Advancements in Anode Slime Treatment: Effects of pH, Temperature, and Concentration of ClO−/OH− on Selenium Dissolution from Decopperized Anode Slimes
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Decopperized Anode Slimes
2.2. Leaching Experiments
3. Results
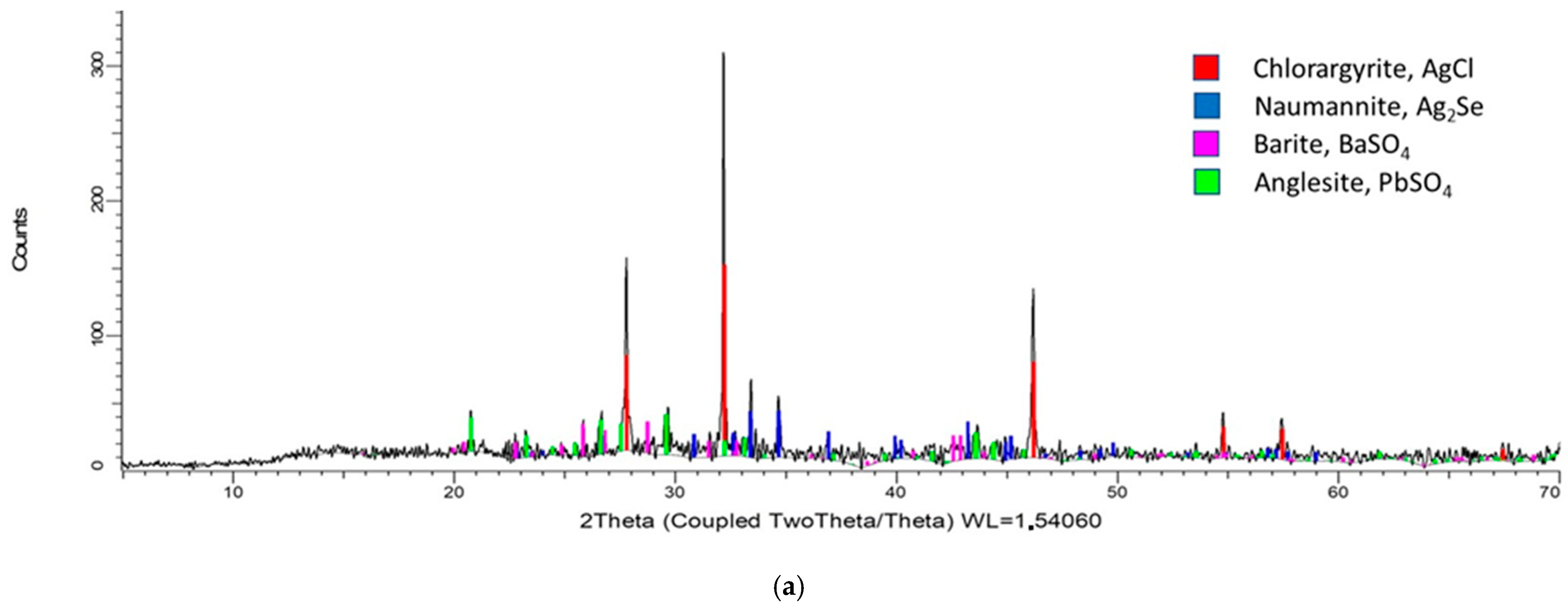
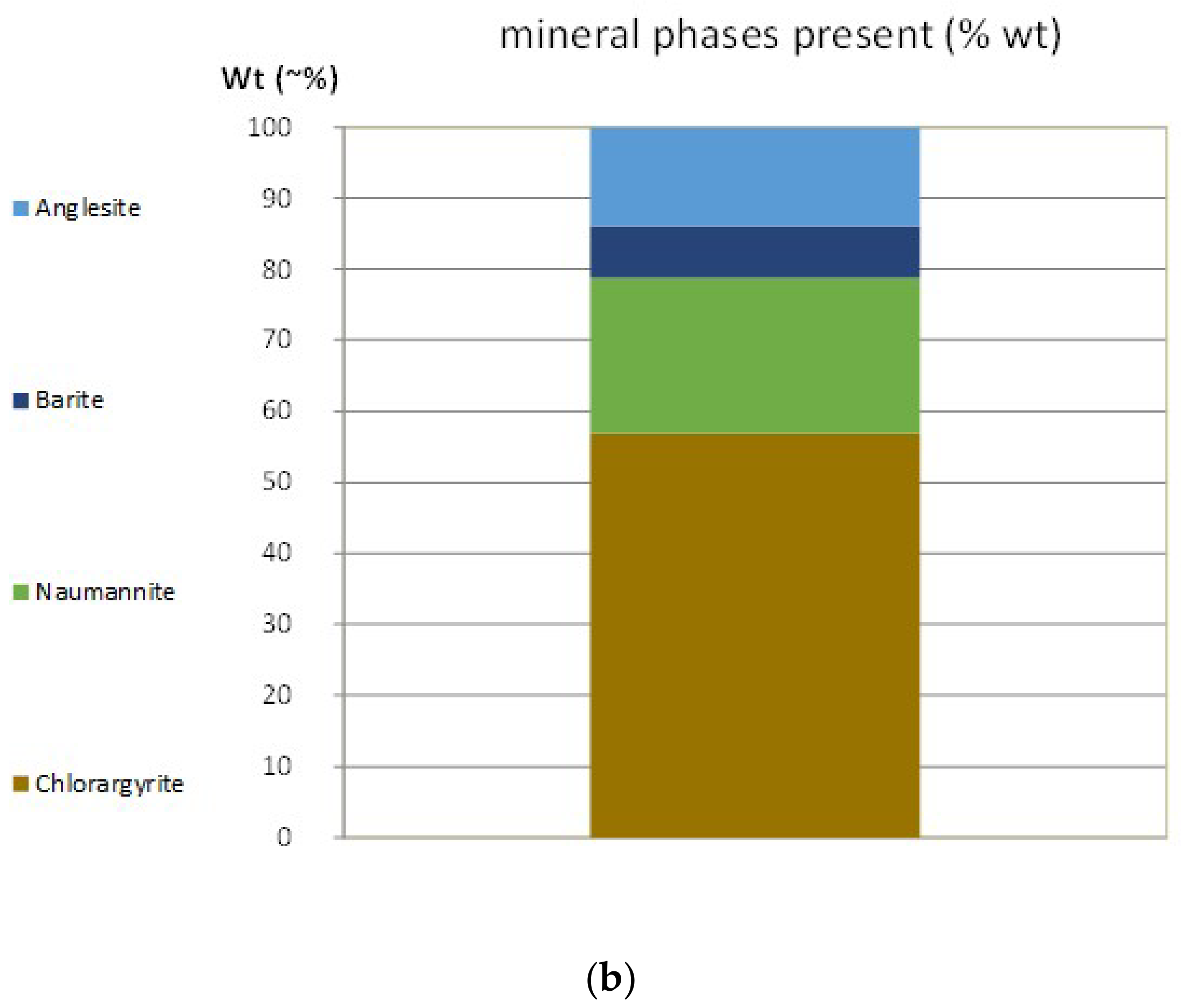
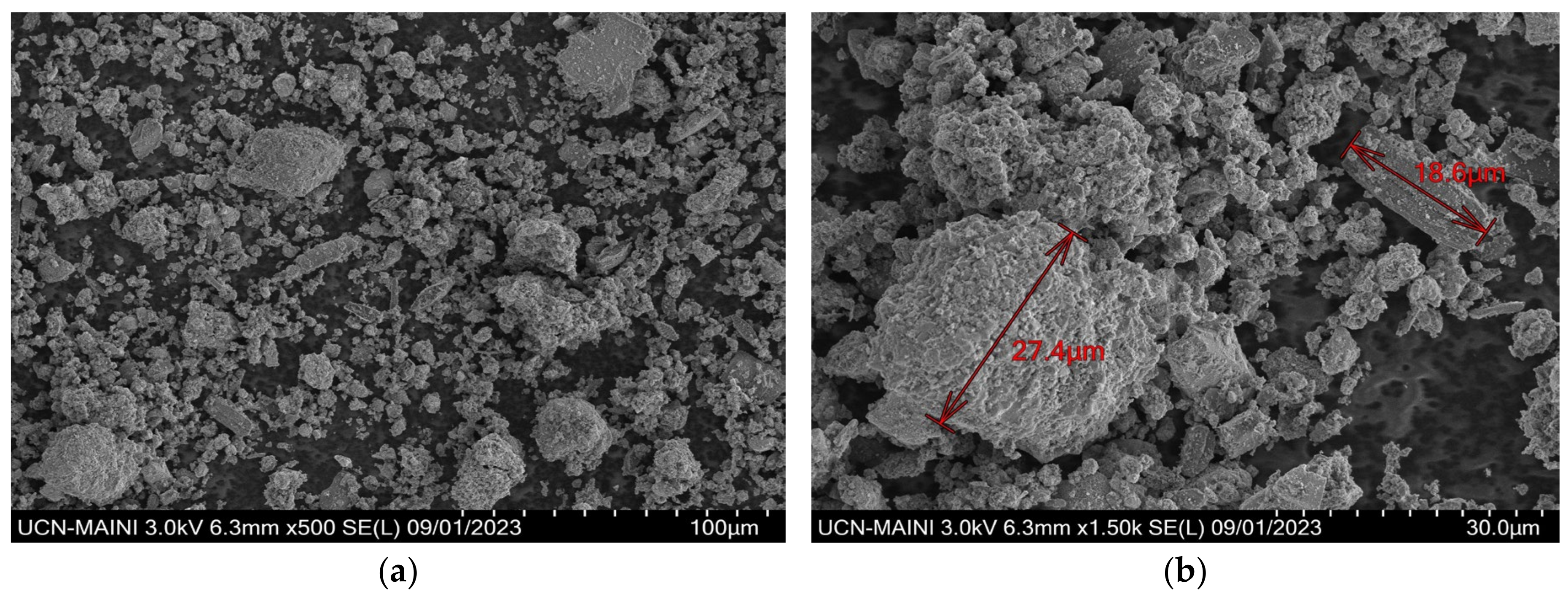
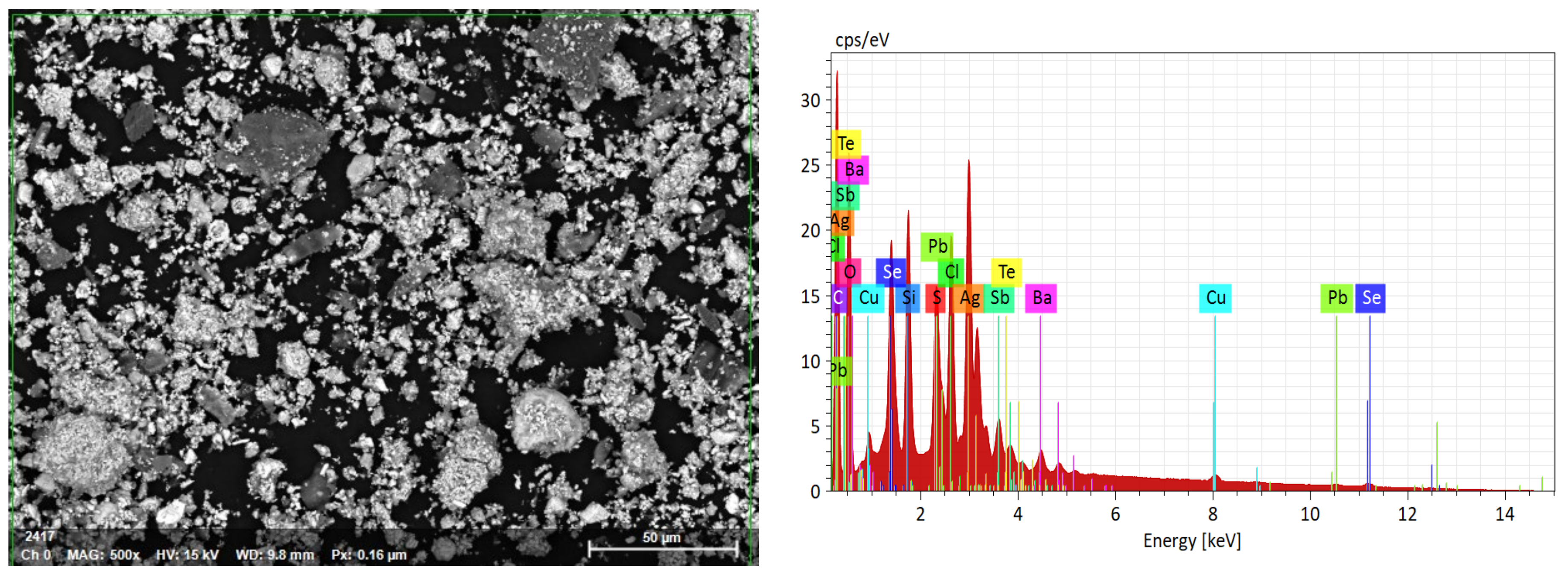
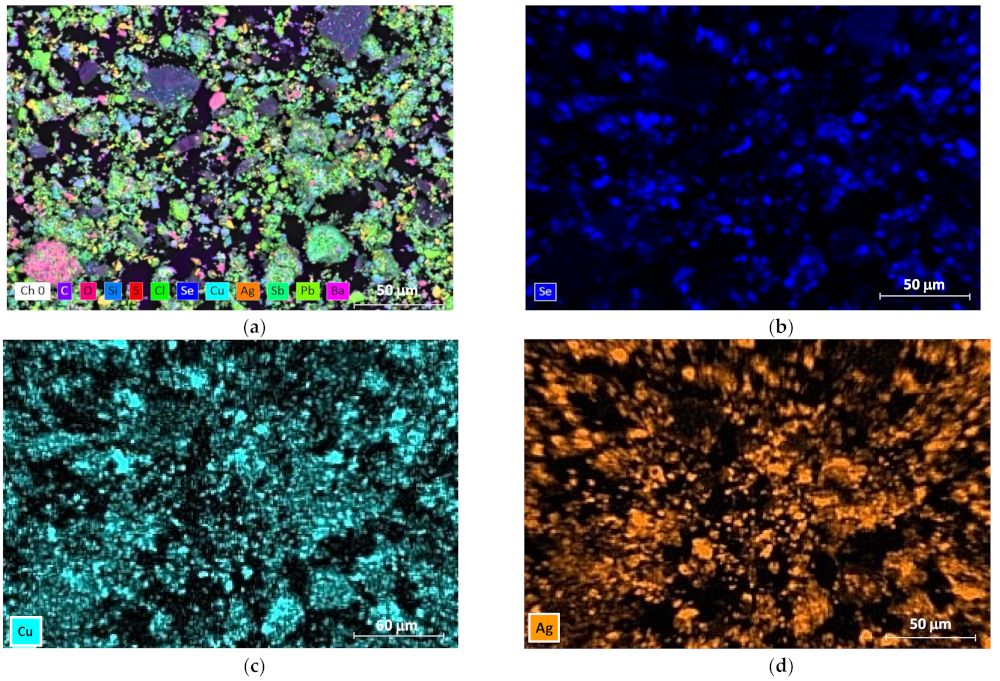
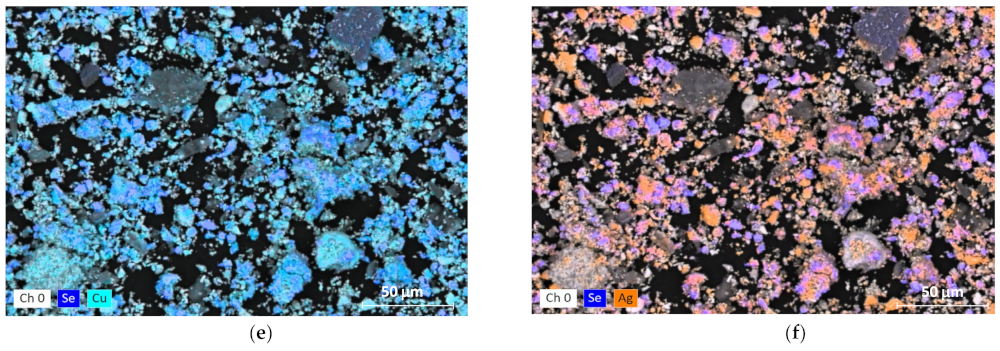
3.1. Mineralogical Characterization
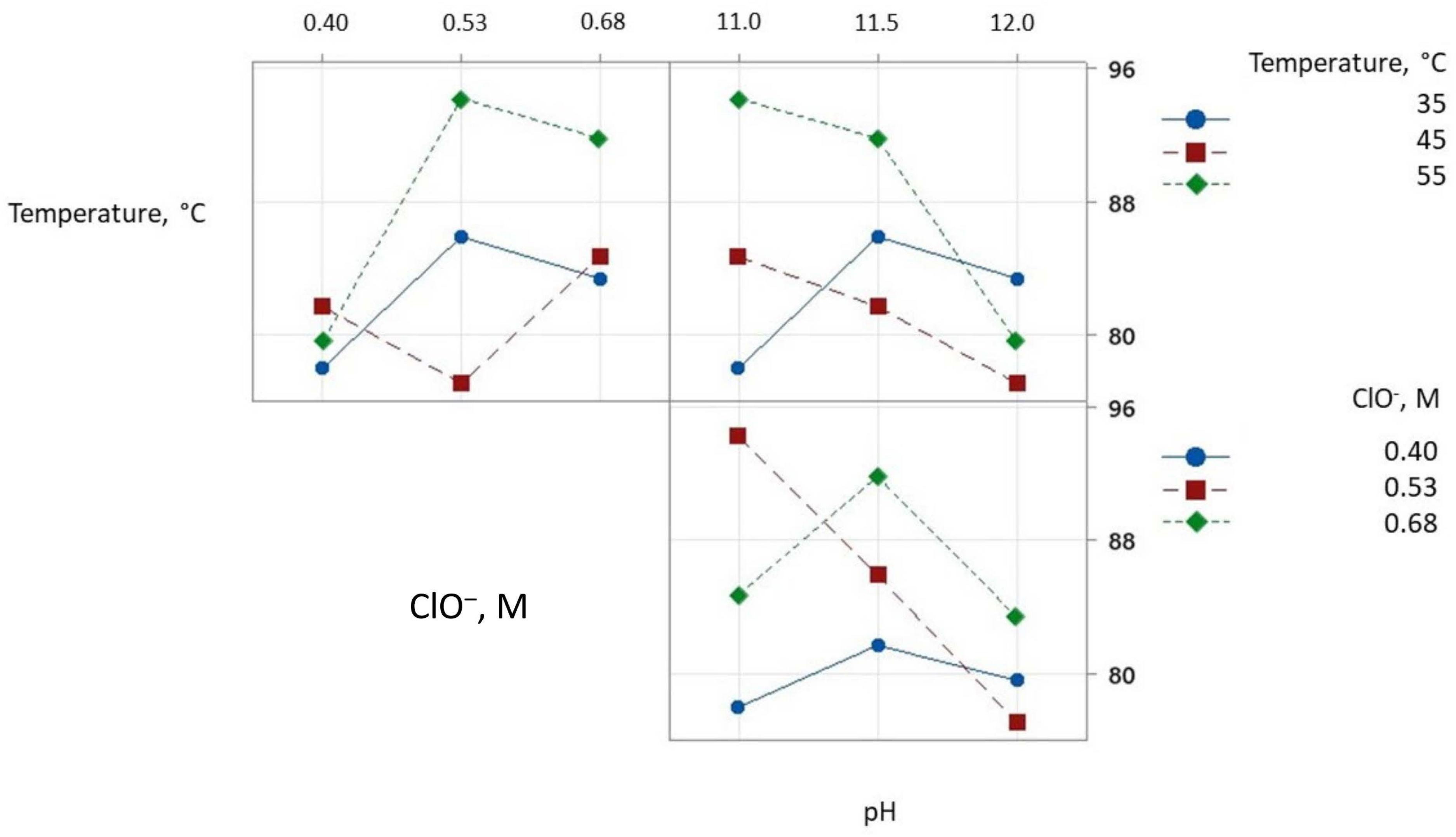
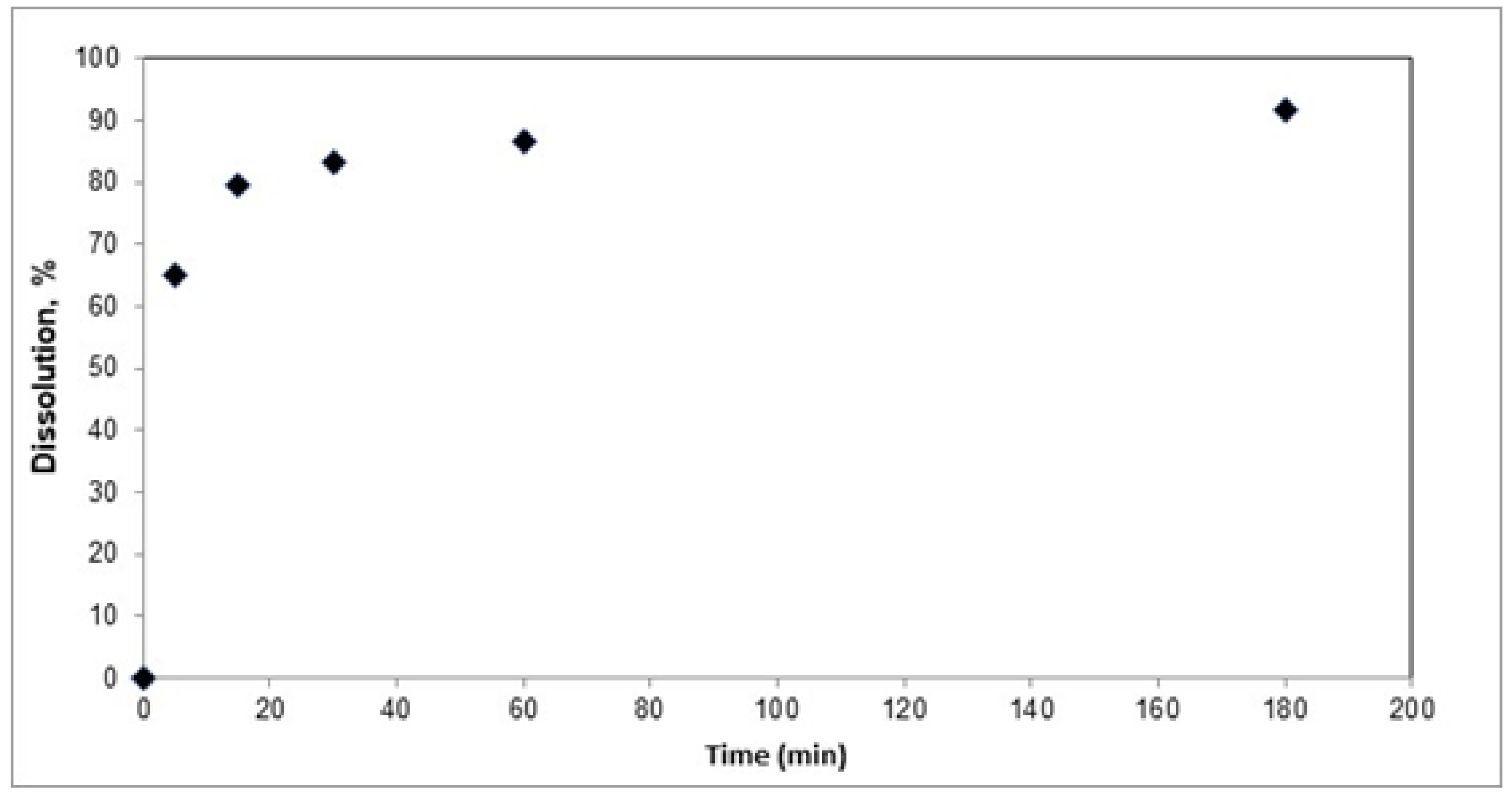
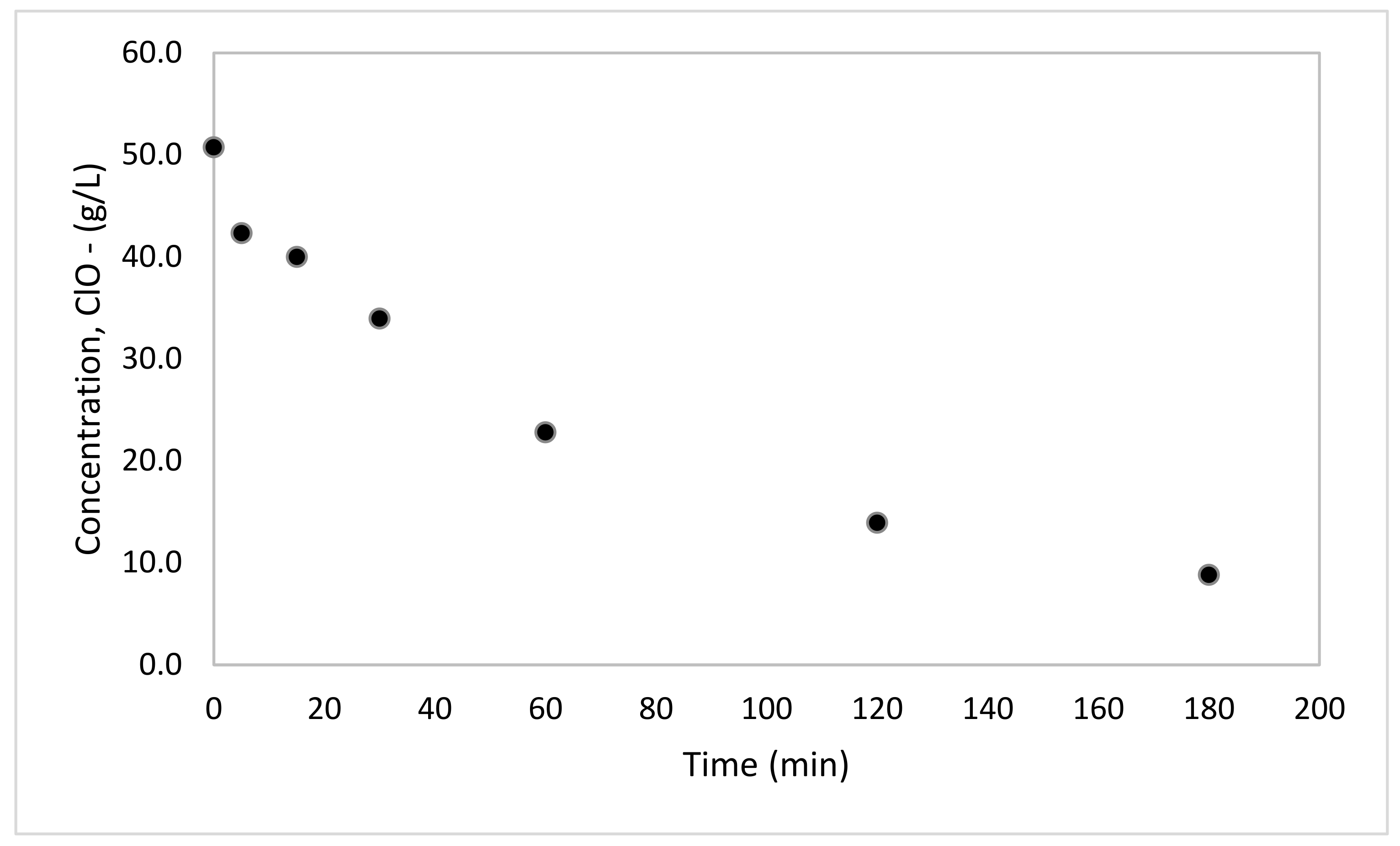
3.2. Selenium Leaching
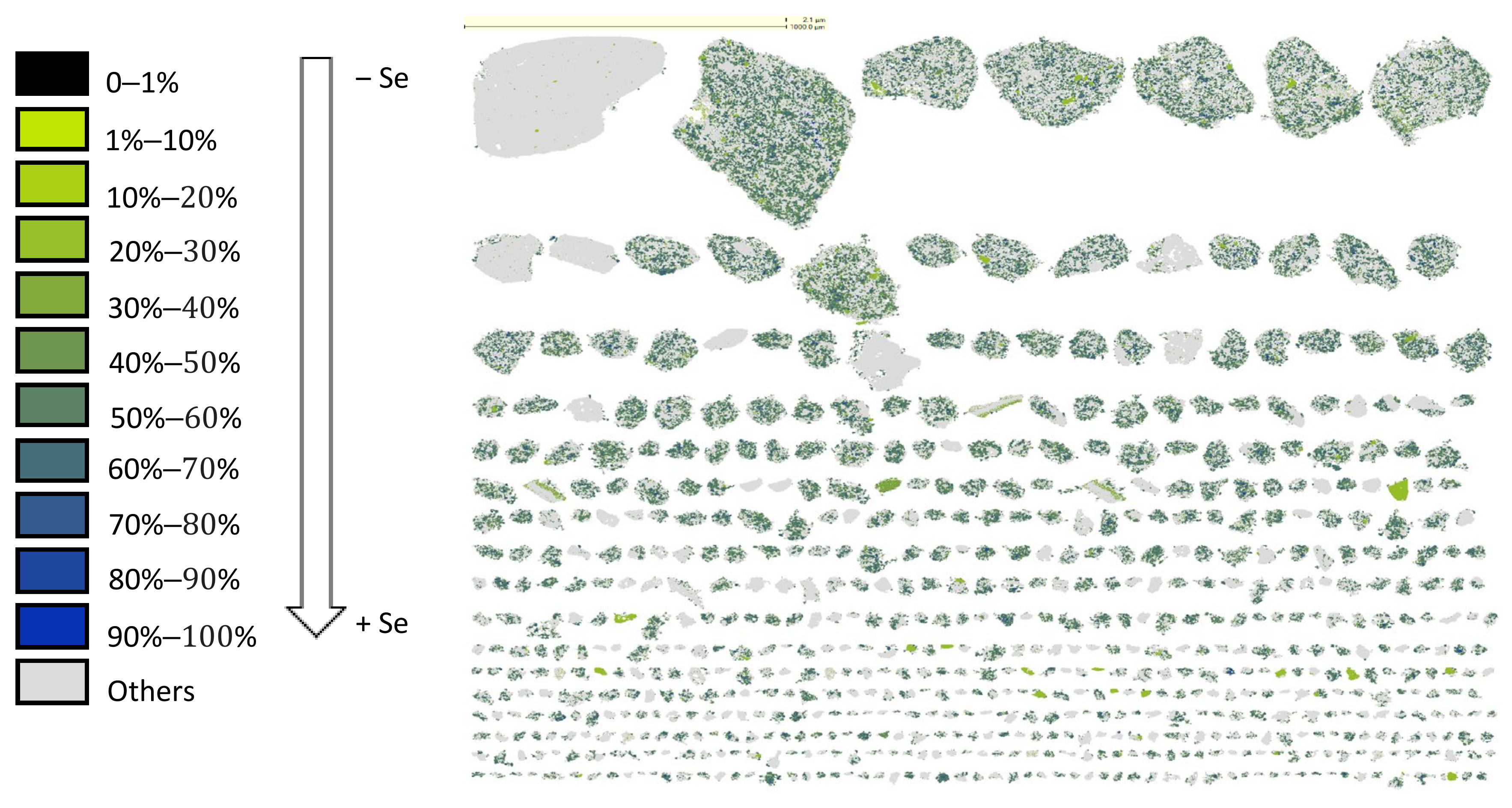
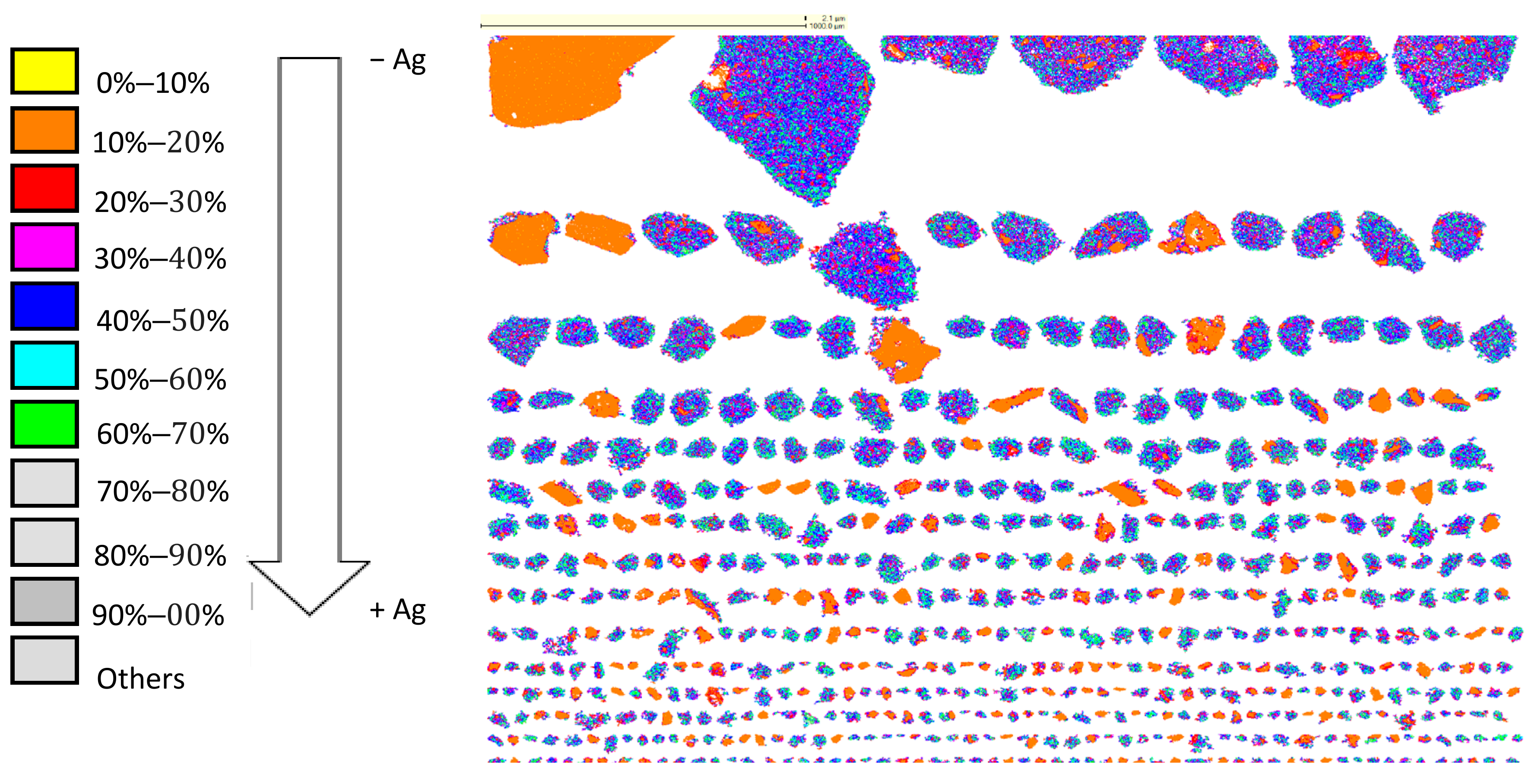
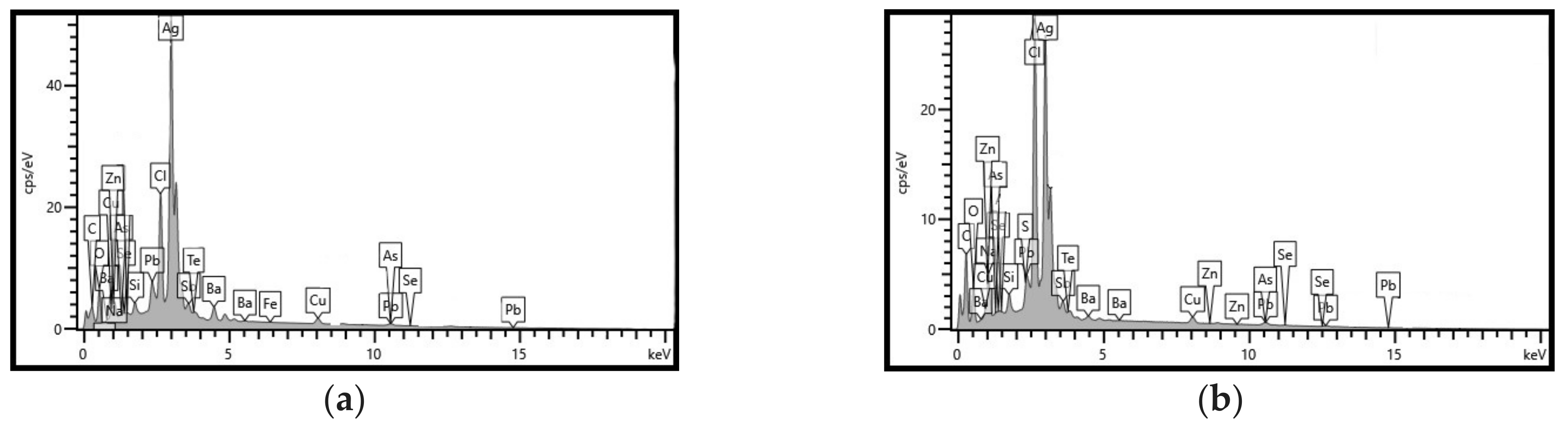
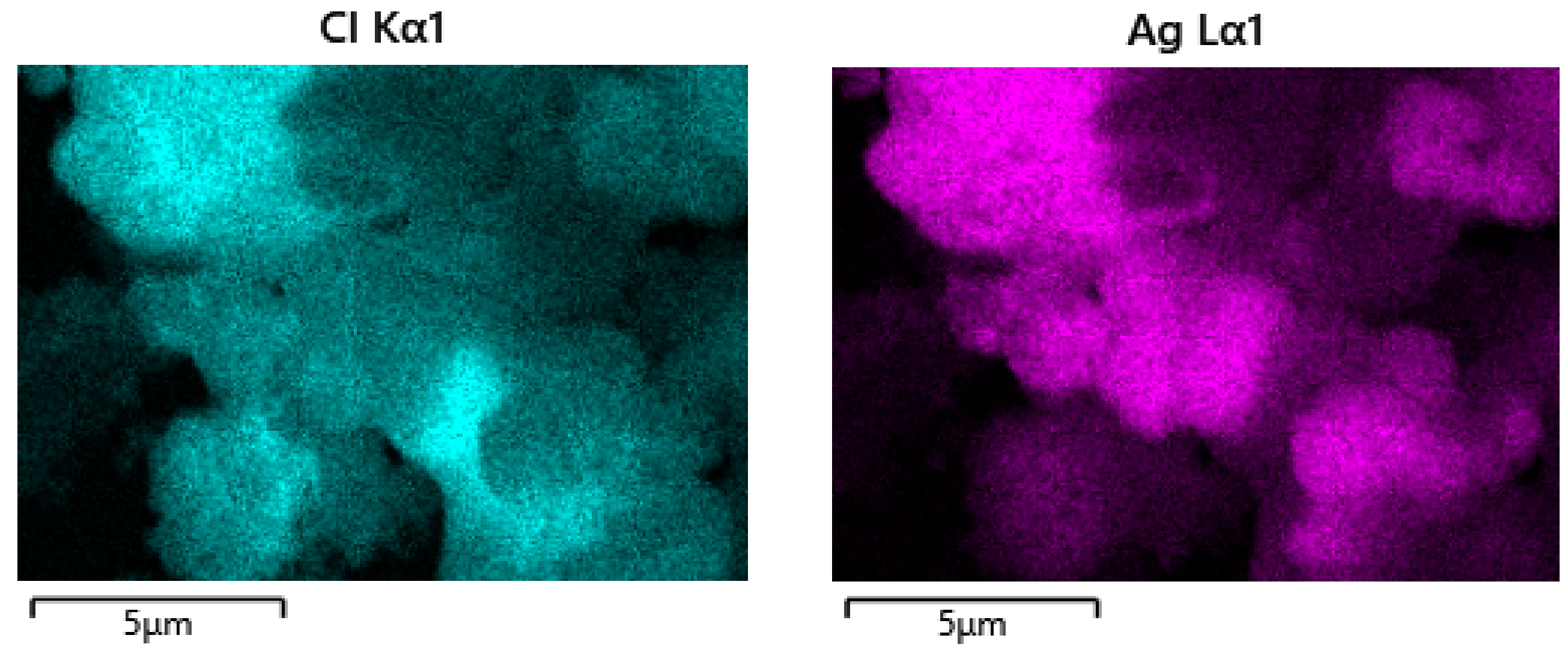
3.3. Characterization of Leaching Residue Using SEM/EDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, S.M.; McCullough, E.A. Critical Minerals: A Review of Elemental Trends in Comprehensive Criticality Studies. Resour. Policy 2018, 59, 192–199. [Google Scholar] [CrossRef]
