Reassessment of Heavy Metal Adsorption Performance in Halloysite Clay Nanotubes: Geographical Variation and Structure–Activity Relationship
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
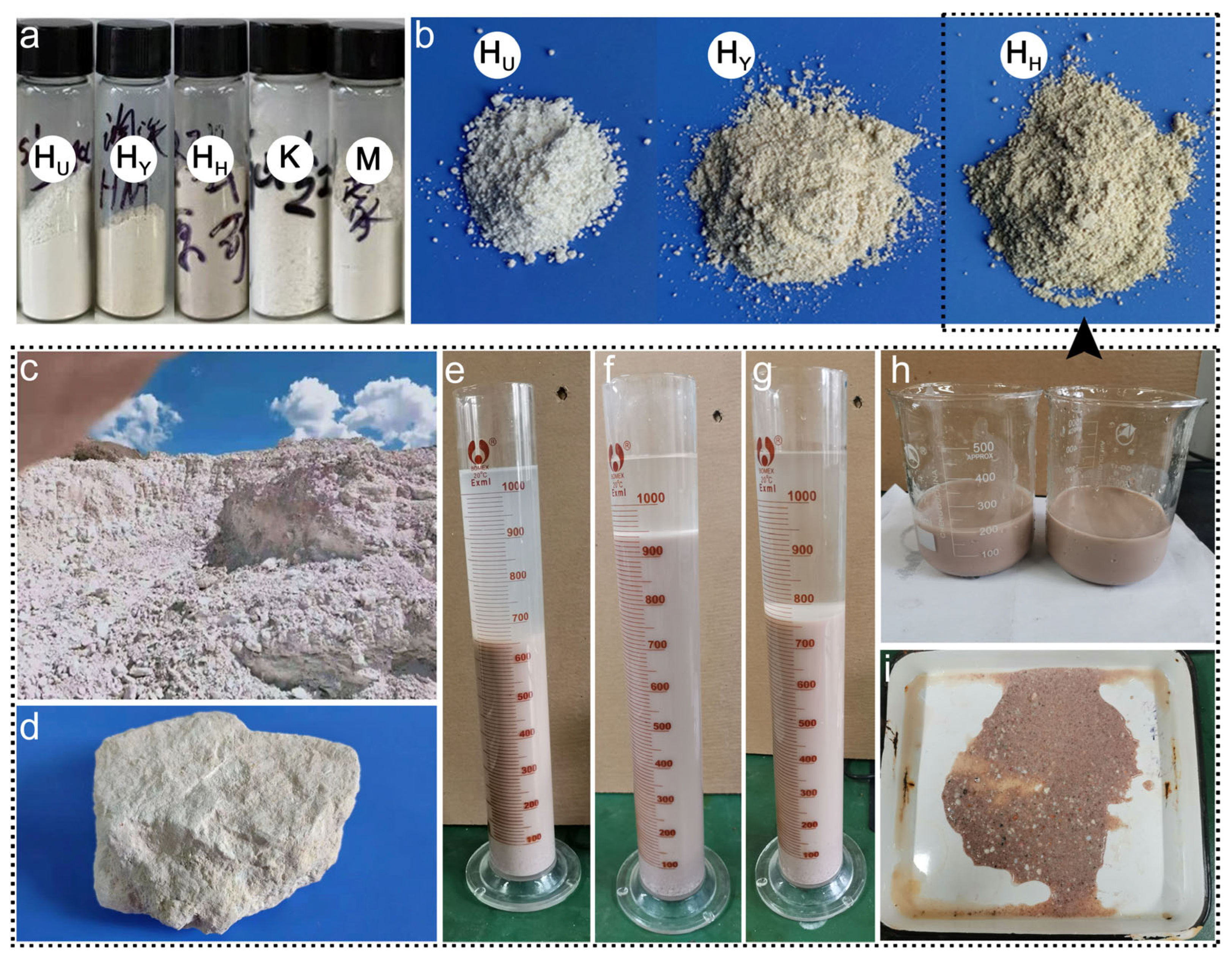
2.2. Mineral Purification
2.3. Structural Characterization and Compositional Analysis of Minerals
2.4. pH Boundary Experiment (Blank Experiment)
2.5. Adsorption Equilibrium Experiment
3. Results
3.1. Structural Characterization of Minerals
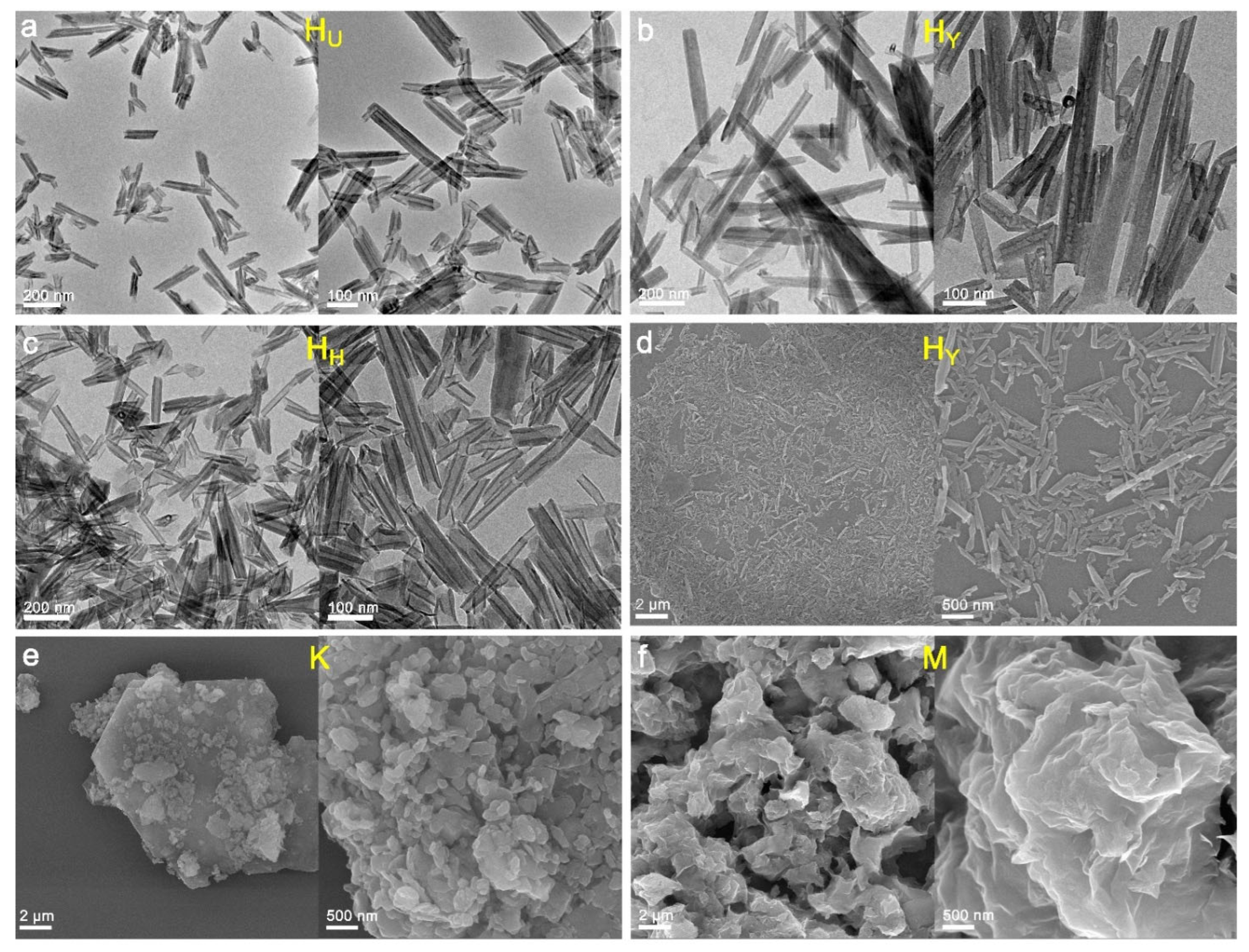
3.1.1. TEM and SEM
3.1.2. BET
3.2. Mineral Composition Analysis
3.2.1. XRF
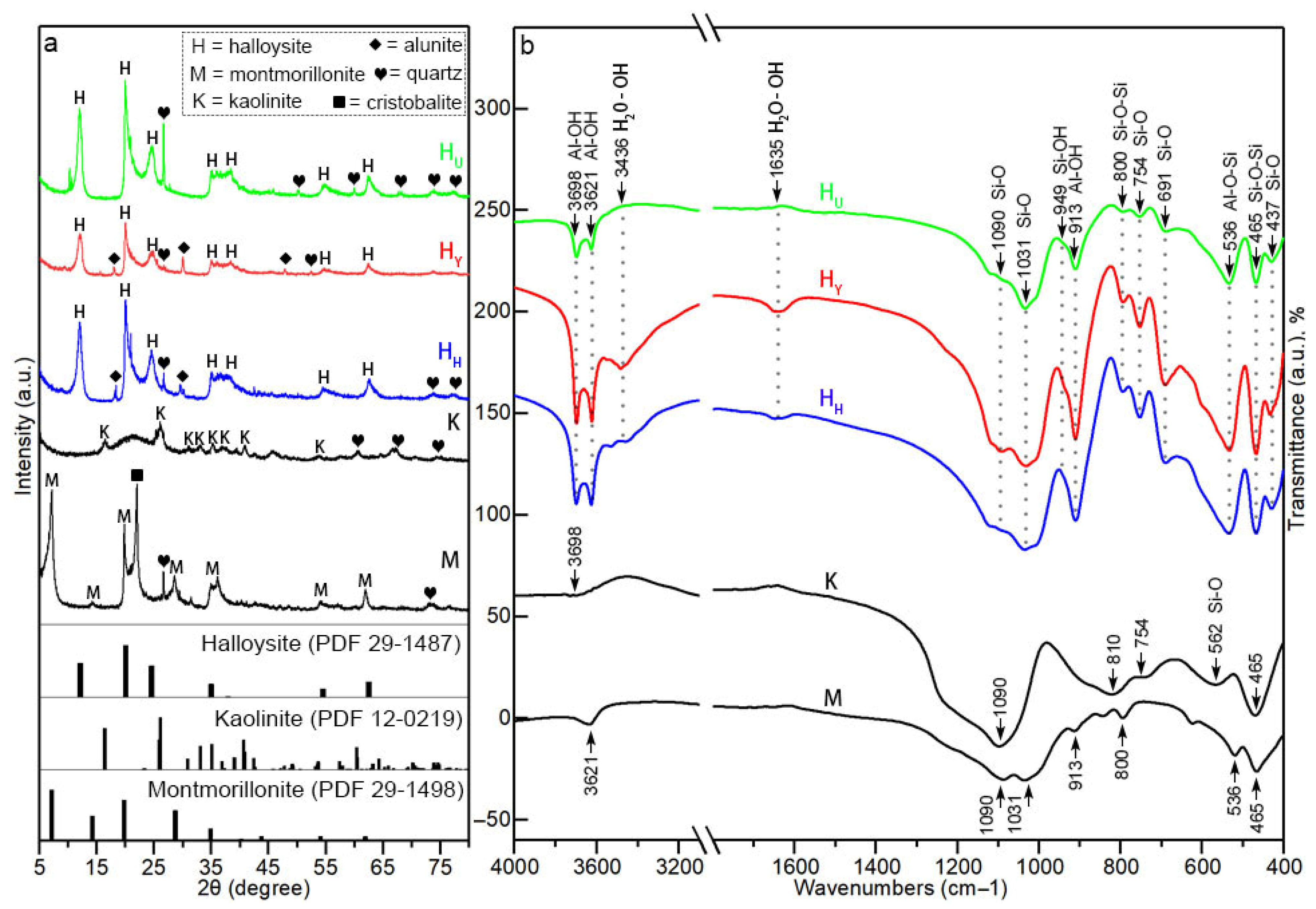
3.2.2. XRD
3.2.3. FTIR
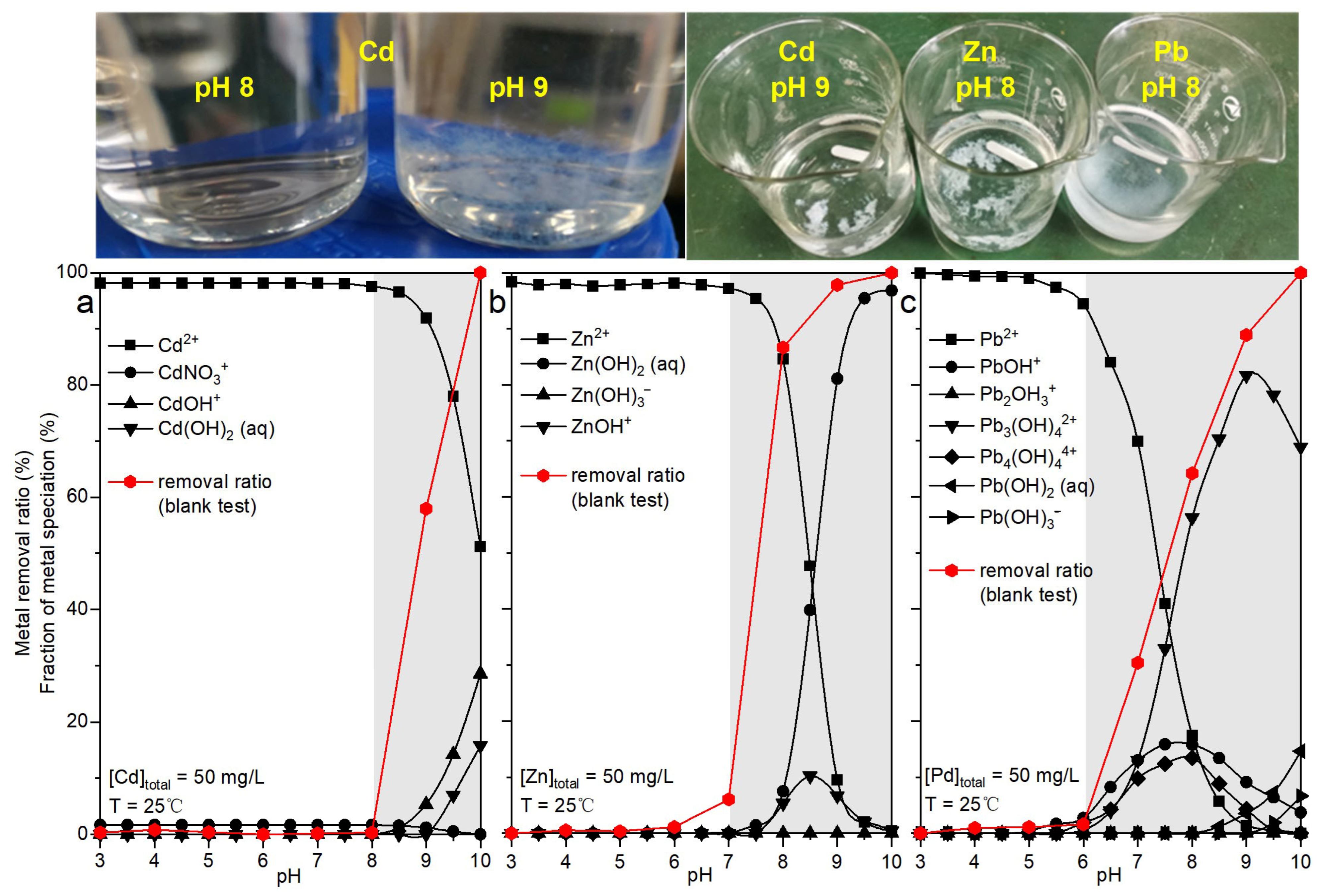
3.3. pH Boundary Determination
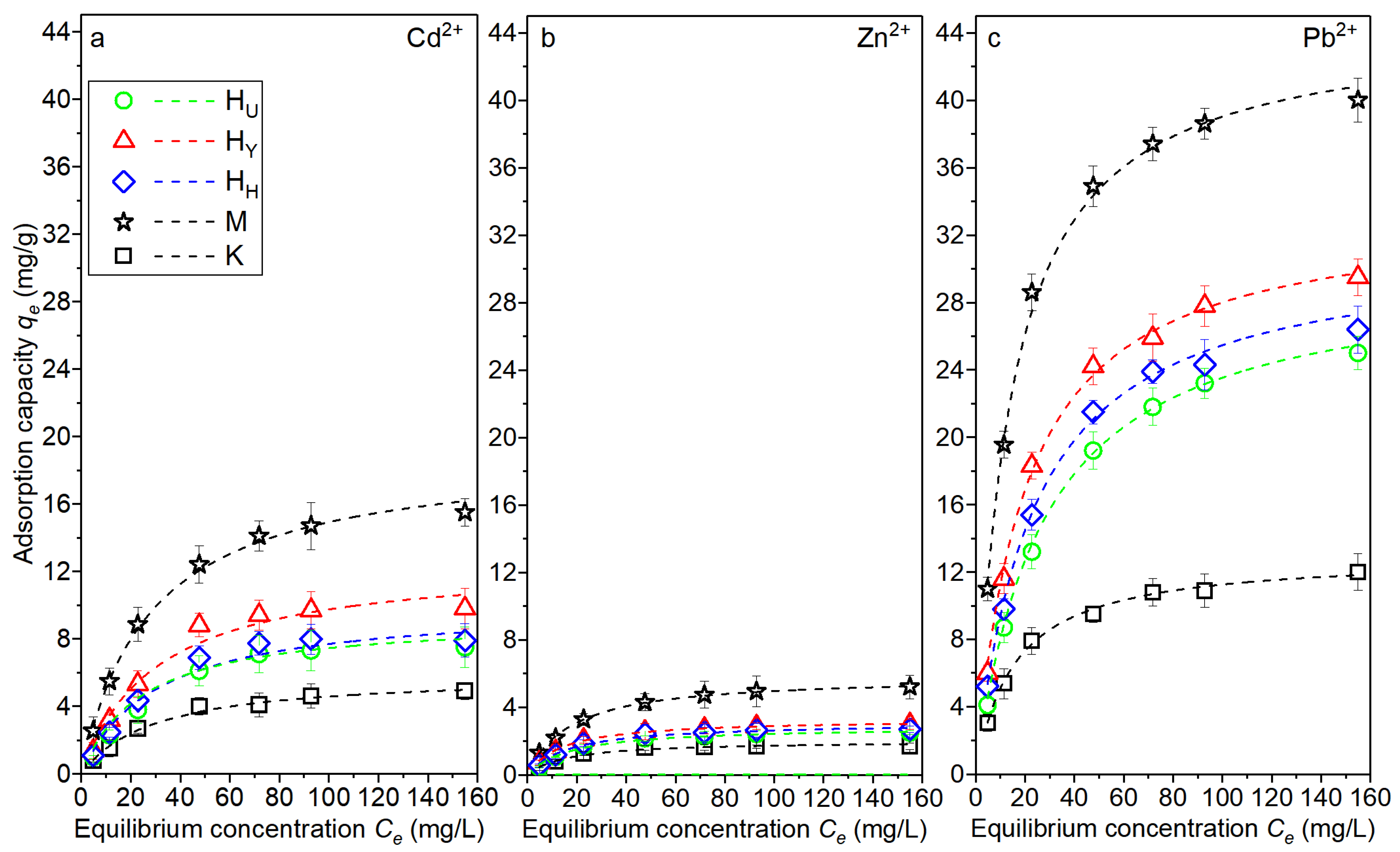
3.4. Adsorption Capacity Measurement
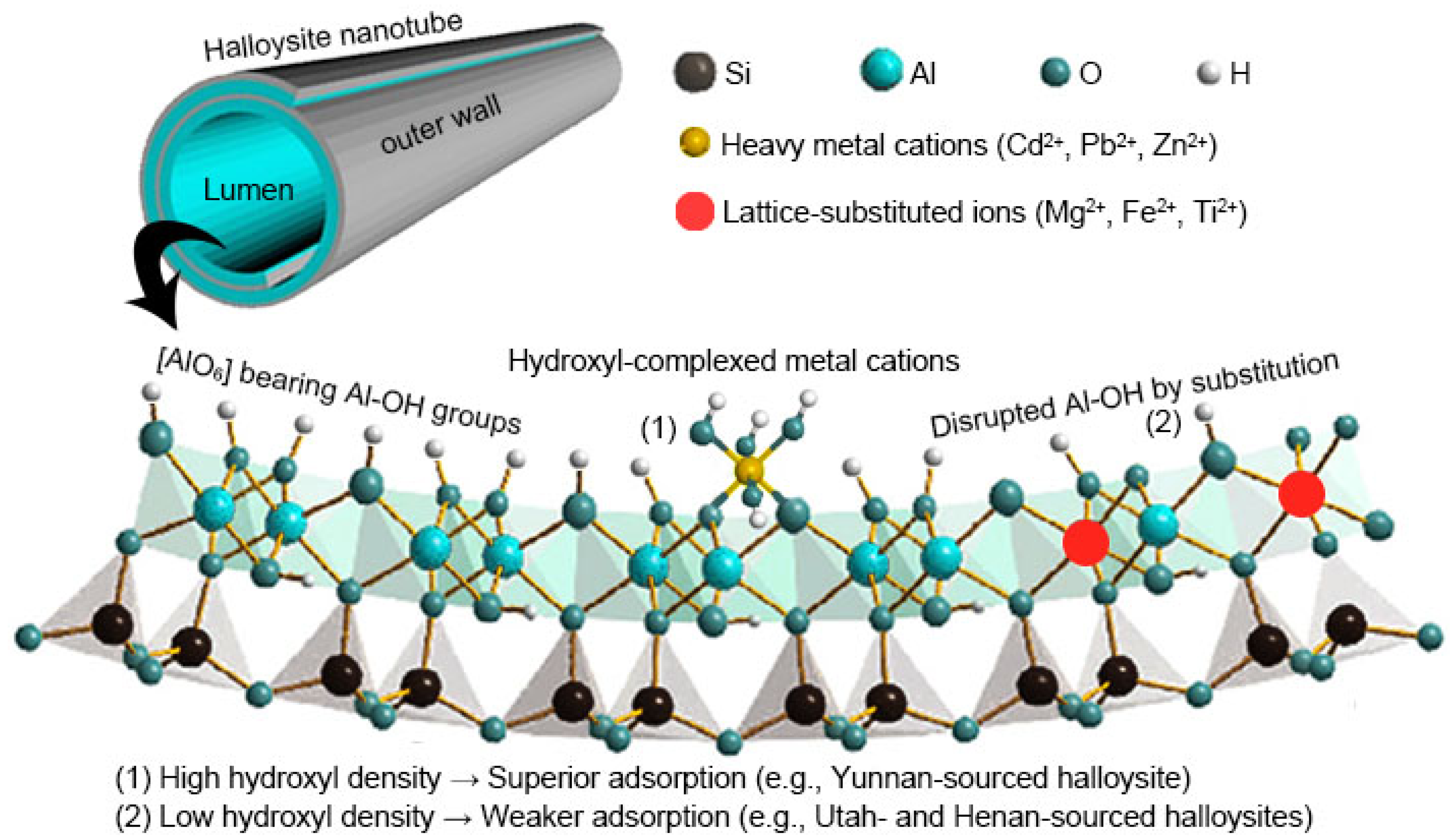
3.5. Structure–Activity Relationship Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Guan, X.; Yuan, X.; Zhao, Y.; Bai, J.; Li, Y.; Cao, Y.; Chen, Y.; Xiong, T. Adsorption behaviors and mechanisms of Fe/Mg layered double hydroxide loaded on bentonite on Cd(II) and Pb(II) removal. J. Colloid Interface Sci. 2022, 612, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.; Guan, X.; Bai, J.; Wang, H. One-pot synthesis of siliceous ferrihydrite−coated halloysite nanorods in alkaline medium: Structure, properties and cadmium adsorption performance. J. Colloid Interface Sci. 2023, 636, 435–449. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Yuan, X.Z.; Wu, Z.B.; Zeng, G.M.; Jiang, L.B.; Peng, X.; Li, H. Adsorption behavior and mechanism of Mg/Fe layered double hydroxide with Fe3O4-carbon spheres on the removal of Pb(II) and Cu(II). J. Colloid Interface Sci. 2019, 536, 440–455. [Google Scholar] [CrossRef]
- Borisover, M.; Davis, J.A. Adsorption of inorganic and organic solutes by clay minerals. In Developments in Clay Science; Tournassat, C., Steefel, C.I., Bourg, I.C., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 33–70. [Google Scholar]
- Yuan, G.D.; Theng, B.K.G.; Churchman, G.J.; Gates, W.P. Clays and clay minerals for pollution control. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 587–644. [Google Scholar]
