Enrichment Regularity of Indium in the Dulong Mineral Processing Plant, Yunnan Province, China
Abstract
1. Introduction
| Mine | Vein Type | Carrier Minerals | Grade/ppm | Reserves/t | References |
|---|---|---|---|---|---|
| Inner Mongolia, dajing | tin-polymetallic veins | copper and zinc sulfides and sulfosalts | 0.5–296 | 768 | [18,19] |
| Inner Mongolia, Meng’entaolegai | quartz-sulfide veins | sphalerite | 9–295 | >500 | [20] |
| Guangxi dachang | Volcanic polymetallic massive sulfides | Marmatite | 0.2–673 | >5000 | [21] |
| Yunnan dulong | Sn-Zn polymetallic ore | Marmatite and chalcopyrite | 0.74–4572 | 7000 | [8] |
| Yunnan gejiu | Zn-enriched sulfide ores | Sphalerite and chalcopyrite | 198.3–1570 | >4000 | [15] |
| Hunan qibaoshan | quartz-sulfide ores (a Sn-Poor Skarn Deposit) | sphalerite-pyrite ores | 28.9–203 | 585 | [22] |
| Hunan yejiwei | skarn-type Cu–Sn ores | sphalerite, chalcopyrite, and stannite | 2.3–214 | >806 | [23] |
| Fujian zhongjia | polymetallic sulfide-magnetite ore | sphalerite, Galena and Magnetite | 22–1346 | 569 | [24] |

2. Materials and Methods
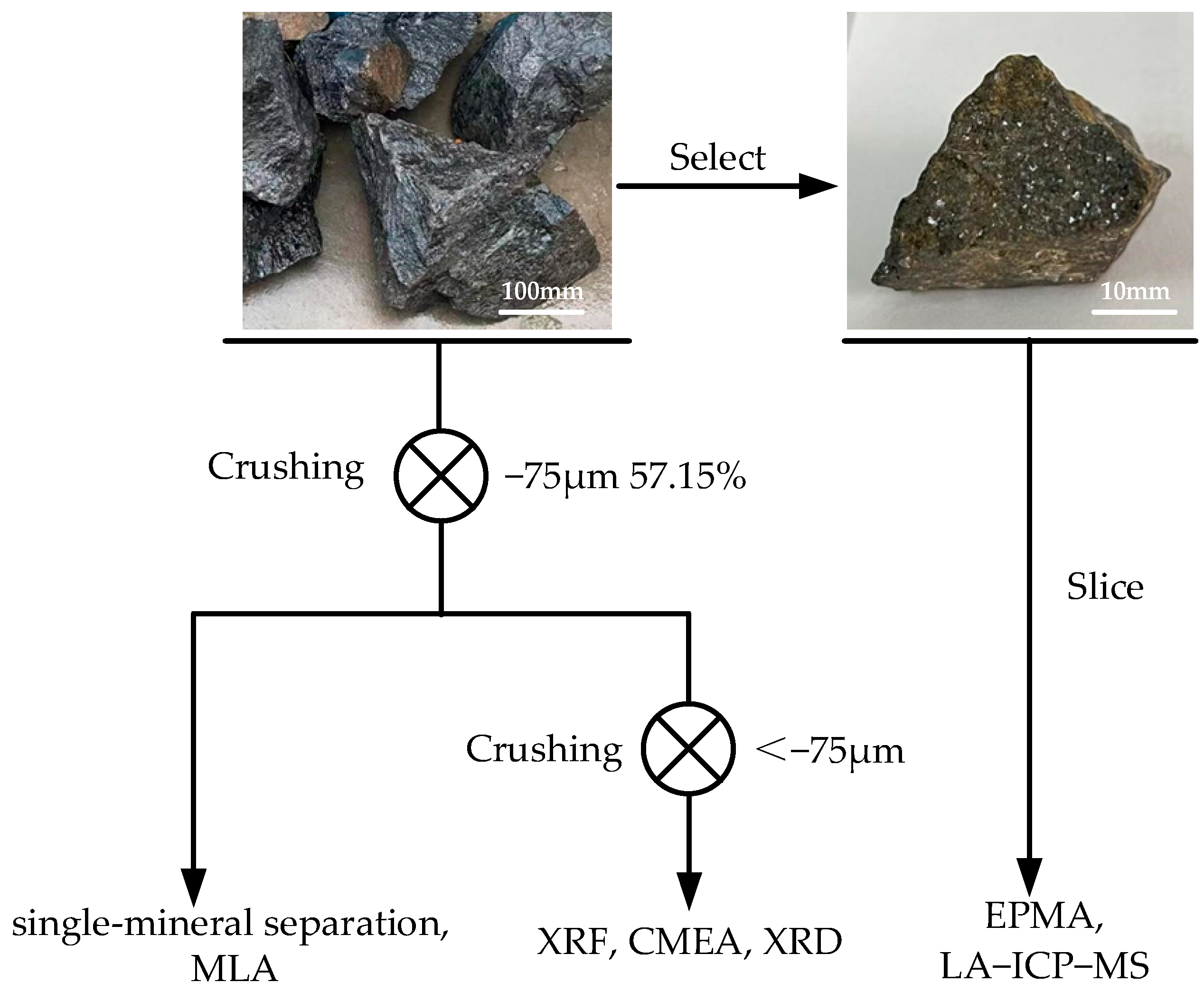
2.1. Sample Handling and Experimental Procedures
2.2. Analytical Methods
2.2.1. Chemical Multi-Element Analysis
2.2.2. Mineral Characterization
2.2.3. Electron Probe Microanalysis
2.2.4. Laser Ablation Inductively Coupled Plasma Mass Spectrometry
3. Results and Discussion
3.1. Elemental and Mineralogical Analysis
3.1.1. Ore Major Element Composition
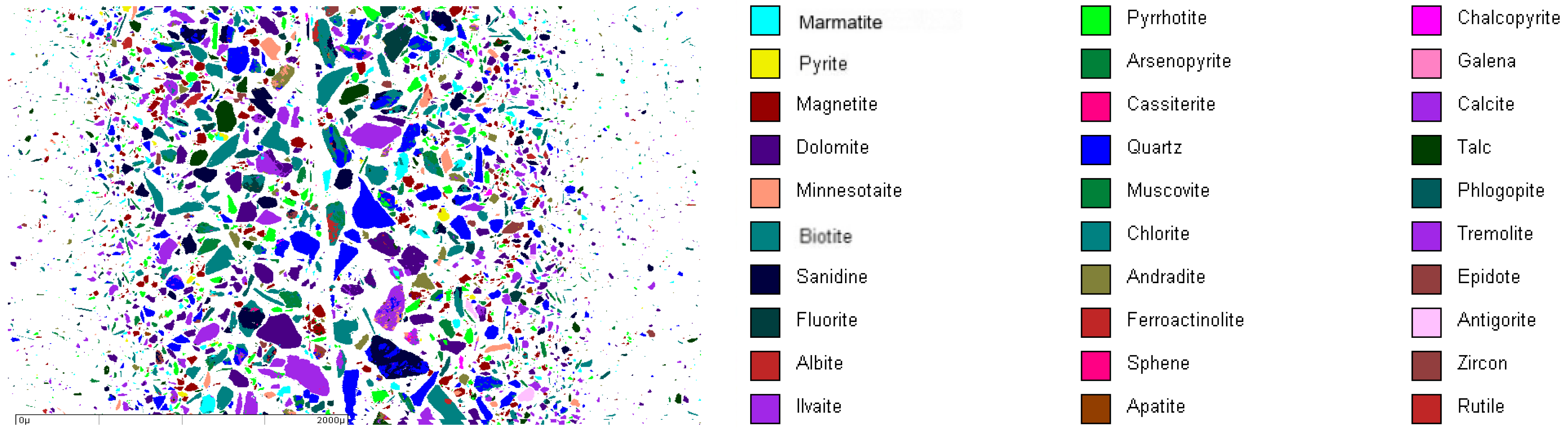
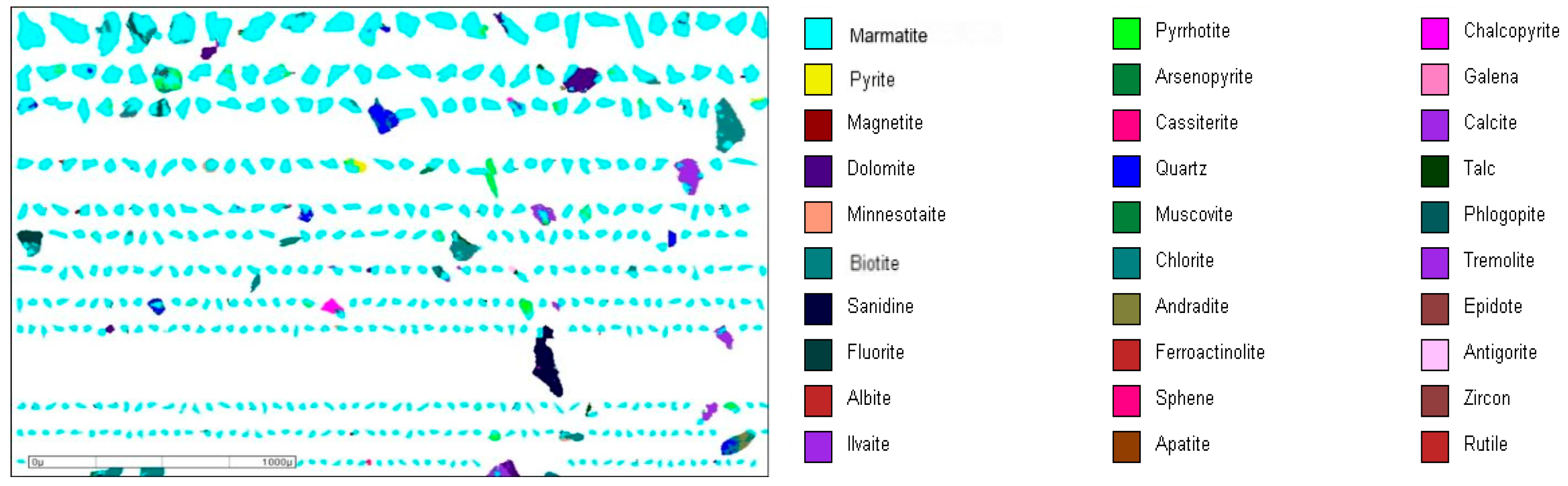
3.1.2. Ore Mineral Composition
3.1.3. Grain Size Distribution of Major Minerals
3.2. Content and Distribution of Indium in Carrier Minerals
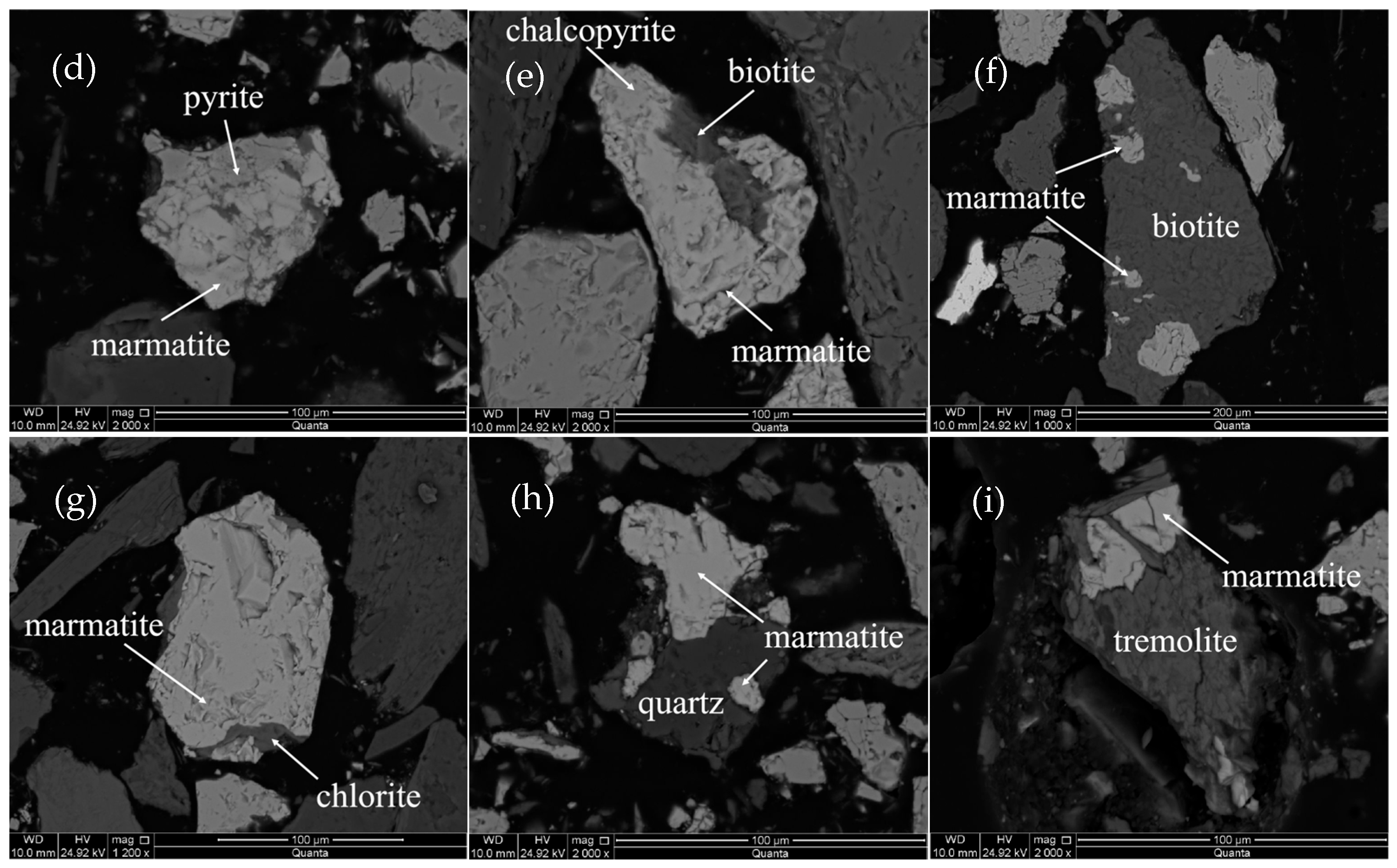
3.3. Textural Characteristics and Substitution Mechanism of Indium-Bearing Marmatite
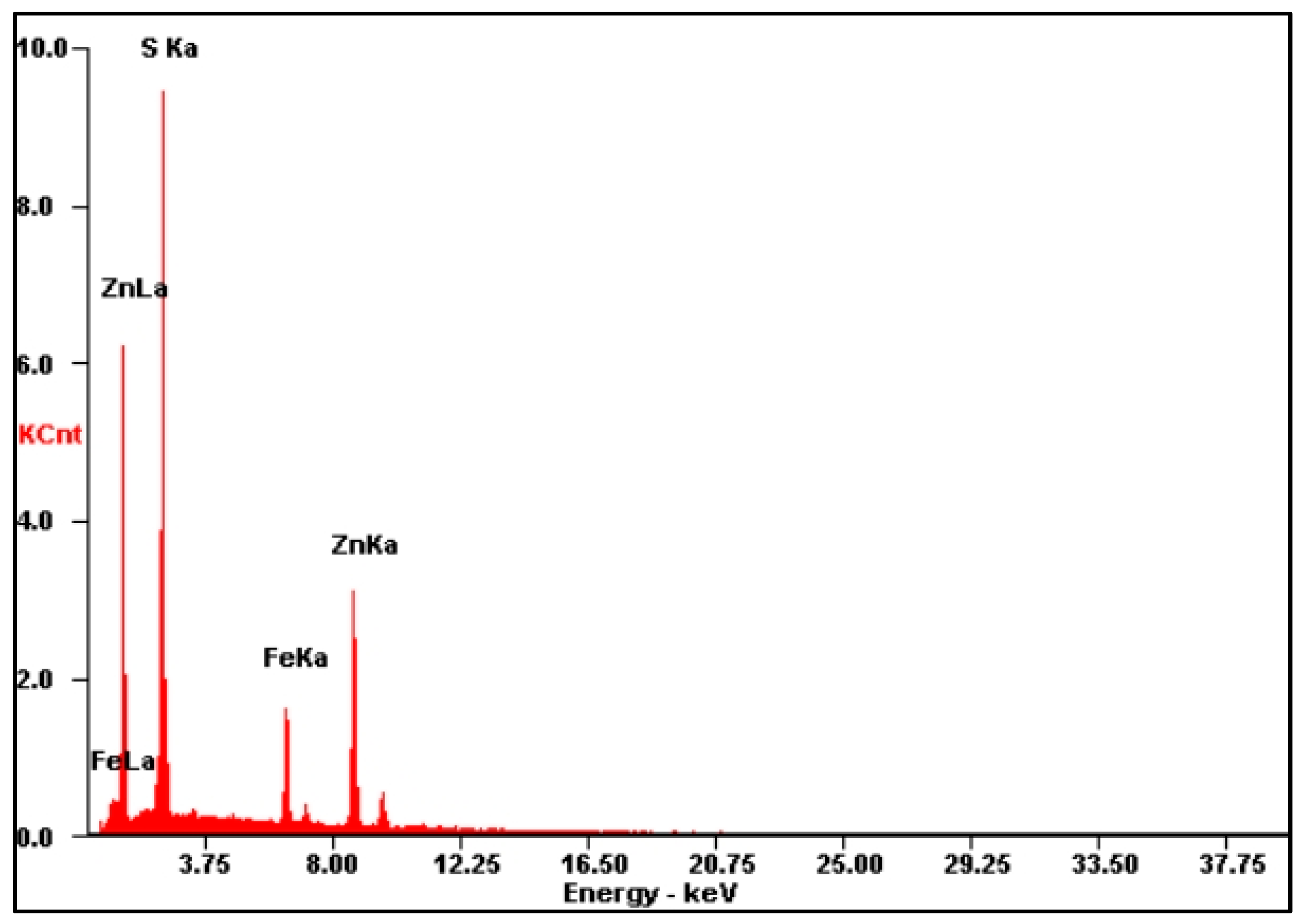
3.3.1. Textural Characteristics of Indium-Bearing Marmatite
3.3.2. Substitution Mechanism of Indium in Marmatite
3.4. Enrichment Regularity of Indium in the Dulong Mineral Processing Plant
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, J.; Li, X.; Luo, F.; Liu, W.; Tang, X.; Wang, M.; Li, Y. Security evaluation of China’s indium industrial chain: Perspective on substance flow throughout the whole life cycle. Sustain. Prod. Consum. 2024, 47, 557–569. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; Fang, L.; Zhao, H.; Li, D.; Lu, Z.; Zhou, S. Influence of post-grown treatments on CuInS2 thin films prepared by sulphurization of Cu-In films. Rare Met. 2008, 27, 490–495. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, Y.; Wang, W.; Li, B.; Zhang, Y.; Zuo, T. Recycling indium from waste LCDs: A review. Resour. Conserv. Recycl. 2015, 104, 276–290. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Chen, W.; Wang, M. Mapping the upstream journey of China’s indium: A trade-linked substance flow analysis. J. Clean. Prod. 2022, 380, 135051. [Google Scholar] [CrossRef]
