The Middle–Late Permian to Late Cretaceous Mediterranean-Type Karst Bauxites of Western Iran: Authigenic Mineral Forming Conditions and Critical Raw Materials Potential
Abstract
1. Introduction
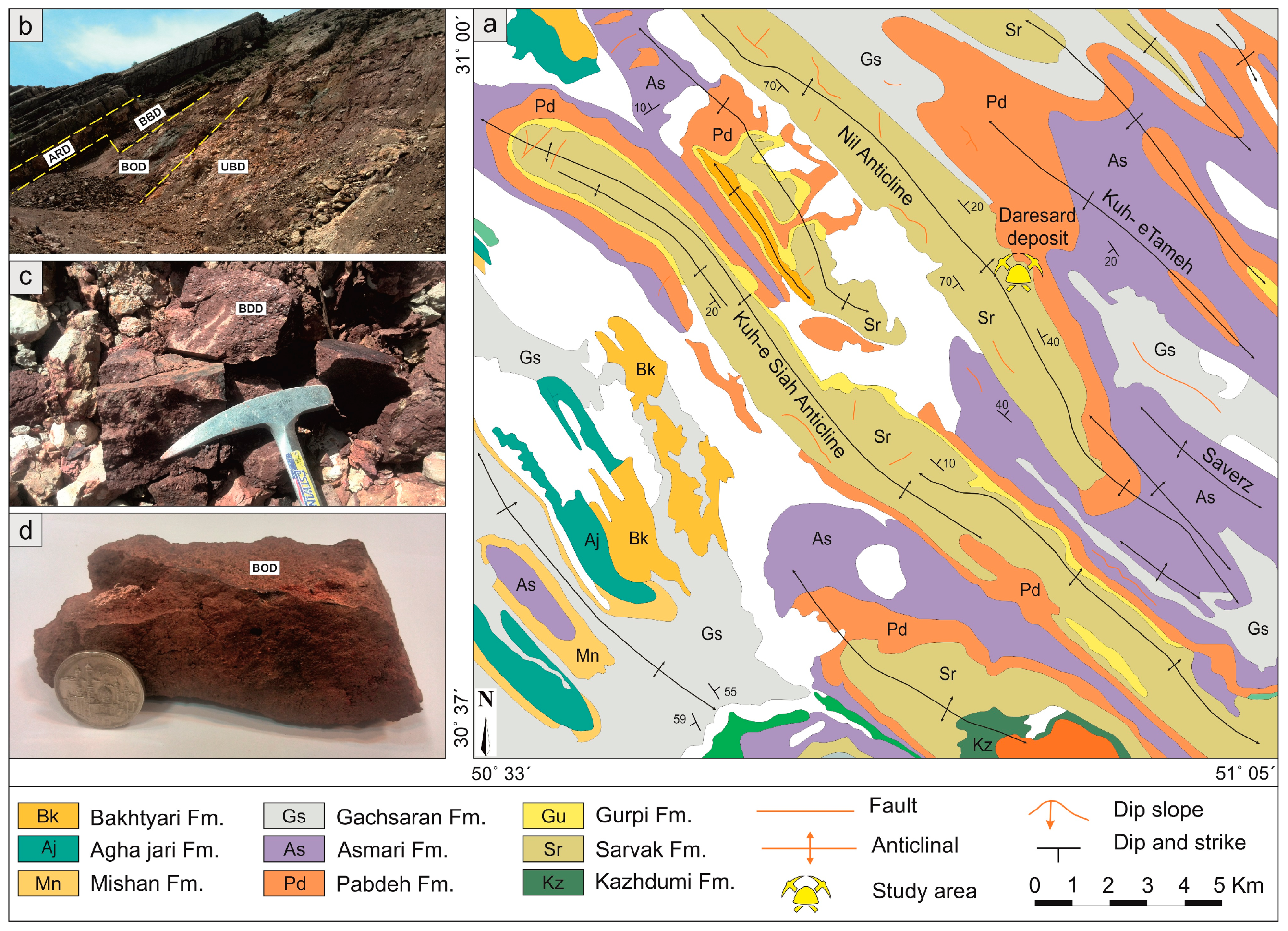
2. Geological Setting
2.1. The Middle–Late Permian Ruteh Formation and Yakshawa Deposit Geology
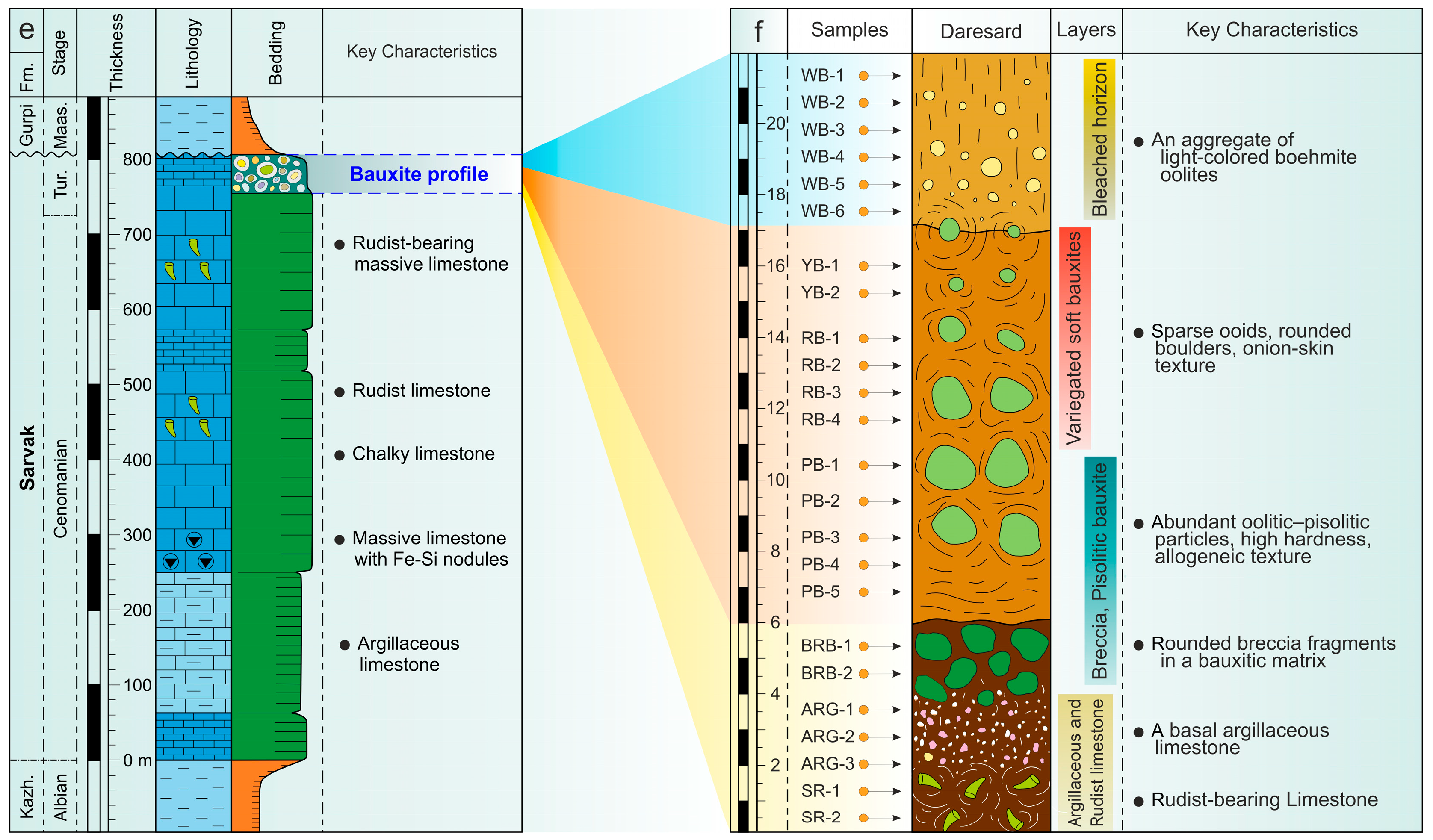
2.2. The Late Cretaceous Sarvak Formation and Daresard Deposit Geology
3. Materials and Methods
