1. Introduction
The Platreef Platinum-Group Element (PGE) ore deposit, a significant component of South Africa’s Bushveld Complex, is recognized for its diverse mineralogy and complex geology. Processing Platinum Group Minerals (PGMs) from this deposit poses considerable challenges due to the variability in mineral associations, textures, and liberation characteristics. This complexity has direct implications for flotation kinetics, recovery rates, and consequently, the operational performance and design of metallurgical plants.
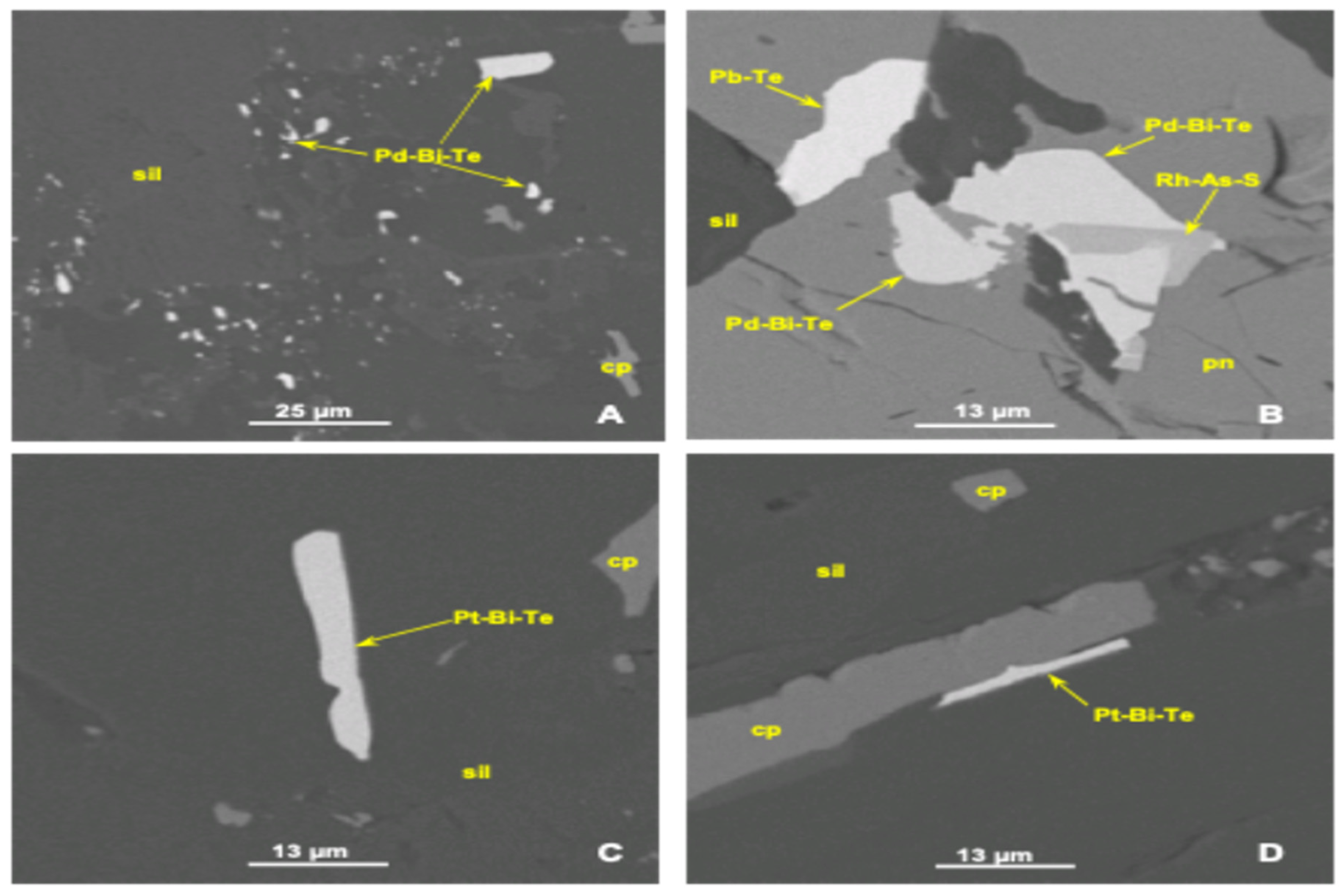
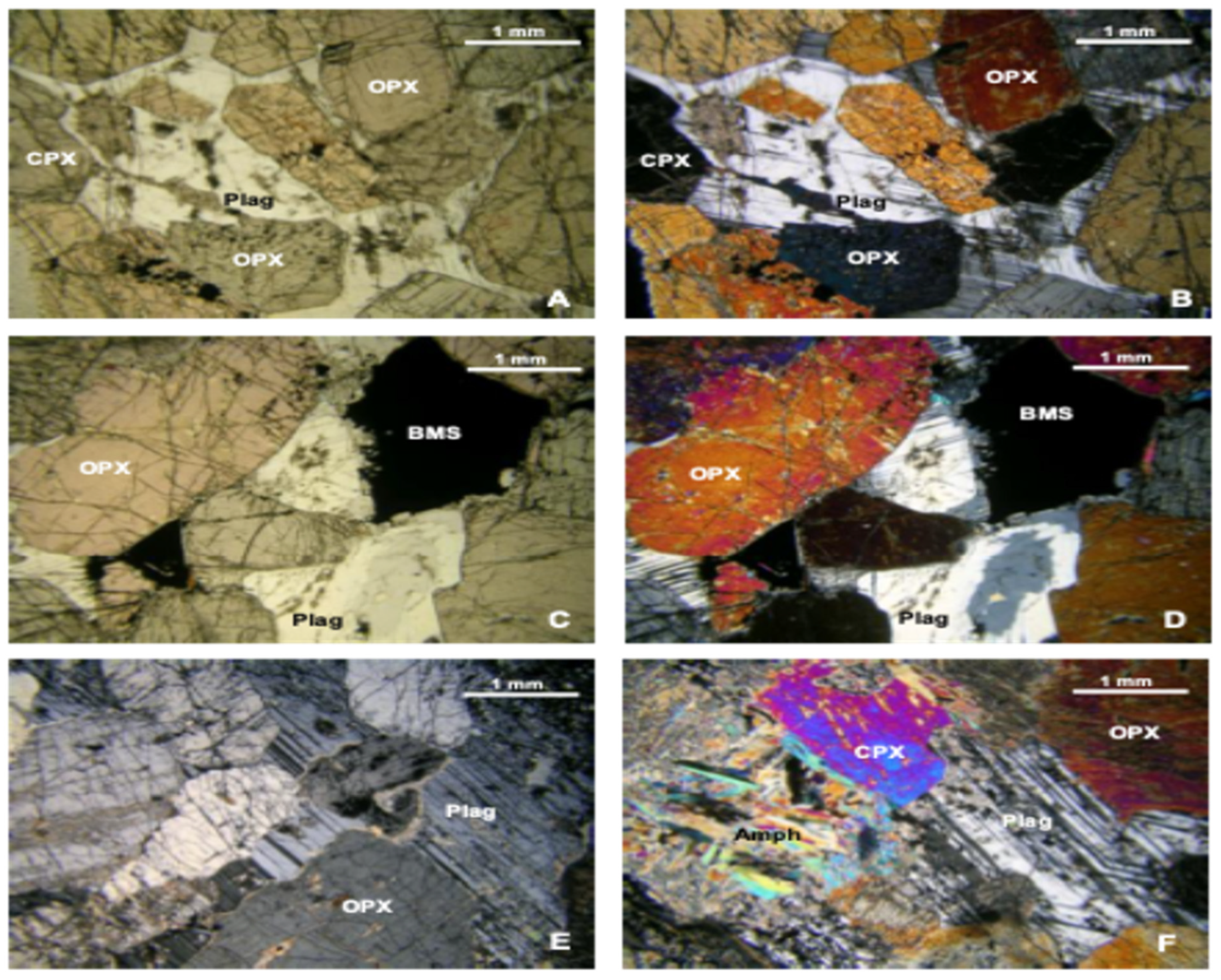
The Platreef orebody exhibits specific mineralogical complexities characterized by the presence of various PGM minerals, including arsenides and tellurides. Due to their unique mineralogical traits, minerals such as chalcopyrite