Recycling and Mineral Evolution of Multi-Industrial Solid Waste in Green and Low-Carbon Cement: A Review
Abstract
1. Introduction
2. Methodology
3. Overview of Industrial Solid Waste
3.1. Classification of Industrial Solid Waste
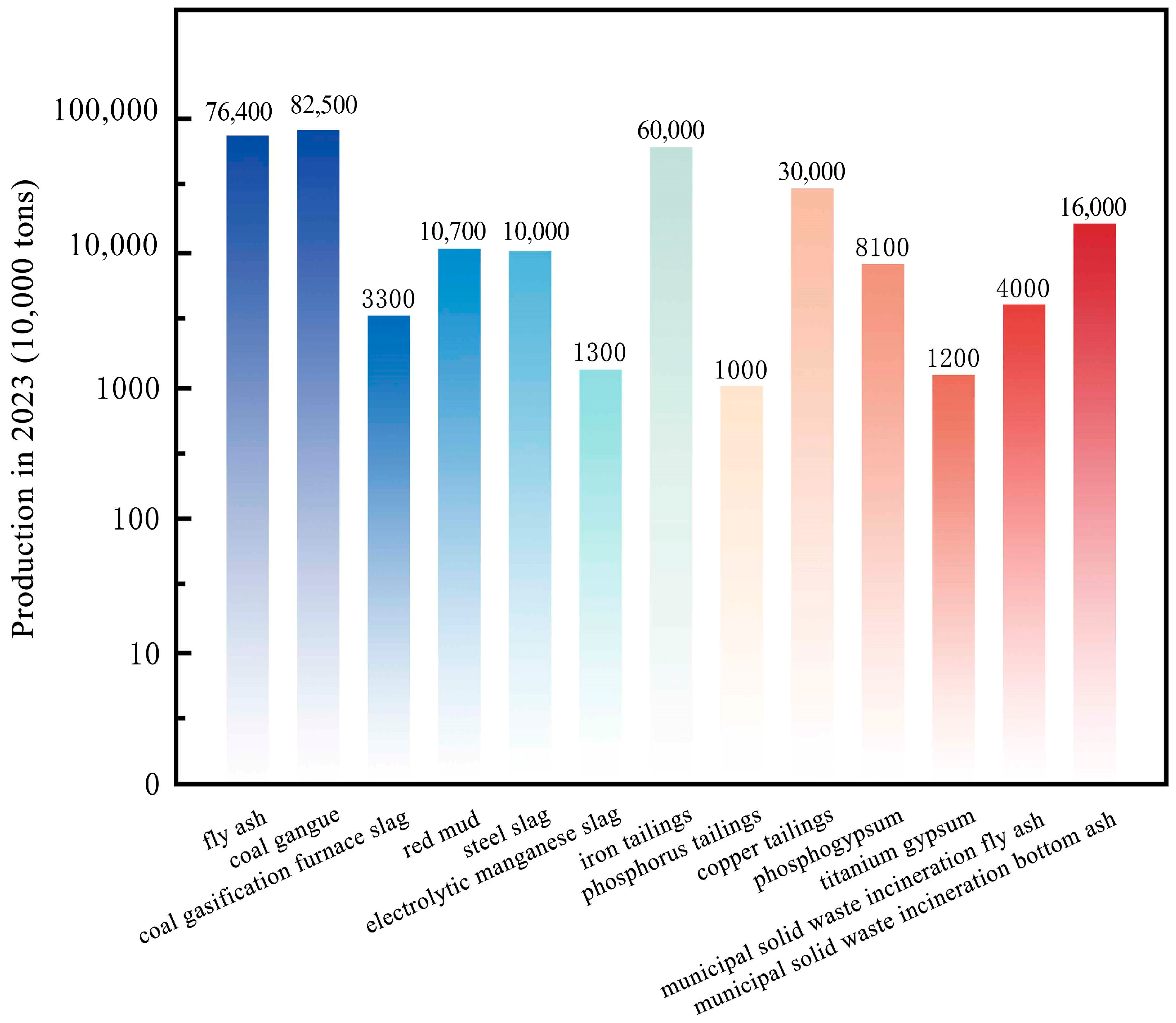
3.2. Production of Industrial Solid Waste in China
4. Application of Coal-Based Solid Waste in Cement
4.1. Application of Fly Ash in Cement
4.2. Application of Coal Gangue in Cement
4.3. Application of Coal Gasification Furnace Slag in Cement
5. Application of Metallurgical Slag in Cement
5.1. Application of Red Mud in Cement
5.2. Application of Steel Slag in Cement
5.3. Application of Electrolytic Manganese Slag in Cement
6. Application of Tailings in Cement
6.1. Application of Iron Tailings in Cement
6.2. Application of Phosphorus Tailings in Cement
6.3. Application of Copper Tailings in Cement
7. Application of Industrial Byproduct Gypsum in Cement
7.1. Application of Phosphogypsum in Cement
7.2. Application of Titanium Gypsum in Cement
8. Application of Municipal Solid Waste Incineration in Cement
8.1. Application of Municipal Solid Waste Incineration Bottom Ash in Cement
8.2. Application of Municipal Solid Waste Incineration Fly Ash in Cement
9. Environmental Performance Evaluation of Cement Prepared from Industrial Solid Waste
10. Conclusions and Prospects
- Economical and efficient pretreatment methods. Pretreatment is typically required prior to the use of industrial solid waste in cement production, due to their complex composition and significant regional variability. However, current technologies such as high-temperature calcination and mechanical grinding are often hindered by high energy consumption and operational costs. Therefore, advancing precise activation and synergistic activation technologies based on the phase composition characteristics of solid waste should be the primary focus of future efforts. At the same time, it is essential to minimize secondary pollution and carbon emissions during the pretreatment process.
- Co-utilization of multiple types of industrial solid waste. The resource utilization of single-type solid waste is often constrained by limited reactive components, high pretreatment costs, and complex processing routes. In contrast, cements prepared with multiple types of industrial waste generally exhibit superior performance compared to those derived from a single waste source. It is thus recommended to fully leverage the characteristics of various reactive components and their synergistic effects. Accurate proportioning is critical for achieving high solid waste replacement levels while maintaining excellent cement properties.
- Mechanical strength and durability as fundamental evaluation criteria. Cement is frequently exposed to harsh service conditions—such as high temperatures, freezing environments, and rainwater erosion—that can significantly affect its long-term stability and structural safety. Therefore, cement produced using industrial solid waste must comply with national and regional standards. It is recommended to simulate actual loading conditions in engineering environments and integrate advanced materials characterization techniques to thoroughly investigate the evolution of the cement’s internal microstructure under prolonged mechanical stress.
- Environmental performance of cement. Although several studies have assessed the environmental performance of industrial waste-based cement, the potential risks associated with heavy metals and radioactive elements cannot be completely ruled out. Future research should adopt more advanced analytical techniques, expand the range of tested samples, and implement long-term, quantitative monitoring programs to ensure environmental safety.
- Policy and regulatory guidance. Detailed regulations should be established for the production, quality control, and engineering application of industrial waste-based cement products, based on comprehensive laboratory data and long-term monitoring from engineering demonstrations. Offering appropriate tax incentives and financial support to relevant enterprises is also essential. Furthermore, mechanisms for interdepartmental information sharing should be improved to enhance coordinated governance through multi-actor, multi-level, and cross-regional cooperation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhai, D. The development of China’s solid waste comprehensive utilization industry: Policy evolution and model innovation. Price Theory Pract. 2024, 95–101. [Google Scholar]
