Machine Learning-Based Analysis of Heavy Metal Migration Under Acid Rain: Insights from the RF and SVM Algorithms
Abstract
1. Introduction
2. Materials and Methods
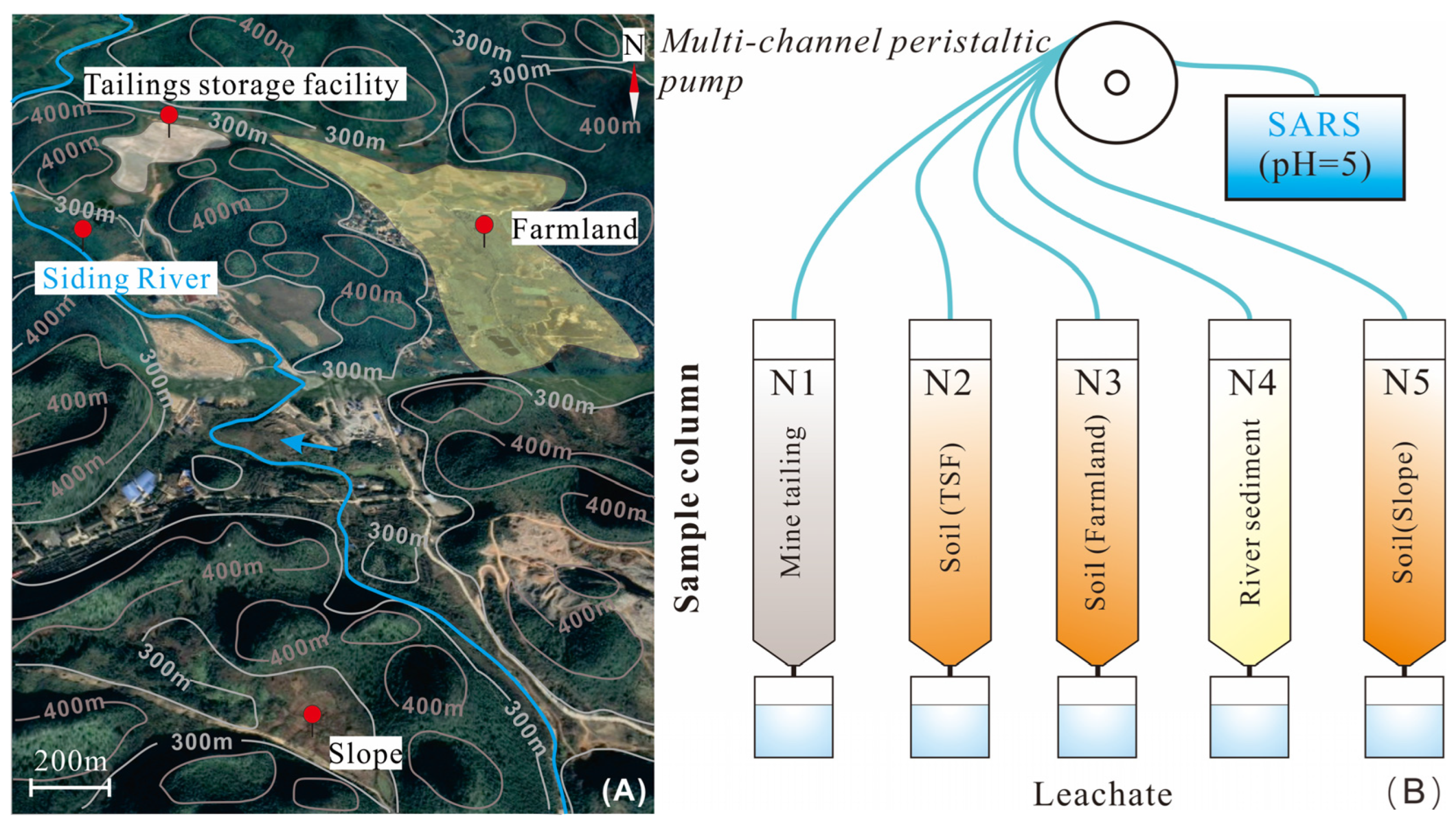
2.1. Study Area and Sample Collection
2.2. Experimental Methods
2.2.1. Preparation of Simulated Acid Rain Solution (SARS)
2.2.2. Column Leaching Test
2.2.3. Analytical Methods
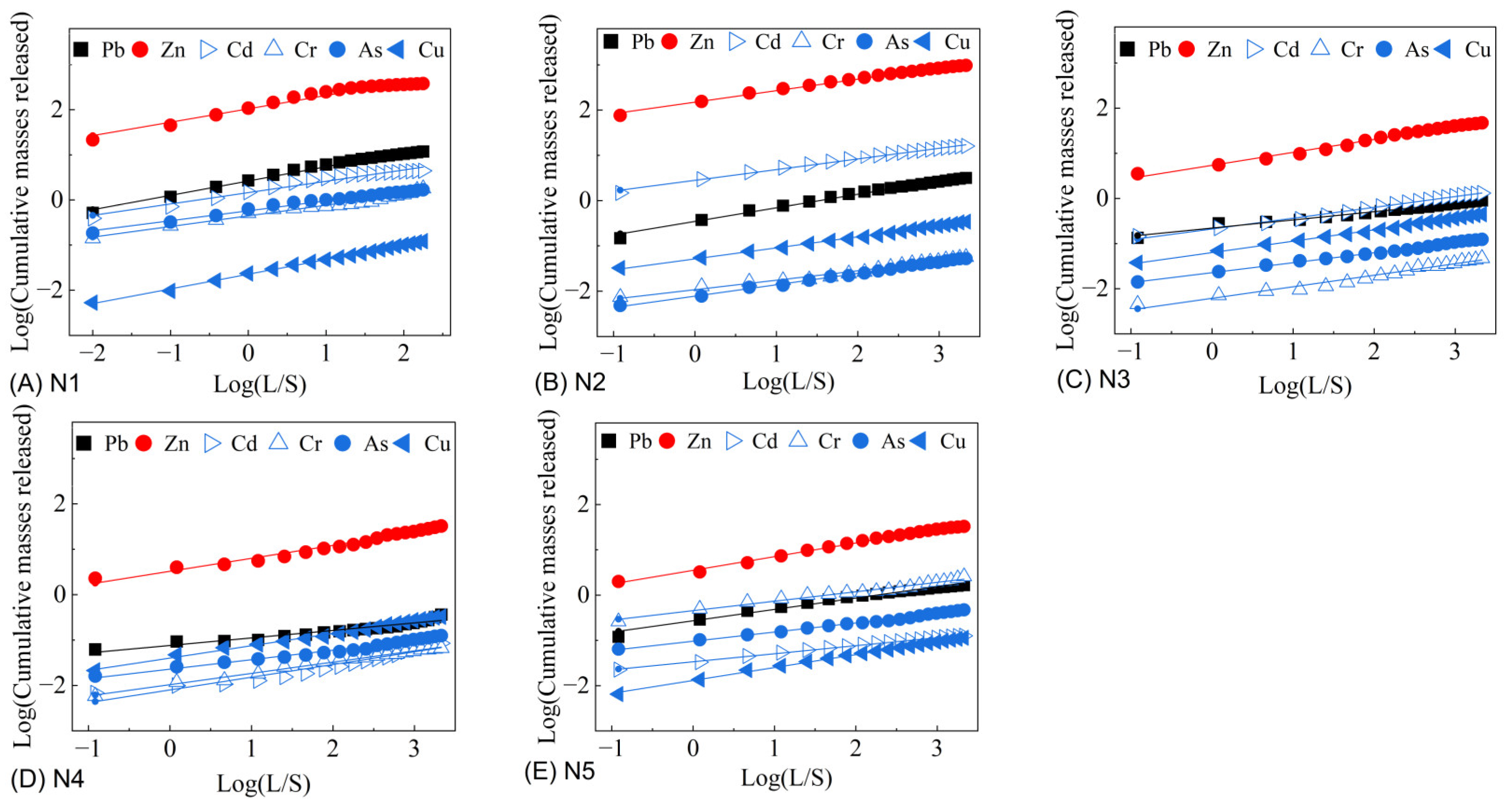
2.3. The Cumulative Release of Heavy Metals
2.4. Environmental Risk Assessment
2.5. Machine Learning Methods
2.6. Statistical Analyses
3. Results and Discussion
3.1. Mineralogical Characteristics of the Samples
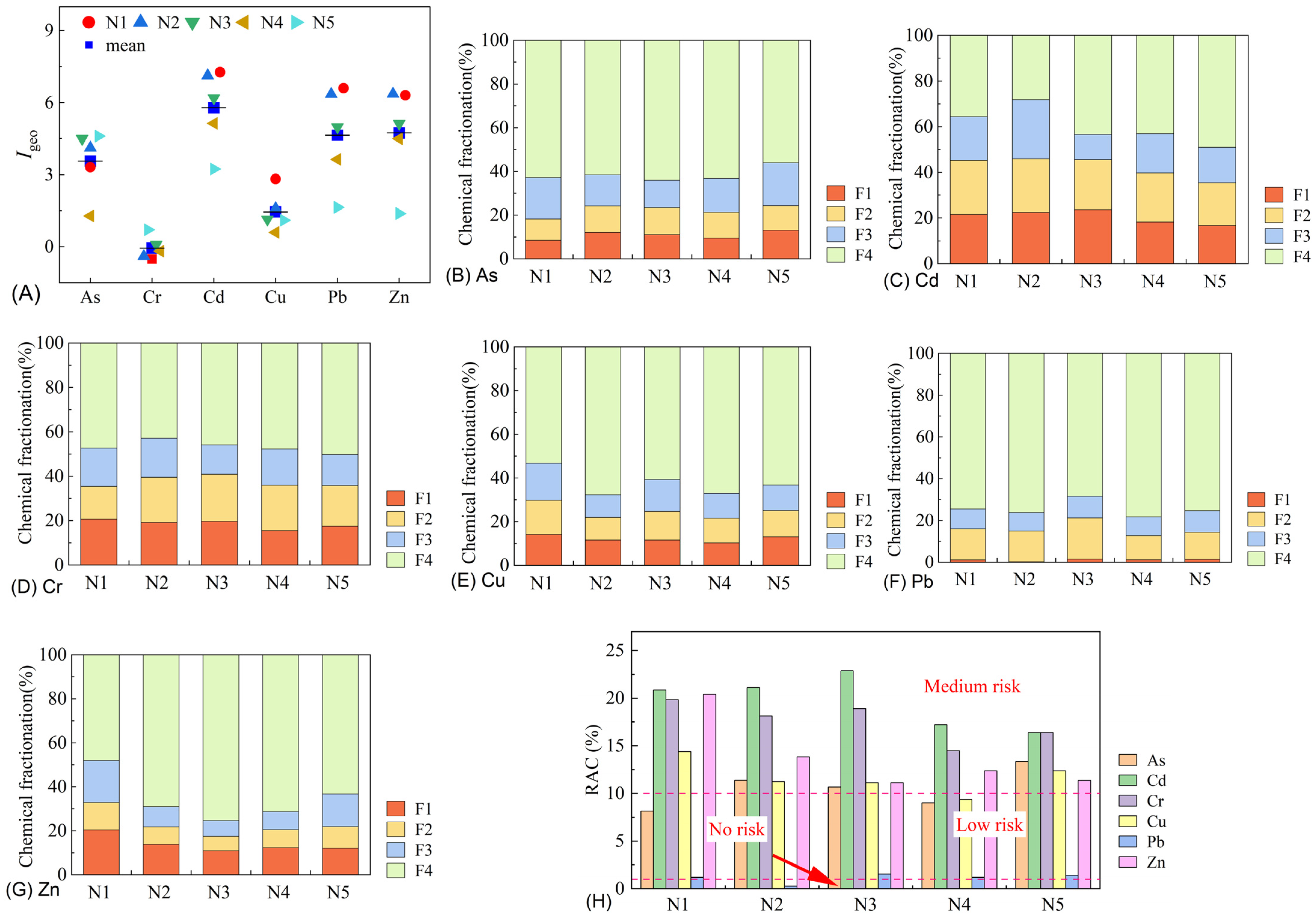
3.2. Heavy Metal Contamination Characteristics of the Samples
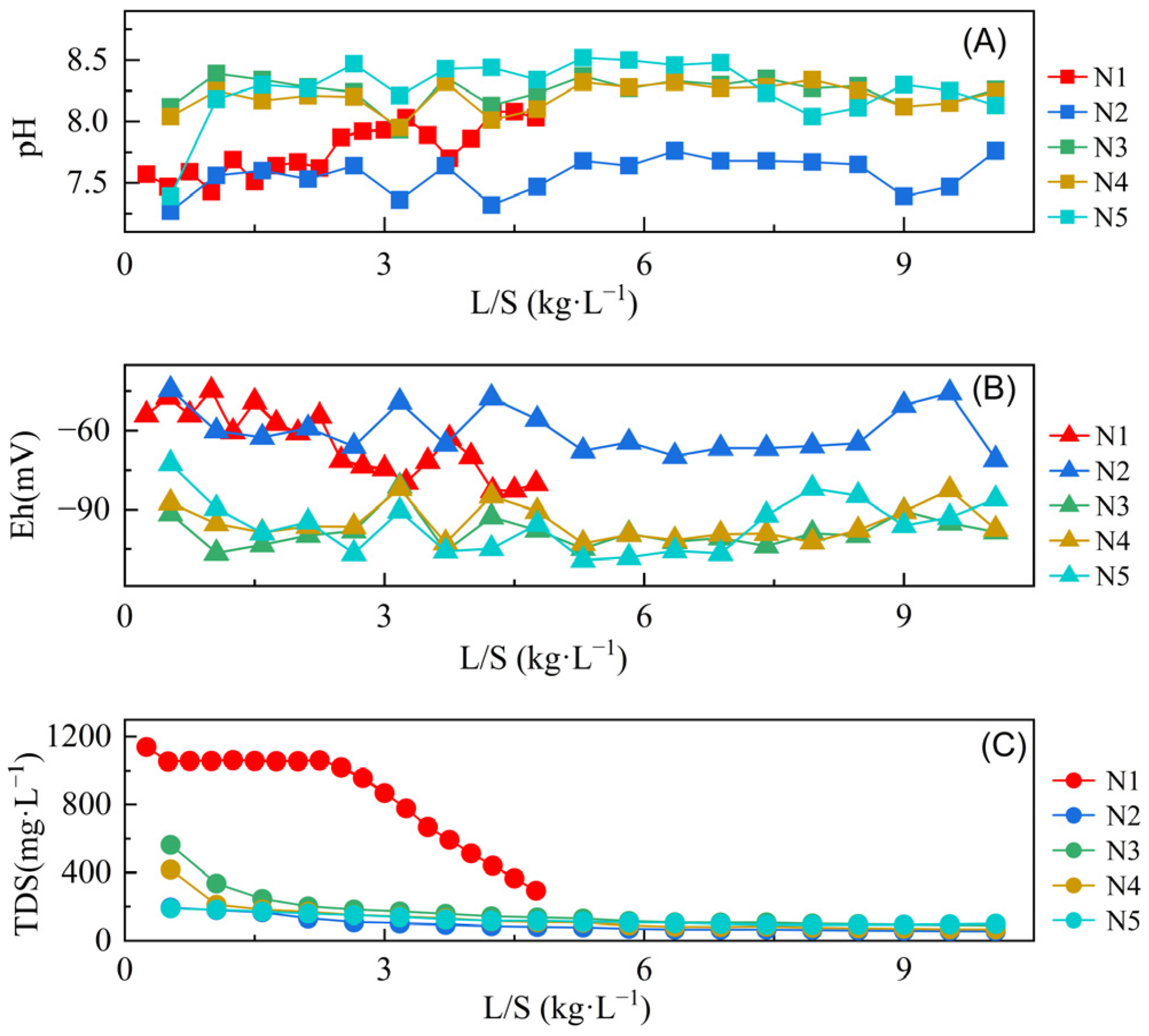
3.3. The pH, Eh, and TDS Characteristics of Sample Leachates
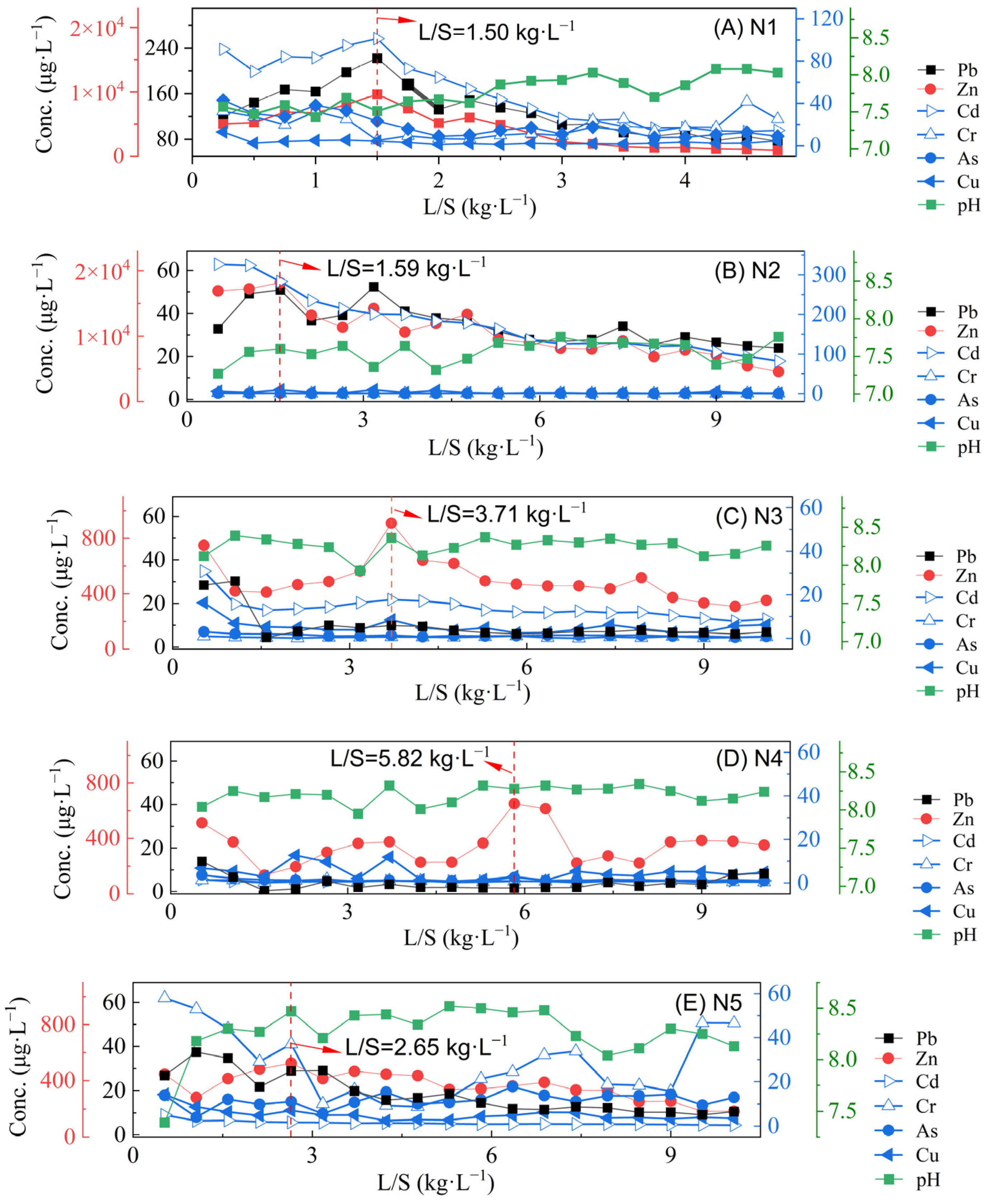
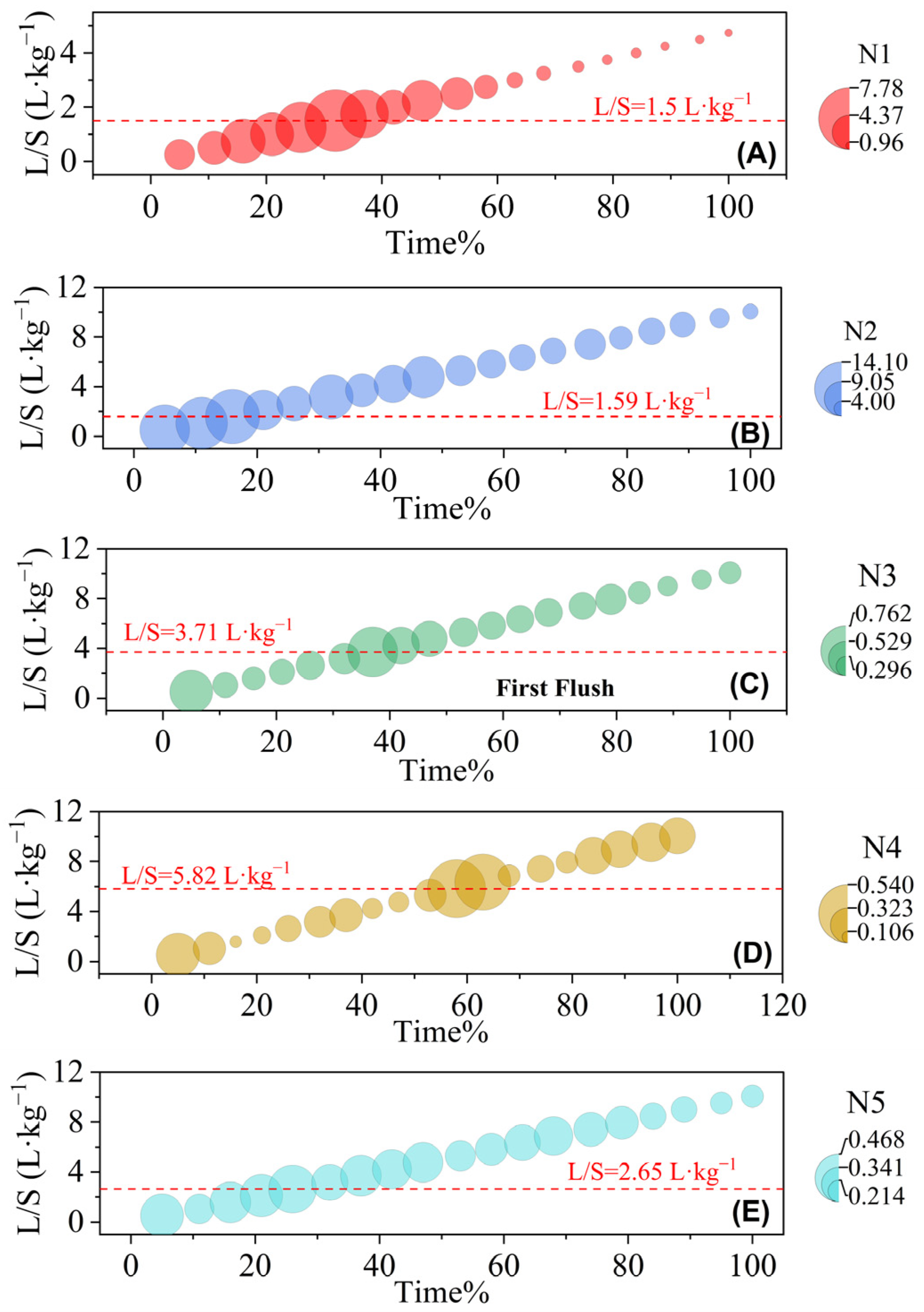
3.4. Heavy Metal Concentrations and Cumulative Release Characteristics in Leachates
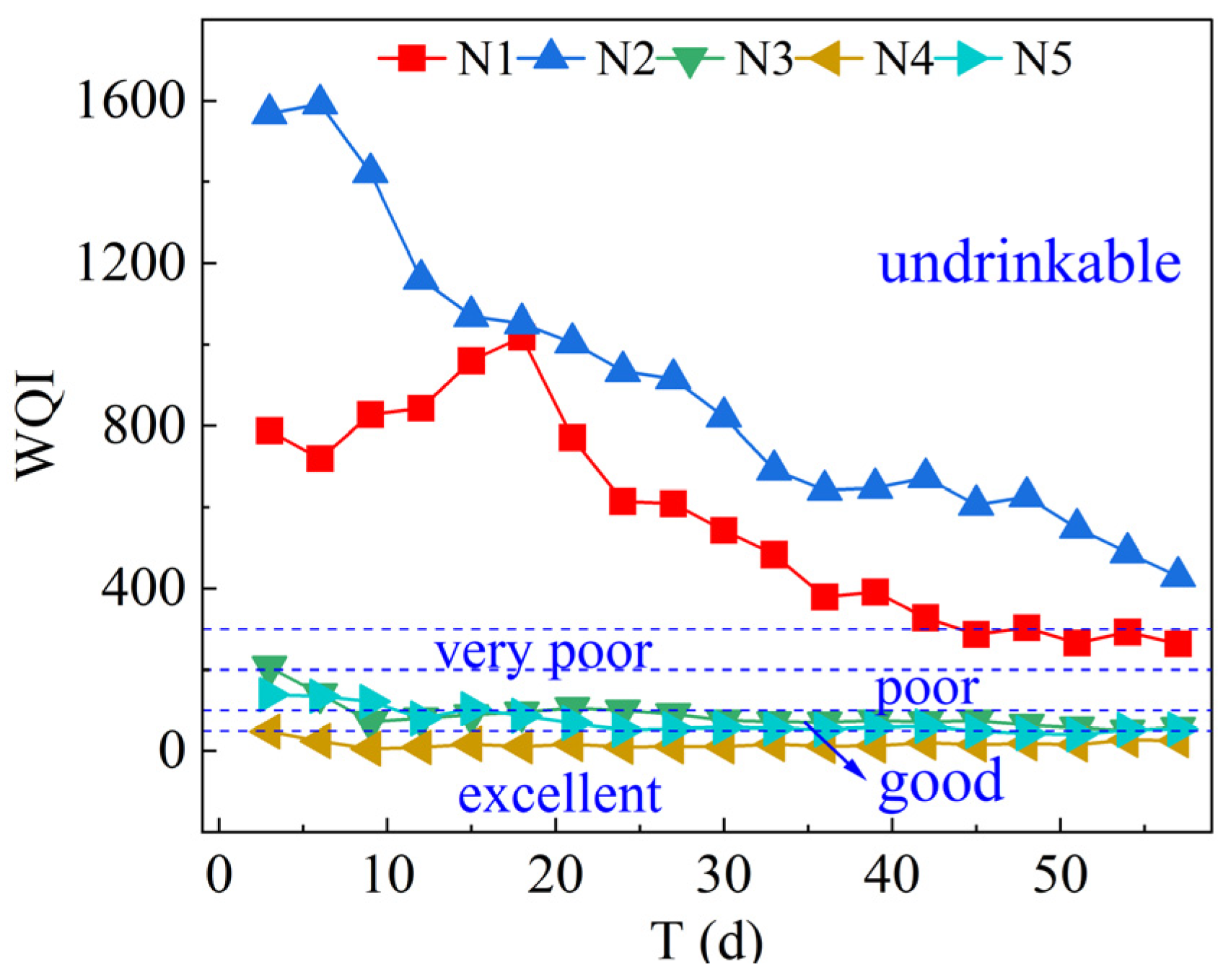
3.5. Environmental Impact Assessment of Leachates
3.6. Correlation Analysis and Machine Learning-Based Prediction of Heavy Metal Migration
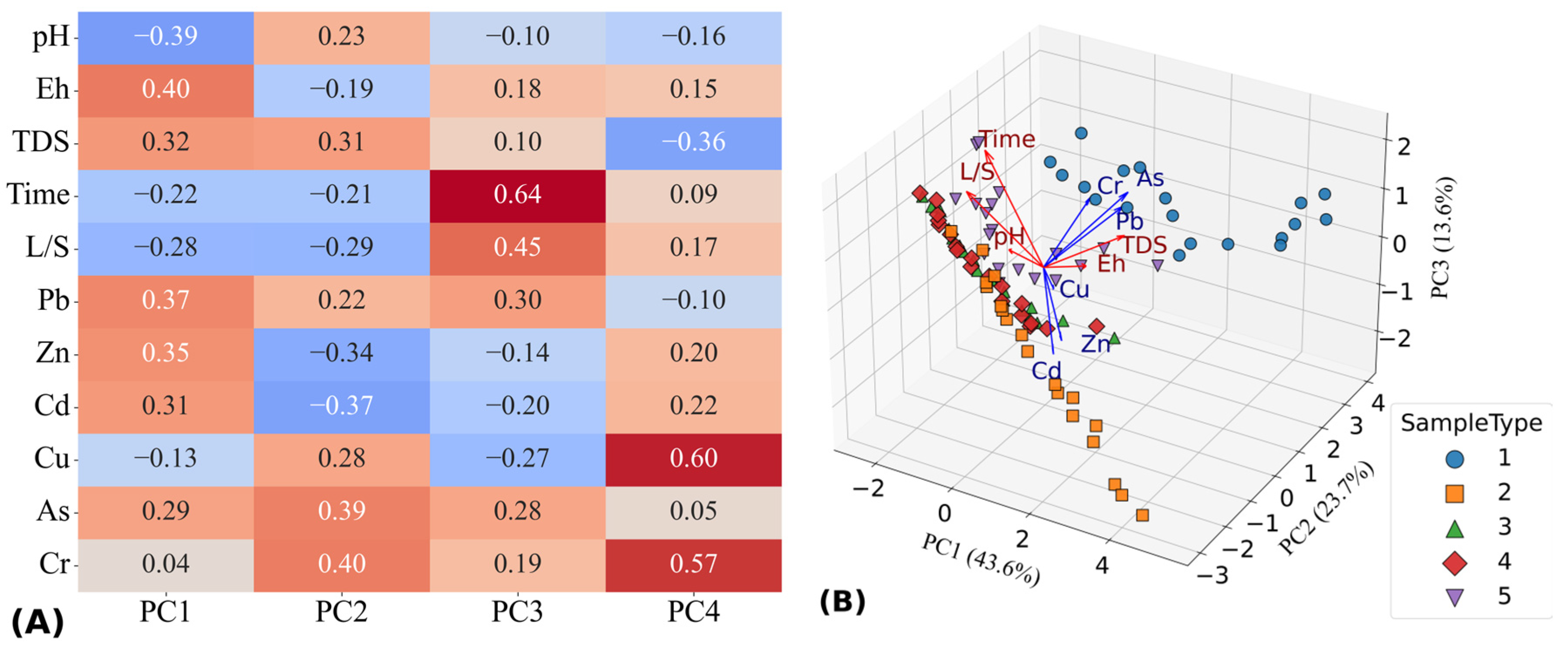
3.6.1. Correlation Analysis
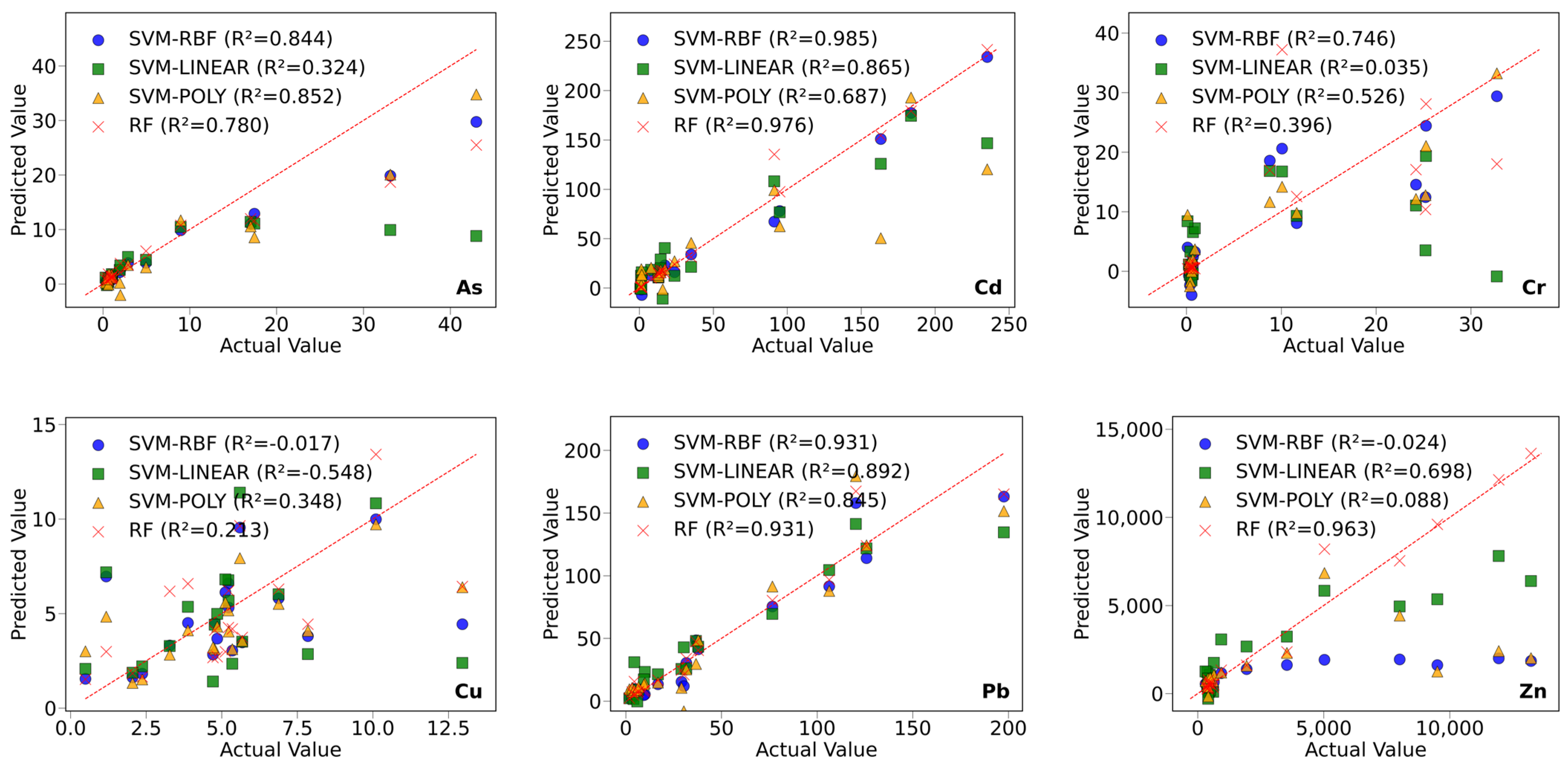
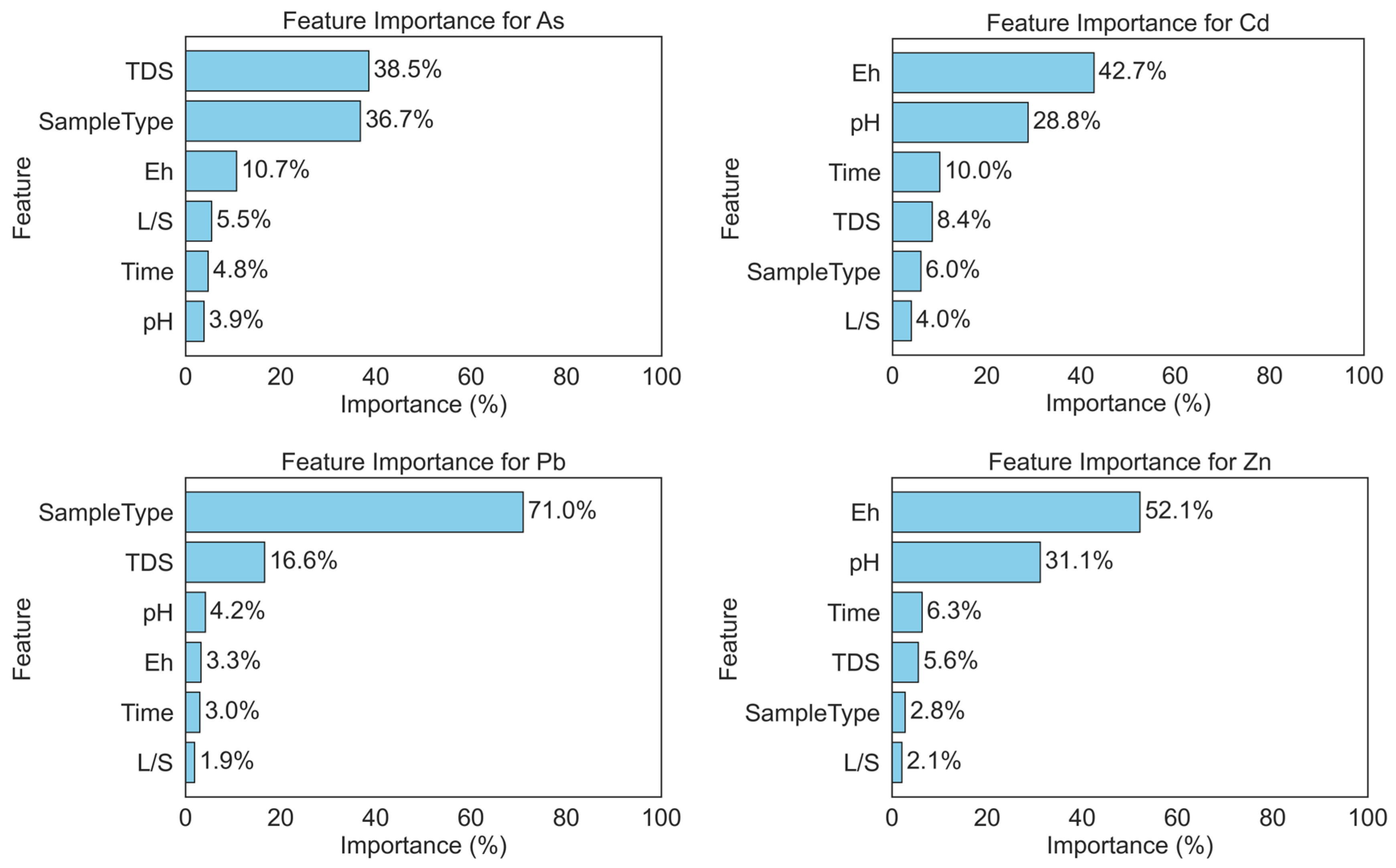
3.6.2. Prediction of Heavy Metal Concentrations Based on Machine Learning
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prakash, J.; Agrawal, S.B.; Agrawal, M. Global Trends of Acidity in Rainfall and Its Impact on Plants and Soil. J. Soil Sci. Plant Nutr. 2023, 23, 398–419. [Google Scholar] [CrossRef] [PubMed]
- Nieva, N.E.; Borgnino, L.; García, M.G. Long term metal release and acid generation in abandoned mine wastes containing metal-sulphides. Environ. Pollut. 2018, 242, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Bu, C.; Li, X.; Li, Q.; Li, L.; Wu, P. Spatiotemporal distributions, sources, and health risks of heavy metals in an acid mine drainage (AMD)-contaminated karst river in southwest China. Appl. Water Sci. 2024, 14, 251. [Google Scholar] [CrossRef]
- Saedpanah, S.; Amanollahi, J. Environmental pollution and geo-ecological risk assessment of the Qhorveh mining area in western Iran. Environ. Pollut. 2019, 253, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, C.; Petrakis, M.; Psiloglou, B.; Lianou, M. Modelling of light pollution in suburban areas using remotely sensed imagery and GIS. J. Environ. Manag. 2006, 79, 57–63. [Google Scholar] [CrossRef]