- Zhao, P.; Zajacz, Z.; Grondahl, C.; Tsay, A.; Mao, J.; Cheng, Q.; Yuan, S. Indium partitioning between silicate melts and magmatic fluids: Implications for indium ore genesis and the tracing of magma degassing. Geochim. Cosmochim. Acta 2024, 374, 146–155. [Google Scholar] [CrossRef]
- Song, H.; Wang, C.; Sun, K.; Geng, H.; Zuo, L. Material efficiency strategies across the industrial chain to secure indium availability for global carbon neutrality. Resour. Policy 2023, 85, 103895. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–51. [Google Scholar]
- Zheng, Y.; Guo, C.; Wang, D.; Zhao, T.; Wang, Y. Temporal and spatial distribution, genetic type, and metallogenic mechanism of indium (gallium, germanium, cadmium) associated deposits in China: Overview and expectation. Acta Geol. Sin. 2023, 97, 3569–3603. [Google Scholar]
- Murakami, H.; Ishihara, S. Trace elements of Indium-bearing sphalerite from tin-polymetallic deposits in Bolivia, China and Japan: A femto-second LA-ICPMS study. Ore Geol. Rev. 2013, 53, 223–243. [Google Scholar] [CrossRef]
- Ivashchenko, V. Geology, geochemistry and mineralogy of indium resources at Pitkäranta Mining District, Ladoga Karelia, Russia. J. Geochem. Explor. 2022, 240, 107046. [Google Scholar] [CrossRef]
- Tong, X.; Song, S.; He, J.; Lopez-Valdivieso, A. Flotation of indium-beard marmatite from multi-metallic ore. Rare Met. 2008, 27, 107–111. [Google Scholar] [CrossRef]
- Xu, L.; Chen, G.; Zhang, X.; Yang, Y.; Leng, C.; Yang, C.; Tian, Y.; Zhao, Z. Waste ITO target recycling for efficient indium recovery through a closed-loop process. J. Environ. Chem. Eng. 2024, 12, 112136. [Google Scholar] [CrossRef]
- Lin, S.; Mao, J.; Chen, W.; Shi, L. Indium in mainland China: Insights into use, trade, and efficiency from the substance flow analysis. Resour. Conserv. Recycl. 2019, 149, 312–321. [Google Scholar] [CrossRef]
- Zhou, Y.; Rechberger, H.; Li, J.; Li, Q.; Wang, G.; Chen, S. Dynamic analysis of indium flows and stocks in China: 2000–2018. Resour. Conserv. Recycl. 2021, 167, 105394. [Google Scholar] [CrossRef]
- Li, Y.; Tao, Y.; Zhu, F.; Liao, M.; Xiong, F.; Deng, X. Distribution and existing state of indium in the Gejiu Tin polymetallic deposit, Yunnan Province, SW China. Chin. J. Geochem. 2015, 34, 469–483. [Google Scholar] [CrossRef]
