Kinetic Control of Oxygenated Apatites: Dynamic Operation of a Pilot-Scale Precipitation Reactor for Bone-Mimetic Biomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis Conditions
2.3. Precipitate Treatment
2.4. Characterization Methods
3. Results
3.1. Study of the Physicochemical Characteristics of the Products Obtained as a Function of Reactor Operating Time
3.1.1. Study by Chemical Analysis
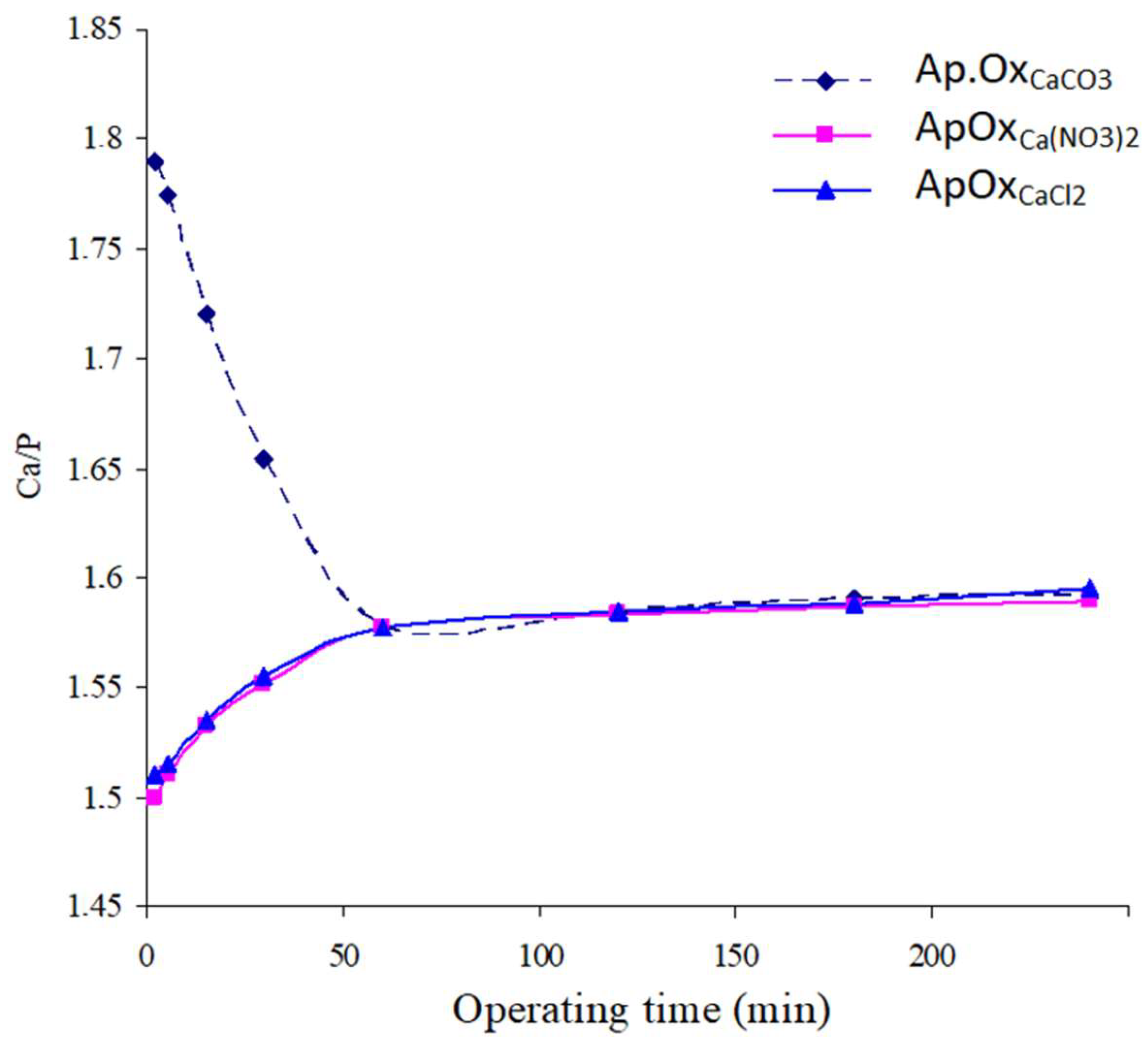
Variation of the Solids’ Ca/P Atomic Ratio as a Function of Time
- In the case of Ap.OxCaCO3, the Ca/P atomic ratio decreases rapidly with increasing reactor operating time, stabilizing at approximately 1.578. The precipitate formed at the first moments of the reaction (2 min), with a ratio of 1.79, contains calcium carbonate. During the reactor operating time, the latter reacts with phosphorus to give an apatite with a ratio of 1.578.
- In the case of Ap.OxCa(NO3)2 or Ap.OxCaCl2, the solids’ Ca/P atomic ratio increases continuously until it reaches a value of 1.577 after one hour. Beyond this time, the Ca/P ratio shows a slight increase.
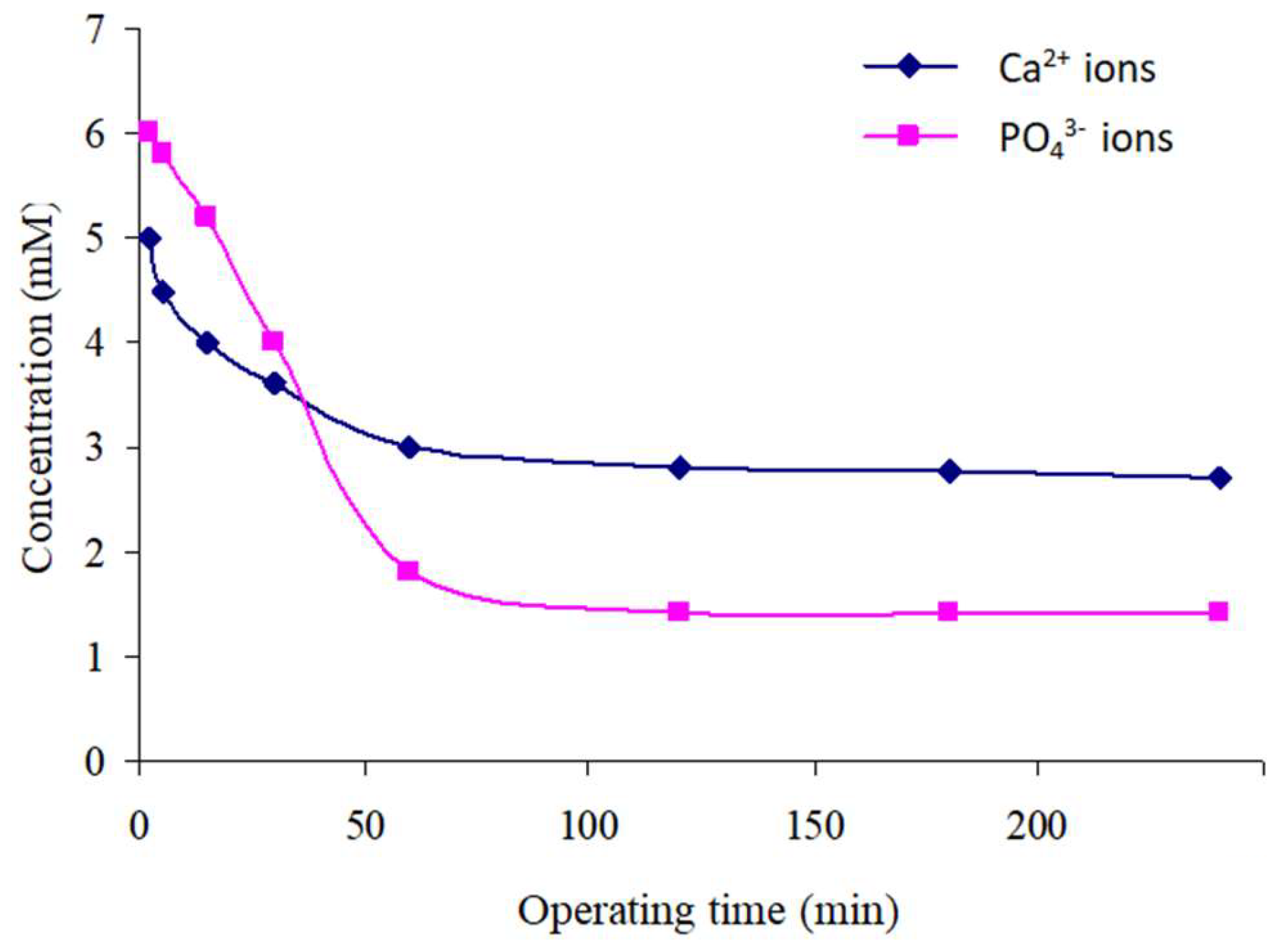
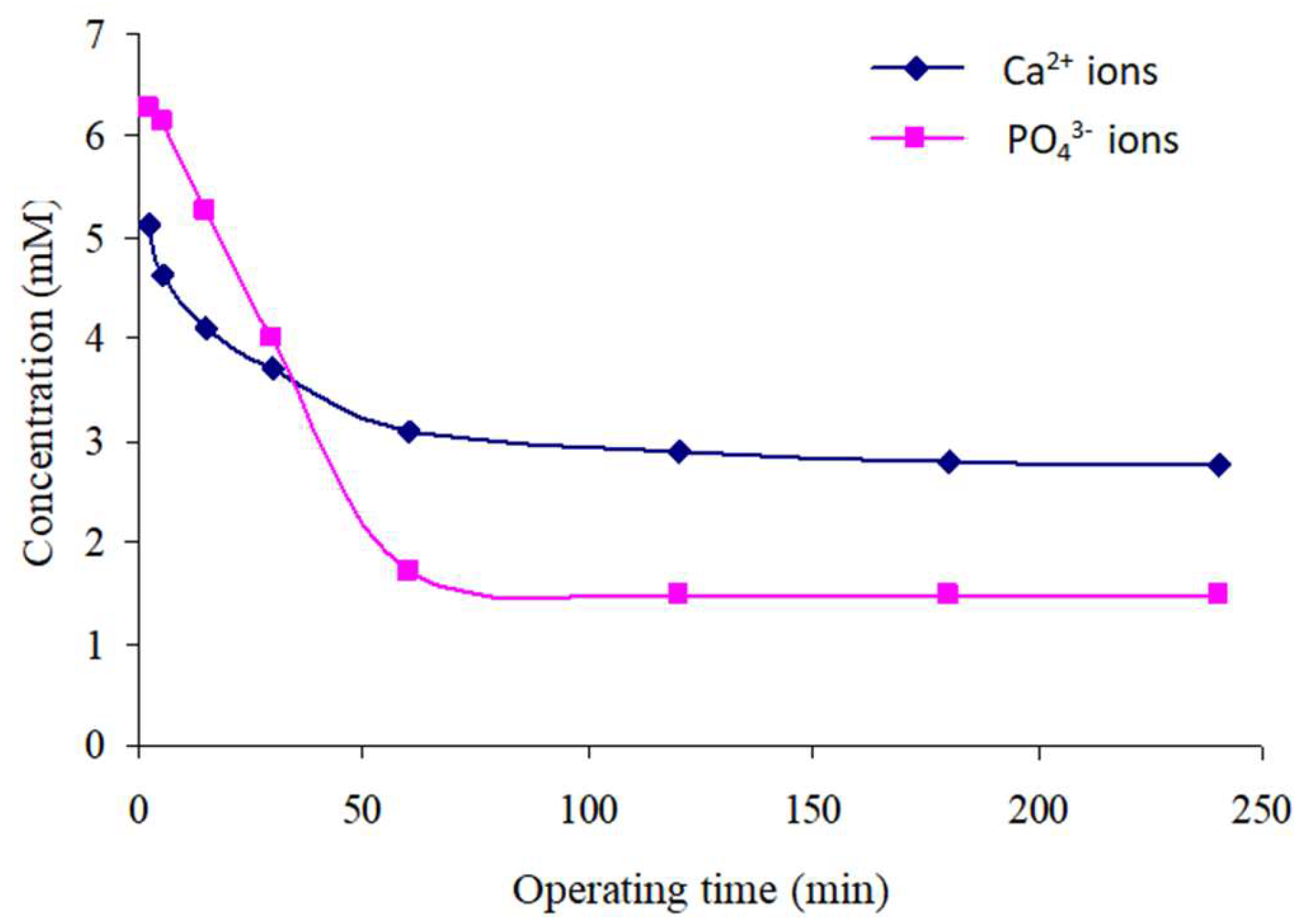
Variation in the Concentrations of Free Ca2+ and PO43− Ions in Solution over Time
- The first stage represents the initial moments of precipitation and extends until approximately 2 min. We observe a sudden increase in Ca2+ ion concentrations, followed by a rapid decrease within the first 15 min.
- The second stage, located beyond 15 min, experiences a continuous increase in Ca2+ ion concentration according to a logarithmic law. This concentration becomes almost stable at 14.6 mM after approximately 1 h of reactor functioning.
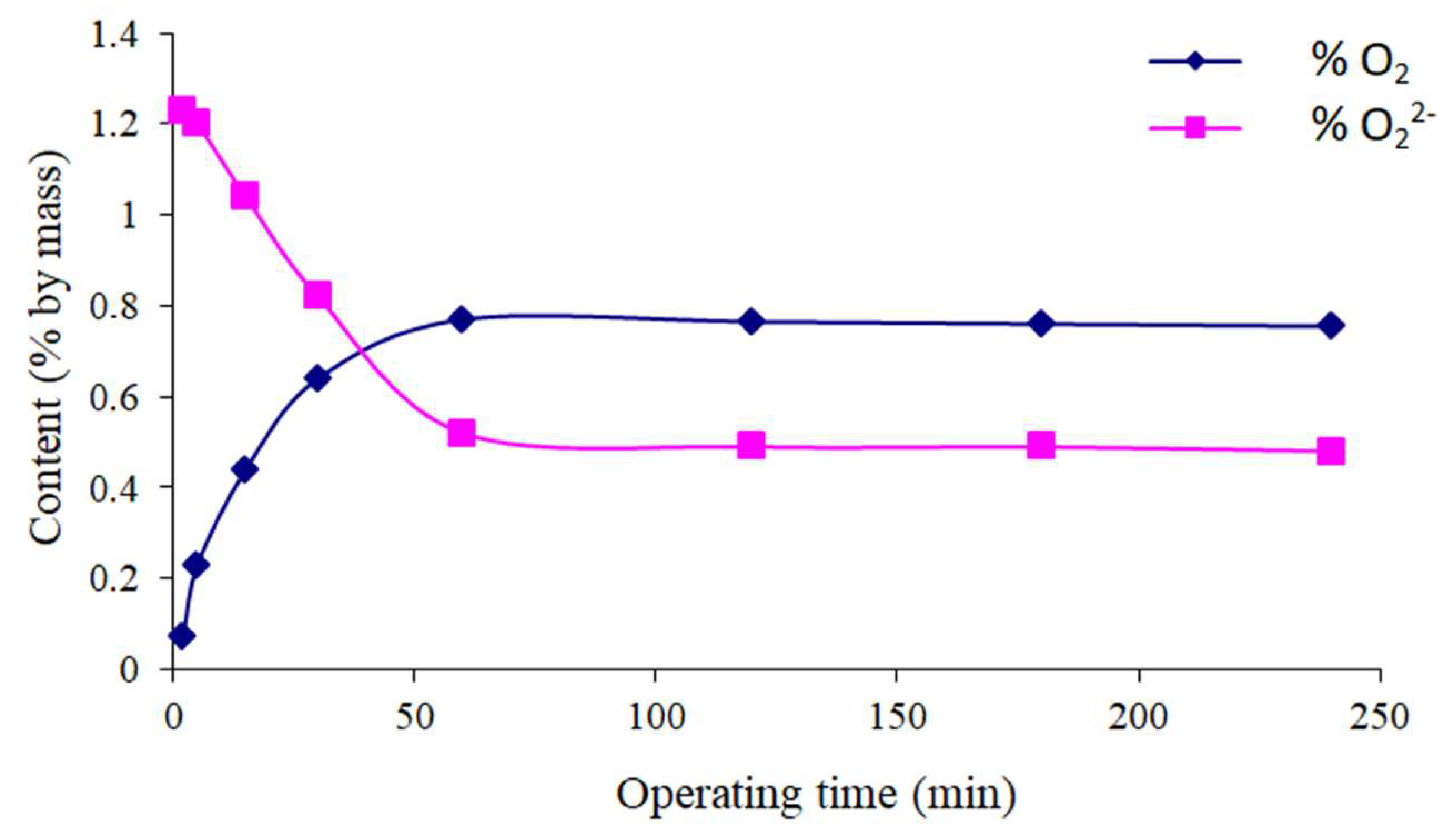
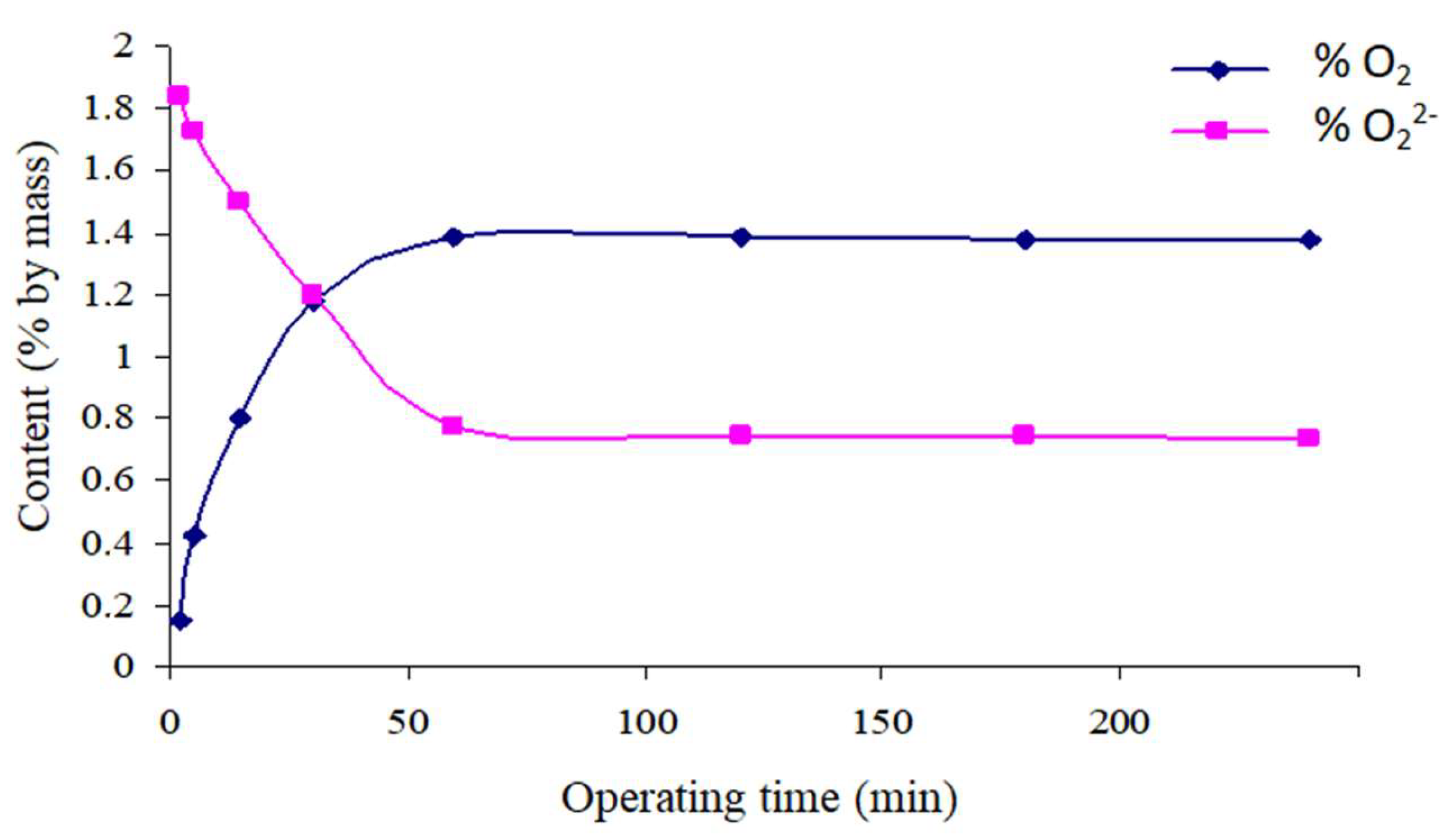
Variation of the Oxygenated Species Content of Solids as a Function of Time
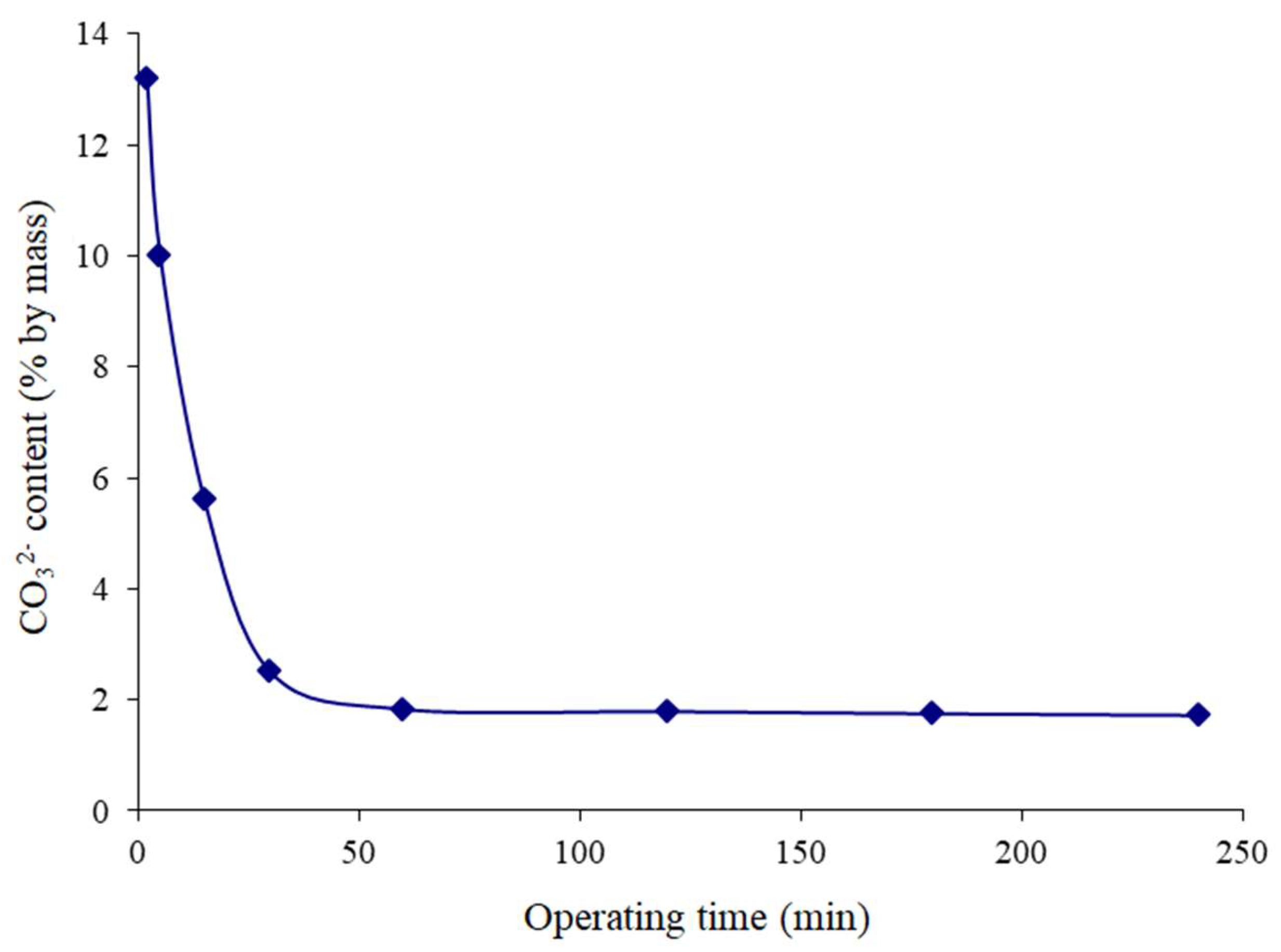
Variation in the Solid’s Carbonate Ion Content Prepared from Calcium Carbonate as a Function of Time
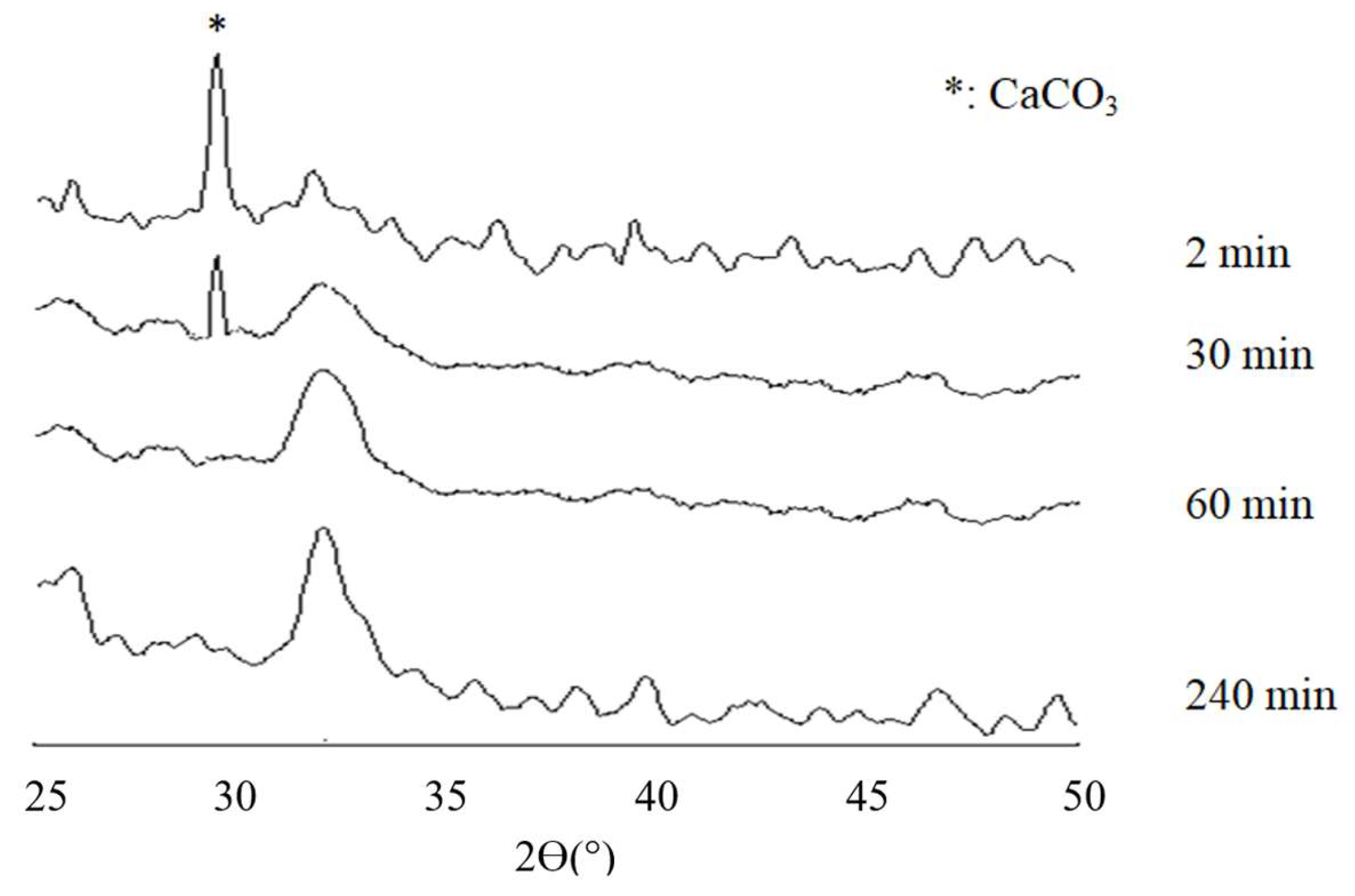
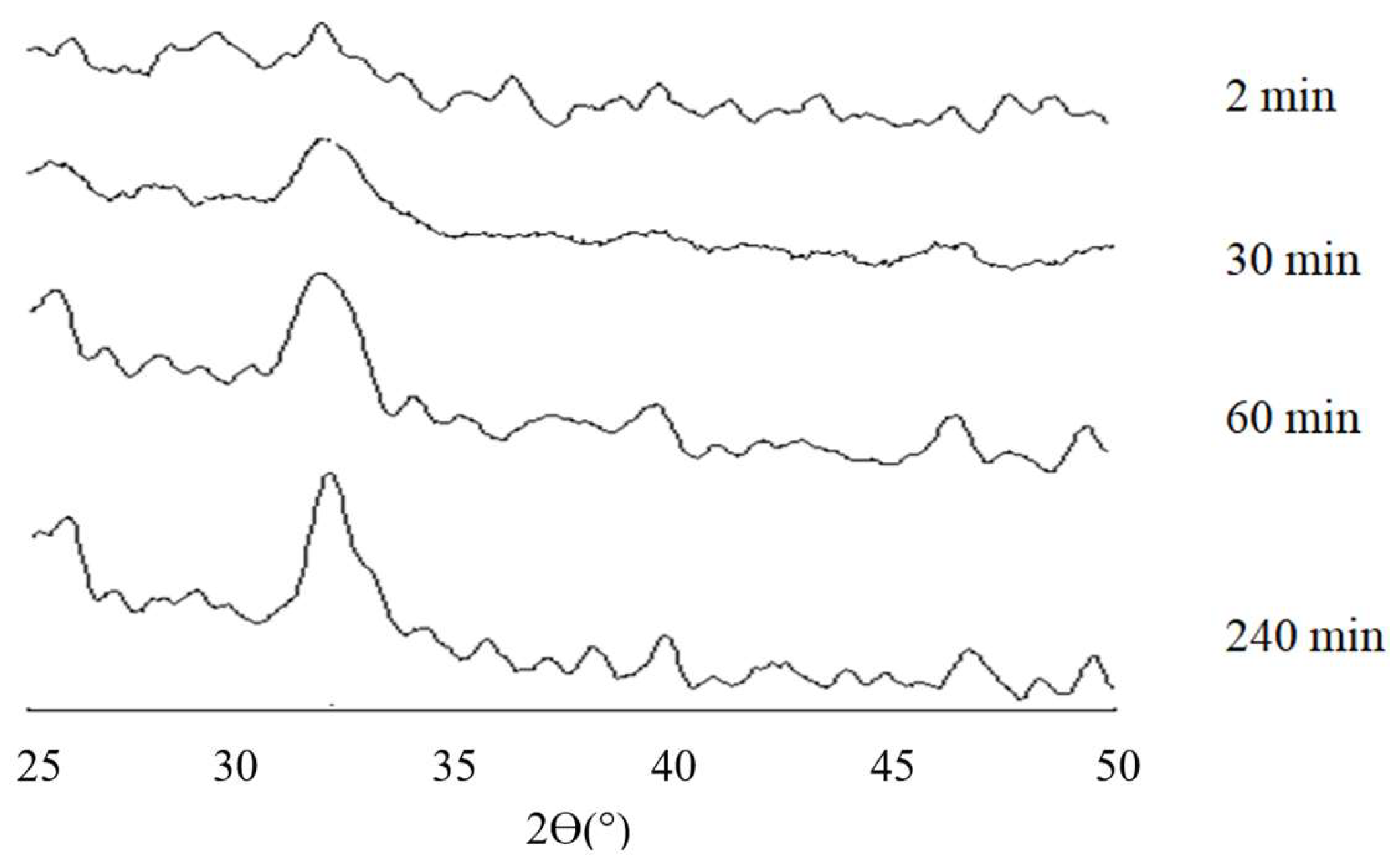
3.2. X-Ray Diffraction Analysis
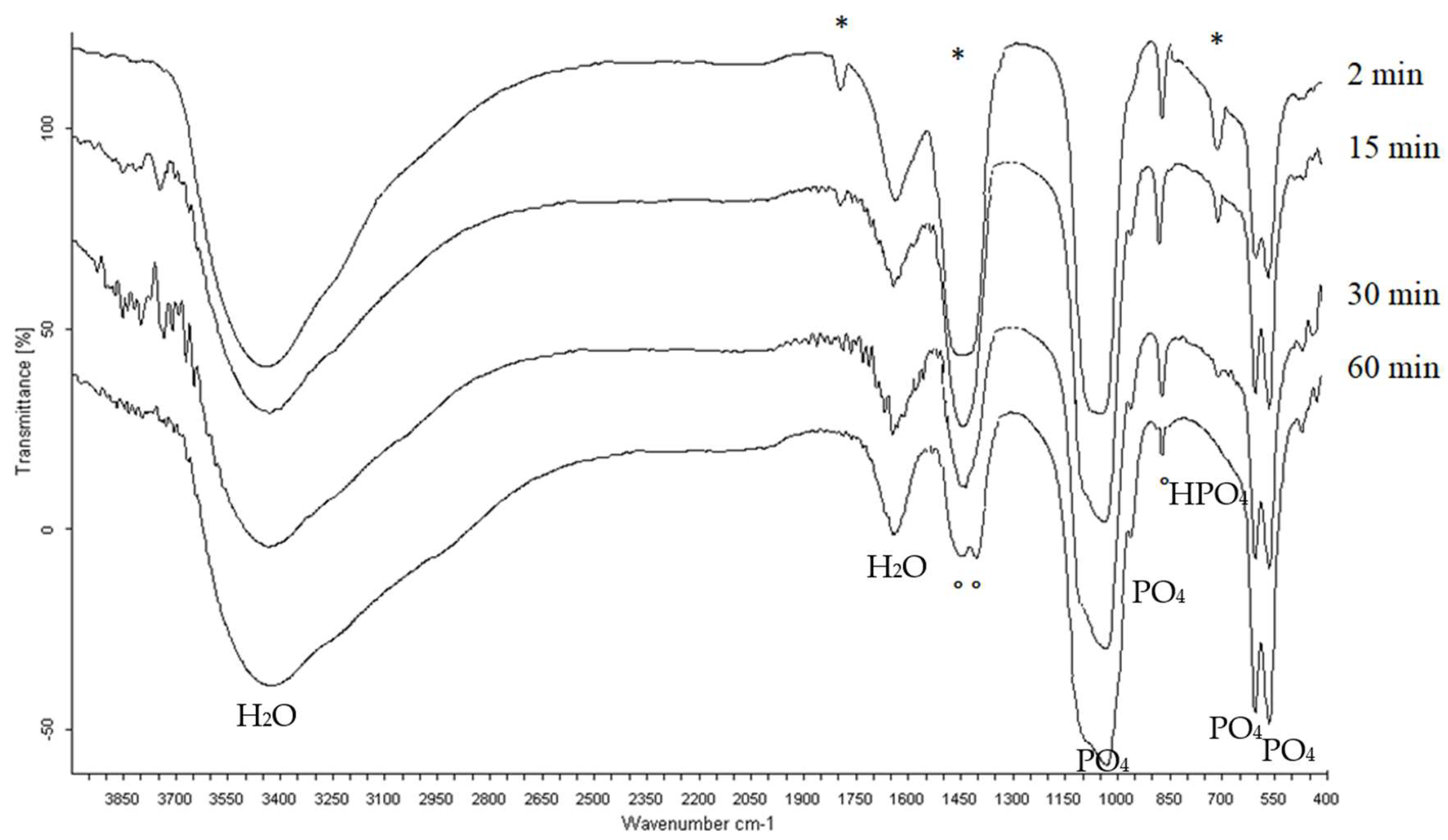
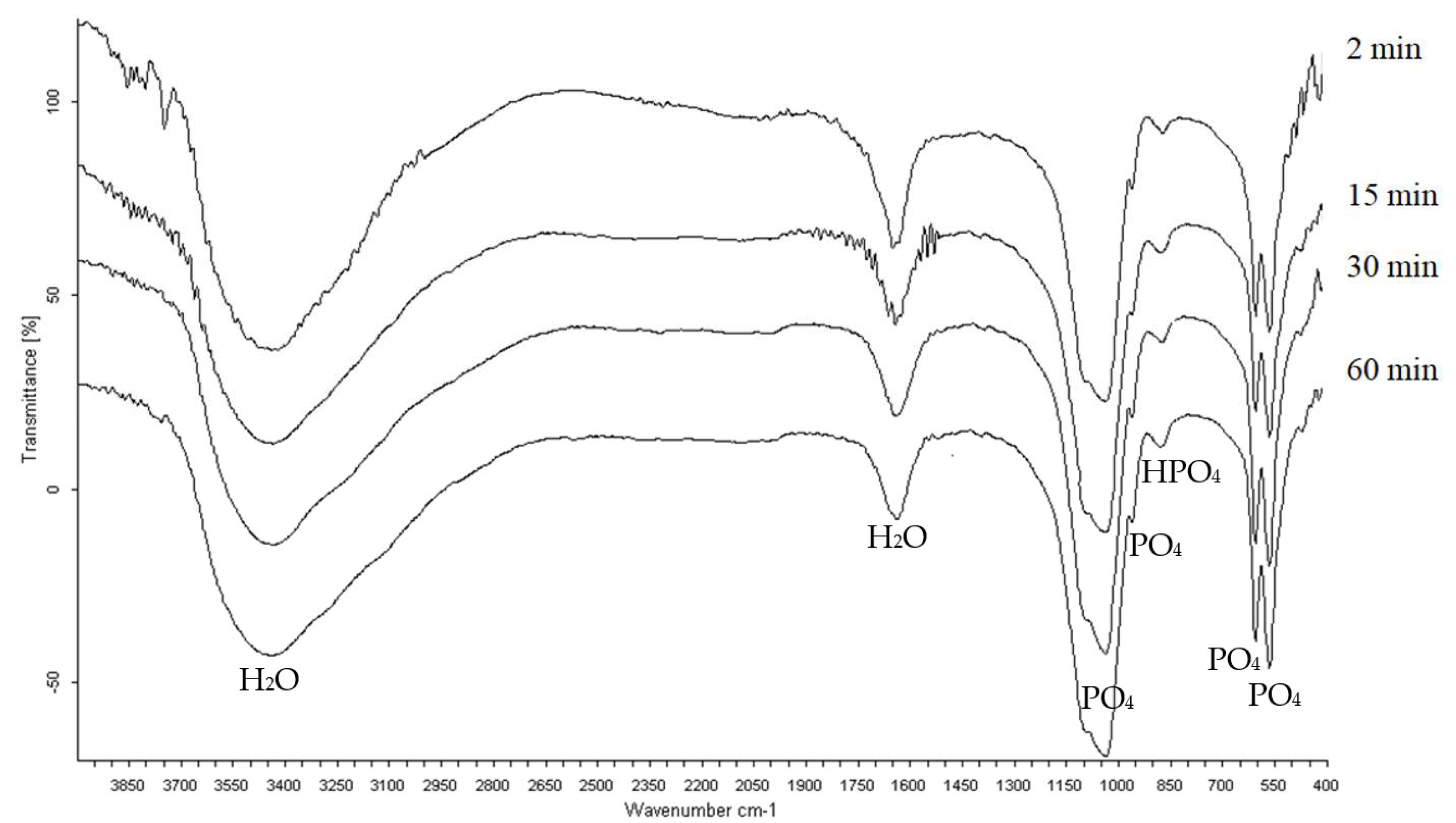
3.3. Infrared Spectrometry Analysis
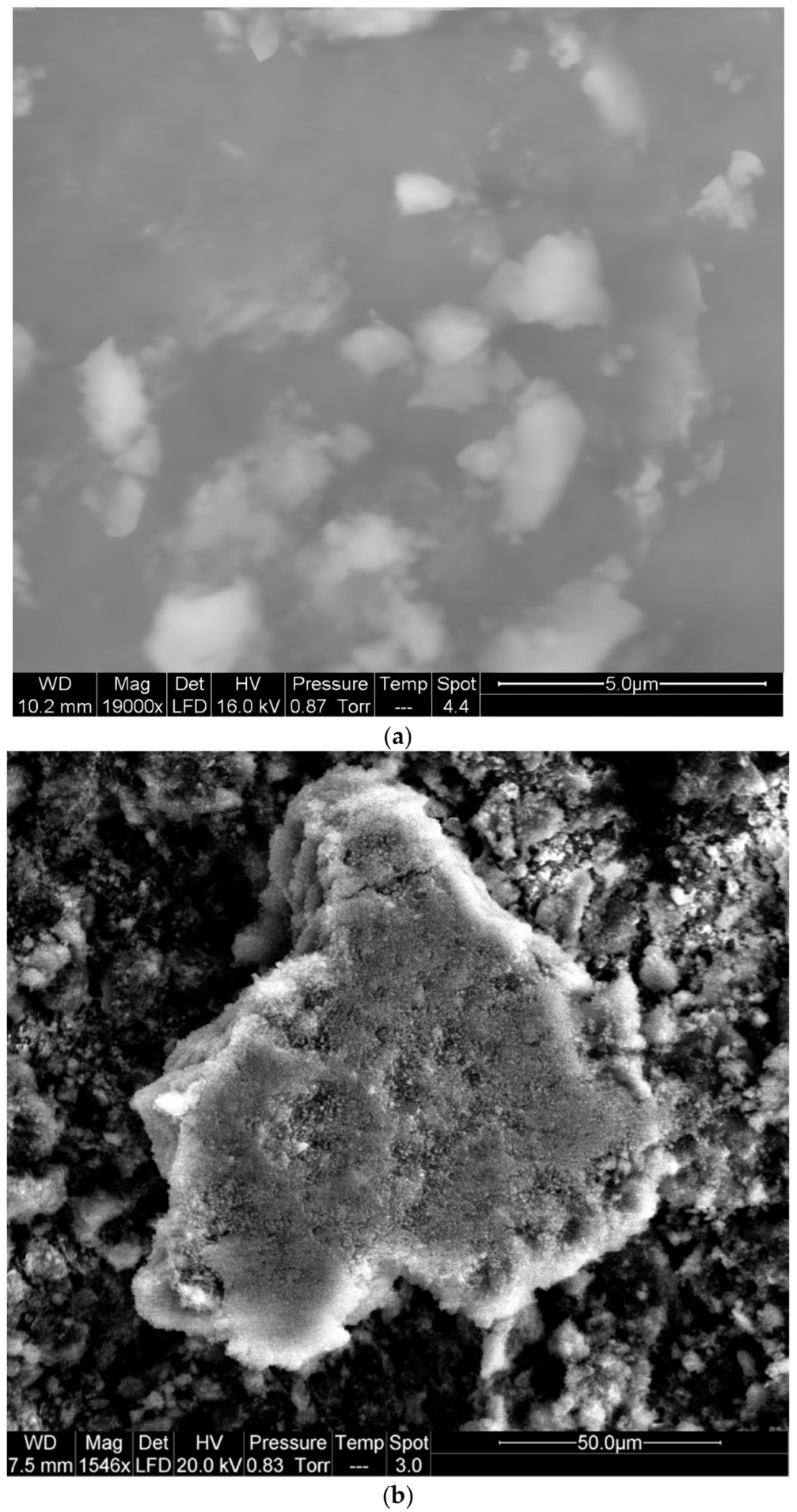
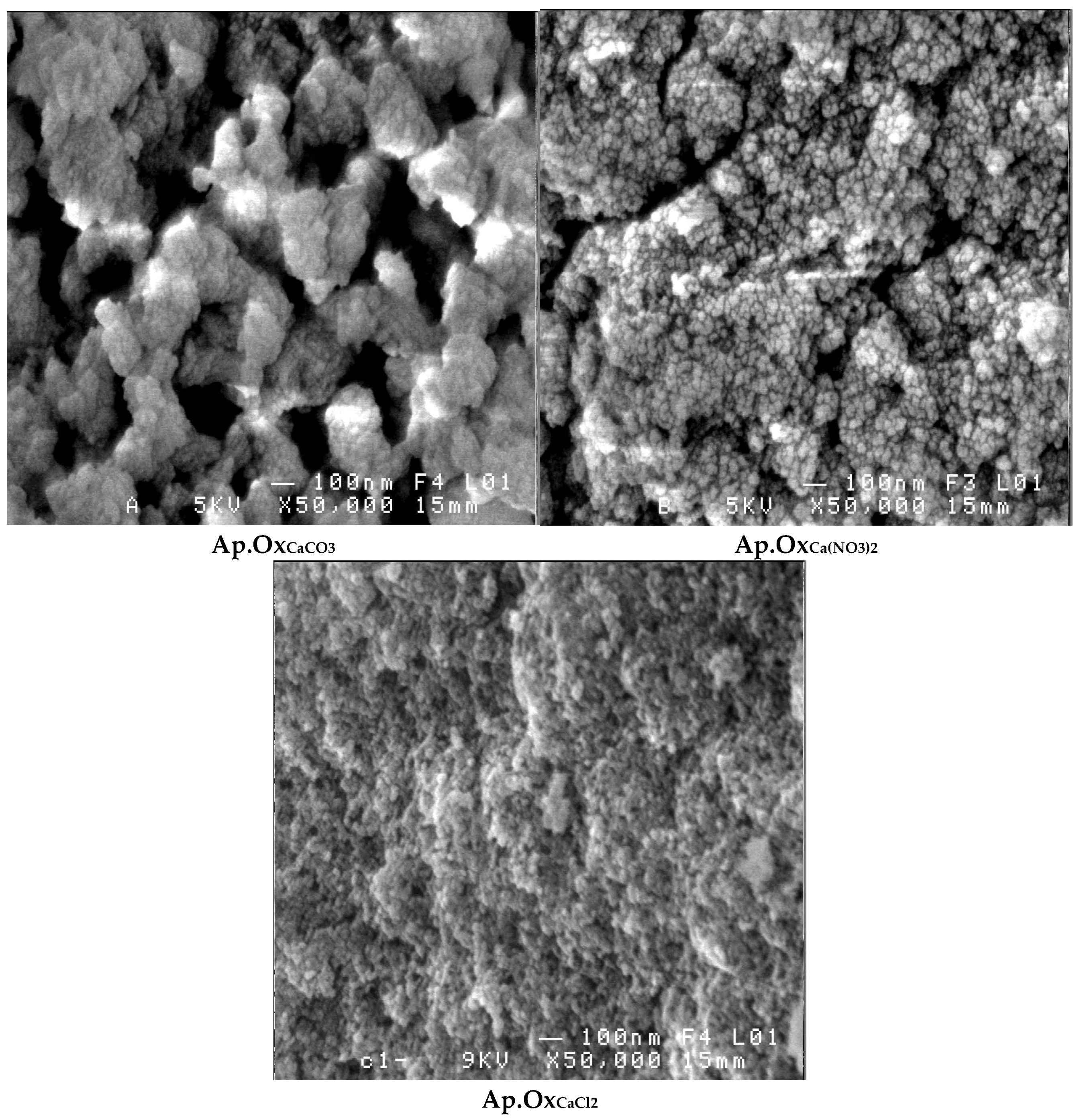
3.4. Scanning Electron Microscopy Analysis
4. Discussion
5. Conclusions
6. Future Work
