Abstract
The objective of the present work was to investigate a possible processing route for a currently discarded material, niobium mineral tailings containing rare earth elements, from the largest niobium producer worldwide (CBMM), for use as a raw material for valuable products such as ferroniobium and rare earth concentrate. A study was conducted on the kinetics of the carbothermic reduction from hematite to magnetite (magnetizing roasting). Thermogravimetric tests performed in duplicate or triplicate were conducted at three different temperatures (700 °C, 800 °C and 900 °C) for 1 h with two times the stoichiometric quantity of the reductant (charcoal). Scanning electron microscopy (SEM) and X-ray diffraction (XRD) showed small amounts of magnetite in the samples reduced at 700 °C and 800 °C. At 900 °C, in accordance with the XRD analysis (Rietveld), almost all hematite was reduced to magnetite. The kinetic model that showed the best fitting was the Ginstling–Brounshtein model. The apparent activation energy was evaluated to be 206 kJ/mol, which is similar to the values reported in the literature for the activation energy of the Boudouard reaction.
1. Introduction
The first step in the production of FeNb by CBMM, the largest niobium producer in the world, is the concentration process. It follows the steps within the flowchart presented in Figure 1. The ore contains approximately 2.5% Nb2O5. After milling, magnetic separation and flotation, a concentrate with ~50% Nb2O5 is obtained. The floated concentrate then undergoes several pyrometallurgical refining steps until FeNb (65% Nb, 35% Fe) is produced.
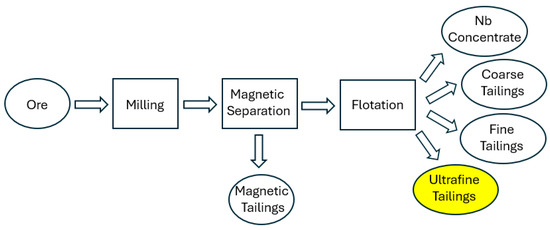
Figure 1.
Simplified flowchart of the production of niobium oxide concentrate.
The annual production of FeNb is around 100 kt and the approximate amount of ultrafine tailings generated is 13 t/t FeNb. This material, which presently is used in dams, contains ~50% iron oxides (goethite and hematite), ~6% total rare earth oxides and ~1.5% niobium oxide. The disposal of these tailings is a significant environmental burden, as well as a waste of valuable mineral resources.
Above 300 °C, goethite loses its hydroxyl group, transforming into hematite [1]. The carbothermic reduction from hematite to magnetite is extensively reported in the literature [2,3,4,5,6]. The high magnetic susceptibility of magnetite could allow its more efficient separation from the other elements of interest (niobium and rare earths) through the application of low-intensity magnetic fields [7]. Niobium and rare earth oxides would remain in the non-magnetic fraction. In a hypothetical scenario of 100% yield, the recovery of this material could represent an annual added production of 683 kt of iron ore (as magnetite), 97 kt of rare earth concentrate (80% total rare earth oxides, mostly Cerium and Lanthanum) and 20 kt de FeNb without needing to mine more material.
Magnetite (Fe3O4) can be produced by reduction reactions (1)–(3) [2,3,4,5,6], given below:
3Fe2O3(s) + C(s) = 2Fe3O4(s) + CO(g)
3Fe2O3(s) + CO(g) = 2Fe3O4(s) + CO2(g)
6Fe2O3(s) + C(s) = 4Fe3O4(s) + CO2(g)
Reactions (1) and (3) depend on the contact between the carbon and iron oxide particles. The CO2 formed through reactions (2) and (3) may regenerate to CO through the Boudouard reaction, reaction (4), given as follows:
C(s) + CO2(g) = 2CO(g)
The formation of CO through the Boudouard reaction is favored with the increase in temperature, as shown in Figure 2 [3] and Figure 3. Since gas–solid contact (CO(g)–Fe2O3(s)), represented by reaction (2), is naturally greater than solid–solid contact (C(s)–Fe2O3(s)), represented by reactions (1) and (3), the reduction by carbon occurs mainly by the gas–solid contact presented in reaction (2) [4].