- Bessaha, F.; Marouf-Khelifa, K.; Batonneau-Gener, I.; Khelifa, A. Characterization and application of heat-treated and acid-leached halloysites in the removal of malachite green: Adsorption, desorption, and regeneration studies. Desalination Water Treat. 2016, 57, 14609–14621. [Google Scholar] [CrossRef]
- Bessaha, G.; Bessaha, F.; Mahrez, N.; Boucifa, F.; Çoruhb, A.; Khelifa, A. Enhancement of the comprehensive performance of tetracycline adsorption by halloysite nanotubes: Kinetics, mechanism, and reusability study. Desalination Water Treat. 2024, 320, 100695. [Google Scholar] [CrossRef]
- Lvov, Y.; Wang, W.C.; Zhang, L.Q.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.; Jiang, L.; Dai, H.; Zhao, Y.; Guan, X.; Bai, J.; Wang, H. Manipulation of the halloysite clay nanotube lumen for environmental remediation: A review. Environ. Sci. Nano 2022, 9, 841–866. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Wilson, I.R. Kaolin and halloysite deposits of China. Clay Miner. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, M.; Liu, X.; Huang, C.; Gu, Z.; Cao, Y. Evaluation of toxicity of halloysite nanotubes and multi-walled carbon nanotubes to endothelial cells in vitro and blood vessels in vivo. Nanotoxicology 2020, 14, 1017–1038. [Google Scholar] [CrossRef]
- Hu, T.; Gui, Z.; Gong, J.; Rong, R.; Wang, X.; Tan, W.; Wang, Z.; Xu, X. INOS-mediated acute stomach injury and recovery in mice after oral exposure to halloysite nanotubes. Environ. Pollut. 2020, 258, 113758. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, P.; Matusik, J. Halloysite composites with Fe3O4 particles: The effect of impregnation on the removal of aqueous Cd(II) and Pb(II). Mineralogia 2017, 48, 107–125. [Google Scholar] [CrossRef][Green Version]
- Maziarz, P.; Matusik, J. The effect of acid activation and calcination of halloysite on the efficiency and selectivity of Pb(II), Cd(II), Zn(II) and As(V) uptake. Clay Miner. 2016, 51, 385–394. [Google Scholar] [CrossRef]
- Matusik, J.; Wscislo, A. Enhanced heavy metal adsorption on functionalized nanotubular halloysite interlayer grafted with aminoalcohols. Appl. Clay Sci. 2014, 100, 50–59. [Google Scholar] [CrossRef]
- Dong, Y.H.; Liu, Z.J.; Chen, L. Removal of Zn(II) from aqueous solution by natural halloysite nanotubes. J. Radioanal. Nucl. Chem. 2012, 292, 435–443. [Google Scholar] [CrossRef]