and chalcocite display differential flotation behaviors. The textural variability further complicates flotation, as fine-grained PGMs exhibit slower flotation kinetics than their coarser counterparts. This necessitates fine grinding, yet finer particles risk entrainment of hydrophilic gangue minerals, negatively impacting the flotation process efficiency.
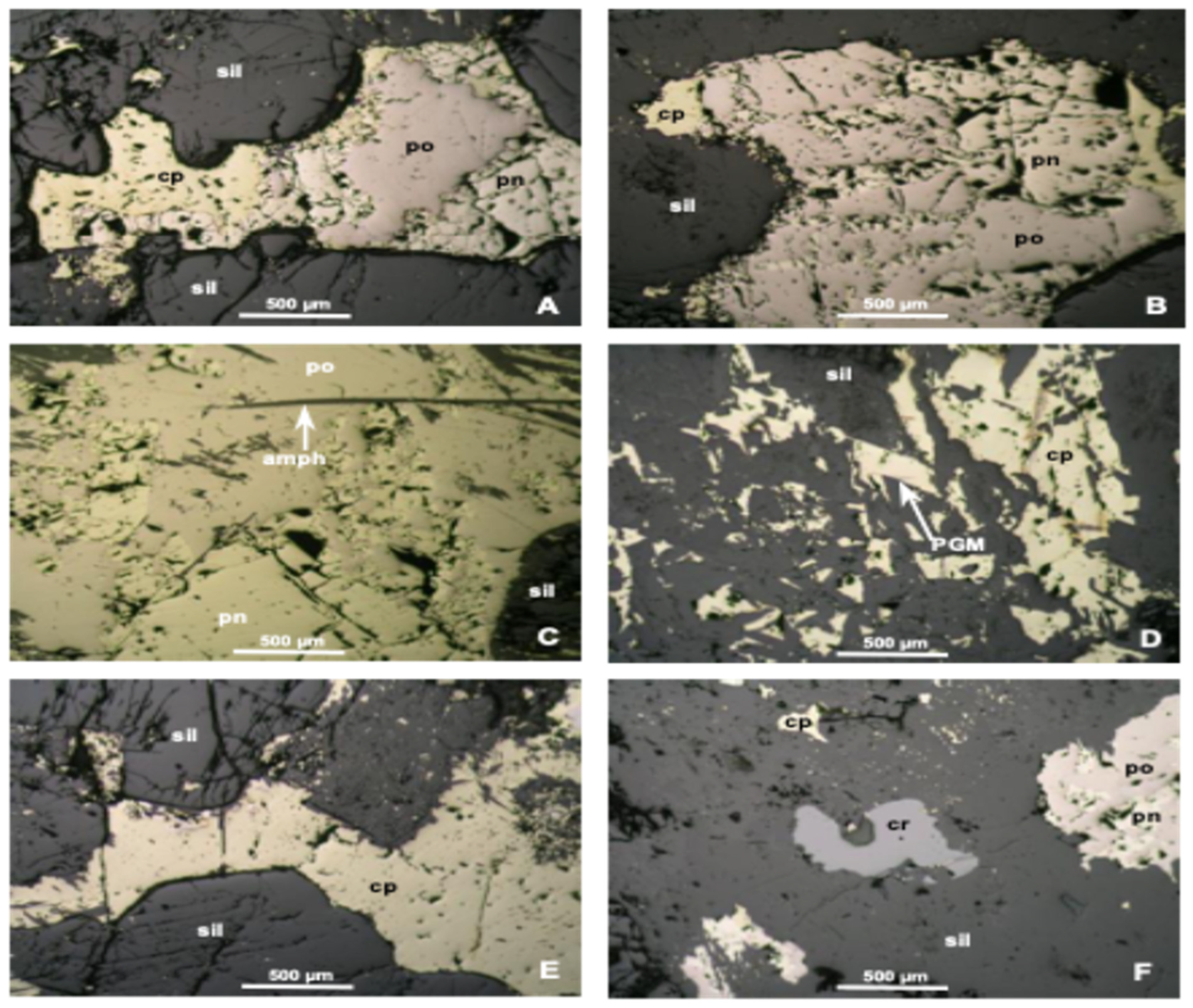
Mineralogical variability notably influences flotation kinetics, primarily through its impact on mineral liberation and association degree. PGMs that are closely associated with base metal sulfides (BMSs) tend to exhibit efficient flotation due to their hydrophobic nature [
1,
2]. Conversely, PGMs locked within gangue minerals or finely disseminated within silicates and oxides present significantly lower flotation recoveries [
1,
2].
