Crystal Chemistry and Thermodynamic Properties of Mineralogically Probable Phosphate Ca2.62Cu1.94Co1.44(PO4)4—Structurally Related to Natural Arsenate Zubkovaite
Abstract
1. Introduction
2. Materials and Methods
2.1. Flux Synthesis
2.2. X-Ray Energy-Dispersive Analysis
2.3. Single-Crystal X-Ray Diffraction and Crystal Structure Determination
3. Results and Discussion
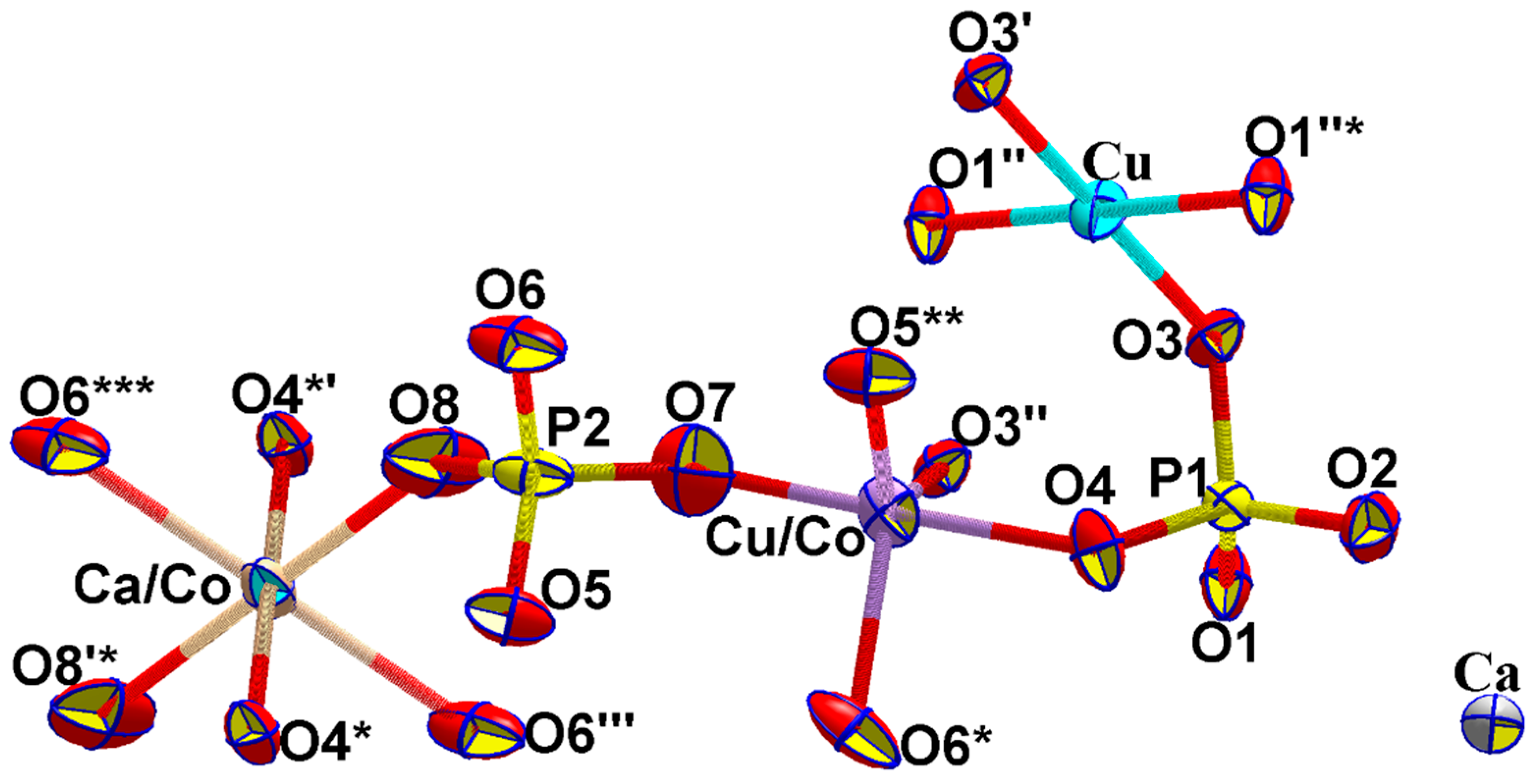
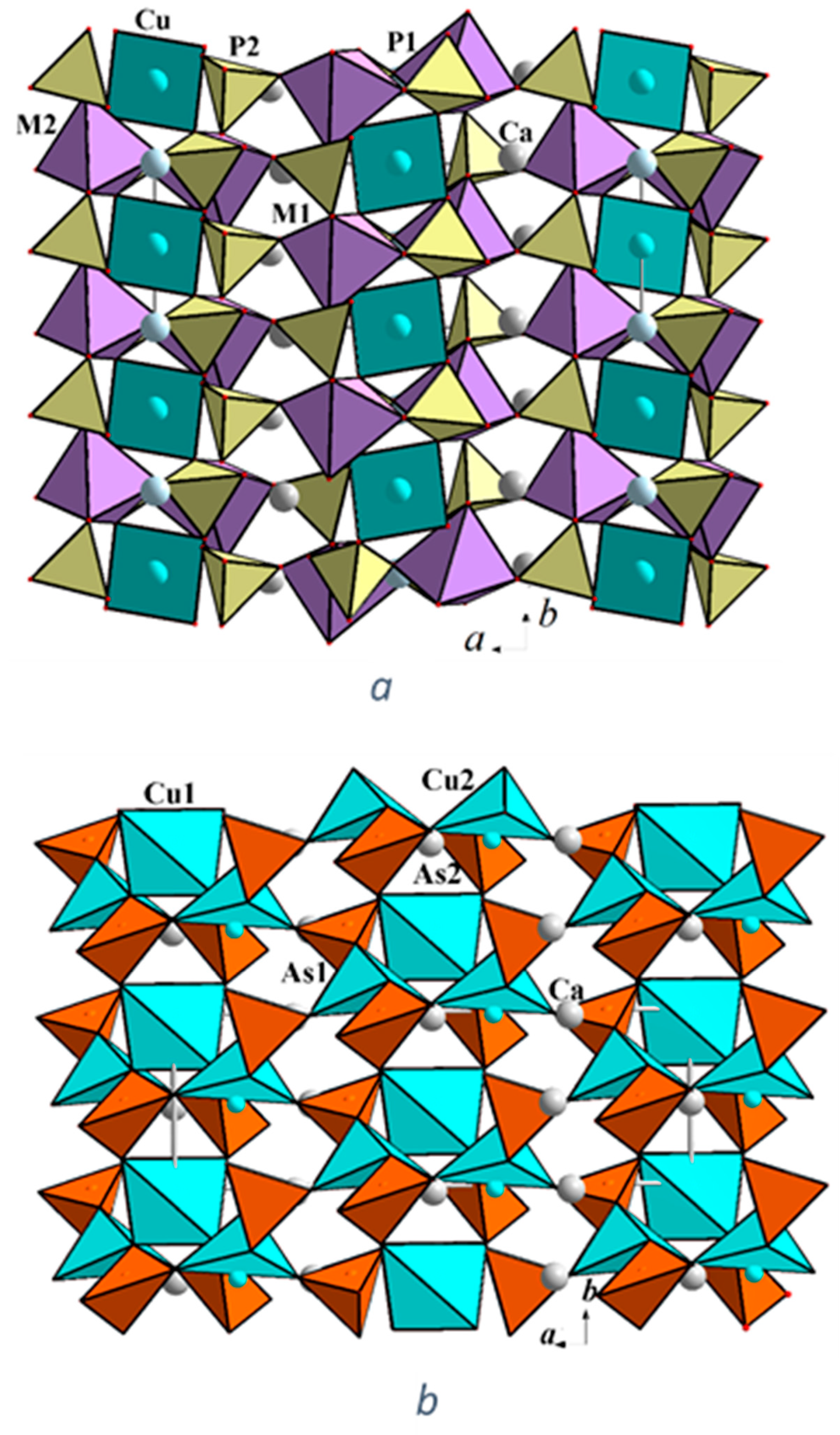
3.1. Interatomic Distances and Crystal Structure Description
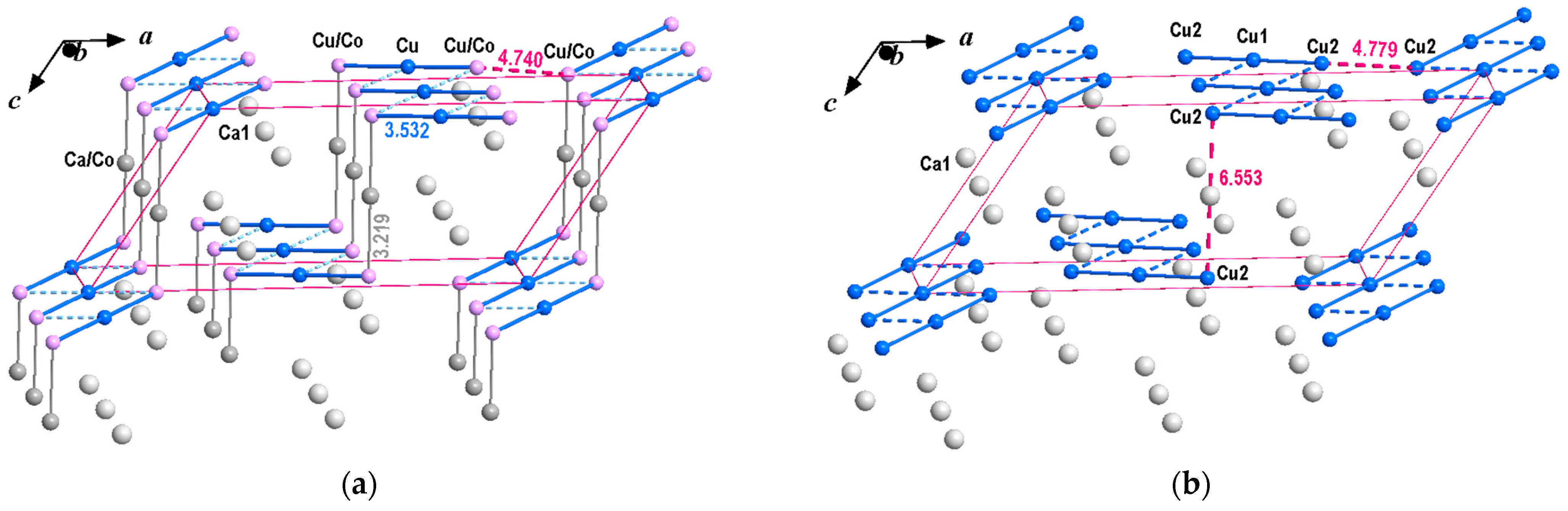
3.2. Crystal Chemical Relations
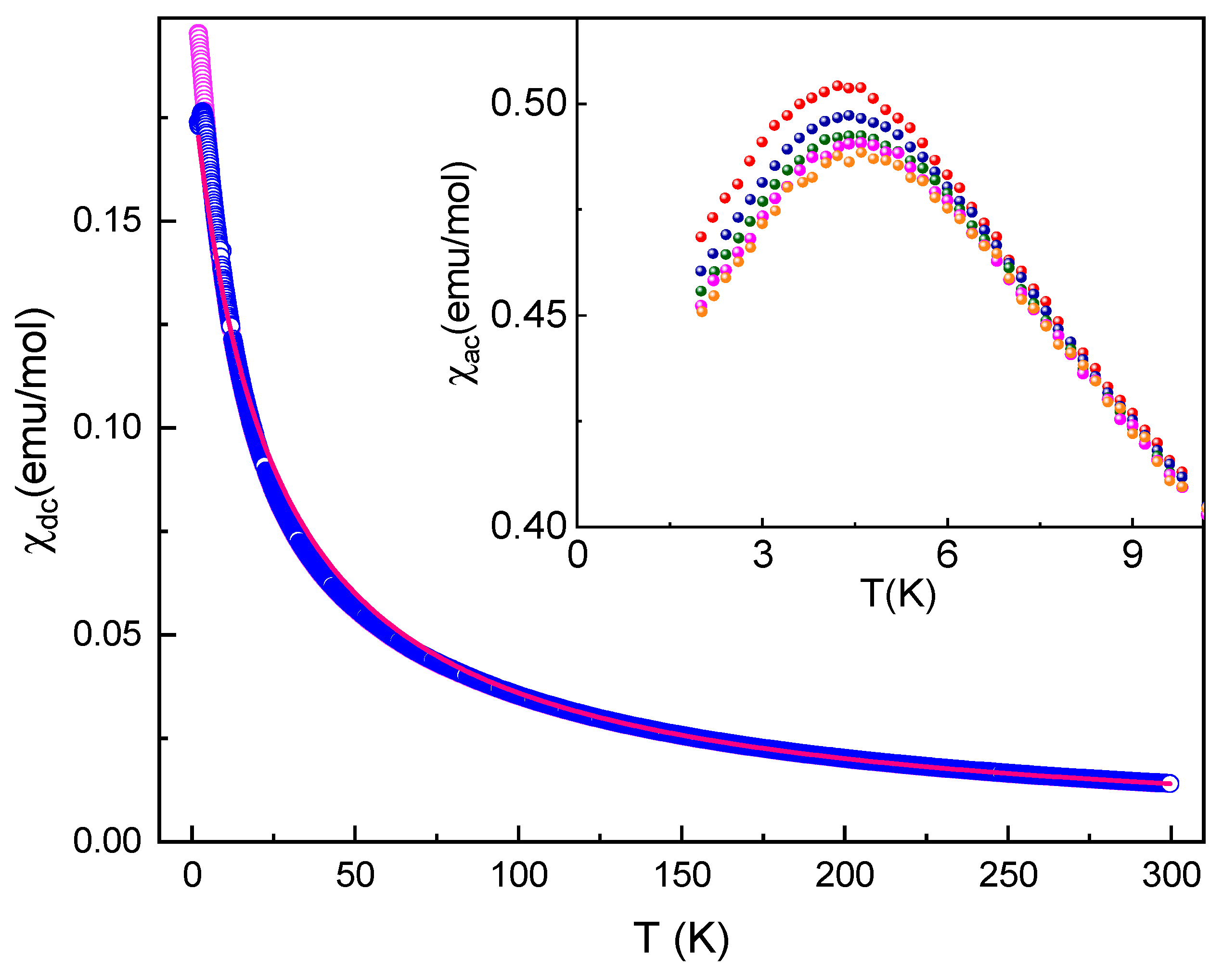
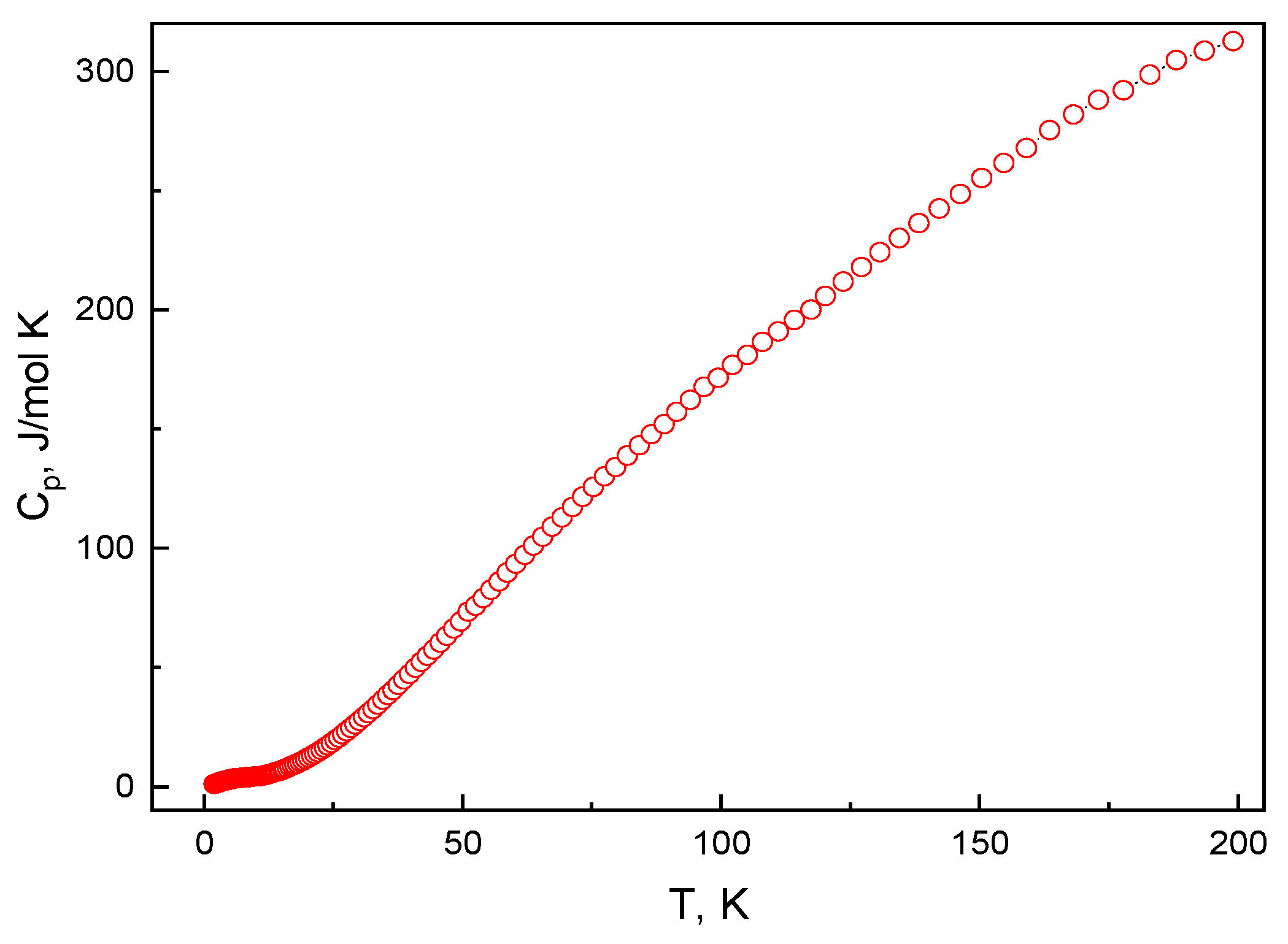
3.3. Thermodynamic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christy, A.G. Causes of anomalous mineralogical diversity in the Periodic Table. Miner. Mag. 2015, 79, 33–49. [Google Scholar] [CrossRef]
- Hazen, R.M.; Hystad, G.; Downs, R.T.; GoLden, J.J.; Pires, A.J.; Grew, E.S. Earth’s “missing” minerals. Am. Mineral. 2015, 100, 2344–2347. [Google Scholar] [CrossRef]
- Hystad, G.; Downs, R.T.; Hazen, R.M. Mineral Species Frequency Distribution Conforms to a Large Number of Rare Events Model: Prediction of Earth’s Missing Minerals. Math. Geosci. 2015, 47, 647–661. [Google Scholar] [CrossRef]
- Hummer, D.R. The Carbon Mineral Challenge: A worldwide effort to find Earth’s missing carbon minerals. Aust. J. Min. 2019, 20, 55–63. [Google Scholar]
- Hazen, R.M.; Morrison, S.M. On the paragenetic modes of minerals: A mineral evolution perspective. Am. Mineral. 2022, 107, 1262–1287. [Google Scholar] [CrossRef]
- Pyatenko, Y.A. Mineralogically probable and unlikely crystal structures. Int. Geol. Rev. 1984, 26, 40–46. (In Russian) [Google Scholar] [CrossRef]
- Lu, A. Mineral evolution heralds a new era for mineralogy. Am. Mineral. 2022, 107, 1217–1218. [Google Scholar] [CrossRef]