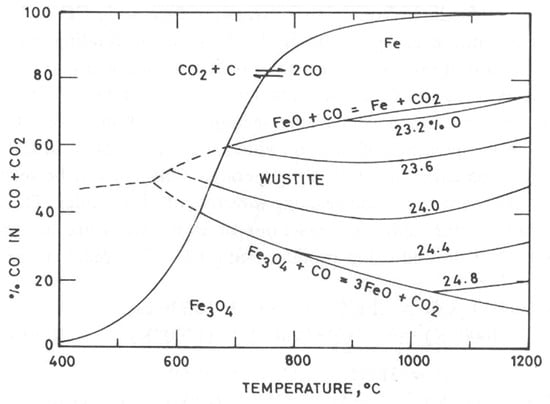
Figure 2.
Variation in the CO/CO2 ratio as a function of temperature for the reduction of iron oxides and the Boudouard reaction [3].
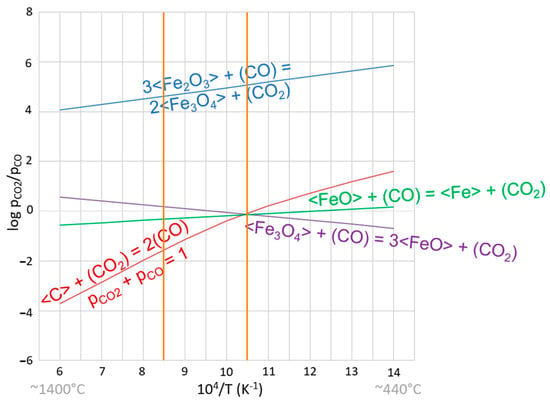
Figure 3.
Gaseous equilibrium relations pCO2/pCO as a function of temperature for iron oxides and the Boudouard reaction. Vertical orange lines correspond to the temperature range in the trials. Thermodynamic data were obtained from the Thermo-Calc database SSUB6.
Figure 3 indicates an upper temperature limit for the process of reducing hematite to magnetite close to 650 °C and a narrow temperature range in which wustite (FeO) is the most stable phase, between approximately 650 °C and 690 °C. Above that, Fe may be formed. In Figure 3, the vertical orange lines correspond to the temperature range of the trials (700–900 °C).
These reactions are illustrated in Figure 4.
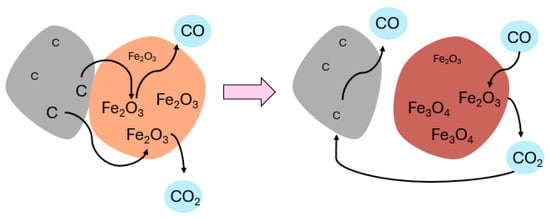
Figure 4.
Illustration of simultaneous reactions which may occur during the magnetizing roasting of iron ore with carbon as a reductant.
In the present study, temperatures from 700 °C to 900 °C were tested, even though it was known that phases such as FeO and Fe could be formed, since the goal was to determine parameters that would make the reduction to magnetite feasible in an industrial environment. Thus, thermodynamics, as well as kinetics, are important.
Table 1 and Table 2 summarize previous kinetic studies on iron ore reduction using solid and gaseous reductants. The range of activation energy (Ea) calculated in these studies ranges from 37.8 kJ/mol to 352.2 kJ/mol for solid reductants and from 9.97 kJ/mol to 96 kJ/mol for gaseous reductants. Ea with gaseous reductants was generally lower than with solid reductants. Lower values of Ea indicate that processes using gaseous reductants are controlled by physical phenomena (diffusion or viscous flow), while higher Ea indicates control by chemical reaction [4].
The focus of the existing literature was mainly the direct reduction from hematite to iron, not the carbothermic reduction from hematite to magnetite. Besides this, most of the authors [8,9,10,11,12] have worked with high-purity iron oxide sources, except for Boari [13] and Daza [14], who both used samples that were very similar to the object of the present study. This is the knowledge gap that this present study addresses: the understanding of the kinetics of magnetizing roasting using an actual mineral sample of iron oxide.

Table 1.
Comparison of energy of activation and mechanisms of reduction of iron oxides in the literature, using solid reductants.
Table 1.