- Chiew, C.S.C.; Yeoh, H.K.; Pasbakhsh, P.; Krishnaiah, K.; Poh, P.E.; Tey, B.T.; Chan, E.S. Halloysite/alginate nanocomposite beads: Kinetics, equilibrium and mechanism for lead adsorption. Appl. Clay Sci. 2016, 119, 301–310. [Google Scholar] [CrossRef]
- Wang, P.L.; Tang, Y.Y.; Liu, Y.S.; Wang, T.; Wu, P.F.; Lu, X.Y. Halloysite nanotube@carbon with rich carboxyl groups as a multifunctional adsorbent for the efficient removal of cationic Pb(II), anionic Cr(VI) and methylene blue (MB). Environ. Sci. Nano 2018, 5, 2257–2268. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Cadmium sorption and desorption in soils: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 489–533. [Google Scholar] [CrossRef]
- Farinmade, A.; Ojo, O.F.; Trout, J.; He, J.; John, V.; Blake, D.A.; Lvov, Y.M.; Zhang, D.; Nguyen, D.; Bose, A. Targeted and stimulus-responsive delivery of surfactant to the oil-water interface for applications in oil spill remediation. ACS Appl. Mater. Interfaces 2020, 12, 1840–1849. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Joo, Y.; Sim, J.H.; Jeon, Y.; Lee, S.U.; Sohn, D. Opening and blocking the inner-pores of halloysite. Chem. Commun. 2013, 49, 4519–4521. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, D.; Zhou, Y.; Huang, Y.; Tie, B.; Lei, M. Mechanisms of arsenate and cadmium co-immobilized on ferrihydrite inferred from ternary surface configuration. Chem. Eng. J. 2021, 424, 130410. [Google Scholar] [CrossRef]
- Keeling, J.L.; Self, P.G.; Raven, M.D. Halloysite in Cenozoic sediments along the Eucla Basin margin. Math. Eng. Sci. Aerosp. 2010, 59, 25–28. [Google Scholar]
- Salata, R.; Melo, V.F.; Batista, L.F.A.; Abate, G.; Azevedo, A.C. Atrazine adsorption and desorption on functionalized montmorillonite: Aluminum-pillared and lithium saturated. J. Environ. Sci. Health 2022, 57, 980–988. [Google Scholar] [CrossRef]
- Olusegun, S.J.; Mohallem, N.D.S. Comparative adsorption mechanism of doxycycline and Congo red using synthesized kaolinite supported CoFe2O4 nanoparticles. Environ. Pollut. 2020, 260, 114019. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Biswas, B.; Warr, L.N.; Hilder, E.F.; Goswami, N.; Rahman, M.M.; Churchman, J.G.; Vasilev, K.; Pan, G.; Naidu, R. Biocompatible functionalisation of nanoclays for improved environmental remediation. Chem. Soc. Rev. 2019, 48, 3740–3770. [Google Scholar] [CrossRef]