- Benites, D.; Torró, L.; Vallance, J.; Laurent, O.; Valverde, P.E.; Kouzmanov, K.; Chelle-Michou, C.; Fontboté, L. Distribution of indium, germanium, gallium and other minor and trace elements in polymetallic ores from a porphyry system: The Morococha district, Peru. Ore Geol. Rev. 2021, 136, 104236. [Google Scholar] [CrossRef]
- Gao, L.S.; Wen, H.J.; Zhu, C.W.; Nie, X.; Chen, A.B.; Yang, G.S. Study on the occurrence state of indium in sphalerite of Dulong Sn-Zn-In polymetallic deposit, Southwest China. Acta Geochim. 2023, 42, 572–582. [Google Scholar] [CrossRef]
- Ishihara, S.; Qin, K.Z.; Wang, Y.W. Resource evaluation of indium in the Dajing tin-polymetallic deposits, Inner Mongolia, China. Resour. Geol. 2008, 58, 72–79. [Google Scholar] [CrossRef]
- Ye, Z.; Xu, J.; Zhao, J.F.; Chen, S.Y.; Chen, J.W.; Liu, W.Y. Mineralogy of indium mineralization in the Dajing Sn-Cu polymetallic deposit in Inner Mongolia. Ore Geol. Rev. 2023, 159, 105564. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, X.Q.; He, Y.H.; Jiang, J.J.; Wang, D.P. Indium enrichment in the Meng’entaolegai Ag-Pb-Zn deposit, Inner Mongolia, China. Resour. Geol. 2006, 56, 337–346. [Google Scholar] [CrossRef]
- Cheng, Y.S. Occurrence characteristics and enrichment regularity of indium in pyrite: A case study of Dachang tin ore-field. Trans. Nonferrous Met. Soc. China 2016, 26, 2197–2208. [Google Scholar] [CrossRef]
- Liu, J.P. Indium Mineralization in a Sn-Poor Skarn Deposit: A Case Study of the Qibaoshan Deposit, South China. Minerals 2017, 7, 76. [Google Scholar] [CrossRef]
- Liu, J.P.; Rong, Y.A.; Gu, X.P.; Shao, Y.J.; Lai, J.Q.; Chen, W.K. Indium Mineralization in the Yejiwei Sn-Polymetallic Deposit of the Shizhuyuan Orefield, Southern Hunan, China. Resour. Geol. 2018, 68, 22–36. [Google Scholar] [CrossRef]
- Mao, G.; Zhu, Y. The occurrence and comprehensive recovery assessment of In and Cd in the Zhongjia iron deposit, Fujian. Contrib. Geol. Miner. Resour. Res. 2013, 28, 58–64. [Google Scholar]
- Xu, B.; Jiang, S.-Y.; Wang, R.; Ma, L.; Zhao, K.-d.; Yan, X. Late Cretaceous granites from the giant Dulong Sn-polymetallic ore district in Yunnan Province, South China: Geochronology, geochemistry, mineral chemistry and Nd–Hf isotopic compositions. Lithos 2015, 218–219, 54–72. [Google Scholar] [CrossRef]
- Xing, B.; Mao, J.; Xiao, X.; Liu, H.; Jia, F.; Wang, S.; Huang, W.; Li, H. Genetic discrimination of the Dingjiashan Pb-Zn deposit, SE China, based on sphalerite chemistry. Ore Geol. Rev. 2021, 135, 104212. [Google Scholar] [CrossRef]