- Müller, D.; Groves, D.I.; Santosh, M.; Yang, C.-X. Critical Metals: Their Applications with Emphasis on the Clean Energy Transition. Geosystems Geoenviron. 2025, 4, 100310. [Google Scholar] [CrossRef]
- Li, Y.; Baker, J.; Fang, Y.; Cao, H.; Pleydell-Pearce, C.; Watson, T.; Chen, S.; Zhao, G. Comparative Environmental Impacts Analysis of Technologies for Recovering Critical Metals from Copper Anode Slime: Insights from LCA. Environ. Chem. Ecotoxicol. 2025, 7, 275–285. [Google Scholar] [CrossRef]
- Rao, S.; Liu, Y.; Wang, D.; Cao, H.; Zhu, W.; Yang, R.; Duan, L.; Liu, Z. Pressure Leaching of Selenium and Tellurium from Scrap Copper Anode Slimes in Sulfuric Acid-Oxygen Media. J. Clean. Prod. 2021, 278, 123989. [Google Scholar] [CrossRef]
- Zeng, Y.; Zou, J.; Liao, C.; Liu, F.; Zhou, X. Selective Separation and Recovery of Selenium from Copper Anode Slime by Compound Leaching Followed by Sulfate Roasting. Miner. Eng. 2022, 186, 107749. [Google Scholar] [CrossRef]
- Khakmardan, S.; Rezai, B.; Abdollahzadeh, A.; Ghorbani, Y. From Waste to Wealth: Unlocking the Value of Copper Anode Slimes through Systematic Characterization and Pretreatment. Miner. Eng. 2023, 200, 108141. [Google Scholar] [CrossRef]
- Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; King, S.A.; Liebezeit, V.; Slattery, A.D. Detailed Characterisation of Precious Metals and Critical Elements in Anode Slimes from the Olympic Dam Copper Refinery, South Australia. Miner. Eng. 2024, 206, 108539. [Google Scholar] [CrossRef]
- Melo, E.; Hernández, M.-C.; Benavente, O.; Quezada, V. Selenium Dissolution from Decopperized Anode Slimes in ClO−/OH− Media. Minerals 2022, 12, 1228. [Google Scholar] [CrossRef]
- Li, B.; Deng, J.; Jiang, W.; Zha, G.; Yang, B. Removal of Arsenic, Lead and Bismuth from Copper Anode Slime by a One-Step Sustainable Vacuum Carbothermal Reduction Process. Sep. Purif. Technol. 2023, 310, 123059. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, G.; Xu, W.; Wang, X.; Song, J.; Zhu, T. Study on Oxidative Leaching of Multi-Component in Copper Anode Slime Enhanced by Hydrogen Peroxide. Sep. Purif. Technol. 2025, 361, 131635. [Google Scholar] [CrossRef]
- Melo Aguilera, E.; Hernández Vera, M.C.; Viñals, J.; Graber Seguel, T. Characterization of Raw and Decopperized Anode Slimes from a Chilean Refinery. Metall. Mater. Trans. B 2016, 47, 1315–1324. [Google Scholar] [CrossRef]
