Characterization and Suitability for Ceramics Production of Clays from Bustos, Portugal
Abstract
1. Introduction
2. Materials and Methods
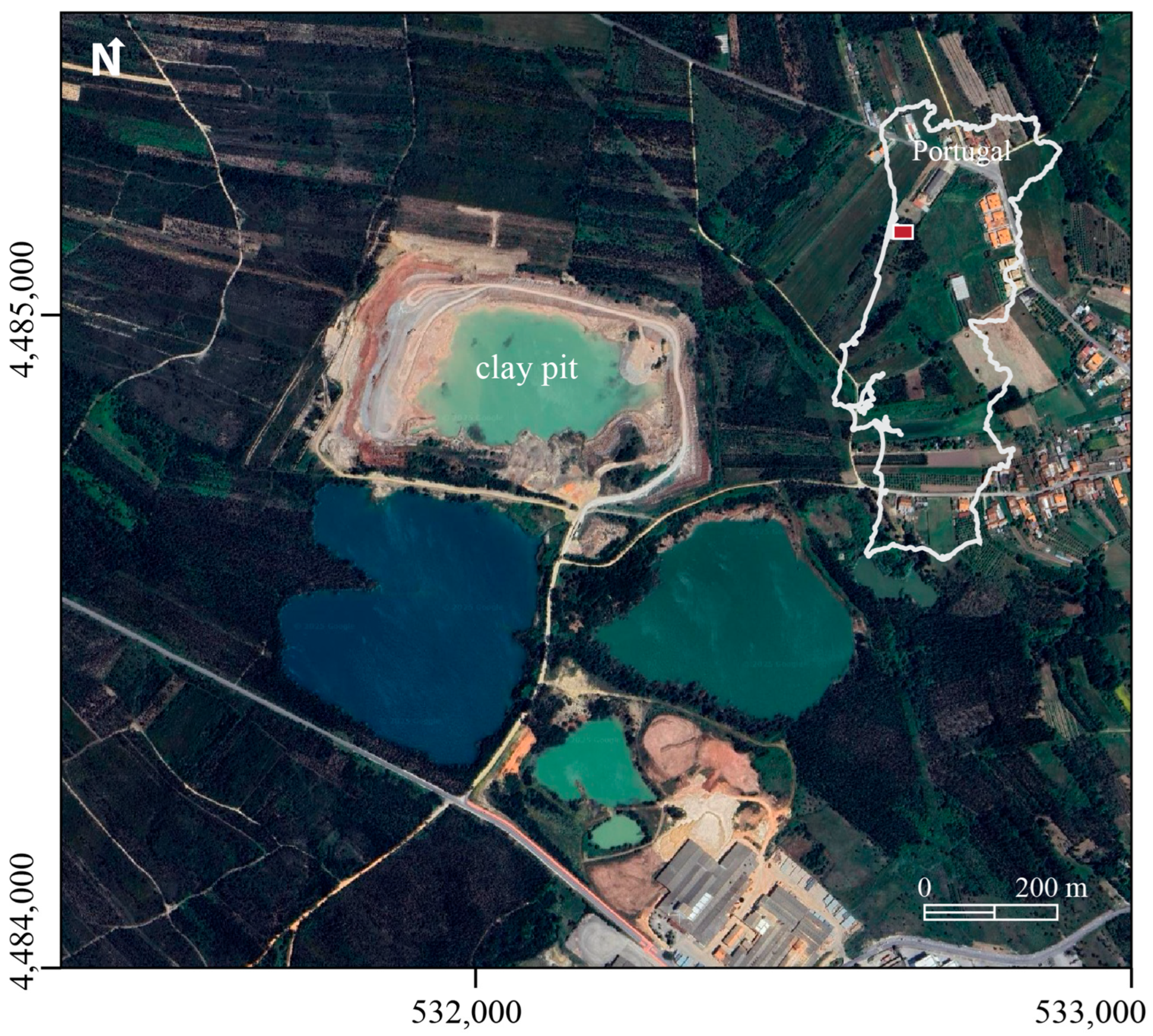
2.1. Study Area Context
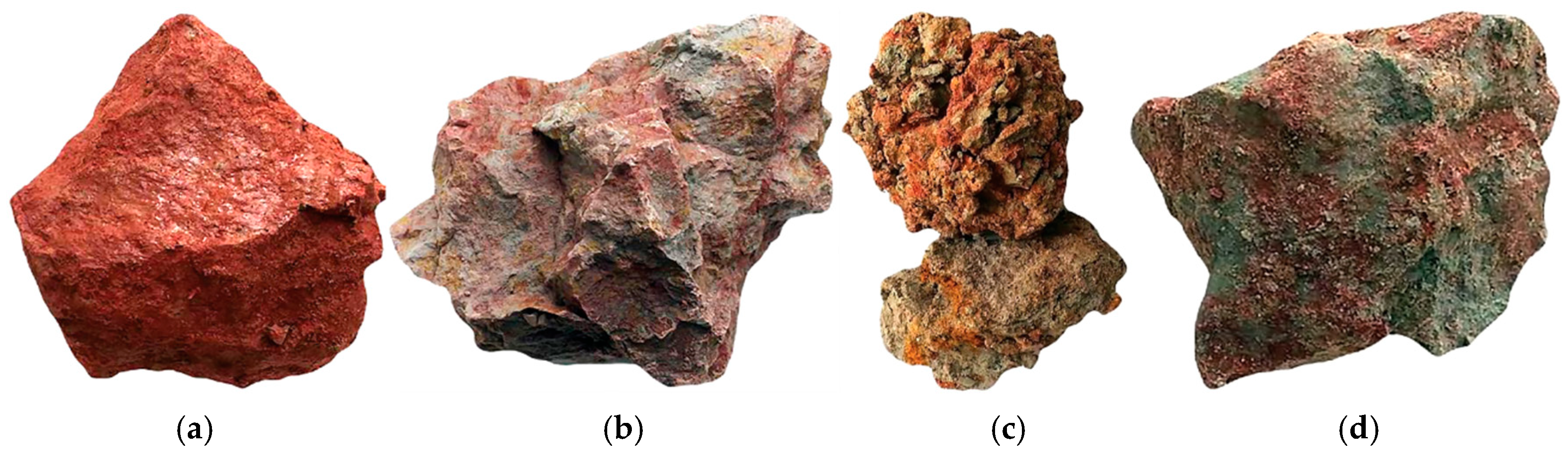
2.2. Samples Description and Analysis
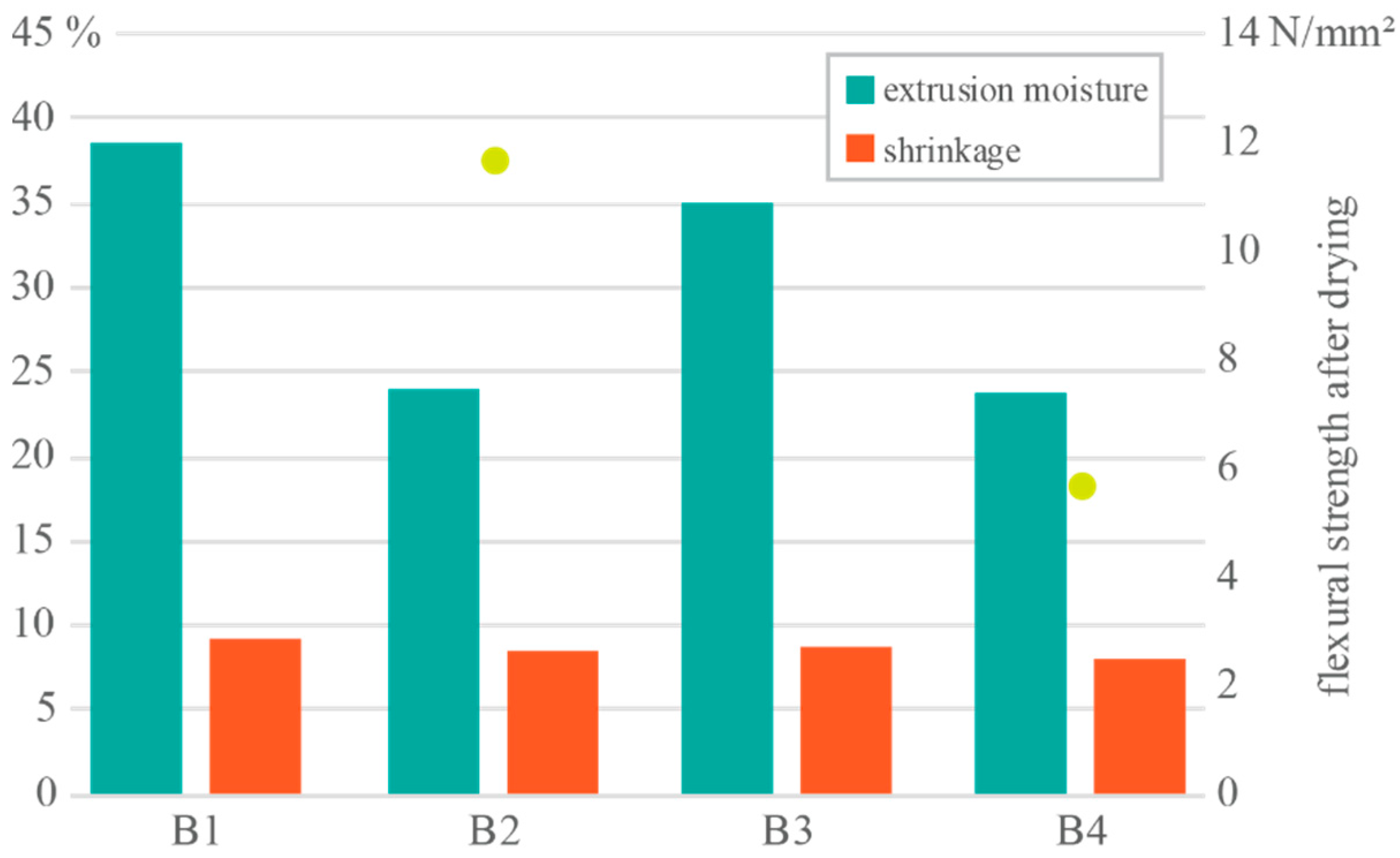