- Fan, M.-S.; Ni, P.; Pan, J.-Y.; Wang, G.-G.; Ding, J.-Y.; Chu, S.-W.; Li, W.-S.; Huang, W.-Q.; Zhu, R.-Z.; Chi, Z. Rare disperse elements in epithermal deposit: Insights from LA–ICP–MS study of sphalerite at Dalingkou, South China. J. Geochem. Explor. 2023, 244, 107124. [Google Scholar] [CrossRef]
- Xu, J.; Cook, N.J.; Ciobanu, C.L.; Li, X.F.; Kontonikas-Charos, A.; Gilbert, S.; Lv, Y.H. Indium distribution in sphalerite from sulfide-oxide-silicate skarn assemblages: A case study of the Dulong Zn-Sn-In deposit, Southwest China. Miner. Depos. 2021, 56, 307–324. [Google Scholar] [CrossRef]
- Celep, O.; Yazici, E.Y.; Altinkaya, P.; Deveci, H. Characterization of a refractory arsenical silver ore by mineral liberation analysis (MLA) and diagnostic leaching. Hydrometallurgy 2019, 189, 105106. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Wilson, S.A.; Ridley, W.I.; Koenig, A.E. Development of sulfide calibration standards for the laser ablation inductively-coupled plasma mass spectrometry technique. J. Anal. At. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, C.; He, X.; Meng, L.; Xu, J.; Liu, W. A synthesis of the geology, spatial–temporal distribution and enrichment mechanism of granite-related indium deposits in China. Ore Geol. Rev. 2022, 146, 104932. [Google Scholar] [CrossRef]
- Chen, C.; Meng, L.; Xu, J.; Zhan, Q.; Zhao, T. Texture and geochemistry of sphalerite from the Chitudian Pb-Zn-Ag deposit, southern margin of the North China Craton: Implications for the enrichments of Cd, Ga, and In. Ore Geol. Rev. 2023, 156, 105392. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Kelvin, M.; Whiteman, E.; Petrus, J.; Leybourne, M.; Nkuna, V. Application of LA-ICP-MS to process mineralogy: Gallium and germanium recovery at Kipushi copper-zinc deposit. Miner. Eng. 2022, 176, 107322. [Google Scholar] [CrossRef]
- Ivashchenko, V.I. Indium Distribution in Sphalerites from Pitkaranta Mining District (South Karelia, Russia). Geol. Ore Depos. 2022, 64, 562–574. [Google Scholar] [CrossRef]
- Bauer, M.E.; Seifert, T.; Burisch, M.; Krause, J.; Richter, N.; Gutzmer, J. Indium-bearing sulfides from the Hammerlein skarn deposit, Erzgebirge, Germany: Evidence for late-stage diffusion of indium into sphalerite. Miner. Depos. 2019, 54, 175–192. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Williams, T. The mineralogy and mineral chemistry of indium in sulphide deposits and implications for mineral processing. Hydrometallurgy 2011, 108, 226–228. [Google Scholar] [CrossRef]