- Hazen, R.M.; Hystad, G.; Golden, J.J.; Hummer, D.R.; Liu, C.; Downs, R.T.; Morrison, S.M.; Ralph, J.; Grew, E.S. Cobalt mineral ecology. Am. Mineral. 2017, 102, 108–116. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Mikhailova, Y.A.; Selivanova, E.A.; Krivovichev, S.V. Pakhomovskyite, Co3(PO4)2·8H2O, a new mineral species from Kovdor, Kola Peninsula, Russia. Can. Mineral. 2006, 44, 117–123. [Google Scholar] [CrossRef]
- Dumasa, E.; Taulelleb, F.; Férey, G. Synthesis and crystal structure of the synthetic analogue of mineral minyulite K[Al2F(H2O)4(PO4)2]. Structural correlations with AlPO4-CJ2. Solid State Sci. 2001, 3, 613–621. [Google Scholar] [CrossRef]
- Krickl, R.; Wildner, M. Crystal chemistry of synthetic Co- and Ni-analogues of natrochalcite– the shortest known hydrogen bonds among mineral-type compounds Part I: Single-crystal X-ray structures. Eur. J. Mineral. 2007, 19, 805. [Google Scholar] [CrossRef]
- Samburov, G.O.; Kalashnikova, G.O.; Panikorovskii, T.L.; Kasikov, A.; Selivanova, E.; Bocharov, V.N.; Bazai, A.V.; Bernadskaya, D.; Yakovenchuk, V.N.; Krivovichev, S.V. A synthetic analog of the mineral ivanyukite: Sorption behavior to lead cations. Crystals 2022, 12, 311. [Google Scholar] [CrossRef]
- Nekrasova, D.O.; Siidra, O.I.; Zaitsev, A.N.; Ugolkov, V.; Colmont, M.; Charkin, D.O.; Mentré, O.; Chen, R.; Kovrugin, V.M.; Borisov, A.S. A fumarole in a one-pot: Synthesis, crystal structure and properties of Zn-and Mg-analogues of itelenite and a synthetic analogue of glikinite. Phys. Chem. Miner. 2021, 48, 6. [Google Scholar] [CrossRef]
- Cumby, J.; Bayliss, R.D.; Berry, F.J.; Greaves, C. Synthetic analogues of Fe(II)–Fe(III) minerals containing a pentagonal ‘Cairo’ magnetic lattice. Dalton Trans. 2016, 45, 11801. [Google Scholar] [CrossRef]