- Chen, A.; Peng, Z.; Hwang, J.-Y.; Ma, Y.; Liu, X.; Chen, X. Recovery of Silver and Gold from Copper Anode Slimes. JOM 2015, 67, 493–502. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. Mineralogical Characterization of a Copper Anode and the Anode Slimes from the La Caridad Copper Refinery of Mexicana de Cobre. Metall. Mater. Trans. B 2005, 36, 229–240. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. Mineralogical Characterization of Anode Slimes—9. The Reaction of Kidd Creek Anode Slimes with Various Lixiviants. Can. Metall. Q. 1993, 32, 267–279. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. The Mineralogy of Copper Electrorefining. JOM 1990, 42, 39–44. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. Mineralogical Characterization of Anode Slimes—II. Raw Anode Slimes from Inco’s Copper Cliff Copper Refinery. Can. Metall. Q. 1988, 27, 97–105. [Google Scholar] [CrossRef]
- Liu, J.; Hong, Y.; Lin, G.; Wang, S.; Zhang, L. Ultrasound Assisted Sodium-Chlorate Leaching of Selenium from Copper Anode Slime. Hydrometallurgy 2023, 222, 106195. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, C.; Liang, J.; Liu, M.; Xiao, H.; Liu, F.; Zeng, Y. Selective Separation and Efficient Extraction of Tellurium and Gold from Complex Copper Anode Slime. Sep. Purif. Technol. 2025, 362, 131625. [Google Scholar] [CrossRef]