- Schiavo, M.; Giambastiani, B.M.; Greggio, N.; Colombani, N.; Mastrocicco, M. Geostatistical assessment of groundwater arsenic contamination in the Padana Plain. Sci. Total Environ. 2024, 931, 172998. [Google Scholar] [CrossRef]
- Hong, T.; Wang, Z.; Luo, X.; Zhang, W. State-of-the-art on research and applications of machine learning in the building life cycle. Energy Build. 2020, 212, 109831. [Google Scholar] [CrossRef]
- Huang, G.; Guo, Y.; Chen, Y.; Nie, Z. Application of Machine Learning in Material Synthesis and Property Prediction. Materials 2023, 16, 5977. [Google Scholar] [CrossRef]
- Vinuesa, R.; Brunton, S. Enhancing computational fluid dynamics with machine learning. Nat. Comput. Sci. 2021, 2, 358–366. [Google Scholar] [CrossRef]
- Bellinger, C.; Mohomed Jabbar, M.S.; Zaïane, O.; Osornio-Vargas, A. A systematic review of data mining and machine learning for air pollution epidemiology. BMC Public Health 2017, 17, 907. [Google Scholar] [CrossRef]
- Kida, M.; Pochwat, K.; Ziembowicz, S. Assessment of machine learning-based methods predictive suitability for migration pollutants from microplastics degradation. J. Hazard. Mater. 2024, 461, 132565. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, C.; Chen, H.; Yue, Y.; Zhang, W.; Zhang, M.; Qi, X.; Fu, Z. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef]
- Zaw, K.; Peters, S.G.; Cromie, P.; Burrett, C.; Hou, Z. Nature, diversity of deposit types and metallogenic relations of South China. Ore Geol. Rev. 2007, 31, 3–47. [Google Scholar] [CrossRef]
- Li, W.; Zhu, T.; Yang, H.; Zhang, C.; Zou, X. Distribution characteristics and risk assessment of heavy metals in soils of the typical karst and non-karst areas. Land 2022, 11, 1346. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512, 143–153. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.; Wang, H.; Hu, Y.; Cheng, H. Potential risk, leaching behavior and mechanism of heavy metals from mine tailings under acid rain. Chemosphere 2024, 350, 140995. [Google Scholar] [CrossRef]
- Qin, S.; Li, X.; Huang, J.; Li, W.; Wu, P.; Li, Q.; Li, L. Inputs and transport of acid mine drainage-derived heavy metals in karst areas of Southwestern China. Environ. Pollut. 2024, 343, 123243. [Google Scholar] [CrossRef]
