1. Introduction
The Lower Paleozoic of the Bohai Bay Basin constitutes a key target for hydrocarbon exploration in China [
1,
2]. Karst reservoirs are widely developed in this interval, and authigenic calcite is one of the most representative diagenetic minerals within these reservoirs [
3]. As the most common mineral in the Earth’s crust, calcite plays a crucial role in reservoir prediction due to its properties. For instance, its high solubility promotes the development of reservoirs with enhanced porosity and permeability [
4], while the temperature, salinity, and other parameters of diagenetic fluid recorded in calcite provide valuable insights into the timing, nature, and evolution of fluid activity [
5,
6]. Previous studies have shown that calcite in the Lower Paleozoic karst reservoirs of the Bohai Bay Basin formed in at least three distinct phases [
7,
8]. However, research on the origins of these calcites remains limited. Most existing studies have focused primarily on hydrothermal calcite, with systematic investigations of other genesis types still at an early stage [
9,
10,
11]. The Lower Paleozoic in this region has undergone multiple phases of complex uplift-subsidence tectonics and hydrocarbon charging events [
12,
13]. As a result, calcite preserved in pores and fractures records abundant information on diagenetic fluid activity, hydrocarbon migration, and tectonic evolution. Therefore, advancing our understanding of the genesis of authigenic calcite is of significant scientific value for analyzing reservoir characteristics, interpreting tectonic evolution, and reconstructing hydrocarbon migration patterns in the Lower Paleozoic of the Bohai Bay Basin.
In reservoir studies, carbon and oxygen isotope (δ
13C and δ
18O) analyses are essential for elucidating the formation mechanisms of carbonate cements. This approach not only effectively distinguishes calcite of different genesis but also provides key insights into diagenetic temperature, fluid salinity, fluid sources, and diagenetic processes [
14,
15,
16,
17,
18]. As such, δ
13C and δ
18O analyses hold significant scientific and practical value in deciphering the formation mechanisms of calcite [
19,
20]. However, in practice applications, isotopic analyses can be influenced by multiple factors, such as the mixing of fluids from different sources and temperature-related isotope fractionation, resulting in data complexity and interpretative uncertainty [
21,
22]. Therefore, to improve the reliability of isotopic interpretations, it is often necessary to integrate multiple geochemical approaches, particularly elemental analyses and fluid inclusion studies [
23]. The combined application of these methods allows for a more comprehensive understanding of calcite formation mechanisms and the diagenetic environmental conditions that they reflect.
Based on petrographic observations combined with isotopic and various chemical analytical techniques, this study systematically investigates the characteristics of authigenic calcite in the Lower Paleozoic reservoirs of the Bozhong Sag in the Bohai Sea. The objectives are as follows: (1) to summarize the geological significance of δ13C and δ18O in diagenesis and to explore the material sources of the authigenic calcite and the conditions of the diagenetic fluids and (2) to preliminarily establish the genesis types of authigenic calcite in the Lower Paleozoic karst reservoirs of the Bohai Sea region, which will provide a scientific foundation for further research on the genesis and geological significance of calcite in the Lower Paleozoic of the Bohai Bay Basin.
2. Geological Background
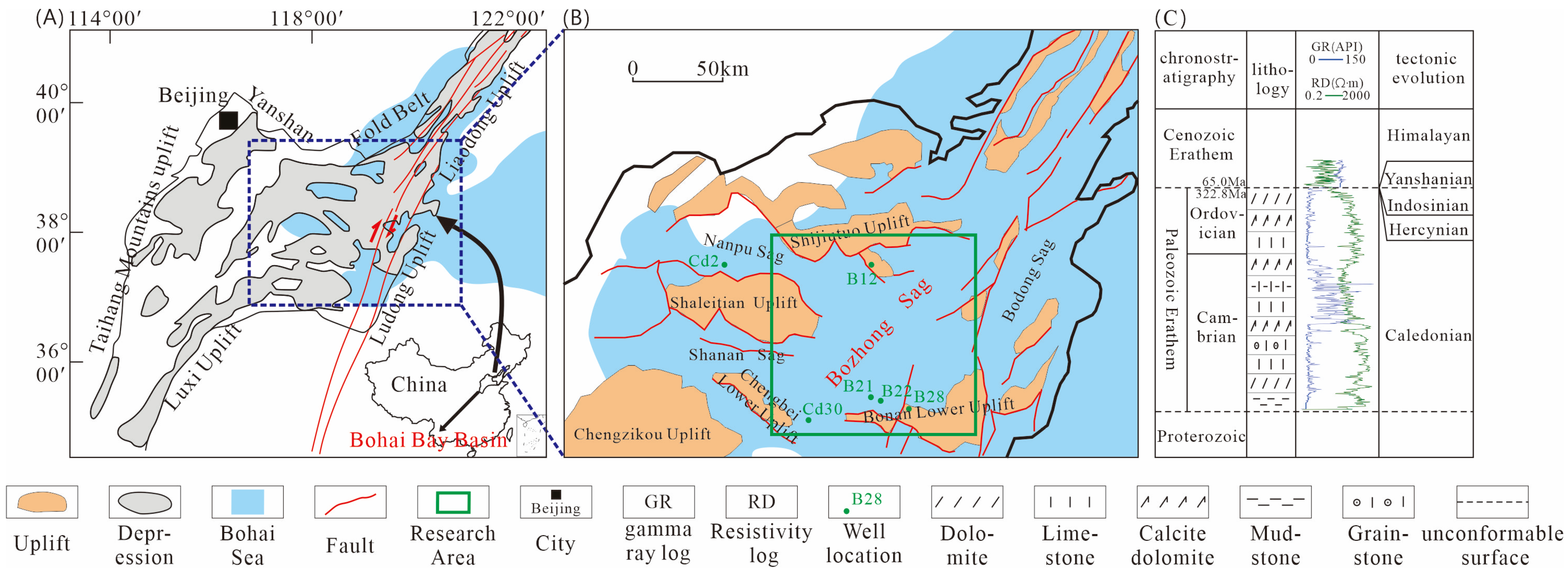
The Bozhong Sag, located at the core of the Bohai Sea structural unit, is the deepest depression in the Bohai Bay Basin [
24], covering an exploration area of 8634 km
2 (
Figure 1). Tectonic evolution studies reveal that since the Late Ordovician Caledonian orogeny, the region has successively experienced several tectonic superposition, including the Hercynian, Indosinian, Yanshanian, and Himalayan [
13]. Repeated tectonic uplifts have kept the North China Craton in a prolonged state of continental erosion, with the most intense erosion occurring during the Mesozoic Yanshanian movement.
This coupled tectonic–sedimentary regime strongly influenced the Lower Paleozoic carbonate strata, which underwent intense surficial karstification, forming a regionally developed paleoweathering crust-type karst reservoir system [
3,
11,
25]. Today, only the Cambrian and Lower Ordovician strata are preserved in the study area. The Cambrian unconformably overlies the pre-Cambrian metamorphic basement, while the Paleogene directly unconformably overlies the Ordovician (
Figure 1).
As an important hydrocarbon-rich sag in the Bohai Bay Basin, the Bozhong sag represents a typical epicontinental carbonate platform depositional system in the Lower Paleozoic [
26,
27]. This unit comprises several sedimentary subfacies, including micritic limestone from tidal flat and lagoon, dolomicrite from restricted platform, oolitic/bioclastic grainstone from open platform, and sparry grainstone from platform margins [
3].
Diagenesis studies reveal that the study area experienced a complex sequence of diagenetic events. During the syndiagenetic stage, meteoric freshwater dissolution occurred. During the early diagenetic stage, dolomitization improved the matrix porosity. During the mesodiagenetic stage, tectonic activity generated extensive fractures. During the epidiagenetic stage, meteoric water leaching led to the development of karstic reservoirs. In the late diagenetic stage, silicification and calcite cementation significantly reduced porosity. Notably, multiple episodes of calcite cementation—including isopachous rim cementation, syntaxial cementation, and vein-like filling—are primary contributors to reservoir quality degradation. Importantly, tectonic reactivation during the Himalayan phase generated micro-fractures that served as pathways for late-stage acidic fluids, which played a significant constructive role in the development of secondary dissolution porosity [
3,
25,
28].
3. Methods
In this study, over 150 samples from eight wells were selected and prepared as thin sections (one-third stained, polished, and uncovered). Petrographic observation under a microscope was carried out to analyze the various types and diagenetic stages of calcite cementation. Based on these observations, more than 20 samples were chosen for geochemical analysis, these samples include micritic calcite as well as authigenic calcite of different cementation types. The experimental work included isotopic analysis, fluid inclusion studies, cathodoluminescence (CL), and electron probe microanalysis (EPMA).
Specifically, the selected samples were ground into 0.05 mm-thick slices and observed using a ZEISS Axio Imager, M2m polarizing microscope. Target areas were marked and pre-screened under CL before isotopic and EPMA testing. All experiments were conducted at the CNOOC Experimental Center (Tianjin, China).
In situ carbon and oxygen isotope analyses were performed using a Teledyne CETAC Fusions CO2 laser sampling system (Teledyne CETAC, Omaha, NE, USA) coupled with a Thermo Scientific DELTA V isotope ratio mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). The results, calibrated to the PDB standard, had a precision better than 0.1‰ and a spatial resolution of 100–150 µm. Fluid inclusion analyses employed a Linkam-TH600 heating/cooling stage and a ZEISS Axio Scope, A1 microscope (Carl Zeiss AG, Oberkochen, Baden-Württemberg, Germany), with a temperature accuracy of ±1 °C. CL observations were conducted using a RELION CL VI system (RELION INDUSTRIES, Bedford, MA, USA) with a ZEISS Axio Imager A2 microscope (Carl Zeiss AG, Oberkochen, Baden-Württemberg, Germany). EPMA was carried out using a Shimadzu EPMA-1720 (Shimadzu Corporation, Kyoto Prefecture, Japan), offering 1 µm spatial resolution, ~0.01% detection limits, and relative errors of 0.1%–1% for major elements. Laboratory conditions were maintained at ~26 °C and 40%–50% humidity.