- Irrgang, N.; Monneron-Enaud, B.; Möckel, R.; Schlömann, M.; Höck, M. Economic feasibility of the co-production of indium from zinc sulphide using bioleaching extraction in Germany. Hydrometallurgy 2021, 200, 105566. [Google Scholar] [CrossRef]






| Element | Fe | Si | Ca | Mg | Al | Zn | S | K | F | As | Mn |
| Content/% | 11.00 | 10.00 | 7.00 | 5.00 | 4.00 | 2.00 | 2.00 | 0.80 | 0.80 | 0.30 | 0.30 |
| Element | Cu | Sn | Ti | P | Rb | Cl | Bi | Sr | Zr | Ga | Y |
| Content/% | 0.20 | 0.10 | 0.10 | 0.03 | 0.02 | 0.008 | 0.005 | 0.005 | 0.005 | 0.002 | 0.002 |
| Compound | In * | SiO2 | Fe2O3 | CaO | Al2O3 | MgO | ZnS | CuS | SnO2 | K2O | MnO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content/% | 43.87 | 28.20 | 17.97 | 11.10 | 7.49 | 7.26 | 4.04 | 0.23 | 0.22 | 1.36 | 0.39 |
| Serial Number | Mineral | Molecular Formula | Content/% | Serial Number | Mineral | Molecular Formula | Content/% |
|---|---|---|---|---|---|---|---|
| 1 | Marmatite | (Zn, Fe) S | 5.14 | 16 | Biotite | K{(Mg < 2\3, Fe > 1\3)3[AlSi3O10](OH)2} | 3.77 |
| 2 | Pyrrhotite | Fe1-xS | 6.12 | 17 | Chlorite | (Mg, Fe, Al)3(OH)6{(Mg, Fe, Al)3[(Si, Al)6O10(OH)2]} | 11.47 |
| 3 | Chalcopyrite | CuFeS2 | 0.66 | 18 | Tremolite | Ca2Mg5[Si4O11]2(OH)2 | 1.63 |
| 4 | Pyrite | FeS2 | 1.02 | 19 | Sanidine | K[AlSi3O8] | 3.43 |
| 5 | Arsenopyrite | FeAsS | 0.98 | 20 | Andradite | Ca3Fe2[SiO4]3 | 5.42 |
| 6 | Galena | PbS | 0.02 | 21 | Epidote | Ca2FeAl2[SiO4][Si2O7]O(OH) | 2.36 |
| 7 | Magnetite | Fe3O4 | 11.01 | 22 | Fluorite | CaF2 | 1.44 |
| 8 | Cassiterite | SnO2 | 0.28 | 23 | Ferroactin-olite | Ca2(MgFe)5[Si4O11]2(OH)2 | 0.53 |
| 9 | Calcite | Ca[CO3] | 11.02 | 24 | Antigorite | Mg6[Si4O10](OH)8 | 0.37 |
| 10 | Dolomite | CaMg[CO3]2 | 9.07 | 25 | Albite | Na[AlSi3O8] | 0.27 |
| 11 | Quartz | SiO2 | 11.12 | 26 | Sphene | CaTiSiO5 | 0.25 |
| 12 | talc | Mg3[Si4O10](OH)2 | 3.30 | 27 | Zircon | Zr[SiO4] | 0.05 |
| 13 | Minnesotaite | Fe3[Si4O10](OH)2 | 1.96 | 28 | Ilvaite | CaFe3(SiO4)2OH | 0.46 |
| 14 | Muscovite | K{Al2[AlSi3O10](OH)2} | 5.21 | 29 | Apatite | Ca5(PO4)3(F, Cl, OH) | 0.15 |
| 15 | Hlogopite | K{(Mg > 2\3, Fe < 1/3)3[AlSi3O10](OH)2} | 1.39 | 30 | Rutile | TiO2 | 0.10 |
| Particle Size (μm) | Raw Ore | Marmatite | Pyrrhotite | Magnetite | Chalcopyrite | Arsenopyrite | Pyrite | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | Cumulative (%) | Content (%) | Cumulative (%) | Content (%) | Cumulative (%) | Content (%) | Cumulative (%) | Content (%) | Cumulative (%) | Content (%) | Cumulative (%) | Content (%) | Cumulative (%) | |
| +300 | 1.33 | 1.33 | — | — | — | — | — | — | — | — | — | — | — | — |
| −300 + 212 | 5.26 | 6.58 | — | — | — | — | — | — | — | — | — | — | — | — |
