2001, 74, 479–486. [Google Scholar] [CrossRef]
- Nassrallah-Aboukais, N.; Boughriet, A.; Gengembre, L.; Aboukais, A. Manganese(II)/vaterite/water systems-Spectroscopic and thermodynamic study. J. Chem. Soc. Faraday Trans. 1998, 94, 2399–2405. [Google Scholar] [CrossRef]
- Maruyama, K.; Kagi, H.; Komatsu, K.; Yoshino, T.; Nakano, S. Pressure-induced phase transitions of vaterite, a metastable phase of CaCO3. J. Raman Spectrosc. 2017, 48, 1449–1453. [Google Scholar] [CrossRef]
- Nassrallah-Aboukais, N.; Boughriet, A.; Gengembre, L.; Aboukais, A. Raman spectroscopic studies of carbonates part I: High-pressure and high-temperature behaviour of calcite, magnesite, dolomite and aragonite. Phys. Chem. Miner. 1993, 20, 1–18. [Google Scholar]
- Wehrmeister, U.; Soldati, A.L.; Jacob, D.E.; Häger, T.; Hofmeister, W. Raman spectroscopy of synthetic, geological and biological vaterite: A Raman spectroscopic study. J. Raman Spectrosc. 2010, 41, 193–201. [Google Scholar] [CrossRef]
- Arabit, J.; Prentice, D.; Luong, J.; Bouissonnié, A.; Govindhakannan, J.; Rosner, F.; Simonetti, D.; La Plante, E.; Gadt, T.; Sant, G. Isopropanol Mediates the Rapid and Selective Synthesis of Vaterite during Ambient Carbonation. ACS Sustain. Chem. Eng. 2025, 13, 7549–7561. [Google Scholar] [CrossRef]
- Arabit, J.; Prentice, D.; Luong, J.; Bouissonnié, A.; Govindhakannan, J.; Rosner, F.; Simonetti, D.; La Plante, E.; Gadt, T.; Sant, G. Sustainable utilization of magnesium slag through carbonation: A pathway to high-value products with vaterite calcium carbonate. J. Sustain. Cem. Based Mater. 2025, 14, 89–102. [Google Scholar]
- Kitamura, M. Crystallization and transformation mechanism of calcium carbonate polymorphs and the effect of magnesium ion. J. Colloid Interface Sci. 2001, 236, 318–327. [Google Scholar] [CrossRef]
- Hadiko, G.; Han, Y.S.; Fuji, M.; Takahashi, M. Effect of magnesium ion on the precipitation of hollow calcium carbonate by bubble templating method. Key Eng. Mater. 2006, 317, 65–68. [Google Scholar] [CrossRef]
- Song, X.; Tang, Z.; Hua, X.; Li, D.; Li, M.; Bu, X. The role of NH+ on controlling the pure vaterite transformations. J. Mol. Liq. 2024, 407, 125229. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, J.; Teng, H.; Becker, U. Growth process and crystallographic properties of ammonia-induced vaterite. Am. Mineral. 2012, 97, 1437–1445. [Google Scholar] [CrossRef]
- Kogo, M.; Umegaki, T.; Kojima, Y. Effect of pH on formation of single-phase vaterite. J. Cryst. Growth 2019, 517, 35–38. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Ren, Y.; Yan, H.; Wang, M.; Wang, D.; Lu, X.Y.; Wang, B.; Fan, T.; Guo, H. Controllable synthesis of all the anhydrous CaCO3 polymorphs with various morphologies in CaCl2-NH3-CO2 aqueous system. Powder Technol. 2018, 333, 410–420. [Google Scholar] [CrossRef]
- Li, H.; Yao, Q.Z.; Wang, F.P.; Huang, Y.R.; Fu, S.Q.; Zhou, G.T. Insights into the formation mechanism of vaterite mediated by a deep-sea bacterium Shewanella piezotolerans WP3. Geochim. Cosmochim. Acta 2019, 256, 35–48. [Google Scholar] [CrossRef]
- Hou, W.; Feng, Q. Morphology and formation mechanism of vaterite particles grown in glycine-containing aqueous solutions. Mater. Sci. Eng. C 2006, 26, 644–647. [Google Scholar] [CrossRef]
- Lu, H.; Lutz, H.; Roeters, S.J.; Hood, M.A.; Schäfer, A.; Munoz-Espi, R.; Berger, R.; Bonn, M.; Weidner, T. Calcium-Induced Molecular Rearrangement of Peptide Folds Enables Biomineralization of Vaterite Calcium Carbonate. J. Am. Chem. Soc. 2018, 140, 2793–2796. [Google Scholar] [CrossRef]