- Chen, Q.Y.; Chen, P.; Chu, Z. Riddled with challenges: Are zero-waste cities transitioning to circular economies? Insights from Chinese cities. J. Environ. Manag. 2025, 385, 125662. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, Y.; Zheng, Y.; Gong, X. Research progress on low-carbon technologies in cement manufacturing industry. Sci. Technol. Rev. 2024, 42, 21–30. [Google Scholar]
- Quan, S.; Han, X. Cement production pollution control and low-carbon environmental protection development path exploration. Leather Manuf. Environ. Technol. 2024, 5, 95–97. [Google Scholar]
- Gu, J.; Liu, X.; Zhang, Z. Road base materials prepared by multi-industrial solid wastes in China: A review. Constr. Build. Mater. 2023, 373, 130860. [Google Scholar] [CrossRef]
- Herath, C.; Gunasekara, C.; Law, D.W.; Setunge, S. Performance of high volume fly ash concrete incorporating additives: A systematic literature review. Constr. Build. Mater. 2020, 258, 120606. [Google Scholar] [CrossRef]
- Xu, G.; Shi, X. Characteristics and applications of fly ash as a sustainable construction material: A state-of-the-art review. Resour. Conserv. Recycl. 2018, 136, 95–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, T.C. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials-A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Wu, H.; Chen, C.; Song, W.; Hou, W. High-capacity utilization of coal gangue as supplementary cementitious material, geopolymer, and aggregate: A review. Constr. Build. Mater. 2024, 435, 136857. [Google Scholar] [CrossRef]
- Patangia, J.; Saravanan, T.J.; Kabeer, K.I.S.A.; Bisht, K. Study on the utilization of red mud (bauxite waste) as a supplementary cementitious material: Pathway to attaining sustainable development goals. Constr. Build. Mater. 2023, 375, 131005. [Google Scholar] [CrossRef]
- Salim, M.U.; Mosaberpanah, M.A.; Danish, A.; Ahmad, N.; Khalid, R.A.; Moro, C. Role of bauxite residue as a binding material and its effect on engineering properties of cementitious Composites: A review. Constr. Build. Mater. 2023, 409, 133844. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Shi, C.; Pan, S.Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Martins, A.C.P.; De Carvalho, J.M.F.; Costa, L.C.B.; Andrade, H.D.; De Melo, T.V.; Ribeiro, J.C.L.; Pedroti, L.G.; Peixoto, R.A.F. Steel slags in cement-based composites: An ultimate review on characterization, applications and performance. Constr. Build. Mater. 2021, 291, 123265. [Google Scholar] [CrossRef]
- Gencel, O.; Karadag, O.; Oren, O.H.; Bilir, T. Steel slag and its applications in cement and concrete technology: A review. Constr. Build. Mater. 2021, 283, 122783. [Google Scholar] [CrossRef]
- Amjad, H.; Abd-Elaal, E.S.; Ma, X.; Benn, T.; Fisher, M. A critical review of iron ore tailings as cement and aggregate substitutes for robust infrastructure: Mechanical, durability, and socio-economic impacts. J. Clean. Prod. 2025, 492, 144853. [Google Scholar] [CrossRef]
- Johansson, L.; Bahrami, A.; Wallhagen, M.; Cehlin, M. A comprehensive review on properties of tailings-based low-carbon concrete: Mechanical, environmental, and toxicological performances. Dev. Built Environ. 2024, 18, 100428. [Google Scholar] [CrossRef]
- Qu, F.; Zhang, Y.; Li, M.; Dong, W.; Li, W.; Tsang, D.C.W. Resource recycling of industrial waste phosphogypsum in cementitious materials: Pretreatment, properties, and applications. J. Environ. Manag. 2025, 376, 124291. [Google Scholar] [CrossRef]
- Murali, G.; Azab, M. Recent research in utilization of phosphogypsum as building materials. J. Mater. Res. Technol. 2023, 25, 960–987. [Google Scholar] [CrossRef]
- Clavier, K.A.; Paris, J.M.; Ferraro, C.C.; Townsend, T.G. Opportunities and challenges associated with using municipal waste incineration ash as a raw ingredient in cement production-a review. Resour. Conserv. Recycl. 2020, 160, 104888. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Xie, G.; Li, Z.; Fan, X.; Zhang, W.; Xing, F.; Tang, L.; Ren, J. Resource utilization of municipal solid waste incineration fly ash-cement and alkali-activated cementitious materials: A review. Sci. Total Environ. 2022, 852, 158254. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, D.; Dong, L.; Xu, Y.; Liu, J. Pollution status and environmental sound management (ESM) trends on typical general industrial solid waste. Procedia Environ. Sci. 2016, 31, 615–620. [Google Scholar] [CrossRef]
