Abstract
Here, we report the discovery of silica- and sulfur-enriched deposits forming on the seafloor off Zannone Island (western Mediterranean Sea), where hydrothermal activity is ongoing. Our multidisciplinary investigation reveals that these deposits form through the interplay between hydrothermal processes and microbial activity. The deposits result from a dynamic equilibrium involving microbial mediation, sedimentation, and episodic lithification, driven primarily by two mineralization pathways: silica and sulfur precipitation. This study provides new insights into the bio-sedimentary processes shaping authigenic crusts in shallow submarine hydrothermal settings, contributing to a broader understanding of mineralization in marine environments influenced by both geological and biological factors.
1. Introduction
Hydrothermal systems are among the most dynamic and chemically diverse environments on Earth, characterized by the circulation of heated fluids through the Earth’s crust and their subsequent discharge onto the seafloor. These systems are important natural laboratories for studying geological, chemical, and biological interactions, particularly the processes that drive mineral precipitation. Mineral formation in hydrothermal contexts can result from a variety of mechanisms, including the rapid cooling of high-temperature fluids, phase separation, mixing with seawater, or biologically mediated processes [1]. Microorganisms can induce mineral precipitation either directly, via metabolic activity (e.g., redox reactions and biomineralization), or indirectly, by serving as nucleation sites through the production of extracellular polymeric substances (EPSs) [2].
These extreme environments commonly support chemosynthetic microbial communities that play a major role in biogeochemical processes [3]. These microbial assemblages play a central role in biogeochemical cycling and can form extensive microbial mats contributing to the accumulation of authigenic mineral deposits, including iron-, manganese-, and phosphorus-rich ores [4,5,6]. Such deposits provide critical archives of microbial activity over geological timescales.
Microbial activity in hydrothermal environments has been shown to mediate the formation of a wide range of minerals, including carbonates, sulfur compounds, silicates, and sulfates, as well as to promote sediment trapping and stabilization, e.g., [7]. These organo-sedimentary structures, formed through the interaction between microorganisms and their environment, are particularly relevant, as they represent some of the earliest evidence of life on Earth, with fossil records extending back over 3.7 billion years [8]. Modern analogs of such structures are found in various hydrothermal and aquatic settings, including geothermal springs [9,10,11], open marine environments [12,13], and inland aquatic systems such as lakes and hypersaline ponds, e.g., [14,15,16]. These deposits exhibit a wide variety of morphologies and textures, from macroscopic domes and pinnacles to microscopic laminae and filaments, e.g., [17]. In rare cases, exceptional environmental conditions, such as rapid silica deposition, can lead to the preservation of entire microbial cells and EPS networks [18], offering valuable windows into ancient microbial ecosystems.
Moreover, their relevance has extended beyond Earth, as similar processes and mineral assemblages may occur on other planetary bodies, particularly Mars, where evidence of past hydrothermal activity and silica-rich outcrops has been documented [19,20]. Consequently, organo-sedimentary deposits formed in hydrothermal settings are increasingly viewed as high-priority analogs for astrobiological research [21,22].
Despite the growing recognition of the Zannone Giant Pockmark (ZGP) as an active hydrothermal system [23] located off Zannone Island in the western Pontine Archipelago (western Mediterranean Sea), a detailed characterization of the silica- and sulfur-rich authigenic deposits and their association with microbial processes remains unexplored. Previous research has described the geological, geochemical, and biological setting of the ZGP [24,25,26,27]. Our study fills this gap by providing the first integrated morphological, mineralogical, and geochemical characterization of these crusts, with a focus on microbial signatures and their role in mineralization processes.
These crusts represent unusual silica- and sulfur-rich deposits that preserve microbial signatures associated with low-temperature hydrothermal activity. The results provide compelling new evidence for active biomineralization processes, specifically, microbially mediated silica and sulfur precipitation, within this submarine environment. Notably, the ZGP emerges as a globally unique site, as it represents a relatively shallow marine site (<200 m water depth) where extensive silica- and sulfur-enriched authigenic deposits are actively forming on the seafloor and preserving microbial structures. Our findings contribute to a deeper understanding of hydrothermal mineralization, microbial preservation, and the potential signatures of life in both Earth and extraterrestrial contexts.
Geological Background and Hydrothermal Activity of the Zannone Giant Pockmark (ZGP)
The authigenic crusts analyzed in this study were collected from the seafloor approximately 3 km off the eastern coast of Zannone Island, within a large seafloor depression known as the Zannone Giant Pockmark (ZGP) [25], at water depths ranging from 128 to 135 m. Zannone Island (ZI), together with Ponza and Palmarola islands, forms part of the western Pontine Archipelago in the Tyrrhenian Sea. This area is located on a folded-thrust structural high [28], about 35 km off the Italian mainland (Figure 1a). The geological framework of ZI is complex. The island is primarily composed of volcanic units overlying older, very low-grade metamorphic rocks (i.e., phyllite-, quartzite-, and quartz-rich sandstone of Paleozoic–Triassic age) [29]. These are overlain by black clays, dolomitic limestones, and dolomites of Late Triassic age, followed by Cretaceous–Eocene limestones and marls, and Miocene flysch deposits. Notably, the pre-volcanic substratum is exposed only along the northern cliffs of the island [29].
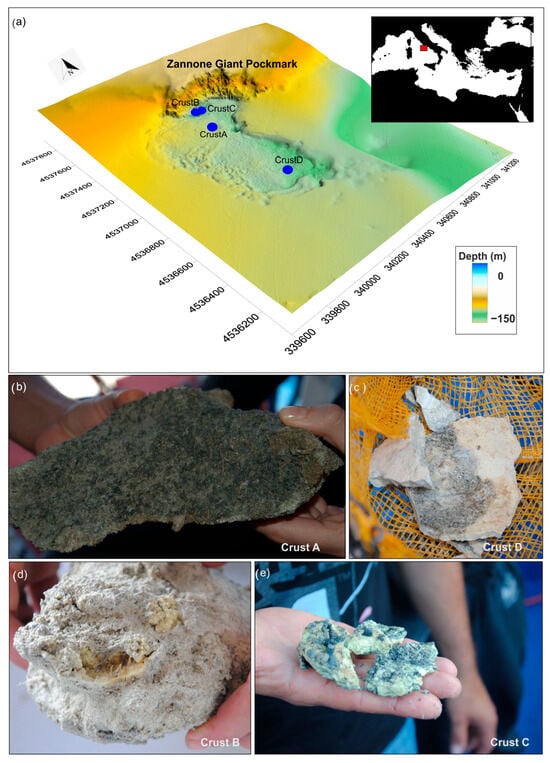
Figure 1.
