Abstract
This study systematically reveals the fundamental mechanisms controlling redox-induced phase transformations occurring in basalt melting processes via integrated high-temperature redox experiments combined with thermodynamic simulations. Our findings demonstrate that oxidizing conditions drive clinopyroxene dissolution and concurrent crystallization of refractory phases—hematite [(Fe,Ti,Al)2O3] and magnesioferrite [(Mg,Fe)(Fe,Al)2O4]—where distinct crystallization pathways govern magnesioferrite morphology evolution. Conversely, reducing environments suppress oxide mineral formation while promoting phase transformation from high-melting-point plagioclase to low-melting-point clinopyroxene solid solutions, thus lowering the system’s liquidus temperature to achieve full melting. This provides a theoretical basis for optimizing energy consumption in basalt fiber production and offers new insights into the effects of material melting temperature.
1. Introduction
Continuous basalt fiber (CBF), derived from basalt through high-temperature melting, exhibits superior performance compared with traditional fibers, making it applicable to various fields such as road construction, energy conservation and environmental protection, aerospace, military equipment, and marine ships [1,2,3,4,5,6,7,8]. The preparation of CBF primarily involves melting basalt, where the melting temperature and duration are essential technological parameters [9,10]. The melting behavior of basalt is largely determined by its chemical and mineral composition [11,12]. Additionally, the presence of a reducing atmosphere can significantly influence the melting temperature and the quality of the final fiber by altering the valence state and content of iron ions.
Research indicates that adding redox agents to basalt raw materials or heat-treating CBF in a reducing atmosphere significantly affects fiber quality and the behavior of iron ions. Specifically, the tensile strength of fibers increased approximately linearly with the rise in Fe2+ content [13,14,15]. The valence state and content of iron ions were critical factors influencing the crystallization behavior of silicate systems. Fe2+ was recognized for its capacity to lower the initial crystallization temperature [15], while Fe3+ functioned as nucleating agents, thereby enhancing crystallization ability [16,17]. Moreover, the crystalline phases present in silicate systems were dependent on the environmental conditions. In oxidizing conditions, feldspar was the dominant crystalline mineral, while pyroxene was more prevalent in reducing conditions [18,19,20,21].
Notably, the majority of studies primarily utilized supplementary redox agents in their experimental designs [13,15,18,22,23], and focusing mainly on basalt in its glassy state. This focus has resulted in neglect of a systematic investigation into the melting process of basalt. Furthermore, to our knowledge, little research has been conducted on the effect of atmospheric changes during the CBF preparation process. During the melting of basalt, variations in oxidizing and reducing atmospheres significantly affect multivalent ions (Fe), and the resulting changes in iron ions in turn exert a pronounced influence on the melting process. Importantly, implementing lower melting temperatures and shortening melting durations in industrial manufacturing processes contribute to a decrease in production costs. Producing CBF under reducing conditions not only has the potential to enhance physical properties but also to lower the melting temperature and duration. Therefore, it is crucial to study the influence of different atmospheric conditions on the melting behavior of basalt.
This study utilizes Cenozoic basalt from Hainan Province, China, as the raw material. Initially, the chemical and mineral compositions of the basalt were analyzed. Subsequently, melting experiments were conducted under varying atmospheric conditions and temperatures. The apparent Gibbs free energy for mineral melting and crystallization processes was calculated, and the results from thermodynamic simulations were compared with experimental findings. These experiments and analyses provide important insights into variations in basalt melting processes and mineral transformation under varying conditions. The findings offer a theoretical foundation for enhancing the production process of continuous basalt fibers.
2. Materials and Methods
2.1. Material
The experimental samples are basalt from the Duowen Formation (Qp2d) of the Pleistocene in northern Hainan Province, China (Figure 1). Two samples, B1 and B2, were selected, both of which are dense, massive basalt (Figure 2).
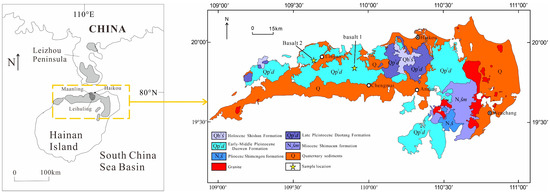
Figure 1.
Sampling positions of basalts (B1,B2) in Hainan Island (adapted from [24]).

Figure 2.
Sample photographs of Basalt (B1,B2).
2.2. Preparation Method of Melting Samples
The melting experiment was conducted using a SAFTherm STM-20-17 high-temperature furnace (SAF Therm, Luoyang, China). Seven grams of 200-mesh basalt powder were placed in a corundum crucible. The samples were heated from 50 °C to the target temperature and held for 1 h. Subsequently, to preserve the molten state, the samples were promptly removed from the furnace and quenched in cold water. Previous research indicated that the primary minerals in basalt typically began to melt at approximately 1100 °C, with a significant liquid phase becoming apparent around 1200 °C [12]. Therefore, the experimental temperatures were set at 1100 °C, 1200 °C, and 1300 °C. To further investigate the influence of atmospheric variations on the high-temperature melting behavior of basalt, an additional temperature point of 1250 °C was included in the experimental design. Detailed information regarding the melting temperature, atmospheric conditions, and experimental number for each sample is provided in Table 1.

Table 1.
Experimental conditions and sample number.
In order to create a reducing atmosphere during the melting process, the basalt raw material was positioned within a small covered crucible, which was subsequently placed inside a larger covered crucible containing an excess of graphite powder (Figure 3). The graphite powder, weighing 15 g and with a particle size of 200 mesh, met the standards for analytical reagents. The atmospheric air is inherently oxidizing. Therefore, no additional conditions were required to maintain an oxidizing atmosphere; the same apparatus could be used without the inclusion of graphite powder. For details on the applicability and theoretical basis of this reduction device, please refer to Supplementary Material S1.
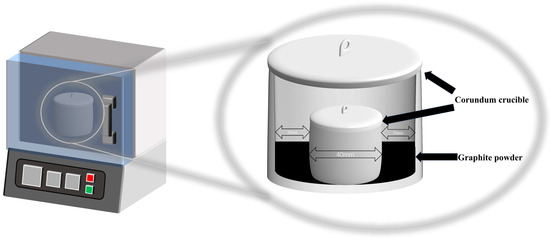
Figure 3.
Schematic diagram of atmosphere reduction device.
2.3. Analytical Methods
2.3.1. ICP-OES Element Analysis
The analysis of major elements in basalt samples was performed utilizing a 5300 DV ICP-OES (PerkinElmer, Waltham, MA, USA) at Chengdu Spectra Analysis Technology Co., Ltd. The findings are presented in Table 2. The Fe2+ content was determined through potassium dichromate oxidation titration, with detailed methodologies provided in article [25]. The Fe3+ content was calculated by subtracting the Fe2+ from the total iron content, with detailed methodologies provided in Supplementary Material S2.

Table 2.
Chemical compositions of basalt B1 and B2 (wt%).