- Future investigations will focus on validating the functional performance of the synthesized oxygenated apatites under conditions relevant to biomedical applications. A key research direction involves evaluating the controlled release behavior of oxygenated species in simulated physiological media, to quantify release kinetics and assess the stability of the oxygenated groups over time [62,63]. In parallel, efforts will be made to enhance the physicochemical properties of the materials through targeted functionalization. This includes the incorporation of bioactive ions, such as strontium (Sr2+) [64], or natural macromolecules like collagen [65], to potentially improve their mechanical strength and bioactivity. Collagen, a major component of the extracellular matrix, is widely recognized for its role in supporting cell adhesion, proliferation, and osteogenic differentiation. Its integration into apatite-based materials has shown promising results in enhancing biomaterial–cell interactions and accelerating bone regeneration in various preclinical models [66,67]. Therefore, functionalizing the oxygenated apatites with collagen may significantly improve their relevance for use as bone scaffolds.
- Another essential direction concerns the biological assessment of the materials, particularly their cytocompatibility, osteoconduction potential, and antiseptic performance [68,69]. These aspects will be investigated through in vitro assays on relevant cell lines and, where appropriate, in vivo studies on animal models to provide a comprehensive understanding of their biomedical relevance. Finally, the feasibility of scaling up the synthesis process will be examined, including the shaping of the materials into granules, scaffolds, or thin coatings [70]. This will require maintaining the structural and functional properties of the apatites during processing, thus paving the way for potential translational applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- LeGeros, R.Z. Calcium phosphates in oral biology and medicine. Monogr. Oral. Sci. 1991, 15, 1–201. [Google Scholar] [PubMed]
- Dorozhkin, S.V. Calcium Orthophosphates: Applications in Nature, Biology, and Medicine; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Jalaludeen, A.M.; Ramakrishnan, R.; Gunasekaran, S.S.; Thajuddin, N.; Selvam, M.K.; Ali, B.M.S.; Dua, R.; Ramakrishnan, P.; Ramesh, M.D.; Vinayagam, S.; et al. Advancements in hydroxyapatite synthesis and surface modifications for emerging biomedical applications. Inorg. Chem. Commun. 2024, 170, 113414. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Ismail, R.; Bayuseno, A.P.; Siregar, J.P.; Cionita, T. Characterization of Hydroxyapatite Extracted from Crab Shell Using the Hydrothermal Method with Varying Holding Times. J. Renew. Mater. 2024, 12, 1145–1163. [Google Scholar] [CrossRef]