δ
13C and δ
18O provide key insights into diagenetic processes. The Z value (Equation (1)), proposed by Keith and Weber [
29], and was used to infer the relative salinity of diagenetic fluid. The paleotemperature of carbonate formation was estimated using Equation (2), developed by Friedman and O’Neil [
30], based on oxygen isotope geothermometer. These parameters were used to reconstruct the temperature and salinity conditions during calcite cementation.
α(calcite–water) = (1 + δ18Ocalcite/1000)/(1 + δ18Owater/1000): isotope fractionation factor;
T: Temperature (°C) of the water during carbonate precipitation;
δ18Ocalcite: in situ laser isotope, PDB standard, ‰;
δ18Owater: oxygen isotope of diagenetic fluid, PDB standard, ‰.
4. Results
4.1. Calcite Occurrence Characteristics
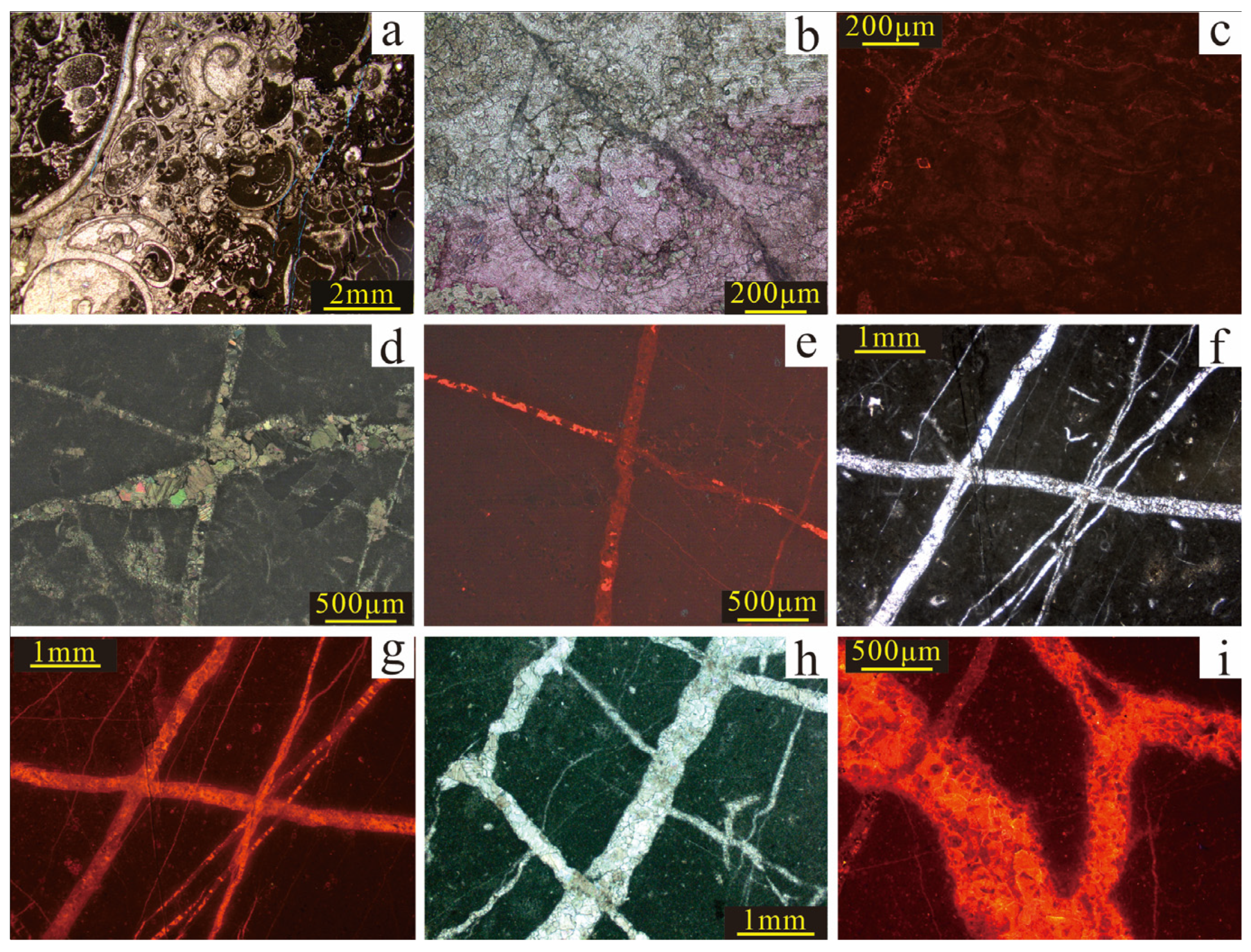
The study area has undergone both karstification and burial diagenesis, resulting in a multi-phase, multi-genetic calcite cementation system. Based on petrographic and CL analysis and considering cement occurrence, crystal morphology, and CL response, three genesis types of calcite cement are identified.
4.1.1. Calcite (C1) in Primary or Early Dissolution Pores
C1 occurs primarily as sparry calcite filling primary intergranular and early dissolution pores. It commonly exhibits fibrous, platy, and coating-like crystal habits (
Figure 2a–c). The calcite is often cross-cut by later fractures, indicating early diagenetic timing. CL reveals non-luminescence to faint luminescence, consistent with high Fe
2+ and low Mn
2+ content, suggesting precipitation in a phreatic environment under near-surface conditions.
4.1.2. Calcite (C2) Characterized by Fracture Fillings
C2 is occurred as a fracture-filling mineral, with cross-cutting relationships indicating at least three distinct cementation phases (
Figure 2f). Early-stage fracture fills display little to no CL (
Figure 2d,e), with the fracture walls showing evidence of dissolution and being commonly cut by subsequent fractures. Later-stage fractures are straighter and show clear tectonic signatures. Due to repeated tectonic activity, fractures intersect each other, and the calcite exhibits a wide range of CL intensities—from weak to strong (
Figure 2d–g)—reflecting multiple fracturing and cementation events.
4.1.3. Karst Collapse Cemented Calcite (C3)
C3 associated with karst collapse is found in reticulate fractures or interclast pores among angular clasts, often showing striated fracture textures (
Figure 2h). This features indicates formation in karst environments. C3 exhibits moderate to strong alternating bright and dark or banded CL intensities (
Figure 2i), which correspond to fluctuations in oxidation–reduction conditions caused by periodic exposure.