- Liao, J.; Hu, C.; Wang, M.; Li, X.; Ruan, J.; Zhu, Y.; Fairchild, I.J.; Hartland, A. Assessing acid rain and climate effects on the temporal variation of dissolved organic matter in the unsaturated zone of a karstic system from southern China. J. Hydrol. 2018, 556, 475–487. [Google Scholar] [CrossRef]
- Santona, L.; Castaldi, P.; Melis, P. Evaluation of the interaction mechanisms between red muds and heavy metals. J. Hazard. Mater. 2006, 136, 324–329. [Google Scholar] [CrossRef]
- Szecsody, J.E.; Truex, M.J.; Qafoku, N.P.; Wellman, D.M.; Resch, T.; Zhong, L. Influence of acidic and alkaline waste solution properties on uranium migration in subsurface sediments. J. Contam. Hydrol. 2013, 151, 155–175. [Google Scholar] [CrossRef]
- Jurjovec, J.; Blowes, D.W.; Ptacek, C.J.; Mayer, K.U. Multicomponent reactive transport modeling of acid neutralization reactions in mine tailings. Water Resour. Res. 2004, 40. [Google Scholar] [CrossRef]
- Dold, B.; Fontboté, L. Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. J. Geochem. Explor. 2001, 74, 3–55. [Google Scholar] [CrossRef]
- Wu, W.; Qu, S.; Nel, W.; Ji, J. The impact of natural weathering and mining on heavy metal accumulation in the karst areas of the Pearl River Basin, China. Sci. Total Environ. 2020, 734, 139480. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, G.; An, J.; Liu, Y. Analysis of acid rain in Liuzhou and its relationship with meteorological conditions. J. Meteorol. Res. Appl. 2018, 39, 75–77+81. [Google Scholar]
- Cao, J.S.; Shao, Z.Z.; Peng, Z.A.; Zheng, H.; Wei, J.; Nong, S.H. Study on fluid inclusions characteristics of the Siding lead-zinc deposit in Guangxi. Miner. Resour. Geol. 2018, 32, 31–38. [Google Scholar]
- Lei, L. Mechanism of heavy metals release from carbonate-type tailings in bufferingperiod: Column leaching test. Acta Petrolog. Miner. 2019, 38, 131–142. [Google Scholar]
- Lei, L.; Song, C.; Wang, F.; Xie, X.; Luo, Y.; Ma, Y. The neutralization capacity and acidification potential of the carbonate-type tailings in north Guangxi and its adjacent areas, South China. J. Miner. Petrol. 2010, 30, 106–113. [Google Scholar]
- Ministry of Environmental Protection of the People’s Republic of China. The Technical Specification for soil Environmental Monitoring HJ/T 166-2004. 2004. Available online: https://www.mee.gov.cn/image20010518/5406.pdf (accessed on 18 June 2025).
