Geogenic and Anthropogenic Origins of Mercury and Other Potentially Toxic Elements in the Ponce Enriquez Artisanal and Small-Scale Gold Mining District, Southern Ecuador
Abstract
1. Introduction
2. Study Area
3. Material and Methods
3.1. Bulk Geochemical Analyses
3.2. Petrography and Mineral Chemistry
3.3. In Situ Trace Element Analysis (LA-ICP-MS)
4. Results
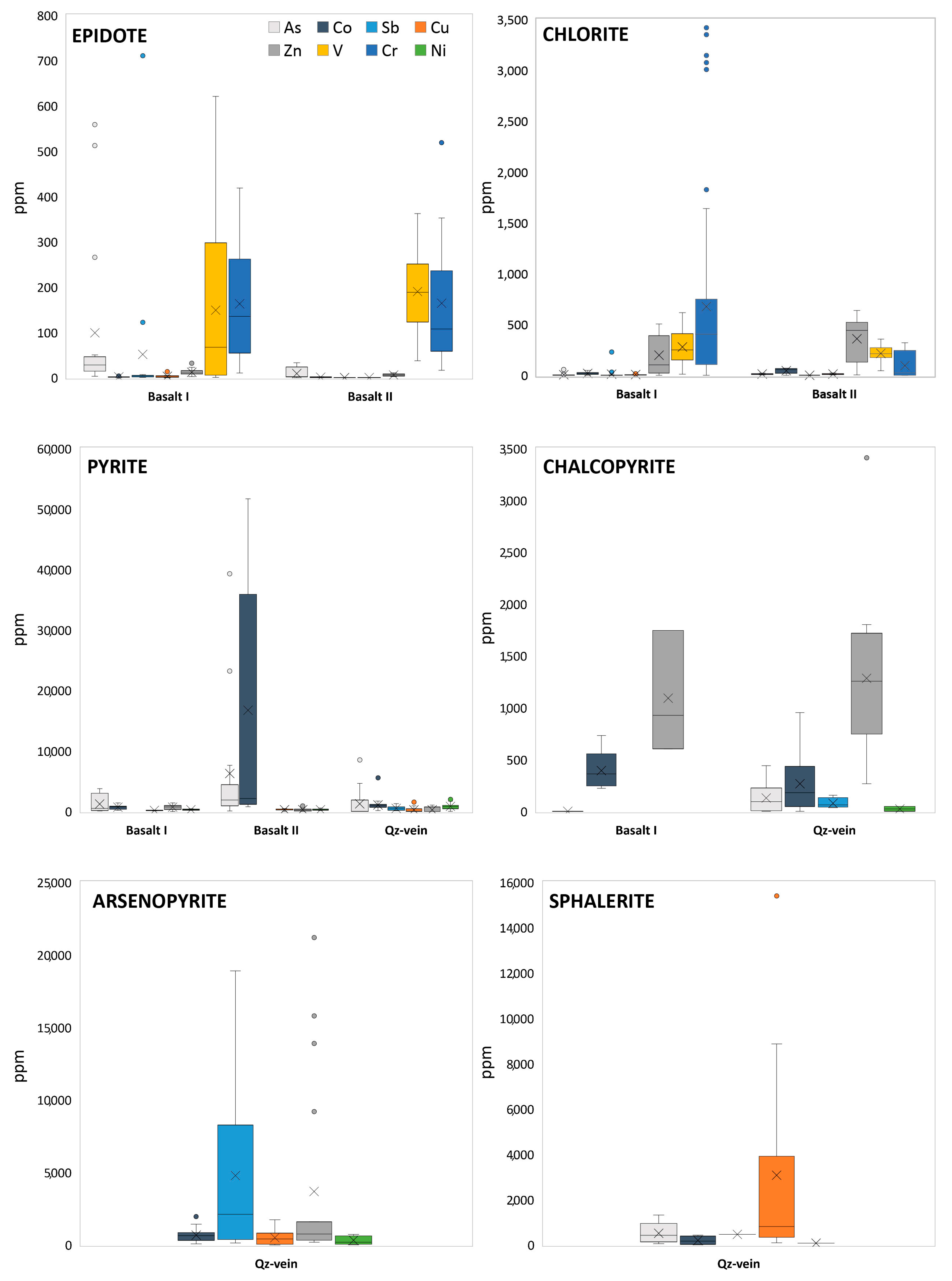
4.1. Petrography and Mineralogy
4.2. Mineral Chemistry of the PTE-Bearing Minerals
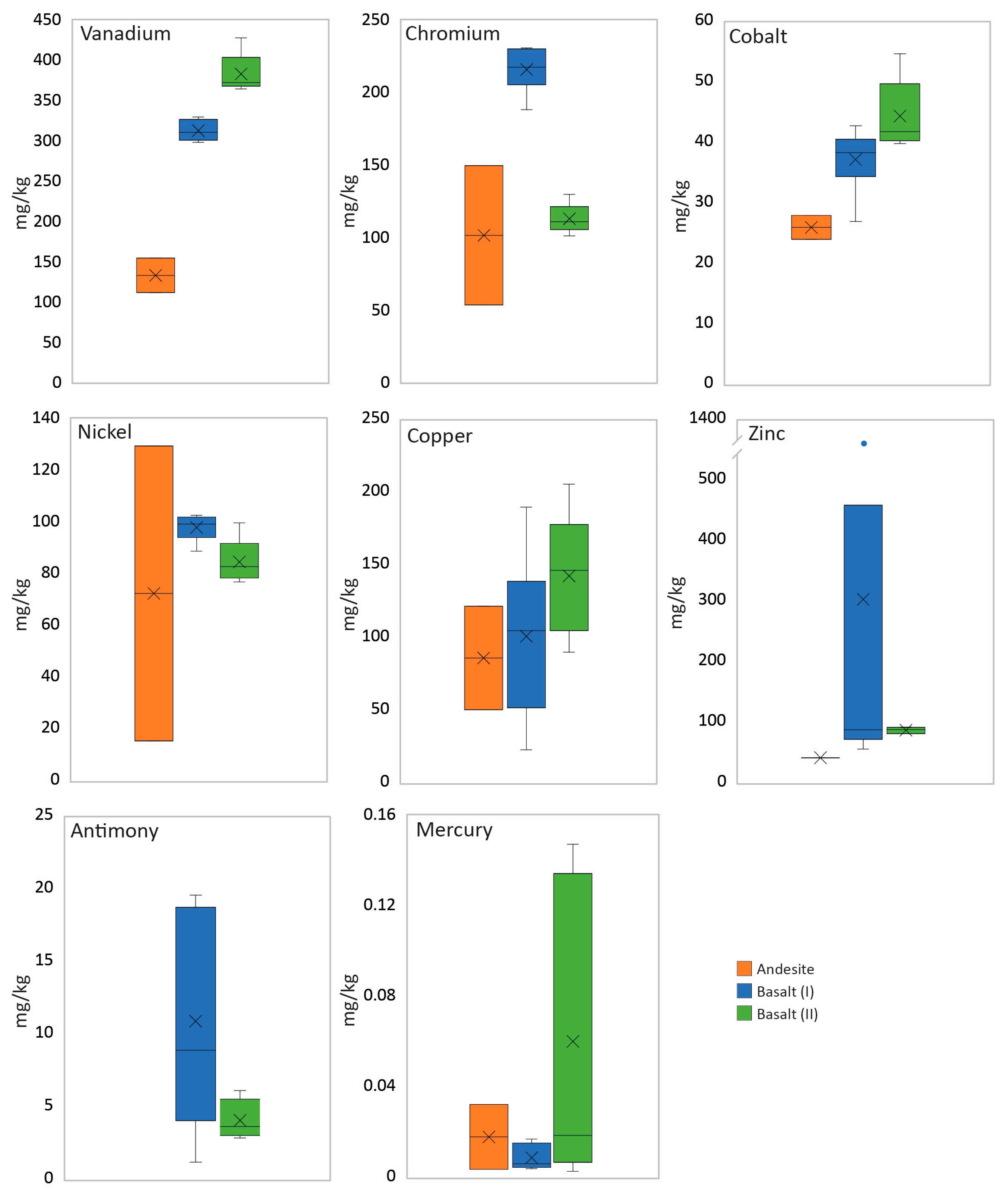
4.3. Bulk Chemistry
5. Discussion
5.1. Chromium, Nickel, Vanadium, and Cobalt
5.2. Mercury
5.3. Arsenic
5.4. Copper, Zinc, and Antimony
5.5. Environmental Implications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donkor, A.K.; Ghoveisi, H.; Bonzongo, J.C.J. Use of metallic mercury in artisanal gold mining by amalgamation: A review of temporal and spatial trends and environmental pollution. Minerals 2024, 14, 555. [Google Scholar] [CrossRef]
- Aldous, A.R.; Tear, T.; Fernandez, L.E. The global challenge of reducing mercury contamination from artisanal and small-scale gold mining (ASGM): Evaluating solutions using generic theories of change. Ecotoxicology 2024, 33, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Garley, M.; Nikliński, J. Mercury exposure and health effects: What do we really know? Int. J. Mol. Sci. 2025, 26, 2326. [Google Scholar] [CrossRef] [PubMed]
- Brocza, F.M.; Rafaj, P.; Sander, R.; Wagner, F.; Jones, J.M. Global scenarios of anthropogenic mercury emissions. Atmosph. Chem. Phys. 2024, 24, 7385–7404. [Google Scholar] [CrossRef]
- Estupiñan, R.; Romero-Crespo, P.; Garcia, M.; Garces, D.; Valverde, P. La minería en Ecuador. Pasado, presente y futuro. Boletín Geol. Y Min. 2021, 132, 533–549. [Google Scholar] [CrossRef]
- Funoh, K.N. The Impacts of Artisanal Gold Mining on Local Livelihoods and the Environment in the Forested Areas of Cameroon; CIFOR: Bogor, Indonesia, 2014; Volume 150, p. 150. [Google Scholar]
- Betancourt, Ó.; Tapia, M.; Méndez, I. Decline of general intelligence in children exposed to manganese from mining contamination in Puyango River Basin, Southern Ecuador. Ecohealth 2015, 12, 453–460. [Google Scholar] [CrossRef]
- Garcés, D.; Sanchez-Palencia, Y.; Jimenez, S.; Llamas, J. Environmental statistical analysis of the basins of the mining district of Ponce Enríquez (Ecuador). In Proceedings of the 24th International Multidisciplinary Scientific GeoConference SGEM 2024, Albena, Bulgaria, 24–30 June 2024; Trofymchuk, O., Rivza, B., Eds.; STEF92 Technology: Sofia, Bulgaria, 2024. [Google Scholar]