3.1. Fieldwork and Sampling
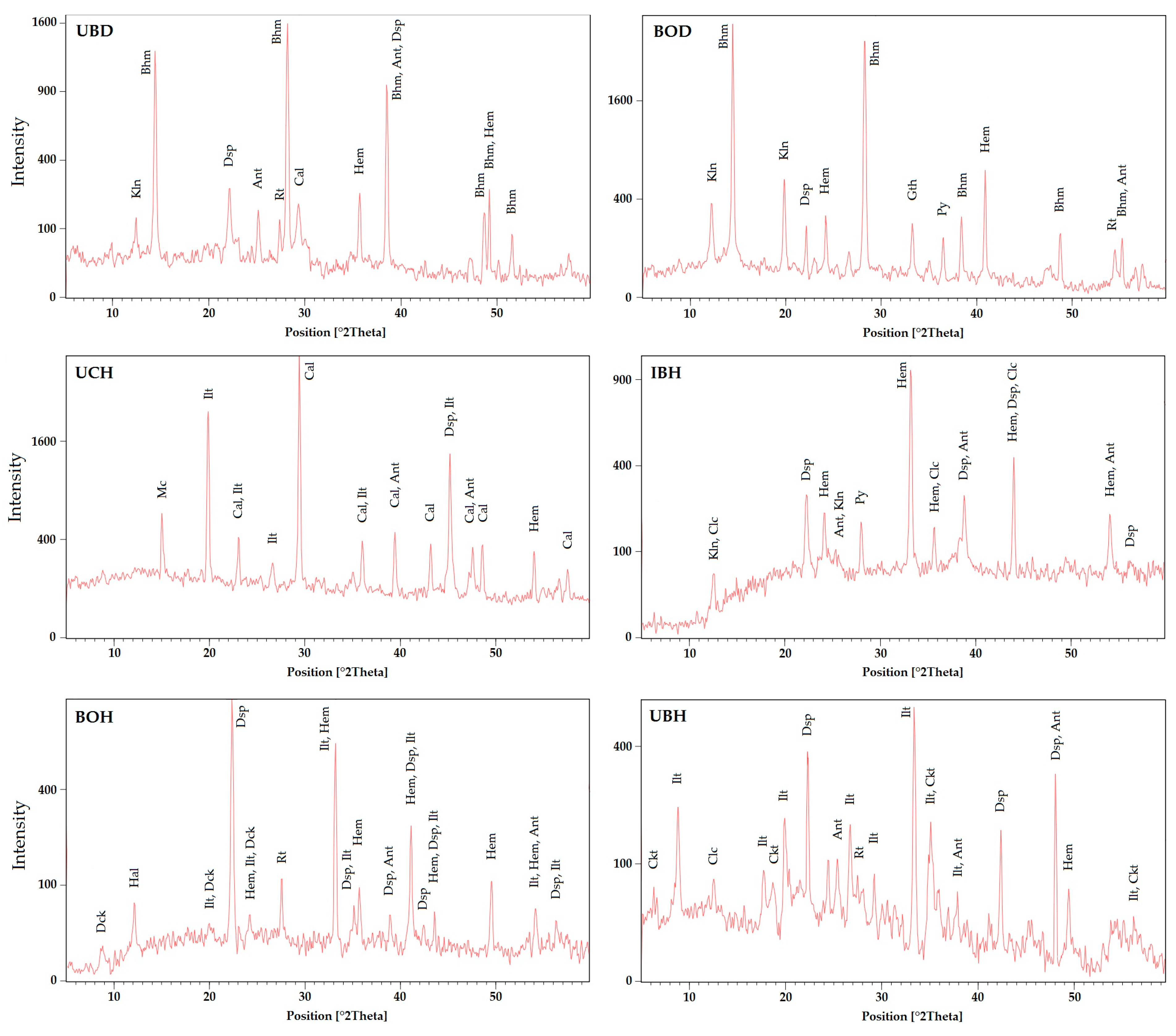
3.2. Mineralogical Analysis
- Oxides/silicates: rutile (Ti Kα), albite (Al Kα, Si Kα), magnetite (Fe Kα), and apatite (Ca Kα, P Kα);
- Fluorides: Fluorite (F Kα), LaF3 (La Lα), EuF3 (Eu Lα), and ThF4 (Th Mα);
- REEs: CeO2 (Ce Lα), PrSi2 (Pr Lα), and NdSi2 (Nd Lα);
- Synthetic standards: YAG (Y3Al5O12; Y Lα) and Gd3Ga5O12 (Gd Lα);
- Accessory minerals: Baddeleyite (Zr Lα)
3.3. Geochemical Analysis