- Mysore, T.H.M.; Patil, A.Y.; Hegde, C.; Sudeept, M.A.; Kumar, R.; Soudagar, M.E.M.; Fattah, I.M.R. Apatite insights: From synthesis to biomedical applications. Eur. Polym. J. 2024, 209, 112842. [Google Scholar] [CrossRef]
- Lin, F.; Basem, A.; Khaddour, M.H.; Salahshour, S.; Li, W.; Sabetvand, R. Advancing mechanical and biological characteristics of polymer-ceramic nanocomposite scaffolds for sport injuries and bone tissue engineering: A comprehensive investigation applying finite element analysis and artificial neural network. Ceram. Int. 2025, 51, 12758–12773. [Google Scholar] [CrossRef]
- Maria, S.V.; Malaiappan, S. Biocompatibility and Periodontal Regenerative Potential of Hydroxyapatite Nanoparticles from Portunus Sanguinolentus Shells: A Crystallographic, Morphological, And Molecular Gene Expression Analysis. J. Dent. 2025, 157, 105762. [Google Scholar] [CrossRef]
- Otsuka, Y.; Rawal, A.; Koshy, P.; Ben-Nissan, B.; Kono, H.; Kikuchi, M. Structural transformations in mechanochemically-synthesized strontium-doped hydroxyapatite. Ceram. Int. 2025. In Press. [Google Scholar] [CrossRef]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31 (Suppl. 4), 37–47. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today. 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent advances in hydroxyapatite-based biocomposites for bone tissue regeneration in orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for drug delivery and tissue engineering: The role of genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Kareem, R.O.; Bulut, N.; Kaygili, O. Hydroxyapatite biomaterials: A comprehensive review of their properties, structures, medical applications, and fabrication methods. J. Chem. Rev. 2024, 6, 1–26. [Google Scholar] [CrossRef]
- Santoro, A.; Voto, A.; Fortino, L.; Guida, R.; Laudisio, C.; Cillo, M.; D’Ursi, A.M. Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes. Int. J. Mol. Sci. 2025, 26, 3085. [Google Scholar] [CrossRef]
- Simpson, D.R. Oxygen-rich apatite. Am. Mineral. 1969, 54, 560–562. [Google Scholar]
- Rey, C. Study of the Relationships Between Apatites and Molecular Compounds. Ph.D. Thesis, National Polytechnic Institute of Toulouse, Toulouse, France, 1984. (In French). [Google Scholar]
- Ledard, C.; Benque, E.; Lacout, J.L.; Rey, C. Bony or dental filling biomaterials and processes for preparation thereof. US Patent 5141561, 25 August 1992. [Google Scholar]
- Belouafa, S.; Chaair, H.; Digua, K.; Sallek, B.; Mountacer, H. New synthesis process of a carbonated apatite with antiseptic properties. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 2679–2687. (In French) [Google Scholar] [CrossRef]