- The Ministry of Ecology and Environment. China’s Annual Report on Ecological and Environmental Statistics 2023; China Environment Publishing Group: Beijing, China, 2024. [Google Scholar]
- Zou, Y.; Song, Q.; Zhang, P.; Xu, S.; Bao, J.; Xue, S.; Qin, L.; Wang, H.; Lin, L.; Liu, C. Research status of building materials utilization and CO2 curing technology on typical coal-based solid waste: A critical review. J. CO2 Util. 2024, 84, 102860. [Google Scholar] [CrossRef]
- Das, D.; Rout, P.K. A review of coal fly ash utilization to save the environment. Water Air Soil Pollut. 2023, 234, 128. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T.; Krupa, A.; Marchewicz, A.; Czech, T.; Sliwinski, L.; Boryczko, G. Hybrid electrostatic filtration system for fly ash particles emission control. J. Electrost. 2021, 114, 103628. [Google Scholar] [CrossRef]
- Jahami, A.; Issa, C.A. Exploring the use of mixed waste materials (MWM) in concrete for sustainable Construction: A review. Constr. Build. Mater. 2023, 398, 132476. [Google Scholar] [CrossRef]
- Moghaddam, F.; Sirivivatnanon, V.; Vessalas, K. The effect of fly ash fineness on heat of hydration, microstructure, flow and compressive strength of blended cement pastes. Case Stud. Constr. Mater. 2019, 10, e00218. [Google Scholar] [CrossRef]
- Glinicki, M.A.; Jóźwiak-Niedźwiedzka, D.; Dąbrowski, M. The influence of fluidized bed combustion fly ash on the phase composition and microstructure of cement paste. Materials 2019, 12, 2838. [Google Scholar] [CrossRef]
- Xiao, M.; Xi, J.; Qiu, P.; Deng, C.; Li, F.; Wei, J.; Gao, P.; Yu, Q. Evaluation of tensile properties and cracking potential evolution of fly ash-cement mortar at early age based on digital image correlation method. Constr. Build. Mater. 2024, 412, 134855. [Google Scholar] [CrossRef]
- Wu, J.; Qi, F.; Zhang, J.; Chen, Z.; Wang, H.; Liu, Q. Modeling of effect of fly ash amount on microstructure and chloride diffusivity of blended fly ash-cement systems. Constr. Build. Mater. 2024, 443, 137711. [Google Scholar] [CrossRef]
- Mishra, G.; Danoglidis, P.; Shah, S.P.; Konsta-Gdoutos, M. Optimization of biochar and fly ash to improve mechanical properties and CO2 sequestration in cement mortar. Constr. Build. Mater. 2023, 392, 132021. [Google Scholar] [CrossRef]
- Yaofei, L.; Xingchen, Z.; Ke, Z. Coal gangue in asphalt pavement: A review of applications and performance influence. Case Stud. Constr. Mater. 2024, 20, e03282. [Google Scholar] [CrossRef]
- Zhou, S.; Dong, J.; Yu, L.; Xu, C.; Jiao, X.; Wang, M. Effect of activated coal gangue in North China on the compressive strength and hydration process of cement. J. Mater. Civ. Eng. 2019, 31, 04019022. [Google Scholar] [CrossRef]
- Shao, Z.; Cao, M. Hydration mechanism of limestone calcined clay cement containing calcined coal gangue. Constr. Build. Mater. 2024, 438, 136906. [Google Scholar] [CrossRef]
- Li, L.; Shao, X.; Ling, T.C. Life cycle assessment of coal gangue composite cements: From sole OPC towards low-carbon quaternary binder. J. Clean. Prod. 2023, 414, 137674. [Google Scholar] [CrossRef]
- Yuan, N.; Zhao, A.; Hu, Z.; Tan, K.; Zhang, J. Preparation and application of porous materials from coal gasification slag for wastewater treatment: A review. Chemosphere 2022, 287, 132227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, X.; Xu, X. Physical-chemical performance of coal gasification slag with calcination activation utilized as supplementary cementitious material. Constr. Build. Mater. 2024, 451, 138776. [Google Scholar] [CrossRef]
- Fu, B.; Cheng, Z.; Wang, D.; Li, N. Investigation on the utilization of coal gasification slag in Portland cement: Reaction kinetics and microstructure. Constr. Build. Mater. 2022, 323, 126587. [Google Scholar] [CrossRef]
- Luo, F.; Jiang, Y.; Wei, C. Potential of decarbonized coal gasification residues as the mineral admixture of cement-based material. Constr. Build. Mater. 2021, 269, 121259. [Google Scholar] [CrossRef]