2.3.2. X-Ray Diffraction
The mineralogical composition and crystalline structure of both the raw material and the melted samples were analyzed at the Chengdu University of Technology’s X-Ray Powder Diffraction Lab using a D8 ADVANCE XRD (BRUKER, Berlin, Germany). The testing conditions included a voltage of 40 kV and a current of 40 mA, with a diffraction slit of 0.6 mm, with a Cu target as the X-ray source. Various scanning rates were utilized depending on the sample type: 6°/min for raw materials, 0.8°/min for melting samples with significant diffraction peaks, and 0.2°/min for melting samples with a higher liquid phase, specifically B1-O-1300, B2-O-1300, B1-R-1200, B2-R-1200, B1-R-1250, and B2-R-1250. Quantitative phase analysis was conducted using TOPAS software (V6.0) (Bruker AXS, Germany) through Rietveld refinement methodology.
2.3.3. Scanning Electron Microscopy
The crystal morphology and composition of the melting samples were examined at the Scanning Electron Microscope Laboratory of Chengdu University of Technology using an FEI Nova NanoSEM 45 0 (FEI, Hillsboro, OR, USA) and EDAX AXF-650 APOLLO (EDAX, Berwyn, IL, USA). The instrument was operated at an accelerating voltage of 30 kV and a beam current of 140 μA. Due to the non-conductive nature of the basalt glass samples, a gilding process was required prior to SEM analysis.
3. Results
3.1. Raw Material Analysis
The chemical compositions of the two basalt samples are presented in Table 2. However, slight weathering in sample B1 caused the formation of a small amount of montmorillonite, leading to a minor Loss on Ignition (LOI). The test result error is less than 1%.
The primary minerals in both samples are plagioclase and clinopyroxene, with ilmenite as an accessory mineral (Figure 4). The quantitative analysis of the samples is presented in Table 3.
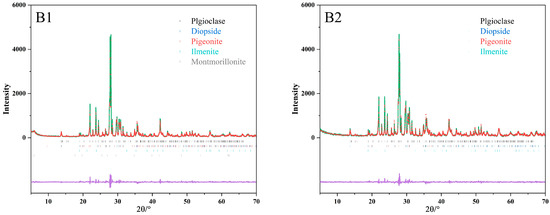
Figure 4.
Rietveld refinements of basalt samples (B1, B2), where vertical bars (in the middle of the figure) denote Bragg’s reflections of the crystalline phases. Calculated (Ic) and observed (Io) patterns are shown with red data points and green line, respectively. Difference between calculated and observed patterns (Ic–Io) is shown with purple line.

Table 3.
XRD analysis result of basalt B1 and B2 (%).
3.2. Variation in Valence States of Iron
To evaluate the reducing capability of the experimental apparatus, this study compared the valence distribution and content differences of iron elements in molten samples between the experimental group (reducing atmosphere) and control group (oxidizing atmosphere) under 1250 °C conditions (Supplementary Material S2, Table 1). As shown in Figure 5 (including error analysis), the reducing group exhibited a significant increase in Fe2+ proportion, while the oxidizing group was predominantly composed of Fe3+. These results demonstrate that the experimental apparatus effectively regulates reaction atmospheres, promoting Fe3+ conversion to Fe2+ under reducing conditions, while facilitating Fe3+ formation under oxidizing conditions. The experimental data conclusively validate the system’s capacity to create reductive environments, providing empirical evidence for its application in high-temperature reduction processes.
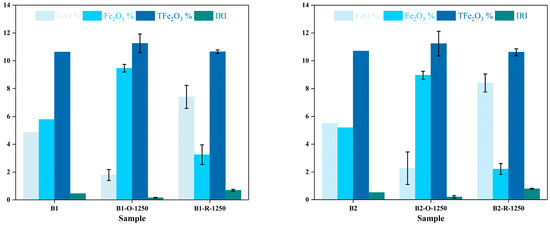
Figure 5.
Effect of Redox Atmosphere on Iron Oxidation States in Basaltic Melts (Data from Experimental and Control Groups).
3.3. Melting Samples Analysis
Figure 5 presents images of basalt samples B1 and B2, which were cooled after melting under varying atmospheric conditions and temperatures, along with their corresponding SEM images. In an oxidizing atmosphere, the samples exhibit notable changes as the temperature increases. At 1100 °C (Figure 6A), the sample appears brick red with a loose granular texture. As the temperature rises to 1200 °C (Figure 6B), the sample transitions to a glassy state, although fine-grained minerals remain visible on its surface. By 1250 °C (Figure 6C), there is a significant decline in crystalline minerals. Ultimately, at 1300 °C (Figure 6D), the sample is fully melted.
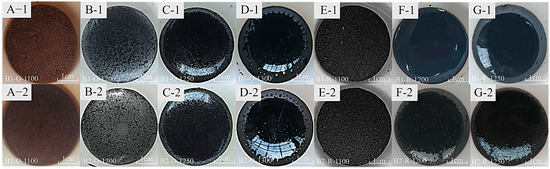
Figure 6.
Melting samples under oxidizing and reducing atmospheres at 1100–1300 °C (A-1–G-1) correspond to B1 samples, and (A-2–G-2) correspond to B2 samples.
Conversely, under a reducing atmosphere, the melting characteristics differ. At 1100 °C (Figure 6E), the sample appears entirely black. As the temperature increases to 1200 °C (Figure 6F), the molten sample exhibits almost a glassy phase. Unfortunately, the results of the XRD analysis (Figure 7) indicate the presence of a minor quantity of minerals. However, a significant transformation occurs at 1250 °C (Figure 6G), where the sample is fully melted into a liquid phase, and the surface displays a glassy luster.
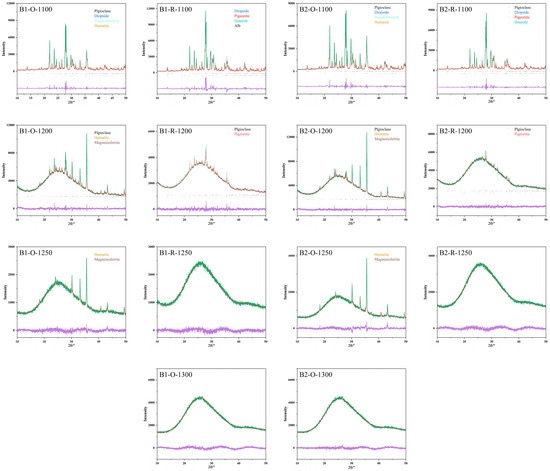
Figure 7.
XRD patterns of melting samples at 1100–1300 °C under different atmospheres. (B1-O,B1-R,B2-O,B2-R) respectively show the melting results of basalt (B1,B2) under oxidizing and reducing atmospheres. Vertical bars (in the middle of the figure) denote Bragg’s reflections of the crystalline phases. Calculated (Ic) and observed (Io) patterns are shown with red data points and green line, respectively. Difference between calculated and observed patterns (Ic–Io) is shown with purple line.
Figure 7 displays the XRD patterns of basalt samples B1 and B2, which were cooled after melting under different atmospheric conditions and temperatures. Under oxidizing conditions, when the basalt sample was heated to 1100 °C, plagioclase, clinopyroxene, pseudobrookite (formed through oxidation of ilmenite), and hematite were identified. Detailed characterization of the ilmenite oxidation process can be found in reference [26]. As the temperature increased to 1200 °C, clinopyroxene completely vanished, and magnesioferrite emerged. Further heating to 1250 °C led to the disappearance of plagioclase, leaving only minor amounts of hematite and magnesioferrite. At 1300 °C, the sharp peaks were no longer present, signifying that the basalt had entirely melted into disordered amorphous glass structures.