- Belouafa, S.; Chaair, H.; Digua, K.; Oudadesse, H.; Sallek, B.; Mountacer, H. Use of experimental designs for the modeling of the preparation of a calcium phosphate with antiseptic properties for biomedical applications. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 337–349. (In French) [Google Scholar] [CrossRef]
- Belouafa, S.; Chaair, H.; Digua, K.; Sallek, B.; Essadani, A.; Oudadesse, H. Synthesis, characterization and thermal behaviour of a Phosphocalcic Oxygenated Apatite. J. Adv. Mater. Covina 2007, 139–142. [Google Scholar]
- Chaair, H.; Belouafa, S.; Digua, K.; Sallek, B.; Oudadesse, H.; Mouhir, L. Advanced statistical optimization of parameters of synthesis process of oxygenated carbonated apatite. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 2752–2768. [Google Scholar] [CrossRef]
- Belouafa, S.; Chaair, H.; Loukili, H.; Digua, K.; Sallek, B. Characterization of antiseptic apatite powders prepared at biomimetics temperature and pH. Mater. Res. 2008, 11, 93–96. [Google Scholar] [CrossRef]
- Chroqui, W.; Akhiyat, I.; Belouafa, S.; Chaair, H.; Digua, K.; Benhayoune, H.; Sallek, B. Optimization of the synthesis of antiseptic-loaded phosphocalcic apatites prepared from calcium nitrate and phosphoric acid. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 2220–2228. (In French) [Google Scholar] [CrossRef]
- Vandecandelaere, N.; Bosc, F.; Rey, C.; Drouet, C. Peroxide-doped apatites: Preparation and effect of synthesis parameters. Powder Technol. 2014, 255, 3–9. [Google Scholar] [CrossRef]
- Yahyaoui, R.; Azzaoui, K.; Lamhamdi, L.; Mejdoubi, E.; Elabed, S.; Hammouti, B. Preparation of oxygenated apatite from hydrolysis of cured brushite cement in aqueous medium. Der Pharm. Chem. 2014, 6, 133–138. [Google Scholar]
- Belouafa, S.; Faouzi, F.E.; Sallek, B.; Digua, K.; Zaoui, F.; Rifki, C.; Chaair, H. Study of the influence of synthesis parameters on the chemical composition of oxygenated carbonated apatites. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 1346–1353. (In French) [Google Scholar] [CrossRef]
- Lamhamdi, A.; Azzaouia, K.; Mejdoubia, E.; Hammouti, B.; Elansaria, L.; Jabria, M.; Berrabah, M.B. Elbali. Modeling and optimization of the synthesis of oxygenated apatite by hydrolysis of dicalcium phosphate dihydrate (DCPD) using the Box-Behnken design. Der Pharm. Chem. 2015, 7, 46–52. [Google Scholar]
- Jerdioui, S.; Elansari, L.L.; Mejdoubi, E.; Azzaoui, K.; Lamhamdi, A.; Jodeh, S.; Hamed, O.; Tahani, A.; Zenkouar, M.; Zarrouk, A.; et al. Physicochemical study of the effect of maturation time on the chemical composition of an oxygenated apatite. J. Mater. 2017, 8, 1211–1219. [Google Scholar]