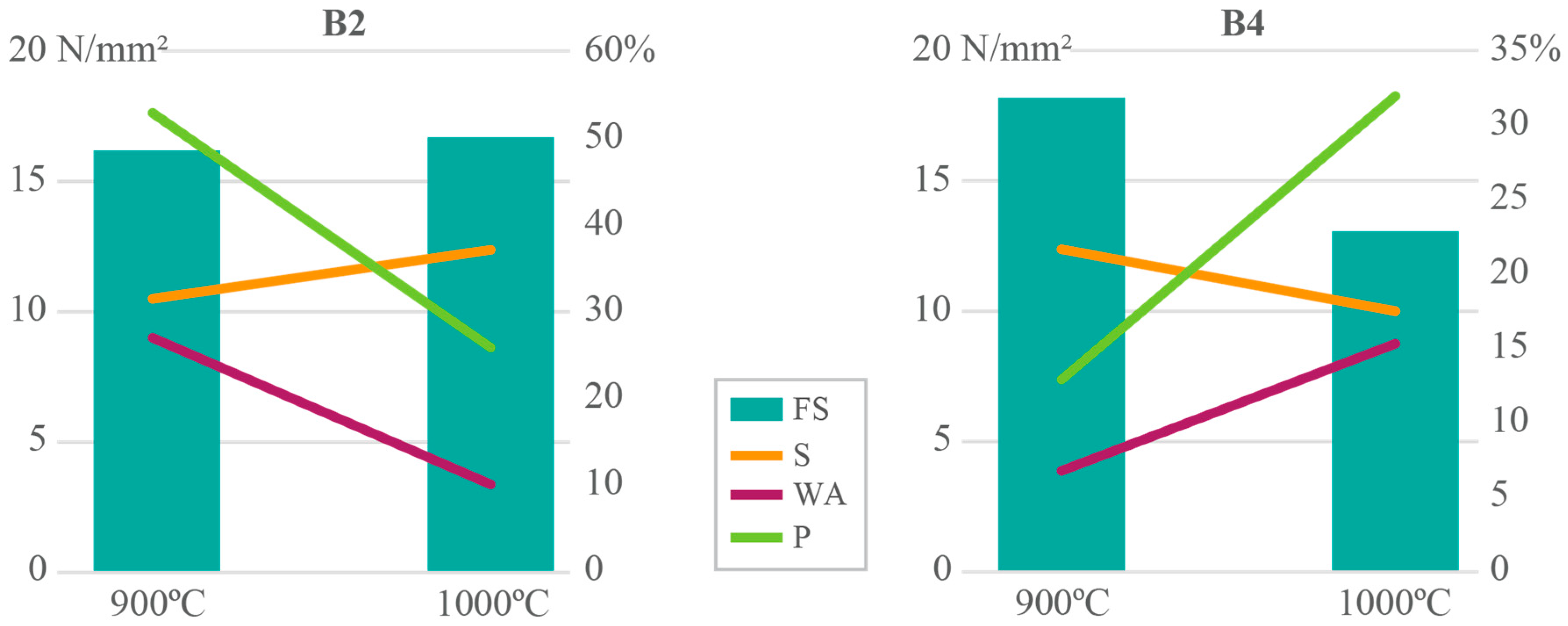
3. Results and Discussion
Results Integration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bergaya, F.; Lagaly, G. General Introduction: Clays, Clay Minerals, and Clay Science. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1–18. [Google Scholar] [CrossRef]
- Aras, A. The change of phase composition in kaolinite- and illite-rich clay-based ceramic bodies. Appl. Clay Sci. 2004, 24, 257–269. [Google Scholar] [CrossRef]
- Thomas, R. High Temperature Processing of Kaolinitic Materials. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2010. Available online: http://etheses.bham.ac.uk/id/eprint/6075 (accessed on 3 April 2025).