Under reducing conditions, the melting process of basalt was characterized by gradual reduction and the disappearance of mineral diffraction peaks with increasing temperature. At 1200 °C, ilmenite that had not undergone reduction disappeared (with no detectable diffraction peaks corresponding to reduction products such as rutile (TiO2) or metallic Fe [27]). Complete melting of all mineral phases was achieved at 1250 °C.
To evaluate the reliability of the experimental results, repeated experiments were conducted using identical basalt under consistent conditions to establish a control group, with XRD experiments being replicated. The results of this control group are presented in Supplementary Material S3. Phase variations and content changes observed in the control group results were closely aligned with those from the initial experiment, demonstrating the reliability of the experimental findings.
Figure 8 shows the SEM results of the molten samples from B1 and B2 under different conditions. As the temperature rises, mineral content decreases. A sample at 1200 °C in a reducing atmosphere has lower mineral content than one in an oxidative atmosphere. The reducing sample becomes fully glassy at 1250 °C, while the oxidative sample requires 1300 °C.
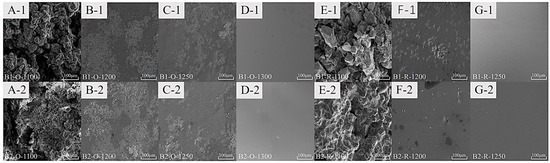
Figure 8.
Surface morphology of melting sample ((A-1–G-1) corresponds to B1 samples, (A-2–G-2) corresponds to B2 samples, with (A–D) indicating oxidizing conditions and (E–G) indicating reducing conditions).
4. Thermodynamic Calculation
With rising temperatures, the primary minerals in basalt—plagioclase and clinopyroxene—undergo melting reactions. These reactions are detailed in Equations (1) to (11). With increasing temperature, the primary minerals in basalt—plagioclase and clinopyroxene—undergo melting reactions, as described in Equations (1) to (4). Under oxidizing conditions, iron-titanium oxides and potentially involved spinel-group minerals crystallize from the melt, as delineated by chemical reaction Equations (5) and (10). Under reducing conditions, the primary reactions encompass the melting of plagioclase and clinopyroxene, alterations in the valence states of iron ions, and the formation of Ca-Tschermak (11). The study’s calculations are conducted under isobaric conditions with varying temperatures. The corresponding apparent Gibbs free energy calculations, which are crucial for understanding these reactions, are comprehensively detailed in Equations (12) to (26) [28,29,30].
NaAlSi3O8(s) = 2.5SiO2(l) + 0.5Al2O3(l) + 0.5Na2SiO3(l)
CaAl2Si2O8(s) = SiO2(l) + Al2O3(l) + CaSiO3(l)
CaFeSi2O6(s) = 0.5SiO2(l) + 0.5Fe2SiO4(l) + CaSiO3(l)
CaMgSi2O6(s) = 0.5SiO2(l) + 0.5Mg2SiO4(l) + CaSiO3(l)
Fe2SiO4(l) + 0.5O2(g) = Fe2O3(s) + SiO2(l)
Fe2SiO4(l) + TiO2(g) = FeTiO3(s) + SiO2(l)
0.5Mg2SiO4(l) + Fe2SiO4(l) + 0.5O2(g) = MgFe2O4(s) + 1.5SiO2(l)
3Fe2SiO4(l) + O2(g) = 2FeFe2O4(s) + 3SiO2(l)
Fe2SiO4(l) + 2Al2O3(l) = 2FeAl2O4(s) + SiO2(l)
Mg2SiO4(l) + 2Al2O3(l) = 2MgAl2O4(s) + SiO2(l)
Al2O3(l) + CaSiO3(l) = CaAl2SiO6(s)
At standard atmospheric pressure, the apparent free energy of the chemical reaction is defined by the following:
The calculation of apparent free energy for each substance is categorized into minerals (), liquids (), and gases ().
In the process of mineral melting, hematite, magnesioferrite and magnetite involve polymorphic transitions. The apparent free energy of polymorphic transitions is added to the free energy function given by Equation (12). Note that if T is greater than the transition temperature, , the integrations in (19) and (20) are performed between and , under standard atmospheric pressure .
The free energy function at temperatures above Tλ can be computed with the following:
, , and are enthalpy, third law entropy, and molar volume, respectively, at the reference pressure () and temperature (), is the liquid temperature, is the phase transition entropy, is the liquid phase heat capacity, is the coefficient of substance i in the reaction formula, k0, k1, k2, k3, a, b, c and d are molar heat capacity polynomial coefficients, and are the coefficients of thermal expansion and compressibility respectively multiplied by molar volume, which are the relevant thermodynamic calculation parameters to see Table 4, Table 5 and Table 6. , and represent the enthalpy, entropy, and heat capacity associated with polymorphic transitions at the reference pressure () and temperature (). (usually 298.15 K) and denote the temperatures of first-order and second-order (lambda) polymorphic transitions, respectively, and are the parameters for polymorphic transitions, as shown in Table 7.

Table 4.
Thermodynamic data for various mineral end-members [28,29].

Table 5.
Thermodynamics data for melt components [28,29].

Table 6.
Thermodynamics data for gas [30].

Table 7.
Thermodynamics data for gas [29].
Figure 9 presents the calculated results of the apparent Gibbs free energy for the aforementioned reactions. The melting reaction temperatures for NaAlSi3O8, CaAl2Si2O8, CaMgSi2O6, and CaFeSi2O6 are determined to be 1035.15 K (797 °C), 1226.15 K (953 °C), 1070.15K (982 °C), and 1255.15 K (762 °C), respectively. Therefore, the primary melting temperatures of the principal minerals, plagioclase and clinopyroxene, are affected by their compositional variations, with ranges spanning from 1070.15 K (797 °C) to 1226.15 K (953 °C) for plagioclase and from 1035.15 K (762 °C) to 1255.15 K (982 °C) for clinopyroxene. However, melting remains challenging within the specified temperature range. To address this issue, it is advisable to elevate the temperature beyond the material’s melting point. In the case of silicate minerals, they generally do not melt rapidly until they are heated to approximately 100-150 K above their melting point [31,32]. This additional heating supplies the requisite energy to surmount the thermodynamic and kinetic barriers, thereby enhancing the melting process. It is recommended to maintain the temperature between 1373.15 K (1100 °C) and 1473.15 K (1200 °C) to promote melting [12]. Equations (5)–(10) are pertinent to the reactions occurring in the melt, where the value of is consistently less than zero, indicating a tendency for crystallization within the melt. For details on potential thermodynamic calculations of ilmenite-hematite solid solutions and spinel-group mineral solid solutions, refer to References [33,34,35,36,37].
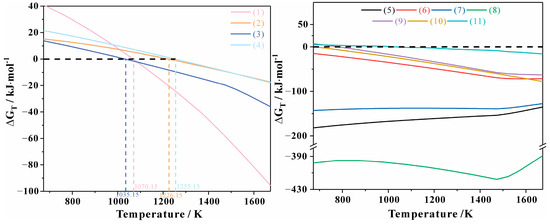
Figure 9.