Comparison of energy of activation and mechanisms of reduction of iron oxides in the literature, using solid reductants.
| Ref. | Reductant | Reduction Step | T (°C) | Ea (kJ/mol) | Reduction Mechanism/ Kinetic Model |
|---|---|---|---|---|---|
| [8] | Graphite | Fe2O3→Fe | 950–1350 | 37.8–237 | Above 1200 °C: heat transport Below 1200 °C: Boudouard reaction, 1st-order reaction |
| [9] | Amorphous Carbon | Fe2O3→Fe | 850–1087 | 301 | Boudouard reaction |
| [13] | Charcoal or coke | Fe2O3→Fe | 800–1000 | 199.6–278.6 | Boudouard reaction/mass transport in the product layer (Ginstling–Brounshtein) |
| [14] | Charcoal or coke | Fe2O3→Fe | 800–1100 | 129.0–352.2 | With charcoal: Boudouard reaction/1st-order reaction With coke: Boudouard reaction/transport in the product layer (Ginstling–Brounshtein) |

Table 2.
Comparison of energy of activation and mechanisms of reduction of iron oxides in the literature, using gaseous reductants.
Table 2.
Comparison of energy of activation and mechanisms of reduction of iron oxides in the literature, using gaseous reductants.
| Ref. | Reductant | Reduction Step | T (°C) | Ea (kJ/mol) | Kinetic Model |
|---|---|---|---|---|---|
| [10] | H2 | Fe2O3→Fe3O4; Fe2O3→Fe | 0–700 | 96; 59–69 | 1st step: unreacted core with kinetic control by chemical reaction 2nd step: random nucleation |
| [11] | CO | Fe2O3→FeO; FeO→Fe; Fe→Fe3C | 800–900 | 9.97; 14.13; 14.65 | Chemical reaction |
| [12] | H2/CO | Fe2O3→Fe | 800–950 | 19.8–42.1 | 1st step: chemical reaction at the oxide/iron interface 2nd step: mixed control 3rd step: transport in the product layer (Ginstling–Brounshtein) |
2. Materials and Methods
The materials used in this study were the ultrafine mineral tailings from CBMM and charcoal. The as-received concentrate was characterized by laser diffraction using Bettersize S3 Plus, (Bettersize, Dandong, China)which indicated that the average size of the particles in the sample (D50) was 5.855 µm. It was also characterized by X-ray fluorescence (XRF), using a spectrometer from Malvern Panalytical (Malvern Panalytical, Malvern, UK), model Zetium; X-ray diffraction (XRD), using a D8 Advance diffractometer from Bruker (Billerica, MA, USA), with CuKα radiation and 1°/min scanning speed reveal; and scanning electron microscopy (SEM) (Bruker, Billerica, MA, USA), using an FEI Quanta 450 FEG (Bruker, Billerica, MA, USA). The following Inorganic Crystal Structure Database (ICSD) files were used to identify the XRD peaks: 071809 (goethite); 066756 (hematite); 036314 (magnetite); 64850 (monazite); 45819 (pyrochlore); 033734 (barite); 031243 (gorceixite); 016331 (quartz); and 044882 (anatase).
Calcination was performed in order to minimize the interference of moisture, volatile content or ash on calculations of reaction fractions during the reduction trials. The concentrate was then calcined at 500 °C for 1 h under a dynamic inert atmosphere (N2) at 2 L/min and characterized again by XRF, XRD and SEM. The charcoal sample had 5.285% moisture, 2.022% ash, 73.467% fixed carbon and 19.226% volatile content as received [10], and was calcined at 1000 °C for 1 h under a dynamic inert atmosphere (N2) at 2 L/min. The calcined tailings and calcined charcoal were kept in sealed bags in a vacuum.
Magnetizing roasting trials were conducted in a vertical tubular furnace, presented in Figure 5.
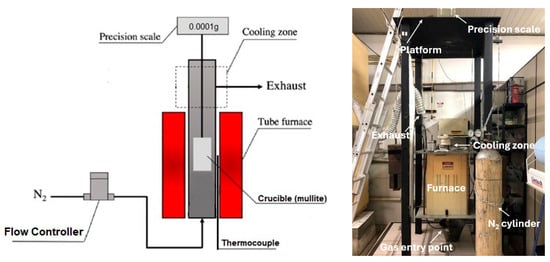
Figure 5.
Schematic representation (left) and picture (right) of the tubular furnace in the Reduction and Smelting Laboratory at the Metallurgical and Materials Engineering Department of the University of Sao Paulo.
The stoichiometric amount of carbon was calculated based on reaction (1) and on the hematite content analyzed by XRD and XRF. An excess of reductant (2× the stoichiometric amount, approximately 4% in the mixture) was used to guarantee availability of reductant for the carbothermic reduction of the iron oxide, in case other unpredicted reactions would take place.