(a) Three-dimensional shaded relief map of the seafloor surrounding the Zannone Giant Pockmark (ZGP) with location of the sampled authigenic crusts. (b–e) Macroscopic samples of the analyzed authigenic crusts.
The ZGP is part of a recently identified shallow-water, high-temperature hydrothermal field surrounding ZI, covering an area of approximately 60 km2 [23]. Geomorphological analyses indicate that the ZGP comprises at least three major craters, each associated with distinct eruptive events that contributed to its formation. The hydrothermal venting areas within the ZGP display a range of morphologies, including small cones, depressions, mounds, terraced and domal crusts, chimneys (both active and inactive), and widespread white bacterial mats [25].
Hydrothermal activity in the ZGP is characterized by the release of high-temperature fluids dominated by CO2 (over 90% by volume), with minor contributions of CH4 and H2S [23,30]. Fluid discharge occurs in various forms, including diffuse seepage creating pockmarked seafloor, continuous or intermittent bubble streams from isolated or clustered vents, and fluid plumes associated with authigenic crusts. The measured vent fluid temperatures near the seafloor reach approximately 60 °C [25], while geochemical models estimate equilibrium temperatures ranging from 150 to 180 °C [23]. pH values measured at depths between 50 and 134 m range from 8.04 to 8.19 [30]. Isotopic analyses of CO2 and helium indicate a substantial mantle-derived component, with δ13C values ranging from −6 to 0‰ and 3He/4He ratios (Ra) between 3.0 and 3.8 [23,30].
2. Materials and Methods
2.1. Sample Collection and Processing
Sediment samples and remotely operated vehicle (ROV) videos were acquired during a research cruise conducted in 2014 by the National Research Council–Institute of Environmental Geology and Geoengineering (CNR-IGAG), aboard the R/V Urania. Authigenic crusts were recovered from the seafloor within the ZGP, at water depths ranging from 128 to 135 m (Table 1).

Table 1.
List of the analyzed crusts sampled within the ZGP.
In the laboratory, the authigenic crust samples were initially examined under an optical microscope to obtain a general overview of their textures and to select distinct morphological fractions. Selected fragments were then analyzed using X-ray Powder Diffraction (XRPD) and observed with Scanning Electron Microscopy equipped with Energy Dispersive X-ray Spectroscopy (SEM-EDS). These techniques were employed to investigate the structural, morphological, mineralogical, and biological characteristics of the samples. The analytical procedures are detailed below.
2.2. X-Ray Powder Diffraction, Chemical, and Isotope Analyses
X-ray Powder Diffraction (XRPD) analyses were carried out using a Bruker D5000 diffractometer at the Department of Earth Sciences, Sapienza University of Rome. The instrument operated with CuKα radiation (λ = 1.5418 Å) at 40 kV and 30 mA. Data were collected with a step size of 0.0250° and a count time of 2 s per step. Diffraction peak positions were identified by comparison with standard reference patterns from the ICDD-JCPDS database.
Major element compositions and δ34S isotopic analyses were performed at the Activation Laboratories Ltd. (Actlabs), Ancaster, Canada, by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-OES) following the ‘WRA + Trace 4 Lithoresearch’ protocol and by Elemental Analysis–Isotope Ratio Mass Spectrometry (EA-IRMS) following the ‘Code 10A’ protocol, respectively. Detailed descriptions of the analytical procedures are available on the laboratory’s website: http://www.actlabs.com (accessed on 18 June 2025).
2.3. Scanning Electron Microscopy (SEM-EDS)
Scanning Electron Microscopy (SEM) combined with Energy Dispersive X-ray Spectrometry (EDS) was employed to investigate the morphological and compositional characteristics of the ZGP crusts, with particular attention to microstructural features and potential biological evidence. A combined qualitative and semi-quantitative approach was adopted.
Observations were conducted using two different instruments: a FEI Quanta 400 SEM and a ZEISS SEM with a thermionic source and LaB6 crystal cathode associated with an Oxford X-Max EDS spectrometer with an 80 mm2 detector. The instruments are located at the Microanalysis and Electron Microscopy Laboratory (LaM2), jointly operated by Department of Earth Sciences, Sapienza University of Rome, and the Institute of Environmental Geology and Geoengineering of the National Research Council.
Whole sediment samples were mounted on 12.5 mm aluminum SEM stubs using carbon adhesive tabs and subsequently coated with a thin layer (15–30 nm) of gold using an Emitech K550X sputter coater. The coating process was performed under standard conditions, with a cycle time of less than four minutes.
SEM imaging and EDS analyses were performed under vacuum mode, using an accelerating voltage between 15 and 30 kV. The working distance was adjusted between 8.5 and 16 mm to optimize focus and image resolution, which ranged from 30 to 200 µm. SEM-EDS analysis provided detailed information on sample chemistry, microstructure, and the presence of biological inclusions. Additionally, elemental X-ray maps (1024 × 768 resolution pixels) were acquired to assess the spatial distribution of key elements, particularly silicon (Si) and sulfur (S), within selected areas of the samples. The maps were performed under the following operating conditions: working distance 8.5 mm, 20 KV acceleration voltage, 50 pA probe current, process time 3, and acquisition time 3 min.
3. Results
3.1. Zannone Crusts: Morphology and Distribution
The authigenic crusts analyzed in this study were collected from within the ZGP, at water depths ranging from 128 to 135 m (Figure 1). These crusts are lithified sediments exhibiting a variety of morphologies, ranging from flange-like to dome-shaped forms (Figure 2).
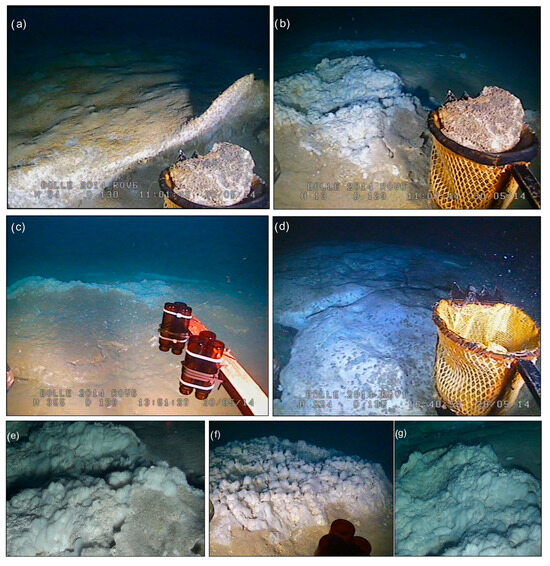
Figure 2.