Gibbs apparent free energy of reaction (1) to (11).
5. Discussion
5.1. Influence of Atmosphere on Melting Temperature of Basalt
Based on the experimental and control group results, the phase and content changes of basalt during its melting process under different atmospheres were analyzed, with the results shown in Figure 10. Both basalt samples began to exhibit a significant presence of the liquid phase at 1100 °C under varying atmospheric conditions. However, there is a notable difference in temperature when the liquid content reaches 100%. In an oxidizing environment, the liquid phase composition attained 100% at 1300 °C, while in a reducing environment, this occurred at 1250 °C.
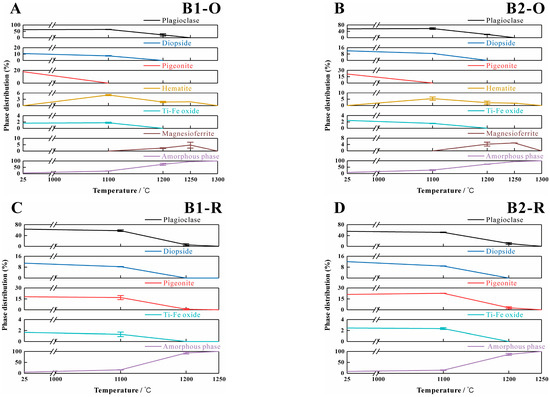
Figure 10.
Crystalline and amorphous phase variations in Basalt 1 and Basalt 2 under varying atmospheric conditions with error analysis. (A,C) Phase evolution profiles of Sample B1 under redox regimes. (B,D) Phase evolution profiles of Sample B2 under redox regimes. (O-oxidation; R-reduction).
To further investigate the impact of atmospheric conditions on the melting process of basalt, thermodynamic simulations were conducted using the software FactSage 8.3 (GTT-Technologies, Herzogenrath, Germany and Thermfact/CRCT, Montreal, QC, Canada). The major element composition of the two samples, as detailed in Table 2, was selected for the simulations. The simulation conditions included a temperature range from 400 °C to 1400 °C, with equilibrium points established at every 100 °C interval. The pressure was maintained at 101.325 kPa, equivalent to standard atmospheric pressure. For the oxidation condition, the atmosphere was set to 21% O2 and 79% N2, while for the reduction condition, the atmosphere was set to 21% CO and 79% N2. The FToxid oxide database was utilized for these simulations. The results of these simulations are presented in Figure 11.
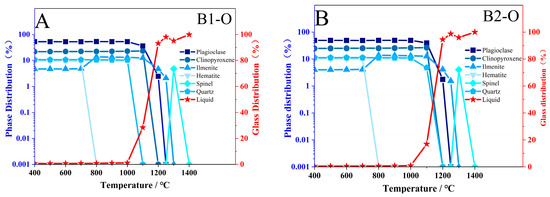
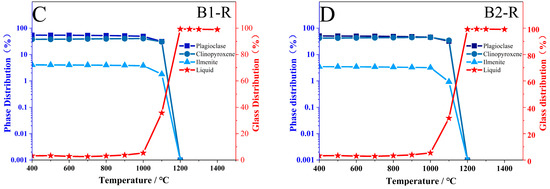
Figure 11.
Simulation Results of Samples B1 and B2 Using Thermodynamic Software Factsage. (A,B) oxidation; (C,D) reduction.
Figure 11 shows the simulation results; the numerical simulation and experimental results are consistent in phase in terms of phase transformation. In an oxidizing atmosphere, iron-containing oxides form during melting, whereas in a reducing atmosphere, the melting temperature decreases.
This temperature decline results from the inhibition of Fe2+ oxidation to Fe3+, which limits the crystallization of high-melting-point minerals such as hematite and magnesioferrite. Additionally, experiments and simulations interestingly demonstrate that the melting behavior of clinopyroxene and plagioclase is significantly influenced by different atmospheric conditions. In experimental results, the melting trend of plagioclase becomes more pronounced under reducing conditions (Figure 12A). This trend is further supported by simulation results, which indicate a decrease in the disappearance temperature of plagioclase to 1200 °C (Figure 12B). Conversely, under oxidizing conditions, the melting of clinopyroxene becomes more significant (Figure 12C), while simulations show a decrease in its content (Figure 12D). It is essential to examine the alterations in the primary mineral contents of basalt, as plagioclase and clinopyroxene play a crucial role in influencing both the initial liquidus temperature and the melting temperature. Specifically, these temperatures tend to increase with a higher content of plagioclase and a lower content of clinopyroxene [12].
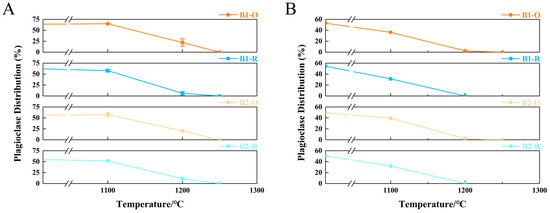
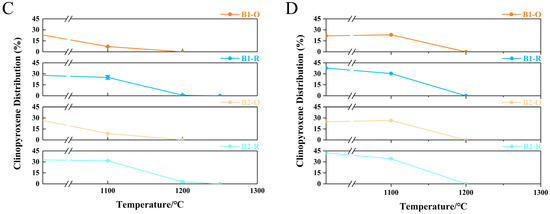
Figure 12.
The plagioclase and clinopyroxene content changes with temperature under varying atmospheric conditions (A,C) Experimental results; (B,D) Simulation results.
Therefore, melting basalt in a reducing atmosphere not only inhibits the crystallization of high-melting-point minerals (hematite and magnesioferrite) but also promotes the melting of plagioclase (a high-melting-point mineral). Based on XRD, SEM, and simulation results, compared to an oxidizing atmosphere, the melting temperature of basalt under reducing conditions can be effectively reduced by approximately 50 °C.
5.2. The Influence of Redox Atmosphere on the Crystallization Behavior of Basalt Materials During the Melting Process
The melting processes of basalt exhibit significant variations under different atmospheric conditions. Under oxidizing conditions, the primary rock-forming mineral clinopyroxene in basalt undergoes preferential melting, while hematite and magnesioferrite crystallize within the basaltic melt. In contrast, reducing conditions promote enhanced melting susceptibility of plagioclase, another principal rock-forming mineral in basaltic systems.
5.2.1. Influence of the Oxidizing Atmosphere on Basalt Melting Process
The discussion on melting mechanisms primarily encompasses two aspects: structural mechanisms and compositional mechanisms. The structural mechanism refers to crystal disintegration induced by crystal defects under external conditions [38,39,40], while the compositional mechanism involves elemental migration through dissolution/melting and diffusion processes [41,42,43,44]. Under the combined effects of these two mechanisms, clinopyroxene exhibits enhanced melting susceptibility under oxidizing conditions.
Recent studies propose that redox reactions can induce dislocation generation [45]. Significantly influenced by oxidation, Fe2+ in the clinopyroxene lattice oxidizes to Fe3+ during melting, as shown in Equation (27) [46]. This defect generation intensifies crystal disintegration and facilitates the breakage of bridging oxygen bonds, thereby promoting the melting of iron- and magnesium-rich clinopyroxene (pigeonite) (Figure 13①).
