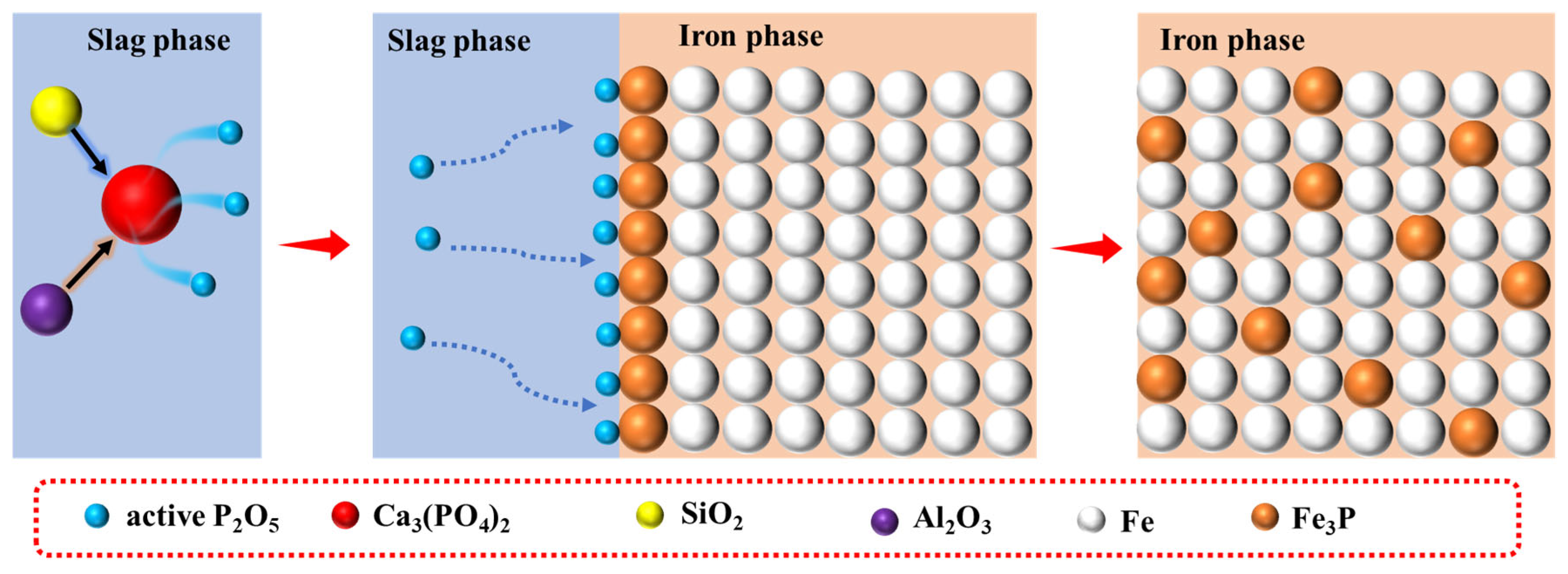
1. Introduction
As the world’s largest steel producer, China faces critical supply chain vulnerabilities due to over 80% reliance on imported iron ore, threatening its steel industry’s sustainability [
1,
2]. Developing domestic low-grade iron resources thus becomes strategically imperative. High-phosphorus oolitic iron ore (HPOIO) constitutes a substantial reserve, with proven global deposits exceeding 20 billion tonnes—over 7 billion tonnes located in China [
3,
4,
5]. Conventional mineral processing techniques, however, suffer from extended flowsheets, high operational costs, inefficient dephosphorization, and carbon-intensive operations, resulting in chronic underutilization of these resources.
Characterized as a refractory low-grade material, HPOIO typically contains 20%–50% Fe and 0.1%–1.5% P [
6,
7]. Its concentric oolitic texture incorporates hematite, quartz, chlorite, and apatite. Complex mineral intergrowth and ultrafine dissemination (0–3 μm) impede conventional beneficiation, while elevated phosphorus levels prevent production of high-grade, low-phosphorus iron concentrates [
8,
9]. Systematic development of alternative techniques—including conventional processing, magnetizing roasting-magnetic separation (MR-MS), direct reduction-magnetic separation (DR-MS), and hydrometallurgical methods—aims to overcome these limitations.