- Mestanza-Ramón, C.; Jiménez-Oyola, S.; Montoya, A.V.G.; Vizuete, D.D.C.; D’Orio, G.; Cedeño-Laje, J.; Straface, S. Assessment of Hg Pollution in Stream Waters and Human Health Risk in Areas Impacted by Mining Activities in the Ecuadorian Amazon. Environ. Geochem. Health 2023, 10, 7183–7197. [Google Scholar] [CrossRef]
- Vangsnes, G.F. The meanings of mining: A perspective on the regulation of artisanal and small-scale gold mining in southern Ecuador. Extr. Ind. Soc. 2018, 5, 317–326. [Google Scholar]
- Williams, T.M.; Dunkley, P.N.; Cruz, E.; Acitimbay, V.; Gaibor, A.; Lopez, E.; Aspden, J.A. Regional geochemical reconnaissance of the Cordillera Occidental of Ecuador: Economic and environmental applications. Appl. Geochem. 2000, 15, 531–550. [Google Scholar] [CrossRef]
- Appleton, J.D.; Williams, T.M.; Orbea, H.; Carrasco, M. Fluvial Contamination Associated with Artisanal Gold Mining in the Ponce Enríquez, Portovelo-Zaruma and Nambija Areas, Ecuador. Water Air Soil Poll. 2001, 131, 19–39. [Google Scholar] [CrossRef]
- Tarras-Wahlberg, N.H. Environmental management of small-scale and artisanal mining: The Portovelo-Zaruma goldmining area, southern Ecuador. J. Environ. Manag. 2002, 65, 165–179. [Google Scholar] [CrossRef]
- Jiménez-Oyola, S.; García-Martínez, M.J.; Ortega, M.F.; Chavez, E.; Romero, P.; García-Garizabal, I.; Bolonio, D. Ecological and probabilistic human health risk assessment of heavy metal (loid) s in river sediments affected by mining activities in Ecuador. Environ. Geochem. Health 2021, 43, 4459–4474. [Google Scholar] [CrossRef]
- Pekey, H. The distribution and sources of heavy metals in Izmit Bay surface sediments affected by a polluted stream. Mar. Poll. Bull. 2006, 52, 1197–1208. [Google Scholar] [CrossRef]
- Wu, J.; Huang, C. Machine learning-supported determination for site-specific natural background values of soil heavy metals. J. Hazard Mat. 2025, 487, 137276. [Google Scholar] [CrossRef] [PubMed]
- Skála, J.; Grygar, T.M.; Achasova, A. Novel definition of local baseline values for potentially toxic elements in Czech farmland using adaptive spatial weighting. Appl. Geochem. 2024, 170, 106082. [Google Scholar] [CrossRef]
- Armiento, G.; Barsanti, M.; Caprioli, R.; Chiavarini, S.; Conte, F.; Crovato, C.; Spaziani, F. Heavy metal background levels and pollution temporal trend assessment within the marine sediments facing a brownfield area (Gulf of Pozzuoli, Southern Italy). Environ. Monit. Assess. 2022, 194, 814. [Google Scholar] [CrossRef] [PubMed]