| −212 + 150 | 10.50 | 17.08 | — | — | 2.00 | 2.00 | 2.18 | 2.18 | — | — | — | — | — | — |
| −150 + 106 | 12.41 | 29.49 | 6.25 | 6.25 | 2.17 | 4.17 | 4.82 | 7.00 | 7.06 | 7.06 | 10.73 | 10.73 | 4.35 | 4.35 |
| −106 + 75 | 13.36 | 42.85 | 11.53 | 17.78 | 14.80 | 18.97 | 8.95 | 15.95 | 15.60 | 22.66 | 7.13 | 17.86 | 16.33 | 20.69 |
| −75 + 53 | 13.50 | 56.35 | 16.47 | 34.25 | 15.86 | 34.83 | 16.84 | 32.79 | 22.28 | 44.94 | 9.86 | 27.72 | 13.47 | 34.16 |
| −53 + 38 | 11.88 | 68.23 | 15.31 | 49.57 | 14.92 | 49.75 | 16.68 | 49.47 | 6.96 | 51.90 | 12.54 | 40.26 | 19.87 | 54.03 |
| −38 + 27 | 10.54 | 78.77 | 16.18 | 65.75 | 14.50 | 64.26 | 17.01 | 66.48 | 15.37 | 67.26 | 17.36 | 57.62 | 15.14 | 69.17 |
| −27 + 19 | 7.96 | 86.72 | 12.61 | 78.36 | 12.71 | 76.97 | 12.70 | 79.18 | 9.14 | 76.40 | 16.41 | 74.03 | 11.46 | 80.63 |
| −19 + 13.5 | 5.39 | 92.11 | 8.79 | 87.15 | 9.39 | 86.36 | 9.14 | 88.32 | 8.48 | 84.88 | 10.76 | 84.79 | 8.87 | 89.50 |
| −13.5 + 9.6 | 3.67 | 95.78 | 5.36 | 92.51 | 6.55 | 92.91 | 6.03 | 94.34 | 6.59 | 91.46 | 6.50 | 91.29 | 4.19 | 93.68 |
| −9.6 + 6.8 | 2.42 | 98.20 | 4.09 | 96.61 | 4.06 | 96.97 | 3.54 | 97.88 | 4.65 | 96.11 | 4.93 | 96.23 | 3.54 | 97.22 |
| −6.8 + 4.8 | 1.44 | 99.64 | 2.59 | 99.19 | 2.33 | 99.31 | 1.65 | 99.53 | 2.93 | 99.04 | 2.99 | 99.21 | 2.05 | 99.28 |
| −4.8 + 3.4 | 0.34 | 99.97 | 0.73 | 99.93 | 0.61 | 99.91 | 0.41 | 99.94 | 0.86 | 99.90 | 0.69 | 99.90 | 0.63 | 99.91 |
| −3.4 | 0.03 | 100.00 | 0.07 | 100.0 | 0.09 | 100.0 | 0.06 | 100.0 | 0.10 | 100.0 | 0.10 | 100.0 | 0.09 | 100.0 |
| Particle Size (μm) | Quartz | Muscovite | Chlorite | Calcite Garnet | Dolomite | Calcite | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cont-Ent (%) | Cumulative (%) | Cont-Ent (%) | Cumulative (%) | Cont-Ent (%) | Cumulative (%) | Cont-Ent (%) | Cumulative (%) | Cont-Ent (%) | Cumulative (%) | Cont-Ent (%) | Cumulative (%) | |
| +300 | 5.70 | 5.70 | — | — | — | — | — | — | — | — | — | — |
| −300 + 212 | 11.94 | 17.64 | — | — | 1.35 | 1.35 | 4.49 | 4.49 | 1.74 | 1.74 | 6.74 | 6.74 |
| −212 + 150 | 13.85 | 31.49 | 5.76 | 5.76 | 11.91 | 13.26 | 10.23 | 14.72 | 8.95 | 10.69 | 12.55 | 19.29 |
| −150 + 106 | 13.09 | 44.58 | 12.71 | 18.46 | 10.74 | 24.00 | 13.98 | 28.70 | 11.37 | 22.06 | 14.72 | 34.02 |
| −106 + 75 | 11.85 | 56.42 | 12.79 | 31.26 | 15.02 | 39.01 | 13.16 | 41.87 | 13.32 | 35.38 | 13.06 | 47.08 |
| −75 + 53 | 14.95 | 71.37 | 12.69 | 43.95 | 12.71 | 51.72 | 10.78 | 52.64 | 19.18 | 54.57 | 12.90 | 59.98 |
| −53 + 38 | 9.81 | 81.18 | 11.26 | 55.21 | 11.17 | 62.89 | 12.67 | 65.32 | 14.50 | 69.06 | 11.86 | 71.84 |