Current research on high-phosphorus oolitic iron ore (HPOIO) predominantly focuses on iron enrichment and phosphorus removal, aiming to produce low-phosphorus iron raw materials suitable for conventional blast furnace processes. However, micron-scale mineral dissemination in HPOIO presents dual challenges: existing grinding technologies fail to achieve effective mineral liberation, while excessively fine particles substantially increase grinding energy consumption. Furthermore, such ultrafine particles promote severe slime coating during separation, collectively resulting in inefficient beneficiation [
10,
11]. These factors constrain traditional mineral processing through high grinding energy demands, low iron recovery, and inadequate dephosphorization, ultimately preventing production of high-grade, low-phosphorus iron concentrates.
Although the MR-MS process enhances mineral processability by converting hematite to magnetite or γ-hematite via reduction roasting, it merely induces phase transformation without disrupting the original oolitic texture. Consequently, iron minerals remain intricately intergrown with phosphorus-bearing phases, yielding concentrates with persistently elevated phosphorus content [
12,
13]. In contrast, DR-MS employs high-temperature roasting to dismantle the oolitic structure and facilitate metallic iron aggregation. This enables secondary phase reconstruction between metallic and slag phases, permitting subsequent production of low-phosphorus iron powder through grinding and magnetic separation [
14,
15]. Nevertheless, DR-MS implementation requires roasting temperatures exceeding 1200 °C alongside substantial calcium/sodium-based dephosphorization reagents to inhibit phosphorus migration into metallic iron, imposing stringent limitations due to prohibitive energy consumption and production costs.
Hydrometallurgical methods (acid/bioleaching) selectively dissolve phosphorus minerals from HPOIO to prepare low-phosphorus iron products. However, these approaches encounter significant challenges, including excessive reagent consumption, protracted microbial cultivation periods, low processing efficiency, and acidic effluent pollution [
16,
17]. Collectively, these constraints compromise both technical feasibility and economic viability.
While phosphorus detrimentally compromises steel plasticity, impact toughness, weldability, and low-temperature performance, it delivers unique functional value in specialized materials [
18,
19]. High-phosphorus weathering steel Q295GNH (0.08%–0.15% P) forms dense protective layers through homogeneous dissolution, exhibiting 2–8 times greater atmospheric corrosion resistance than carbon steel while providing high strength, superior cold formability, and exceptional impact fatigue resistance for marine and automotive applications [
20,
21]. High-phosphorus cast iron (0.35%–0.65% P) demonstrates 1–3 times higher wear resistance than standard gray iron, serving ideally for machine tool guides and piston rings [
22,
23]. Medium- (0.7%–1.0% P) and high-phosphorus (2.0%–2.5% P) railway brake blocks leverage phosphorus-enhanced tribological properties for braking system applications [
24,
25], collectively validating phosphorus’s critical functional role in advanced materials.
This study innovatively proposes a pre-reduction and smelting separation (PR-SS) process that directly produces multifunctional Fe–P alloy from HPOIO. By circumventing conventional dephosphorization steps, PR-SS enables simultaneous iron and phosphorus enrichment, establishing an efficient pathway for strategic high-value utilization of HPOIO. Compared to traditional blast furnace ironmaking, this electric furnace-based process significantly reduces carbon emissions by eliminating the need for coke combustion. The compact process flow further enhances operational efficiency, utilizing coal, biocoke, or conventional coke as reductors without requiring sintering or coking operations. Consequently, PR-SS achieves substantially lower production and capital investment costs, representing a promising short-process alternative for Fe–P alloy synthesis.
2. Materials and Methods
2.1. Materials
The detailed chemical compositions of the two ores used in this experiment are presented in
Table 1. The oolitic magnetite concentrate (OMC) was upgraded from the raw HPOIO ore via magnetizing roasting in a rotary kiln (850 °C, 15 min, 3% coal addition) followed by wet ball milling (95% of product <0.045 mm) and wet drum magnetic separation (1000 Gs magnetic field intensity). The raw HPOIO ore exhibited a total iron (TFe) grade of only 35.04%. After beneficiation, the TFe grade of OMC increased to 52.06%, yet it still contained high levels of SiO
2 (16.32%), Al
2O
3 (9.86%), and P (0.37%). Due to its low iron grade and elevated phosphorus content, this ore is unsuitable for conventional Blast Furnace-Basic Oxygen Furnace (BF-BOF) processes and fails to meet industrial requirements. Consequently, exploring efficient non-blast furnace ironmaking technologies is critically imperative.