- Naanaai, L.; Azzaoui, K.; Lamhamdi, A.; Mejdoubi, E.; Lakrat, M.; Jodeh, S. Study and optimization of oxygenated apatite obtained by dissolution-reprecipitation of hydroxyapatite in a solution of hydrogen peroxide. Chem. Afr. 2020, 3, 227–235. [Google Scholar] [CrossRef]
- Belouafa, S.; Chaair, H.; Digua, K. Application of statistical experimental design and surface plot technique to optimize oxygenated apatite synthesis. Adv. Mater. Sci. Eng. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Ana, I.D.; Lestari, A.; Lagarrigue, P.; Soulie, J.; Anggraeni, R.; Maube-Bosc, F.; Thouron, C.; Duployer, B.; Tenailleau, C.; Drouet, C. Safe-by-design antibacterial peroxide-substituted biomimetic apatites: Proof of concept in tropical dentistry. J. Funct. Biomater. 2022, 13, 144. [Google Scholar] [CrossRef]
- Jerdioui, S.; Elansari, L.L.; Bouammali, H.; Azzaoui, K.; Sabbahi, R.; Hammouti, B.; Mejdoubi, M. Effect of calcium/phosphorus ratio on the chemical and structural properties of oxygenated apatite synthesized by neutralization. Moroc. J. Chem. 2024, 12, 145–156. [Google Scholar] [CrossRef]
- Jerdioui, S.; Bouammalia, H.; Mejdoubi, E.; Touzani, R.; Azzaoui, K.; Hammouti, B.; Sabbahi, R.; Nandiyanto, A.B.D.; Elansari, L.L. Physico-chemical characteristics of Ca/P ratio on the composition and structure of oxygenated apatite. Commun. Sci. Technol. 2024, 9, 100–106. [Google Scholar] [CrossRef]
- Jiang, S.; Zheng, Y.; Xia, H.; Liu, Z.; Rao, S.; Wang, Y.; Sun, H.; Lu, X.; Xie, C. Oxygen-Releasing Hydrogels for Tissue Regeneration. Adv. NanoBiomed Res. 2024, 4, 2300133. [Google Scholar] [CrossRef]
- Zoneff, E.; Wang, Y.; Jackson, C.; Smith, O.; Duchi, S.; Onofrillo, C.; Farrugia, B.; Moulton, S.E.; Williams, R.; Parish, C.; et al. Controlled oxygen delivery to power tissue regeneration. Nat. Commun. 2024, 15, 4361. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Yi, J. Biomaterials Designed to Modulate Reactive Oxygen Species for Enhanced Bone Regeneration in Diabetic Conditions. J. Funct. Biomater. 2024, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; Giustetto, P.; Rolfo, A.; Caniggia, I.; Balbis, S.; Guiot, C.; Cavalli, R. Microbubble-Mediated Oxygen Delivery to Hypoxic Tissues as a New Therapeutic Device. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; IEEE: New York, NY, USA, 2008. [Google Scholar]
- Sokolova, M.; Putnins, A.; Kreicbergs, I.; Locs, J. Scale-up of wet precipitation calcium phosphate synthesis. Key Eng. Mater. 2014, 604, 216–219. [Google Scholar] [CrossRef]