- Wang, X.M.; Cheng, H.J.; Chai, P.C.; Bian, J.H.; Wang, X.M.; Liu, Y.; Yin, X.B.; Pan, S.D.; Pan, Z.J. Pore characterization of different clay minerals and its impact on methane adsorption capacity. Energy Fuels 2020, 34, 12204–12214. [Google Scholar] [CrossRef]
- Wang, W.B.; Wang, A.Q. Nanoscale clay minerals for functional ecomaterials: Fabrication, applications, and future trends. In Handbook of Ecomaterials; Martínez, L.M.T., Ed.; Springer: Cham, Switzerland, 2019; pp. 2409–2490. [Google Scholar]
- Liu, X.; Lu, X.; Sprik, M.; Cheng, J.; Meijer, E.J.; Wang, R. Acidity of edge surface sites of montmorillonite and kaolinite. Geochim. Cosmochim. Acta 2013, 117, 180–190. [Google Scholar] [CrossRef]
- Deng, L.L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.M.; Chen, F.R.; Liu, Z.W. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Won, J.; Wirth, X.; Burns, S.E. An experimental study of cotransport of heavy metals with kaolinite colloids. J. Hazard. Mater. 2019, 373, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Struijk, M.; Rocha, F.; Detellier, C. Novel thio-kaolinite nanohybrid materials and their application as heavy metal adsorbents in wastewater. Appl. Clay Sci. 2017, 150, 192–201. [Google Scholar] [CrossRef]
- Ruyter-Hooley, M.; Johnson, B.B.; Morton, D.W.; Angove, M.J. The adsorption of myo-inositol hexaphosphate onto kaolinite and its effect on cadmium retention. Appl. Clay Sci. 2017, 135, 405–413. [Google Scholar] [CrossRef]
- Tunega, D.; Zaoui, A. Mechanical and bonding behaviors behind the bending mechanism of Kaolinite clay layers. J. Phys. Chem. C 2020, 124, 7432–7440. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Ouyang, J. Physicochemical properties of halloysite. In Developments in Clay Science; Yuan, P., Thill, A., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 67–91. [Google Scholar]
- Ramanayaka, S.; Sarkar, B.; Cooray, A.T.; Ok, Y.S.; Vithanage, M. Halloysite nanoclay supported adsorptive removal of oxytetracycline antibiotic from aqueous media. J. Hazard. Mater. 2020, 384, 121301. [Google Scholar] [CrossRef]