The laser particle size distribution characteristics of the OMC are illustrated in
Figure 1a. The cumulative particle sizes D
50 and D
90 were measured as 17.891 μm and 48.511 μm, respectively, which means that the particle size of OMC was very fine.
Figure 1b,c present the XRD patterns and SEM-EDS results of OMC, respectively, with
Table 2 summarizing the point-scan EDS data. Microstructural analysis reveals that the ore primarily consists of magnetite ((Fe, Al)
3O
4), hematite ((Fe, Al)
2O
3), amorphous silicate (4FeO·Al
2O
3·3SiO
2), quartz (SiO
2), and fluorapatite (Ca
5(PO
4)
3F). Magnetite is intergrown with silicates, quartz, and fluorapatite, forming intricate concentric banded structures. This configuration encapsulates iron minerals within layered matrices with ultrafine particle sizes (predominantly <5 μm), rendering effective liberation of magnetite monomers challenging. Critically, the iron-bearing silicate gangue phase (4FeO·Al
2O
3·3SiO
2) is inevitably discarded into tailings during conventional beneficiation. As magnetic separation, gravity concentration, and flotation cannot recover iron from this phase, the overall iron recovery in the final concentrate is substantially reduced. Furthermore, extensive isomorphous substitution of aluminum in iron mineral lattices impedes Fe-Al separation.