- Wojasin, M.; Latocha, J.; Liszewska, P.; Makowski, Ł.; Sobieszuk, P.; Ciach, T. Scaled-up 3D-printed reactor for precipitation of lecithin-modified hydroxyapatite nanoparticles. Ind. Eng. Chem. Res. 2021, 60, 12944–12955. [Google Scholar] [CrossRef]
- Kovrlija, I.; Menshikh, K.; Marsan, O.; Rey, C.; Combes, C.; Locs, J.; Loca, D. Exploring the formation kinetics of octacalcium phosphate from alpha-tricalcium phosphate: Synthesis scale-up, determination of transient phases, their morphology and biocompatibility. Biomolecules 2023, 13, 462. [Google Scholar] [CrossRef]
- Posner, A.S.; Betts, F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res. 1975, 8, 273–281. [Google Scholar] [CrossRef]
- Heughebaert, J.C.; Montel, G. Conversion of amorphous tricalcium phosphate into apatitic tricalcium phosphate. Calcif. Tissue Int. 1982, 34, S103–S108. [Google Scholar]
- LeGeros, R.Z. Calcium phosphate materials in restorative dentistry: A review. Adv. Dent. Res. 1988, 2, 164–180. [Google Scholar] [CrossRef]
- Lagno, F.; Rocha, S.D.F.; Katsarou, L.; Demopoulos, G.P. Supersaturation-controlled synthesis of dicalcium phosphate dihydrate and nanocrystalline calcium-deficient hydroxyapatite. Ind. Eng. Chem. Res. 2012, 51, 6605–6612. [Google Scholar] [CrossRef]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef]
- Nikolenko, M.V.; Vasylenko, K.V.; Myrhorodska, V.D.; Kostyniuk, A.; Likozar, B. Synthesis of calcium orthophosphates by chemical precipitation in aqueous solutions: The effect of the acidity, Ca/P molar ratio, and temperature on the phase composition and solubility of precipitates. Processes. 2020, 8, 1009. [Google Scholar] [CrossRef]
- Meyer, J.L.; Lee, K.E.; Bergert, J.H. The inhibition of calcium oxalate crystal growth by multidentate organic phosphonates: Effect of pH. Calcif. Tissue Res. 1977, 23, 83–86. [Google Scholar] [CrossRef]
- Gee, A.; Dietz, V.R. Determination of phosphate by differential spectrophotometry. Anal. Chem. 1953, 25, 1320–1324. [Google Scholar] [CrossRef]
- Trombe, J.C.; Montel, G. Some features of the incorporation of oxygen in different oxidation states in the apatitic lattice-I on the existence of calcium and strontium oxy apatites. J. Inorg. Nucl. Chem. 1978, 40, 15–21. [Google Scholar] [CrossRef]
- López-Lara, T.; Castaño, V.M. Time Dependency of CaO-Treated Expansive Soils. Electron. J. Geotech. Eng. 2001, 6, 1–9. [Google Scholar]
- Heughebaert, J.C. Contribution to the Study of the Evolution of Amorphous Precipitated Calcium Orthophosphates into Apatitic Orthophosphates. Ph.D. Thesis, National Polytechnic Institute of Toulouse, Toulouse, France, 1977. (In French). [Google Scholar]