Different PGMs, such as Pt, Pd, and additionally Au, exhibit distinct flotation behaviors, with Pt generally showing the fastest floatability, followed by Pd and Au [
3]. The observed PGM mineral species are michenerite, merenskyite, kotulskite (Pd-Bi-Te), maslovite, moncheite (Pt-Bi-Te), sperrylite (Pt-As), platarsite (Pt-As-S), hollingworthite (Rh-As-S), isoferroplatinum (Pt-Fe), stibiopalladinite, mertieite, sudburyite (Pd-Sb), gold (Au > 90 mass%) (Au-Ag). Previous studies, such as those by Doubra et al. [
3], confirm that flotation performance variability across PGMs such as Pt, Pd, and Au correlates strongly with their specific mineralogical associations, highlighting the criticality of understanding detailed mineralogical characteristics at the Mode of Occurrence (MOC) level.
Flotation remains the primary beneficiation method for recovering Platinum Group Metals (PGMs) from complex ores. Its efficiency, however, is governed not only by circuit configuration (e.g., rougher, scavenger, cleaner stages), reagent regimes, pulp density, and water chemistry, but also by the inherent mineralogical characteristics of the ore [
4,
5]. While operational parameters are commonly optimized to improve recovery, the integration of geometallurgical variables, such as mineral associations, grain size, liberation, and MOC, into flotation modelling remains limited.
Among kinetic models, the Kelsall model is widely used for characterizing flotation behavior due to its ability to distinguish between fast-floating and slow-floating mineral fractions. It has been applied extensively to sulfide ores, including copper, nickel, and PGMs, in studies seeking to model recovery curves and optimize flotation performance [
6,
7,
8]. For instance, Kelsall-based models have been used to evaluate circuit configuration impacts (rougher-cleaner loops), reagent dosage strategies (xanthates, dithiophosphates, frothers), and varying pulp densities (typically between 25%–40%) on kinetic responses [
9,
10]. These applications commonly use empirical flotation data under well-controlled experimental conditions but tend to treat the ore as a bulk entity without discriminating based on mineralogical textures [
11,
12].
Past studies highlight the shortcomings of purely empirical or bulk-parameter models when applied to geologically complex deposits. Bahrami et al. [
13] and O’Connor [
14] demonstrated that textural characteristics such as intergrowth, mineral associations, and liberation significantly influence flotation rates and recovery trajectories, effects that are obscured when kinetic parameters are derived solely from bulk assay data. More recent work by Tijsseling [
15] and Bahrami et al. [
13] demonstrates that textural characteristics, such as liberation, association, and Mode of Occurrence, significantly influence flotation recovery trends, outcomes that are often overlooked when kinetic parameters are fitted using bulk assay data alone. Despite these insights, flotation models remain largely empirical in PGM systems, with limited incorporation of mineralogical constraints into parameter estimation frameworks. Despite this, few models incorporate these mineralogical constraints into parameter estimation, particularly in PGM systems where discrete particle tracking and textural classification remain underutilized.
This study addresses this gap by presenting a mineralogically informed adaptation of the Kelsall model, linking kinetic parameters directly to mineralogical classes defined by MOC and liberation. Flotation tests were performed on Platreef ore under standardized rougher flotation conditions (35% solids, pH~9, SIBX 120 g/t, Sendep 30E 300 g/t, and Senfroth 522 80 g/t) to isolate the effect of ore mineralogy. The model is calibrated using Particle Swarm Optimization (PSO), enabling robust kinetic parameter estimation across multiple lithologies and textures. This work aims to demonstrate that integrating mineralogical attributes into flotation models not only improves recovery prediction accuracy but also enhances their applicability in geometallurgical frameworks, circuit design, and strategic mine planning.
Traditionally, flotation kinetics have been modelled using several approaches, primarily based on first-order kinetics, rate constant distribution models, and graphical and computational methods. The Kelsall model is a well-known example that incorporates both fast- and slow-floating mineral fractions, each with its own rate constant [
16,
17]. Equation (1) is the unmodified Kelsall model with the basic assumption that the flotation process follows first-order kinetics, with the basic assumption that the mechanism of bubble–particle attachment recovers minerals and the recovery rate is proportional to the concentration of floatable particles. The Kelsall model incorporates fast- and slow-floating components that are the most widely used to describe the flotation kinetics.
where:
R is the maximum recovery at time, t
Kf is the fast-flotation rate
Ks is the slow-flotation rate
θ is the fraction of the slow-floating component
The unmodified Kelsall model constraints are given in Equations (2)–(4). Equation (2) represents the fast flotation rate constant constraints, a critical parameter that controls several aspects, such as recovery rate, cell design configuration, reagent dosage, and process control. This also includes the control of the liberated and locked particles of valuable minerals, respectively. Equation (3) represents the constraints of the slow flotation rate constant, which controls the extended residence time and influences the larger cell configuration, type, and reagent amount. Equation (4) represents the slow-floating fraction constraints. It represents a non-binding constraint that allows the slow fraction to satisfy a set of minimum and maximum values. Equation (5) represents the fast–slow flotation fraction constraints, while Equation (6) represents the non-negativity constraints.