4Fe2++O2 → 4Fe3++2O2−
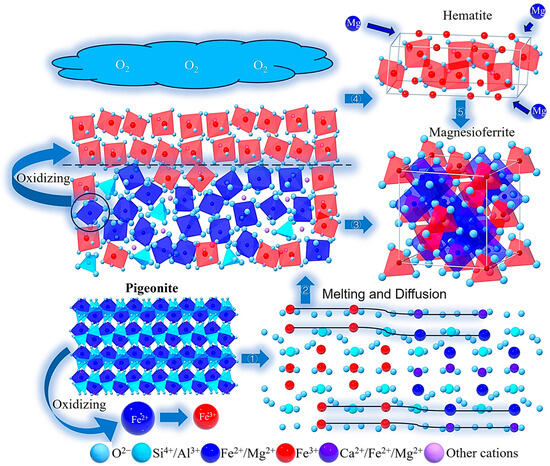
Figure 13.
Schematic Diagrams and SEM Images of Pigeonite Melting and Hematite-Magnesioferrite Crystallization Mechanisms.
The diffusion in silicate minerals can be categorized into volume diffusion and grain-boundary diffusion [41,42,43,44], where grain-boundary diffusion includes elemental migration at mineral-melt interfaces. During clinopyroxene melting, locally Fe-Mg-enriched melts form (Figure 13②). However, the iron and magnesium elements in the molten liquid phase are subsequently consumed to form magnesioferrite (as evidenced by octahedral granular magnesioferrite crystals observed on clinopyroxene surfaces with distinct cleavage planes in Figure 14A). The depletion of Fe-Mg elements promotes diffusion processes, thereby accelerating clinopyroxene melting.
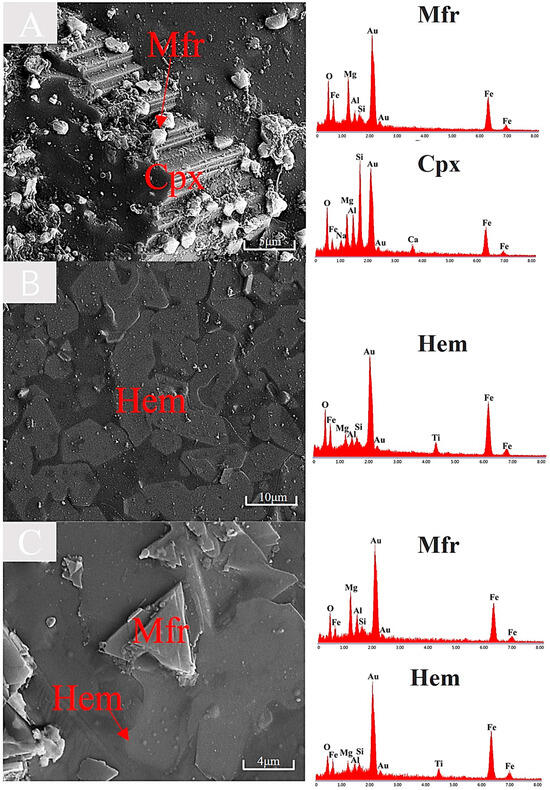
Figure 14.
SEM images of minerals under oxidizing atmosphere. (A) Melting of clinopyroxene and crystallization of magnesioferrite at 1200 °C. (B) Crystallization of hematite at 1200 °C. (C) Crystallization of magnesioferrite and hematite at 1250 °C.
Therefore, synthesizing the discussion of these two melting mechanisms, we conclude that the oxidation of Fe2+ to Fe3+ within the lattice under oxidizing conditions facilitates metal cations in overcoming lattice constraints. Simultaneously, the migration of Fe-Mg elements further accelerates the melting of Fe-Mg-rich clinopyroxene (pigeonite). As a result, at a temperature of 1100 °C the iron-rich pigeonite disappears, and the calcium-rich diopside remains, as illustrated in Figure 7 and Figure 10.
As confirmed by previous studies [47,48,49,50], iron in basaltic melts undergoes oxidative enrichment and subsequently crystallizes as hematite (Figure 13④). This process is quantitatively described by the chemical equilibria in Equations (5) and (6). Combined with Figure 14B,C, it is evident that hematite crystallized during the melting of basalt contains minor titanium and aluminum. Our experimental results demonstrate that sheet-shaped hematite microcrystals form through this crystallization mechanism at both 1200 °C (B) and 1250 °C (C), with their characteristic morphology documented in Figure 13. Magnesioferrite exhibits an octahedral shape (Figure 14A) or a triangular shape (Figure 14C), with their distinct morphologies being linked to their respective formation processes. In melts derived from clinopyroxene, Fe2+ is oxidized to Fe3+, accompanied by localized enrichment of Fe and Mg, leading to the formation of octahedral granular magnesioferrite (Figure 13③). The triangular sheet magnesioferrite is formed by the counter-diffusion process of Mg2+ within a hematite lattice (Figure 13⑤), and the chemical transformation is depicted in Equation (28) [51]. Additionally, combined with Figure 14B,C, it is evident that the magnesioferrite crystallized during the melting of basalt contains minor aluminum.
3Fe2O3 + 2Mg2+ → 2MgFe2O4 + 2Fe2+ + 0.5O2
The iron-rich liquid phase present in the air is susceptible to oxidation, which facilitates the formation of hematite and magnesioferrite [15]. Similarly, basalt, characterized as a silicate system abundant in iron and aluminum, inherently faces challenges in avoiding crystallization. This crystallization tendency is evident both during cooling [52,53,54] and even during the melting process.
5.2.2. Influence of the Reducing Atmosphere on Basalt Melting Process
In a reducing atmosphere, plagioclase melts more easily because it transforms into clinopyroxene. Specifically, anorthite, a component of plagioclase, can decompose into Al- and Ca-rich clinopyroxene, known as the Ca-Tschermak molecule, as described in Equation (24) [52,53,54]. Ca-Tschermak molecule tends to form the stable solid solution of clinopyroxene [55], which can be synthesized at approximately 1200 °C under standard atmospheric pressure [56]. During our process, plagioclase incorporates into the diopside solid solution in the form of a Ca-Tschermak molecule, as illustrated in Figure 15 and represented by chemical Formulas (29) and (30). Therefore, at 1100 °C, clinopyroxene appears on the surface of plagioclase as short columnar microcrystals (Figure 16). During silicate depolymerization, the structural transition from framework silicates to chain silicates can occur [57], in which Mg2+ plays a critical role, as evidenced by the preferential incorporation of Mg2+ into chain-structured environments. Under high-temperature conditions, the four-membered rings within the framework structure of the plagioclase lattice undergo breakdown. This depolymerization exposes more non-bridging oxygen, which subsequently binds with metallic cations. The main cation in plagioclase are Al3+ and Ca2+, which contribute to the formation of a Ca-Tschermak molecule. However, plagioclase also contains smaller amounts of divalent cations, such as Mg2+ and Fe2+. These mental cations can integrate into crystal structures, with Mg2+ particularly favoring incorporation into chain silicates [57]. For details on potential thermodynamic calculations of Diopside-Ca-Tschermak solid solutions, refer to References [58,59]. Moreover, The oxidation of mental cations is difficult to occur under reducing atmospheric conditions. This reductive environment facilitates the development of chain silicates, such as clinopyroxene solid solutions. This observation corroborates the hypothesis proposed in Section 5.1: the enhanced basalt melting observed under reducing conditions is linked to plagioclase melting phenomena. This phenomenon occurs because the transformation of plagioclase (a high-melting-point mineral) into clinopyroxene (a low-melting-point mineral) renders basalt more susceptible to melting.