- Piro, O.E.; Baran, E.J. Crystal chemistry of organic minerals–salts of organic acids: The synthetic approach. Crystallogr. Rev. 2018, 24, 149–175. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Urusov, V.S. The genetic crystal chemistry of pegmatite phosphates. Mosc. Univ. Geol. Bull. 1996, 51, 18. [Google Scholar]
- Kiriukhina, G.; Nesterova, V.; Yakubovich, O.; Volkov, A.; Dimitrova, O.; Trigub, A.; Lyssenko, K. Mixed valanced V3+, V2+ phosphate Na7V4(PO4)6: A structural analogue of mineral yurmarinite. Minerals 2022, 12, 1517. [Google Scholar] [CrossRef]
- Yakubovich, O.; Kiriukhina, G.; Simonov, S.; Volkov, A.; Dimitrova, O. Na3(VO)(PO4)(CO3): A synthetic member of the bradleyite phosphate carbonate family with a new type of crystal structure. CrystEngComm 2023, 25, 4024–4032. [Google Scholar] [CrossRef]
- Yakubovich, O.; Kiriukhina, G.; Nesterova, V.; Volkov, A.; Fedotov, S.; Dimitrova, O. Crystal Chemistry of a New Mineral-like Phosphate Na6.9Ni2+0.9V3+4.3Al0.8(PO4)8(H2O)2 in the Series of α-CrPO4 Derivatives. Minerals 2025, 15, 3. [Google Scholar] [CrossRef]
- Shvanskaya, L.; Yakubovich, O.; Krikunova, P.; Kiriukhina, G.; Ivanova, A.; Volkov, A.; Dimitrova, O.; Borovikova, E.; Volkova, O.; Vasiliev, A. Nonstoichiometric Ellenbergerite-Type Phosphates: Hydrothermal Synthesis, Crystal Chemistry, and Magnetic Behavior. Inorg. Chem. 2022, 61, 4879. [Google Scholar] [CrossRef]
- Shvanskaya, L.V.; Krikunova, P.V.; Vasilchikova, T.M.; Borovikova, E.Y.; Volkova, O.S.; Vasiliev, A.N. Crystal structure, infrared spectroscopy and thermodynamic properties of a manganese member of the ellenbergerite family. New J. Chem. 2024, 48, 1952. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849. [Google Scholar] [CrossRef]