- Deng, L.L.; Yuan, P.; Liu, D.; Du, P.X.; Zhou, J.M.; Wei, Y.F.; Song, Y.R.; Liu, Y.Q. Effects of calcination and acid treatment on improving benzene adsorption performance of halloysite. Appl. Clay Sci. 2019, 181, 105240. [Google Scholar] [CrossRef]
- Chaudhari, S.; Baek, M.H.; Kwon, Y.; Shon, M.; Nam, S.; Park, Y. Surface-modified halloysite nanotube-embedded polyvinyl alcohol/polyvinyl amine blended membranes for pervaporation dehydration of water/isopropanol mixtures. Appl. Surf. Sci. 2019, 493, 193–201. [Google Scholar] [CrossRef]
- Yu, J.Y.; Niedenthal, W.; Smarsly, B.M.; Natile, M.M.; Huang, Y.X.; Carraro, M. Au nanoparticles supported on piranha etched halloysite nanotubes for highly efficient heterogeneous catalysis. Appl. Surf. Sci. 2021, 546, 149100. [Google Scholar] [CrossRef]
- Yilmaz, R.B.; Bayram, G.; Yilmazer, U. Effect of halloysite nanotubes on multifunctional properties of coaxially electrospun poly(ethylene glycol)/polyamide-6 nanofibrous thermal energy storage materials. Thermochim. Acta 2020, 690, 178673. [Google Scholar] [CrossRef]
- Qin, C.; Yuan, X.; Xiong, T.; Tan, Y.Z.; Wang, H. Physicochemical properties, metal availability and bacterial community structure in heavy metal-polluted soil remediated by montmorillonite-based amendments. Chemosphere 2020, 261, 128010. [Google Scholar] [CrossRef]
- Shaban, M.; Sayed, M.I.; Shahien, M.G.; Abukhadra, M.R.; Ahmed, Z.M. Adsorption behavior of inorganic- and organic-modified kaolinite for Congo red dye from water, kinetic modeling, and equilibrium studies. J. Sol-Gel Sci. Technol. 2018, 87, 427–441. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Paikaray, S.; Essilfie-Dughan, J.; Hendry, M.J. Ionic substitution of Mg2+ for Al3+ and Fe3+ with octahedral coordination in hydroxides facilitate precipitation of layered double hydroxides. Geochim. Cosmochim. Acta 2018, 220, 217–234. [Google Scholar] [CrossRef]
- Pimentel, C.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Effect of iron isomorphic substitution in Mg:Al and Zn:Al-layered double-hydroxide structures by means of first principle calculations. ACS Earth Space Chem. 2022, 6, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Ucarli, O.; Yayintas, O.T.; Engin, M.S.; Cay, S.; Saglikoglu, G.; Yilmaz, S. Investigation of competitive and noncompetitive adsorption of some heavy metals ions on Leucodon sciuroides (Hedw.) Schwagr. Langmuir 2020, 36, 8265–8271. [Google Scholar] [CrossRef] [PubMed]