CaAl2Si2O8(s) → CaAl2SiO6(s) + SiO2
4(Ca,Mg,Fe)(Al,Si)4O8(s) + 2CaSiO3(l)→
3CaAl2SiO6·(Ca,Mg,Fe)Al2SiO6·Ca(Mg,Fe)Si2O6(s) + 4SiO2(l)
3CaAl2SiO6·(Ca,Mg,Fe)Al2SiO6·Ca(Mg,Fe)Si2O6(s) + 4SiO2(l)
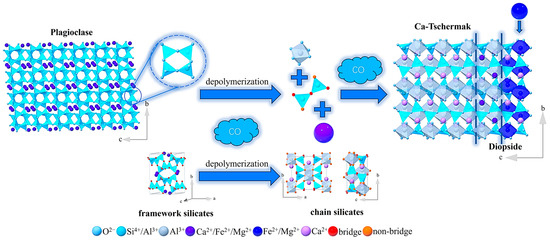
Figure 15.
Crystallization diagrams of the diopside solid solution.
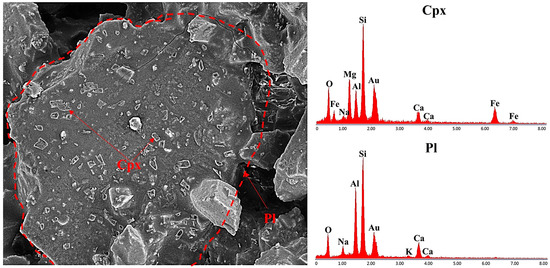
Figure 16.
SEM images of plagioclase and clinopyroxene under reducing atmosphere at 1100 °C.
Under reducing conditions, the melting capacity of clinopyroxene is slightly constrained. This constraint is primarily due to the following two factors: the conversion of some plagioclase into clinopyroxene and the suppression of the transformation from Fe2+ to Fe3+ by the reducing environment. The CO atmosphere significantly influences the valence state of iron ions [60]. Notably, Fe2+ exhibits a greater tendency to integrate into the crystalline structure of clinopyroxene than Fe3+ [18]. The preference for Fe2+ incorporation restricts the release of iron ions from the clinopyroxene lattice, affecting the overall melting process of clinopyroxene.
6. Conclusions
- In an oxidizing atmosphere, the melting process is hindered due to the crystallization of high-melting-point minerals such as hematite and magnesioferrite. Conversely, a reducing atmosphere can lower the melting temperature by approximately 50. This decrease in melting temperature is attributed to the suppression of high-melting-point minerals (hematite and magnesioferrite), as well as the transformation of plagioclase into lower-melting-point clinopyroxene;
- Atmospheric conditions influence the melting of major minerals. Clinopyroxene melts more readily under oxidizing conditions, whereas plagioclase melts more easily under reducing conditions;
- Sheet-shaped hematite can crystallize from the liquid phase as Fe2+ oxidizes to Fe3+ at the liquid’s surface. Magnesioferrite crystallization can occur through two mechanisms: one involves the oxidation of Fe2+ and its combination with Mg2+ to form octahedral granular magnesioferrite, while the other involves the counter-diffusion of Mg2+ through the lattice of pre-crystallized hematite. Under reducing conditions, plagioclase partially transforms into the solid solution of diopside-Ca-Tschermak.
Our findings demonstrate that the reduction of melting temperature can effectively decrease energy consumption during CBF production. Furthermore, as evidenced by prior studies, the enhancement of Fe2+ content has been demonstrated to improve the physical properties of resultant fibers [13,14,15]. These mechanistic insights collectively validate that the controlled modulation of redox conditions during basalt melting processes constitutes a strategically advantageous approach for optimizing CBF manufacturing efficiency and product quality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min15060596/s1, S1: Reducing Atmosphere System; S2: Determination of Total Iron and Fe2+; S3: Control Group Experimental Results. References [25,61,62,63,64,65] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, K.S. and G.X.; methodology, K.S. and G.X.; validation, D.W., Z.L. and H.W.; formal analysis, K.S.; investigation, K.S., Z.L., H.W. and H.L.; resources, G.X.; data curation, K.S. and D.W.; writing—original draft preparation, K.S.; writing—review and editing, G.X., D.W., Z.L., H.W., H.L., J.L. (Jie Li) and J.L. (Jiangfan Liang); visualization, K.S.; supervision, G.X.; project administration, G.X. and D.W.; funding acquisition, G.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hainan Key Laboratory of Marine Geological Resources and Environment (Grant No. HNHYD22YHJKF016).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
This work was supported by the Hainan Key Laboratory of Marine Geological Resources and Environment. We would like to thank the Key Laboratory of Tectonic Controls on Mineralization and Hydrocarbon Accumulation, Ministry of Natural Resources, Chengdu University of Technology. Our thanks go to the editor and anonymous reviewers for their constructive feedback and comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, L.; Chu, F.; Tuo, W.; Zhao, X.; Wang, Y.; Zhang, P.; Gao, Y. Review of research on basalt fibers and basalt fiber-reinforced composites in China (I): Physicochemical and mechanical properties. Polym. Polym. Compos. 2021, 29, 1612–1624. [Google Scholar] [CrossRef]
- Jiang, T.; Cao, S.; Yilmaz, E. Exploring Pore Structure Features, Crack Propagation and Failure Behavior of Fiber Reinforced Foam Tail Fill by CT Imaging and 3D Reconstruction. Minerals 2025, 15, 354. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Bella, G.; Valenza, A. A review on basalt fiber and its composites. Compos. Part B 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, W. Mechanical, Chloride Resistance, and Microstructural Properties of Basalt Fiber-Reinforced Fly Ash–Silica Fume Composite Concrete. Minerals 2025, 15, 348. [Google Scholar] [CrossRef]
- Dhand, V.; Mittal, G.; Rhee, K.Y.; Park, S.; Hui, D. A short review on basalt fiber reinforced polymer composites. Compos. Part B 2015, 73, 66–180. [Google Scholar] [CrossRef]
- Lopresto, V.; Leone, C.; De Iorio, I. Mechanical characterization of basalt fiber reinforced plastic. Compos. Part B 2011, 42, 717–723. [Google Scholar] [CrossRef]
- Zhao, X.; Ouyang, J.; Yang, H.; Tan, Q. Effect of Basalt Fibers for Reinforcing Resin-Based Brake Composites. Minerals 2020, 10, 490. [Google Scholar] [CrossRef]
- Liu, J.; Ding, L.; Wang, Q.; Luo, L.; Wang, H. Recycling of arsenic residue to basalt fiber via vitrification. Ceram. Int. 2024, 50, 6622–36630. [Google Scholar] [CrossRef]
- Ivanitskii, S.G.; Gorbachev, G.F. Continuous basalt fibers: Production aspects and simulation of forming processes. I. State of the art in continuous basalt fiber technologies. Powder Metall. Met. Ceram. 2011, 50, 125. [Google Scholar] [CrossRef]