Figure 1d illustrates the oolitic texture and phosphorus distribution in HPOIO: hematite is tightly encapsulated by gangue minerals, forming characteristic ooids, with coarse-grained phosphorus minerals (0–10 μm) concentrated at ooid rims while fine-grained phosphorus phases predominantly reside in ooid cores. These mineralogical characteristics demonstrate significant challenges for mineral liberation and separation via traditional beneficiation approaches.
This study employed coal and coke as reductants for the pre-reduction and smelting separation stages, respectively, both pulverized to <5 mm before use. As detailed in
Table 3, the fixed carbon (F
Cad) contents were 51.55% for coal and 82.81% for coke, with ash (A
ad) contents of 9.44% and 14.81%, volatile matter (V
ad) of 31.90% and 2.25%, and air-dried moisture (M
ad) of 7.11% and 0.13%, respectively. These proximate analysis parameters meet the technical specifications for carbonaceous reductants in the PR-SS process, while the substantially low levels of detrimental impurities (S, P) comply with industrial production standards.
2.2. Experimental Methods
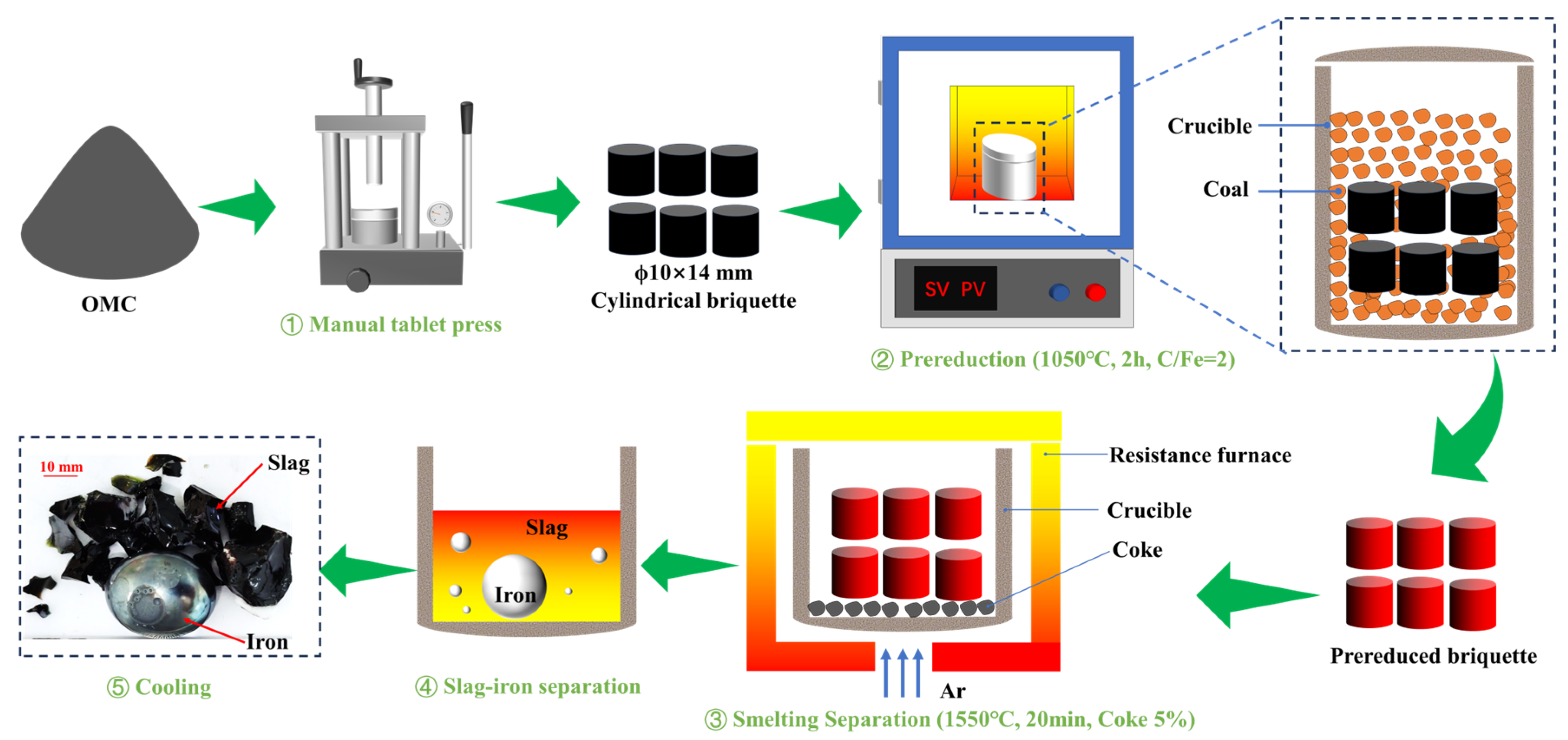
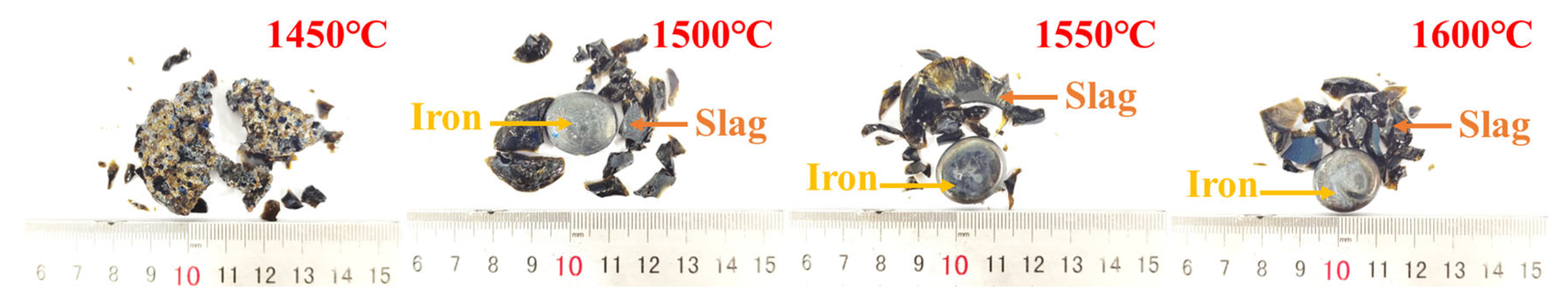
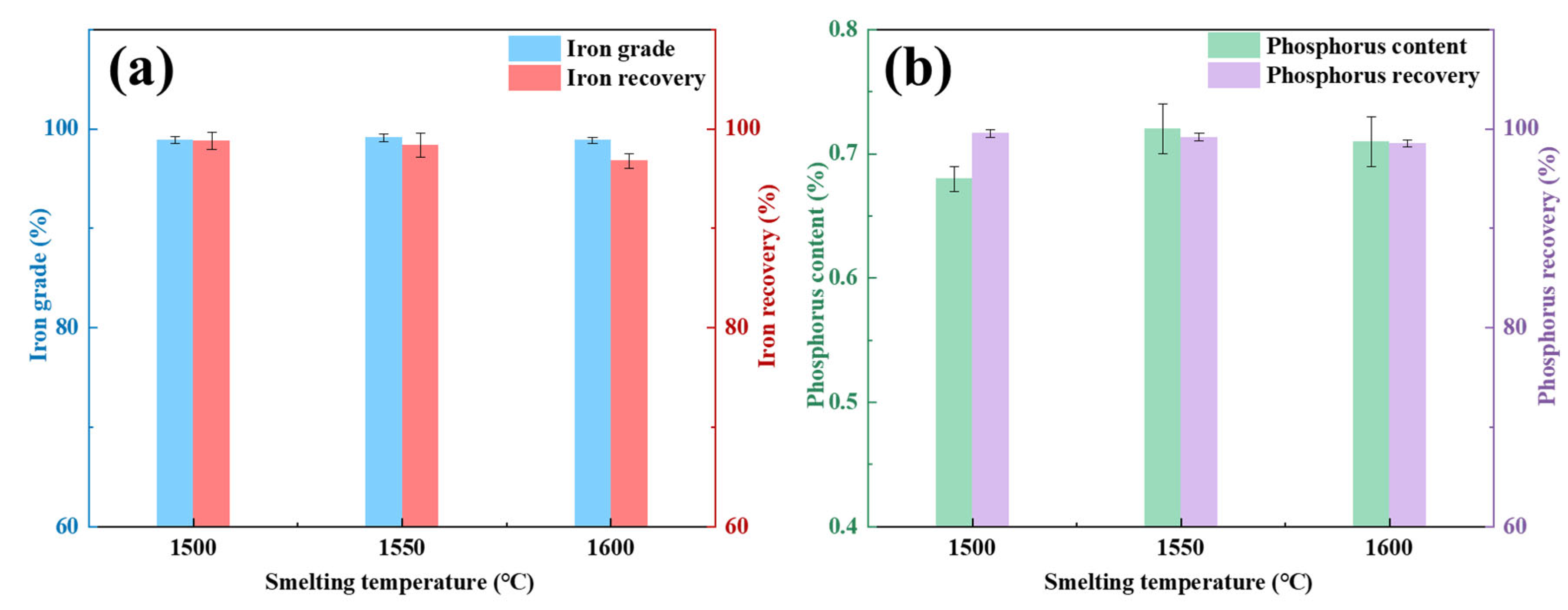
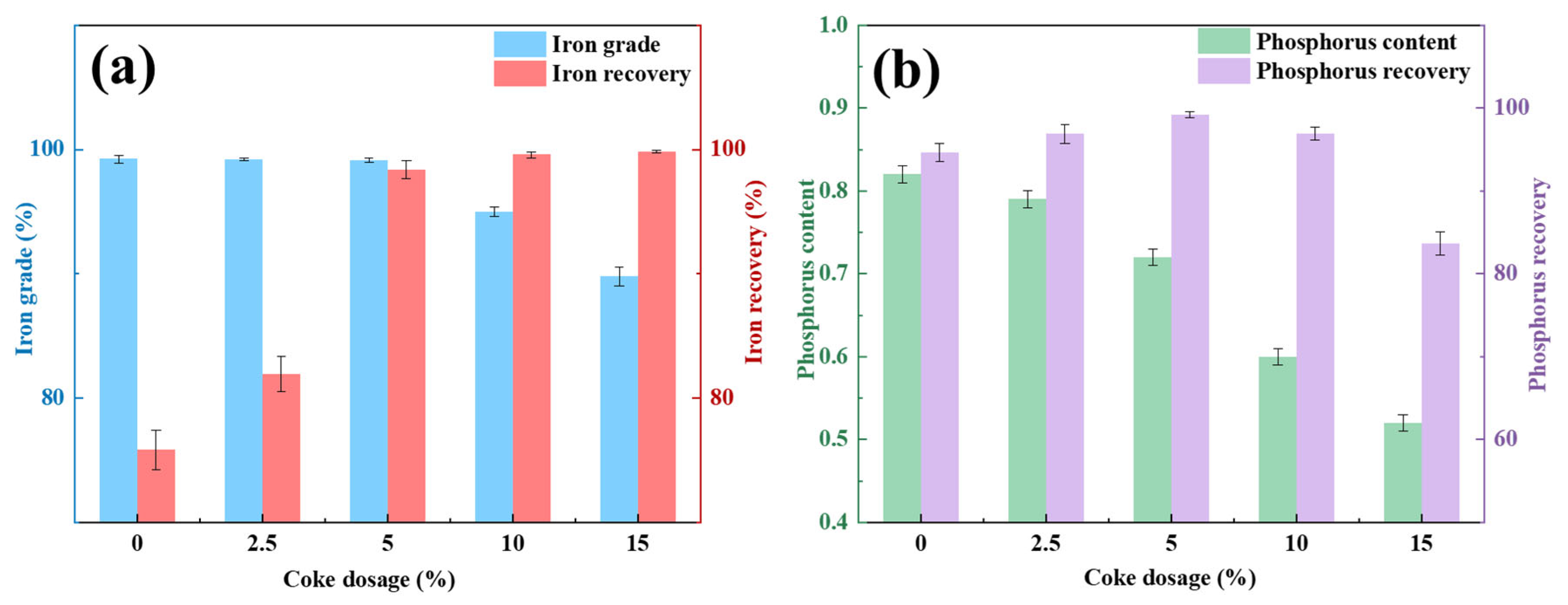