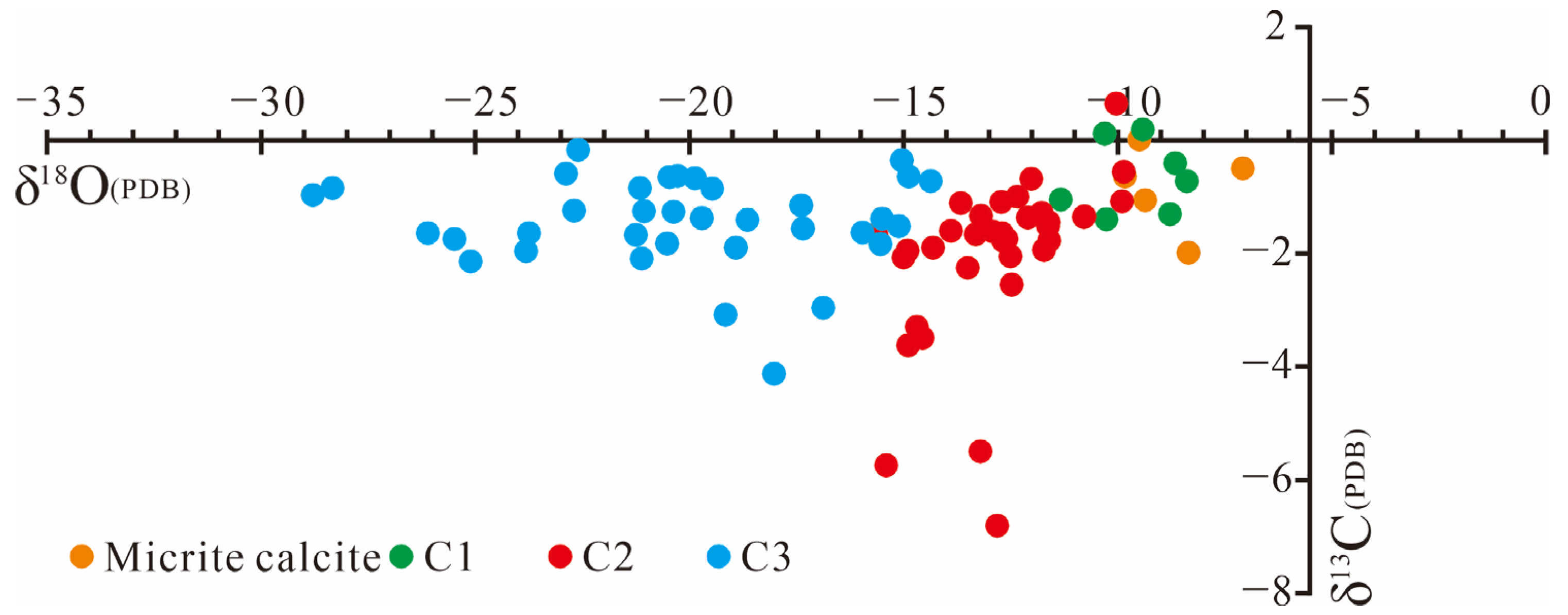
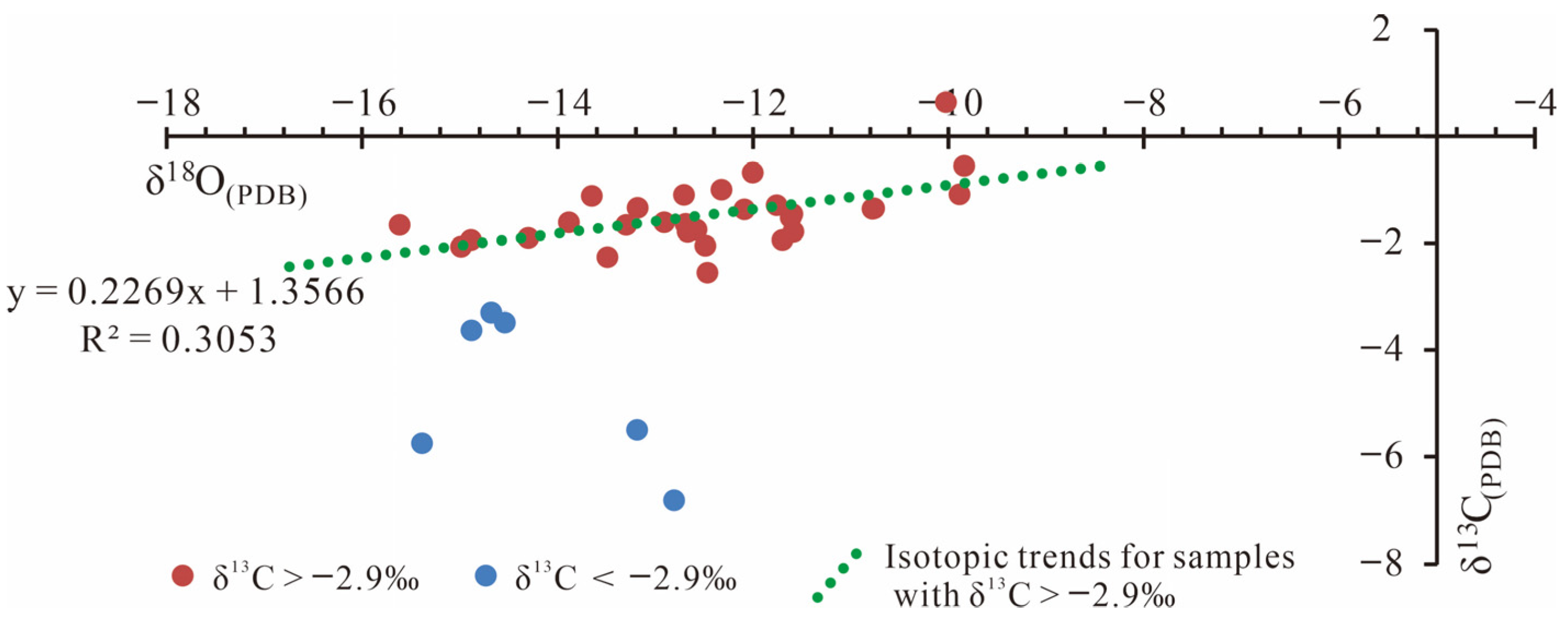
4.2. δ13C and δ18O Distribution Characteristics
The δ
13C of calcite range from −6.809‰ to 0.646‰, while δ
18O vary from −28.787‰ to −7.071‰ (
Figure 3). In situ isotopic analyses were performed on micritic calcite and the three identified calcite types (C1–C3). As an original sedimentary component, micritic calcite shows δ
13C values between −1.99‰ and 0.02‰ and δ
18O from −9.82‰ to −7.07‰, closely approximating the marine carbonate background (δ
13C
PDB ~0‰). These values indicate deposition under normal marine conditions. C1 exhibits δ
13C values from −1.4‰ to 0.187‰ and δ
18O from −11.32‰ to −8.37‰. C2 ranges more widely, with δ
13C between −6.809‰ and 0.646‰ and δ
18O from −15.62‰ to −9.84‰. The broader δ
13C range and localized negative excursions suggest varied fluid sources and multi-phase cementation events. C3 shows δ
13C from −4.129‰ to −0.167‰ and δ
18O from −28.787‰ to −14.348‰, with the pronounced oxygen isotope depletion indicative of meteoric water influence during karst-related diagenesis.
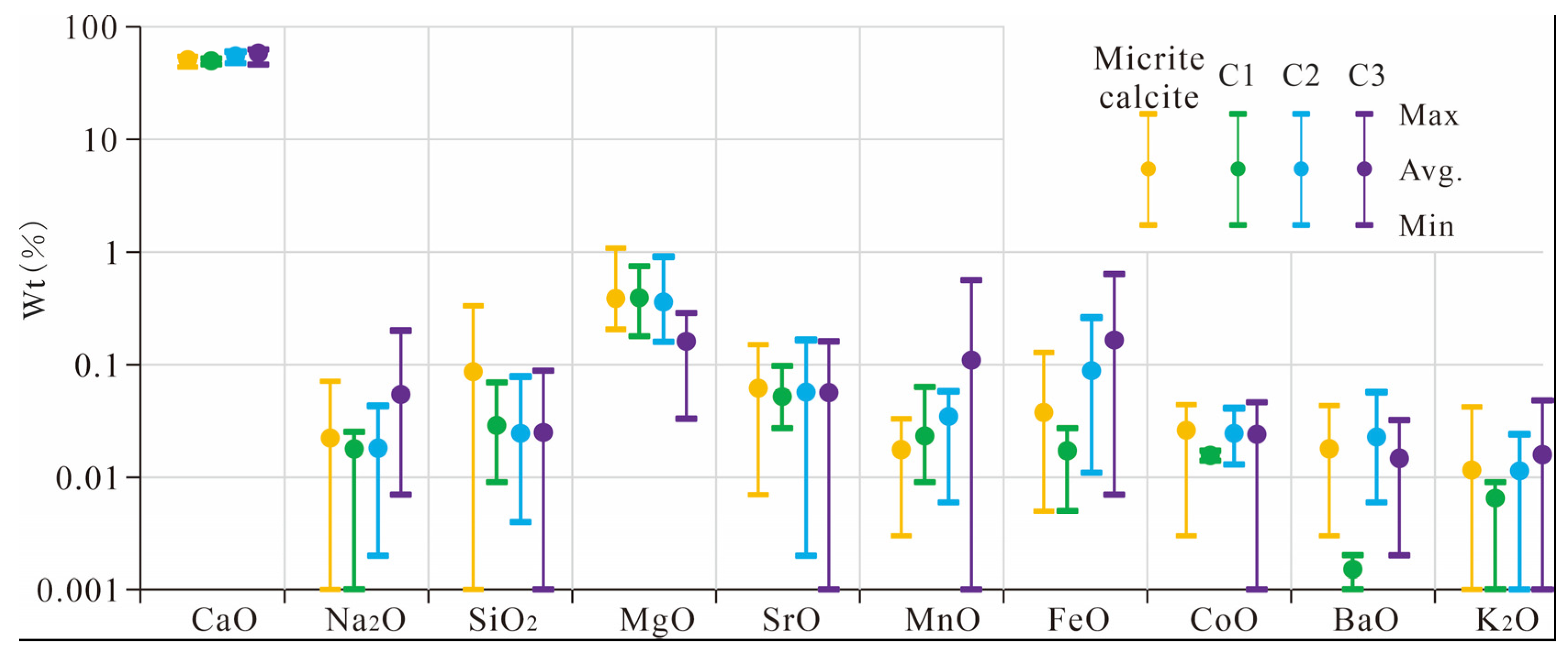
4.3. EPMA Elemental Distribution Characteristics
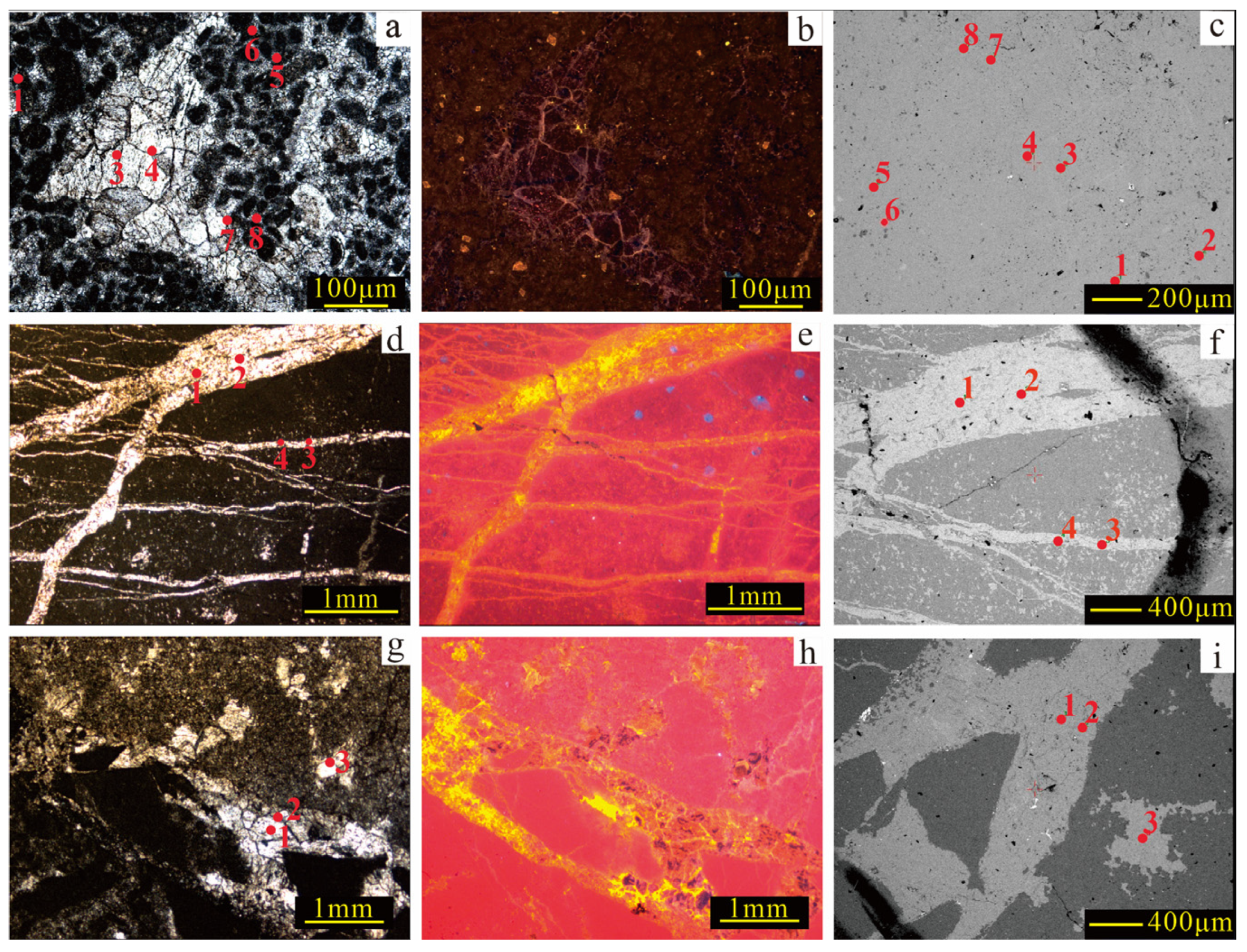
EPMA was conducted on micritic and authigenic calcites with distinct CL and occurrence features (
Figure 4). While C1, C2, and C3 share overall elemental trends, notable differences exist (
Figure 5). Compared to micritic calcite, authigenic calcite exhibits lower SiO
2 concentrations. C1 shows reduced BaO concentrations, while C2 and C3 display broader elemental variability, particularly elevated MnO and FeO levels. These differences suggest that during formation, the authigenic calcite may have undergone different diagenetic fluid interactions, reflecting a complex diagenetic environment and fluid evolution process.