4. Results
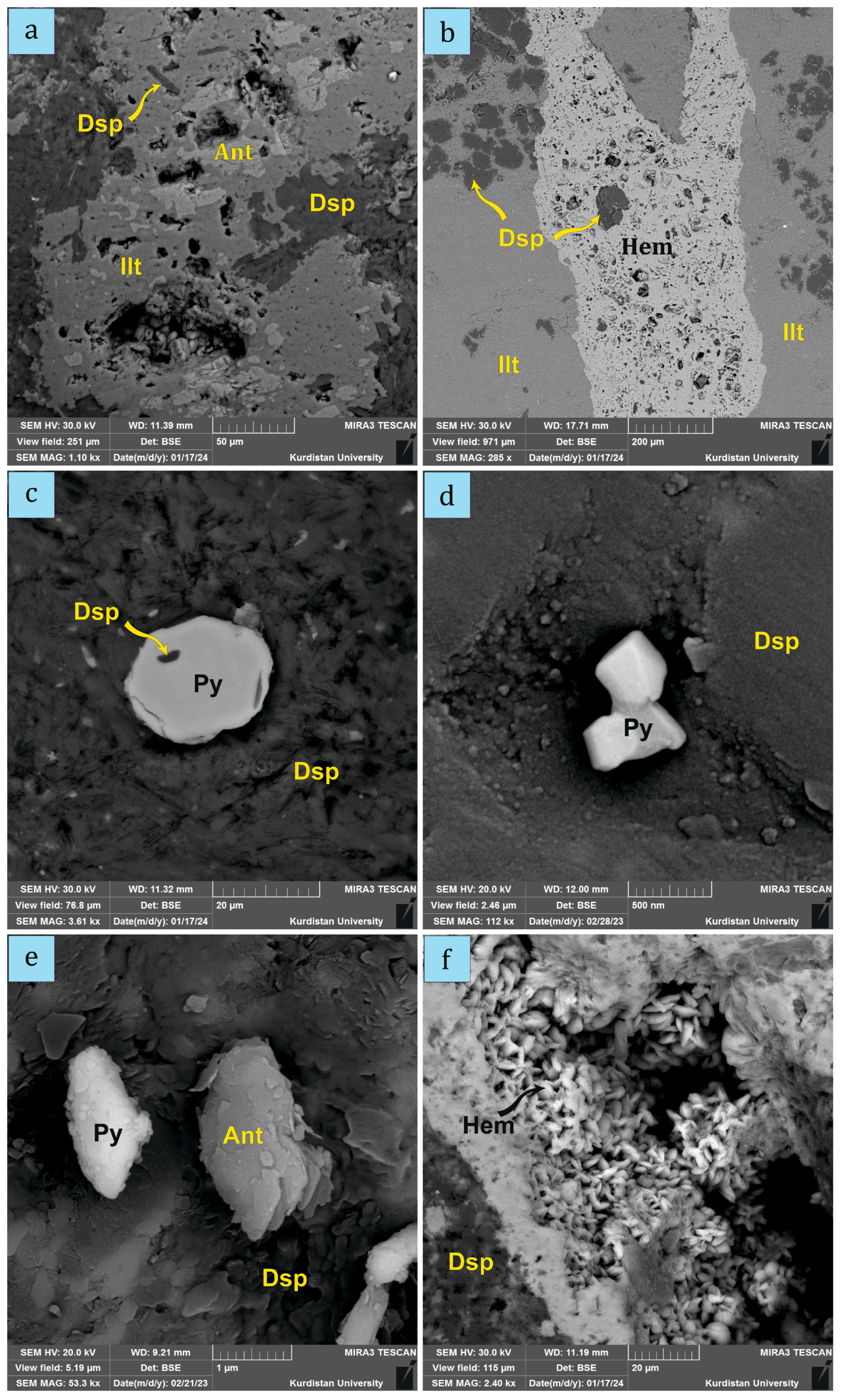
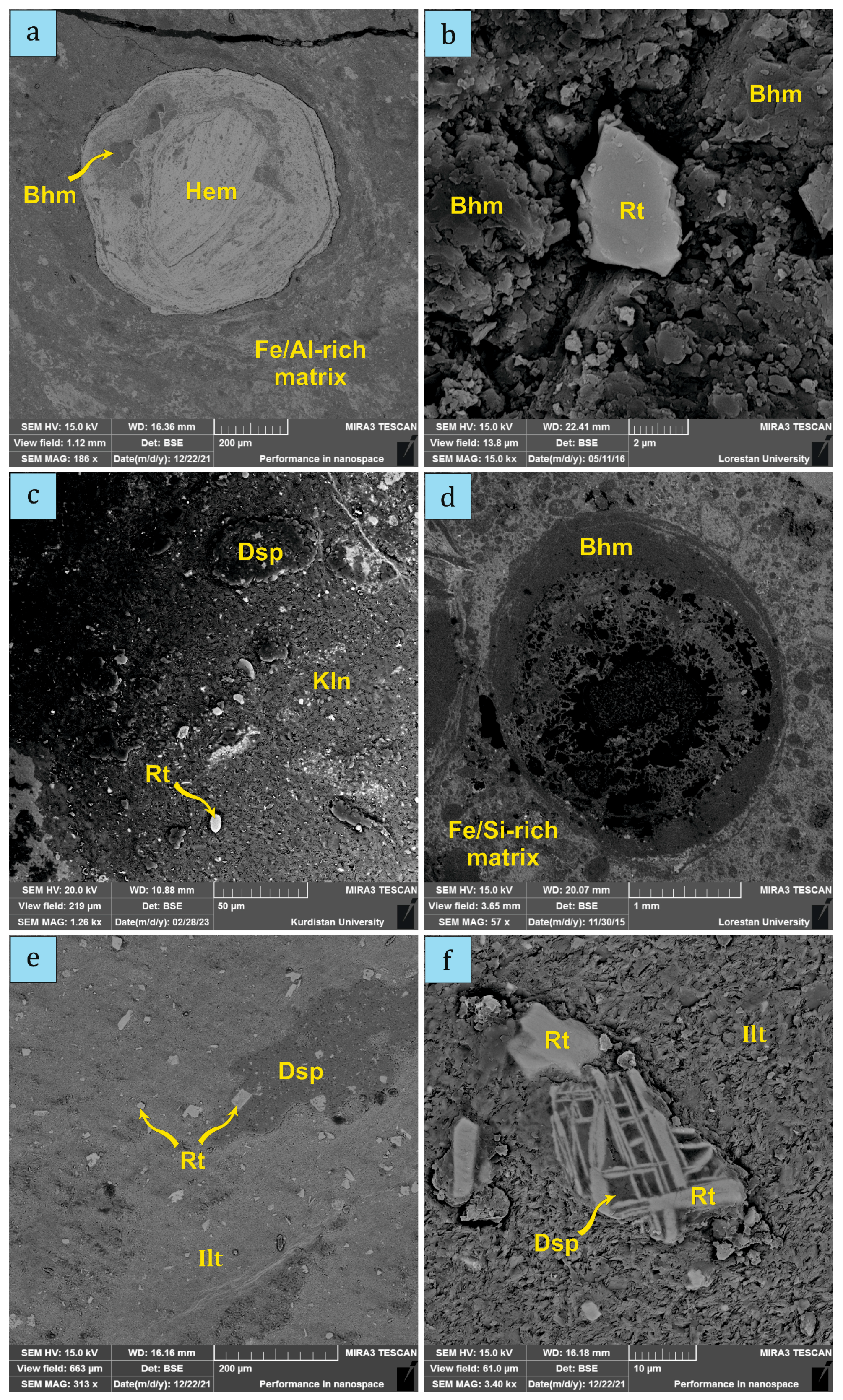
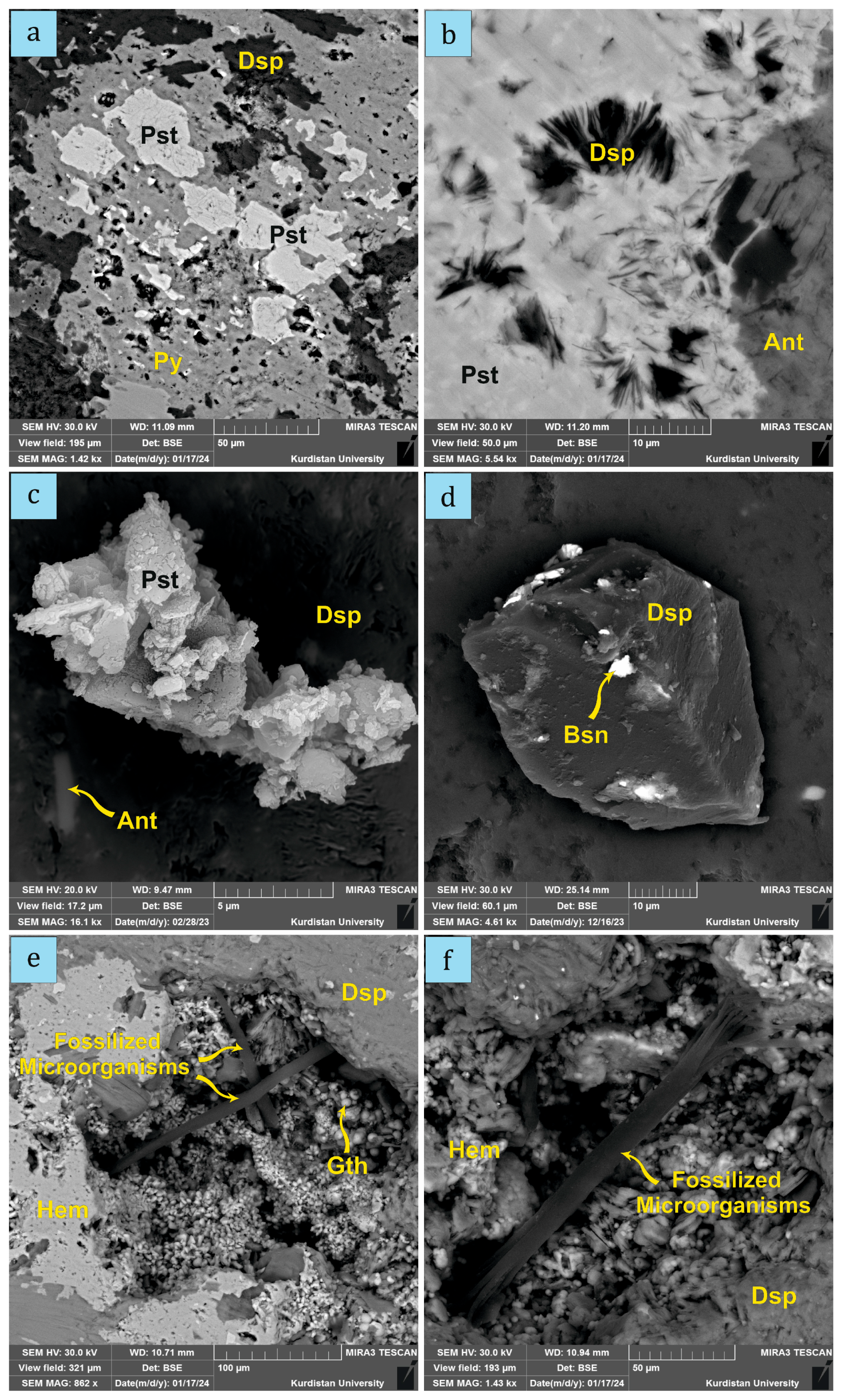
4.1. Mineral Compositions
4.2. The Occurrence of Microorganisms
4.3. Ore Geochemistry
4.3.1. Major Element Geochemistry
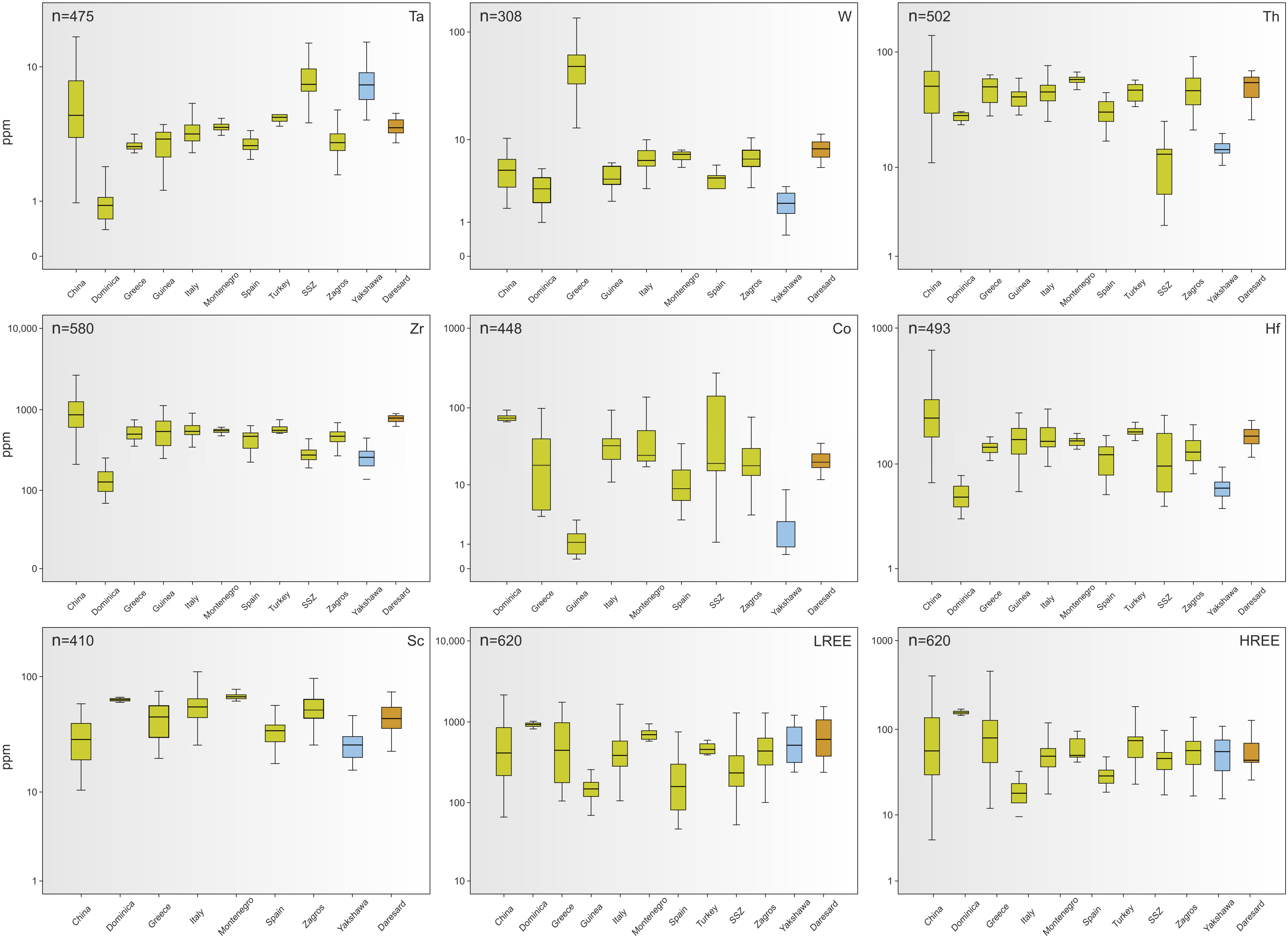
4.3.2. Trace Element Geochemistry
5. Discussion
5.1. Main Authigenic Mineral Forming Conditions
5.2. Deposition Mechanisms of High-Grade Bauxite and Fe-Rich Horizon