- Wu, P.; Liu, X.; Zhang, Z.; Wei, C. Properties of red mud-filled and modified resin composites. Constr. Build. Mater. 2023, 409, 133984. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, Y.; Li, Z.; Li, Y.; Ren, Y. Binary reaction behaviors of red mud based cementitious material: Hydration characteristics and Na+ utilization. J. Hazard. Mater. 2021, 410, 124592. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, S.; Wang, Y.; Shen, P.; Xuan, D.; Guan, X.; Shi, C. Hydration-hardening properties of low-clinker composite cement incorporating carbonated waste sintering red mud and metakaolin. Constr. Build. Mater. 2022, 354, 129171. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, X.; Wei, C.; Zeng, Q.; Ma, S.; Liu, X.; Zhang, Z.; Webeck, E. Synergistic enhancement of converter steelmaking slag, blast furnace slag, bayer red mud in cementitious materials: Strength, phase composition, and microstructure. J. Build. Eng. 2022, 60, 105177. [Google Scholar] [CrossRef]
- Tian, K.; Wang, Y.; Dong, B.; Fang, G.; Xing, F. Engineering and micro-properties of alkali-activated slag pastes with Bayer red mud. Constr. Build. Mater. 2022, 351, 128869. [Google Scholar] [CrossRef]
- Garanayak, L. Strength effect of alkali activated red mud slag cement in ambient condition. Mater. Today Proc. 2021, 44, 1437–1443. [Google Scholar] [CrossRef]
- Song, Q.; Guo, M.Z.; Wang, L.; Ling, T. Use of steel slag as sustainable construction materials: A review of accelerated carbonation treatment. Resour. Conserv. Recycl. 2021, 173, 105740. [Google Scholar] [CrossRef]
- Gao, T.; Dai, T.; Shen, L.; Jiang, L. Benefits of using steel slag in cement clinker production for environmental conservation and economic revenue generation. J. Clean. Prod. 2021, 282, 124538. [Google Scholar] [CrossRef]
- Ji, X.; Hou, J.; Liu, Y.; Liu, J. Effect of CaO-FeO-MnO system solid solution on the hydration activity of tri-component f-CaO in steel slag. Constr. Build. Mater. 2019, 225, 476–484. [Google Scholar] [CrossRef]
- Cao, L.; Shen, W.; Huang, J.; Yang, Y.; Zhang, D.; Huang, X.; Lv, Z.; Ji, X. Process to utilize crushed steel slag in cement industry directly: Multi-phased clinker sintering technology. J. Clean. Prod. 2019, 217, 520–529. [Google Scholar] [CrossRef]
- Hu, Y.; Ren, X.; Luan, Z.; Xiao, Y.; Zhang, W. Performance of a steel slag-based supplementary cementitious material via the synergistic modification of binary fly ash and gypsum system. Constr. Build. Mater. 2023, 405, 133186. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Bolte, G. Composition of the reactivity of engineered slags from bauxite residue and steel slag smelting and use as SCM for Portland cement. Constr. Build. Mater. 2022, 321, 126331. [Google Scholar] [CrossRef]
- Tian, E.; Ren, W.; Zhuang, Y.; Zeng, W. Effect of steel slag powder and stone powder by-product from manufactured sand on the mechanical properties and microstructure of cementitious materials. J. Clean. Prod. 2024, 452, 142128. [Google Scholar] [CrossRef]
- Wang, X.; Sun, D.; Li, J.; Wang, W.; Mao, Y.; Song, Z. Properties and hydration characteristics of an iron-rich sulfoaluminate cementitious material under cold temperatures. Cem. Concr. Res. 2023, 168, 107152. [Google Scholar] [CrossRef]
- Fu, Y.; Qiao, H.X.; Feng, Q.; Chen, K.; Li, Y.; Xue, C.; Zhang, Y. Review of new methods for resource utilisation of electrolytic manganese residue and its application in building materials. Constr. Build. Mater. 2023, 401, 132901. [Google Scholar] [CrossRef]
- Wang, D.; Fang, J.; Wang, Q.; Liu, Y. Utilizing desulphurized electrolytic-manganese residue as a mineral admixture: A feasibility study. Cem. Concr. Compos. 2022, 134, 104822. [Google Scholar] [CrossRef]
- Lan, J.; Sun, Y.; Tian, H.; Zhan, W.; Du, Y.; Ye, H.; Du, D.; Zhang, T.; Hou, H. Electrolytic manganese residue-based cement for manganese ore pit backfilling: Performance and mechanism. J. Hazard. Mater. 2021, 411, 124941. [Google Scholar] [CrossRef]
- Wang, F.; Long, G.; Bai, M.; Wang, J.; Yang, Z.; Zhou, X.; Zhou, J. Cleaner and safer disposal of electrolytic manganese residues in cement-based materials using direct electric curing. J. Clean. Prod. 2022, 356, 131842. [Google Scholar] [CrossRef]