- Chen, T.; Shi, P.; Li, Y.; Duan, T.; Yu, Y.; Li, X.; Zhu, W. Biomineralization of varied calcium carbonate crystals by the synergistic effect of silk fibroin/magnesium ions in a microbial system. Crystengcomm 2018, 20, 2366–2373. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Zhang, Z.; Pan, Z.; Cheng, H.; Cheng, F. Glycine-induced synthesis of vaterite by direct aqueous mineral carbonation of desulfurization gypsum. Environ. Chem. Lett. 2022, 20, 2261–2269. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Gonzalez-Muñoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralization of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yao, Q.Z.; Li, H.; Zhou, G.T.; Sheng, Y.M. Formation of Vaterite Mesocrystals in Biomineral-like Structures and Implication for Biomineralization. Cryst. Growth Des. 2015, 15, 1714–1725. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Wang, S.Q.; Tong, X.Y.; Kang, X. Crystal transformation and self-assembly theory of microbially induced calcium carbonate precipitation. Appl. Microbiol. Biotechnol. 2022, 106, 3555–3569. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, H.; Nie, W.; Sun, M.; Li, X.; Lian, B. Facilitated Mechanism of Biological Vaterite Stability Mediated by Bacillus velezensis and Its Secretions. ACS Earth Space Chem. 2023, 7, 2019–2030. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Ren, K.; Dong, H.; Lian, B. Mechanisms of carbonate precipitation induced by two model bacteria. Chem. Geol. 2023, 628, 121461. [Google Scholar] [CrossRef]
- Debnath, A.; Hazra, C.; Sen, R. Insight into biomolecular interaction-based non-classical crystallization of bacterial biocement. Appl. Microbiol. Biotechnol. 2023, 107, 6683–6701. [Google Scholar] [CrossRef]
- Lv, J.J.; Ma, F.; Li, F.C.; Zhang, C.H.; Chen, J.N. Vaterite induced by &ITLysinibacillus&IT sp GW-2 strain and its stability. J. Struct. Biol. 2017, 200, 97–105. [Google Scholar]
- Clarà Saracho, A.; Haigh, S.K.; Hata, T.; Soga, K.; Farsang, S.; Redfern, S.A.; Marek, E. Characterisation of CaCO3 phases during strain-specific ureolytic precipitation. Sci. Rep. 2020, 10, 10168. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Hu, Q.; Chen, J.; Ji, J.; Teng, H.H. Carbonate biomineralization induced by soil bacterium Bacillus megaterium. Geochim. Cosmochim. Acta 2006, 70, 5522–5535. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem. Geol. 2009, 262, 138–146. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Q.; Chi, W.; Cai, M.; Wang, R.; Fu, Z.; Xie, J.; Zou, Z. Multiple crystallization pathways of amorphous calcium carbonate in the presence of poly(aspartic acid) with a chain length of 30. Crystengcomm 2022, 24, 4809–4818. [Google Scholar] [CrossRef]
- Hoffmann, T.D.; Reeksting, B.J.; Gebhard, S. Bacteria-induced mineral precipitation: A mechanistic review. Microbiology 2021, 167, 001049. [Google Scholar] [CrossRef]
- Zheng, T.W.; Yi, H.H. Formation and Stabilization of Vaterite Aggregate Grooves with Aspartic Acid (Asp) by Bubbling CO2 into a Ca(OH) 2 Suspension. Cryst. Res. Technol. 2021, 56, 11. [Google Scholar]
- Dupont, L.; Portemer, F.; Figlarz, M. Synthesis and study of a well crystallized CaCO3 vaterite showing a new habitus. J. Mater. Chem. 1997, 7, 797–800. [Google Scholar] [CrossRef]