- Lo Medico, F.; Varrica, D.; Zuccolini, M.V.; Miola, M.; Scopelliti, G.; Alaimo, M.G. Geochemical baseline values and spatial distribution of major, trace, and rare earth elements in unpolluted soils of the Sicily region (Italy). Environ. Geochem. Health 2025, 47, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kierczak, J.; Pietranik, A.; Pędziwiatr, A. Ultramafic geoecosystems as a natural source of Ni, Cr, and Co to the environment: A review. Sci. Total Environ. 2021, 755, 142620. [Google Scholar] [CrossRef]
- Marescotti, P.; Comodi, P.; Crispini, L.; Gigli, L.; Zucchini, A.; Fornasaro, S. Potentially toxic elements in ultramafic soils: A study from metamorphic ophiolites of the Voltri Massif (Western Alps, Italy). Minerals 2019, 9, 502. [Google Scholar] [CrossRef]
- Koner, S.; Chen, J.S.; Rathod, J.; Hussain, B.; Hsu, B.M. Unravelling the ultramafic rock-driven serpentine soil formation leading to the geo-accumulation of heavy metals: An impact on the resident microbiome, biogeochemical cycling and acclimatized eco-physiological profiles. Environ. Res. 2023, 216, 114664. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, T.; Perkins, R.B.; Zhu, J.; Zhu, Z.; Xiong, Y.; Ning, Z. Geogenic cadmium pollution and potential health risks, with emphasis on black shale. J. Geochem. Explor. 2017, 176, 42–49. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Yang, Z.; Zhang, Q.; Ji, J. Enrichment and source identification of Cd and other heavy metals in soils with high geochemical background in the karst region, Southwestern China. Chemosphere 2020, 245, 125620. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, X.; Liu, Z.; Li, G. Pollution and potential ecological risk evaluation of heavy metals in the sediments around Dongjiang Harbor, Tianjin. Procedia Environ. Sci 2010, 2, 729–736. [Google Scholar] [CrossRef]
- Manga, V.E.; Agyingi, C.M.; Suh, C.E. Trace element soil quality status of Mt. Cameroon soils. Adv. Geol. 2014, 2014, 894103. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Rama Gayatri, S.; Venkateswarlu, B.; Jakkula, V.S.; Wani, S.P.; Kundu, S.; Gopala Krishna, G. Heavy metals concentration in soils under rainfed agro-ecosystems and their relationship with soil properties and management practices. Int. J. Environ. Sci. Tech. 2014, 11, 1959–1972. [Google Scholar] [CrossRef]
- Hošek, M.; Pavlíková, P.; Šoltýs, M.; Tůmová, Š.; Matys Grygar, T. Distinguishing Geogenic Load and Anthropogenic Contribution to Soil Contamination in Mineralised Mountain Landscape of Ore Mountains (Czech Republic) Using Cumulative Distribution Functions. Land 2024, 13, 218. [Google Scholar] [CrossRef]