However, the current models have limitations, primarily due to their inability to comprehensively integrate the complex mineralogical characteristics at the level of liberation classes based on the MOC for complex ore bodies. They simplify assumptions and assume that the rate constant for both fast-floating and slow-floating particles remains constant throughout the flotation process. They do not account for particle entrainment, which can occur due to water turbulence. Previous studies, such as Alvarez-Silva et al. [
18], compared kinetic models using relatively simple mineral systems (e.g., pyrite and calcite). While it provides a good benchmark for model performance, it lacks validation against ores with heterogeneous mineralogy, intergrowths, or textural complexity. Such variability can lead to deviations in flotation behavior from model predictions, particularly when mineral associations influence surface chemistry or bubble–particle interactions. Bu et al. [
16] explored a range of kinetic models and rate constant distributions; their work is largely based on batch flotation tests conducted under controlled conditions. These models often assume homogeneity in particle behavior, which does not hold in complex ores where liberation varies significantly across size fractions and mineral associations. Consequently, their models may not accurately capture the behavior of complex orebodies with variable liberation characteristics, leading to an over- or underestimation of recovery and rate constants. Vinnett et al. [
19] introduce a useful method for analyzing flotation rate distributions across size classes; it does not explicitly integrate liberation data or mineralogical associations into the analysis. In complex ores, particle size alone is not a sufficient predictor of flotation behavior—liberation degree and mineral locking play a critical role. Without incorporating these factors, the model may misinterpret erratic performance as size-related rather than liberation-driven.
This leads to inadequate predictions of flotation kinetics for complex ore bodies exhibiting variability in the liberation classes.
The modified Kelsall model has demonstrated potential in capturing differential flotation kinetics by explicitly accounting for fast and slow flotation fractions of minerals. Previous research, such as Doubra et al., has noted its effectiveness [
3]. Additional studies have reinforced its utility in modeling complex flotation systems, particularly in the recovery of Platinum Group Metals (PGMs). For instance, Chipise et al. applied the five-parameter modified Kelsall model to scavenger bank flotation data in a PGM concentrator and found that it provided the best fit for describing the kinetics of slow-floating mineral species [
20]. Similarly, Ramlall and Loveday compared several kinetic models for UG2 platinum ore and concluded that the modified Kelsall model, especially when combined with entrainment modeling, offered superior predictive accuracy for both valuable and gangue minerals [
21]. Furthermore, research on Platreef ore flotation has shown that the model can be used to develop empirical correlations for Pt, Pd, and Au recoveries, enhancing process optimization and control [
20] and other studies examining PGM recovery. Despite this, a significant gap remains: the model’s limited capability to fully integrate detailed mineralogical attributes such as the MOC classifications, particularly for PGMs that do not precisely fall into the fast or slow flotation categories.
Advancements in flotation prediction increasingly emphasize integrating detailed mineralogical data, including liberation characteristics, mineral associations, and grain size distributions. Recent research, such as Vinnett et al. [
19], highlights the importance of explicitly incorporating MOC classifications into kinetic models to enhance predictive accuracy. Previous studies have utilized SEM and Microsoft spreadsheets using Visual Basic Macros (VBMs) to classify each PGM bearing particle into one of the six predefined MOCs, as demonstrated in
Table 1 [
22,
23].
The Positive Matrix Factorization (PMF) approach and machine learning techniques have shown promise. Yet, these methods often still rely on simplified mineralogical categorizations, not fully resolving the complexities inherent in Platreef-type mineralogical variability.
Optimization techniques, particularly Particle Swarm Optimization (PSO), hold significant promise for addressing the inherent complexities associated with flotation processes. In this study, the PSO was employed to calibrate flotation kinetic parameters. PSO is a population-based, metaheuristic optimization algorithm inspired by the collective social behavior observed in bird flocks and fish schools [
24]. The method uses a set of candidate solutions called “particles” that explore the multidimensional parameter space by adjusting their positions based on their own experience and that of neighboring particles. Through iterative updates, PSO effectively balances exploration and exploitation to identify optimal or near-optimal solutions, making it well-suited for complex, nonlinear problems such as flotation kinetics parameter estimation. Its advantages include simplicity, computational efficiency, and a reduced tendency to become trapped in local minima compared to traditional gradient-based methods.
The capability of PSO to manage nonlinear and multidimensional optimization challenges has been extensively demonstrated, notably through its application in optimizing flotation parameters to enhance recoveries and concentrate grades [
25]. Recent research further supports this, highlighting PSO’s efficiency in predictive modeling and optimization in flotation circuits [
26,
27,
28]. Furthermore, the integration of machine learning with traditional flotation models has proven beneficial, enabling improved prediction accuracy and deeper understanding of flotation kinetics, particularly in ores exhibiting complex mineralogical characteristics [
29,
30]. Thus, PSO has emerged as a method suitable for predicting flotation kinetics, offering significant operational advantages for processing intricate mineralogical compositions.
Integrating flotation kinetics into the early stages of plant design is crucial for optimizing the flotation process and ensuring efficient mineral recovery and the scale-up of laboratory results to full-scale plant operation. Flotation kinetics, which describes the rate at which particles are recovered in the flotation process, is influenced by various factors such as particle size, bubble–particle interactions, liberation, and the hydrodynamic conditions within the flotation cell. By incorporating these kinetics into the design phase, engineers can make informed decisions about the configuration and operation of flotation circuits, ultimately enhancing the plant’s performance and economic viability.
Moreover, insights from studies such as those by Dunne et al. [
31,
32] and Ramlall et al. [
33] demonstrate the necessity of tailoring flotation circuit designs to each orebody’s specific mineralogical and metallurgical characteristics. They also demonstrate that no one-size-fits-all approach exists for flotation plant design, especially when processing complex ore bodies. While substantial progress has been made, a notable gap remains in the literature regarding the dynamic interplay between mineralogical variability, flotation kinetics, and real-time operational parameters. Existing studies often fall short in simulating the complex effects of mineralogy, particularly MOC and liberation class, on flotation kinetics under variable plant conditions.