- State Administration for Market Regulation of the People’s Republic of China. Chemicals—Testing of Leaching in Soil Columns GB/T 41667-2022. 2022. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=56A59C3129E2D32167AD94F485CB3419 (accessed on 18 June 2025).
- Ministry of Ecology and Environment of the People’s Republic of China. Soil–Determination of pH–Potentiometry HJ 962–2018. 2018. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201808/W020180815584753007210.pdf (accessed on 18 June 2025).
- Ministry of Ecology and Environment of the People’s Republic of China. Water Quality—Determination of pH—Electrode Method HJ 1147–2020. 2020. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/xgbzh/202011/W020201127577750190580.pdf (accessed on 18 June 2025).
- Ministry of Environmental Protection of the People’s Republic of China. Water Quality-Determination of Dissolved Oxygen-Electrochemical Probe Method HJ 506–2009. 2009. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/200911/W020111114554879821017.pdf (accessed on 18 June 2025).
- Zhang, C.Y.; Peng, P.A.; Song, J.Z.; Liu, C.S.; Jue, P.; Lu, P.X. Utilization of modified BCR procedure for the chemical speciation of heavymetals in Chinese soil reference material. Ecol. Environ. Sci. 2012, 21, 1881–1884. [Google Scholar]
- Ministry of Environmental Protection of the People’s Republic of China. Water Quality—Determination of 65 Elements—Inductively Coupled Plasma-Mass Spectrometry HJ 700-2014. 2014. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201405/W020140523543423227755.pdf (accessed on 18 June 2025).
- Haris, H.; Looi, L.; Aris, A.; Mokhtar, N.; Ayob, N.A.A.; Yusoff, F.; Salleh, A.; Praveena, S. Geo-accumulation index and contamination factors of heavy metals (Zn and Pb) in urban river sediment. Environ. Geochem. Health 2017, 39, 1259–1271. [Google Scholar] [CrossRef]
- Perin, G.; Craboledda, L.; Lucchese, M.; Cirillo, R.; Dotta, L.; Zanette, M.; Orio, A. Heavy metal speciation in the sediments of Northern Adriatic Sea: A new approach for environmental toxicity determination. Heavy Met. Environ. 1985, 2, 454–456. [Google Scholar]
- Mukherjee, P.; Das, P.K.; Ghosh, P. The extent of heavy metal pollution by chemical partitioning and risk assessment code of sediments of sewage-fed fishery ponds at east Kolkata Wetland, a ramsar site, India. Bull. Environ. Contam. Toxicol. 2022, 108, 731–736. [Google Scholar] [CrossRef]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef]