(a–d) ROV images showing the sampled crusts and the surrounding seafloor. (e–g) HD ROV images showing the microbial assemblages associated with some of the sampled crusts.
Crust A was recovered from a sub-circular, crater-like depression in the northeastern sector of the ZGP, at a water depth of 128 m. As previously reported in [27], Crust A is a lithified crust composed of yellow-brown cemented sand, exhibiting macroscopic evidence of both coated grains and microbial activity, including visible bacterial filaments and dark organic mats (Figure 1b and Figure S1). The coated grains appear as yellow concentric layers of mineral precipitates (primarily silica and sulfur), which may result from repeated cycles of microbial activity and mineral deposition (Figure S1). This crust has a multi-level flange shape and a thickness of approximately 5 cm. The surrounding seafloor is covered with widespread white bacterial aggregates (Figure 2a), often associated with continuous or intermittent bubble streams. The bacterial aggregates form linear and circular patterns with a thickness of about 5–10 cm. Upon closer inspection, these aggregates appear as white gelatinous spheres, collectively forming a sub-circular mat structure on the seafloor (Figure 2e–g).
Crusts B and C (ST2BNR1 and ST2BNR3) were recovered from an elongate depression in the northern sector of the ZGP, at a water depth of 135 m. Crust B was found buried in sandy sediment and is characterized by cemented sandy grains forming a lithified crust, with a core composed of numerous smooth yellow spheres that are cemented together to form a central aggregate structure (Figure 1d). Crust C, recovered from gravelly sandy sediment, consists of a yellow portion and a darker granular section (Figure 1e). The seafloor surrounding both Crust B and Crust C is characterized by intense venting activity, as evidenced by the presence of a large plume reaching heights of 40–70 m from the seafloor, widespread bacterial mats, and multiple bubble streams (Figure 2b,c).
Crust D was recovered from the southern sector of the ZGP, at a water depth of 130 m. Crust D is a homogeneous crust with a thickness of about 0.5 cm, composed of yellow millimetric grains (Figure 1c). Video observations revealed that Crust D represents a small fragment of a larger authigenic dome crust forming a seafloor pavement, which is characterized by active vents and the presence of widespread bacterial mats (Figure 2d).
3.2. Mineral-Chemical Analyses
X-ray diffraction, SEM, and chemical analyses performed on the studied crusts revealed that Crust A, Crust B, and Crust C are sulfur-cemented sandy samples primarily composed of quartz, alkaline feldspars, and plagioclase [27]. Grains of pyroxene and small iron spheres were observed in Crust A, while occasional barite was present in both Crust A and Crust B samples. Crust C also contains clasts of altered rhyolite and quartz–pyrite, in addition to hydrothermal phyllosilicates (illite and kaolinite), gypsum, rutile, and barite crystals (up to 50 µm in size). Moreover, in Crust C, there is clear evidence of organo-mineral aggregates associated with microbial communities.
In contrast, Crust D consists of a cemented sandy crust composed almost entirely of sulfur crystals (Figure 3).
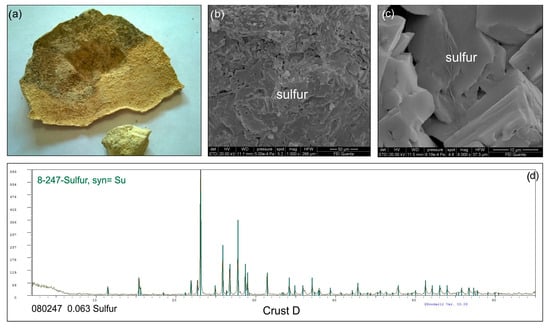
Figure 3.
(a) Macroscopic sample of Crust D. (b,c) SEM images showing the crust is made up exclusively of sulfur crystals; (d) XRD diffractogram of Crust D.
Major element bulk analyses (Table S1) show that SiO2, Al2O3, and alkali oxides are the predominant elements in the sandy samples. These concentrations align with the main mineral assemblages and their relative percentages in each sample. The key difference among the samples lies in the percentage of sulfur cement, as reflected in the varying Loss on Ignition (LOI) values (Table S1). The LOI value for Crust D is nearly 100%, indicating that this crust is almost entirely composed of elemental sulfur (Table S1).
3.3. δ34S Dissolved Sulfur Analysis
The conventional sulfur isotope δ34S data for the Zannone crusts show limited variation, ranging from −2.03‰ to −9.82‰ (Table 2). Notably, Crust C, which has the highest δ34S value (−2.03‰), may reflect a greater contribution from S-bearing mineral phases, such as barite and gypsum, which are present in varying abundances. These minerals are characterized by high positive δ34S values, with barite, for example, exhibiting a δ34S signature of +25.0‰ or higher [31].

Table 2.
Analytical results of the sulfur isotopic composition of the Zannone crusts.
3.4. Zannone Crusts: Biological Evidence
Evidence of biological activity in the ZGP crusts is observed through SEM analysis, which reveals the presence of bacterial filaments forming dense, tangled networks. These filaments vary in length from 20 to 100 µm and in width from 5 to 10 µm (Figure 4a–c). The branching filaments are sometimes composed of a series of well-connected cells (Figure 4c). Additionally, silica-encrusted microbial biofilms are observed (Figure 4d), and the presence of organic material is confirmed by carbon enrichment, as indicated by the EDS spectra. Moreover, the morphological features and localized carbon enrichment observed in SEM-EDS analyses (Figure 4b) suggest the presence of extracellular polymeric substances (EPSs).
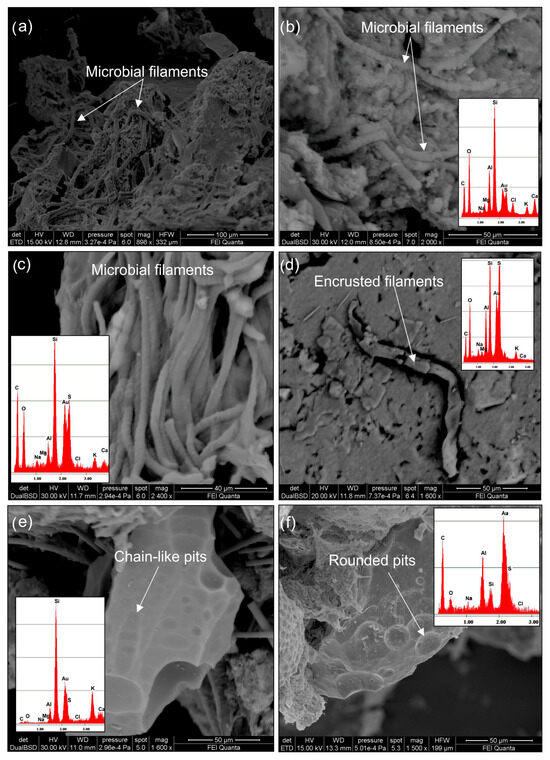
Figure 4.