- Rout, K.; Mohapatra, M.; Anand, S. Line ferrihydrite: Synthesis, characterization and its adsorption behaviour for removal of Pb(II), Cd(II), Cu(II) and Zn(II) from aqueous solutions. Dalton Trans 2012, 41, 3302–3312. [Google Scholar] [CrossRef]
- Hong, C.; Dong, Z.; Zhang, J.; Zhu, L.; Che, L.; Mao, F.; Qiu, Y. Effectiveness and mechanism for the simultaneous adsorption of Pb(II), Cd(II) and As(III) by animal-derived biochar/ferrihydrite composite. Chemosphere 2022, 293, 133583. [Google Scholar] [CrossRef]
- Naidu, R.; Kookana, R.S.; Sumner, M.E.; Harter, R.D.; Tiller, K. Cadmium sorption and transport in variable charge soils: A review. J. Environ. Qual. 1997, 26, 602–617. [Google Scholar] [CrossRef]
- Baeyens, B.; Fernandes, M.M. Adsorption of heavy metals including radionuclides. In Developments in Clay Science; Schoonheydt, R., Johnston, C.T., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 125–172. [Google Scholar]

| Sample Origin | Tag | Primary Morphology | Geometric Dimension 1 | Aspect Ratio | |||
|---|---|---|---|---|---|---|---|
| ID (nm) | OD (nm) | WT (nm) | LR (nm) | ||||
| Utah, USA | HU | Slender, short tube | 10–28 | 13–70 | 10–30 | 100–1000 | 4.3 |
| Yunnan, China | HY | Robust, long tube | 10–35 | 30–91 | 20–50 | 100–3000 | 11.6 |
| Henan, China | HH | Thicker-walled, short tube | 15–32 | 15–80 | 5–30 | 50–2500 | 5.1 |
| Clay Mineral | Tag | Average Pore Size (nm) | Pore Volume (cm3/g) | SSA (m2/g) |
|---|---|---|---|---|
| Halloysite | HU | 10.9 | 0.133 | 48.2 |
| HY | 12.1 | 0.129 | 42.9 | |
| HH | 11.6 | 0.131 | 44.6 | |
| Montmorillonite | K | 28.1 | 0.051 | 17.6 |
| Kaolinite | M | 6.8 | 0.118 | 70.2 |
| Clay Mineral | Main Chemical Composition (%) | Si:Al | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | IL 1 | ||
| HU | 45.19 | 35.43 | 0.33 | 0.26 | 0.12 | 0.07 | 0.07 | 0.02 | 15.70 | 1.08 |
| HY | 43.99 | 38.57 | 0.62 | 0.28 | 0.19 | 0.18 | 0.09 | 0.15 | 17.53 | 0.97 |
| HH | 45.35 | 35.07 | 0.90 | 0.51 | 0.39 | 0.16 | 0.28 | 0.03 | 16.02 | 1.10 |
| K | 45.66 | 38.90 | 0.29 | 0.01 | 0.09 | 0.17 | 0.03 | 0.37 | 13.60 | 1.01 |