- International Tables for Crystallography, 3rd ed.; Prince, E., Ed.; Table 4.2.6.8 and 6.1.14; Kluwer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, 41, 244. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Crystallogr. 2015, B71, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Randenburg, K. DIAMOND; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Pekov, I.V.; Lykova, I.S.; Agakhanov, A.A.; Belakovskiy, D.I.; Vigasina, M.F.; Britvin, S.N.; Turchkova, A.G.; Sidorov, E.G.; Scheidl, K.S. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. XII. Zubkovaite, Ca3Cu3(AsO4)4. Mineral. Mag. 2019, 83, 879. [Google Scholar] [CrossRef]
- Osterloh, D.; Miiller-Buschbaum, H. Zur Kenntnis eines Calcium-Kupfer-Orthoarsenats: Ca1.5Cu1.5(AsO4)2. J. Alloys Compounds. 1994, 206, 155. [Google Scholar] [CrossRef]
- Anderson, J.B.; Kostiner, E.; Ruszala, F.A. The crystal structure of Ca3Cu3(PO4)4. J. Solid State Chem. 1981, 39, 29. [Google Scholar] [CrossRef]
- Eder, F.; Weil, M. Crystal structure of Zn2(HTeO3)(AsO4). Acta Cryst. 2021, E77, 555–558. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Belakovskiy, D.I.; Yapaskurt, V.O.; Vigasina, M.F.; Sidorov, E.G.; Pushcharovsky, D.Y. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. II. Ericlaxmanite and kozyrevskite, two natural modifications of Cu4O(AsO4)2. Mineral. Mag. 2015, 79, 1737. [Google Scholar] [CrossRef]
- Elliott, P.; Kolitsch, U.; Willis, A.C.; Libowitzky, E. Description and crystal structure of domerockite, Cu4(AsO4)(AsO3OH)(OH)3·H2O, a new mineral from the Dome Rock Mine, South Australia. Mineral. Mag. 2013, 77, 509–522. [Google Scholar] [CrossRef]
- Matsuda, M.; Kakurai, K.; Belik, A.A.; Azuma, M.; Takano, M.; Fujita, M. Magnetic excitations from the linear Heisenberg antiferromagnetic spin trimer system A3Cu3(PO4)4 (A =Ca, Sr, and Pb). Phys. Rev. B. 2005, 71, 144411. [Google Scholar] [CrossRef]