SEM images showing biological evidence characteristics of the Zannone crusts referred to as Crust A and Crust C. (a–c) Microbial filaments forming dense tangled networks, sometimes characterized by a series of well-visible connected cells. (d) Silica-encrusted microbial organism (e,f) microbial dissolution pits characterized by shapes varying from elongate to sub-rounded or rounded.
SEM observations also provide indirect evidence of biological activity, as evidenced by sulfur and silica minerals exhibiting extensive dissolution pits on their surfaces. These microbe–mineral traces show shapes ranging from elongated to sub-rounded or rounded (Figure 4e,f). Chain-like formations of dissolution pits are also visible (Figure 4e), with sizes ranging from 2 to 15 µm. In some cases, these pits overlap, creating interconnected channels.
Finally, SEM observations reveal an interaction between microbial filaments and the deposition of both silicon and sulfur minerals. To further investigate this precipitation, we analyzed specific elemental maps for subsamples of Crust A, which highlight the deposition of these minerals on the microbial filaments (Figure 5). These maps show that both silicon and sulfur minerals occur on the exterior of the filaments related to the microbial mat. These minerals are widespread throughout the analyzed crusts.
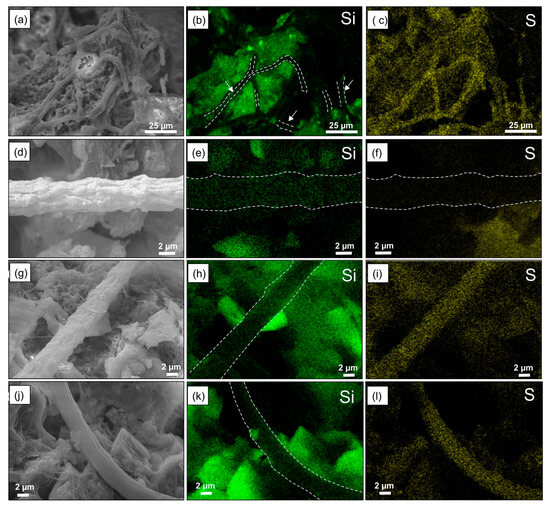
Figure 5.
(a,d,g,j) Back-scattered images and (b,e,h,k) X-ray elemental maps (512 × 400 pixel mapping resolution) showing Si and S minerals (c,f,i,l) precipitates on isolated microbial filaments and in correspondence with microbial network areas of the different areas of the Crust A.
4. Discussion
This study presents the first comprehensive analysis of morphology, mineralogical composition, geochemistry, and microbial signatures of authigenic crusts collected from the Zannone Giant Pockmark (ZGP), a shallow-water hydrothermal system located off the eastern coast of Zannone Island (western Mediterranean Sea). This section discusses the formation processes and implications of our findings in the context of microbial–mineral interactions and their relevance to both modern and ancient biosignatures.
4.1. Interpretation and Formation Processes of the ZGP Crusts
Based on integrated geological, chemical, mineralogical, and morphological data, we interpret the ZGP crusts as modern organo-sedimentary structures enriched in silica and sulfur minerals. These crusts preserve clear microbial signatures and likely result from the interplay between microbial communities, sediment accumulation, and episodic mineral precipitation. Two dominant mineralization pathways are evident: (i) the ongoing silicification process and (ii) the precipitation of native sulfur.
Based on ROV observations showing a close spatial association between microbial mats and mineralized crusts, we propose that lithification results from both abiotic and biotic processes. Abiotic precipitation of silica and sulfur is possible, driven by fluid cooling and seawater mixing, whereas microbial mats contribute to biotic lithification by serving as nucleation sites and altering geochemical conditions (e.g., redox gradients and pH) through metabolic activity. Ongoing fluid emissions not only supply mineralizing agents but also provide nutrients that sustain microbial activity, which in turn enhances lithification processes. This cyclic feedback mechanism reflects a dynamic system in which microbial growth and mineral precipitation are interlinked.
The compositional diversity of the analyzed crusts, comprising quartz, feldspars, pyroxenes, sulfur, barite, gypsum, and hydrothermal phyllosilicates, reflects the geochemical complexity of the ZGP environment [23]. High-temperature CO2-rich hydrothermal fluids facilitate the formation of varied mineral phases, with local conditions (e.g., redox gradients, microbial communities, and vent activity) determining the dominance of specific pathways.
For instance, Crust D, entirely composed of elemental sulfur, likely reflects a more sulfur-rich environment, possibly associated with active venting where sulfur and hydrogen sulfide are abundant. In contrast, crusts like Crust A, Crust B, and Crust C, which contain both sulfur and silica, may represent zones where the interaction between hydrothermal fluids and microbial communities is more pronounced, leading to the co-precipitation of silica and sulfur minerals. As reported by [32], chemoautotrophic pathways and sulfur oxidation processes were overrepresented in the Zannone mat sediment.
4.2. Silicification Pattern of the Mat
The Zannone Giant Pockmark (ZGP) exhibits hydrothermal activity characterized by CO2-dominated fluids (exceeding 90% by volume), with minor contributions of CH4 and H2S [23,30]. Fluid discharge occurs in multiple forms, including diffuse seepage producing a pockmarked seafloor, continuous or intermittent bubble streams from isolated or clustered vent points, and fluid plumes associated with authigenic crusts. The geochemical conditions of the hydrothermal fluids escaping from the ZGP seafloor [23,30] lead to the saturation with respect to SiO2, facilitating silica precipitation [33,34]. Taking this into account, the silica component of the ZGP crusts is interpreted as deriving from both abiotic (sandy silicate grains, predominantly quartz and feldspars) and biogenic silica production [35,36,37,38]. SEM and EDS analyses (Figure 5) reveal microbial filaments encrusted with SiO2, indicating that microorganisms likely serve as templates for silica nucleation and growth. Extracellular polymeric substances (EPSs) possibly play a key role by concentrating on silica and facilitating its precipitation (Figure 6). Differences in Si, O, and C content across microdomains suggest various stages of silica precipitation from initial coating to complete mineral envelopment of microbial structures. SEM imaging revealed that microbial filaments, typically 20–100 µm in length, play a key role in mineralization. Silicium and sulfur precipitation on these filaments induce the growth of mineral mats, indicating an active role of the microbial community in mineral deposition. While we cannot directly confirm biologically induced silica precipitation, the association between microbial structures and silica encrustation supports a model in which microbial surfaces contribute to mineral formation under favorable geochemical conditions. These findings are consistent with prior studies emphasizing microbial mediation as a critical driver of mineral formation in hydrothermal and extreme environments, e.g., [3,6,7,9].