- He, D.; Chen, M.; Liu, H.; Wang, J. Preparation of activated electrolytic manganese residue-slag-cement ternary blended cementitious material: Hydration characteristics and carbon reduction potential. Constr. Build. Mater. 2024, 425, 135990. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, K.; Su, Y.; Shi, Y. An evaluation of iron ore tailings characteristics and iron ore tailings concrete properties. Constr. Build. Mater. 2021, 286, 122968. [Google Scholar] [CrossRef]
- Liu, J.; An, S.; Zhang, Y. Mechanism of regulating the mechanical properties and paste structure of supersulfated cement through ultrafine iron tailings powder. Cem. Concr. Compos. 2023, 140, 105061. [Google Scholar] [CrossRef]
- Zhang, B.; Muhammad, F.; Yu, T.; Fahimizadeh, M.; Hassan, M.A.S.; Liang, J.; Ning, X.; Yuan, P. Harnessing iron tailings as supplementary cementitious materials in Limestone Calcined Clay Cement (LC3): An innovative approach towards sustainable construction. Constr. Build. Mater. 2024, 453, 139111. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Zhang, Q.; Yang, L.; Cao, J. Influence of phosphorus tailings fineness on the hydration process and physical properties of ordinary Portland cement. Constr. Build. Mater. 2024, 417, 135349. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, J.; Gbozee, M. Influences of phosphate tailings on hydration and properties of Portland cement. Constr. Build. Mater. 2015, 98, 593–601. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Li, Q.; Song, J. Hydration properties of Portland cement-copper tailing powder composite binder. Constr. Build. Mater. 2020, 251, 118882. [Google Scholar] [CrossRef]
- Jian, S.; Gao, W.; Lv, Y.; Tan, H.; Li, X.; Li, B.; Huang, W. Potential utilization of copper tailings in the preparation of low heat cement clinker. Constr. Build. Mater. 2020, 252, 119130. [Google Scholar] [CrossRef]
- Pei, T.; Zheng, Y.; Wang, Y.; Zhang, D.; Zhang, P.; Cui, S.; Zheng, Y.; Zhao, S. Utilization of copper tailings in the preparation of low-calcium Portland cement clinker and carbonation-hardening mechanism. Constr. Build. Mater. 2024, 457, 139362. [Google Scholar] [CrossRef]
- Cheng, Y.; Qi, R.; Hou, J.; Huang, Q. Feasibility study on utilization of copper tailings as raw meal and addition for low carbon Portland cement production. Constr. Build. Mater. 2023, 382, 131275. [Google Scholar] [CrossRef]
- Wu, F.; Ren, Y.; Qu, G.; Liu, S.; Chen, B.; Liu, X.; Zhao, C.; Li, J. Utilization path of bulk industrial solid waste: A review on the multi-directional resource utilization path of phosphogypsum. J. Environ. Manag. 2022, 313, 114957. [Google Scholar] [CrossRef]
- Rosales, J.; Pérez, S.M.; Cabrera, M.; Gázquez, M.J.; Bolivar, J.P.; De Brito, J.; Agrela, F. Treated phosphogypsum as an alternative set regulator and mineral addition in cement production. J. Clean. Prod. 2020, 244, 118752. [Google Scholar] [CrossRef]
- Liu, S.; Ouyang, J.; Ren, J. Mechanism of calcination modification of phosphogypsum and its effect on the hydration properties of phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2020, 243, 118226. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Ren, C.; Yao, Y.; Wang, W. Recycling phosphogypsum as a sole calcium oxide source in calcium sulfoaluminate cement and its environmental effects. J. Environ. Manag. 2020, 271, 110986. [Google Scholar] [CrossRef]
- Dong, M.; Li, J.S.; Lang, L.; Chen, X.; Jin, J.; Ma, W. Recycling thermal modified phosphogypsum in calcium sulfoaluminate cement: Evolution of engineering properties and micro-mechanism. Constr. Build. Mater. 2023, 373, 130823. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, K.; Yang, Y.; Chang, J. Investigation on the preparation of low carbon cement materials from industrial solid waste phosphogypsum: Clinker preparation, cement properties, and hydration mechanism. J. Clean. Prod. 2024, 452, 142203. [Google Scholar] [CrossRef]
- Wu, S.; Yao, Y.; Yao, X.; Ren, C.; Li, J.; Xu, D.; Wang, W. Co-preparation of calcium sulfoaluminate cement and sulfuric acid through mass utilization of industrial by-product gypsum. J. Clean. Prod. 2020, 265, 121801. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Q.; Li, C.; Pan, H. Optimization design and mechanical properties of multi-component ecological gelling materials. J. Clean. Prod. 2024, 443, 140970. [Google Scholar] [CrossRef]