- Sun, S.S.; Ao, M.; Geng, K.R.; Chen, J.Q.; Deng, T.H.B.; Li, J.J.; Qiu, R.L. Enrichment and speciation of chromium during basalt weathering: Insights from variably weathered profiles in the Leizhou Peninsula, South China. Sci. Total Environ. 2022, 822, 153304. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Yamamoto, T.; Arima, T.; Mufalo, W.; Igarashi, T. Frequency distribution of naturally occurring arsenic leached from excavated rocks of Hokkaido Shinkansen tunnels between Shin-Hakodate-Hokuto and Oshamambe. Soils Found. 2024, 64, 101445. [Google Scholar] [CrossRef]
- Fulignati, P.; Mulas, M.; Villalta Echeverria, M.D.P.; Fornasaro, S.; Larreta, E.; Mendoza Arteaga, P.L.; Gioncada, A. The propylitic alteration in the Ponce Enriquez Gold Mining district, Azuay province, Ecuador: Genetic constraints from a mineral chemistry and fluid inclusions study. Front. Earth Sci. 2023, 11, 1255712. [Google Scholar] [CrossRef]
- Cooperative Minera Bella Rica. Available online: https://bellarica.org/index.php/desarollo-tecnico/96-la-cooperativa-bella-rica-y-su-ambito-tecnico-minero (accessed on 1 February 2025).
- Velásquez-López, P.C.; Veiga, M.M.; Klein, B.; Shandro, J.A.; Hall, K. Cyanidation of mercury-rich tailings in artisanal and small-scale gold mining: Identifying strategies to manage environmental risks in Southern Ecuador. J. Clean. Prod. 2011, 19, 1125–1133. [Google Scholar] [CrossRef]
- Gonçalves, A.O.; Marshall, B.G.; Kaplan, R.J.; Moreno-Chavez, J.; Veiga, M.M. Evidence of reduced mercury loss and increased use of cyanidation at gold processing centers in southern Ecuador. J. Clean. Prod. 2017, 165, 836–845. [Google Scholar] [CrossRef]
- Vallejo, C.; Spikings, R.A.; Horton, B.K.; Luzieux, L.; Romero, C.; Winkler, W.; Thomsen, T.B. Late Cretaceous to Miocene stratigraphy and provenance of the coastal forearc and Western Cordillera of Ecuador: Evidence for accretion of a single oceanic plateau fragment. In Andean Tectonics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 209–236. [Google Scholar]
- Prodeminca, B. Prospecto beroen. Campo Mineral. Molleturo 2000, 2, 114. [Google Scholar]
- Jaillard, E.; Ordoñez, M.; Suárez, J.; Toro, J.; Iza, D.; Lugo, W. Stratigraphy of the late Cretaceous–Paleogene deposits of the Cordillera Occidental of central Ecuador: Geodynamic implications. J. S. Am. Earth Sci. 2004, 17, 49–58. [Google Scholar] [CrossRef]
- Pesantes, A.A.; Carpio, E.P.; Vitvar, T.; López, M.M.M.; Menéndez-Aguado, J.M. A multi-index analysis approach to heavy metal pollution assessment in river sediments in the Ponce Enríquez Area, Ecuador. Water 2019, 11, 590. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal At. Spectrom 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Hastie, A.R.; Kerr, A.C.; Pearce, J.A.; Mitchell, S.F. Classification of altered volcanic island arc rocks using immobile trace elements: Development of the Th–Co discrimination diagram. J. Pet. 2007, 48, 2341–2357. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Hu, Z.; Gao, S. Upper crustal abundances of trace elements: A revision and update. Chem. Geol. 2008, 253, 205–221. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Declercq, J.; Saldi, G.D.; Gislason, S.R.; Schott, J. Olivine dissolution rates: A critical review. Chem. Geol. 2018, 500, 1–19. [Google Scholar] [CrossRef]
- Wen, D.; Liu, H.; Guo, Y.; Zeng, Q.; Wu, M.; Liu, R.S. Disorder–order conversion-induced enhancement of thermal stability of pyroxene near-infrared phosphors for light-emitting diodes. Angew. Chem. Int. Ed. 2023, 61, e202204411. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K. Potentially toxic elements in the environment–a review of sources, sinks, pathways and mitigation measures. Rev. Environ. Health 2024, 39, 561–575. [Google Scholar] [CrossRef]
- Dupla, X.; Möller, B.; Baveye, P.C.; Grand, S. Potential accumulation of toxic trace elements in soils during enhanced rock weathering. Eur. J. Soil Sci. 2023, 74, e13343. [Google Scholar] [CrossRef]
- Meloni, F.; Dinelli, E.; Cabassi, J.; Nisi, B.; Montegrossi, G.; Rappuoli, D.; Vaselli, O. Provenance and distribution of potentially toxic elements (PTEs) in stream sediments from the eastern Hg-district of Mt. Amiata (central Italy). Environ. Geochem. Health 2025, 47, 123. [Google Scholar] [CrossRef] [PubMed]
- Palme, H.; O'Neill, H.S.C.; Benz, W. Evidence for collisional erosion of the Earth. In Proceedings of the 45th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 17–21 March 2014; p. 1741. [Google Scholar]
- Nitschke, N.; Guédron, S.; Tessier, E.; Tisserand, D.; Campillo, S.; Amouroux, D. Evaluation of the Hg Contamination from Gold Mining in French Guiana at the Watershed Scale Using Hg Isotopic Composition in River Sediments. ACS EST Water 2024, 4, 3443–3452. [Google Scholar] [CrossRef]
- Ure, A.M.; Berrow, M.L. The Elemental Constituents of Soils; Royal Society of Chemistry: London, UK, 1982. [Google Scholar]
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259–284. [Google Scholar] [CrossRef]
- Yokobori, N.; Igarashi, T.; Yoneda, T. Leaching characteristics of heavy metals from mineralized rocks located along tunnel construction sites. In Engineering Geology for Society and Territory-Volume 6: Applied Geology for Major Engineering Projects; Springer International Publishing: Cham, Switzerland, 2014; pp. 429–433. [Google Scholar]
- Tabelin, C.B.; Igarashi, T. Mechanisms of arsenic and lead release from hydrothermally altered rock. J. Hazard Mat. 2009, 169, 980–990. [Google Scholar] [CrossRef]