- State Administration for Market Regulation of the People’s Republic of China. Standards for Drinking Water Quality GB 5749-2022. 2022. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=99E9C17E3547A3C0CE2FD1FFD9F2F7BE (accessed on 18 June 2025).
- Wasana, H.M.S.; Perera, G.D.R.K.; Gunawardena, P.D.S.; Fernando, P.S.; Bandara, J. WHO water quality standards Vs Synergic effect(s) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Sci. Rep. 2017, 7, 42516. [Google Scholar] [CrossRef] [PubMed]
- Probst, P.; Wright, M.; Boulesteix, A. Hyperparameters and tuning strategies for random forest. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2018, 9, e1301. [Google Scholar] [CrossRef]
- Tharwat, A. Parameter investigation of support vector machine classifier with kernel functions. Knowl. Inf. Syst. 2019, 61, 1269–1302. [Google Scholar] [CrossRef]
- Contreras, P.; Orellana-alvear, J.; Muñoz, P.; Bendix, J.; Célleri, R. Influence of Random Forest Hyperparameterization on Short-Term Runoff Forecasting in an Andean Mountain Catchment. Atmosphere 2021, 12, 238. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Liu, X. Grid search with a weighted error function: Hyper-parameter optimization for financial time series forecasting. Appl. Soft Comput. 2024, 154, 111362. [Google Scholar] [CrossRef]
- Ali, A.; Gravino, C. An empirical comparison of validation methods for software prediction models. J. Softw. Evol. Process 2021, 33, e2367. [Google Scholar] [CrossRef]
- Boni, M.; Mondillo, N.; Balassone, G. Zincian dolomite: A peculiar dedolomitization case? Geology 2011, 39, 183–186. [Google Scholar] [CrossRef]
- Boni, M.; Mondillo, N.; Balassone, G.; Joachimski, M.; Colella, A. Zincian dolomite related to supergene alteration in the Iglesias mining district (SW Sardinia). Int. J. Earth Sci. 2012, 102, 61–71. [Google Scholar] [CrossRef]
- Ye, Z.; Zhou, J.; Liao, P.; Finfrock, Y.; Liu, Y.; Shu, C.; Liu, P. Metal (Fe, Cu, and As) transformation and association within secondary minerals in neutralized acid mine drainage characterized using X-ray absorption spectroscopy. Appl. Geochem. 2022, 139, 105242. [Google Scholar] [CrossRef]
- Moncur, M.C.; Ptacek, C.J.; Blowes, D.W.; Peterson, R.C. The occurrence and implications of efflorescent sulfate minerals at the former Sherritt–Gordon Zn–Cu Mine, Sherridon, Manitoba, Canada. Can. Mineral. 2015, 53, 961–977. [Google Scholar] [CrossRef]