Given these gaps and challenges, this study seeks to comprehensively understand how detailed mineralogical characteristics influence flotation kinetics in complex PGM orebodies. By integrating precise mineralogical data into the modified Kelsall kinetic model and employing PSO for optimization, the research intends to significantly enhance the prediction accuracy and optimization efficiency of flotation processes for Platreef ores.
This study aims to do the following:
Assess the challenges posed by mineralogical variability in PGM flotation;
Evaluate the influence of mineralogical attributes on the flotation performance of PGM metals;
Integrating mineralogical attributes into the flotation kinetics model;
Predict the kinetics indices of PGM metals using the Modified Kelsall model.
4. Discussion
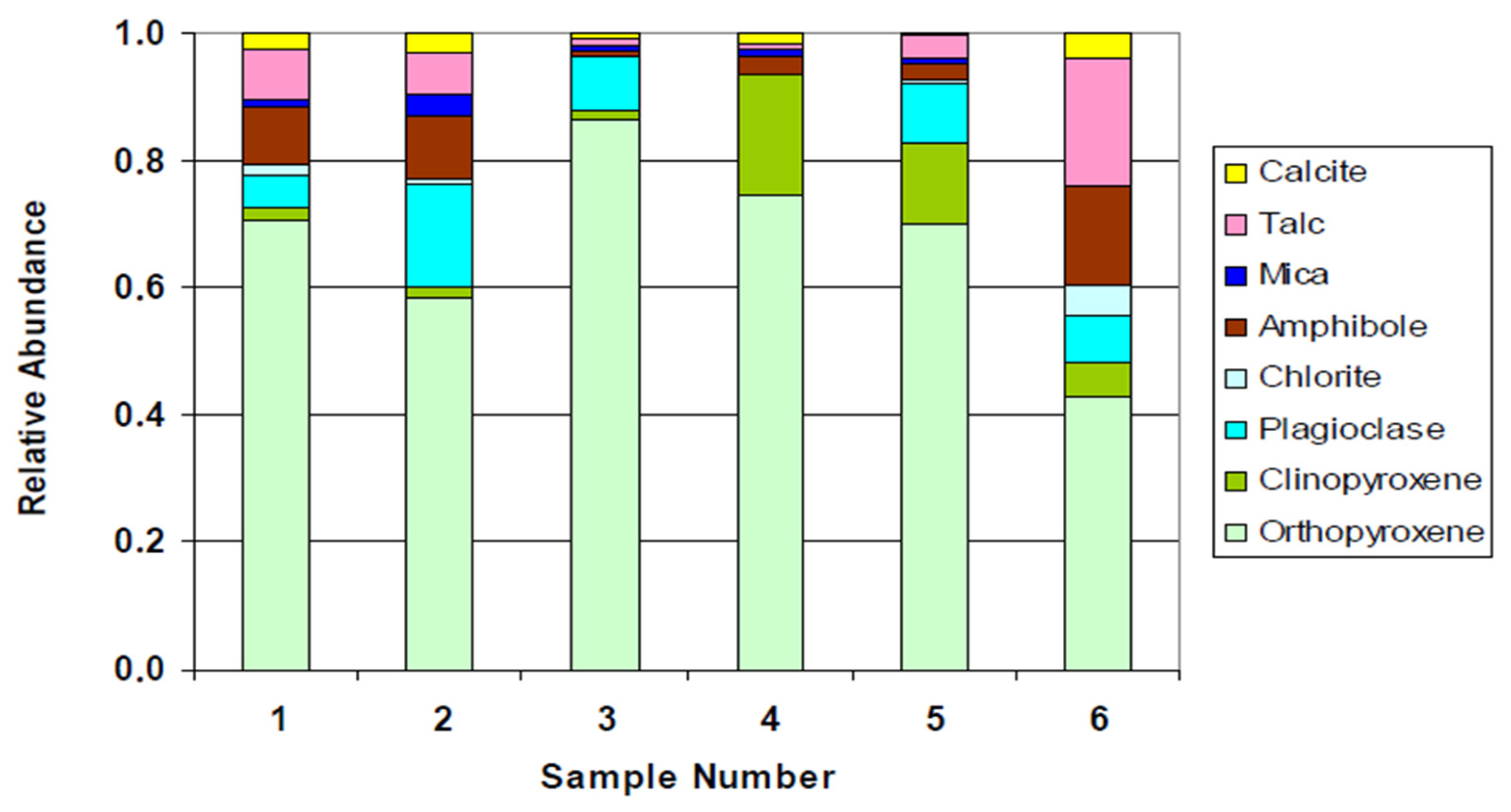
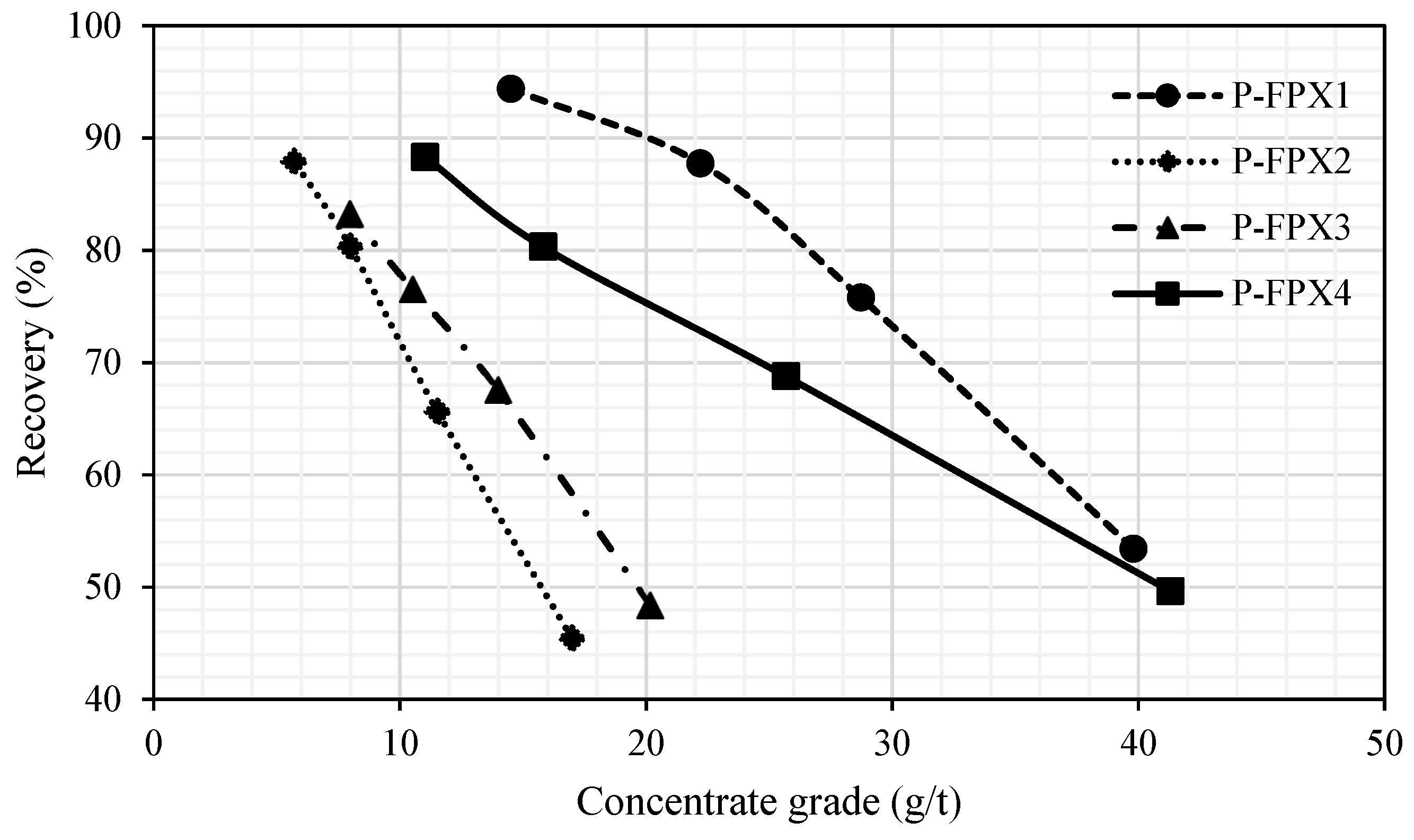
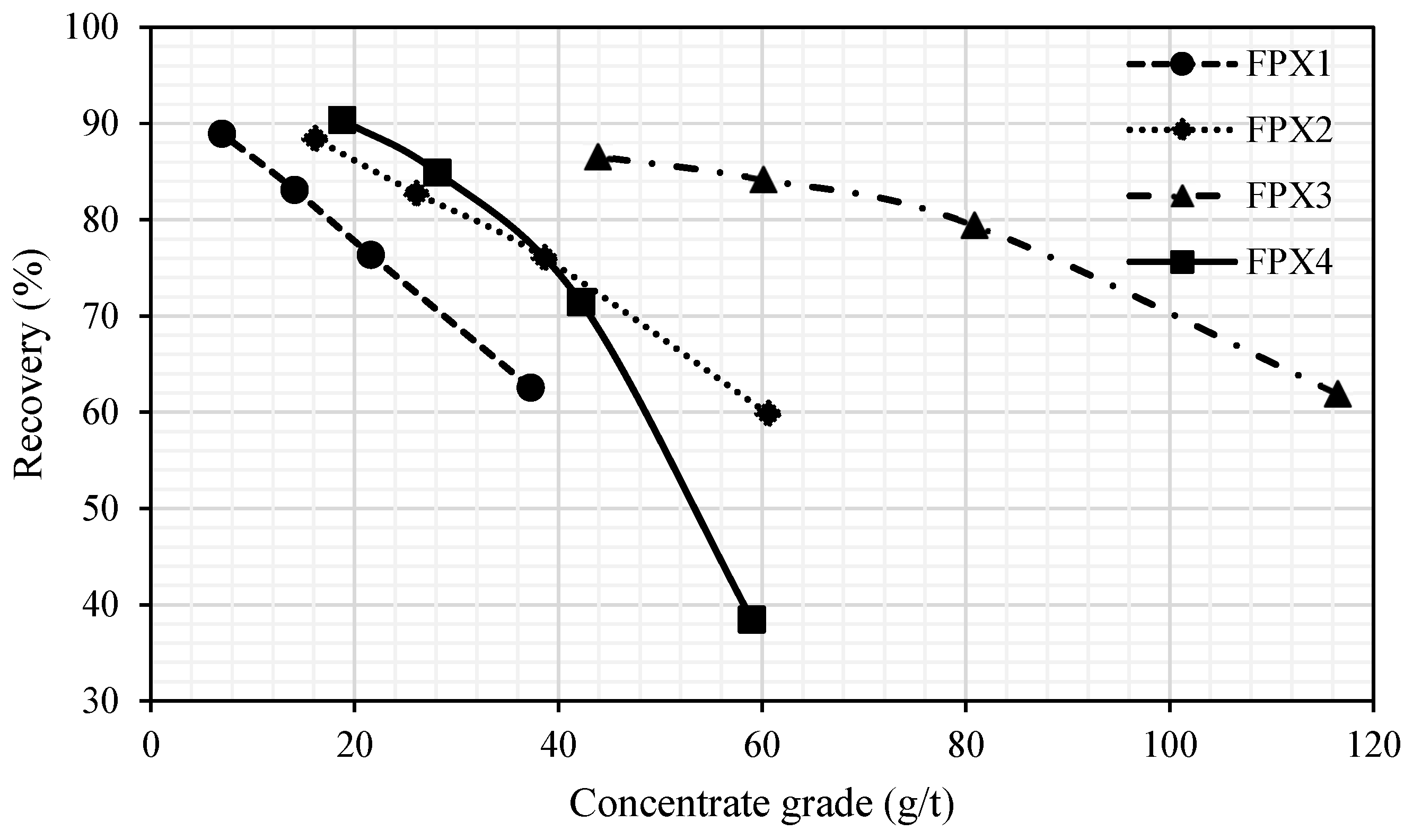
This study reveals substantial mineralogical variability between P-FPX and FPX lithologies and their direct influence on flotation performance. FPX samples consistently outperformed P-FPX in terms of cumulative recovery (e.g., FPX4 = 90.35%) and concentrate grade (FPX3 = 116.5 g/t at 1 min), attributable to higher proportions of liberated PGMs (up to 90.6% in FPX1) and simpler mineral associations. In contrast, P-FPX samples, particularly P-FPX2 and P-FPX3, contained a greater abundance of PGMs in locked or complex modes of occurrence (e.g., SAG, G classes), which showed suppressed flotation kinetics (Kf < 0.09 min−1) and anomalous slow-floating behavior (Ks up to 8.67 min−1).