5. Source of Fluids Forming Calcite Veins
5.1. Applicability of Geochemical Data
The δ13C and δ18O are influenced by variations in both the depositional environment and diagenetic processes. Micritic calcite, serving as the original sedimentary component, is theoretically the most strongly affected by external diagenetic conditions. To assess the extent of post-depositional alteration and ensure the reliability of the isotopic measurements, a comparative isotopic analysis of micritic calcite was performed.
The isotopic composition of micritic calcite in the study area generally aligns with that of global Cambrian–early Ordovician bioclasts, with δ
18O primarily between −7‰ and −10‰ and δ
13C mainly between −2‰ and 0‰. These values are also consistent with the carbon and oxygen isotopic characteristics of Cambrian–Ordovician limestone samples from the Bohai Bay Basin [
31,
32]. Additionally, the isotopic Z-value of the micritic calcite range from 119.08 to 122.76, with an average of 121.21, further supporting deposition in a marine environment. Importantly, δ
13C and δ
18O show no significant covariation (
Figure 3), and δ
18O are all greater than −10‰, which further confirms the stability of the diagenetic environment. Overall, the isotopic data from the selected samples effectively represent the diagenetic conditions, providing a reliable basis for investigating diagenetic processes and fluid evolution.
5.2. Diagenetic Fluid Characteristics of C1
The EPMA element distribution pattern of C1 is similar to that of micritic calcite but with a significant decrease of BaO. The comparative analysis of ionic radii indicates that Ba cannot be easily substituted for Ca or Mg in carbonate minerals due to its relatively large radius. In micritic calcite, Ba primarily exists in an adsorbed state within the sediment; thus, during the carbonate cementation—when external fluid sources are absent—the BaO content inevitably decreases.
The isotopic analysis reveals that the δ
13C and δ
18O of C1 clearly overlap with the distribution range of calcite formed in the syngenetic diagenetic environment (
Figure 3). Its Z value (ranging from 119.33 to 123.00, with an average of 121.19) is comparable to that of micritic calcite. This further confirms that C1 formed in a hypersaline environment and is highly correlated with the syngenetic diagenetic setting. Using an estimated δ
18O
SMOW of −1.4‰ for Ordovician seawater as the diagenetic fluid [
33], the calculated formation temperature of C1 ranges from 52 to 72 °C, consistent with cementation during the syngenetic-to-early-burial-diagenetic stage (typically <85 °C).
5.3. Diagenetic Fluid Characteristics of C2
Compared to the elemental distribution of C1, C2 exhibits a broader SrO range and elevated BaO content. The increased Ba concentration and high ω(Mn)/ω(Sr) ratios (average 3.797; range: 0.092–26.560) suggest involvement of non-marine or hydrothermal fluids [
11,
34]. While, the elemental distribution pattern of C2 shows some similarity to that of micritic calcite and C1, reflecting the important role of sedimentary fluids during its formation, C2 also exhibits a relatively wide variation in δ
13C and δ
18O, a feature that further supports the multi-source origins of the diagenetic fluids.
Veizer et al. reported that the carbon isotopes of Cambrian–Ordovician carbonate sediments are greater than −2.9‰ [
31]. In this study, the samples with δ
13C greater than −2.9‰ have Z values ranging from 115.60 to 123.63, with an average of 118.11, while those with δ
13C
PDB less than −2.9‰ have Z values ranging from 106.98 to 113.24, with an average of 110.49. This significant difference in Z values indicates that the diagenetic process of C2 involved fluids of varying salinities, further revealing the multi-source origins of the diagenetic fluids.
For samples with δ
13C greater than −2.9‰, a significant positive correlation between δ
13C and δ
18O is observed (
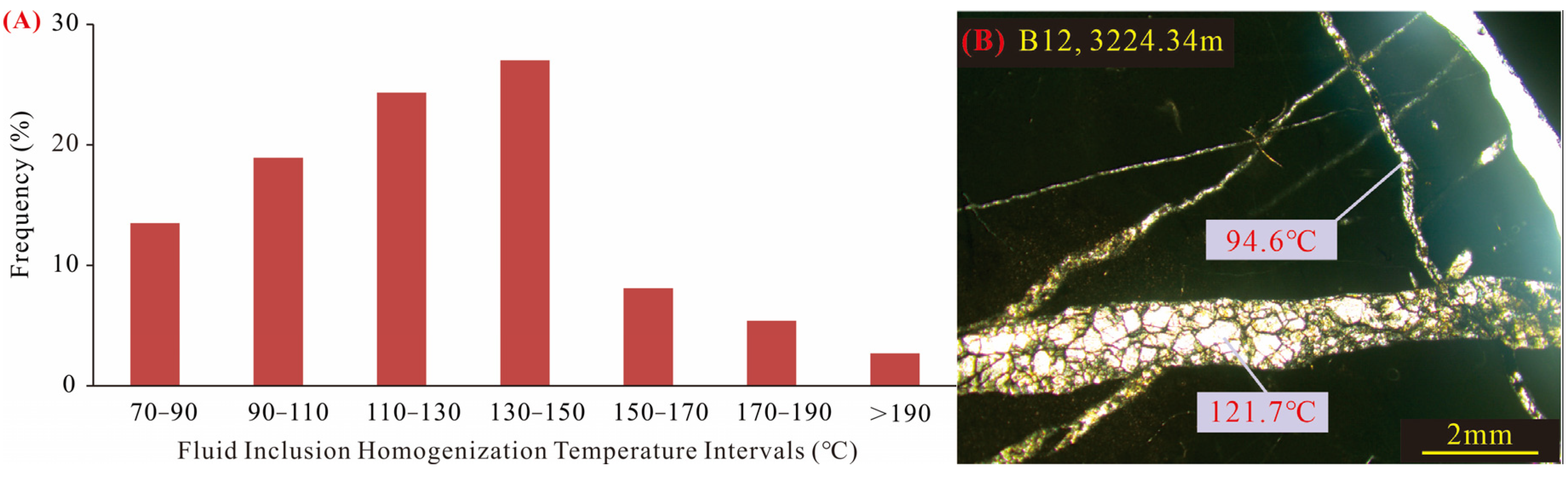
Figure 6), with a correlation coefficient of approximately 0.55. This manifests as a covariance line with synchronous negative shifts. Such a pattern reflects the diagenetic changes occurring during the burial-heating process, largely because oxygen isotope exchange reactions are highly sensitive to temperature—resulting in a typically wider range of oxygen isotope variation compared to carbon isotopes. The carbon isotope values range from −2.55‰ to 0.646‰, with an average of −1.474‰, falling within the expected range for calcite under a Lower Paleozoic sedimentary background. Based on fluid inclusion microthermometry (
Figure 7), the diagenetic temperatures associated with burial heating are estimated to range from 70 °C to 150 °C. Using the corresponding δ
18O (−15.62‰ to −9.84‰), the calculated δ
13O
SMOW of the diagenetic fluid ranges from −0.21‰ to 2.02‰. This range aligns well with δ
13O
SMOW (approximately −5‰ to 4‰) observed in formations from China’s major oil and gas basins that are effectively sealed and remain unaffected by external fluids [
35]. In summary, the carbon isotopic characteristics of calcite with δ
13C greater than −2.9‰ indicate that the carbon source is derived from sedimentary fluids entrapped within the reservoir. This further demonstrates the fluid isolation during burial diagenesis and its controlling effect on diagenetic evolution.