Figure 2 delineates the experimental procedure of the PR-SS process. Initially, OMC powder was compacted into cylindrical briquettes (Φ10 mm × 14 mm) using a PC-12 manual pellet press. A 50 g briquette was homogeneously blended with coal and loaded into a 200 mL alumina crucible for pre-reduction roasting at 1050 °C for 2 h with a C/Fe mass ratio of 2.0 in a KSL-1200X-J muffle furnace (Kejing Instrument, Hefei, China). Following pre-reduction, the briquette was mixed with coke at predetermined ratios in a dedicated alumina crucible and subjected to smelting separation in a VSF-type electric resistance furnace (atmosphere-controllable chamber furnace). Notably, the entire smelting separation process was conducted under an argon atmosphere. Upon reaching the designated smelting duration, the crucible was extracted, cooled to ambient temperature in argon, and manually disintegrated to segregate slag and metallic iron products. The metallization degree of pre-reduced briquettes was calculated using Equation (1), while iron and phosphorus recoveries in the smelting metal phase were determined via Equations (2) and (3), respectively.
where
is the metallization rate and
MFe and
TFe are the metallic iron and total iron contents in the sample, respectively.
where
and
represent the Fe recovery and P recovery (%), respectively;
m represents the weight of the metallic iron (g);
C and
D represent the Fe grade and P grade of the metallic iron (%), respectively;
M represents the weight of the feed (g); and
R and
K represent the Fe grade and P grade of the feed (%), respectively.