| M | 56.21 | 21.76 | 0.75 | 2.99 | 4.99 | 0.26 | 0.22 | 0.32 | 13.36 | 2.20 |
| Wavenumber (cm−1) | Assignment | Wavenumber (cm−1) | Assignment |
|---|---|---|---|
| 3698 | Inner-surface Al–OH stretch | 800 | Si–O–Si bend |
| 3621 | Inner Al–OH stretch | 754 | Transverse Si–O stretch |
| 3436 | Adsorbed H2O–OH stretch | 691 | Transverse Si–O stretch |
| 1635 | Adsorbed H2O–OH bend | 562 | Si–O stretch |
| 1090/1031 | In-plane Si–O stretch | 536 | Al–O–Si bend |
| 949 | Si–OH bend | 465 | Si–O–Si bend |
| 913 | Inner Al–OH bend | 437 | Si–O bend |
| Metal Ions | Clay | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| KL (L/mg) | qmax (mg/g) | R2 | n | Kf (mg/g)(L/mg)1/n | R2 | ||
| Cd2+ | HU | 0.04 | 7.8 | 0.933 | 3.7 | 12.7 | 0.954 |
| HY | 0.03 | 10.1 | 0.891 | 3.3 | 13.8 | 0.925 | |
| HH | 0.03 | 8.2 | 0.884 | 3.5 | 12.4 | 0.917 | |
| K | 0.01 | 5.2 | 0.912 | 7.3 | 10.2 | 0.935 | |
| M | 0.06 | 15.8 | 0.931 | 2.9 | 14.8 | 0.957 | |
| Zn2+ | HU | 0.04 | 2.7 | 0.938 | 4.1 | 12.0 | 0.945 |
| HY | 0.04 | 3.2 | 0.951 | 3.7 | 12.2 | 0.935 | |
| HH | 0.03 | 2.9 | 0.924 | 3.9 | 11.9 | 0.958 | |
| K | 0.02 | 1.8 | 0.903 | 8.2 | 9.8 | 0.864 | |
| M | 0.07 | 5.4 | 0.959 | 3.1 | 13.9 | 0.991 | |
| Pb2+ | HU | 0.07 | 25.5 | 0.942 | 4.2 | 13.5 | 0.966 |
| HY | 0.06 | 30.6 | 0.947 | 3.6 | 13.7 | 0.974 | |
| HH | 0.06 | 27.4 | 0.952 | 4.0 | 13.1 | 0.961 | |
| K | 0.04 | 12.3 | 0.936 | 6.8 | 10.8 | 0.944 | |
| M | 0.09 | 42.7 | 0.915 | 2.7 | 17.7 | 0.926 | |
| Metal Ions | Halloysite Mining Location | Adsorption Experimental Conditions | qmax (mg/g) | Ref. |
|---|---|---|---|---|
| Cd2+ | Lower Silesia, Poland | pH = 5.0, 25 °C | 1.2 | [15] |
| Lower Silesia, Poland | pH = 5.0, 25 °C | 2.1 | [16] | |
| Lower Silesia, Poland | pH = 5.0 | 0.5 | [17] | |
| Utah, USA (HU) | pH = 5.5, 25 °C, ionic strength of 0.001 M | 7.8 | This study | |
| Yunnan, China (HY) | pH = 5.5, 25 °C, ionic strength of 0.001 M | 10.1 | This study | |
| Hennan, China (HH) | pH = 5.5, 25 °C, ionic strength of 0.001 M | 8.2 | This study | |
| Zn2+ | Lower Silesia, Poland | pH = 5.0, 25 °C | 1.8 | [16] |
| Lower Silesia, Poland | pH = 5.0 | 0.1 | [17] | |
| Guangzhou, China | pH = 6.0, 20 °C, ionic strength of 0.01 M | 9.8 | [18] | |