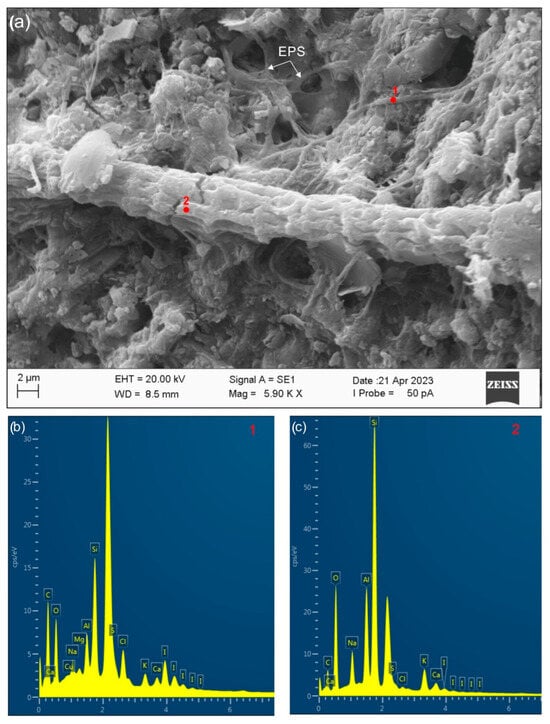
Figure 6.
(a) Secondary electron images (corresponding to Crust A) showing different intensities of encrustation of microbial filaments by SiO2. Filaments with different SiO2 covers are also shown by the EDS patterns (b,c). Evidence of widespread EPS is also observed.
Moreover, the widespread presence of microbial dissolution pits on silica surfaces, often interpreted as biosignatures, reinforces the active role of microbial communities not only in silica deposition but also in its mobilization and redistribution [38]. These pits vary in morphology (elongated, rounded, and sub-rounded) and are thought to reflect microbe–mineral interactions that shape mineral textures. The detection of organic material enriched in carbon on mineral surfaces corroborates the active participation of microorganisms in the silicification process.
While the role of biofilms in sediment stabilization is well documented, e.g., [39,40], occurrences of silica-rich bio-sedimentary structures are rare. Such formations are significant because they represent environments in which the earliest microbial life has been preserved [41]. Organo-deposits, such as those observed in the ZGP, are often considered analogs for early Earth ecosystems, as they provide a window into the types of microbial communities that may have existed billions of years ago. Furthermore, the similarity between these modern deposits and the microbial life preserved in ancient rocks offers a potential model for detecting past life on Mars [42,43,44,45].
4.3. Sulfur Precipitation Process
In the ZGP authigenic deposits, the native sulfur formation is supposed to be the product of multiple processes. Sulfur isotope data (δ34S), ranging from −2.03‰ to −9.82‰, provide important insights into the sulfur cycle in this hydrothermal setting. These ranges of values suggest that both biotic and abiotic sources (hydrothermal fluids and microbial activity) are involved in sulfur origin. Microbial sulfate reduction typically produces strongly negative δ34S values (−5‰ to −40‰), e.g., [46,47]), while abiotic processes, such as magmatic SO2 disproportionation or H2S oxidation, often yield values between 0‰ and −10‰ [48,49]. The most negative value in our dataset (−9.82‰) may thus reflect microbial sulfate reduction. However, given the isotopic overlap between biotic and abiotic processes, these data alone cannot conclusively confirm a microbial origin. Nevertheless, the combined evidence of isotopic depletion, the widespread presence of microbial filaments and extracellular polymeric substances (EPSs), and sulfur encrustation observed via SEM supports a scenario in which microbial activity contributes to sulfur cycling at ZGP. Conversely, the less negative values (e.g., −2.03‰) may result from abiotic processes or mixing with sulfur-bearing phases such as barite, which can have δ34S values of +25‰ or higher.
Specifically, the abiotic process involves the ascent of high-temperature fluids, likely sourced from a buried magma chamber [23,30]. As these fluids migrate toward the seafloor, they become enriched in sulfur species, enabling the precipitation of elemental sulfur upon cooling and mixing with seawater [48,49,50,51]. This mechanism is characteristic of volcanic-hydrothermal systems. The second pathway is biologically mediated. This is suggested by images extracted from videos and secondary electron images observations. In detail, video data highlights the widespread occurrence of both fresh biomass and white mineral deposition associated with the Zannone crusts, testifying to an ongoing growing process of such structure. White bacterial aggregates are commonly observed on and around the crusts, displaying two primary morphologies: (i) spherical aggregates and (ii) filamentous and mat-forming structures. These microbial structures form gelatinous mats, resembling similar formations documented in the shallow-water cave off Paxos Island (Greece) and at the deep-sea Storegga Slide (Norway) [52]. In both environments, large sulfide-oxidizing bacteria have been linked to elemental sulfur production and reduced sulfidic sediments [52,53,54,55,56]. The mat-forming structures, visible on video images as white filamentous, correspond to sulfide-oxidizing bacteria as reported by [32]. Ref. [52] reports the availability of the electron donor of hydrogen sulfide as the main environmental factor selecting the presence of these bacteria. At the ZGP, such aggregations are always found around the main active vent points (associated or not with analyzed crusts), as widely documented by ROV videos. This evidence also supports the idea that such bacterial aggregates can form in areas where hydrogen sulfide reaches the sediment surface and suggests the occurrence of these bacterial aggregates as a proxy for the detection of hydrogen sulfide sources.
The negative δ34S values measured in the ZGP crusts provide further insight into sulfur origin and cycling. Although such values are often interpreted as signatures of microbial sulfate reduction [57], they are not exclusive indicators of biological processes. Similar δ34S depletions can arise from abiotic mechanisms, including disproportionation of magmatic SO2 or oxidation of H2S during fluid boiling [58]. Therefore, while negative δ34S values alone do not conclusively confirm biogenic sulfur formation, they are consistent with microbial involvement, particularly when considered alongside microbial filaments encrusted with sulfur and spatial associations with active venting.
We propose that microbial sulfate reduction is likely contributing to sulfur deposition in some crusts. In this process, microorganisms utilize sulfate as an electron acceptor, reducing it to sulfide, which can then precipitate as elemental sulfur [47,58]. This hypothesis is strongly supported by SEM observations showing microbial filaments closely associated with sulfur crystals. The correlation between sulfur isotope signatures and direct biological evidence reinforces the interpretation that microbial processes significantly influence the geochemistry and mineralogy of the sulfur-rich deposits at ZGP.