Grain size distribution also contributed to flotation outcomes, with over 85% of PGMs across all samples being >3 μm, which is generally considered favorable for flotation [
23]. Nonetheless, fine PGMs locked in silicates continued to pose recovery challenges, confirming that mineral association exerts a stronger control over floatability than grain size alone.
The variations in flotation kinetics observed between FPX and P-FPX lithologies can be partially explained by phenomenological factors rooted in mineral surface properties. Sulfide minerals, especially liberated base metal sulfides such as pentlandite and chalcopyrite, exhibit inherent hydrophobicity under typical flotation conditions due to their favorable electrochemical surface characteristics [
24]. This contributes to their rapid attachment to air bubbles, yielding higher fast-floating fractions (∅
f) and elevated
Kf values in FPX samples. In contrast, PGMs locked within silicate or oxide gangue phases, as commonly seen in P-FPX samples, exhibit significantly lower surface hydrophobicity, often requiring prolonged flotation time or surface activation for recovery [
42]. Furthermore, the presence of surface coatings, such as iron oxides or slimes, can passivate particle surfaces and hinder collector adsorption, as noted in previous studies on Bushveld ores [
43,
44,
45]. These textural and mineral-chemical complexities provide a mechanistic basis for the suppressed kinetics observed in P-FPX samples and reinforce the need for tailored reagent regimes and conditioning protocols.
These findings align with prior work by Corin et al. [
46], who demonstrated that mineral liberation and PGM association with sulfides are primary drivers of flotation efficiency in Bushveld-type ores. Similarly, (a) Becker et al. [
11] and O’Connor et al. [
12] noted that complex gangue interactions such as those with talc, feldspar, and amphibole can dilute concentrate grade and hinder bubble–particle attachment, as reflected here by the lower recovery observed in P-FPX3 (83.25%).
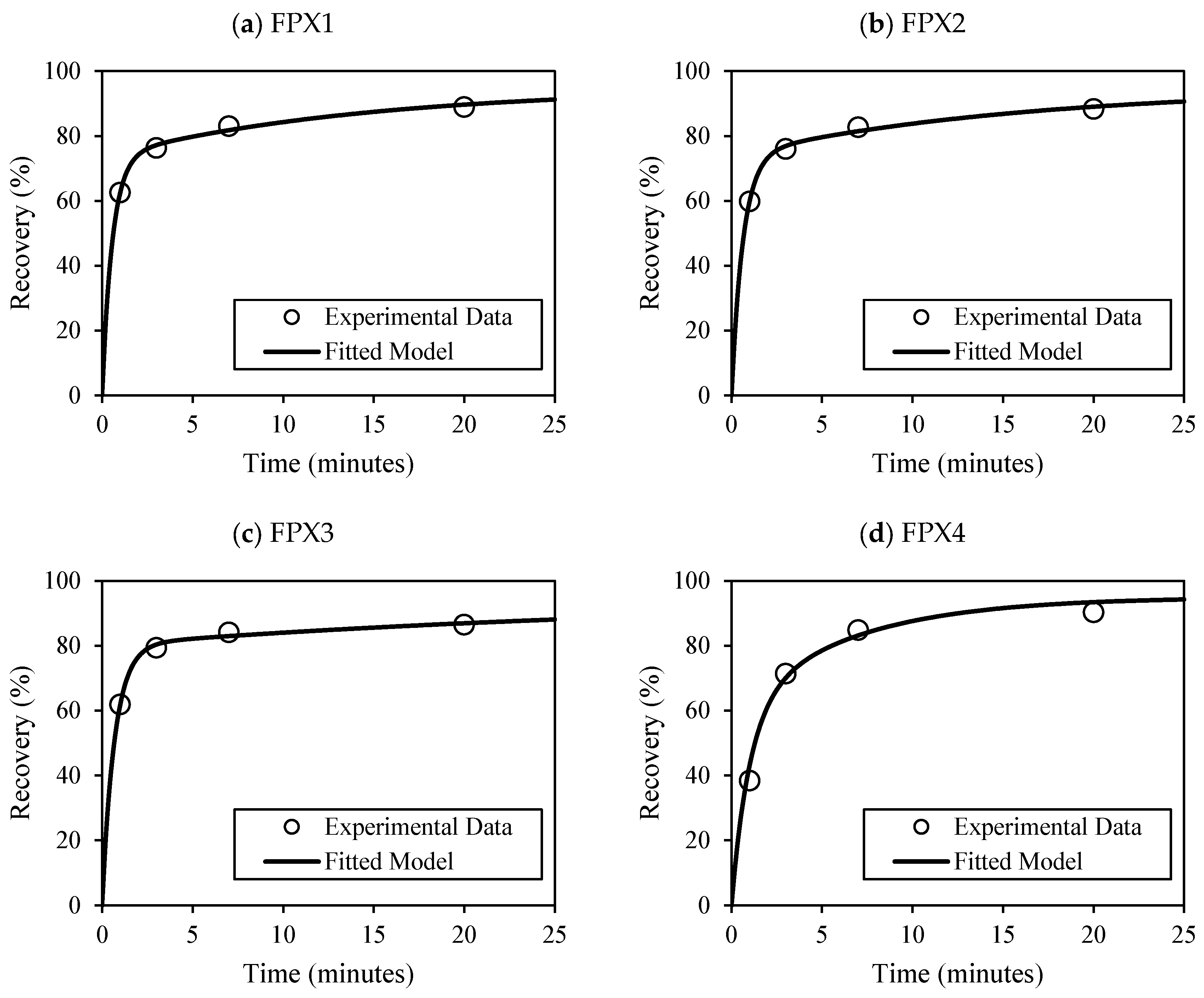
The modified Kelsall model, enhanced with MOC classes and optimized using PSO, successfully modeled dual-component flotation kinetics, achieving R
2 values above 0.97 across all samples. Fast-floating fractions (∅
f = 0.77–0.84) and high
Kf values (1.45–1.78 min
−1) were linked to L and SL MOC classes, corroborating earlier kinetic modeling studies by Ross [
6] and Hay and Rule [
7]. Conversely, samples dominated by G or SAG classes required longer flotation times and were associated with higher model error (e.g., P-FPX4 = 57.3), indicating the need for improved classification or advanced machine learning techniques to better capture complex textural effects [
47,
48].