- Belik, A.A.; Matsuo, A.; Azuma, M.; Kindo, K.; Takano, M. Long-range magnetic ordering of S= 1/2 linear trimers in A3Cu3(PO4)4 (A= Ca, Sr, and Pb). J. Solid State Chem. 2005, 178, 709–714. [Google Scholar] [CrossRef]
- Drillon, M.; Belaiche, M.; Legoll, P.; Aride, J.; Boukhari, A.; Moqine, A. 1D ferrimagnetism in copper (II) trimetric chains: Specific heat and magnetic behavior of A3Cu3(PO4)4 with A= Ca, Sr. J. Magn. Mater. 1993, 128, 83–92. [Google Scholar] [CrossRef]
- Drillon, M.; Coronado, E.; Belaiche, M.; Carlin, R.L. Low-dimensional magnetic systems; from 1D to 3D ferrimagnets. J. Appl. Phys. 1988, 63, 3551–3553. [Google Scholar] [CrossRef]
- Boukhari, A.; Moqine, A.; Flandrois, S. A new linear trimeric magnetic ion in (Ca, Sr)3Cu3 (PO4)4 phosphates. Mater. Res. Bull. 1986, 21, 395–400. [Google Scholar] [CrossRef]
- Pomjakushin, V.Y.; Furrer, A.; Sheptyakov, D.V.; Pomjakushina, E.V.; Conder, K. Crystal and magnetic structures of the spin-trimer compounds Ca3Cu3− xNix(PO4)4 (x= 0, 1, 2). Phys. Rev. B. 2007, 76, 174433. [Google Scholar] [CrossRef]


| Crystal Data | |
|---|---|
| Chemical formula | Ca5.25Co2.89Cu3.86(PO4)8 |
| Mr | 1385.75 |
| Crystal system, space group | Monoclinic, P21/n |
| a, b, c (Å) | 8.8040 (2), 4.8970 (1), 14.5772 (3) |
| β (°) | 93.993 (2) |
| V (Å3) | 626.94 (2) |
| Z, calculated density, g/cm3 | 1, 3.670 |
| µ (mm−1) | 6.81 |
| Crystal size (mm) | 0.31 × 0.13 × 0.04 |
| Data collection | |
| Radiation type | MoKα, graphite monochromator |
| Temperature (K) | 293 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 5597, 1825, 1694 |
| Rint | 0.027 |
| (sin θ/λ)max (Å−1) | 0.703 |
| Refinement | |
| Refinement method | Full-matrix least-squares on F2 |