Trials were carried out in duplicate or triplicate, under nitrogen atmosphere (0.08 m3/h), with 5 g samples of the mixture of calcined tailings and charcoal. The tested variable was temperature. Samples were put in alumina crucibles at 700 °C, 800 °C and 900 °C and were left at the target temperature for 1 h. The loss of mass was registered every 2 min 30 s.
For each trial, the reaction fraction (α) was calculated considering loss on ignition in the calcined mineral sample and the reduction of hematite to magnetite. α was calculated according to Equation (5).
where
mi = initial mass
mt = mass after reduction for time t
max = maximum possible loss of mass after reduction to hematite
The maximum possible loss of mass was calculated according to Equation (6).
where
MCO = molecular mass of CO
%Fe2O3 = percentage of this oxide in the calcined tailings
mt = mass of calcined tailings in the initial sample
%Cfixed = percentage of fixed C in calcined reductant
mr = mass of calcined reductant in the initial sample
MFe2O3 = molecular mass of this oxide
MC = molecular mass of C
LOIt = loss on ignition in calcined tailings
For the kinetic analysis, 4 possible models, shown in Table 3, were considered:

Table 3.
Kinetic models analyzed.
Loss of mass data from the trials in the vertical tubular furnace were collected every 2 min 30 s and analyzed. The comparison of the kinetic models was performed through the analysis of 12 graphs plotted on Excel.
3. Results and Discussion
After calcination, all goethite was transformed to hematite, as shown by the DRX results in Table 4. XRF results of the samples as received and after calcination presented in Table 5 show that 2.82% loss on ignition (LOI) remained in the in the calcined sample, which may be explained by the presence of gorceixite and pyrochlore, both of which are mineral phases that contain hydroxyl groups. The goodness of fit (GOF) for the XRD of the as-received and calcined samples were, respectively, 1.14 and 1.27, which indicates a very good fit of the spectra to the reference ICSD files, since 1.0 is equivalent to a perfect refinement and values under 5.0 reflect optimized refinement [15].

Table 4.
XRD analysis (Rietveld method) of a.r. and calcined samples.

Table 5.
XRF analysis (wt%) of as-received (a.r.) and calcined samples.
Figure 6 shows backscattered electron images of sample as-received and after calcination. Semi-quantitative energy dispersion spectroscopy (EDS) analysis was carried out on particles of the calcined sample, as presented in Table 6, which shows particles rich in Nb (Figure 6, point 1, likely pyrochlore), rare earths (Figure 6, area 2, probably monazite) and iron with impurities (Figure 6, point 3).
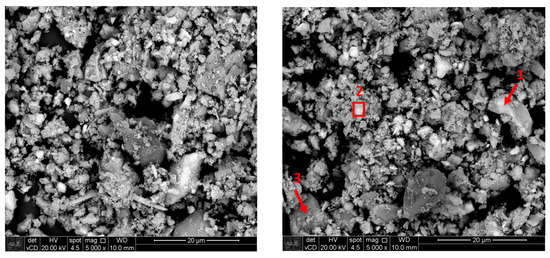
Figure 6.
Backscattered electron images of a.r. (left) and calcined (right) samples.

Table 6.
EDS chemical analysis of selected points/areas of the calcined sample (% at.).
Macroscopic images from the raw material and products are shown in Figure 7. The colors of these materials are related to the typical color of goethite, hematite and magnetite, which are yellowish brown, light to dark red, and black, respectively [16]. Goethite is the major mineral phase on the as-received tailings, according to the XRD results in Table 5. The XRD results presented in Figure 8 and Figure 9 and Table 7 show that hematite is the major mineral phase in the calcined tailings, as well as in the products of reduction at 700 °C and 800 °C, while the product of reduction at 800 °C already presents a quantifiable amount of magnetite, making it a darker shade of brown. Finally, the major mineral phase in the product of reduction at 900 °C is magnetite (Table 7), which makes it the darkest color of the group, black.

Figure 7.
General appearance of the raw material and products.
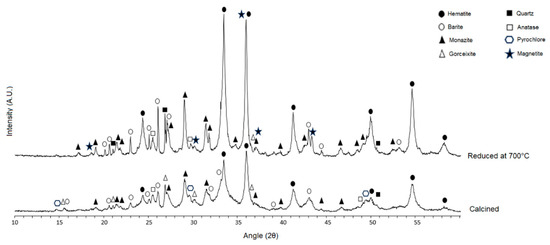
Figure 8.