Several flotation kinetics parameters derived in this study exhibited characteristics indicative of mathematical artefacts, which diminish their physical interpretability. This phenomenon can be attributed primarily to the intrinsic complexity and mineralogical variability of the ore, where multiple MOCs with diverse liberation and flotation behaviors complicate parameter estimation. Moreover, measurement noise and variability inherent in flotation test data contribute to parameter uncertainty, often leading the optimization routine to fit noise rather than true kinetic behavior.
From a practical plant perspective, the kinetic parameters derived in this study offer critical insight for tailoring flotation circuits to lithological domains. For instance, the significantly faster flotation kinetics and high fast-floating fractions observed in FPX samples (e.g., ∅f = 0.77–0.84; Kf = 1.45–1.78 min−1) suggest that these ores can be effectively treated using standard residence times and conventional reagent regimes. In contrast, P-FPX ores, characterized by slower kinetics and higher proportions of locked PGMs, may require circuit modifications such as increased flotation residence time, staged regrinding, or customized reagents targeting gangue depression and selective collector adsorption.
An important but often overlooked variable is water quality. In this study, tap water was used throughout flotation testing to reflect site-relevant operational conditions. While this introduces a realistic context, it also adds uncontrolled variability in parameters such as ionic strength, pH buffering, and hardness, all of which can influence collector adsorption, frother performance, and gangue depression [
46,
49]. Although no major inconsistencies were observed in replicate tests, future work should include water chemistry profiling and controlled experiments to isolate and quantify these effects for more robust kinetic modeling and improved plant predictability.
Importantly, the study demonstrates that lithology-specific flotation strategies are essential: P-FPX ores, due to their complex associations, may benefit from finer grinding and targeted reagents, while FPX ores can be efficiently processed using standard circuits. This geometallurgical insight supports the integration of mineralogical attributes into flotation kinetics for improved recovery prediction, process design, and dynamic plant control, particularly at the DFS stage.
In practice, flotation plant operators and metallurgists rarely have access to detailed mineralogical models in real time. However, this research emphasizes the strategic value of integrating mineralogical inputs at the design and geometallurgical modeling stages, especially when defining spatial domains, blending strategies, and flotation circuit configuration. For example, predicting the floatability index based on MOC distributions can support ore classification during mine planning or short-term stockpile management. In feasibility studies, the kinetic parameters derived can feed into dynamic simulation platforms like JKSimFloat or Outotec HSC Sim, enabling predictive evaluations of throughput, concentrate grade, and recovery under varying feed scenarios.
Overall, the integration of comprehensive mineralogical characterization, systematic flotation testing, and rigorous kinetic modeling provides a robust and quantitative framework to inform circuit design and optimize flotation performance across mineralogically diverse ore types. By directly correlating key mineralogical parameters, such as liberation size distributions, mineral associations, and modes of occurrence, with flotation kinetics and recovery metrics, this approach enables the development of predictive models that more accurately capture the complexity and variability of real-world ores. This methodology aligns with the principles of modern geometallurgy, as emphasized by Mwanga et al. [
50] and Lois-Morales et al. [
51] supports improved process design, control strategies, and long-term operational efficiency in the context of resource variability.
Such integration supports the rational design and optimization of flotation circuits by enabling targeted adjustments in grinding, reagent schemes, and operational conditions tailored to specific lithological domains. The use of advanced optimization algorithms, including PSO, further refines kinetic parameter estimation, enhancing model robustness and predictive capability.
This framework is consistent with contemporary geometallurgical paradigms, which advocate for the synergistic coupling of geological, mineralogical, and metallurgical datasets to drive data-informed process design and control. The application of this integrated methodology at the feasibility and design stages offers improved confidence in plant performance forecasting, facilitates dynamic circuit adjustments to accommodate ore variability, and ultimately contributes to enhanced recovery efficiency and concentrate quality.
The results presented demonstrate the necessity of incorporating mineralogical complexity into flotation kinetic models to capture the effects of heterogeneous mineral associations and surface properties. This integrated geometallurgical approach has broad applicability for optimizing flotation performance in a range of polymetallic and complex sulfide ore systems, thereby advancing process understanding and supporting more effective mineral processing operations.









 PGM
PGM  BMS
BMS  GANGUE
GANGUE