- Rautaray, D.; Ahmad, A.; Sastry, M. Biosynthesis of CaCO3 Crystals of Complex Morphology Using a Fungus and an Actinomycete. J. Am. Chem. Soc. 2003, 125, 14656–14657. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Matijevic, E. Homogeneous Precipitation of Calcium Carbonates by Enzyme Catalyzed Reaction. J. Colloid Interface Sci. 2001, 238, 208–214. [Google Scholar] [CrossRef]
- Ueyama, N.; Takahasi, K.; Onoda, A.; Okamura, T.A.; Yamamoto, H. Tight binding of poly(carboxylate) ligand to calcium carbonate with intramolecular NH…O hydrogen bond. Macromol. Symp. 2002, 186, 129–134. [Google Scholar] [CrossRef]
- Takahashi, K.; Doi, M.; Kobayashi, A.; Taguchi, T.; Onoda, A.; Okamura, T.A.; Yamamoto, H.; Ueyama, N. Formation of 6-, 7- or 8-membered ring intra-side-chain NH O hydrogen bond toward Ca-binding oxyanion in poly(allylaminocarboxylate) ligands stabilizes CaCO3 vaterite crystals. J. Cryst. Growth 2004, 263, 552–563. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Influence of the primary structure of enzymes on the formation of CaCO3 polymorphs:: A comparison of plant (Canavalia Ensiformis) and bacterial (Bacillus Pasteurii) ureases. Langmuir 2005, 21, 8876–8882. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Decho, A.W. A laboratory investigation of cyanobacterial extracellular polymeric secretions (EPS) in influencing CaCO3 polymorphism. J. Cryst. Growth 2002, 240, 230–235. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. The role of bacterial extracellular polymeric substances in geomicrobiology. Chem. Geol. 2014, 386, 115–132. [Google Scholar] [CrossRef]
- Chen, X.; Xin, M.; Li, M.; Chen, Z.; Chen, Z. Biomimetic Synthesis of Vaterite by N-Succinyl-OHydroxypropyl Sulfonated Chitosan. Chin. J. Mater. Res. 2016, 30, 31–37. [Google Scholar]
- Jin, B.; Wang, S.; Lei, Y.; Jia, H.; Niu, Q.; Dapaah, M.F.; Gao, Y.; Cheng, L. Green and effective remediation of heavy metals contaminated water using CaCO3 vaterite synthesized through biomineralization. J. Environ. Manag. 2024, 353, 120136. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, B.; Ping, H.; Fu, Z.; Li, Y.; Wang, W.; Wang, H.; Wang, Y.; Zhang, J.; Zhang, F. Template-free synthesis of hierarchical porous calcium carbonate microspheres for efficient water treatment. RSC Adv. 2016, 6, 472–480. [Google Scholar] [CrossRef]
- Liu, R.; Lian, B. Non-competitive and competitive adsorption of Cd2+, Ni2+, and Cu2+ by biogenic vaterite. Sci Total Environ. 2019, 659, 122–130. [Google Scholar] [CrossRef]
- Dang, H.C.; Yuan, X.; Xiao, Q.; Xiao, W.X.; Luo, Y.K.; Wang, X.L.; Song, F.; Wang, Y.Z. Facile batch synthesis of porous vaterite microspheres for high efficient and fast removal of toxic heavy metal ions. J. Environ. Chem. Eng. 2017, 5, 4505–4515. [Google Scholar] [CrossRef]
- Lin, P.Y.; Wu, H.M.; Hsieh, S.L.; Li, J.S.; Dong, C.; Chen, C.W.; Hsieh, S. Preparation of vaterite calcium carbonate granules from discarded oyster shells as an adsorbent for heavy metal ions removal. Chemosphere 2020, 254, 126903. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Yu, S.H.; Jiang, H.F.; Yao, Q.Z.; Fu, S.Q.; Zhou, G.T. Performance and mechanism of simultaneous removal of Cd(II) and Congo red from aqueous solution by hierarchical vaterite spherulites. Appl. Surf. Sci. 2018, 444, 224–234. [Google Scholar] [CrossRef]
- Zhang, H. Preparation of Vaterite-Type Calcium Carbonate and Its Adsorption Behavior and Mechanism of Phosphorus and Lead in Water. Master’s Thesis, Inner Mongolia University of Science & Technology, Baotou, China.