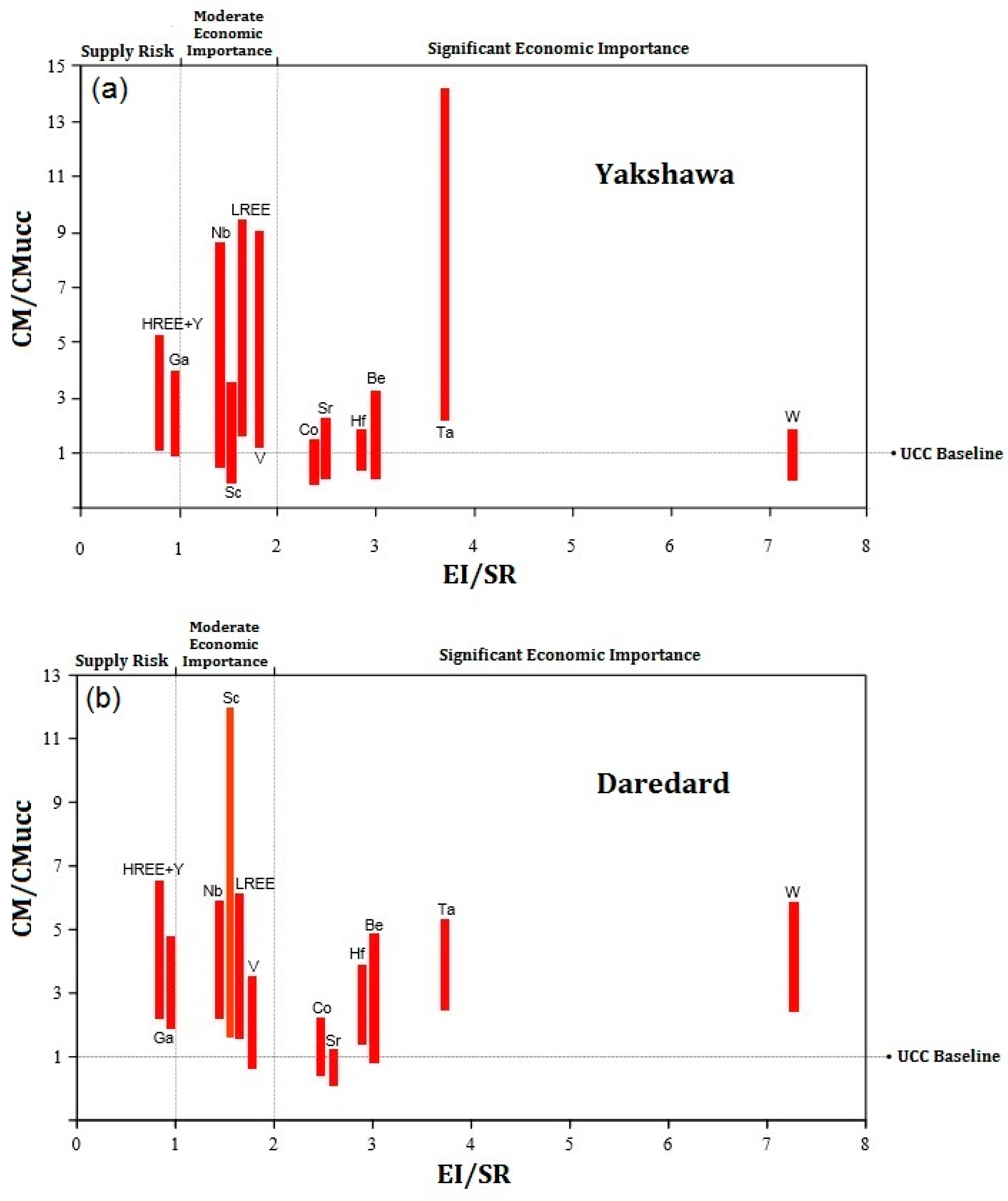
5.3. Evaluation of the Critical Raw Materials Profitability
6. Conclusions
- Authigenic illite in Yakshawa forms via isochemical reactions (kaolinite/K-feldspar dissolution), with SEM confirming illite replacement of kaolinite. Burial depth enhances illite crystallinity, producing pseudohexagonal morphologies, while kaolinite originates from acidic weathering of feldspars and later silicification of Al-hydroxides.