The results of this study offer insights into the processes forming authigenic deposits in active hydrothermal environments.
The discovery of microbial signatures preserved within the ZGP silica and sulfur-rich deposits adds novel information regarding mineralization processes in this hydrothermal system and contributes to our broader knowledge of microbial activity in such an extreme environment.
5. Conclusions
This study provides new insights into the morphological, mineralogical, geochemical, and biological characteristics of authigenic crusts formed in the Zannone Giant Pockmark (ZGP), a shallow-water hydrothermal system in the western Mediterranean Sea. Our integrated analysis demonstrates that these crusts represent modern organo-sedimentary deposits enriched in silica and sulfur and actively influenced by microbial processes. The evidence of encrusted microbial filaments encrusted, the widespread occurrence of extracellular polymeric substances (EPSs), and the presence of biologically shaped dissolution pits all point to a significant role for microbial communities in mediating mineral precipitation. These findings underscore the importance of microbe mineral interactions (co-precipitation of both silica and elemental sulfur) in the stabilization and lithification of microbial mats.
The ZGP stands out as a globally unique environment: a relatively shallow marine hydrothermal field (<200 m water depth) where extensive, microbially influenced silica and sulfur-rich crusts are actively forming on the seafloor. These crusts preserve key biosignatures and offer a compelling modern analog for ancient hydrothermal systems where early life may have emerged and been preserved in the geologic record.
Beyond its regional significance, the ZGP hydrothermal system contributes to a broader understanding of early diagenetic processes such as microbially driven silica and sulfur precipitation, mat stabilization, and early lithification, which are critical to the preservation of biosignatures in marine hydrothermal environments. The preservation of biosignatures in this environment has implications for interpreting ancient organo-deposits and for astrobiological exploration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min15080794/s1, Figure S1. Macroscopic samples of Crust A showing evidence of (a) dark organic material and (b) presence of bacterial filaments and yellow coated grains; Table S1. Chemical analysis of major element contents (wt%) in the Zannone crusts.
Author Contributions
M.I. and E.M. collected the data and designed this study. M.P., T.R., M.I. and L.D.B. prepared the samples and performed the SEM analyses. A.M.C. and C.P. prepared the samples and performed the X-ray diffraction analyses. M.I. and E.M. made the analyses of ROV images. A.M.C., C.P. and T.R. contributed to the classification and geochemistry of the samples. M.I. and L.D.B. contributed to the analysis of the biological evidence. M.I. contributed to the editing of the present versions of this manuscript. All authors participated in the writing of the present versions of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The data used in this study were collected by CNR-IGAG (Rome, Italy) aboard of the Urania and Maria Grazia research vessels. We would like to thank the captains, crews, and participants of the oceanographic cruises. Three anonymous reviewers are thanked for fruitful and constructive comments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ZGP | Zannone Giant Pockmark |
| EPSs | Extracellular Polymeric Substances |
References
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef]
- López-García, P.; Kazmierczak, J.; Benzerara, K.; Kempe, S.; Guyot, F.; Moreira, D. Bacterial diversity and carbonate precipitation in the giant microbialites from the highly alkaline Lake Van, Turkey. Extremophiles 2005, 9, 263–274. [Google Scholar] [CrossRef]
- Dick, G.J. The microbiomes of deep-sea hydrothermal vents: Distributed globally, shaped locally. Nat. Rev. Microbiol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentary deposits of benthic microbial communities. Palaios 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Riding, R. Classification of microbial carbonates. In Calcareous Algae and Stromatolites; Springer: Berlin/Heidelberg, Germany, 1991; pp. 21–51. [Google Scholar]
- Dong, H.; Huang, L.; Zhao, L.; Zeng, Q.; Liu, X.; Sheng, Y.; Shi, L.; Wu, G.; Jiang, H.; Li, F.; et al. A critical review of mineral–microbe interaction and co-evolution: Mechanisms and applications. Natl. Sci. Rev. 2022, 9, nwac128. [Google Scholar] [CrossRef]
- Douglas, S.; Beveridge, T.J. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 1998, 26, 79–88. [Google Scholar] [CrossRef]
- Nutman, A.P.; Bennett, V.C.; Friend, C.R.; Van Kranendonk, M.J.; Chivas, A.R. Rapid emergence of life shown by discovery of 3700-million-year-old microbial structures. Nature 2016, 537, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Pepe-Ranney, C.; Berelson, W.M.; Corsetti, F.A.; Treants, M.; Spear, J.R. Cyanobacterial construction of hot spring siliceous stromatolites in Yellowstone National Park. Environ. Microbiol. 2012, 14, 1182–1197. [Google Scholar] [CrossRef]
- Coman, C.; Chiriac, C.M.; Robeson, M.S.; Ionescu, C.; Dragos, N.; Barbu-Tudoran, L.; Andrei, A.Ş.; Banciu, H.L.; Sicora, C.; Podar, M. Structure, mineralogy, and microbial diversity of geothermal spring microbialites associated with a deep oil drilling in Romania. Front. Microbiol. 2015, 6, 253. [Google Scholar] [CrossRef]