- Villafane, O.R.S.; Igarashi, T.; Kurosawa, M.; Takase, T. Comparison of potentially toxic metals leaching from weathered rocks at a closed mine site between laboratory columns and field observation. Appl. Geochem. 2012, 27, 2271–2279. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Basri, A.H.M.; Igarashi, T.; Yoneda, T. Removal of arsenic, boron, and selenium from excavated rocks by consecutive washing. Water Air Soil Poll. 2012, 223, 4153–4167. [Google Scholar] [CrossRef]
- Reich, M.; Deditius, A.P.; Chryssoulis, S.; Li, J.W.; Ma, C.Q.; Parada, M.A.; Barra, F.; Mittermayr, F. Pyrite as a record of hydrothermal fluid evolution in a porphyry copper system: A SIMS/EMPA trace element study. Geochim. Cosmochim. Acta 2013, 104, 42–62. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Qian, G.; Brugger, J.; Testamale, D.; Skiner, W.; Pring, A. Formation of As(II)—Pyrite during experimental replacement of magnetite under hydrothermal conditions. Geochim. Cosmochim. Acta 2013, 100, 1–10. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Ewing, R.C.; Kesler, S.E. Nanoscale “liquid” inclusions of As-Fe-S in arsenian pyrite. Am. Miner. 2009, 94, 391–394. [Google Scholar] [CrossRef]
- Morales-Simfors, N.; Bundschuh, J. Arsenic-rich geothermal fluids as environmentally hazardous materials–A global assessment. Sci. Total Environ. 2022, 817, 152669. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Mukherjee, A.; Bhattacharya, P.; Bundschuh, J. An appraisal of the principal concerns and controlling factors for Arsenic contamination in Chile. Sci. Rep. 2023, 13, 11168. [Google Scholar] [CrossRef]
- Bavi, H.; Gharaie, M.H.M.; Moussavi-Harami, R.; Zand-Moghadam, H.; Mahboubi, A.; Tohidi, M.R. Spatial dispersion hot spots of contamination and human health risk assessments of PTEs in surface sediments of streams around porphyry copper mine, Iran. Environ. Geochem. Health 2023, 45, 3907–3931. [Google Scholar] [CrossRef]
- Tian, Y.; Mao, J.; Jian, W.; Wang, Y.; Feng, R.; Ye, H.; Wang, P. Recognition of the Xiayu intermediate-sulfidation epithermal Ag-Pb-Zn-Au (-Cu) mineralization in the East Qinling polymetallic ore belt, China: Constraints from geology and geochronology. Ore Geol. Rev. 2023, 156, 105398. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, T.; Wang, F.; Fan, Y.; Fu, P.; Kong, F. Distribution of Co, Se, Cd, In, Re and other critical metals in sulfide ores from a porphyry-skarn system: A case study of Chengmenshan Cu deposit, Jiangxi, China. Ore Geol. Rev. 2023, 158, 105520. [Google Scholar] [CrossRef]
- Castellón, C.I.; Toro, N.; Gálvez, E.; Robles, P.; Leiva, W.H.; Jeldres, R.I. Froth flotation of chalcopyrite/pyrite ore: A critical review. Materials 2022, 15, 6536. [Google Scholar] [CrossRef]