- Dou, H.; Wang, Y.; Bai, J.; Kong, L.; Bai, Z.; Li, H.; Guo, Z.; Li, W. Effect of melt homogenization on the structure and properties of zirconium-rich basalt fibers. Ceram. Int. 2024, 50, 16940–16949. [Google Scholar] [CrossRef]
- Novitskii, A.G. High-Temperature Heat-Insulating Materials Based on Fibers from Basalt-Type Rock Materials. Refract. Ind. Ceram. 2004, 45, 144–146. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Hui, D.; Chen, M.; Wu, Z. Study of melting properties of basalt based on their mineral components. Compos. Part B 2017, 16, 53–60. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Q.; Wang, Q.; Xiao, J.; Liu, J.; Ding, L.; Jiang, W. Effect of the Iron Reduction Index on the Mechanical and Chemical Properties of Continuous Basalt Fiber. Materials 2019, 12, 2472. [Google Scholar] [CrossRef]
- Gutnikov, S.I.; Manylov, M.S.; Lipatov, Y.V.; Lazoryak, B.I.; Pokholok, K.V. Effect of the reduction treatment on the basalt continuous fiber crystallization properties. J. Non-Cryst. Solids 2013, 368, 45–50. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Luo, L.; Yan, T.; Liu, J.; Ding, L.; Jiang, W. Effects of high-temperature treatment and iron reduction index on tensile strength of basalt continuous fiber. J. Non-Cryst. Solids 2021, 564, 120836. [Google Scholar] [CrossRef]
- Wang, S.; Kuang, F.; Yan, Q.; Ge, C.; Qi, L. Crystallization and infrared radiation properties of iron ion doped cordierite glass-ceramics. J. Alloys Compd. 2011, 509, 2819–2823. [Google Scholar] [CrossRef]
- Sharma, K.; Deo, M.N.; Kothiyal, G.P. Effect of iron oxide addition on structural properties of calcium silico phosphate glass/glass-ceramics. J. Non-Cryst. Solids 2012, 358, 1886–1891. [Google Scholar] [CrossRef]
- Sørensen, P.M.; Pind, M.; Yue, Y.Z.; Rawlings, R.D.; Boccaccini, A.R.; Nielsen, E.R. Effect of the redox state and concentration of iron on the crystallization behavior of iron-rich aluminosilicate glasses. J. Non-Cryst. Solids 2005, 351, 1246–1253. [Google Scholar] [CrossRef]
- Du, Y.; Li, B.; Zhang, X.; Jia, X. Influence of Content and Valence of Iron on Crystallization Characteristics and Properties of Baiyunebo Tailing Glass-Ceramics. J. Synthetic Cryst. 2013, 42, 2170–2176. [Google Scholar] [CrossRef]
- Karamanov, A.; Pisciella, P.; Pelino, M. The crystallisation kinetics of iron rich glass in different atmospheres. J. Eur. Ceram. Soc. 2000, 20, 2233–2237. [Google Scholar] [CrossRef]
- Karamanov, A.; Pelino, M. Crystallization phenomena in iron-rich glasses. J. Non-Cryst. Solids 2001, 281, 139–151. [Google Scholar] [CrossRef]
- Manylov, M.S.; Gutnikov, S.I.; Lipatov, Y.V.; Malakho, A.P.; Lazoryak, B.I. Effect of deferrization on continuous basalt fiber properties. Mendeleev Commun. 2015, 25, 386–388. [Google Scholar] [CrossRef]
- El-Shennawi, A.W.A.; Morsi, M.M.; Abdel-Hameed, S.A.M. Effect of fluoride nucleating catalysts on crystallization of cordierite from modified basalt-based glasses. J. Eur. Ceram. Soc. 2007, 27, 1829–1835. [Google Scholar] [CrossRef]
- Liu, J.Q.; Ren, Z.Y.; Nichols, A.R.L.; Song, M.S.; Qian, S.P.; Zhang, Y.; Zhao, P.P. Petrogenesis of Late Cenozoic basalts from North Hainan Island: Constraints from melt inclusions and their host olivines. Geochim. Cosmochim. Acta 2015, 152, 89–121. [Google Scholar] [CrossRef]
- Guo, Y.F.; Guo, X.M. Formation of [Mg1-x, Fex]O•Fe2O3 in Solid-state Reactions between MgO and Fe2O3 in the Fe2O3-rich System. ISIJ Int. 2017, 57, 228–235. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Y.; Wei, F. Phase Transitions and Reaction Mechanism of Ilmenite Oxidation. Met. Mater. Trans. A 2010, 41, 1338–1348. [Google Scholar] [CrossRef]
- Chen, F.; Lv, W.; Zhou, G.; Liu, Z.; Chu, M.; Lv, X. Effects of pre-oxidation on the hydrogen-rich reduction of Panzhihua ilmenite concentrate powder: Reduction kinetics and mechanism. Int. J. Hydrogen Energy 2023, 48, 13415–13429. [Google Scholar] [CrossRef]
- Ghiorso, M.S.; Sack, R.O. Chemical mass transfer in magmatic processes IV. A revised and internally consistent thermodynamic model for the interpolation and extrapolation of liquid-solid equilibria in magmatic systems at elevated temperatures and pressures. Contr. Contrib. Mineral. Petrol. 1995, 119, 197–212. [Google Scholar] [CrossRef]
- Berman, R.G. Internally-consistent thermodynamic data for minerals in the system Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-SiO2-TiO2-H2O-CO2. J. Petrol. 1988, 29, 445–522. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R. An enlarged and updated internally consistent thermodynamic dataset with uncertainties and correlations: The system K2O–Na2O–CaO–MgO–MnO–FeO–Fe2O3–Al2O3–TiO2–SiO2–C–H2–O2. J. Metamorph. Geol. 1990, 8, 89–124. [Google Scholar] [CrossRef]
- Johnson, L.A.; McCauley, R.A. The thermal behavior of albite as observed by DTA. Thermochim. Acta 2005, 437, 134–139. [Google Scholar] [CrossRef]
- Navrotsky, A.; Ziegler, D.; Oestrike, R.; Maniar, P. Calorimetry of silicate melts at 1773 K: Measurement of enthalpies of fusion and of mixing in the systems diopside-anorthite-albite and anorthite-forsterite. Contrib. Mineral. Petrol. 1989, 101, 122–130. [Google Scholar] [CrossRef]
- Guimarães, W.G.; de Lima, G.F.; Duarte, H.A. Exploring the effect of isomorphically Al-substituted on the thermodynamical and structural properties of hematite and goethite—A DFT investigation. Hydrometallurgy 2023, 221, 106125. [Google Scholar] [CrossRef]
- Ghiorso, M.S. Thermodynamic properties of hematite—ilmenite—geikielite solid solutions. Contr. Mineral. Petrol. 1990, 104, 645–667. [Google Scholar] [CrossRef]
- McKibbin, S.J.; O’Neill, H.S.C. Petrogenesis of D’Orbigny-like angrite meteorites and the role of spinel in the angrite source. Meteorit. Planet. Sci. 2018, 53, 306–325. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels: Crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Min. 1983, 68, 181–194. [Google Scholar]