- Bradley, J.A.; Daille, L.K.; Trivedi, C.B.; Bojanowski, C.L.; Stamps, B.W.; Stevenson, B.S.; Nunn, H.S.; Johnson, H.A.; Loyd, S.J.; Berelson, W.M.; et al. Carbonate-rich dendrolitic cones: Insights into a modern analog for incipient microbialite formation, Little Hot Creek, Long Valley Caldera, California. npj Biofilms Microbiomes 2017, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Planavsky, N.; Ginsburg, R.N. Taphonomy of modern marine Bahamian microbialites. Palaios 2009, 24, 5–17. [Google Scholar] [CrossRef]
- Khodadad, C.L.; Foster, J.S. Metagenomic and metabolic profiling of nonlithifying and lithifying stromatolitic mats of Highborne Cay, The Bahamas. PLoS ONE 2012, 7, e38229. [Google Scholar] [CrossRef]
- Kempe, S.; Kazmierczak, J.; Landmann, G.; Konuk, T.; Reimer, A.; Lipp, A. Largest known microbialites discovered in Lake Van, Turkey. Nature 1991, 349, 605–608. [Google Scholar] [CrossRef]
- Proemse, B.C.; Eberhard, R.S.; Sharples, C.; Bowman, J.P.; Richards, K.; Comfort, M.; Barmuta, L.A. Stromatolites on the rise in peat-bound karstic wetlands. Sci. Rep. 2017, 7, 15384. [Google Scholar] [CrossRef]
- Ingrassia, M.; Conte, A.M.; Perinelli, C.; Aldega, L.; Di Bella, L.; Mazzoni, C.; Fazi, S.; Falese, F.G.; Ruspandini, T.; Piacentini, A.; et al. Experimental vs. Natural Mineral Precipitation in Modern Microbialites: The Case Study of the Alkaline Bagno Dell’acqua Lake (Pantelleria Island, Italy). Minerals 2024, 14, 1013. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Awramik, S.M. Microbialite morphostratigraphy as a tool for correlating Late Cambrian–Early Ordovician sequences. J. Geol. 2000, 108, 171–180. [Google Scholar] [CrossRef]
- Golubic, S.; Hofmann, H.J. Comparison of Holocene and mid-Precambrian Entophysalidaceae (Cyanophyta) in stromatolitic algal mats: Cell division and degradation. J. Paleontol. 1976, 50, 1074–1082. [Google Scholar]
- Rice, M.S.; Bell, J.F.; Cloutis, E.A.; Wang, A.; Ruff, S.W.; Craig, M.A.; Bailey, D.T.; Johnson, J.R.; de Souza, P.A.; Farrand, W.H. Silica-rich deposits and hydrated minerals at Gusev Crater, Mars: Vis-NIR spectral characterization and regional mapping. Icarus 2010, 205, 375–395. [Google Scholar] [CrossRef]
- Rogers, E.R.; Qualizza, B.R.; Heidenreich, J.R.; Dawson, H.G.; Horgan, B.H. Silica-Bearing Mounds and Strata in the Southwest Melas Basin, Valles Marineris, Mars: Evidence for a Hydrothermal Origin. J. Geophys. Res. Planets 2023, 128, e2023JE007881. [Google Scholar] [CrossRef]
- Ruff, S.W.; Farmer, J.D. Silica deposits on Mars with features resembling hot spring biosignatures at El Tatio in Chile. Nat. Commun. 2016, 7, 13554. [Google Scholar] [CrossRef]
- Rizzo, V. Why should geological criteria used on Earth not be valid also for Mars? Evidence of possible microbialites and algae in extinct Martian lakes. Int. J. Astrobiol. 2020, 19, 283–294. [Google Scholar] [CrossRef]
- Martorelli, E.; Italiano, F.; Ingrassia, M.; Macelloni, L.; Bosman, A.; Conte, A.M.; Beaubien, S.E.; Graziani, S.; Sposato, A.; Chiocci, F.L. Evidence of a shallow water submarine hydrothermal field off Zannone Island from morphological and geochemical characterization: Implications for Tyrrhenian Sea Quaternary volcanism. J. Geophys. Res. Solid Earth 2016, 121, 8396–8414. [Google Scholar] [CrossRef]
- Ingrassia, M.; Di Bella, L.; Chiocci, F.L.; Martorelli, E. Influence of fluid emissions on shallow-water benthic habitats of the Pontine Archipelago (Tyrrhenian Sea, Italy). Alp. Mediterr. Quat. 2015, 28, 99–110. [Google Scholar]
- Ingrassia, M.; Martorelli, E.; Bosman, A.; Macelloni, L.; Sposato, A.; Chiocci, F.L. The Zannone Giant Pockmark: First evidence of a giant complex seeping structure in shallow-water, central Mediterranean Sea, Italy. Mar. Geol. 2015, 363, 38–51. [Google Scholar] [CrossRef]
- Di Bella, L.; Ingrassia, M.; Frezza, V.; Chiocci, F.L.; Martorelli, E. The response of benthic meiofauna to hydrothermal emissions in the Pontine Archipelago, Tyrrhenian Sea (central Mediterranean Basin). J. Mar. Syst. 2016, 164, 53–66. [Google Scholar] [CrossRef]
- Conte, A.M.; Di Bella, L.; Ingrassia, M.; Perinelli, C.; Martorelli, E. Alteration and Mineralization Products of the Zannone Giant Pockmark (Zannone Hydrothermal Field, Central Tyrrhenian Sea). Minerals 2020, 10, 581. [Google Scholar] [CrossRef]
- Bartole, R.; Savelli, D.; Tramontana, M.; Wezel, F.C. Structural and sedimentary features in the Tyrrhenian margin off Campania, Southern Italy. Mar. Geol. 1984, 55, 163–180. [Google Scholar] [CrossRef]
- De Rita, D.; Funiciello, R.; Pantosti, D.; Salvini, F.; Sposato, A.; Velonà, M. Geological and structural characteristics of the Pontine Islands (Italy) and implications with the evolution of the Tyrrhenian margin. Mem. Della Soc. Geol. Ital. 1986, 36, 55–65. [Google Scholar]
- Italiano, F.; Romano, D.; Caruso, C.; Longo, M.; Corbo, A.; Lazzaro, G. Magmatic signature in submarine hydrothermal fluids vented offshore Ventotene and Zannone Islands (Pontine Archipelago, Central Italy). Geofluids 2019, 2019, 8759609. [Google Scholar] [CrossRef]
- Paytan, A.; Mearon, S.; Cobb, K.; Kastner, M. Origin of marine barite deposits: Sr and S isotope characterization. Geology 2002, 30, 747–750. [Google Scholar] [CrossRef]
- Rastelli, E.; Corinaldesi, C.; Dell’ANno, A.; Tangherlini, M.; Martorelli, E.; Ingrassia, M.; Chiocci, F.L.; Martire, M.L.; Danovaro, R. High potential for temperate viruses to drive carbon cycling in chemoautotrophy-dominated shallow-water hydrothermal vents. Environ. Microbiol. 2017, 19, 4432–4446. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry; John Wiley & Sons Ltd.: New York, NY, USA, 1981. [Google Scholar]
- Herzig, P.M.; Hannington, M.D. Polymetallic massive sulfides at the modern seafloor a review. Ore Geol. Rev. 1995, 10, 95–115. [Google Scholar] [CrossRef]