- Bidari, E.; Aghazadeh, V. Pyrite oxidation in the presence of calcite and dolomite: Alkaline leaching, chemical modeling and surface characterization. Trans. Nonferrous Met. Soc. China 2018, 28, 1433–1443. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, M.; Zhao, C. The study of geochemical background and base line for 54 chemical indicators in Chinese soil. Earth Sci. Front. 2024, 31, 380–402. [Google Scholar]
- Mbey, J.A.; Thomas, F.; Razafitianamaharavo, A.; Caillet, C.; Villiéras, F. A comparative study of some kaolinites surface properties. Appl. Clay Sci. 2019, 172, 135–145. [Google Scholar] [CrossRef]
- Lim, J.E.; Ahmad, M.; Lee, S.S.; Shope, C.L.; Hashimoto, Y.; Kim, K.; Usman, A.R.; Yang, J.E.; Ok, Y.S. Effects of lime-based waste materials on immobilization and phytoavailability of cadmium and lead in contaminated soil. Clean–Soil Air Water 2013, 41, 1235–1241. [Google Scholar] [CrossRef]
- Luo, K.; Liu, H.; Liu, Q.; Tu, Y.; Yu, E.; Xing, D. Cadmium accumulation and migration of 3 peppers varieties in yellow and limestone soils under geochemical anomaly. Environ. Technol. 2022, 43, 10–20. [Google Scholar] [CrossRef]
- Sorrentino, G.P.; Guimarães, R.; Valentim, B.; Bontempi, E. The influence of liquid/solid ratio and pressure on the natural and accelerated carbonation of alkaline wastes. Minerals 2023, 13, 1060. [Google Scholar] [CrossRef]
- Bonnissel-gissinger, P.; Alnot, M.; Ehrhardt, J.; Behra, P. Surface oxidation of pyrite as a function of pH. Environ. Sci. Technol. 1998, 32, 2839–2845. [Google Scholar] [CrossRef]
- Daniels, W.L.; Zipper, C.E.; Orndorff, Z.; Skousen, J.; Barton, C.D.; Mcdonald, L.M.; Beck, M.A. Predicting total dissolved solids release from central Appalachian coal mine spoils. Environ. Pollut. 2016, 216, 371–379. [Google Scholar] [CrossRef]
- Marguš, M.; Morales-reyes, I.; Bura-nakić, E.; Batina, N.; Ciglenečki, I. The anoxic stress conditions explored at the nanoscale by atomic force microscopy in highly eutrophic and sulfidic marine lake. Cont. Shelf Res. 2015, 109, 24–34. [Google Scholar] [CrossRef]
- Skousen, J.G.; Sexstone, A.; Ziemkiewicz, P.F. Acid mine drainage control and treatment. Reclam. Drastically Disturb. Lands 2000, 41, 131–168. [Google Scholar]
- Standardization Administration of the People’s Republic of China. Standard for Groundwater Quality GB/T 14848-2017. 2017. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=F745E3023BD5B10B9FB5314E0FFB5523 (accessed on 18 June 2025).