- Marinoni, N.; Levy, D.; Dapiaggi, M.; Pavese, A.; Smith, R.I. In situ high-temperature X-ray and neutron powder diffraction study of cation partitioning in synthetic Mg(Fe0.5Al0.5)2O4 spinel. Phys. Chem. Miner. 2011, 38, 11–19. [Google Scholar] [CrossRef]
- Kosterlitz, J.M.; Thouless, D.J. Ordering, metastability and phase transitions in two-dimensional systems. J. Phys. C Solid State Phys. 1973, 6, 1181–1203. [Google Scholar] [CrossRef]
- Zhang, Y. Diffusion in Minerals and Melts: Theoretical Background. Rev. Mineral. Geochem. 2010, 72, 5–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, B. Diffusive Mg isotope fractionation in silicate melts during mineral dissolution. Chem. Geol. 2025, 681, 122720. [Google Scholar] [CrossRef]
- Pertermann, M.; Hirschmann, M.M. Partial melting experiments on a MORB-like pyroxenite between 2 and 3 GPa: Constraints on the presence of pyroxenite in basalt source regions from solidus location and melting rate. J. Geophys. Res. 2003, 108, 2125. [Google Scholar] [CrossRef]
- Li, H.; Ma, R.; Liang, Y.; Chi, X.; Dong, C. Melting sequence and dynamics of the major minerals of gabbro in the process of dynamic melting. Acta Petrol. Sin. 2005, 21, 1749–1758. [Google Scholar]
- Burakovsky, L.; Preston, D.L. Analysis of dislocation mechanism for melting of elements. Solid State Commun. 2000, 115, 341–345. [Google Scholar] [CrossRef]
- Liu, K.; Chen, H. Statistical mechanics of melting mediated by two types of defects. Phys. Lett. A 2008, 372, 1109–1113. [Google Scholar] [CrossRef]
- Sun, X.; Wu, D.; Zou, L.; House, S.D.; Chen, X.; Li, M.; Zakharov, D.N.; Yang, J.C.; Zhou, G. Dislocation-induced stop-and-go kinetics of interfacial transformations. Nature 2022, 607, 708–713. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Z.; Tong, X.; Guo, L.; Miao, S.; Jiang, L.; Li, Y.; Li, H.; Liu, C. Effects of raw material homogenization on the structure of basalt melt and performance of fibers. Ceram. Int. 2022, 48, 11998–12005. [Google Scholar] [CrossRef]
- Xing, D.; Chang, C.; Xi, X.-Y.; Hao, B.; Zheng, Q.; Gutnikov, S.I.; Lazoryak, B.I.; Ma, P.-C. Morphologies and mechanical properties of basalt fibre processed at elevated temperature. J. Non-Crystalline Solids 2022, 582, 121439. [Google Scholar] [CrossRef]
- Liu, H.-P.; Huang, X.-F.; Ma, L.-P.; Chen, D.-L.; Shang, Z.-B.; Jiang, M. Effect of Fe2O3 on the crystallization behavior of glass-ceramics produced from naturally cooled yellow phosphorus furnace slag. Int. J. Miner. Met. Mater. 2017, 24, 316–323. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Marcial, J.; McCloy, J. Crystallization of iron-containing sodium aluminosilicate glasses in the NaAlSiO4-NaFeSiO4 join. J. Geophys. Res. Solid Earth 2017, 122, 2504–2524. [Google Scholar] [CrossRef]
- Yue, Y.; Korsgaard, M.; Kirkegaard, L.F.; Heide, G. Formation of a Nanocrystalline Layer on the Surface of Stone Wool Fibers. J. Am. Ceram. Soc. 2009, 92, 62–67. [Google Scholar] [CrossRef]
- Deraz, N.M.; Alarifi, A. Novel preparation and properties of magnesioferrite nanoparticles. J. Anal. Appl. Pyrolysis 2012, 97, 55–61. [Google Scholar] [CrossRef]
- Pakhomova, A.; Simonova, D.; Koemets, I.; Koemets, E.; Aprilis, G.; Bykov, M.; Gorelova, L.; Fedotenko, T.; Prakapenka, V.; Dubrovinsky, L. Polymorphism of feldspars above 10 GPa. Nat. Commun. 2020, 11, 2721. [Google Scholar] [CrossRef]
- Mccarthy, T.C.; Patiño Douce, A.E. Empirical calibration of the silica–Ca-tschermak’s–anorthite (SCAn) geobarometer. J. Metamorph. Geol. 1998, 16, 675–686. [Google Scholar] [CrossRef]
- Ma, C.; Simon, S.B.; Rossman, G.R.; Grossman, L. Calcium Tschermak’s pyroxene, CaAlAlSiO6, from the Allende and Murray meteorites: EBSD and micro-Raman characterization. Am. Mineral. 2009, 94, 1483–1486. [Google Scholar] [CrossRef]
- Salama, S.N.; Darwish, H.; Abo-Mosallam, H.A. Crystallization and properties of glasses based on diopside–Ca-Tschermak’s–fluorapatite system. J. Eur. Ceram. Soc. 2005, 25, 1133–1142. [Google Scholar] [CrossRef]
- Zeng, R. Experimental Studies In Silicate Systems: I. The Undersaturated Part of the System NaAlSiO4-KAlSiO4-SiO2-H2O at PH2O = 5 kb. II. A Study of Diopside Solid Solution in the System CaMgSi2O6-CaAl2SiO6 at 1 Atmosphere. Ph.D. Thesis, The University of Manchester, Manchester, UK, 1983. ProQuest Dissertations & Theses. Available online: https://www.proquest.com/dissertations-theses/experimental-studies-silicate-systems-i/docview/2485526967/se-2 (accessed on 18 March 2025).
- Sánchez-Navas, A.; Melchor, S.; Ortega-Castro, J.; Vidal-Daza, I.; Castro, A. A case study of depolymerization in silicates: Melting of quartz and zircon crystallization at high pressure. Chem. Geol. 2024, 661, 122156. [Google Scholar] [CrossRef]
- Wood, B.J. Activity-composition relationships in Ca(Mg,Fe)Si2O6-CaAl2SiO6 clinopyroxene solid solutions. Am. J. Sci. 1979, 279, 854–875. [Google Scholar] [CrossRef]
- Benisek, A.; Etzel, K.; Cemič, L. Thermodynamic mixing behavior of synthetic Ca-Tschermak–diopside pyroxene solid solutions: II. Heat of mixing and activity–composition relationships. Phys. Chem. Miner. 2007, 34, 747–755. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-Specific Enhancement of the Carbon–Carbon Dioxide (Boudouard) Reaction. J. Phys. Chem. C 2013, 117, 26871–26880. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Haugen, N.E.L.; Loong, B.K.Y.; Mitchell, R.E. Numerical approaches for thermochemical conversion of char. Prog. Energy Combust. Sci. 2022, 91, 100993. [Google Scholar] [CrossRef]
- Tabereaux, A.T.; Peterson, R.D. Chapter 2.5—Aluminum Production. Treatise Process Metall. 2014, 3, 839–917. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Jian, Z.; Wu, Z.-S. W. Determination of Total Iron and Fe2+in Basalt. Spectrosc. Spectr. Anal. 2015, 35, 2312–2315. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).