For samples exhibiting a negative carbon isotope shift (δ
13C
PDB < −2.9‰), the carbon isotopic signature serves as a key indicator of the diagenetic fluids. In this study, we trace the fluid isotopic trends resulting from the mixing of carbon sources with depositional-burial fluids. In nature systems, the isotopic range of carbon in carbon reservoirs is relatively fixed, representative carbon sources for negatively shifted carbon isotopes mainly include atmospheric freshwater, organically derived CO
2 (e.g., from organic matter or thermally induced decarboxylation), and inorganically derived CO
2 (e.g., mantle-derived CO
2) [
36,
37].
In deeply buried hydrocarbon reservoirs, CO₂ produced by the thermal decarboxylation of organic matter and mantle-derived CO₂ are considered the primary contributors for calcite formation [
14]. In the Bohai Bay Basin, the δ
13C of mantle-derived CO₂ are generally between −5.9‰ and −3.2‰ [
38,
39], closely matching the δ
13C observed in these calcite samples (ranging from −6.809‰ to −3.297‰, with an average of −4.746‰). When integrated with temperature data from fluid inclusions (
Figure 7), this isotopic signature suggests that calcite affected by mantle-derived fluids formed under deep burial conditions at temperatures ranging from approximately 150 °C to 212 °C. Based on the δ
18O distribution of these calcites (−15.387‰ to −12.807‰), the deduced diagenetic fluid δ
18O
SMOW is estimated to range from 4.884‰ to 5.997‰. These values are consistent with those of carbonates formed in magmatic environments (5.5‰ to 8.5‰) [
40]. Collectively, these findings indicate that the formation of calcite with strongly negative δ
13C values in the study area was significantly influenced by mantle-derived fluids. This highlights the critical role of deep-sourced materials in driving diagenetic processes during advanced burial stages.
5.4. Diagenetic Fluid Characteristics of C3
C3 displays a marked negative oxygen isotopic shift, which provides critical constraints on the nature of the diagenetic fluids. Typically, the oxygen isotopic compositions of atmospheric freshwater and magma sources show lighter δ
18O
SMOW (with atmospheric precipitation ranging from −55 to +10‰ and magma sources from +5.5 to +8.5‰). However, the minimum δ
18O
PDB of C3 is −28.787, which is notably lower than that of magma sources. In domestic karst reservoirs, carbonates formed by freshwater leaching generally exhibit lower δ
18O
PDB [
15,
41]. Furthermore, the Z values for C3 range from 110.42 to 116.03, with an average of 113.50, indicating that the diagenetic fluid during C3 formation had a relatively low mineralization. It is therefore inferred that the ion source for C3 formation was supplied by atmospheric freshwater.
Geochemical data reveal that relative to the elemental partitioning pattern of micritic calcite (
Figure 8), C3 exhibits a pronounced enrichment in Mn and Fe, accompanied by a depletion in Mg and Si. In natural waters, silicon is primarily present as undissociated siloxane molecules, while Mg
2+ is prone to isomorphic substitution with Fe
2+ and Mn
2+. Moreover, compared with modern seawater, continental freshwater contains relatively lower concentrations of Mg and Sr but higher levels of Mn and Fe [
42,
43]. In the study area, the MnO content in C3 ranges from 0.001% to 0.558% (averaging 0.107%), and the FeO ranges from 0.007% to 0.358% (averaging 0.131%); both are higher than the values found in the sedimentary background micritic calcite (0.013% and 0.026% for MnO and FeO, respectively). The CL characteristics of the minerals provide insight into the geochemical nature of the diagenetic fluids. C3 displays intergrown, intercalated, or ring-banded cementation with bright red and very bright orange luminescent phases. This multiphase luminescence directly reflects the spatial segregation of trace elements—particularly Mn
2+ (a luminescence activator) and Fe
2+ (a quencher)—in the diagenetic fluids, indicating the mixing of multiple fluid sources.
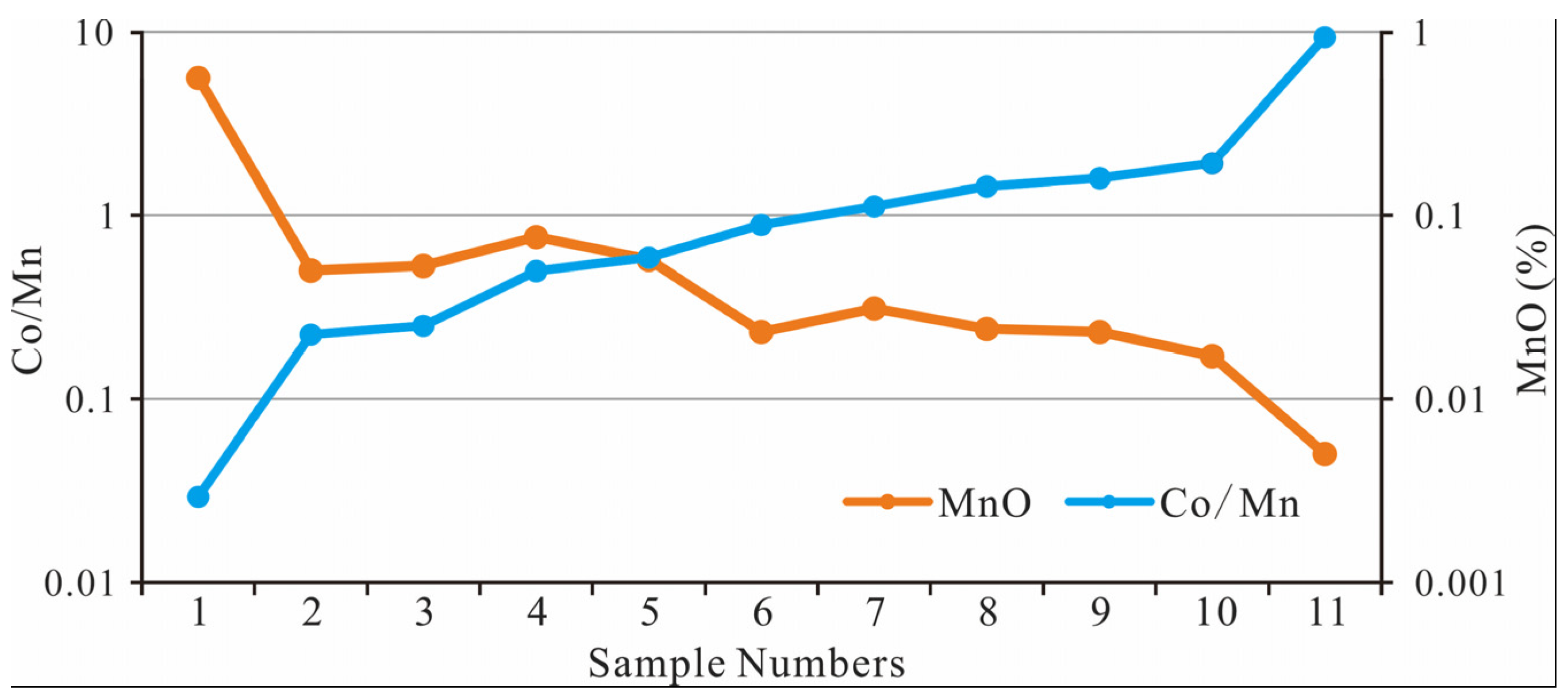
The concentrations of Mn and Co vary in response to redox conditions. Under reducing conditions, the solubility of Mn increases, whereas Co is more readily transported into sediments. Therefore, low Co/Mn ratios indicate an oxidizing environment and can be used as an indirect proxy for the influence of meteoric water. In normal marine settings, the dissolved Co/Mn ratio is approximately 0.187 [
44]. If diagenesis is affected by oxidative conditions, this ratio will decrease. In the C3 samples, the Co/Mn ratio ranges from 0.029 to 9.343. The lower values clearly reflect oxidation of the cement, which indirectly indicates the influence of meteoric karst water.
Compared to seawater, river water—representing meteoric karst water—contains significantly higher concentrations of dissolved Mn. For example, the Mn concentration in seawater is about 0.182 nmol/kg, whereas in river water it reaches approximately 149 nmol/kg [
44]. As such, Mn concentrations can serve as an indicator of meteoric water input. With increasing proportions of freshwater in mixed-source fluids, the mass fraction of MnO increases, while the Co/Mn ratio decreases accordingly. These geochemical characteristics suggest that the diagenetic fluids in the C3 samples were derived from surface karst water—a mixture of meteoric water and calcium carbonate dissolution water.
6. Origin and Geological Significance of Calcite
Based on the above analysis, the diagenetic characteristics of different types of calcite in the study area have been summarized (
Table 1). During the syngenetic-to-early-diagenetic stage, the pore water expelled by mechanical compaction, similar to sedimentary fluids, is a high-salinity solution that provides the material foundation for calcium carbonate precipitation. The solubility of calcium carbonate decreases with increasing temperature, which leads to the cementation of C1 during burial and temperature increase.