| Absorption correction, Tmin/Tmax | Gaussian, 1.0/0.77 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.038, 0.074, 1.28 |
| No. of reflections/parameters | 1825/124 |
| Extinction coefficient | 0.0020 (3) |
| Δρmax, Δρmin (e Å−3) | 0.77, −0.77 |
| x/a | y/b | z/c | Ueq | Occ. (<1) | |
|---|---|---|---|---|---|
| Co1 | 0.32038 (6) | 0.47909 (11) | 0.62037 (4) | 0.01357 (18) | 0.53 (3) |
| Cu1 | 0.32038 (6) | 0.47909 (11) | 0.62037 (4) | 0.01357 (18) | 0.47 (3) |
| Cu2 | 0.5 | 0.0 | 0.5 | 0.01053 (18) | |
| Ca1 | 0.04023 (9) | 0.53530 (17) | 0.23560 (6) | 0.01270 (19) | |
| Ca2 | 0.5 | 0.0 | 0.0 | 0.0117 (3) | 0.62 (1) |
| Co2 | 0.5 | 0.0 | 0.0 | 0.0117 (3) | 0.38 (1) |
| P1 | 0.34151 (11) | 0.49025 (19) | 0.40507 (7) | 0.0074 (2) | |
| P2 | 0.37855 (12) | 0.4895 (2) | 0.84517 (8) | 0.0120 (2) | |
| O1 | 0.4000 (3) | 0.7875 (6) | 0.39869 (19) | 0.0116 (6) | |
| O2 | 0.2570 (3) | 0.3997 (6) | 0.3174 (2) | 0.0129 (6) | |
| O3 | 0.4886 (3) | 0.3084 (6) | 0.41840 (19) | 0.0093 (5) | |
| O4 | 0.2486 (3) | 0.4496 (6) | 0.48902 (19) | 0.0138 (6) | |
| O5 | 0.2121 (3) | 0.5751 (6) | 0.8572 (2) | 0.0155 (6) | |
| O6 | 0.3936 (4) | 0.1799 (6) | 0.8634 (2) | 0.0189 (7) | |
| O7 | 0.4144 (4) | 0.5377 (7) | 0.7437 (2) | 0.0213 (7) | |
| O8 | 0.4925 (4) | 0.6575 (7) | 0.9030 (3) | 0.0248 (8) |
| P1—Tetrahedron | P2—Tetrahedron | Cu2—Square Planar |
|---|---|---|
| P1—O2 1.500 (3) | P2—O8 1.509 (3) | Cu2—O3 1.921 (3) × 2 |
| O4 1.532 (3) | O6 1.543 (3) | O1 1.964 (3) × 2 |
| O1 1.549 (3) | O5 1.546 (3) | <Cu2—O> 1.943 |
| O3 1.572 (3) | O7 1.551 (3) | |
| <P1—O> 1.538 | <P2—O> 1.537 | |
| M1—five-vertex polyhedron | M2—octahedron | Ca1—polyhedron |
| M1—O7 1.947 (3) | M2—O8 2.192 (3) × 2 | Ca1—O2 2.278 (3) |
| O4 1.980 (3) | O4 2.219 (3) × 2 | O7 2.374 (3) |
| O5 2.029 (3) | O6 2.315 (3) × 2 | O1 2.393 (3) |
| O3 2.089 (3) | <M2—O> 2.242 | O6 2.566 (3) |