- Wei, C.; Lu, Y.; Liu, X.; Zhang, Z.; Wu, P.; Gu, J. Harmless disposal of phosphogypsum synergized red mud: Harmful element control and material utilization. J. Environ. Chem. Eng. 2024, 12, 113660. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Huang, D.; Huang, Q.; Li, X.; Shui, Z. Safe environmentally friendly reuse of red mud modified phosphogypsum composite cementitious material. Constr. Build. Mater. 2023, 368, 130348. [Google Scholar] [CrossRef]
- Isteri, V.; Ohenoja, K.; Hanein, T.; Kinoshita, H.; Kletti, H.; Rössler, C.; Tanskanen, P.; Illikainen, M.; Fabritius, T. Ferritic calcium sulfoaluminate belite cement from metallurgical industry residues and phosphogypsum: Clinker production, scale-up, and microstructural characterisation. Cem. Concr. Res. 2022, 154, 106715. [Google Scholar] [CrossRef]
- Lin, Q.; Zhen, X.; Rong, Y.; Li, Y.; Zhang, H.; Zhang, Q.; Yao, Z.; Yao, K. Investigation on mechanical and microstructure properties of silt improved by titanium gypsum-based stabilizer. Materials 2022, 16, 271. [Google Scholar] [CrossRef]
- Chang, N.; Li, H.; Liu, W.; Zhang, D.; Zheng, W.; Wan, Z.; Wu, X.; Luo, Z. Phase evolution and mechanical performance of red mud-gypsum waste derived activator composite cementitious materials exposed to various Ca/Si and Al/S ratios. Constr. Build. Mater. 2024, 412, 134807. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, S. Effects of titanium gypsum on high calcium fly ash system: Physical and microscopic properties, hydration, and Fe transformation. J. Build. Eng. 2023, 78, 107653. [Google Scholar] [CrossRef]
- Tian, Y.; Bourtsalas, A.C.T.; Kawashima, S.; Ma, S.; Themelis, N.J. Performance of structural concrete using Waste-to-Energy (WTE) combined ash. Waste Manag. 2020, 118, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Kleib, J.; Aouad, G.; Abriak, N.E.; Benzerzour, M. Production of Portland cement clinker from French municipal solid waste incineration bottom ash. Case Stud. Constr. Mater. 2021, 15, e00629. [Google Scholar] [CrossRef]
- Chen, B.; Perumal, P.; Illikainen, M.; Ye, G. A review on the utilization of municipal solid waste incineration (MSWI) bottom ash as a mineral resource for construction materials. J. Build. Eng. 2023, 71, 106386. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Shen, Z.L.; Deng, J. Effects of pre-curing temperatures on the mechanical properties and microstructural development of impregnated municipal solid waste incineration bottom ash mortar. Constr. Build. Mater. 2023, 369, 130600. [Google Scholar] [CrossRef]
- Yao, J.; Song, H.; Li, Y.; Cui, Y.; Chai, M.; Ling, W. Mechanism of macro-and microscopic performance of cement mortars influenced by municipal solid waste incineration bottom ash as sand substitution. Constr. Build. Mater. 2023, 397, 132317. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, H.; Zhang, W.; Xie, G.; Liu, J.; Xing, F. Influence of municipal solid waste incineration bottom ash on the hydration and carbonation behavior of reactive magnesia cement. Chem. Eng. J. 2024, 502, 158059. [Google Scholar] [CrossRef]
- Huang, B.; Gan, M.; Ji, Z.; Fan, X.; Zhang, D.; Chen, X.; Sun, Z.; Huang, X.; Fan, Y. Recent progress on the thermal treatment and resource utilization technologies of municipal waste incineration fly ash: A review. Process Saf. Environ. Prot. 2022, 159, 547–565. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Kumar, M.S. An overview of environmental sustainability in cement and steel production. J. Clean. Prod. 2019, 231, 856–871. [Google Scholar] [CrossRef]
- Bogush, A.A.; Stegemann, J.A.; Zhou, Q.; Wang, Z.; Zhang, B.; Zhang, T.; Zhang, W.; Wei, J. Co-processing of raw and washed air pollution control residues from energy-from-waste facilities in the cement kiln. J. Clean. Prod. 2020, 254, 119924. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, H.; Wang, W.; Jia, M.; Che, X. Pretreatment of municipal solid waste incineration fly ash and preparation of solid waste source sulphoaluminate cementitious material. J. Hazard. Mater. 2020, 385, 121580. [Google Scholar] [CrossRef]
- Wang, S.; Pan, H.; Xiao, C.; Zhao, Q.; Wang, J. Preparation and mix proportion optimization of red mud-fly ash-based cementitious material synergistic activated by carbide slag and MSWIFA. Constr. Build. Mater. 2024, 415, 135032. [Google Scholar] [CrossRef]