Following the deposition of Lower Paleozoic carbonates, the Bohai Bay Basin underwent multiple stages of tectonic uplift, particularly during the Yanshanian orogeny, which led to intense karstification and the development of karst-related pores and fractures. Surface karst water, composed of meteoric water and calcium carbonate dissolution water, infiltrated deep into the ancient karst layers, reaching the phreatic zones, and even deeper. These phreatic zones are located in the groundwater’s saturation zone, where CO
2 saturation led to the cementation of C3 [
45].
During the Himalayan tectonic period, the basin underwent multiple stages of rifting, subsidence, and strike-slip movements, which were accompanied by prolific fracture development. The Shahejie and Dongying formations in the BoZhong sag developed high-quality hydrocarbon source rocks, with the hydrocarbon generation threshold around 3100 m [
46]. Under the conditions of moderate to deep burial, the source rocks underwent intense water–rock reactions due to acid generation, and diagenetic fluids migrated along dominant pathways, forming C2 cement filling fractures.
Since the Pliocene, deep large faults have connected mantle-derived inorganic CO2 with diagenetic fluids, providing the ionic source for the formation of hydrothermal calcite.
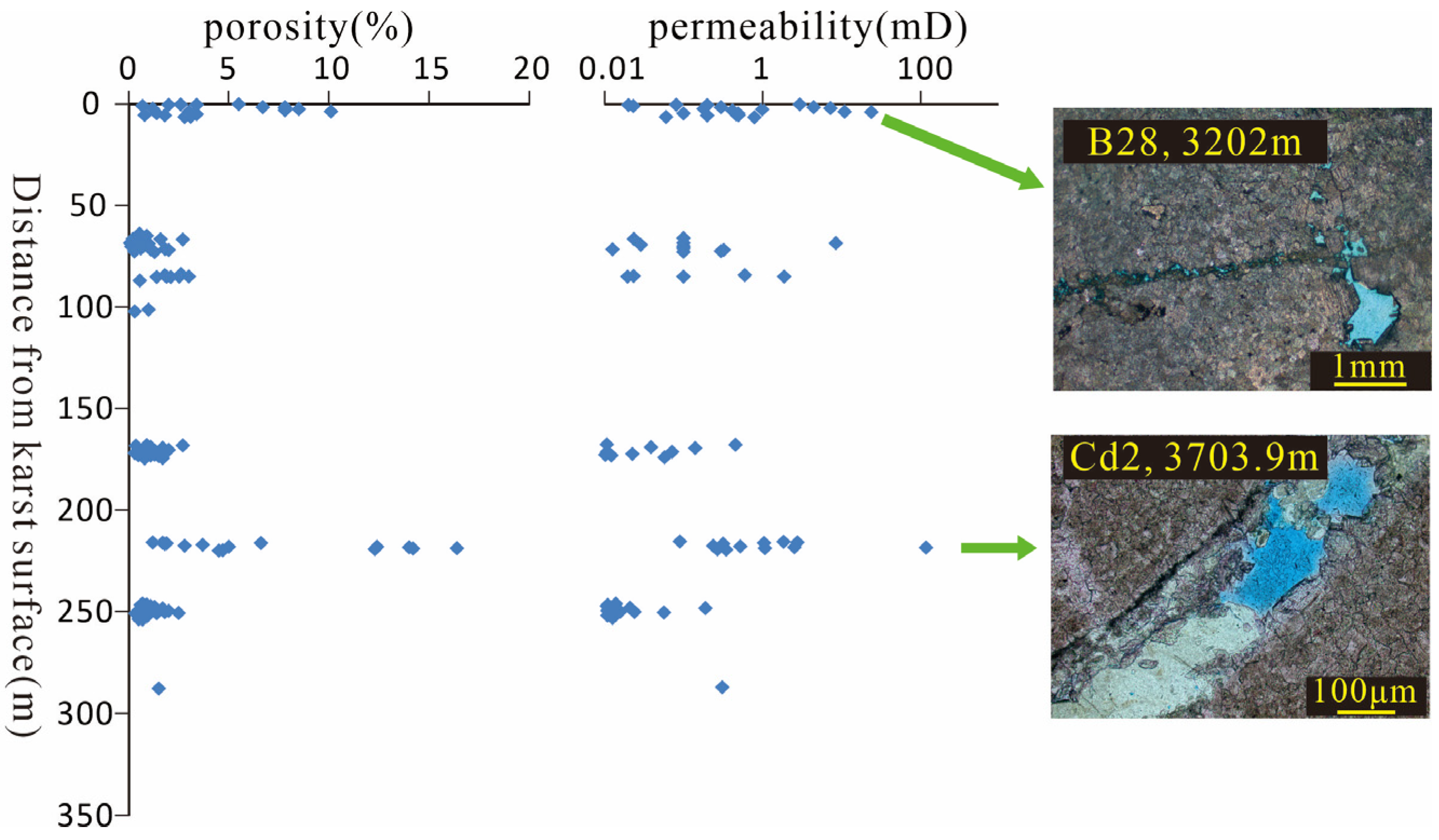
In the Bohai Sea area, the primary fractures and pores of the Lower Paleozoic carbonate reservoirs are commonly filled by early calcite due to the influence of pore water and burial heating and have generally lost their porosity. The formation of C3 confirms the development of karst reservoirs in buried hills. The intense karstification in the study area led to the formation of developed karst-type reservoirs, which are often characterized by accelerated drilling rates, enlargement of the wellbore, and mud loss during geological drilling near unconformities. Additionally, the physical properties show a decreasing trend in porosity with increasing depth (
Figure 9). Under the microscope, the development of networked fractures confirms that karstification plays a significant role in the formation of buried-hill karst-type reservoirs (
Figure 9). The development of C2 indicates that tectonic activity since the Cenozoic has created multiple generations of intersecting fractures. Data statistics reveal anomalously high permeability and porosity values in deep strata (
Figure 9). Reservoir profiles in the BZ28-1 area show that faults cut through deep strata, creating developed fractured reservoir spaces [
47]. In the case of the ancient buried-hill oil and gas reservoir in Bohai Zhong 21–22 [
39], hydrocarbons and mantle-derived gas have been injected, suggesting that after fractures were filled by C2, effective pore spaces still developed, providing favorable pathways for the migration and accumulation of oil, gas, and mantle-derived fluids.
7. Conclusions
C1 formed during the syn-depositional-to-early-diagenetic stage, under the influence of pore fluid and burial-induced temperature increase, resulting in the formation of sparry calcite that filled both primary intergranular pores and early diagenetic dissolution pores.
The cementation of C2 is controlled by multiple fluid sources. For calcite with carbon isotope values greater than −2.9‰, its formation mechanism is similar to that of C1, where diagenetic ions sequestered in the strata are released into pores and fractures under the influence of burial heating and related environmental changes, precipitating as calcite cement. In contrast, for calcite with carbon isotope values lower than −2.9‰, the supply of mantle-derived fluids, together with the mixing of formation-trapped fluids, provides the necessary material for calcite cementation.
During its geological history, the study area underwent intense surficial karstification, during which the mixing of meteoric freshwater with ions released from karst processes supplied the material for C3 cementation.
The development of C2 and C3 in the study area demonstrates that the formation of reservoirs is influenced by tectonic fracturing and karstification. Karstification has created significant karstic reservoir spaces near unconformity surfaces, which serve as the primary reservoirs. Tectonic activity has formed fractures that provide essential storage space for the formation of inner reservoirs.
Author Contributions
Conceptualization, X.S. and Y.H. (Yahao Huang); Methodology, X.S., Y.H. (Yahao Huang) and Y.H. (Yinjun He); Software, Y.H. (Yinjun He); Validation, P.H.; Formal analysis, X.S. and Y.H. (Yahao Huang); Investigation, X.S.; Resources, X.S. and C.Z.; Data curation, X.S. and Y.H. (Yinjun He); Writing—original draft, X.S.; Writing—review & editing, X.S.; Visualization, P.H.; Supervision, T.Z., P.H. and C.Z.; Project administration, T.Z.; Funding acquisition, T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Project of the Engineering and Technology Branch of CNOOC Energy TechDrilling and Production Company, Tianjin, China (grant number: GCJSXMHT–2318).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that this study received funding from CNOOC Energy TechDrilling and Pro-duction Company. The funder was not involved in the study design, collection, analysis, interpre-tation of data, the writing of this article or the decision to submit it for publication.