- Cooper, W.C. The Treatment of Copper Refinery Anode Slimes. JOM 1990, 42, 45–49. [Google Scholar] [CrossRef]
- Ludvigsson, B.M.; Larsson, S.R. Anode Slimes Treatment: The Boliden Experience. JOM 2003, 55, 41–44. [Google Scholar] [CrossRef]
- Komori, K.; Ito, S.; Okada, S.; Iwahori, S. Hydrometallurgical process of precious metal in Naoshima smelter and refinery. In Proceedings of the Copper 2010, Hamburg, Germany, 6–10 June 2010; pp. 1403–1411. [Google Scholar]
- Kim, D.; Wang, S. Developments in Copper Anode Slimes-Wet Chlorination-Processing; Copper: Hamburg, Germany, 2010. [Google Scholar]
- Dönmez, B.; Çelik, C.; Çolak, S.; Yartaşi, A. Dissolution Optimization of Copper from Anode Slime in H2 SO4 Solutions. Ind. Eng. Chem. Res. 1998, 37, 3382–3387. [Google Scholar] [CrossRef]
- Amer, A.M. Processing of Copper Anodic-Slimes for Extraction of Valuable Metals. Waste Manag. 2003, 23, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, Y.; Yu, Y.; Fu, G.; Liu, Y.; Sun, Z.; Ye, S. Enhanced Selective Recovery of Selenium from Anode Slime Using MnO2 in Dilute H2SO4 Solution as Oxidant. J. Clean. Prod. 2019, 209, 494–504. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, J.; Chen, Y.; Li, Q.; Yang, Y. Comprehensive Recoveries of Selenium, Copper, Gold, Silver and Lead from a Copper Anode Slime with a Clean and Economical Hydrometallurgical Process. Chem. Eng. J. 2020, 393, 124762. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Tong, L.; Jin, Z.; Yin, L.; Chen, G. Leaching Kinetics of Selenium from Copper Anode Slimes by Nitric Acid-Sulfuric Acid Mixture. Trans. Nonferrous Met. Soc. China 2018, 28, 186–192. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.L.; Yu, Y.; Fu, G.Y.; Han, P.W.; Sun, Z.H.I.; Ye, S.F. An Environmentally Friendly Process to Selectively Recover Silver from Copper Anode Slime. J. Clean. Prod. 2018, 187, 708–716. [Google Scholar] [CrossRef]
- Burriel, F.; Lucena, F.; Arribas, S.; Hernández, J. Química Analítica Cualitativa, 18th ed.; Thomson Editores: Madrid, Spain, 2008; pp. 567–570. [Google Scholar]
- Hernández, M.C.; Benavente, O.; Roca, A.; Melo, E.; Quezada, V. Selective Leaching of Arsenic from Copper Concentrates in Hypochlorite Medium. Minerals 2023, 13, 1372. [Google Scholar] [CrossRef]