- Hu, J.; Niu, R.; Liu, J.; Zhang, W.; Liu, J.; Xing, F. The hydration and heavy metal immobilization performance of the CO2-active incineration fly ash-calcined clay-cement (CFC3) composite low-carbon cementitious system. Cem. Concr. Compos. 2024, 149, 105528. [Google Scholar] [CrossRef]
- Song, X.; Liang, Z.; Luo, W.; Huang, D.; Yang, Z.; Xu, P.; Hu, J.; Hou, H.; Liu, B.; Yang, J. Life cycle assessment of cement manufacture solely from industrial solid waste. Acta Sci. Circumstantiae 2021, 41, 5190–5199. [Google Scholar]


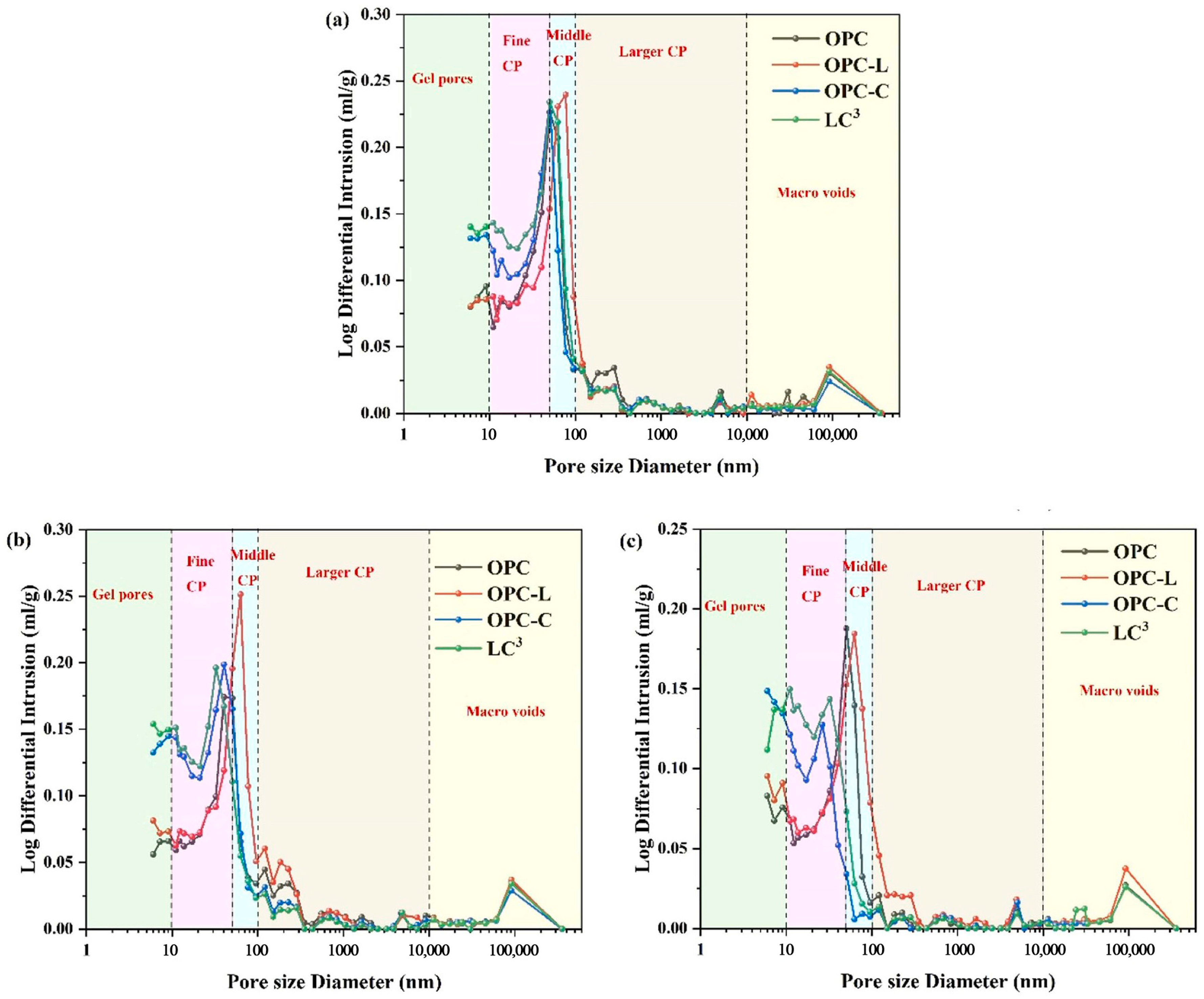
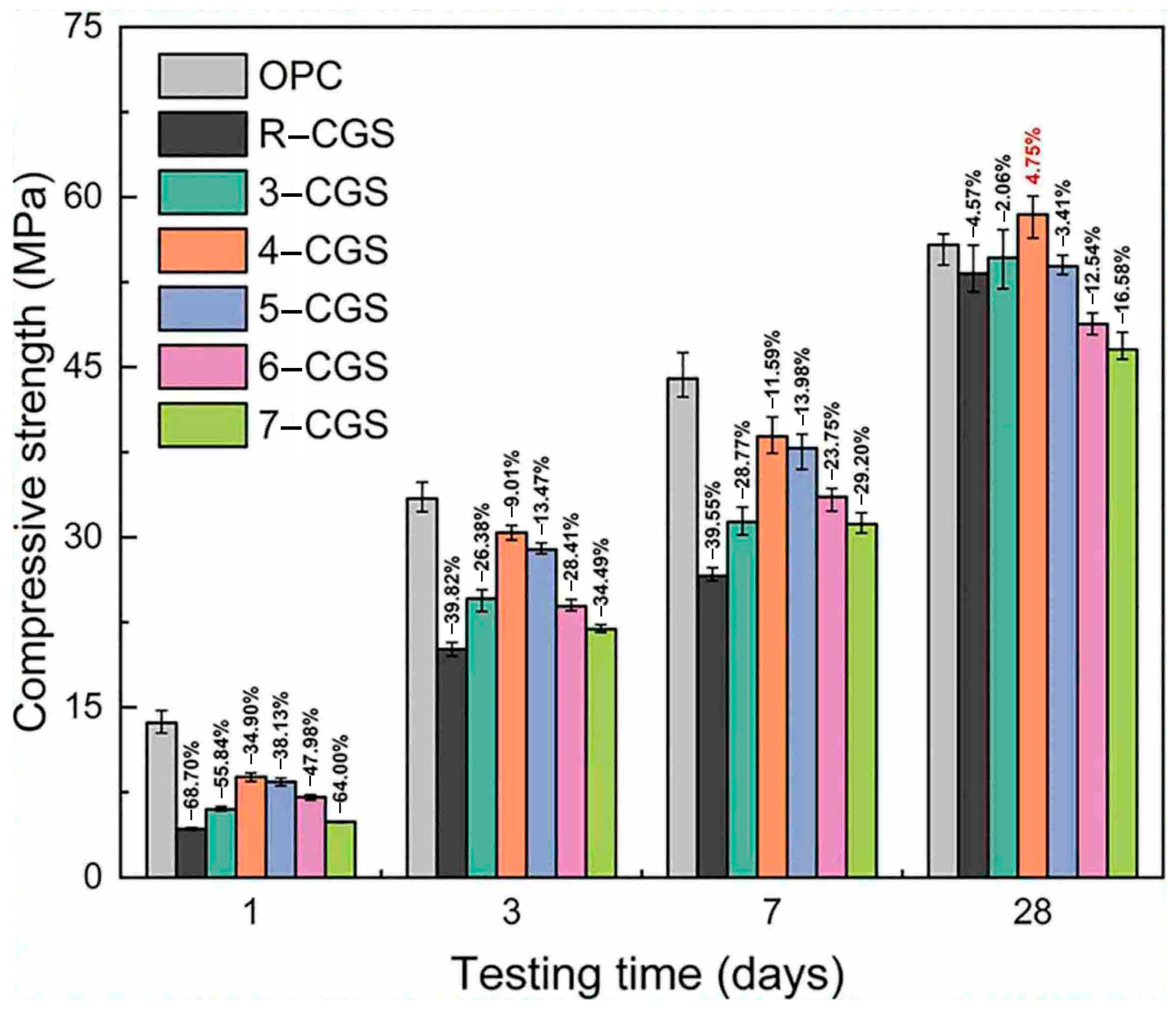
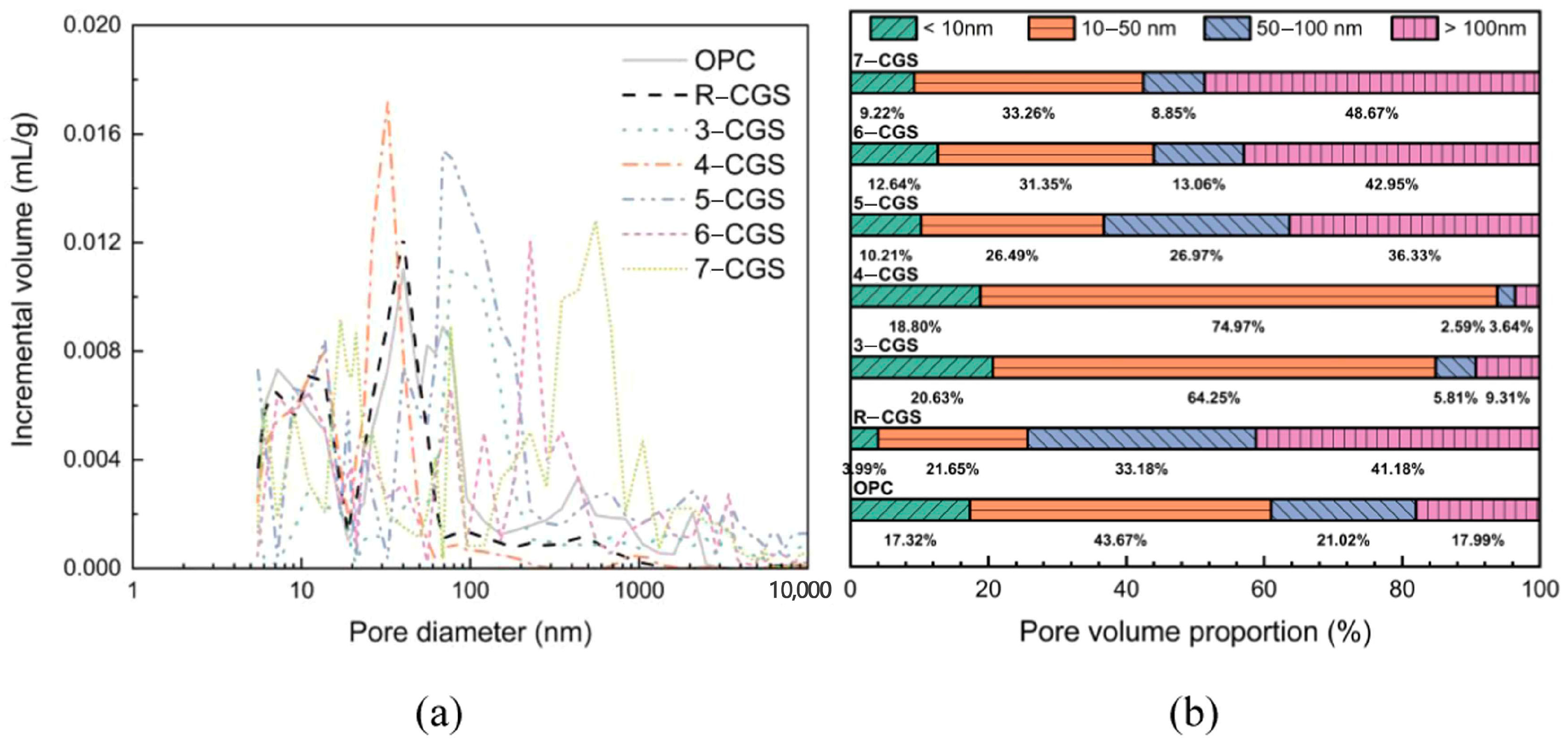
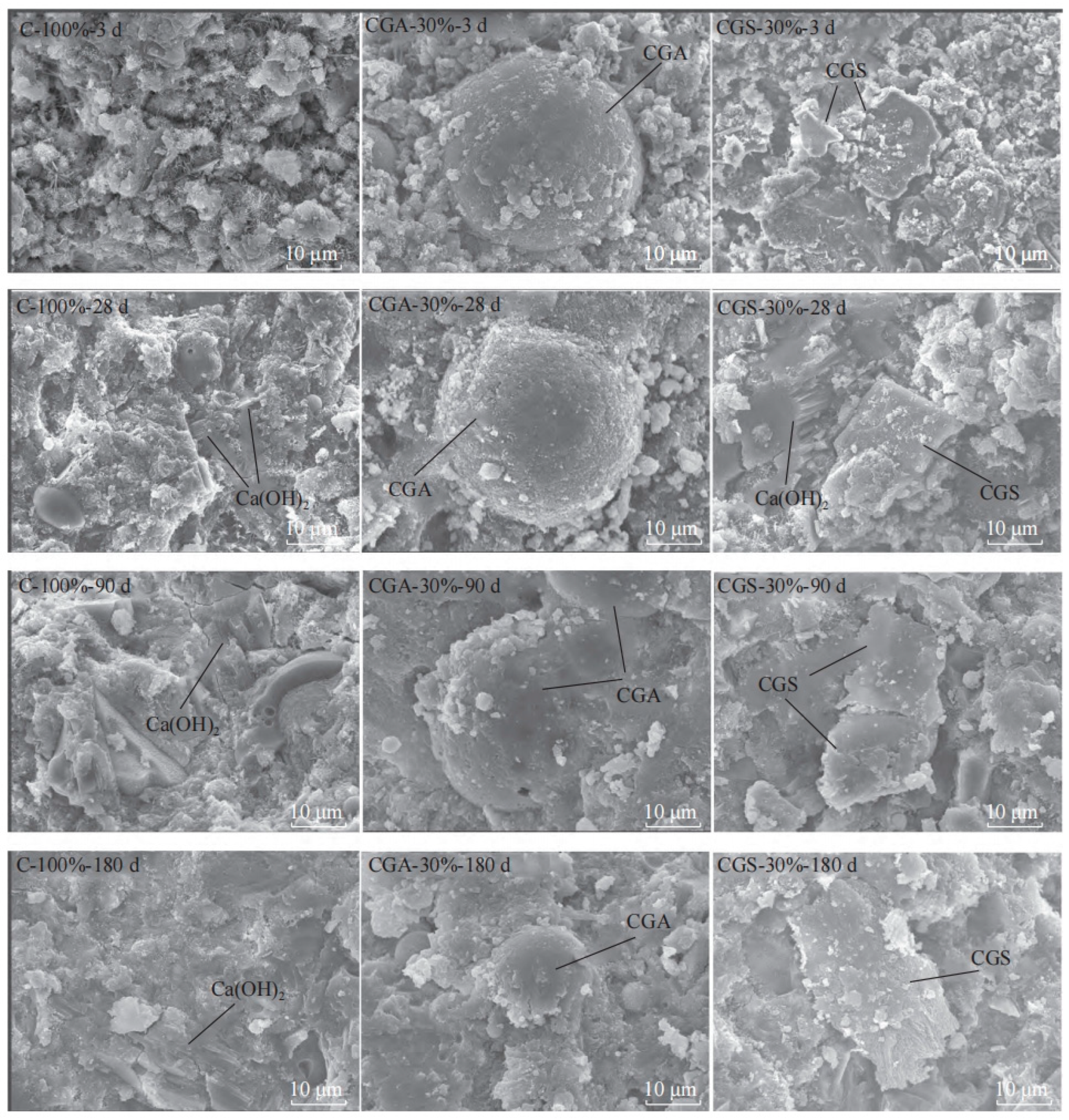
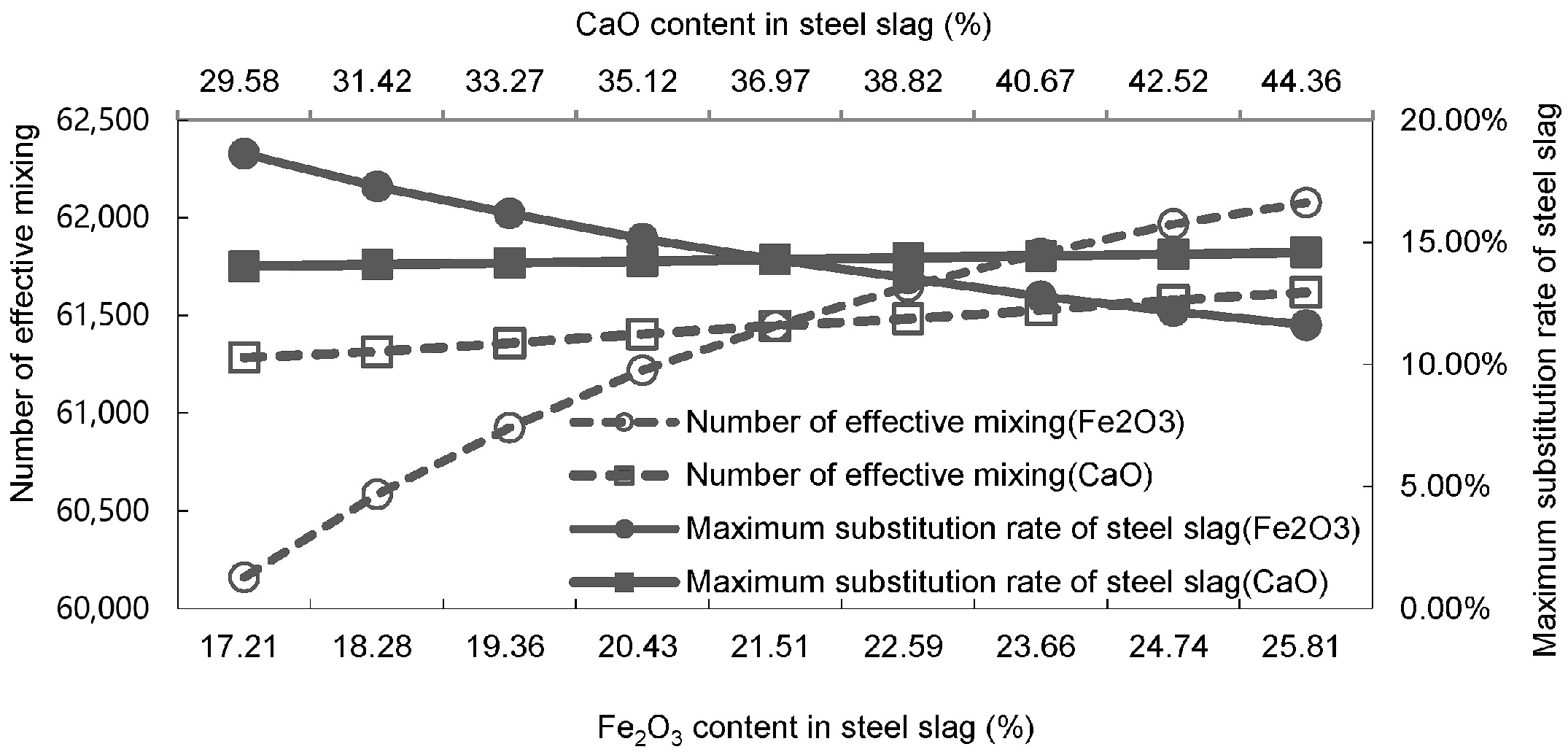
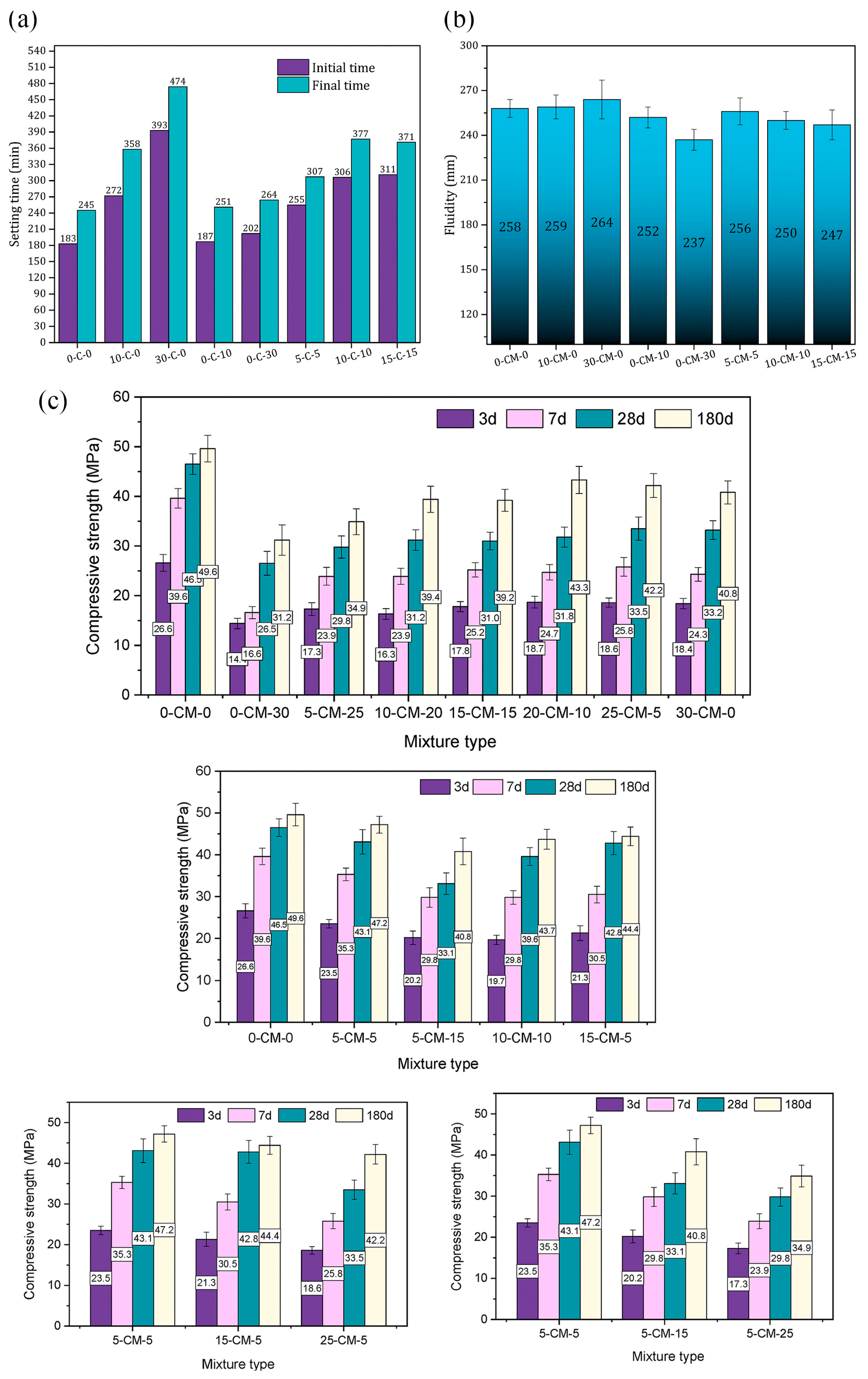



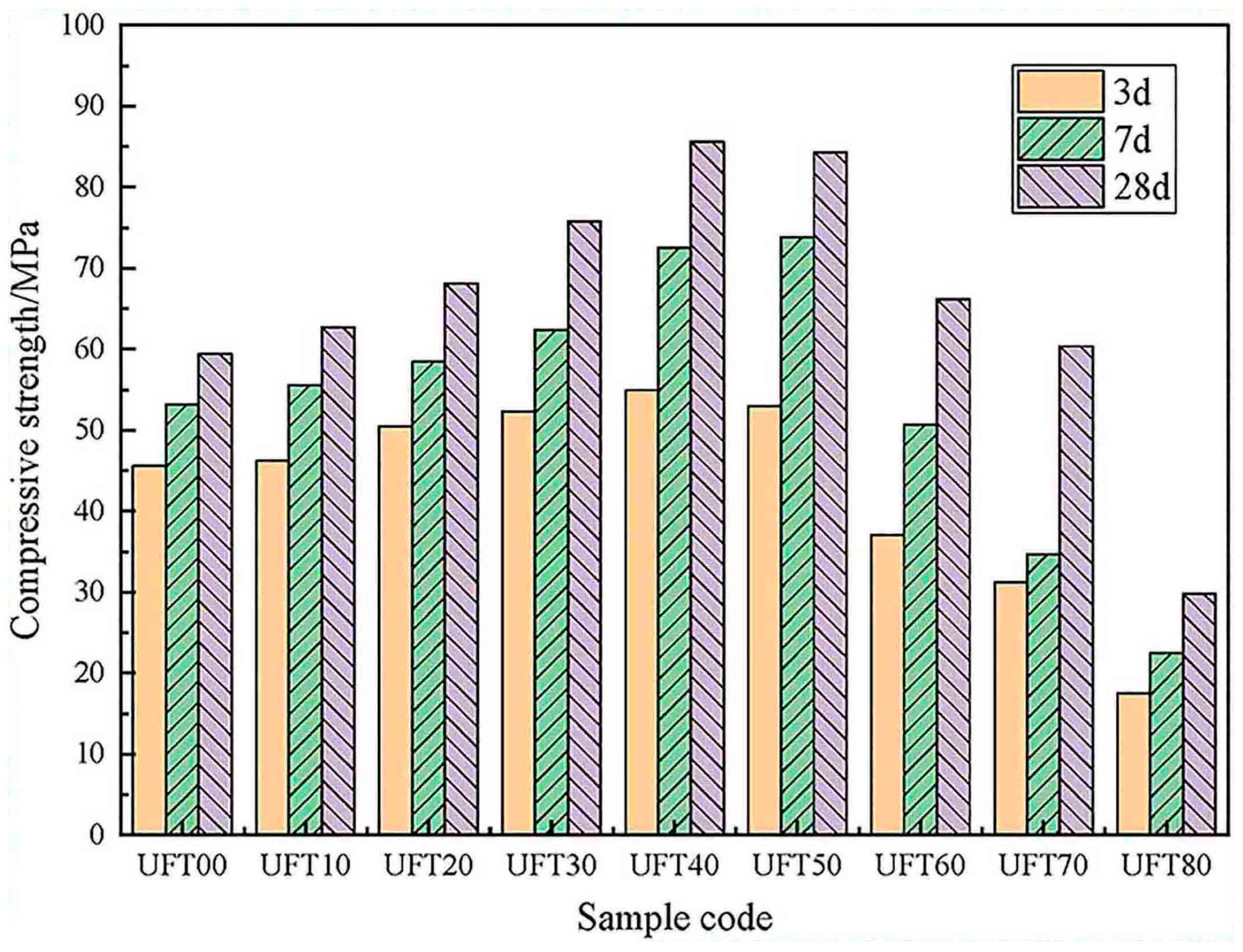

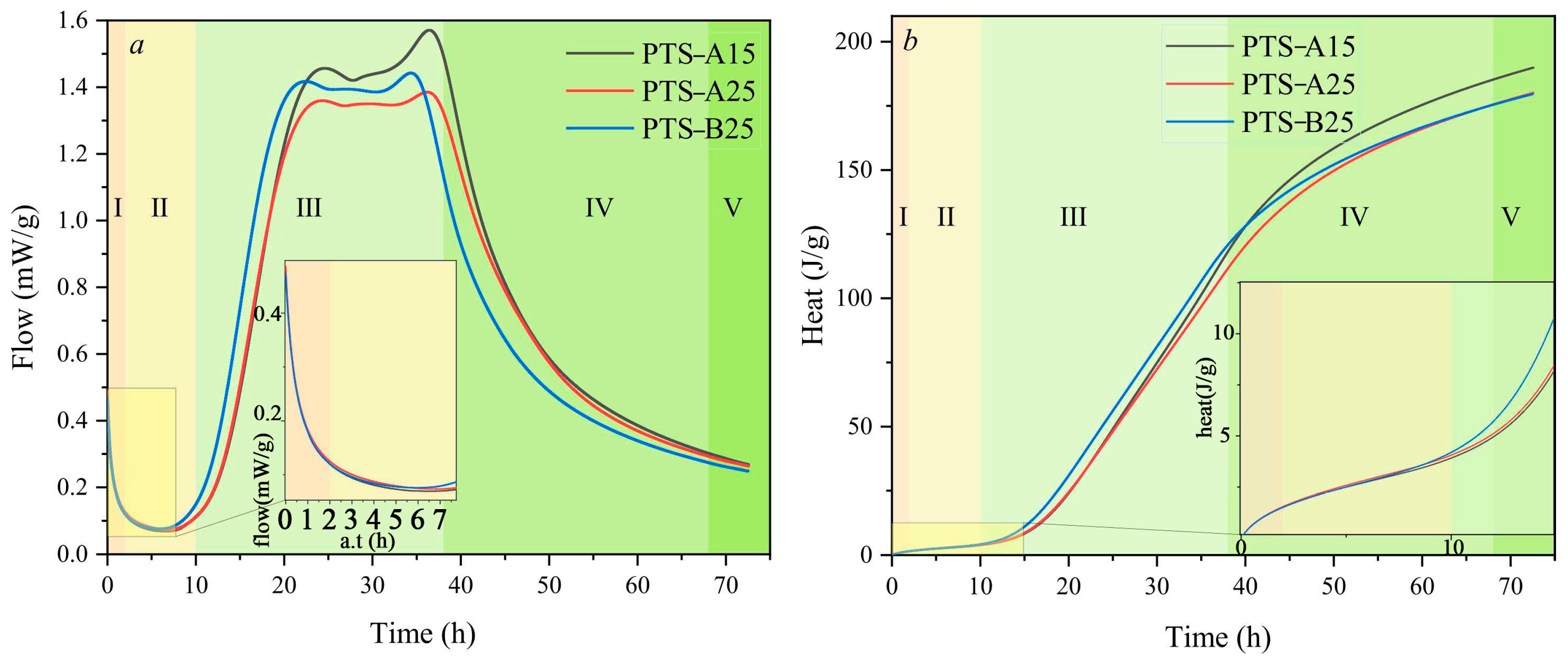
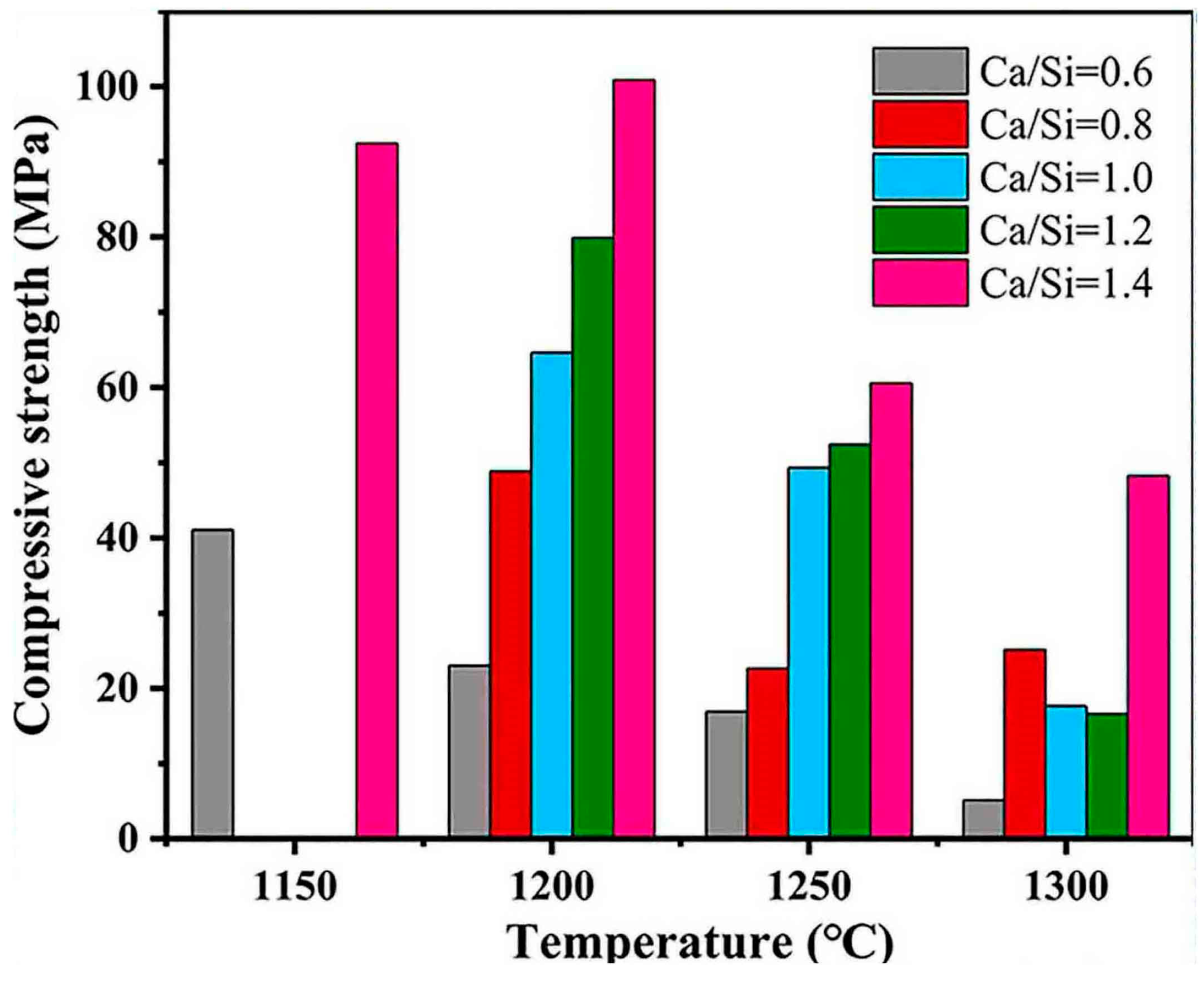

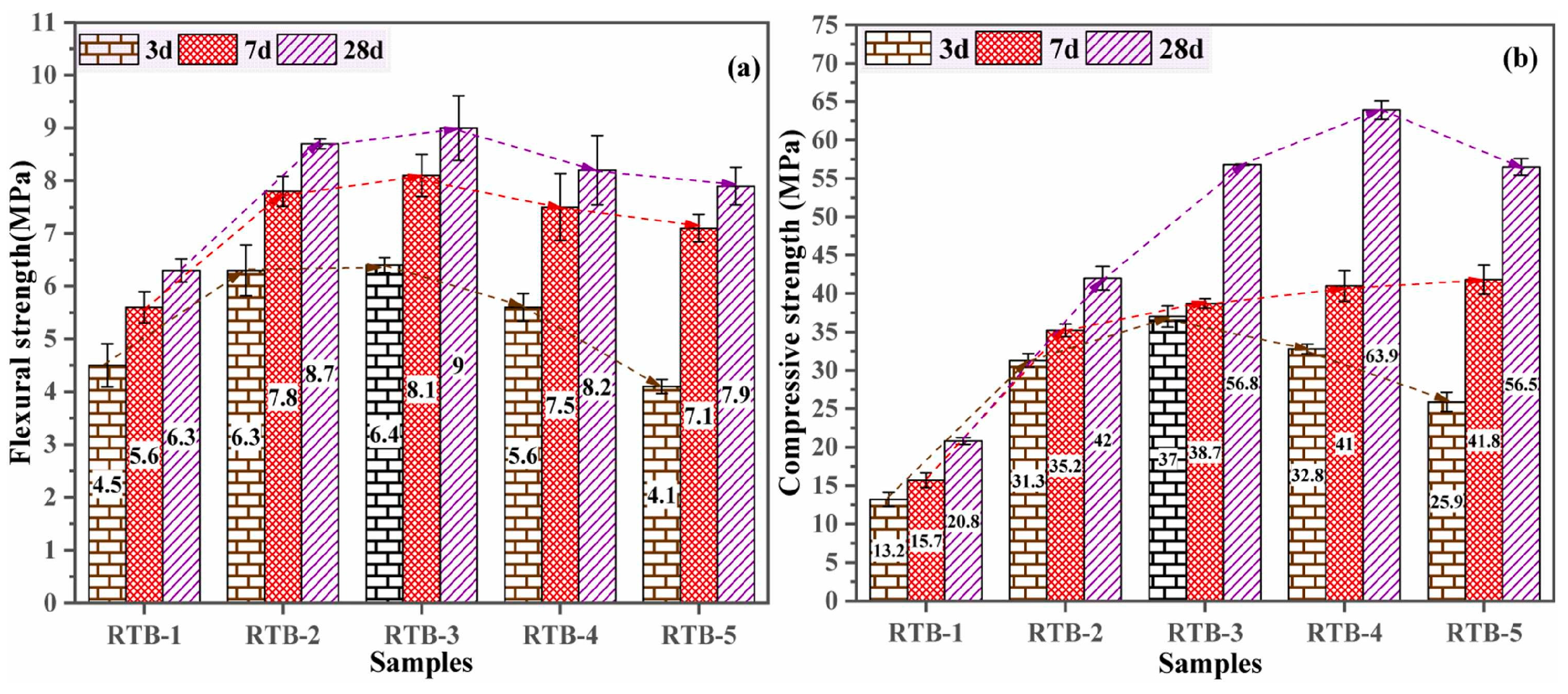
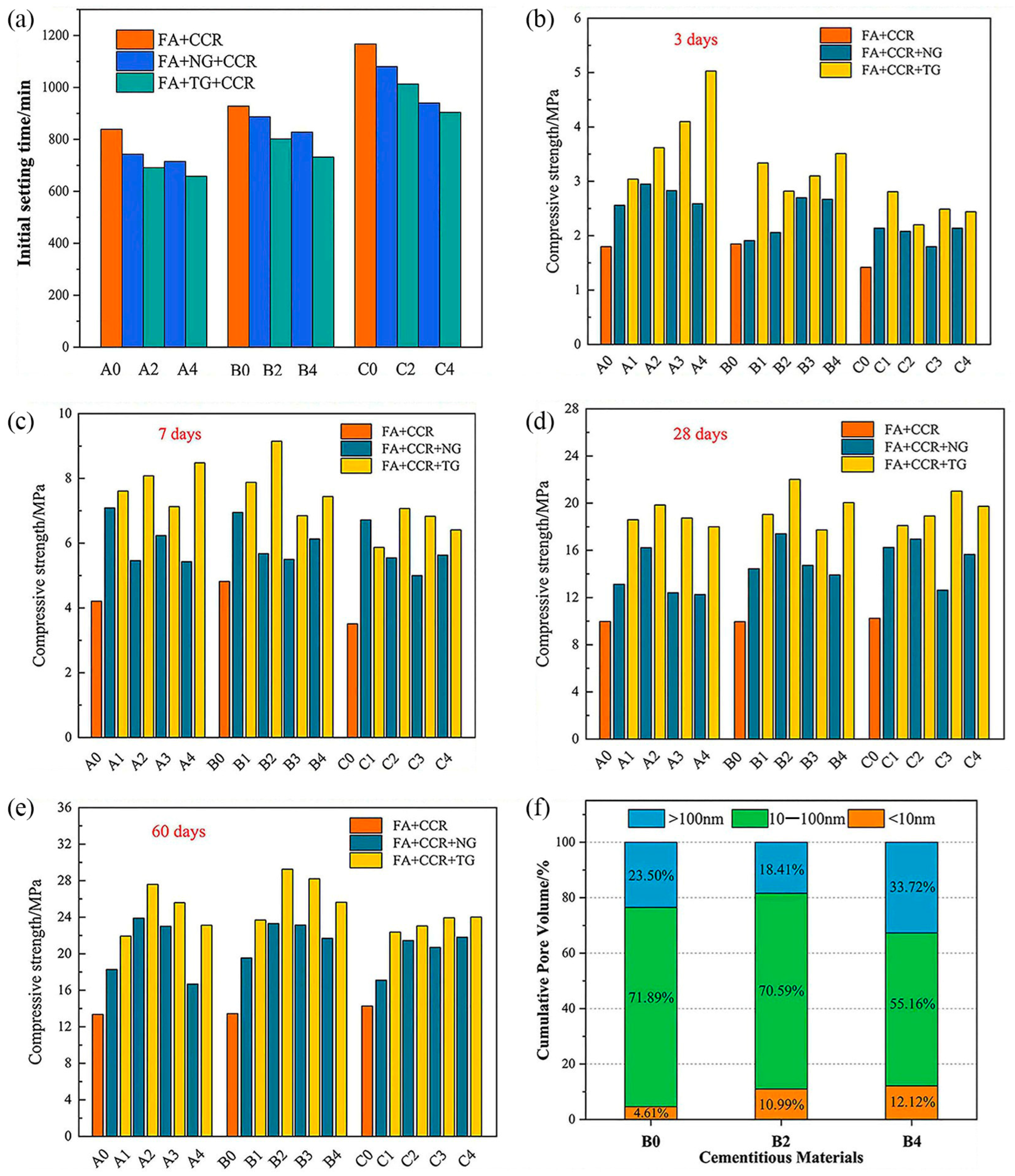

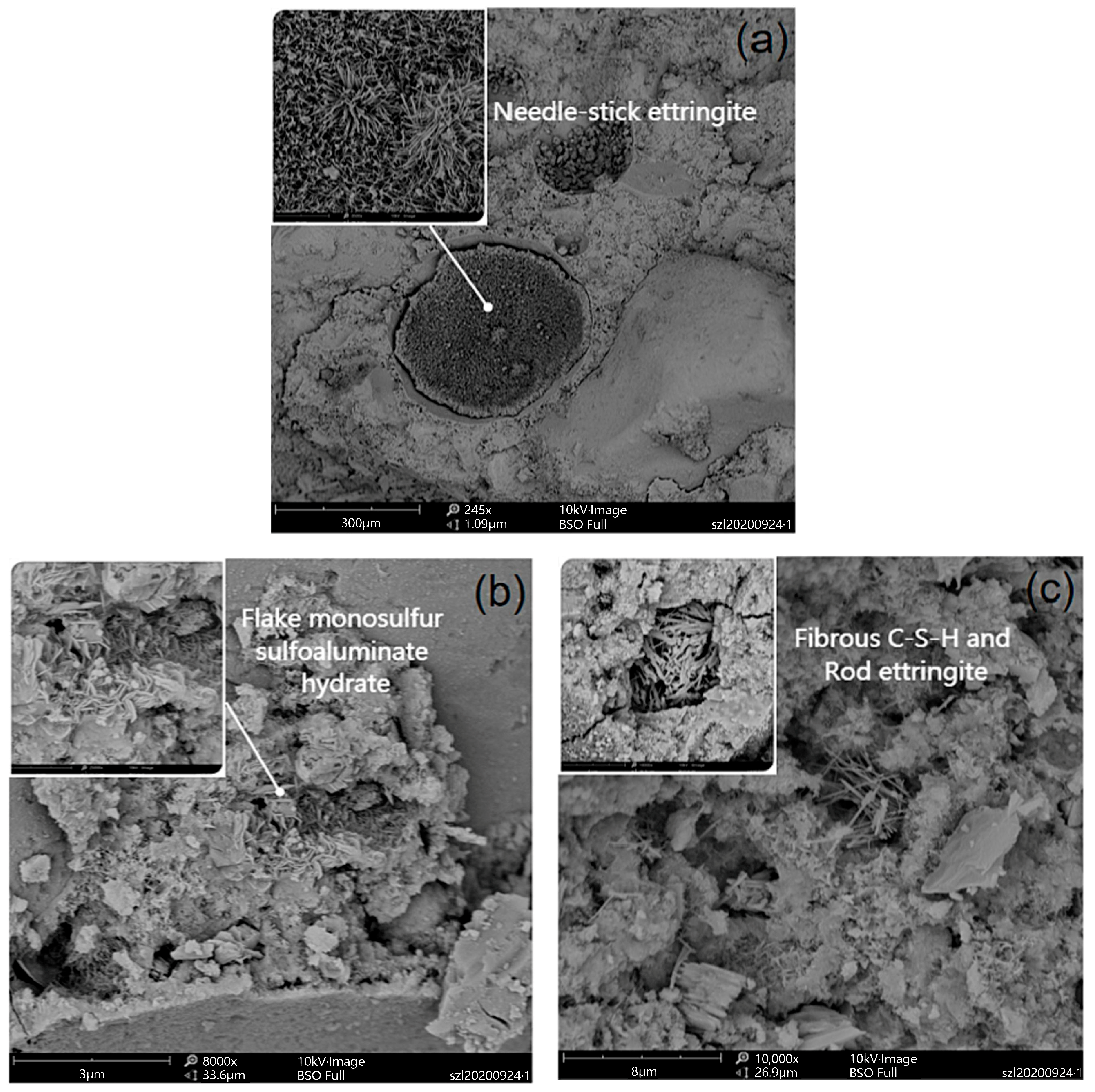

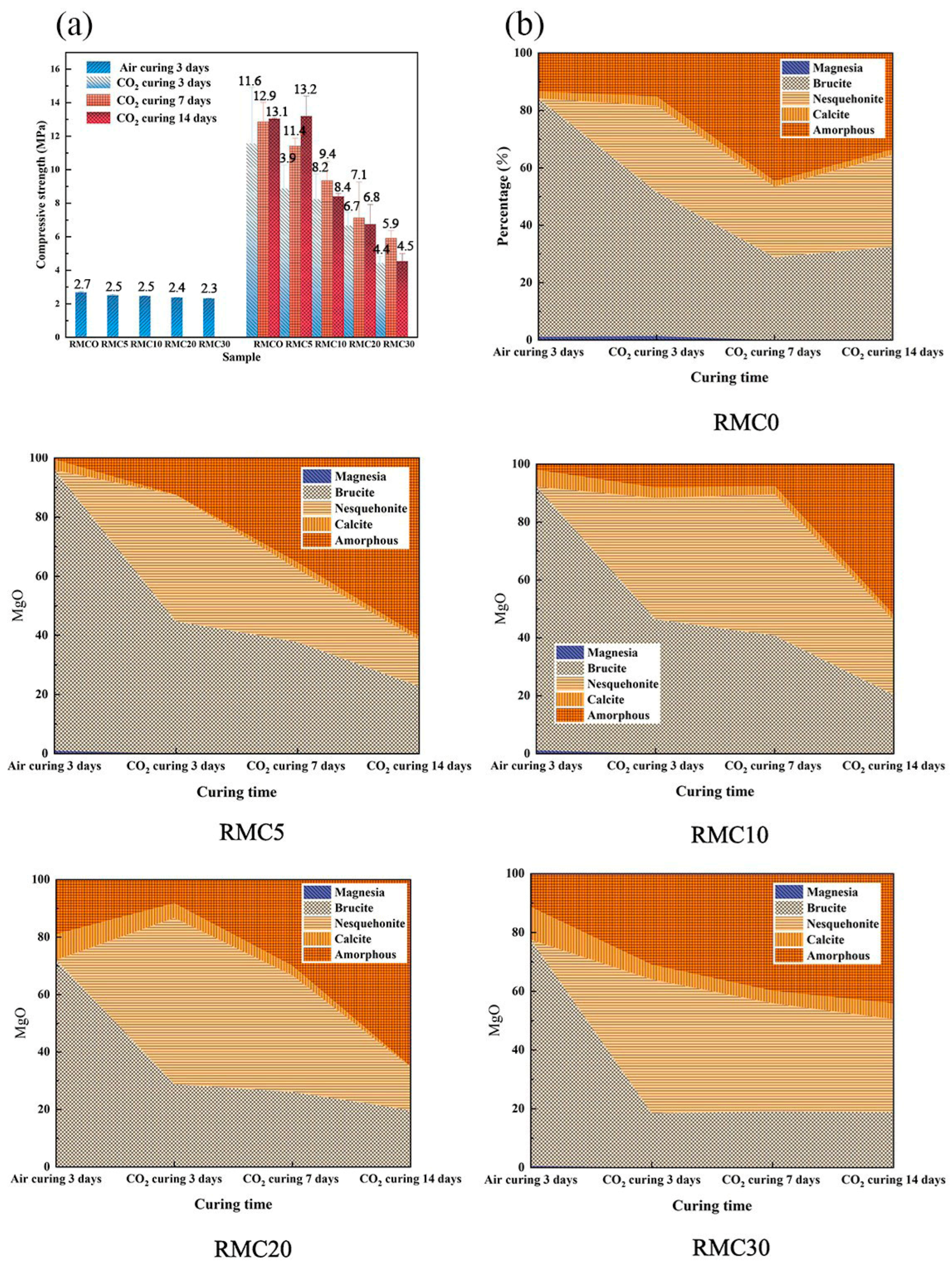
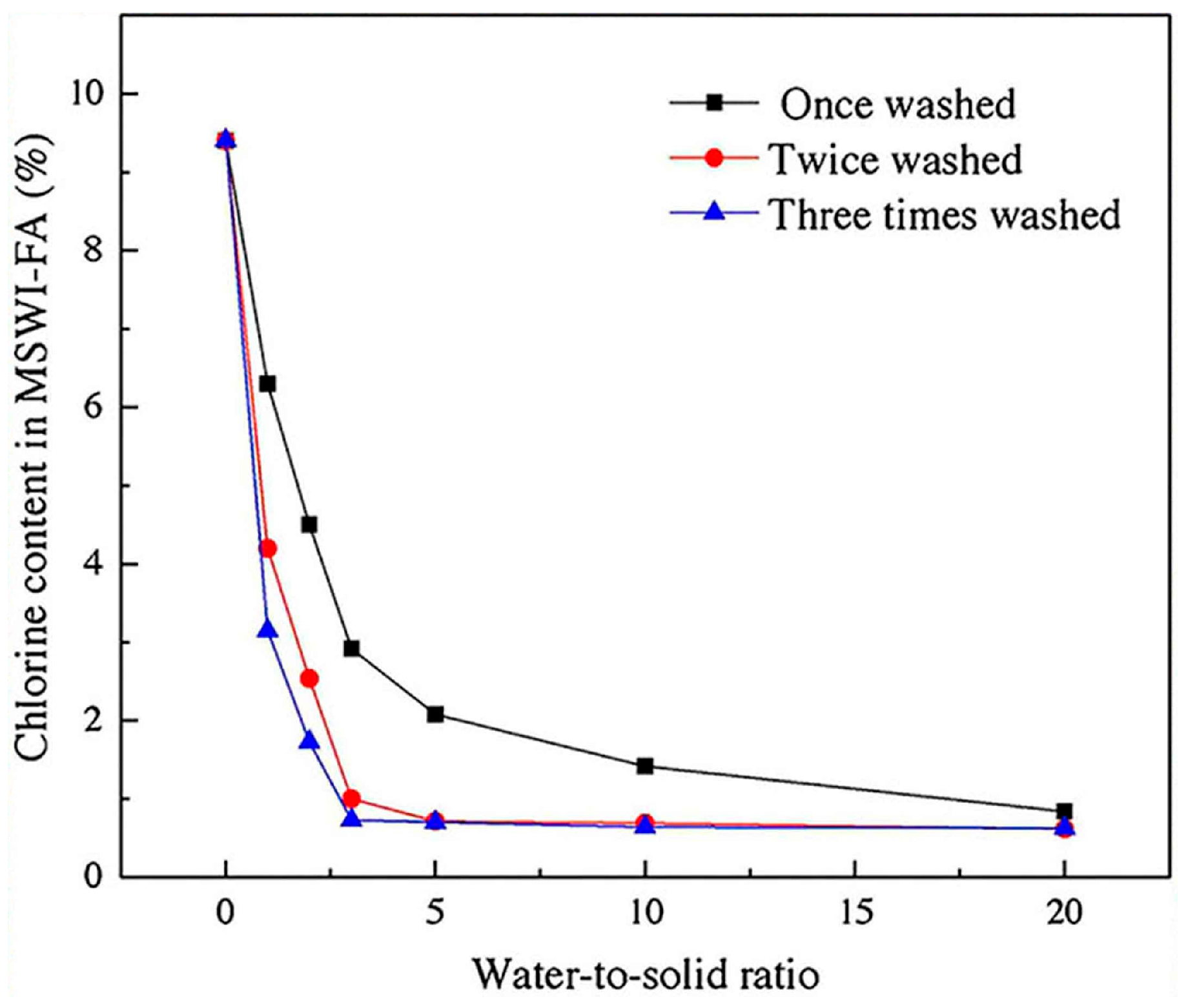
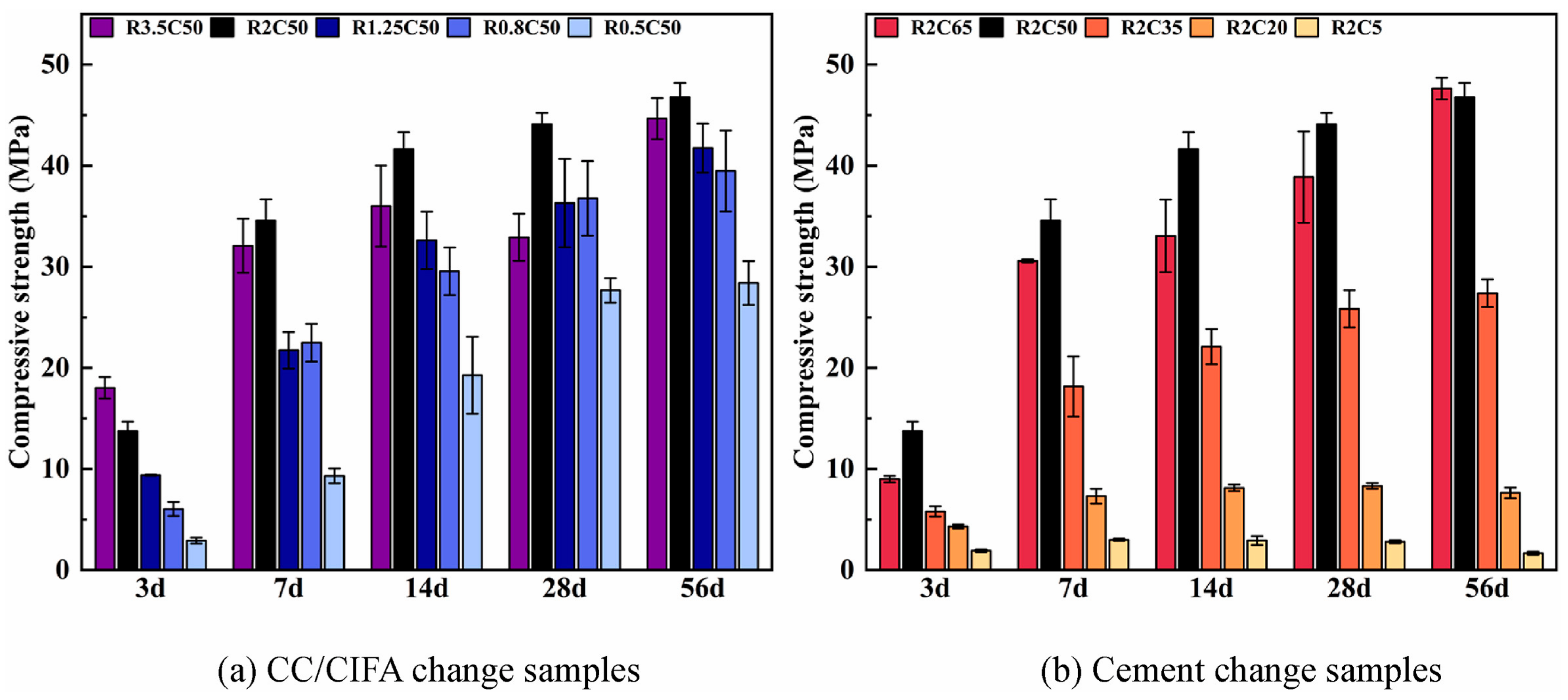
| Proportioning of Raw Materials | Density, g/cm3 | Specific Surface Area, cm2/kg | Setting Time, min | Compressive Strength, MPa | ||
|---|---|---|---|---|---|---|
| Initial | Final | 3 d | 28 d | |||
| Red mud–Cement = 20:80 [41] | — | — | — | — | 21.10 | 35.50 |
| Red mud and metakaolin composite–Cement = 50:50 [42] | 3.12 | 355 | 99 | 158 | 27.00 | 50.30 |
| Red mud–Slag cement = 8:92 [43] | 2.42 | — | — | — | 22.90 | 56.30 |
| Red mud–Alkali-activated slag cement = 40:60 [44] | — | — | 49 | 62 | 60.00 | 89.93 |
| Pretreated red mud–Slag cement = 50:50 [45] | — | — | — | — | 43.39 | 68.79 |
| Proportioning of Raw Materials | Compressive Strength MPa | |
|---|---|---|
| 3 d | 28 d | |
| 2.5% of washed and filtered phosphogypsum in ordinary Portland cement [69] | 20.30 | 53.60 |
| 15% of calcined phosphogypsum in supersulfated cement [70] | 87.60 | 99.30 |
| 72% of phosphogypsum in calcium sulfoaluminate cement [71] | 86.70 | 112.60 |
| 10% of thermally modified phosphogypsum in calcium sulfoaluminate cement [72] | 46.50 | 88.40 |
| 20% of phosphogypsum in calcium sulfoaluminate cement [73] | 28.10 | 41.59 |
| 73% of phosphogypsum in cement co-produced with sulfuric acid [74] | 13.00 | 28.95 |
| 4% of phosphogypsum in eco-cement [75] | — | 45.80 |
| Multiple Solid Waste Systems | Performance Difference Compared with Single Solid Waste |
|---|---|
| 5% biochar + 10% fly ash + cement [26] | Flexural strength and elastic modulus improve significantly with the addition of biochar. |
| 25% steel slag powder + 15% limestone powder + cement [52] | The activity index is higher. |
| 15% electrolytic manganese slag + 15% slag + cement [58] | The compressive strength increased by 83% compared with the use of electrolytic manganese slag alone. |
| 40% slag powder + 40% iron tailings powder + cement [60] | The incorporation of iron tailings resulted in a 20.4% and 44.1% increase in compressive strength at 3 and 28 days, respectively. |
| 20% red mud + phosphogypsum + blast furnace slag + cement [76] | The soluble phosphorus is transformed into inert material and is completely stabilized when red mud dosing reaches 20%. The slow setting is significantly improved, resulting in an increase in 3-day compressive strength. |
| 46.9% phosphogypsum + 26.5% red mud + 4.1% fly ash + 12.2% quicklime + 10.2% cement [77] | Under the alkaline stimulation of materials such as red mud and quicklime, silicon ions and aluminum ions in industrial solid waste such as fly ash are activated to exhibit high activity, which can react with OH− and Ca2+ in the system to form Ca5Si6O16(OH)·4H2O and CaSiO3·nH2O gels. Some active aluminumions can react with OH−, SO42− and Ca2+ to form 3CaO·Al2O3·3CaSO4·32H2O. |
| 12.5% titanium gypsum + fly ash + calcium carbide residue [81] | The setting time of fly ash–carbide slag composite binders is effectively shortened, compressive strength is improved, pore structure is optimized, and the overall performance of the material is enhanced when titanium gypsum is used as an additive. |
| Classification | Industrial Solid Waste | Main Chemical Composition | Typical Substitution Level | Compressive Strength (28 d) | Durability | Environmental Benefits | Potential Risks and Recommended Treatments | Reference |