References
- Zhao, X.; Pu, X.; Jiang, W.; Zhou, L.; Jin, F.; Xiao, D.; Fu, L.; Li, H. An exploration breakthrough in Paleozoic petroleum system of Huanghua Depression in Dagang Oilfield and its significance. Pet. Explor. Dev. 2019, 46, 621–632. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, J.; Yang, H.; Ye, T. New fields, new types and resource potentials of oil-gas exploration in Bohai Sea. Acta Pet. Sin. 2024, 45, 163–182. [Google Scholar]
- Song, X. Characteristics of Lower Paleozoic Fractured-Vuggy Reservoirs in the BZ28-1 Area, Bonan Low Uplift, Bohai Bay Basin. Master’s thesis, Chengdu University of Technology, Chengdu, China, 2017. [Google Scholar]
- Yuan, G.H.; Cao, Y.C.; Gluyas, J.; Wang, Y.Z.; Liu, K.Y.; Xi, K.L.; Yang, T.; Wang, J. How important is carbonate dissolution in buried sandstones: Evidences from petrography, porosity, experiments, and geochemical calculations. Pet. Sci. 2019, 16, 729–751. [Google Scholar]
- Shi, S.; Hu, S.; Liu, W.; Xu, Z.; Li, B.; Wu, N. Distinguishing Paleokarst Period by Integrating Carbon-Oxygen Isotopes and Fluid Inclusion Characteristics. Nat. Gas Geosci. 2015, 26, 208–217. [Google Scholar]
- Liu, A.; Zhou, P.; Chen, X.; Cai, Q.; Li, H.; Miao, F.; Peng, Z.; Huang, H. Evaluation of shale gas preservation conditions using calcite vein inclusions and C/O isotopes: A case study on the Cambrian strata of Middle Yangtze area. Nat. Gas Ind. 2021, 41, 47–55. [Google Scholar]
- Cheng, X. Dissolution Mechanism and the Origin of High Quality Reservoirs of the Lower Paleozoic Carbonate Rocks in Jiyang and Huanghua Area. Ph.D. thesis, China University of Petroleum (East China), Qingdao, China, 2020. [Google Scholar]
- Zhang, X. Effects of the Lower Paleozoic Diagenetic Fluid Evolution on Reservoirs in Southwest Dongying Depression. Master’s thesis, China University of Petroleum, Beijing, China, 2022. [Google Scholar]
- Jin, Q.; Mao, J.; Du, Y.; Huang, X. Fracture filling mechanisms in the carbonate buried-hill of Futai Oilfield in Bohai Bay Basin, East China. Pet. Explor. Dev. 2015, 42, 454–462. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Liu, C.; Dong, D.; Gao, Z. Hydrothermal fluid activity and the quantitative evaluation of its impact on carbonate reservoirs: A case study of the Lower Paleozoic in the west of Dongying sag, Bohai Bay Basin, East China. Pet. Explor. Dev. 2016, 43, 359–366. [Google Scholar] [CrossRef]
- Liu, C.; Ma, J.; SHALAKE, S.; Liu, Z.; Xu, X. Geochemical evidences of hydrothermal fluid alterations of Ordovician paleokarst reservoirs in Shaleitian area. Fault-Block Oil Gas Field 2021, 28, 620–624. [Google Scholar]
- Jiang, Y.; Ye, T.; Zhang, S.; Liu, H. Enrichment characteristics and main controlling factors of hydrocarbon in buried hill of Bohai Bay Basin. J. China Univ. Pet. (Ed. Nat. Sci.) 2015, 39, 20–29. [Google Scholar]
- Zhang, G.; Tong, D.; Chen, K.; Liu, H.; Fang, X. Tectonic evolution and source rocks development of the super oil-rich Bohai Bay Basin, East China. Pet. Explor. Dev. 2024, 51, 1008–1023. [Google Scholar] [CrossRef]
- Nitta, M.; Inoue, A. Carbon and Oxygen Isotope Compositions of Calcite and Rhodochrosite from Geothermal Exploratory Drills THand THnear the Toyoha Deposit, Hokkaido, Japan. Resour. Geol. 2011, 61, 159–173. [Google Scholar] [CrossRef]
- Meng, Q.; Zhu, D.; Hu, W.; Jin, Z. Dissolution-filling mechanism of atmospheric precipitation controlled by both thermodynamics and kinetics. Sci. China Earth Sci. 2013, 43, 1797–1806. [Google Scholar] [CrossRef]
- Liu, S.; Huang, S.; Shen, Z.; Lv, Z.; Song, R. Diagenetic fluid evolution and water-rock interaction model of carbonate cements in sandstone: An example from the reservoir sandstone of the Fourth Member of the Xujiahe Formation of the Xiaoquan-Fenggu area, Sichuan Province, China. Sci. China: Earth Sci. 2014, 44, 1077–1092. [Google Scholar] [CrossRef]
- Swart, P.K. The geochemistry of carbonate diagenesis: The past, present and future. Sedimentology 2015, 62, 1233–1304. [Google Scholar]
- Han, J.; You, D.; Qian, Y.; Dong, S.; Peng, S. Constraints on carbonate diagenetic fluid properties by microzone in situ analysis of carbon and oxygen isotopes: A case study of Cambrian-Ordovician, Tarim Basin. Pet. Geol. Exp. 2023, 45, 135–144. [Google Scholar]
- Fu, S.; Wang, Z.; Zang, Y.; Wang, A.; Kong, H.; Fang, C. Origin of Carbonate Cements in Reservoir Rocks and Its Petroleum Geologic Significance: Eboliang structure belt, northern margin of Qaidam Basin. Acta Sedimentol. Sin. 2015, 33, 991–999. [Google Scholar]
- Zhang, Z.; Pang, J.; Yang, Y.; Cao, Y.; Qi, M.; Zhang, Y.; Zhang, L.; Ma, S. Carbon and oxygen isotope characteristics and genesis of carbonate cements in sandstone of the 4th Member of the Xujiahe Formation in the central western Sichuan depression, Sichuan basin, China. Acta Geol. Sin. 2022, 96, 2094–2106. [Google Scholar]
- Mohammedyasin, M.S.; Magnall, J.M.; Gleeson, S.A.; Schulz, H.M.; Scicchitano, M.R. Carbon and oxygen isotope microanalysis of calcite in the Permian Kupferschiefer system, Saale subbasin, eastern Germany. Chem. Geol. (Incl. Isot. Geosci.) 2023, 642, 19. [Google Scholar] [CrossRef]
- Wang, G.; Hao, F.; Zou, H.; Li, P. Influence of thermochemical sulfate reduction on oxygen isotopic composition of calcite cements in carbonates of the Triassic Feixianguan and Permian Changxing formations in the Sichuan Basin, China. Front. Earth Sci. 2023, 10, 1030472. [Google Scholar] [CrossRef]
- Xi, K.; Cao, Y.; Liu, K.; Wu, S.; Yuan, G.; Zhu, R.; Zhao, Y.; Hellevang, H. Geochemical constraints on the origins of calcite cements and their impacts on reservoir heterogeneities: A case study on tight oil sandstones of the Upper Triassic Yanchang Formation, southwestern Ordos Basin, China. AAPG Bull. 2019, 103, 2447–2485. [Google Scholar] [CrossRef]
- Pang, X.; Guo, Y.; Jiang, F.; Yin, X.; Ma, X.; Guo, E. High-quality source rocks and their distribution prediction in the Bohai Sea waters. Oil Gas Geol. 2009, 30, 393–397. [Google Scholar]
- Zhao, G.; Wang, Q.; Jin, X.; Wang, F.; Liu, X.; Bai, B. Diagenesis of the Ordovician Carbonates in Bozhong Depression, Bohai Sea Area. Geol. Sci. Technol. Inf. 2015, 34, 1–7. [Google Scholar]
- Hua, X.; Li, H.; Sun, X.; Mao, L.; Wang, B. The Paleozoic sedimentary microfacies and its control on karst reservoir in southwestern Bozhong sag, Bohai Bay Basin. J. Palaeogeogr. 2017, 19, 1013–1022. [Google Scholar]
- Xie, Y.; Zhang, G.; Shen, P.; Liu, L.; Huang, S.; Chen, S.; Yang, S. Formation conditions and exploration direction of large gas field in Bozhong sag of Bohai Bay Basin. Acta Pet. Sin. 2018, 39, 1199–1210. [Google Scholar]
- Zhao, G.; Wang, Q.; Yang, B.; Wang, X.; Bai, B.; Wan, L. Dissolution mechanism analysis of Ordovician carbonates under burial environment of Bozhong Sag, Bohai Sea area. Nat. Gas Geosci. 2016, 27, 111–120. [Google Scholar]
- Keith, M.H.; Weber, J.N. Isotopic composition and environmental classification of selected limestones and fossils. Geochim. Cosmochim. Acta 1964, 28, 1787–1816. [Google Scholar] [CrossRef]
- Friedman, I.; O’Nei, J.R. Compilation of Stable Isotope Fractionation Factors of Geochemical Interest. In Data of Geochemistry, 6th ed.; Geological Survey Professional Paper 440-KK; US Government Printing Office: Washington, DC, USA, 1977. [Google Scholar]
- Veizer, J.; Ala, D.; Azmy, K.; Bruckschen, P.; Buhl, D.; Bruhn, F.; Carden, G.A.F.; Diener, A.; Stefan, E.; Godderis, Y.; et al. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999, 161, 59–88. [Google Scholar]
- Wang, D.; Feng, X. Research on Carbon and Oxygen Geochemistry of Lower Paleozoic in North China. Acta Geol. Sin. 2002, 76, 400–408. [Google Scholar]
- Goldberg, S.L.; Present, T.M.; Finnegan, S.; Bergmann, K.D. A high-resolution record of early Paleozoic climate. Proc. Natl. Acad. Sci. USA 2021, 118, e2013083118. [Google Scholar] [CrossRef]
- Li, h.; Li, G.; Luo, Y.; Fu, H.; Gao, Y.; Zhu, Y.; Zhang, W. Analysis of characteristics of deep hydrothermal fluids in Ordovician carbonate reservoir in Tahe area. J. Northeast Pet. Univ. 2014, 38, 12–21. [Google Scholar]
- Li, W.; Qin, S. Characteristics of trace elements and hydrogen and oxygen isotopes in the formation water of the Xujiahe Formation, Sichuan Basin. Acta Pet. Sin. 2012, 33, 55–63. [Google Scholar]
- Liu, J.; He, M.; Li, Z.; Liu, Y.; Li, Z.; Zhang, G.; Yang, W.; Yang, A. Oxygen and Carbon Isotopic Geochemistry of Baiyangping Silver_Copper Polymetallic Ore Concentration Area in Lanping Basin of Yunnan Province and Its Significance. Miner. Depos. 2004, 23, 1–10. [Google Scholar]
- Liu, Y.; Gao, Z.; Yang, C.; Deng, M.; Liu, J.; Zhao, F. Carbon and oxygen isotopic compositions of hydrothermal calcites from the Mengxing Pb-Zn deposit, western Yunnan and their significances. Acta Mineral. Sin. 2021, 41, 57–68. [Google Scholar]
- Li, L.; Zhong, D.; Yang, C.; Zhao, L. Faults role in formation and distribution of the mantle derived carbon dioxide gas pools: Case study of the Jiyang Depression in Bohai Bay Basin, China. Acta Petrol. Sin. 2016, 32, 2209–2216. [Google Scholar]
- Zhou, X.; Zhang, R.; Li, H.; Wang, B.; Guo, Y. Major controls on natural gas accumulations in deep-buried hills in Bozhong Depression, Bohai Bay Basin. J. China Univ. Pet. (Ed. Nat. Sci.) 2017, 41, 42–50. [Google Scholar]
- Tong, H. The Oxygen and Carbon Isotopic Compositions and the Diagenetic Fluid of Feixianguan Carbonates in Early Triassic, Eastern Sichuan Basin. Master’s thesis, Chengdu University of Technology, Chengdu, China, 2010. [Google Scholar]
- Liu, D.; Sun, X.; Li, Z.; Tang, N.; Tan, Y.; Liu, B. Analysis of carbon and oxygen isotope on the Ordovician dolostones in the Ordos Basin. Pet. Geol. Exp. 2006, 28, 155–161. [Google Scholar]
- Liu, Y. Geochemistry of Element; Science Press: Beijing, China, 1984. [Google Scholar]
- Huang, S. Cathodoluminescence and diagenetic alteration of marine carbonate minerals. Sediment. Geol. Tethyan Geol. 1990, 10, 9–15. [Google Scholar]
- Tim, S.; Sander, V.D.B.; Alexander, J.D.; Gert-Jan, R. Definition of new trace-metal proxies for the controls on organic matter enrichment in marine sediments based on Mn, Co, Mo and Cd concentrations. Chem. Geol. 2016, 441, 235–245. [Google Scholar]
- Ren, Y.; Du, Y.; Guo, X.; Wang, G. Characteristics and distribution of Paleogene high-quality source rocks in Bozhong sag. Pet. Geol. Recovery Effic. 2015, 22, 5–13. [Google Scholar]
- Wang, J. Karst Palaeogeomorphology and Reservoir Characteristics of Ordovician Weathered Crust in Eastern Ordos Basin. Ph.D. Thesis, Northwest University, Xi’an, China, 2011. [Google Scholar]
- Li, Y.; Huang, Z. Oil and Gas sources in BZ28-1 field, Bonan Uplift, Bohai Sea. China Offshore Oil Gas (Geol.) 2001, 15, 83–88. [Google Scholar]
Figure 1.