- Diaspore forms through both gibbsite transformation (evidenced by syngenetic diaspore-rutile assemblages) and direct precipitation from Al-rich solutions in microbial-mediated, reducing karst environments (pH 7–8), as supported by its paragenesis with pyrite, LREE-fluorocarbonates (e.g., parisite), and elevated TOC (0.08–0.57%).
- Sulfate-reducing bacteria (e.g., Desulfovibrio) drive Fe3⁺→Fe2⁺ reduction under high-TOC conditions, forming Fe-rich horizons via pyrite pseudomorphs (goethite) and microveins. Subsequent oxidative weathering enriches Al-hydroxides in upper profile zones, creating high-grade bauxite.
- Microbial activity (framboidal pyrite, microfossils) and organic matter (TOC up to 0.57%) critically control mineral paragenesis (diaspore, REE phases) and Fe/Al fractionation, alongside abiotic factors (pH, Eh, burial depth).
- The bauxitization process enriches critical metals (especially Ta, LREE, and Sc) in the studied karst bauxites, controlled by host minerals (P-rich/fluorocarbonates for REE+Y; Fe/Ti/Al oxides for others), paleoclimate, and organic-mediated redox processes, highlighting their potential as valuable CRM sources.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mongelli, G.; Mameli, P.; Sinisi, R.; Buccione, R.; Oggiano, G. REES and other critical raw materials in Cretaceous Mediterranean-type bauxite: The case of the Sardinian ore (Italy). Ore Geol. Rev. 2021, 139, 104559. [Google Scholar] [CrossRef]
- Jaroni, M.; Friedrich, B.; Letmathe, P. Criticality of Rare Earth Metals: A European Analysis; Working Paper; RWTH Aachen University: Aachen, Germany, 2017. [Google Scholar]
- Reinhardt, N.; Proenza, J.A.; Villanova-de-Benavent, C.; Aiglsperger, T.; Bover-Arnal, T.; Torro, L.; Salas, R.; Dziggel, A. Geochemistry and mineralogy of rare earth elements (REE) in bauxitic ores of the Catalan Coastal Range, NE Spain. Minerals 2018, 8, 562–586. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Mongelli, G. Geology, geochemistry, and genesis of REY minerals of the late Cretaceous karst bauxite deposits, Zagros Simply Folded Belt, SW Iran. J. Geochem. Explor. 2022, 240, 107030. [Google Scholar] [CrossRef]
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Zhang, S.; Liu, J.; Xiao, K. Geologic characteristics and potential of bauxite in China. Ore Geol. Rev. 2020, 120, 103278. [Google Scholar] [CrossRef]
- Bilal, A.; Yang, R.; Lenhardt, N.; Han, Z.; Luan, X. The Paleocene Hangu formation: A key to unlocking the mysteries of Paleo-Tethys tectonism. Mar. Pet. Geol. 2023, 157, 106508. [Google Scholar] [CrossRef]
- Horbe, A.M.C.; da Costa, M.L. Geochemical evolution of a lateritic Sn–Zr–Th–Nb–Y–REE-bearing ore body derived fromapogranite: The case of Pitinga, Amazonas-Brazil. J. Geochem. Explor. 1999, 66, 339–351. [Google Scholar] [CrossRef]
- Mutakyahwa, M.K.D.; Ikingura, J.R.; Mruma, A.H. Geology and geochemistry of bauxite deposits in Lushoto District, Usambara Mountains, Tanzania. J. Afr.Earth Sci. 2003, 36, 357–369. [Google Scholar] [CrossRef]
- Bogatyrev, B.A.; Zhukov, V.V.; Tsekhovsky, Y.G. Formation conditions and regularities of the distribution of large and superlarge bauxite deposits. Lithol. Min. Resour. 2009, 44, 135–151. [Google Scholar] [CrossRef]
- Bárdossy, G. Karst Bauxites, Bauxite Deposits on Carbonate Rocks. In Developments in Economic Geology 14; Elsevier: Amsterdam, The Netherlands, 1982; Volume 14, pp. 1–441. [Google Scholar]
- Zamanian, H.; Ahmadnejad, F.; Zarasvandi, A. Mineralogical and geochemical investigations of the Mombi bauxite deposit, Zagros Mountains, Iran. Chem. Erde 2016, 76, 13–37. [Google Scholar] [CrossRef]
- Putzolu, F.; Piccolo Papa, A.; Mondillo, N.; Boni, M.; Balassone, G.; Mormone, A. Geochemical characterization of bauxite deposits from the Abruzzi Mining District (Italy). Minerals 2018, 8, 298. [Google Scholar] [CrossRef]
- Liu, X.F.; Wang, Q.F.; Zhao, L.H.; Peng, Y.B.; Ma, Y.; Zhou, Z.H. Metallogeny of the large-scale Carboniferous karstic bauxite in the Sanmenxia area, southern part of the North China Craton, China. Chem. Geol. 2020, 556, 119851. [Google Scholar] [CrossRef]
- Sun, X.; Yang, S.; Liu, X.; Zhao, L.; Liu, L.; Zhang, Q.; Feng, Y.; Wang, W. Metallogenic process of Permian Taiping karstic bauxite deposit in Youjiang Basin, China. Ore Geol. Rev. 2023, 152, 105258. [Google Scholar] [CrossRef]
- Taghipour, B.; Jahangirzadeh, S.; Mondillo, N. The mineralogy and geochemistry of the Pirashkaft bauxite, Zagros belt, Iran. J. Geochem. Explor. 2024, 259, 107394. [Google Scholar] [CrossRef]
- Torro, L.; Proenza, J.A.; Aiglsperger, T.; Bover-Arnal, T.; Villanova-de-Benavent, C.; Rodríguez-García, D.; Ramírez, A.; Rodríguez, J.; Mosquea, L.A.; Salas, R. Geological, geochemical and mineralogical characteristics of REE-bearing Las Mercedes bauxite deposit, Dominican Republic. Ore Geol. Rev. 2017, 89, 114–131. [Google Scholar] [CrossRef]
- Luo, C.; Liang, P.; Yang, R.; Gao, J.; Chen, Q.; Mo, H. Mineralogical and Geochemical Constraints on the Occurrence Forms of REEs in Carboniferous Karst Bauxite, Central Guizhou Province, Southwest China: A Case Study of Lindai Bauxite. Minerals 2023, 13, 320. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Mongelli, G.; Parkalian, H.; Haghighi, H.; Sharifi, M. The Middle-Late Permian Yakshawa and Late Cretaceous Daresard bauxite deposits, western Iran. J. Asian Earth Sci. 2025, 277, 106397. [Google Scholar] [CrossRef]