- Teague, A.J.; Van Deventer, J.S.J.; Swaminathan, C. The effect of copper activation on the behaviour of free and refractory gold during froth flotation. Int. J. Min. Proc. 2000, 59, 113–130. [Google Scholar] [CrossRef]
- Reimann, C.; Garrett, R.G. Geochemical background—Concept and reality. Sci. Total Environ. 2005, 350, 12–27. [Google Scholar] [CrossRef]
- Muller, G. Index of geo-accumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Saleem, M.; Iqbal, J.; Akhter, G.; Shah, M.H. Fractionation, bioavailability, contamination and environmental risk of heavy metals in the sediments from a freshwater reservoir, Pakistan. J. Geochem. Explor. 2018, 184, 199–208. [Google Scholar] [CrossRef]
- Hasan, M.; Kausar, D.; Akhter, G.; Shah, M.H. Evaluation of the mobility and pollution index of selected essential/toxic metals in paddy soil by sequential extraction method. Ecotox. Environ. Safe 2018, 147, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Kříbek, B.; Majer, V.; Knésl, I.; Nyambe, I.; Mihaljevič, M.; Ettler, V.; Sracek, O. Concentrations of arsenic, copper, cobalt, lead and zinc in cassava (Manihot esculenta Crantz) growing on uncontaminated and contaminated soils of the Zambian Copperbelt. J. Afr. Earth Sci 2014, 99, 713–723. [Google Scholar] [CrossRef]
- Akopyan, K.; Petrosyan, V.; Grigoryan, R.; Melkomian, D.M. Assessment of residential soil contamination with arsenic and lead in mining and smelting towns of northern Armenia. J. Geochem. Explor. 2018, 184, 97–109. [Google Scholar] [CrossRef]
- Fornasaro, S.; Ghezzi, L.; Arrighi, S.; Shukurov, N.; Petrov, M.; Tomei, A.; Petrini, R. Geochemistry and risk assessment of potentially toxic elements in surface river sediments (Chirchik-Akhangaran basin, Uzbekistan). Environ. Geochem. Health 2025, 47, 127. [Google Scholar] [CrossRef]
- Githaiga, K.B.; Njuguna, S.M.; Yan, X. Local geochemical baselines reduce variation caused by the use of different conservative elements in predicting Cu and Zn enrichment in agricultural soils, Kenya. Chem. Afr. 2021, 4, 869–880. [Google Scholar] [CrossRef]
- Correa-Burrows, J.P.; Navarrete-Calvo, Á.; Valenzuela-Díaz, M.J.; Zapata-Aguiló, V.A.; Montserrat, S.; Navarro-Valdivia, L.; Caraballo, M.A. The role of local geochemical and mineralogical backgrounds as essential information to build efficient sediment quality guidelines at high-mountainous hydrothermally-altered basins (Mapocho basin, Chile). Sci. Total Environ. 2021, 785, 147266. [Google Scholar] [CrossRef]