- Guidry, S.A.; Chafetz, H.S. Anatomy of siliceous hot springs: Examples from Yellowstone National Park, Wyoming, USA. Sediment. Geol. 2003, 157, 71–106. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Meloche, J.D.; Fyfe, W.S.; Murray, R.G.E. Diagenesis of metals chemically complexed to bacteria: Laboratory formation of metal phosphates, sulfides, and organic condensates in artificial sediments. Appl. Environ. Microbiol. 1983, 45, 1094–1108. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, J.; Xu, J.; Yin, J. Effects of surface topography of SiO2 particles on the heterogeneous condensation process observed by environmental scanning electron microscopy. Aerosol Sci. Technol. 2021, 55, 920–929. [Google Scholar] [CrossRef]
- Sauro, F.; Cappelletti, M.; Ghezzi, D.; Columbu, A.; Hong, P.-Y.; Zowawi, H.M.; Carbone, C.; Piccini, L.; Vergara, F.; Zannoni, D.; et al. Microbial diversity and biosignatures of amorphous silica deposits in orthoquartzite caves. Sci. Rep. 2018, 8, 17569. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef]
- Chen, X.D.; Zhang, C.K.; Zhou, Z.; Gong, Z.; Zhou, J.J.; Tao, J.F.; Paterson, D.M.; Feng, Q. Stabilizing effects of bacterial biofilms: EPS penetration and redistribution of bed stability down the sediment profile. J. Geophys. Res. Biogeosciences 2017, 122, 3113–3125. [Google Scholar] [CrossRef]
- Farmer, J.D.; Marais, D.J.D. Exploring for a record of ancient Martian life. J. Geophys. Res. Planets 1999, 104, 26977–26995. [Google Scholar] [CrossRef] [PubMed]
- Milliken, R.; Swayze, G.; Arvidson, R.; Bishop, J.; Clark, R.N.; Ehlmann, B.; Green, R.; Grotzinger, J.; Morris, R.; Murchie, S.; et al. Opaline silica in young deposits on Mars. Geology 2008, 36, 847–850. [Google Scholar] [CrossRef]
- Squyres, S.W.; Arvidson, R.E.; Ruff, S.; Gellert, R.; Morris, R.V.; Ming, D.W.; Crumpler, L.; Farmer, J.D.; Marais, D.J.D.; Yen, A.; et al. Detection of silica-rich deposits on Mars. Science 2008, 320, 1063–1067. [Google Scholar] [CrossRef]
- Skok, J.R.; Mustard, J.F.; Ehlmann, B.L.; Milliken, R.E.; Murchie, S.L. Silica deposits in the Nili Patera caldera on the Syrtis Major volcanic complex on Mars. Nat. Geosci. 2010, 3, 838–841. [Google Scholar] [CrossRef]
- Ruff, S.W.; Farmer, J.D.; Calvin, W.M.; Herkenhoff, K.E.; Johnson, J.R.; Morris, R.V.; Rice, M.S.; Arvidson, R.E.; Bell, J.F., III; Christensen, P.R.; et al. Characteristics, distribution, origin, and significance of opaline silica observed by the Spirit rover in Gusev crater, Mars. J. Geophys. Res. Planets 2011, 116. [Google Scholar] [CrossRef]
- Canfield, D.E. Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochim. Cosmochim. Acta 2001, 65, 1117–1124. [Google Scholar] [CrossRef]
- Rouxel, O.; Ono, S.; Alt, J.; Rumble, D.; Ludden, J. Sulphur isotope evidence for microbial sulfate reduction in altered oceanic basalts at ODP Site 801. Earth Planet. Sci. Lett. 2008, 268, 110–123. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Chemical composition of volcanic gases. In Monitoring and Mitigation of Volcano Hazards; Springer: Berlin/Heidelberg, Germany, 1996; pp. 221–256. [Google Scholar]
- Kusakabe, M.; Komoda, Y.; Takano, B.; Abiko, T. Sulphur isotopic effects in the disproportionation reaction of sulphur dioxide in hydrothermal fluids: Implications for the δ34S variations of dissolved bisulfate and elemental sulphur from active crater lakes. J. Volcanol. Geotherm. Res. 2000, 97, 287–307. [Google Scholar] [CrossRef]
- de Ronde, C.E.; Massoth, G.J.; Butterfield, D.A.; Christenson, B.W.; Ishibashi, J.; Ditchburn, R.G.; Hannington, M.D.; Brathwaite, R.L.; Lupton, J.E.; Kamenetsky, V.S.; et al. Submarine hydrothermal activity and gold-rich mineralization at Brothers Volcano, Kermadec Arc, New Zealand. Miner. Depos. 2011, 46, 541–584. [Google Scholar] [CrossRef]
- Seewald, J.S.; Reeves, E.P.; Bach, W.; Saccocia, P.J.; Craddock, P.R.; Shanks, W.C.; Sylva, S.P.; Pichler, T.; Rosner, M.; Walsh, E. Submarine venting of magmatic volatiles in the eastern Manus Basin, Papua New Guinea. Geochim. Cosmochim. Acta 2015, 163, 178–199. [Google Scholar] [CrossRef]
- Grünke, S.; Felden, J.; Lichtschlag, A.; Girnth, A.C.; de Beer, D.; Wenzhöfer, F.; Boetius, A. Niche differentiation among mat-forming, sulfide-oxidizing bacteria at cold seeps of the Nile Deep Sea Fan (Eastern Mediterranean Sea). Geobiology 2011, 9, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.; Nelson, D.C. The genera beggiatoa and thioploca. Prokaryotes 2006, 6, 784–810. [Google Scholar]
- Ahmad, A.; Barry, J.P.; Nelson, D.C. Phylogenetic affinity of a wide, vacuolate, nitrate-accumulating Beggiatoa sp. from Monterey Canyon, California, with Thioploca spp. Appl. Environ. Microbiol. 1999, 65, 270–277. [Google Scholar] [CrossRef]
- Mills, H.J.; Martinez, R.J.; Story, S.; Sobecky, P.A. Identification of members of the metabolically active microbial populations associated with Beggiatoa species mat communities from Gulf of Mexico cold-seep sediments. Appl. Environ. Microbiol. 2004, 70, 5447–5458. [Google Scholar] [CrossRef] [PubMed]
- de Beer, D.; Sauter, E.; Niemann, H.; Kaul, N.; Foucher, J.P.; Witte, U.; Schlüter, M.; Boetius, A. In situ fluxes and zonation of microbial activity in surface sediments of the Håkon Mosby Mud Volcano. Limnol. Oceanogr. 2006, 51, 1315–1331. [Google Scholar] [CrossRef]
- Taylor, C.D.; Wirsen, C.O. Microbiology and ecology of filamentous sulphur formation. Science 1997, 277, 1483–1485. [Google Scholar] [CrossRef]
- Peters, M.; Strauss, H.; Petersen, S.; Kummer, N.-A.; Thomazo, C. Hydrothermalism in the Tyrrhenian Sea: Inorganic and microbial sulphur cycling as revealed by geochemical and multiple sulphur isotope data. Chem. Geol. 2011, 280, 217–231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).