- Khosravi, M.; Vérard, C.; Abedini, A. Palaeogeographic and geodynamic control on the Iranian karst-type bauxite deposits. Ore Geol. Rev. 2021, 139, 104589. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Mongelli, G. Geochemistry of Upper Cretaceous bauxite deposits, Zagros Fold Thrust Belt, SW Iran. Sediment. Geol. 2023, 454, 106461. [Google Scholar] [CrossRef]
- Abedini, A.; Mongelli, G.; Khosravi, M. Geochemical constraints on the middle Triassic Kani Zarrineh karst bauxite deposit, Irano-Himalayan belt, NW Iran. Ore Geol. Rev. 2021, 133, 104099. [Google Scholar] [CrossRef]
- Berberian, M.; King, G.C. Towards a palaeogeography and tectonics evolution of Iran. Can. J. Earth Sci. 1981, 18, 210–265. [Google Scholar] [CrossRef]
- Agard, P.; Omrani, J.; Jolivet, L.; Mouthereau, F. Convergence history across Zagros (Iran): Constraints from collisional and earlier deformation. Int. J. Earth Sci. 2005, 94, 401–419. [Google Scholar] [CrossRef]
- Omrani, J.; Agard, P.; Whitechurch, H.; Benoit, M.; Prouteau, G.; Jolivet, L. Arc-magmatism and subduction history beneath the Zagros Mountains, Iran. Lithos 2008, 106, 380–398. [Google Scholar] [CrossRef]
- Taghipour, B.; Ahmadnejad, F. Platinum-Group Elements Geochemistry and Chromian Spinel Composition in Podiform Chromitites and Associated Peridotites from the Cheshmeh-Bid Deposit, Neyriz, Southern Iran. Acta Geol. Sin. 2018, 92, 183–209. [Google Scholar] [CrossRef]
- Kordi, M. Sedimentary basin analysis of the Neo-Tethys and its hydrocarbon systems in the Southern Zagros fold-thrust belt and foreland basin. Earth-Sci. Rev. 2019, 191, 1–11. [Google Scholar] [CrossRef]
- Dilek, Y.; Imamverdiyev, N.; Altunkaynak, S. Geochemistry and tectonics of Cenozoic volcanism in the Lesser Caucasus (Azerbaijan) and the peri-Arabian region. Int. Geol. Rev. 2010, 52, 536–578. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Zamanian, H.; Sameti, M. Geochemistry and Economic Potential of the Samen Granitoid Intrusion, Northwestern Iran. N. Jb. Miner. Abh. 2017, 194, 175–203. [Google Scholar] [CrossRef]
- Shabanian, R.; Tehrani, K.K.; Momeni, I. Stratigraphy and Micropaleontology of the Permian Rocks in NW Iran. Sci. Q. J. Geosci. 2007, 16, 98–109. [Google Scholar]
- Moghaddas, Y.M.; Mahari, R.; Shabanian, R.; Najafzadeh, A. Facies analysis, depositional environment and sequence stratigraphy of the Permian Ruteh Formation in north of Mahabad (NW Iran). Iran. J. Earth Sci. 2020, 14, 58–77. [Google Scholar]
- Hajikazemi, E.; Al-Aasm, I.S.; Coniglio, M. Diagenetic history and reservoir properties of the Cenomanian-Turonian carbonates in southwestern Iran and the Persian Gulf. Mar. Pet. Geol. 2017, 88, 845–857. [Google Scholar] [CrossRef]
- Ghazban, F. Petroleum Geology of the Persian Gulf; University of Tehran Press: Tehran, Iran, 2007; Volume 706. [Google Scholar]
- Hajikazemi, E.; Al-Aasm, I.S.; Coniglio, M. Subaerial exposure and meteoric diagenesis of the Cenomanian-Turonian Upper Sarvak Formation, southwestern Iran. Geol. Soc. Spec. Publ. 2010, 330, 253–272. [Google Scholar] [CrossRef]
- Van Buchem, F.S.P.; Razin, P.; Homewood, P.W.; Oterdoom, W.H.; Philip, J. Stratigraphic organization of carbonate ramps and organic-rich intrashelf basins: Natih Formation (Middle cretaceous) of northern Oman. AAPG Bull. 2002, 86, 21–53. [Google Scholar]
- Vincent, B.; Van Buchem, F.S.P.; Bulot, L.G.; Jalali, M.; Swennen, R.; Hosseini, S.A.; Baghbani, D. Depositional sequences, diagenesis and structural control of the Albian to Turonian carbonate platform systems in coastal Fars (SW Iran). Mar. Pet. Geol. 2015, 63, 46–67. [Google Scholar] [CrossRef]
- Mehrabi, H.; Rahimpour-Bonab, H.; Hajikazemi, E. Controls on depositional facies in Upper Cretaceous carbonate reservoirs in the Zagros area and the Persian Gulf, Iran. Facies 2015, 61, 23. [Google Scholar] [CrossRef]
- Bétard, F.; Caner, L.; Gunnell, Y.; Bourgeon, G. Illite neoformation in plagioclase during weathering: Evidence from semi-arid Northeast Brazil. Geoderma 2009, 152, 53–62. [Google Scholar] [CrossRef]
- Bjørlykke, K. Clay mineral diagenesis in sedimentary basins—A key to the prediction of rock properties. Ex. North Sea Basin. Clay Miner. 1998, 33, 15–34. [Google Scholar] [CrossRef]
- Schleicher, A.M.; Warr, L.N.; Kober, B.; Laverret, E.; Clauer, N. Episodic mineralization of hydrothermal illite in the Soultz-sous-Forêts granite (Upper Rhine Graben, France). Contrib. Mineral. Petrol. 2006, 152, 349–364. [Google Scholar] [CrossRef]
- Huggett, J.; Cuadros, J.; Gale, A.S.; Wray, D.; Adetunji, J. Low temperature, authigenic illite and carbonates in a mixed dolomite-clastic lagoonal and pedogenic setting, Spanish Central System, Spain. Appl. Clay Sci. 2016, 132, 296–312. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Wang, Q.; Deng, J.; Liu, X.; Wang, J. Mineralogical and geochemical features of karst bauxites from Poci (western Henan, China). Ore Geol. Rev. 2019, 105, 295–309. [Google Scholar] [CrossRef]
- Lanson, B.; Beaufort, D.; Berger, G.; Bauer, A.; Cassagnabère, A.; Meunier, A. Authigenic kaolin and illitic minerals during burial diagenesis of sandstones: A review. Clay Miner. 2002, 37, 1–22. [Google Scholar] [CrossRef]
- Velde, B.; Meunier, A. The Origin of Clay Minerals in Soils and Weathered Rocks; Springer: Berlin, Heidelberg, 2008; 406p. [Google Scholar]
- Essington, M.E. Soil and Water Chemistry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; 656p. [Google Scholar]