- He, X.; Hu, M.; Cui, Y.; Wang, X.; Lian, B. Simulated Experimental Study on the Removal of Methylene Blue-Cu(II) Composite Pollution by Magnetized Vaterite. Minerals 2024, 14, 1142. [Google Scholar] [CrossRef]
- Sawada, K.; Yoshida, S.; Suzuki, T. Adsorption Of Phosphate On Vaterite. J. Chem. Soc. Faraday Trans. 1992, 88, 2227–2231. [Google Scholar] [CrossRef]
- Chong, K.Y.; Chia, C.H.; Zakaria, S.; Sajab, M.S. Vaterite calcium carbonate for the adsorption of Congo red from aqueous solutions. J. Environ. Chem. Eng. 2014, 2, 2156–2161. [Google Scholar] [CrossRef]
- Nagpal, M.; Kakkar, R. Selective adsorption and separation of toxic cationic dyes using hierarchically porous SDBS modified vaterite microspheres (Hr-SMV). J. Phys. Chem. Solids 2020, 146, 109598. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, P.; An, X.; Wang, X.; Li, S.; Lian, B. Vaterite Synthesized by Waste Liquid of Extracting Chitin from Crab Shells and the Mineral Loading for Doxorubicin Hydrochloride. Minerals 2022, 12, 1608. [Google Scholar] [CrossRef]
- Song, X.; Tuo, Y.; Li, D.; Hua, X.; Wang, R.; Xue, J.; Yang, R.; Bu, X.; Luo, X. A Green Approach to Preparing Vaterite CaCO3 for Clean Utilization of Steamed Ammonia Liquid Waste and CO2 Mineralization. Sustainability 2023, 15, 13275. [Google Scholar] [CrossRef]
- Li, S.Y.; Lian, B. Application of Calcium Carbonate as a Controlled Release Carrier for Therapeutic Drugs. Minerals 2023, 13, 1136. [Google Scholar] [CrossRef]
- Grigorieva, D.V.; Mikhalchik, E.V.; Balabushevich, N.G.; Mosievich, D.V.; Murina, M.A.; Panasenko, O.M.; Sokolov, A.V.; Gorudko, I.V. Effect of Biopolymers and Functionalized by Them Vaterite Microparticles on Platelet Aggregation. J. Evol. Biochem. Physiol. 2024, 60, 1221–1233. [Google Scholar] [CrossRef]
- Fu, L.H.; Qi, C.; Hu, Y.R.; Mei, C.G.; Ma, M.G. Cellulose/vaterite nanocomposites: Sonochemical synthesis, characterization, and their application in protein adsorption. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 96, 426–435. [Google Scholar] [CrossRef]
- Peng, C.Y.; Zhao, Q.H.; Gao, C.Y. Sustained delivery of doxorubicin by porous CaCO3 and chitosan/alginate multilayers-coated CaCO3 microparticles. Colloids Surf. A-Physicochem. Eng. Asp. 2010, 353, 132–139. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Filatova, L.Y.; Kirzhanova, E.A.; Mikhalchik, E.V.; Volodkin, D.; Vikulina, A.S. Hybrid Mucin-Vaterite Microspheres for Delivery of Proteolytic Enzyme Chymotrypsin. Macromol. Biosci. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Schlicht, S.; Campbell, J.; Weber, A.; Westhoff, J.; Volodkin, D.; Fischer, D.; Drummer, D.; Vikulina, A. Vaterite-based in situ surface modification and process-dependent biocompatibility of laser sintered polypropylene. J. Mater. Res. Technol. JMRT 2024, 32, 3447–3455. [Google Scholar] [CrossRef]
- Fujikura, K.; Obata, A.; Lin, S.; Jones, J.R.; Law, R.V.; Kasuga, T. Preparation of Electrospun Poly(Lactic Acid)-Based Hybrids Containing Siloxane-Doped Vaterite Particles for Bone Regeneration. J. Biomater. Sci. Polym. Ed. 2012, 23, 1369–1380. [Google Scholar] [CrossRef]