| Utah, USA (HU) | pH = 5.5, 25 °C, ionic strength of 0.001 M | 2.7 | This study | |
| Yunnan, China (HY) | pH = 5.5, 25 °C, ionic strength of 0.001 M | 3.2 | This study | |
| Hennan, China (HH) | pH = 5.5, 25 °C, ionic strength of 0.001 M | 2.9 | This study | |
| Pb2+ | Lower Silesia, Poland | pH = 5.0, 25 °C | 7.5 | [15] |
| Lower Silesia, Poland | pH = 5.0, 25 °C | 8.1 | [16] | |
| Lower Silesia, Poland | pH = 5.0 | 8.1 | [17] | |
| New Zealand | pH = 5.0, 25 °C | 84.0 | [19] | |
| Hebei, China | pH = 6.0, 25 °C | 11.2 | [20] | |
| Utah, USA (HU) | pH = 5.5, 25 °C, ionic strength of 0.001 M | 25.5 | This study | |
| Yunnan, China (HY) | pH = 5.5, 25 °C, ionic strength of 0.001 M | 30.6 | This study | |
| Hennan, China (HH) | pH = 5.5, 25 °C, ionic strength of 0.001 M | 27.4 | This study |
| Halloysite | Morphology | SSA (m2/g) | Si:Al | FTIR Transmittance Reduction at Al–OH (%) | qmax (mg/g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 3698 cm−1 | 3621 cm−1 | 913 cm−1 | Cd2+ | Zn2+ | Pb2+ | ||||
| HU | Short tube | 48.2 | 1.08 | 20 | 19 | 29 | 7.8 | 2.7 | 25.5 |
| HY | Long tube | 42.9 | 0.97 | 67 | 68 | 76 | 10.1 | 3.2 | 30.6 |
| HH | Short tube | 44.6 | 1.10 | 50 | 52 | 62 | 8.2 | 2.9 | 27.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yuan, X.; Wei, X.; Long, Y. Reassessment of Heavy Metal Adsorption Performance in Halloysite Clay Nanotubes: Geographical Variation and Structure–Activity Relationship. Minerals 2025, 15, 739. https://doi.org/10.3390/min15070739
Li Y, Yuan X, Wei X, Long Y. Reassessment of Heavy Metal Adsorption Performance in Halloysite Clay Nanotubes: Geographical Variation and Structure–Activity Relationship. Minerals. 2025; 15(7):739. https://doi.org/10.3390/min15070739
Chicago/Turabian StyleLi, Ying, Xingzhong Yuan, Xiuying Wei, and Yao Long. 2025. "Reassessment of Heavy Metal Adsorption Performance in Halloysite Clay Nanotubes: Geographical Variation and Structure–Activity Relationship" Minerals 15, no. 7: 739. https://doi.org/10.3390/min15070739
APA StyleLi, Y., Yuan, X., Wei, X., & Long, Y. (2025). Reassessment of Heavy Metal Adsorption Performance in Halloysite Clay Nanotubes: Geographical Variation and Structure–Activity Relationship. Minerals, 15(7), 739. https://doi.org/10.3390/min15070739