Diffractogram of the reduction product at 700 °C compared to calcined concentrate.
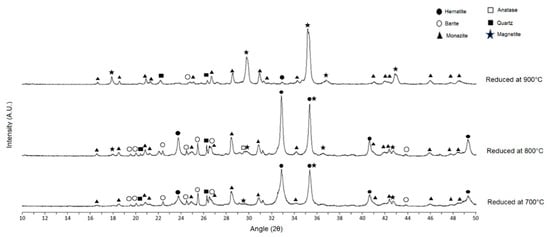
Figure 9.
Diffractogram of the reduction products at 700 °C, 800 °C and 900 °C.

Table 7.
Quantitative XRD (Rietveld method) of the magnetizing roasting products, compared to the calcined tailings (in %).
XRD analysis reveals that the characteristic peaks of magnetite start to appear in the reduction products at 700 °C (Figure 8), at 2θ equal to 18.3°, 30.1°, 35.4°, 37.0 and 43.0°. These characteristic peaks appear with higher intensity in the samples reduced at 800 °C and 900 °C (Figure 9).
Quantitative XRD by Rietveld method presented in Table 7 add to the analysis of the spectra. The GOF for samples reduced at 700 °C, 800 °C and 900 °C was 1.27, 1.19 and 1.67, respectively, all equivalent to optimized refinement [15]. The quantitative XRD indicated that 7.5% magnetite in the sample was reduced at 800 °C, and 79.0% magnetite in the sample was reduced at 900 °C. In the sample reduced at 700 °C, there was no quantifiable magnetite, probably because its content was below the method’s detection limit (<1%). It is possible that if the reduction tests had been performed for longer periods of time, quantifiable amounts of magnetite could be detected in the magnetizing roast product at 700 °C. From Figure 2 and Figure 3, no thermodynamic issue can be seen in reducing hematite to magnetite at 700 °C, but because of the low temperature, the reaction rate was very low; thus, less magnetite was formed.
Figure 10 shows the reduction products reacted at 700 °C, 800 °C and 900 °C. The chemical compositions of some regions of this figure are presented in Table 8. At 700 °C, point 2 could be magnetite, since this phase typically contains 57% at., although it was not detected in the XRD, probably due to the detection limit of the technique. Points 1 and 3 are probably pyrochlore and monazite, respectively. At 800 °C, point 1 is probably ilmenite. Points 2 and 3 could be wustite (Fe/O~1). Finally, at 900 °C, point 1 could be pyrochlore, point 2 could be iron silicate and point 3 could be wustite (which typically contains 50% at. O and 50% at. Fe).
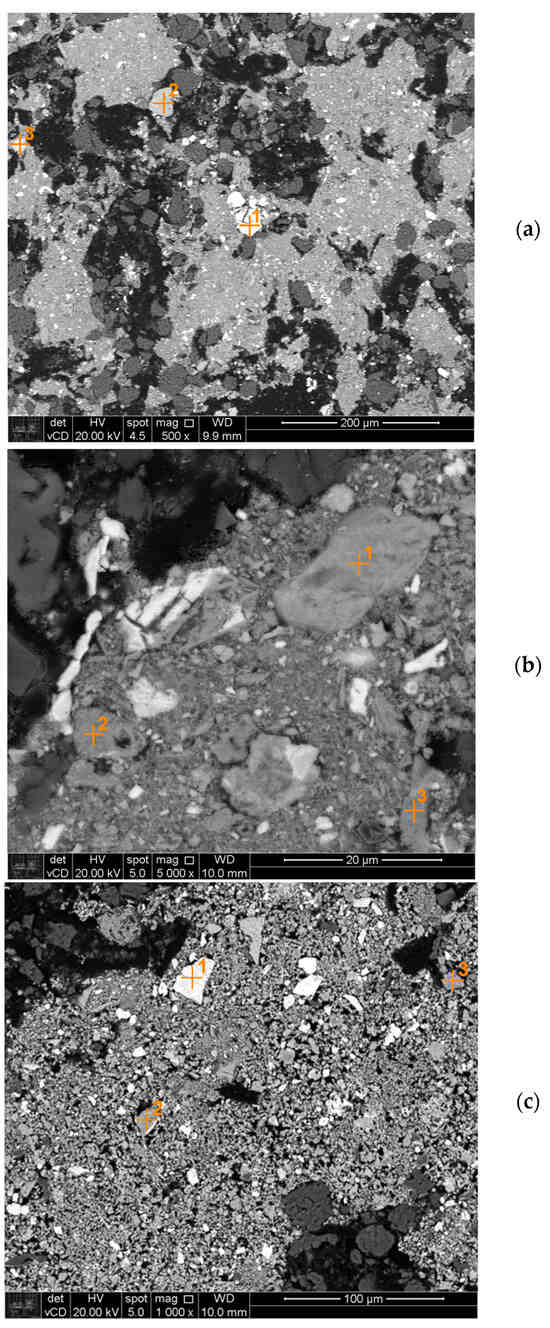
Figure 10.