- Mascarenhas, R.B.; Gloaguen, T.V.; Hadlich, G.M.; Gomes, N.S.; da Conceição Almeida, M.; de Souza Souza, E.; Santos, J.A.G. The challenge of establishing natural geochemical backgrounds in human-impacted mangrove soils of Northeastern Brazil. Chemosphere 2025, 376, 144261. [Google Scholar] [CrossRef]






| V | Cr | Co | Ni | Cu | Zn | As | Sb | Pb | Hg | |
|---|---|---|---|---|---|---|---|---|---|---|
| Andesite | ||||||||||
| PE02 | 111 | 150 | 24 | 130 | 50 | 40 | <5 | <0.5 | <5 | 0.032 |
| PE07 | 154 | 53 | 28 | 15 | 122 | 40 | nd | <0.5 | nd | 0.003 |
| Basalt (I) | ||||||||||
| PE01 | 331 | 231 | 43 | 102 | 122 | 80 | nd | <0.5 | nd | 0.014 |
| PE06 | 303 | 189 | 37 | 96 | 61 | 76 | nd | 1.1 | nd | 0.004 |
| PE10 | 327 | 232 | 38 | 103 | 94 | 93 | nd | 8.9 | nd | 0.004 |
| PE13 | 318 | 225 | 40 | 102 | 116 | 1358 | nd | 18.0 | nd | 0.017 |
| PE15 | 305 | 212 | 39 | 97 | 22 | 54 | nd | 19.7 | nd | 0.003 |
| PE16 | 299 | 212 | 27 | 89 | 191 | 163 | nd | 6.9 | nd | 0.007 |
| Basalt (II) | ||||||||||
| PE03 | 381 | 101 | 41 | 77 | 147 | 91 | nd | 3.5 | nd | 0.018 |
| PE17 | 373 | 110 | 55 | 100 | 90 | 80 | 13 | 2.8 | <5 | 0.010 |
| PE18 | 366 | 111 | 45 | 83 | 207 | 80 | nd | <0.5 | nd | 0.002 |
| PE19 | 374 | 113 | 40 | 84 | 151 | 87 | nd | 3.7 | nd | 0.148 |
| PE23 | 430 | 130 | 42 | 80 | 120 | 90 | 39 | 6.1 | <5 | 0.122 |
| Qz-vein | ||||||||||
| PE24 | 10 | <20 | 12 | <20 | 210 | 7610 | >2000 | >200 | 5360 | 0.707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornasaro, S.; Fulignati, P.; Gioncada, A.; Garces, D.; Mulas, M. Geogenic and Anthropogenic Origins of Mercury and Other Potentially Toxic Elements in the Ponce Enriquez Artisanal and Small-Scale Gold Mining District, Southern Ecuador. Minerals 2025, 15, 725. https://doi.org/10.3390/min15070725
Fornasaro S, Fulignati P, Gioncada A, Garces D, Mulas M. Geogenic and Anthropogenic Origins of Mercury and Other Potentially Toxic Elements in the Ponce Enriquez Artisanal and Small-Scale Gold Mining District, Southern Ecuador. Minerals. 2025; 15(7):725. https://doi.org/10.3390/min15070725
Chicago/Turabian StyleFornasaro, Silvia, Paolo Fulignati, Anna Gioncada, Daniel Garces, and Maurizio Mulas. 2025. "Geogenic and Anthropogenic Origins of Mercury and Other Potentially Toxic Elements in the Ponce Enriquez Artisanal and Small-Scale Gold Mining District, Southern Ecuador" Minerals 15, no. 7: 725. https://doi.org/10.3390/min15070725
APA StyleFornasaro, S., Fulignati, P., Gioncada, A., Garces, D., & Mulas, M. (2025). Geogenic and Anthropogenic Origins of Mercury and Other Potentially Toxic Elements in the Ponce Enriquez Artisanal and Small-Scale Gold Mining District, Southern Ecuador. Minerals, 15(7), 725. https://doi.org/10.3390/min15070725