- Öztürk, H.; Hanilçi, N.; Cansu, Z.; Kasapçi, C. Formation of Ti-rich bauxite from alkali basalt in continental margin carbonates, Payas region, SE Turkey. Turk. J. Earth Sci. 2021, 30, 116–141. [Google Scholar] [CrossRef]
- Zotov, A.; Mukhamet-Galeev, A.; Schott, J. An experimental study of kaolinite and dickite relative stability at 150-300°C and the thermodynamic properties of dickite. Am. Mineral. 1998, 83, 516–524. [Google Scholar] [CrossRef]
- Bárdossy, G.; Aleva, G.J.J. Lateritic Bauxites; Elsevier: Amsterdam, The Netherlands, 1990; 624p. [Google Scholar]
- Horbe, A.M.C.; Anand, R.R. Bauxite on igneous rocks from Amazonia and southwestern of Australia: Implication for weathering process. J. Geochem. Explor. 2011, 111, 1–12. [Google Scholar] [CrossRef]
- Ling, K.Y.; Zhu, X.Q.; Tang, H.S.; Wang, Z.G.; Yan, H.W.; Han, T.; Chen, W.Y. Mineralogical characteristics of the karstic bauxite deposits in the Xiuwen ore belt, Central Guizhou Province, Southwest China. Ore Geol. Rev. 2015, 65, 84–96. [Google Scholar] [CrossRef]
- Hanilçi, N. Geological and geochemical evolution of the Bolkardagi bauxite deposits, Karaman, Turkey. J. Geochem. Explor. 2013, 133, 118–137. [Google Scholar] [CrossRef]
- Denigres Filho, R.W.N.; Rocha, G.D.A.; Montes, C.R.; Vieira-Coelho, A.C. Synthesis and characterization of boehmites obtained from gibbsite in presence of different environments. Mater. Res. 2016, 19, 659–668. [Google Scholar] [CrossRef]
- Yu, W.; Algeo, T.J.; Yan, J.; Yang, J.; Du, Y.; Huang, X.; Weng, S. Climatic and hydrologic controls on upper Paleozoic bauxite deposits in South China. Earth-Sci. Rev. 2019, 189, 159–176. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. Bio-mineralization and potential biogeochemical processes in bauxite deposits: Genetic and ore quality significance. Miner. Petrol. 2013, 107, 471–486. [Google Scholar] [CrossRef]
- Liu, X.F.; Wang, Q.F.; Zhang, Q.Z.; Yang, S.J.; Liang, Y.Y.; Zhang, Y.; Li, Y.; Guan, T. Genesis of the Permian karstic Pingguo bauxite deposit, western Guangxi, China. Mineral. Deposita. 2017, 52, 1031–1048. [Google Scholar] [CrossRef]
- Liu, T.J.; Wang, X.L.; Wang, Z.T.; Liu, X.F.; Ju, P.C.; Zhong, J.A. Provenance of the Early Permian bauxitic claystone in Huayingshan region, Sichuan Basin, South China. J. Palaeogeogr. 2023, 12, 211–228. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Yang, S.; Ma, X.; Liu, L.; Sun, X. Regional multi-sources of Carboniferous karstic bauxite deposits in North China Craton. Sediment. Geol. 2021, 421, 105958. [Google Scholar] [CrossRef]
- Salamab-Ellahi, S.; Taghipour, B.; Nejadhadad, M. The Role of Organic Matter in the Formation of High-Grade Al Deposits of the Dopolan Karst Type Bauxite, Iran. Minerals 2017, 7, 97. [Google Scholar] [CrossRef]
- Panahi, A.; Young, G.M.; Rainbird, R.H. Behavior of major and trace elements (including REE) during Paleoproterozoic pedogenesis and diagenetic alteration of an Archean granite near Ville Marie, Quebec, Canada. Geochim. Cosmochim. Acta 2000, 64, 2199–2220. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Zamanian, H.; Taghipour, B.; Zarasvandi, A.; Buccione, R. Mineralogical and geochemical evolution of the Bidgol bauxite deposit, Zagros Mountain Belt, Iran. Ore Geol. Rev. 2017, 86, 755–783. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Li, Y.; Wu, C.; Zheng, C. The “coal-bauxite-iron” structure in the ore-bearing rock series as a prospecting indicator for southeastern Guizhou bauxite mines. Ore Geol. Rev. 2013, 53, 145–158. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. The role of microorganisms on the mineralogical and geochemical characteristics of the Parnassos-Ghiona bauxite deposits, Greece. J. Geochem. Explor. 2007, 93, 67–77. [Google Scholar] [CrossRef]
- Kalaitzidis, S.; Siavalas, G.; Skarpelis, N.; Araujo, C.V.; Christanis, K. Late Cretaceous coal overlying karstic bauxite deposits in the Parnassus-Ghiona Unit, Central Greece. Int. J. Coal Geol. 2010, 81, 211–226. [Google Scholar] [CrossRef]
- Seal, R.R. Sulfur Isotope Geochemistry of Sulfide Minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- Chapelle, F.H. Ground-Water Microbiology and Geochemistry; John Wiley & Sons: Hoboken, NJ, USA, 1993; 496p. [Google Scholar]
- Moyano, F.E.; Vasilyeva, N.; Bouckaert, L.; Cook, F.; Craine, J.; Yuste, J.C.; Don, A.; Epron, D.; Formanek, P.; Franzluebbers, A.; et al. The moisture response of soil heterotrophic respiration: Interaction with soil properties. Biogeosciences 2012, 9, 1173–1182. [Google Scholar] [CrossRef]
- Sierra, C.A.; Trumbore, S.E.; Davidson, E.A.; Vicca, S.; Janssens, I.A. Sensitivity of decomposition rates of soil organic matter with respect to simultaneous changes in temperature and moisture. J. Adv. Model. Earth Syst. 2015, 7, 335–356. [Google Scholar] [CrossRef]
- European Commission. Study on the EU’s List of Critical Raw Materials Critical Raw Materials, Factsheets (Final); Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs: Joint Research Centre Directorate GROW.C, JRC.D Unit GROW.C.2 Energy-intensive Industries and Raw Materials, JRC.D.3 Land Resources; European Commission: Brussels, Belgium, 2020; 819p. [Google Scholar]
- McLennan, S.M.; Taylor, S.R.; Hemming, S.R. Composition, differentiation and evolution of continental crust: Constrains from sedimentary rocks and heat flow. In Evolution and Differentiation of the Continental Crust; Brown, M., Rushmer, T., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 92–134. [Google Scholar]