- Assis, C.M.; Vercik, L.C.O.; Santos, M.L.; Fook, M.V.L. Comparison of crystallinity between natural hydroxyapatite and synthetic cp-Ti/HA coatings. Mat. Res. 2005, 8, 207–211. [Google Scholar] [CrossRef]
- Thamaraiselvi, T.V.; Prabakaran, K.; Rajeswari, S. Synthesis of Hydroxyapatite that Mimic Bone Minerology. Trends Biomater. Artif. Organs. 2006, 19, 81–83. [Google Scholar]
- Nadir, S.; Belainass, A.; Irhzo, A.; Lacout, J.L. Synthèse par neutralisation d’une hydroxyapatite pure à partir de l’acide orthophosphorique technique et de la calcite. Phosphorus Sulfur Silicon Relat. Elem. 1996, 112, 33–40. [Google Scholar] [CrossRef]
- Vignoles, M.; Bonel, G.; Holcomb, D.W.; Young, R.A. Influence of preparation conditions on the composition of type B carbonated hydroxyapatite and on the localization of the carbonate ions. Calcif. Tissue Int. 1988, 43, 33–40. [Google Scholar] [CrossRef]
- Eanes, E.; Gillesen, I.; Posner, A. Intermediate states in the precipitation of hydroxyapatite. Nature 1965, 208, 365–367. [Google Scholar] [CrossRef]
- Eanes, E.D.; Meyer, J.L. The maturation of crystalline calcium phosphates in aqueous suspensions at physiologic pH. Calcif. Tissue Res. 1977, 23, 259–269. [Google Scholar] [CrossRef]
- Tomson, M.B.; Nancollas, G.H. Mineralization kinetics: A contant composition approach. Science 1978, 200, 1059–1060. [Google Scholar] [CrossRef]
- Brown, W.E.; Schroeder, L.W.; Ferris, J.S. Interlayering of crystalline octacalcium phosphate and hydroxyapatite. J. Phys. Chem. 1979, 83, 1385–1388. [Google Scholar] [CrossRef]
- Boskey, A.L.; Posner, A.S. Formation of hydroxyapatite at low supersaturation. J. Phys. Chem. 1976, 80, 40–45. [Google Scholar] [CrossRef]
- Guan, Y.; Gao, N.; Niu, H.; Dang, Y.; Guan, J. Oxygen-release microspheres capable of releasing oxygen in response to environmental oxygen level to improve stem cell survival and tissue regeneration in ischemic hindlimbs. J. Control. Release. 2021, 331, 376–389. [Google Scholar] [CrossRef]
- Zeng, Y.; Yan, C.; Chen, G.; Chen, Z.; Wang, F. Advances in oxygen-releasing matrices for regenerative engineering applications. Med. Biol. Eng. Comput. 2025, 1–16. [Google Scholar] [CrossRef]
- Anand, A.; Sengupta, S.; Galusek, D.; Beltrán, A.M.; Galusková, D.; Boccaccini, A.R. A new approach to overcome cytotoxic effects of Cu by delivering dual therapeutic ions (Sr, Cu). J. Trace Elem. Med. Biol. 2025, 87, 127565. [Google Scholar] [CrossRef]