2.3. Analysis Methods
The chemical composition of the samples was analyzed via an X-ray fluorescence spectrometer (ZSX Primus IV, Rigaku, Saitama, Japan) and an inductively coupled plasma spectrometer (iCAP RPplus, Thermo Fisher Scientific, Waltham, MA, USA). A laser particle size analyzer (MS3000, Malvern, Worcestershire, UK) was used to determine the particle size and specific surface area of the sample. The mineral composition of each sample was determined through an advanced X-ray diffractometer (XRD) (D8 DISCOVER, BRUKER, Ettlingen, Germany). Optical microscopy (DMI5000 M, Leica, Karlsruhe, Germany) and field emission scanning electron microscopy (SEM) (MIRA3, Tescan, Brno, Czech Republic) were utilized to analyze the microstructure and crystal-chemical properties of the minerals. An electron probe X-ray microanalyzer (EPMA) (1720T, SHIMADZU, Kyoto, Japan) was used to measure the microelement composition of the sample. X-ray photoelectron spectroscopy (XPS) (K-Alpha, Thermo Fisher Scientific, Waltham, MA, USA) was employed to measure the elemental composition and valence states of the samples. ImageJ 1.44Plus (National Institutes of Health, Bethesda, MD, USA) was used to calculate the grain size of the crystals, and FactSage 8.3 (Thermfact/CRCT, Montreal, QC, Canada) was used to calculate the thermodynamic reactions and equilibrium phase diagrams.