- Mongelli, G.; Buccione, R.; Sinisi, R. Genesis of autochthonous and allochthonous Apulian karst bauxites (Southern Italy): Climate constraints. Sediment. Geol. 2015, 325, 168–176. [Google Scholar] [CrossRef]
- Liu, X.F.; Wang, Q.F.; Deng, J.; Zhang, Q.Z.; Sun, S.L.; Meng, J.Y. Mineralogical and geochemical investigations of the Dajia Salento-type bauxite deposits, western Guangxi, China. J. Geochem. Explor. 2010, 105, 137–152. [Google Scholar] [CrossRef]
- Hreus, S.; Výravský, J.; Cempírek, J.; Breiter, K.; Galiová, M.V.; Krátký, O.; Šešulka, V.; Skoda, R. Scandium distribution in the world-class Li-Sn-W Cínovec greisen-type deposit. Ore Geol. Rev. 2021, 139, 104433. [Google Scholar] [CrossRef]



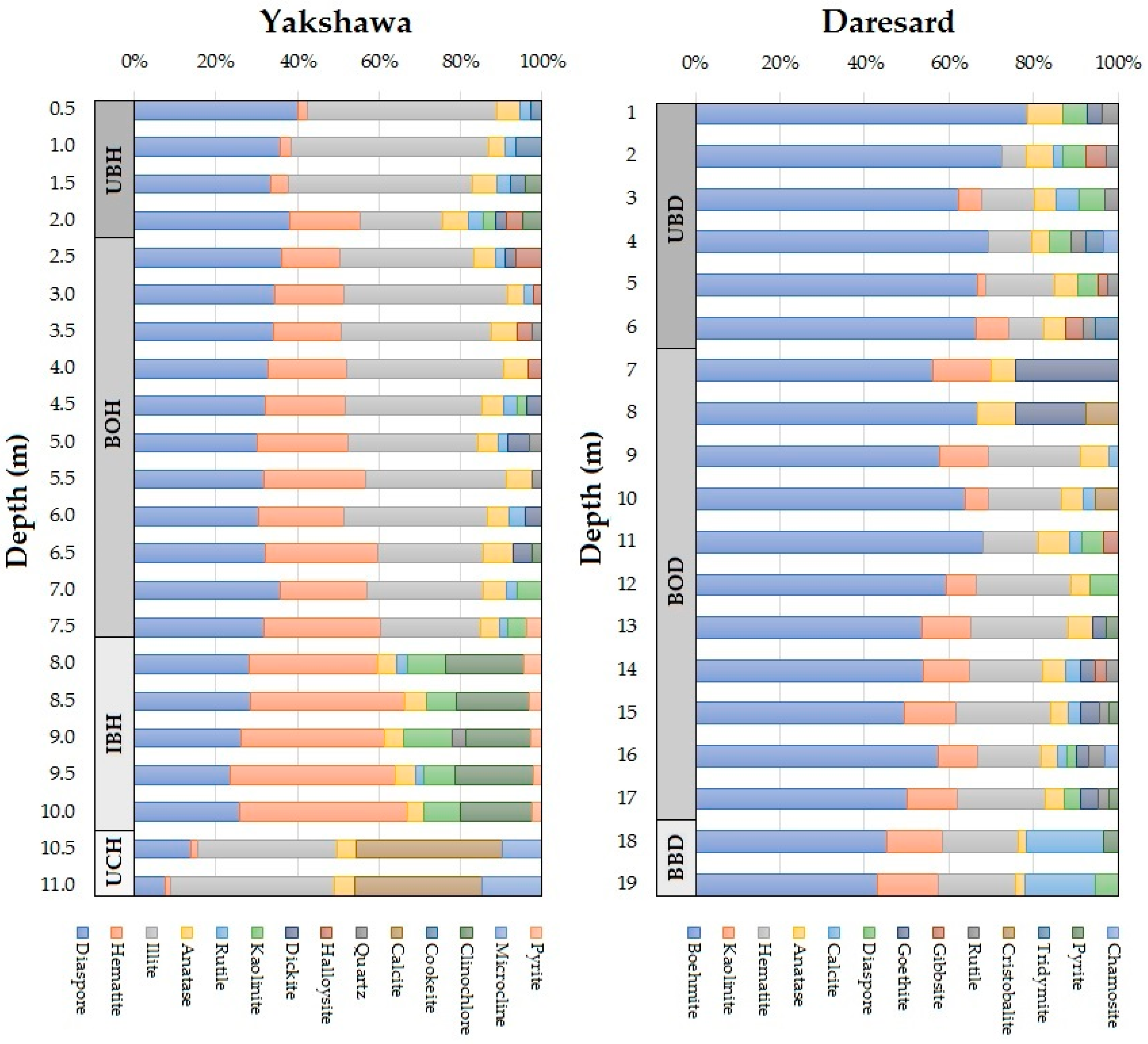





| Deposit | Yakshawa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minerals | Dsp | Hem | Ilt | Ant | Rt | Kln | Dck | Hal | Qz | Cal | Ckt | Clc | Mc | Py |
| UBH1 | 40.2 | 2.2 | 46.5 | 5.6 | 2.9 | - | - | - | - | - | 2.6 | - | - | - |
| UBH2 | 35.8 | 2.8 | 48.3 | 4 | 2.6 | - | - | - | - | - | 6.5 | - | - | - |
| UBH3 | 33.6 | 4.1 | 45.4 | 5.9 | 3.2 | - | - | - | - | - | 3.6 | 4.2 | - | - |
| UBH4 | 38.1 | 17.4 | 20.1 | 6.4 | 3.5 | 3 | 2.8 | 4.1 | - | - | - | 4.6 | - | - |
| BOH-1 | 36.1 | 14.2 | 32.9 | 5.6 | 2.3 | - | 2.5 | 6.4 | - | - | - | - | - | - |
| BOH-2 | 34.5 | 16.8 | 40.3 | 4.1 | 2.2 | - | - | 2.1 | - | - | - | - | - | - |
| BOH-3 | 34.2 | 16.5 | 37.1 | 6.3 | - | - | - | 3.5 | 2.4 | - | - | - | - | - |
| BOH-4 | 32.8 | 19.2 | 38.8 | 5.9 | - | - | - | 3.3 | - | - | - | - | - | - |
| BOH-5 | 32.3 | 19.5 | 33.6 | 5.4 | 3.2 | 2.3 | 3.7 | - | - | - | - | - | - | - |
| BOH-6 | 30.1 | 22.3 | 31.8 | 5.2 | 2.1 | - | 5.4 | - | 3.1 | - | - | - | - | - |
| BOH-7 | 31.8 | 25.1 | 34.4 | 6.3 | - | - | - | - | 2.4 | - | - | - | - | - |
| BOH-8 | 30.6 | 20.7 | 35.5 | 5.1 | 3.9 | - | 4.2 | - | - | - | - | - | - | - |
| BOH-9 | 32.2 | 27.4 | 25.9 | 7.3 | - | - | 4.8 | - | - | - | - | 2.4 | - | - |
| BOH-10 | 35.7 | 21.3 | 28.6 | 5.8 | 2.5 | 6.1 | - | - | - | - | - | - | - | - |
| BOH-11 | 31.7 | 28.8 | 24.5 | 4.5 | 2.1 | 4.8 | - | - | - | - | - | - | - | 3.6 |
| IBH-1 | 28.1 | 31.6 | - | 4.7 | 2.8 | 9.3 | - | - | - | - | - | 19.2 | - | 4.3 |
| IBH-2 | 28.4 | 37.9 | - | 5.5 | - | 7.2 | - | - | - | - | - | 17.8 | - | 3.2 |
| IBH-3 | 26.3 | 35.2 | - | 4.4 | - | 12.2 | - | - | 3.4 | - | - | 15.9 | - | 2.6 |
| IBH-4 | 23.6 | 40.5 | - | 4.8 | 2.3 | 7.4 | - | - | - | - | - | 19.3 | - | 2.1 |
| IBH-5 | 25.8 | 41.3 | - | 4.1 | - | 8.9 | - | - | - | - | - | 17.4 | - | 2.5 |
| UCH-1 | 13.8 | 1.6 | 34.5 | 4.6 | - | - | - | - | - | 35.9 | - | - | 9.6 | - |
| UCH-2 | 7.6 | 1.3 | 40.1 | 5.2 | - | - | - | - | - | 31.2 | - | - | 14.6 | - |
| Deposit | Daresard | |||||||||||||
| Minerals | Bhm | Kln | Hem | Ant | Cal | Dsp | Gth | Gbs | Rt | Crs | Trd | Py | Chm | |
| UBD-1 | 78.5 | - | - | 8.4 | - | 5.7 | 3.8 | - | 3.6 | - | - | - | - | |
| UBD-2 | 72.6 | - | 5.7 | 6.5 | 2.3 | 5.4 | - | 4.8 | 2.7 | - | - | - | - | |
| UBD-3 | 62.2 | 5.4 | 12.6 | 5.2 | 5.5 | 5.9 | - | - | 3.2 | - | - | - | - | |
| UBD-4 | 69.4 | - | 10.2 | 4.1 | - | 5.1 | - | - | 3.5 | - | 4.4 | - | 3.3 | |
| UBD-5 | 66.7 | 2.1 | 16.2 | 5.5 | - | 4.8 | - | 2.3 | 2.4 | - | - | - | - | |
| UBD-6 | 66.3 | 7.7 | 8.5 | 5.1 | - | - | - | 4.1 | 3.1 | - | 5.2 | - | - | |
| BOD-1 | 56.2 | 13.8 | - | 5.8 | - | - | 24.2 | - | - | - | - | - | - | |
| BOD-2 | 66.9 | - | - | 8.9 | - | - | 16.7 | - | - | 7.5 | - | - | - | |
| BOD-3 | 57.8 | 11.6 | 21.7 | 6.7 | 2.2 | - | - | - | - | - | - | - | - | |
| BOD-4 | 63.9 | 5.3 | 17.5 | 5.1 | 3 | - | - | - | - | 5.2 | - | - | - | |
| BOD-5 | 68.1 | - | 13.2 | 7.4 | 2.6 | 5.4 | - | 3.3 | - | - | - | - | - | |
| BOD-6 | 59.3 | 7.2 | 22.4 | 4.5 | - | 6.6 | - | - | - | - | - | - | - | |
| BOD-7 | 53.7 | 11.4 | 23.1 | 5.8 | - | - | 3.1 | - | - | - | - | 2.9 | - | |
| BOD-8 | 54.1 | 10.6 | 17.6 | 5.3 | 3.4 | - | 3.5 | 2.7 | 2.8 | - | - | - | - | |
| BOD-9 | 49.5 | 12.2 | 22.4 | 4.2 | 2.7 | - | 4.6 | - | 2.3 | - | - | 2.1 | - | |
| BOD-10 | 57.6 | 9.1 | 15.2 | 3.7 | 2.3 | 2.4 | 2.9 | - | 3.7 | - | - | - | 3.1 | |
| BOD-11 | 50.2 | 11.7 | 21 | 4.4 | - | 3.7 | 4.3 | - | 2.5 | - | - | 2.2 | - | |
| BBD-1 | 45.4 | 13.1 | 17.8 | 2.1 | 18.1 | - | - | - | - | - | - | 3.5 | - | |
| BBD-2 | 42.9 | 14.6 | 18.3 | 2.2 | 16.8 | 5.2 | - | - | - | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadnejad, F.; Mongelli, G.; Rafat, G.; Sharifi, M. The Middle–Late Permian to Late Cretaceous Mediterranean-Type Karst Bauxites of Western Iran: Authigenic Mineral Forming Conditions and Critical Raw Materials Potential. Minerals 2025, 15, 584. https://doi.org/10.3390/min15060584
Ahmadnejad F, Mongelli G, Rafat G, Sharifi M. The Middle–Late Permian to Late Cretaceous Mediterranean-Type Karst Bauxites of Western Iran: Authigenic Mineral Forming Conditions and Critical Raw Materials Potential. Minerals. 2025; 15(6):584. https://doi.org/10.3390/min15060584
Chicago/Turabian StyleAhmadnejad, Farhad, Giovanni Mongelli, Ghazal Rafat, and Mohammad Sharifi. 2025. "The Middle–Late Permian to Late Cretaceous Mediterranean-Type Karst Bauxites of Western Iran: Authigenic Mineral Forming Conditions and Critical Raw Materials Potential" Minerals 15, no. 6: 584. https://doi.org/10.3390/min15060584
APA StyleAhmadnejad, F., Mongelli, G., Rafat, G., & Sharifi, M. (2025). The Middle–Late Permian to Late Cretaceous Mediterranean-Type Karst Bauxites of Western Iran: Authigenic Mineral Forming Conditions and Critical Raw Materials Potential. Minerals, 15(6), 584. https://doi.org/10.3390/min15060584