Backscattered electron image of the product of reduction at 700 °C (a), 800 °C (b) and 900 °C (c).

Table 8.
Simplified EDS chemical analysis of points in Figure 10 (% at.).
The R2 values for the fit of loss of mass data to the four kinetic models are presented in Table 9; most were over 0.9. The R2 values for two models were very close: the shrinking core model with the kinetic control step by mass transport in the product layer, and the shrinking core model with the kinetic control step by mass transport in the gas layer. Ultimately, a decision was made for the shrinking core model with the kinetic control step by mass transport in the product layer (Ginstling–Brounshtein), adhering to the literature where very similar samples were investigated [13,14].

Table 9.
R2 for different kinetic models.
Some kinetic curves are shown in Figure 11. As the reaction at 900 °C was at least one order of magnitude faster than at 800 °C and two orders of magnitude faster than at 700 °C (see Figure 11 and Table 10), there are fewer data points for 900 °C. The precision of the scale was 1 × 10−4 g, so it is not possible to show any error bars.
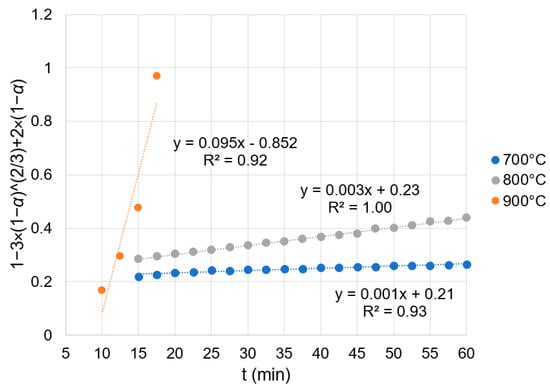
Figure 11.
Kinetic behavior of reduced samples.

Table 10.
Kinetic constant (k) for magnetizing reduction trials.
Table 10 shows the maximum loss of mass and the kinetic constants (k) for each trial. The maximum loss of mass was above the level of loss on ignition in the calcined tailings for all trials, suggesting that a reduction has taken place. At 900 °C, k values are at least one order of magnitude larger than k at 800 °C and two orders of magnitude larger than k at 700 °C, indicating that temperature has a high degree of influence on the rate of reaction.
It is possible that the Boudouard reaction did not occur from 700 °C to 800 °C because the reaction rate might have been low. However, XRD and EDS results have shown that magnetite was formed in all cases, albeit in small amounts at the lower temperatures. In order to evaluate whether the Boudouard reaction took place, for example, a mass spectrometer should be attached to the furnace to measure gas composition.
The Arrhenius plot presented in Figure 12 considered data from the three trials at 700 °C, two trials at 800 °C and two trials at 900 °C.
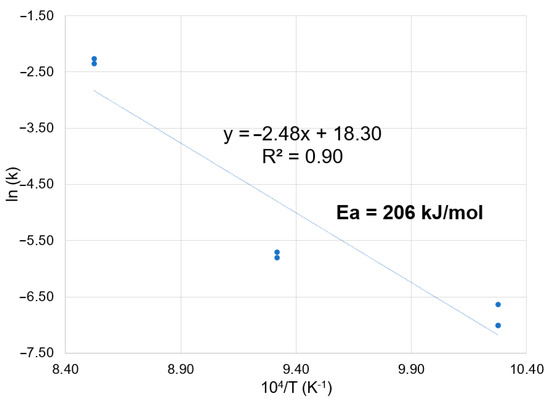
Figure 12.
Arrhenius plot of the magnetizing roasting tests.