| O6 2.152 (3) | O5 2.576 (3) | |
| <M1—O> 2.039 | O3 2.610 (3) | |
| O2 2.676 (3) | ||
| O8 2.922 (4) | ||
| O7 3.022 (3) | ||
| <Ca1—O> 2.602 |
| Atom | M1 | Cu1 | Ca1 | M2 | P1 | P2 | ∑ |
|---|---|---|---|---|---|---|---|
| O1 | 0.463↓2 | 0.298 | 1.202 | 1.96 | |||
| O2 | 0.394; 0.149 | 1.372 | 1.92 | ||||
| O3 | 0.342 | 0.520↓2 | 0.175 | 1.129 | 2.17 | ||
| O4 | 0.453 | 0.374↓2 | 1.258 | 2.09 | |||
| O5 | 0.371 | 0.190 | 1.212 | 1.77 | |||
| O6 | 0.288 | 0.195 | 0.294↓2 | 1.218 | 2.00 | ||
| O7 | 0.494 | 0.312; 0.064 | 1.195 | 2.06 | |||
| O8 | 0.082 | 0.399↓2 | 1.339 | 1.82 | |||
| ∑ | 1.95 | 1.97 | 1.86 | 2.13 | 4.96 | 4.96 |
| Compound | Unit Cell Parameters (a, b, c) Å and Angles, ° | Space Group V, Å3 ρcalc., g/cm3 | Average Cation—Oxygen Distances, Å, and Angles, ° | Synthesis technique/ Natural Genesis | Ref. |
|---|---|---|---|---|---|
| Zubkovaite, Ca3Cu3(AsO4)4 | 16.836 (3) 5.0405 (8) 9.1173 (17) β = 117.39 (1) | C2 687.0 4.161 | <Cu1—O> 1.953 <Ca1—O> 2.641 <Cu2—O> 2.032 <Ca2—O> 2.330 <As1—O> 1.679 <As2—O> 1.666 O-As1-O range: 76.1–134.3 O-As2-O range: 73.3–129.3 | Deposited directly from volcanic gas as a sublimate or crystallized as a result of the interaction between fumarolic gas and basalt scoria at temperatures not lower than 400 °C. | [30] |
| Ca3Cu3(AsO4)4 | 16.609 (9) 5.056 (4) 8.950 (6) β = 117.08 (5) | P21/a 669.16 4.30 | <Cu1—O> 1.945 <Ca1—O> 2.632 <Cu2—O> 2.025 <Ca2—O> 2.202 <As1—O> 1.693 <As2—O> 1.689 O-As1-O range: 105.2–113.5 O-As2-O range: 104.7–113.2 | Prepared by the solid-state reaction of CuO, CaCO3, and 3As2O5·5H2O pressed into tablets, at 980 °C (below the melting point). | [31] |