- Perera, T.; Mcgree, J.; Egodawatta, P.; Jinadasa, K.; Goonetilleke, A. New conceptualisation of first flush phenomena in urban catchments. J. Environ. Manag. 2021, 281, 111820. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Han, Y.; Kang, J.; Kayhanian, M.; Stenstrom, M. Characteristics of Highway Stormwater Runoff in Los Angeles: Metals and Polycyclic Aromatic Hydrocarbons. Water Environ. Res. 2009, 81, 308–318. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Z.; Hu, Y.; Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 2019, 695, 133893. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D’enza, A.I.; Markos, A.; Tuzhilina, E. Principal component analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]
- Jurjovec, J.; Ptacek, C.J.; Blowes, D.W. Acid neutralization mechanisms and metal release in mine tailings: A laboratory column experiment. Geochim. Cosmochim. Acta. 2002, 66, 1511–1523. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Xiao, T.; Zhu, Z.; Ma, L.; Li, H.; Ning, Z. Geogenic pollution, fractionation and potential risks of Cd and Zn in soils from a mountainous region underlain by black shale. Sci. Total Environ. 2020, 760, 143426. [Google Scholar] [CrossRef]
- Markiewicz-patkowska, J.; Hursthouse, A.; Przybyla-kij, H. The interaction of heavy metals with urban soils: Sorption behaviour of Cd, Cu, Cr, Pb and Zn with a typical mixed brownfield deposit. Environ. Int. 2005, 31, 513–521. [Google Scholar] [CrossRef]
- Li, X.; Zhou, T.; Li, Z.; Wang, W.; Zhou, J.; Hu, P.; Luo, Y.; Christie, P.; Wu, L. Legacy of contamination with metal (loid) s and their potential mobilization in soils at a carbonate-hosted lead-zinc mine area. Chemosphere 2022, 308, 136589. [Google Scholar] [CrossRef]
- Stoessell, R.K.; Moore, Y.H.; Coke, J.G. The occurrence and effect of sulfate reduction and sulfide oxidation on coastal limestone dissolution in Yucatan cenotes. Groundwater 1993, 31, 566–575. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, J.; Hu, B.; Wei, W. Adsorption and desorption for dynamics transport of hexavalent chromium (Cr(VI)) in soil column. Environ. Sci. Pollut. Res. 2017, 25, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Ding, Y.; Liu, J.; Jiang, H.; Dong, H. Enhanced oxidation of Cr (III)–Fe (III) hydroxides by oxygen in dark and alkaline environments: Roles of Fe/Cr ratio and siderophore. Environ. Sci. Technol. 2023, 57, 13172–13181. [Google Scholar] [CrossRef]
- Löv, Å.; Sjöstedt, C.; Larsbo, M.; Persson, I.; Gustafsson, J.P.; Cornelis, G.; Kleja, D.B. Solubility and transport of Cr (III) in a historically contaminated soil–evidence of a rapidly reacting dimeric Cr (III) organic matter complex. Chemosphere 2017, 189, 709–716. [Google Scholar] [CrossRef]
- Griffin, R.; Au, A.K.; Frost, R. Effect of pH on adsorption of chromium from landfill-leachate by clay minerals. J. Environ. Sci. Health Part A 1977, 12, 431–449. [Google Scholar] [CrossRef]