Considering all data points, the apparent activation energy for the magnetizing reduction of mineral tailings is equal to 206 kJ/mol. This value falls within the range reported in the literature mentioned in the introduction, and is similar to the values reported within the literature for the activation energy of the Boudouard reaction [17,18].
Although thermodynamically possible at 700 °C and 800 °C, the Boudouard reaction should be very slow or not occur at all, as mentioned before. In this temperature range, the formation of magnetite and wustite may be a result of direct contact between the mineral tailings and the solid reductant and, consequently, the reduction rate is very low. At 900 °C, the Boudouard reaction certainly occurs, so the reduction happens mainly through gaseous intermediates, which substantially increases the rate of hematite reduction, as shown in Figure 11.
Considering only the k values obtained at 700 °C and 800 °C, the apparent activation energy drops to 98 kJ/mol, suggesting that the reduction reaction in lower temperatures is kinetically controlled by a physical process, for example, mass transport or heat transfer.
4. Conclusions
Summarizing the main findings, EDS analysis of the product at 700 °C showed evidence of magnetite formation by magnetizing roasting; at 800 °C, XRD analysis showed magnetite formation and EDS analysis showed particles with chemical composition close to wustite; at 900 °C, particles with chemical composition similar to wustite were identified by EDS analysis and XRD showed major formation of magnetite, with 79% Fe3O4 quantified in the product. Based on an analysis of statistical fit and looking at previous literature, the model of unreacted shrinking core with kinetic control by mass transport in the product layer (Ginstling–Brounshtein) was chosen to describe the kinetics of magnetizing roasting; monazite was preserved in all the products of magnetizing roasting, and the parameters which allowed the highest formation of magnetite were as follows: 900 °C, 1 h of reduction at the target temperature and two times the stoichiometric quantity of the reductant.
In conclusion, considering all the experimental data, the apparent activation energy was calculated as 206 kJ/mol, which suggests that the process of magnetizing roasting is controlled by the Boudouard reaction. At all three temperatures, hematite was reduced to magnetite. However, at lower temperatures, small amounts of magnetite were formed due to kinetic issues. This suggests that at low temperatures (up to 800 °C), the reduction takes place by direct contact of the solid reductant with the iron oxide particles, and at higher temperatures (900 °C), the Boudouard reaction occurs and reduction happens through gaseous intermediates, increasing the rate of reduction substantially.
For future research on this topic, we suggest investigating the reduction at 850 °C as an intermediate temperature, as well as the reductions at 700 °C and 800 °C for longer than 1 h, and tests at 900 °C with larger samples (over 1 kg), in order to prepare material for magnetic separation.
Author Contributions
Conceptualization, F.B.; methodology, V.G.A. and F.B.; software, V.G.A. and F.B.; validation, V.G.A. and F.B.; formal analysis, V.G.A. and F.B.; investigation, V.G.A. and F.B.; resources, V.G.A. and F.B.; data curation, F.B.; writing—original draft preparation, V.G.A.; writing—review and editing, V.G.A. and F.B.; visualization, V.G.A.; supervision, F.B.; project administration, F.B.; funding acquisition, V.G.A. and F.B.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Companhia Brasileira de Metalurgia e Mineração (CBMM) P794 and by the University of Sao Paulo PROEX 0727/2020.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank CBMM’s Center of Technology for providing the mineral sample and the support for characterization and the technicians from the Metallurgical and Materials Engineering Department at the University of Sao Paulo for support in the trials and characterization.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| a.r. | As received |
| CBMM | Companhia Brasileira de Metalurgia e Mineração |
| Ea | Activation energy |
| GOF | Goodness of fit |