- Wakita, T.; Obata, A.; Poologasundarampillai, G.; Jones, J.R.; Kasuga, T. Preparation of electrospun siloxane-poly(lactic acid)-vaterite hybrid fibrous membranes for guided bone regeneration. Compos. Sci. Technol. 2010, 70, 1889–1893. [Google Scholar] [CrossRef]
- Obata, A.; Tokuda, S.; Kasuga, T. Enhanced in vitro cell activity on silicon-doped vaterite/poly(lactic acid) composites. Acta Biomater. 2009, 5, 57–62. [Google Scholar] [CrossRef]
- Schröder, R.; Besch, L.; Pohlit, H.; Panthöfer, M.; Roth, W.; Frey, H.; Tremel, W.; Unger, R.E. Particles of vaterite, a metastable CaCO3 polymorph, exhibit high biocompatibility for human osteoblasts and endothelial cells and may serve as a biomaterial for rapid bone regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 1754–1768. [Google Scholar] [CrossRef]
- Maeda, H.; Maquet, V.; Chen, Q.Z.; Kasuga, T.; Jawad, H.; Boccaccini, A.R. Bioactive coatings by vaterite deposition on polymer substrates of different composition and morphology. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2007, 27, 741–745. [Google Scholar] [CrossRef]
- Yamada, S.; Yamamoto, A.; Kasuga, T. Poly(L-lactic acid)/vaterite composite coatings on metallic magnesium. J. Mater. Sci. Mater. Med. 2014, 25, 2639–2647. [Google Scholar] [CrossRef]
- Obata, A.; Hasegawa, D.; Nakamura, J.; Jones, J.R.; Kasuga, T. Induction of hydroxycarbonate apatite formation on polyethylene or alumina substrates by spherical vaterite particles deposition. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1976–1981. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, F.; Jinag, L.; Lu, B.; Hou, G.; Zhu, J. Production of vaterite via wet carbonation of carbide residue: Enhancing cement properties and CO2 sequestration. Cem. Concr. Compos. 2024, 150, 105549. [Google Scholar] [CrossRef]
- Maeda, H.; Kogo, Y.; Obata, A.; Inukai, K.; Kato, K.; Kasuga, T. Surface modification of poly(L-lactic acid)-vaterite composites on a zirconia substrate by imogolite coating. J. Ceram. Soc. Jpn. 2013, 121, 749–752. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Zhu, J.; Zhao, R.; Guan, X. Process and Mechanism of Vaterite Calcium Carbonate Preparation from Calcium Carbide Slag. J. Build. Mater. 2023, 26, 939–948. [Google Scholar]
- Fan, T.B.; Jia, X.H.; Han, D.X.; Chen, S.; Jiang, Y.; Hu, T.T.; Guo, H.F.; Li, L.; Liu, Y.Y. Study on preparation of vaterite from dolomite by ammonia-alkali method and its mechanism. Inorg. Chem. Ind. 2021, 53, 56–60. [Google Scholar]
- Song, X.H.; Pan, M.Y.; Xu, J.F.; Huang, Z.Y.; Pan, Q.; Li, Q.N. Applications of Vaterite in Drug Loading and Controlled Release. Prog. Biochem. Biophys. 2025, 52, 162–181. [Google Scholar]
- Borkowski, A.; Działak, P.; Berent, K.; Gajewska, M.; Syczewski, M.D.; Słowakiewicz, M. Mechanism of bacteriophage-induced vaterite formation. Sci. Rep. 2024, 14, 20481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).