|---|---|---|---|---|---|---|---|---|
| Aluminosilicate-rich | Fly ash | SiO2, Al2O3, and CaO | 25% | 41.0 MPa | Enhances resistance to chloride penetration and carbonation, and reduces water permeability and capillary sorptivity | Reduces emissions, recovers resources, sequesters carbon dioxide, and reduces cement consumption | Trace heavy metals; control dosage and monitor leaching | [28] |
| Coal gangue | SiO2 and Al2O3 | 30% | 42.3 MPa | Improves with activation | Utilizes mining waste; reduces land pressure and carbon footprint | Low activity; requires calcination or alkali activation | [34] | |
| Coal gasification furnace slag | SiO2, Al2O3, CaO, and Fe2O3 | 30% | 54.0 MPa | Reduces porosity; improves impermeability | Reduces energy and cement demand | Contains unburned carbon; requires decarbonization and ball milling | [37] | |
| Electrolytic manganese slag | SiO2, Al2O3, and Fe2O3 | 10% | 38.0 MPa | Acceptable after sulfate removal and activation | Utilizes Mn-rich industrial waste; cuts cement usage | Contains sulfates and Mn2+; requires detoxification and stabilization | [55] | |
| Iron tailings | SiO2, Al2O3, and Fe2O3 | 28% | 42.0 MPa | Acceptable after fine grinding; dense matrix structure | Reduces raw material consumption; utilizes mining tailings | Heavy metals and sulfides; leaching test and pretreatment required | [61] | |
| Copper tailings | SiO2, Al2O3, and Fe2O3 | 15% | 43.4 MPa | Reduces porosity and densifies structure | Reuses mining waste; reduces land use and CO2 emissions | Contains Cu, Fe; must stabilize to avoid rebar corrosion and leaching | [67] | |
| Calcium-rich | Red mud | CaO, Fe2O3, Al2O3, and SiO2 | 20% | 35.5 MPa | Low permeability; high alkalinity aids in chloride resistance | Solidify highly alkaline waste residue to reduce red mud storage and pollution | Strong alkalinity and heavy metals; must be neutralized | [41] |
| Steel slag | CaO, SiO2, and Fe2O3 | 8% | 60.3 MPa | Enhances sulfate and freeze-thaw resistance | Saves energy and generates economic benefits | High alkalinity and expansion risk; needs fine grinding and stabilization | [47] | |
| Municipal solid waste incineration bottom ash | CaO, SiO2, Al2O3 and Fe2O3 | 25% | 43.0 MPa | Good frost/erosion resistance after Al removal | Reduces landfill pressure; saves cement and CO2 | Al causes expansion; must remove metallic Al | [86] | |
| Phosphorus-/Sulfur-rich | Phosphorus tailings | CaO, MgO, and P2O5 | 30% | 31.0 MPa | Acceptable if stabilized; raw use cause expansion from sulfate salts | Enables phosphorus recovery; reduces hazardous tailing dumps | Radioactivity and soluble salts; must be stabilized and monitored | [63] |
| Phosphogypsum | (CaSO4·2H2O) and SiO2 | 20% | 41.6 MPa | Acceptable after washing or calcination | Replaces natural gypsum; utilizes P-industry by-product | Contains P, F, radioactive elements; must be treated and limited | [73] | |
| Titanium gypsum | SO3, CaO, and Fe2O3 | 20% | 57.0 MPa | Reduces porosity and improve pore distribution | Reduces gypsum extraction | High impurities; requires desulfurization or calcination | [80] | |
| Heavy-metal-/salt-rich | Municipal solid waste incineration fly ash | SiO2, CaO and Al2O3 | 15% | 44.0 MPa | High-temp roasting improves resistance and reduces toxicity | Enables safe reuse of highly toxic incineration ash | Contains heavy metals, persistent organic pollutants; must be incinerated or stabilized | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Z.; Zhang, W. Recycling and Mineral Evolution of Multi-Industrial Solid Waste in Green and Low-Carbon Cement: A Review. Minerals 2025, 15, 740. https://doi.org/10.3390/min15070740
Yue Z, Zhang W. Recycling and Mineral Evolution of Multi-Industrial Solid Waste in Green and Low-Carbon Cement: A Review. Minerals. 2025; 15(7):740. https://doi.org/10.3390/min15070740
Chicago/Turabian StyleYue, Zishu, and Wei Zhang. 2025. "Recycling and Mineral Evolution of Multi-Industrial Solid Waste in Green and Low-Carbon Cement: A Review" Minerals 15, no. 7: 740. https://doi.org/10.3390/min15070740
APA StyleYue, Z., & Zhang, W. (2025). Recycling and Mineral Evolution of Multi-Industrial Solid Waste in Green and Low-Carbon Cement: A Review. Minerals, 15(7), 740. https://doi.org/10.3390/min15070740