| ICSD | Inorganic Crystal Structure Database |
| k | Kinetic constant |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
References
- Gialanella, S.; Girardi, F.; Ischia, G.; Lonardelli, I.; Mattarelli, M.; Montagna, M. On the goethite to hematite phase transformation. J. Therm. Anal. Calorim. 2010, 102, 867–873. [Google Scholar] [CrossRef]
- Uwadiale, G.G.O.O. Magnetizing Reduction of Iron Ores. Miner. Process. Extr. Metall. Rev. 1992, 11, 1–19. [Google Scholar] [CrossRef]
- Biswas, A.K. Principles of Blast Furnace Ironmaking, 1st ed.; Cootha Publishing House: Brisbane, Australia, 1981; pp. 72–76. [Google Scholar]
- Rosenqvist, T. Principles of Extractive Metallurgy, 1st ed.; McGraw-Hill: New York, NY, USA, 1983; pp. 121, 236–270. [Google Scholar]
- Faris, N.; Tardio, J.; Ram, R.; Bhargava, S.; Pownceby, M.I. Investigation into coal-based magnetizing roasting of an iron-rich rare earth ore and the associated mineralogical transformations. Miner. Eng. 2017, 114, 37–49. [Google Scholar] [CrossRef]
- Zhao, B.; Gao, P.; Tang, Z.; Zhang, W. The Efficient Improvement of Original Magnetite in Iron Ore Reduction Reaction in Magnetization Roasting Process and Mechanism Analysis by In Situ and Continuous Image Capture. Minerals 2021, 11, 645. [Google Scholar] [CrossRef]
- Rosenblum, S.; Brownfield, I.K. Magnetic susceptibilities of minerals. In Publications of the U.S. Geological Survey; USGS: Reston, VA, USA, 2000. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; De Carvalho, P.I., Jr.; Mourão, M.B. Cinética da Redução de Pelotas Auto-Redutoras em Elevadas Temperaturas; XXX Seminário de Redução de Minério de Ferro: Belo Horizonte, Brazil, 1999. (In Portuguese) [Google Scholar]
- Rao, Y.K. The Kinetics of Reduction of Hematite by Carbon. Metall. Trans. 1971, 2, 1439–1447. [Google Scholar] [CrossRef]
- Tiernan, M.J.; Barnes, P.A.; Parkes, G.M.B. The Investigation of Kinetic Parameters Using Rate Perturbation and Linear Heating Thermoanalytical Techniques. J. Phys. Chem. B 2001, 105, 220–228. [Google Scholar] [CrossRef]
- Mondal, K.; Lorethova, H.; Hippo, E.; Wiltowski, T.; Lalvani, S.B. Reduction of iron oxide in carbon monoxide atmosphere–reaction controlled kinetics. Fuel Process. Technol. 2004, 86, 33–47. [Google Scholar] [CrossRef]
- Moon, I.; Rhee, C.; Min, D. Reduction of hematite compacts by H2-CO gas mixtures. Steel Res. Process Metall. 1998, 69, 302–306. [Google Scholar] [CrossRef]
- Boari, V.H. Study of Carbothermic Reduction of Iron Oxides Contained in Monazite Concentrate. Avaliação da Redução Carbotérmica dos Óxidos de Ferro Contidos no Concentrado de Monazita. Master’s Thesis, Polytechnic School of the University of Sao Paulo, Sao Paulo, Brazil, 2021. (In Portuguese). [Google Scholar] [CrossRef]
- Daza Prada, I.M. Kinetics Evaluation of the Carbothermic Reduction of Iron Oxides Contained in Monazite Concentrate. Avaliação da Cinética da Redução Carbotérmica dos Óxidos de Ferro Contidos em Concentrado Monazítico. Master’s Dissertation, Polytechnic School of the University of Sao Paulo, Sao Paulo, Brazil, 2022. (In Portuguese). [Google Scholar] [CrossRef]
- Antoniassi, J.L. X-ray Diffraction with Rietveld Method Applied to Bauxites of Porto Trombetas, PA. A Difração de Raios X com o Método de Rietveld Aplicada a Bauxitas de Porto Trombetas, PA. Master’s Dissertation, Polytechnic School of the University of Sao Paulo, Sao Paulo, Brazil, 2010. (In Portuguese). [Google Scholar] [CrossRef]
- Instituto de Geociências da Universidade de São Paulo. Available online: https://didatico.igc.usp.br/minerais/oxidos-hidroxidos/ (accessed on 4 January 2024).
- Mourão, M.B. Analysis of the Iron Ore Reduction Process by Carbon in the Form of Self-Reducing Pellets. Análise do Processo de Redução de Minério de Ferro por Carbono na Forma de Pelotas Auto-Redutoras. Ph.D. Thesis, Polytechnic School of the University of Sao Paulo, Sao Paulo, Brazil, 1988. (In Portuguese). [Google Scholar] [CrossRef]
- Basu, P. Chapter 5—Gasification Theory and Modeling of Gasifiers Biomass Gasification and Pyrolysis. In Biomass Gasification Design Handbook; Academic Press: Cambridge, MA, USA, 2010; pp. 117–165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).