| −38 + 27 | 7.34 | 88.52 | 12.46 | 67.67 | 10.75 | 73.65 | 11.53 | 76.85 | 12.12 | 81.18 | 9.73 | 81.56 |
| −27 + 19 | 4.76 | 93.28 | 11.28 | 78.95 | 9.17 | 82.81 | 7.73 | 84.58 | 8.56 | 89.74 | 6.66 | 88.22 |
| −19 + 13.5 | 3.26 | 96.54 | 8.14 | 87.09 | 6.45 | 89.26 | 5.58 | 90.16 | 4.62 | 94.37 | 4.66 | 92.88 |
| −13.5 + 9.6 | 1.78 | 98.33 | 6.12 | 93.21 | 4.70 | 93.96 | 4.25 | 94.41 | 2.70 | 97.07 | 3.29 | 96.18 |
| −9.6 + 6.8 | 1.02 | 99.34 | 3.76 | 96.97 | 3.27 | 97.23 | 3.15 | 97.56 | 1.68 | 98.75 | 2.22 | 98.40 |
| −6.8 + 4.8 | 0.53 | 99.87 | 2.33 | 99.30 | 2.14 | 99.38 | 1.82 | 99.38 | 0.99 | 99.74 | 1.28 | 99.68 |
| −4.8 + 3.4 | 0.12 | 99.99 | 0.60 | 99.90 | 0.55 | 99.93 | 0.54 | 99.92 | 0.24 | 99.98 | 0.30 | 99.98 |
| −3.4 | 0.01 | 100.00 | 0.10 | 100.00 | 0.07 | 100.00 | 0.08 | 100.00 | 0.02 | 100.00 | 0.02 | 100.00 |
| Mineral | Marmatite | Chalcopyrite | Pyrrhotite | Arsenopyrite and Pyrite |
| Content of In (g/t) | 543.00 | 521.00 | 86.33 | 22.35 |
| Mineral | Silicate Minerals | Carbonate Minerals | Quartz and Other Veinstone | Magnetite |
| Content of In (g/t) | 24.45 | 0.00 | 0.00 | 0.00 |
| Mineral | Content/% | In Grade of Carrier Minerals (g/t) | Distribution of In in Carrier Minerals/% |
|---|---|---|---|
| Marmatite | 5.14 | 543.00 | 63.62 |
| Chalcopyrite | 0.66 | 521.00 | 7.84 |
| Magnetite | 11.01 | 0.00 | 0.00 |
| Pyrrhotite | 6.12 | 86.33 | 4.22 |
| Arsenopyrite, pyrite | 2.00 | 22.35 | 1.02 |
| Silicate minerals | 41.82 | 24.45 | 23.30 |
| Quartz | 11.12 | 0.00 | 0.00 |
| Carbonates | 20.09 | 0.00 | 0.00 |
| Other | 2.70 | 0.00 | 0.00 |
| Raw mineral | 100.00 | 43.87 | 100.00 |
| Number | S/% | Fe/% | Zn/% | Number | S/% | Fe/% | Zn/% |
|---|---|---|---|---|---|---|---|
| 1 | 34.3 | 12.6 | 53.1 | 12 | 33.8 | 12.1 | 54.1 |
| 2 | 31.8 | 10.9 | 57.3 | 13 | 34.1 | 11.1 | 54.8 |
| 3 | 33.9 | 10.7 | 55.4 | 14 | 33.8 | 12.4 | 53.9 |
| 4 | 33.8 | 11.9 | 54.2 | 15 | 34.2 | 10.9 | 54.9 |
| 5 | 34.7 | 9.5 | 55.8 | 16 | 34.4 | 10.3 | 55.3 |
| 6 | 34.2 | 9.0 | 56.8 | 17 | 34.2 | 9.6 | 56.2 |
| 7 | 33.3 | 6.5 | 60.2 | 18 | 34.5 | 7.8 | 57.6 |
| 8 | 35.8 | 13.4 | 50.8 | 19 | 32.9 | 11.1 | 56.1 |
| 9 | 34.0 | 11.7 | 54.3 | 20 | 34.7 | 13.7 | 51.7 |
| 10 | 36.2 | 11.4 | 52.5 | 21 | 33.8 | 10.1 | 56.1 |
| 11 | 33.9 | 11.1 | 55.0 | average value | 34.1 | 10.8 | 55.1 |
| Product | Main Mineral |
|---|---|
| Zinc Concentrate | Marmatite |
| Copper Concentrate | Chalcopyrite |
| Iron Sulfur Concentrate | Pyrrhotite |
| Tin Concentrate | Cassiterite |
| Iron Concentrate | Magnetite |
| Tin Middling | cassiterite |