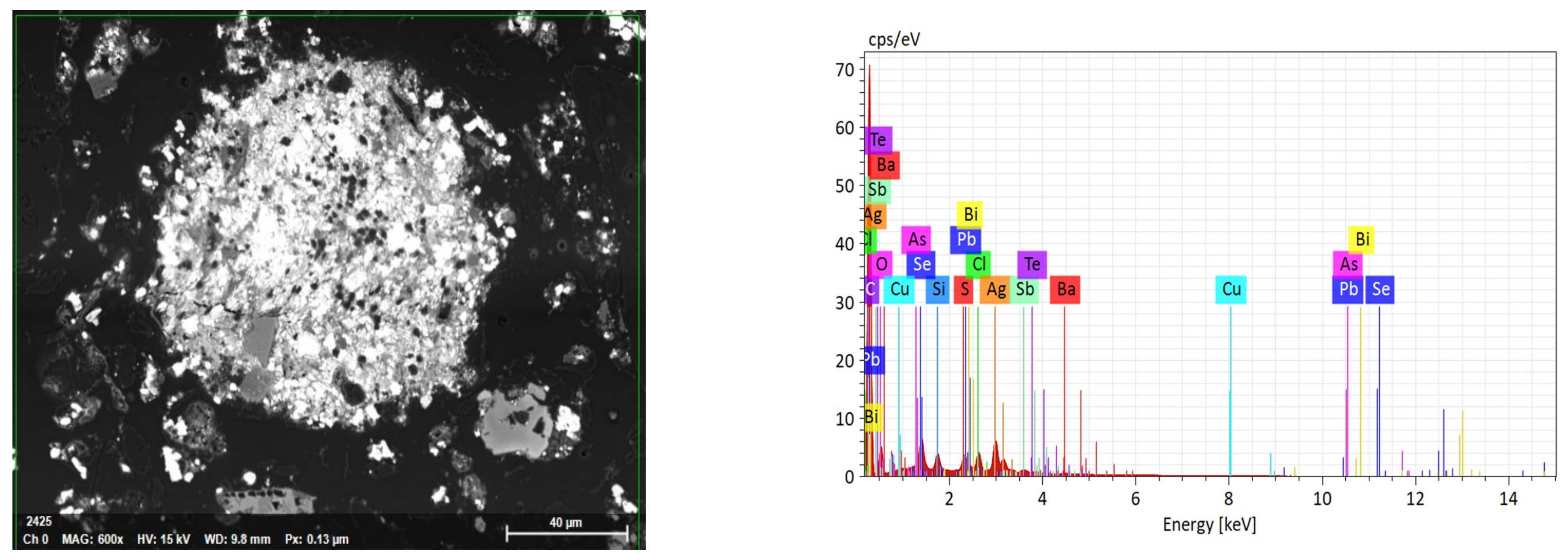


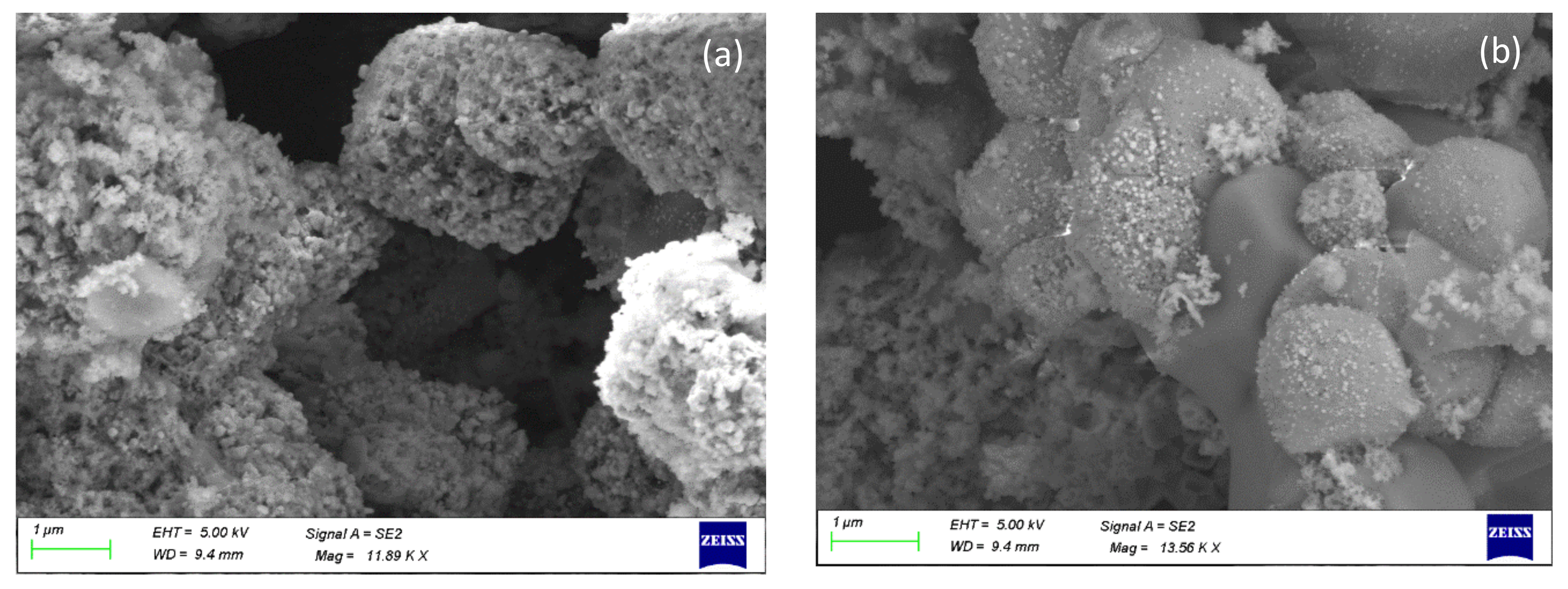
| Factor/Level | (1) | (2) | (3) |
|---|---|---|---|
| Temperature, °C | 35 | 45 | 55 |
| ClO−, M | 0.4 | 0.53 | 0.68 |
| pH | 11 | 11.5 | 12 |
| Element | %Weight | %Atomic |
|---|---|---|
| Ag | 65.48 | 58.13 |
| Se | 34.52 | 41.87 |
| No. Test | Temperature, °C | ClO−, M | pH | Selenium Solution (%) |
|---|---|---|---|---|
| 1 | 35 | 0.40 | 11.0 | 78.0 |
| 2 | 35 | 0.53 | 11.5 | 85.9 |
| 3 | 35 | 0.68 | 12.0 | 83.4 |
| 4 | 45 | 0.40 | 11.5 | 81.7 |
| 5 | 45 | 0.53 | 12.0 | 77.1 |
| 6 | 45 | 0.68 | 11.0 | 84.7 |
| 7 | 55 | 0.40 | 12.0 | 79.6 |
| 8 | 55 | 0.53 | 11.0 | 94.2 |
| 9 | 55 | 0.68 | 11.5 | 91.8 |
| % Selenium Dissolution | |||
|---|---|---|---|
| Level | ClO−, M | pH | Temperature, °C |
| Low (1) | 79.70 | 85.0 | 82.40 |
| Medium (2) | 85.70 | 86.50 | 81.20 |
| High (3) | 88.53 | 86.63 | 80.03 |
| Factor | F | p-Value |
|---|---|---|
| ClO− | 17.74 | 0.05 |
| pH | 6.80 | 0.12 |
| Temperature | 14.72 | 0.06 |
| Element | Weight % | Atomic % |
|---|---|---|
| Cl | 16.50 | 21.55 |
| O | 14.17 | 40.97 |
| Pb | 4.80 | 1.08 |
| Na | 4.12 | 8.32 |
| Sb | 3.73 | 1.43 |
| Cu | 2.28 | 1.66 |
| Ag | 50.04 | 21.48 |