- Peng, T.; Maity, A.; Grande, D.A. Nanotechnologies; Biomaterials, and Scaffolds. In Regenerative Medicine in Sports and Orthopaedics: A New Approach; Springer Nature: Cham, Switzerland, 2025; pp. 435–462. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Tian, Y.; Yang, Y.; Wang, J.; Guo, H.; Banitaba, S.N.; Khademolqorani, S.; Li, J. The Collagen-Based Scaffolds for Bone Regeneration: A Journey through Electrospun Composites Integrated with Organic and Inorganic Additives. Processes 2023, 11, 2105. [Google Scholar] [CrossRef]
- Todd, E.A.; Mirsky, N.A.; Silva, B.L.G.; Shinde, A.R.; Arakelians, A.R.L.; Nayak, V.V.; Marcantonio, R.A.C.; Gupta, N.; Witek, L.; Coelho, P.G. Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions. J. Funct. Biomater. 2024, 15, 280. [Google Scholar] [CrossRef]
- Park, S.Y.; Yi, S.M.; On, S.W.; Che, S.A.; Lee, J.Y.; Yang, B.E. Evaluation of Low-Crystallinity Apatite as a Novel Synthetic Bone Graft Material: In Vivo and In Vitro Analysis. J. Dent. 2025, 154, 105597. [Google Scholar] [CrossRef]
- Khalil, W.A.; Alharbi, H. Comparative analysis of calcium dynamics in regular-set versus fast-set bioceramics: Impact on cytotoxicity and apatite formation. J. Endod. 2025, 51, 325–331. [Google Scholar] [CrossRef]
- Barui, S.; Hadagalli, K.; Mukherjee, S.; Roy, S.; Bhattacharjee, D.; Basu, B. Pilot-scale manufacturing of phase-pure and highly crystalline hydroxyapatite: Lessons learned and process protocols. Int. J. Appl. Ceram. Technol. 2022, 19, 762–772. [Google Scholar] [CrossRef]



| Calcium Salt | T (°C) | D (h) | pH | Ca/P |
|---|---|---|---|---|
| CaCO3 | 40 | 1 | 7.38 | 1.647 |
| Ca(NO3)2 | 40 | 1 | 7.87 | 1.542 |
| CaCl2 | 40 | 1 | 7.87 | 1.513 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belouafa, S.; Berrada, M.; Digua, K.; Chaair, H. Kinetic Control of Oxygenated Apatites: Dynamic Operation of a Pilot-Scale Precipitation Reactor for Bone-Mimetic Biomaterials. Minerals 2025, 15, 700. https://doi.org/10.3390/min15070700
Belouafa S, Berrada M, Digua K, Chaair H. Kinetic Control of Oxygenated Apatites: Dynamic Operation of a Pilot-Scale Precipitation Reactor for Bone-Mimetic Biomaterials. Minerals. 2025; 15(7):700. https://doi.org/10.3390/min15070700
Chicago/Turabian StyleBelouafa, Soumia, Mohammed Berrada, Khalid Digua, and Hassan Chaair. 2025. "Kinetic Control of Oxygenated Apatites: Dynamic Operation of a Pilot-Scale Precipitation Reactor for Bone-Mimetic Biomaterials" Minerals 15, no. 7: 700. https://doi.org/10.3390/min15070700
APA StyleBelouafa, S., Berrada, M., Digua, K., & Chaair, H. (2025). Kinetic Control of Oxygenated Apatites: Dynamic Operation of a Pilot-Scale Precipitation Reactor for Bone-Mimetic Biomaterials. Minerals, 15(7), 700. https://doi.org/10.3390/min15070700