| Ca3Cu3(PO4)4 | 17.619 (2) 4.8995 (4) 8.917 (1) β = 124.08 (1) | P21/a 637.6 3.598 | <Cu1—O> 1.943 <Ca1—O> 2.607 <Cu2—O> 2.029 <Ca2—O> 2.333 <P1—O> 1.538 <P2—O> 1.539 O-P1-O range: 106.1–112.2 O-P2-O range: 105.2–113.6 | Synthesized under hydrothermal conditions at 420 °C and 3.8 kbar using a mixture of hydroxyapatite and Cu3(PO4)2 suspended in 0.1 M H3PO4 mineralizer solution. | [32] |
| Ca2.62Cu1.94Co1.44(PO4)4 | 17.5465 (4) 4.8970 (1) 8.8040 (2) β = 124.03 (1) | P21/a 626.94 3.668 | <Cu1—O> 1.943 <Ca1—O> 2.602 <M1—O> 2.039 <M2—O> 2.242 <P1—O> 1.538 <P2—O> 1.537 O-P1-O range: 105.4–112.4 O-P2-O range: 106.2–113.2 | Flux from a mixture of CaCO3, CuCl2·2H2O, CoCl2·6H2O, and (NH4)2HPO4 at 820 °C. | Our data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakubovich, O.; Kiriukhina, G.; Shvanskaya, L.; Vasiliev, A. Crystal Chemistry and Thermodynamic Properties of Mineralogically Probable Phosphate Ca2.62Cu1.94Co1.44(PO4)4—Structurally Related to Natural Arsenate Zubkovaite. Minerals 2025, 15, 645. https://doi.org/10.3390/min15060645
Yakubovich O, Kiriukhina G, Shvanskaya L, Vasiliev A. Crystal Chemistry and Thermodynamic Properties of Mineralogically Probable Phosphate Ca2.62Cu1.94Co1.44(PO4)4—Structurally Related to Natural Arsenate Zubkovaite. Minerals. 2025; 15(6):645. https://doi.org/10.3390/min15060645
Chicago/Turabian StyleYakubovich, Olga, Galina Kiriukhina, Larisa Shvanskaya, and Alexander Vasiliev. 2025. "Crystal Chemistry and Thermodynamic Properties of Mineralogically Probable Phosphate Ca2.62Cu1.94Co1.44(PO4)4—Structurally Related to Natural Arsenate Zubkovaite" Minerals 15, no. 6: 645. https://doi.org/10.3390/min15060645
APA StyleYakubovich, O., Kiriukhina, G., Shvanskaya, L., & Vasiliev, A. (2025). Crystal Chemistry and Thermodynamic Properties of Mineralogically Probable Phosphate Ca2.62Cu1.94Co1.44(PO4)4—Structurally Related to Natural Arsenate Zubkovaite. Minerals, 15(6), 645. https://doi.org/10.3390/min15060645