| Si | 0.96 | 1.59 |
| S | 0.38 | 0.55 |
| Ba | 1.47 | 0.51 |
| Te | 0.83 | 0.30 |
| Se | 0.34 | 0.21 |
| As | 0.28 | 0.18 |
| Al | 0.09 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, E.; Jaldín, Á. Advancements in Anode Slime Treatment: Effects of pH, Temperature, and Concentration of ClO−/OH− on Selenium Dissolution from Decopperized Anode Slimes. Minerals 2025, 15, 442. https://doi.org/10.3390/min15050442
Melo E, Jaldín Á. Advancements in Anode Slime Treatment: Effects of pH, Temperature, and Concentration of ClO−/OH− on Selenium Dissolution from Decopperized Anode Slimes. Minerals. 2025; 15(5):442. https://doi.org/10.3390/min15050442
Chicago/Turabian StyleMelo, Evelyn, and Álvaro Jaldín. 2025. "Advancements in Anode Slime Treatment: Effects of pH, Temperature, and Concentration of ClO−/OH− on Selenium Dissolution from Decopperized Anode Slimes" Minerals 15, no. 5: 442. https://doi.org/10.3390/min15050442
APA StyleMelo, E., & Jaldín, Á. (2025). Advancements in Anode Slime Treatment: Effects of pH, Temperature, and Concentration of ClO−/OH− on Selenium Dissolution from Decopperized Anode Slimes. Minerals, 15(5), 442. https://doi.org/10.3390/min15050442