- Xu, T.; Nan, F.; Jiang, X.; Tang, Y.; Zeng, Y.; Zhang, W.; Shi, B. Effect of soil pH on the transport, fractionation, and oxidation of chromium(III). Ecotoxicol. Environ. Saf. 2020, 195, 110459. [Google Scholar] [CrossRef]
- Raj, J.; Ananthi, V. Recurrent neural networks and nonlinear prediction in support vector machines. J. Soft Comput. Paradig. 2019, 2019, 33–40. [Google Scholar] [CrossRef]
- Saarela, M.; Jauhiainen, S. Comparison of feature importance measures as explanations for classification models. SN Appl. Sci. 2021, 3, 272. [Google Scholar] [CrossRef]
- Gregorutti, B.; Michel, B.; Saint-Pierre, P. Correlation and variable importance in random forests. Stat. Comput. 2013, 27, 659–678. [Google Scholar] [CrossRef]
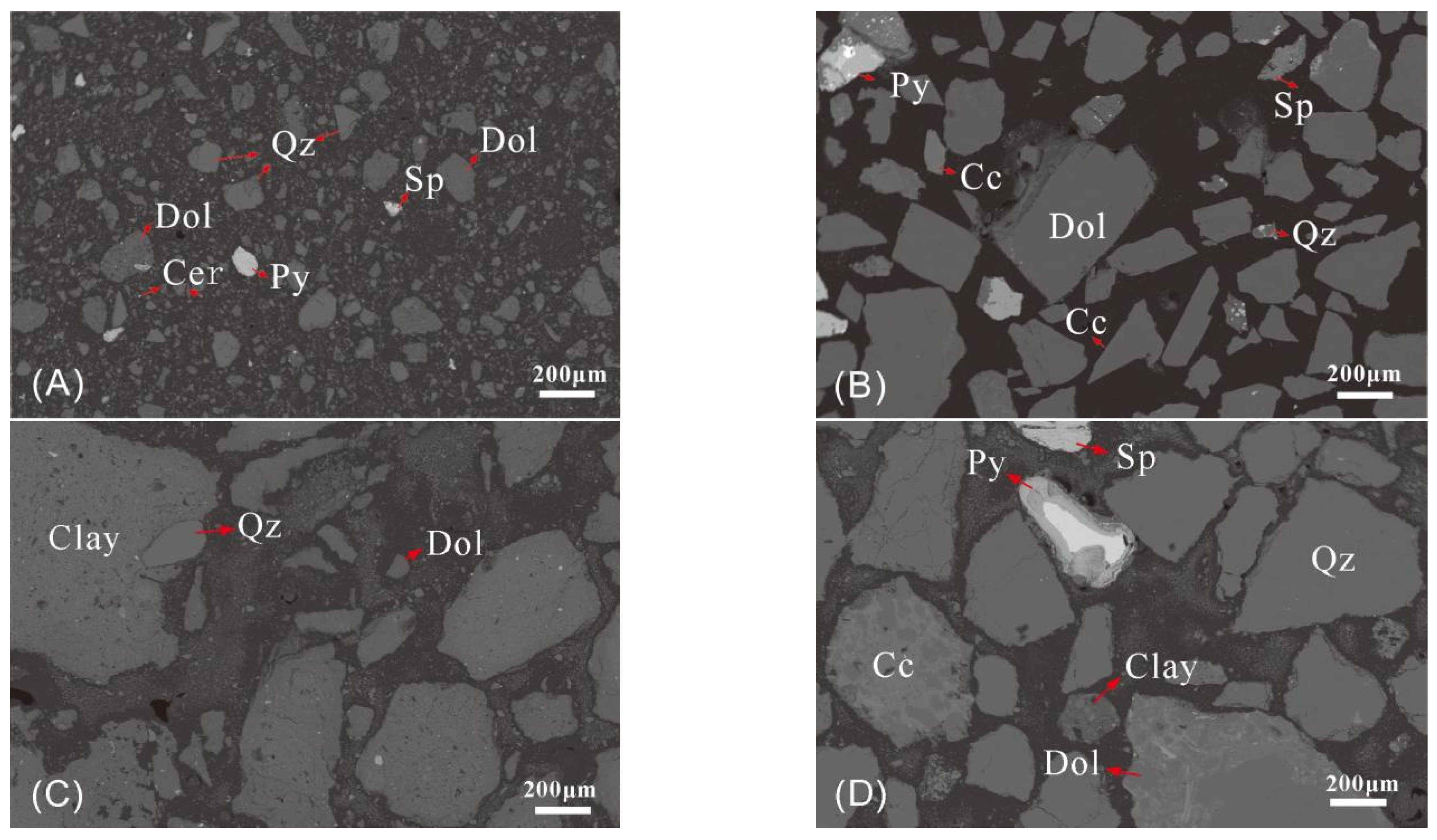
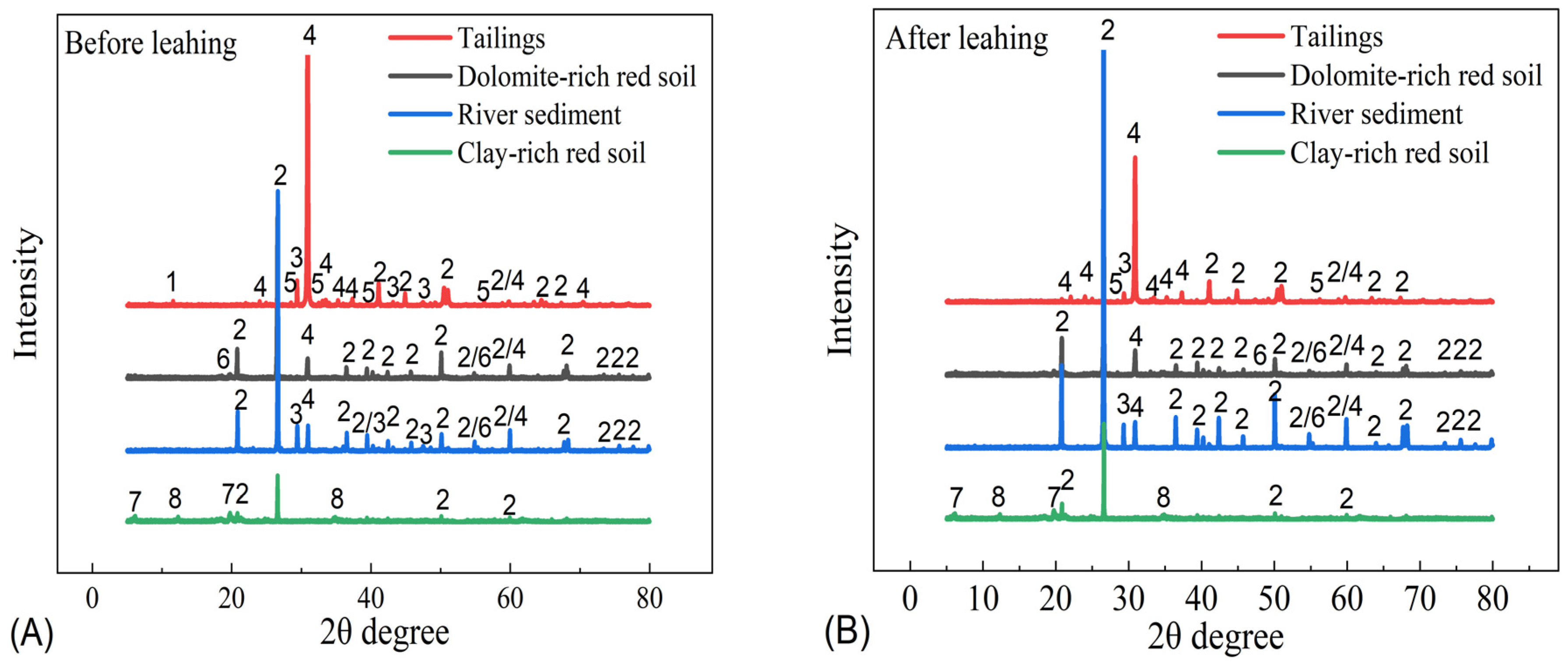
| Sample | N1 * | N2 | N3 | N4 | N5 | |
|---|---|---|---|---|---|---|
| Dolomite | Dol | 50 | 70 | 68 | 18 | 5 |
| Calcite | Cc | 0 | 12 | 9 | 3 | <1 |
| Quartz | Qz | 38 | 8 | 11 | 71 | 0 |
| Clay minerals | Clay | 0 | 1 | 9 | 0 | 93 |
| Pyrite | Sp | 8 | 5 | 2 | 5 | <1 |
| Cerussite | Cer | 1 | 1 | 0 | 0 | 0 |
| Sphalerite | Py | 3 | 3 | 1 | 3 | 0 |
| Sample | Location | Type | pH | ω (%) | As | Cd | Cr | Cu | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | TSK | Tailings | 7.24 | 14.7 | 321 ± 3.42 | 169 ± 5.35 | 117 ± 3.9 | 265 ± 8.42 | 5562 ± 145 | 15362 ± 787 |
| N2 | TSK | Red soil | 7.07 | 18.6 | 563 ± 5.75 | 153 ± 2.46 | 127 ± 4.78 | 113 ± 2.73 | 4677 ± 150 | 16001 ± 429 |
| N3 | Farmland | Red soil | 7.33 | 13.6 | 731 ± 12.5 | 79.9 ± 1.58 | 178 ± 6.95 | 82.8 ± 0.22 | 1808 ± 23.5 | 6786 ± 376 |
| N4 | Siding River | Sediment | 7.82 | 14.7 | 78.7 ± 0.47 | 38.5 ± 0.47 | 147 ± 1.77 | 56.5 ± 1.25 | 711 ± 5.51 | 4389 ± 215 |
| N5 | Slope | Red soil | 7.37 | 21.4 | 786 ± 10.6 | 10.3 ± 0.57 | 272 ± 3.27 | 80.5 ± 2.71 | 178 ± 5.38 | 506 ± 30.5 |
| Average value | 7.37 | 16.6 | 496 ± 3.54 | 90.1 ± 1.23 | 168 ± 2.01 | 120 ± 1.87 | 2587 ± 42.1 | 8609 ± 199 | ||
| Liuzhou [31] | 21.6 | 0.73 | 111 | 25.0 | 38.1 | 129 | ||||
| China [32] | 11 | 0.1 | 61 | 23 | 26 | 74 | ||||
| Risk screening value | 60 | 65 | 5.7 | 18000 | 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Qian, J.; Ji, D. Machine Learning-Based Analysis of Heavy Metal Migration Under Acid Rain: Insights from the RF and SVM Algorithms. Minerals 2025, 15, 663. https://doi.org/10.3390/min15060663
Yao J, Qian J, Ji D. Machine Learning-Based Analysis of Heavy Metal Migration Under Acid Rain: Insights from the RF and SVM Algorithms. Minerals. 2025; 15(6):663. https://doi.org/10.3390/min15060663
Chicago/Turabian StyleYao, Jie, Jianping Qian, and Dongru Ji. 2025. "Machine Learning-Based Analysis of Heavy Metal Migration Under Acid Rain: Insights from the RF and SVM Algorithms" Minerals 15, no. 6: 663. https://doi.org/10.3390/min15060663
APA StyleYao, J., Qian, J., & Ji, D. (2025). Machine Learning-Based Analysis of Heavy Metal Migration Under Acid Rain: Insights from the RF and SVM Algorithms. Minerals, 15(6), 663. https://doi.org/10.3390/min15060663