| Tailings | Carbonates, Magnetite Silicate minerals, Quartz |
| Indicator | Element | Products | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Zinc Concentrate | Copper Concentrate | Tin Concentrate | Tin Middling | Iron Sulfur Concentrate | Iron Concentrate | Tailings | Raw Ore | ||
| Grade/% | Zn | 46.73 | 6.97 | 0.08 | 0.11 | 0.22 | 0.05 | 0.18 | 2.58 |
| Cu | 0.73 | 16.58 | 0.02 | 0.03 | 0.05 | 0.01 | 0.02 | 0.18 | |
| Sn | 0.07 | 0.14 | 38.22 | 2.60 | 0.17 | 0.16 | 0.12 | 0.23 | |
| Fe | 13.93 | 20.47 | 12.93 | 16.99 | 60.25 | 66.22 | 11.62 | 17.26 | |
| S | 33.01 | 24.68 | 3.93 | 5.15 | 9.62 | 2.33 | 2.07 | 4.61 | |
| In | 483.10 | 319.42 | 53.65 | 47.61 | 28.31 | 34.92 | 25.35 | 51.11 | |
| Ag | 60.17 | 771.08 | 3.25 | 4.87 | 8.59 | 3.47 | 3.57 | 12.59 | |
| As | 0.30 | 0.21 | 0.17 | 0.38 | 0.06 | 0.01 | 0.29 | 0.27 | |
| MgO | 1.04 | 5.76 | 1.55 | 5.34 | 2.11 | 1.24 | 7.15 | 6.33 | |
| Recovery/% | Zn | 92.74 | 1.99 | 0.01 | 0.01 | 0.84 | 0.02 | 5.91 | 100.00 |
| Cu | 20.78 | 67.56 | 0.03 | 0.06 | 2.62 | 0.04 | 11.39 | 100.00 | |
| Sn | 1.67 | 0.47 | 43.80 | 3.72 | 7.20 | 0.74 | 43.99 | 100.00 | |
| Fe | 4.12 | 0.87 | 0.20 | 0.32 | 33.59 | 3.98 | 55.78 | 100.00 | |
| S | 36.62 | 3.94 | 0.22 | 0.37 | 20.08 | 0.52 | 37.24 | 100.00 | |
| In | 48.01 | 4.57 | 0.28 | 0.30 | 5.30 | 0.71 | 40.83 | 100.00 | |
| Ag | 24.44 | 45.14 | 0.07 | 0.13 | 6.57 | 0.29 | 23.49 | 100.00 | |
| As | 5.61 | 0.58 | 0.16 | 0.46 | 2.05 | 0.03 | 88.35 | 100.00 | |
| MgO | 0.84 | 0.67 | 0.06 | 0.28 | 3.21 | 0.20 | 93.69 | 100.00 | |
| Yield/% | - | 5.11 | 0.74 | 0.26 | 0.33 | 9.62 | 1.04 | 82.90 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Tong, X.; Xie, X.; Song, Q.; Xie, R.; Han, B.; Fu, H.; Lu, Z. Enrichment Regularity of Indium in the Dulong Mineral Processing Plant, Yunnan Province, China. Minerals 2025, 15, 672. https://doi.org/10.3390/min15070672
Fan P, Tong X, Xie X, Song Q, Xie R, Han B, Fu H, Lu Z. Enrichment Regularity of Indium in the Dulong Mineral Processing Plant, Yunnan Province, China. Minerals. 2025; 15(7):672. https://doi.org/10.3390/min15070672
Chicago/Turabian StyleFan, Peiqiang, Xiong Tong, Xian Xie, Qiang Song, Ruiqi Xie, Bin Han, Haitao Fu, and Zhiming Lu. 2025. "Enrichment Regularity of Indium in the Dulong Mineral Processing Plant, Yunnan Province, China" Minerals 15, no. 7: 672. https://doi.org/10.3390/min15070672
APA StyleFan, P., Tong, X., Xie, X., Song, Q., Xie, R., Han, B., Fu, H., & Lu, Z. (2025). Enrichment Regularity of Indium in the Dulong Mineral Processing Plant, Yunnan Province, China. Minerals, 15(7), 672. https://doi.org/10.3390/min15070672