(
A) A map showing the location of the Bozhong Sag [
13]. (
B) A map showing the main well location distribution of the Bozhong Sag. (
C) A comprehensive stratigraphic map of the study area.
Figure 1.
(
A) A map showing the location of the Bozhong Sag [
13]. (
B) A map showing the main well location distribution of the Bozhong Sag. (
C) A comprehensive stratigraphic map of the study area.
Figure 2.
The plate of authigenic calcite types. (a) Calcite filling in biological cavities, exhibiting a geopetal structure pattern, Ordovician, B28, 3466.5 m. (b) Plate-like calcite filling dissolution pores within grains and intergranular pores, Ordovician, B28, 3650 m. (c) Sparry calcite filling the pore spaces between clastic grains, displaying extremely weak CL, Ordovician, B12, 3212.04 m. (d) Early fractures filled with calcite showing dissolution features, intersected by subsequent fractures, Ordovician, B22, 4366.3 m. (e) In the same field of view as (d), the early calcite shows no CL, while the later calcite shows moderate to strong CL. (f) At least three phases of tectonic fractures intersect, Ordovician, B22, 4361.80 m. (g) In the same field of view as (f), the calcite’s CL intensity ranges from dull to strong. (h) Calcite filling in reticulate and polygonal (turtle-cracked) fractures, Ordovician, B12, 3225 m. (i) Calcite exhibiting alternating bright and dark zones with medium to strong CL, Ordovician, B21, 4889.2 m.
Figure 2.
The plate of authigenic calcite types. (a) Calcite filling in biological cavities, exhibiting a geopetal structure pattern, Ordovician, B28, 3466.5 m. (b) Plate-like calcite filling dissolution pores within grains and intergranular pores, Ordovician, B28, 3650 m. (c) Sparry calcite filling the pore spaces between clastic grains, displaying extremely weak CL, Ordovician, B12, 3212.04 m. (d) Early fractures filled with calcite showing dissolution features, intersected by subsequent fractures, Ordovician, B22, 4366.3 m. (e) In the same field of view as (d), the early calcite shows no CL, while the later calcite shows moderate to strong CL. (f) At least three phases of tectonic fractures intersect, Ordovician, B22, 4361.80 m. (g) In the same field of view as (f), the calcite’s CL intensity ranges from dull to strong. (h) Calcite filling in reticulate and polygonal (turtle-cracked) fractures, Ordovician, B12, 3225 m. (i) Calcite exhibiting alternating bright and dark zones with medium to strong CL, Ordovician, B21, 4889.2 m.
![Minerals 15 00508 g002]()
Figure 3.
δ13C and δ18O distribution characteristics of different calcite types.
Figure 3.
δ13C and δ18O distribution characteristics of different calcite types.
Figure 4.
The typical plate of EPMA locations. (a), (b), and (c) represent, respectively, the plane-polarized light (PPL), CL, and backscattered electron (BSE) of the same field of view; these images show developed clastic grains with calcite filling the intergranular spaces, representing typical EPMA locations for micritic calcite and C1 (B22, 4360.51 m, Ordovician). (d), (e), and (f) represent, respectively, the PPL, CL, and BSE of the same field of view; they exhibit multiple phases of intersecting fractures filled with calcite, representing a typical EPMA location for C2 (Cd2, 3443 m, Ordovician). (g), (h), and (i) represent, respectively, the PPL, CL, and BSE of the same field of view, they show calcite filling in karst breccias, representing a typical EPMA location for C3 (Cd2, 3705.55 m, Ordovician).
Figure 4.
The typical plate of EPMA locations. (a), (b), and (c) represent, respectively, the plane-polarized light (PPL), CL, and backscattered electron (BSE) of the same field of view; these images show developed clastic grains with calcite filling the intergranular spaces, representing typical EPMA locations for micritic calcite and C1 (B22, 4360.51 m, Ordovician). (d), (e), and (f) represent, respectively, the PPL, CL, and BSE of the same field of view; they exhibit multiple phases of intersecting fractures filled with calcite, representing a typical EPMA location for C2 (Cd2, 3443 m, Ordovician). (g), (h), and (i) represent, respectively, the PPL, CL, and BSE of the same field of view, they show calcite filling in karst breccias, representing a typical EPMA location for C3 (Cd2, 3705.55 m, Ordovician).
Figure 5.
Distribution patterns of electron probe element (the total number of samples analyzed was 69).
Figure 5.
Distribution patterns of electron probe element (the total number of samples analyzed was 69).
Figure 6.
Distribution characteristics of δ13C and δ18O in C2.
Figure 6.
Distribution characteristics of δ13C and δ18O in C2.
Figure 7.
(A) Homogenization temperatures of primary brine inclusions trapped in fracture-hosted calcite. (B) A representative image illustrating the mineral characteristics associated with typical fluid inclusions and their corresponding homogenization temperatures.
Figure 7.
(A) Homogenization temperatures of primary brine inclusions trapped in fracture-hosted calcite. (B) A representative image illustrating the mineral characteristics associated with typical fluid inclusions and their corresponding homogenization temperatures.
Figure 8.
Distribution patterns of Co and Mn elements in C3.
Figure 8.
Distribution patterns of Co and Mn elements in C3.
Figure 9.
Characterization of reservoir porosity and permeability with depth.
Figure 9.
Characterization of reservoir porosity and permeability with depth.
Table 1.
Diagenetic indicators of different types of calcite.
Table 1.
Diagenetic indicators of different types of calcite.
| Diagenetic Stage | Syngenetic to Early Diagenesis | Epidiagenetic | Mesogenetic to Telogenetic |
|---|
| Fluid Sources | Seawater, Pore Fluids | Karst Water | Pore Fluids | Hydrothermal Fluids |
|---|
| Geochemical Parameters | The mass fraction of BaO exhibiting a pronounced decrease (compared to micritic calcite); δ13C: −1.40~0.19; δ18O: −11.32~−8.37. | The concentrations of Na, Mn, and Fe ions increase, while those of Mg and Si ions decrease (compared to micritic calcite); δ13C: −4.13~−0.17; δ18O: −28.79~−14.35. | The EPMA elemental distribution pattern is similar to that of micritic calcite; δ13C: −2.55~0.646; δ18O: −15.62~−9.84. | Higher Ba ion concentrations (compared to C1); ω(Mn)/ω(Sr) > 2; δ13C: −6.81~−3.30; δ18O: −15.39~−12.81. |
| Diagenetic phenomena | In primary intergranular pores, C1 occurs as fibrous, platy, or coating, and under cathodoluminescence it either shows no luminescence or only extremely weak luminescence. | C3 that fills reticulate fractures or polygonal (turtle-cracked) fractures formed by karst collapse; and displays alternating bright and dark or banded medium-to-strong cathodoluminescence. | Since the Cenozoic, under the influence of multiple phases of tectonic activity, intersecting fractures and intense water–rock interactions have led to several episodes of calcite cementation. In particular, since the Pliocene, deep major fractures have connected mantle-derived inorganic CO2 with diagenetic fluids, resulting in the formation of hydrothermal calcite. |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).