- Murray, H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Ouahabi, M.; Idrissi, H.; Daoudi, L.; Halim, M.; Fagel, N. Moroccan clay deposits: Physico-chemical properties in view of provenance studies on ancient ceramics. Appl. Clay Sci. 2019, 172, 65–74. [Google Scholar] [CrossRef]
- Ryan, W. Properties of Ceramic Raw Materials; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Besendörfer, G.; Roosen, A. Particle Shape and Size Effects on Anisotropic Shrinkage in Tape-Cast Ceramic Layers. J. Am. Ceram. Soc. 2008, 91, 2514–2520. [Google Scholar] [CrossRef]
- Amorós, J.; Orts, M.; Mestre, S.; Garcia-Tem, J.; Feliu, C. Porous single-fired wall tile bodies: Influence of quartz particle size on tile properties. J. Eur. Ceram. Soc. 2010, 30, 17–28. [Google Scholar] [CrossRef]
- Yariv, S.; Michelian, K. Structure and surface acidity of clay minerals. In Organo-Clay Complexes Interact; Yariv, S., Cross, H., Eds.; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Wiśniewska, K.; Pichór, W.; Kłosek-Wawrzyn, E. Influence of Firing Temperature on Phase Composition and Color Properties of Ceramic Tile Bodies. Materials 2021, 14, 6380. [Google Scholar] [CrossRef]
- Vera, J.; Ancochea, A.; Barnolas Cortinas, A.; Bea Carredo, F.; Calvo Sorando, J. Geología de España; Sociedad Geológica de España e Instituto Geológico y Minero de España: Madrid, Spain, 2004. [Google Scholar]
- Dias, R.; Araújo, A.; Terrinha, P.; Kullberg, J. (Eds.) Geologia de Portugal. Vol. II: Geologia Meso-Cenozóica de Portugal; Escolar Editora: Lisboa, Portugal, 2013; p. 798. (In Portuguese) [Google Scholar]
- Dias, R.; Ribeiro, A. The Ibero-Armorican Arc: A collisional effect against an irregular continent? Tectonophysics 1995, 246, 113–128. [Google Scholar] [CrossRef]
- Rocha, F. Argilas Aplicadas a Estudos Litoestratigráficos e Paleoambientais na Bacia Sedimentar de Aveiro. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 1993. Available online: http://hdl.handle.net/10773/4386 (accessed on 30 March 2025). (In Portuguese).
- Barbosa, B. Geological Map of Portugal (1/50.000), Sheet 16-C, Vagos; Serviços Geológicos de Portugal: Lisbon, Portugal, 1981. [Google Scholar]
- Teixeira, C.; Zbyszewski, G. Geological Ma of Portugal (1/50.000), Sheet 16-A, Aveiro; Serviços Geológicos de Portugal: Lisbon, Portugal, 1976. [Google Scholar]
- Coroado, J. Propriedades Cerâmicas das Argilas das Unidades Litoestratigráficas “Argilas de Aveiro” e “Argilas de Tomar”. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 2000. Available online: http://hdl.handle.net/10773/29844 (accessed on 30 March 2025). (In Portuguese).
- Coroado, J.; Marques, J.; Rocha, F.; Gomes, C. Caracterization and Ceramic Properties of Bustos Clay (Aveiro—Portugal). In Proceedings of the 2nd Mediterranean Clay Meeting, Aveiro, Portugal, 16–19 September 1998; pp. 375–380. [Google Scholar]
- Candeias, C.; Ávila, P.; Alves, C.; Gama, C.; Sequeira, C.; da Silva, E.; Rocha, F. Dust characterization and its potential impact during the 2014–2015 Fogo Volcano eruption (Cape Verde). Minerals 2021, 11, 1275. [Google Scholar] [CrossRef]
- Santos, I. Caracterização das Matérias-Primas Argilosas do Barreiro de Bustos. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 2021. Available online: http://hdl.handle.net/10773/31351 (accessed on 2 March 2025). (In Portuguese).
- Oliveira, A.; Rocha, F.; Rodrigues, A.; Jouanneau, J.; Dias, A.; Weber, O.; Gomes, C. Clay minerals from the sedimentary cover from the Northwest Iberian shelf. Prog. Oceanogr. 2002, 52, 233–247. [Google Scholar] [CrossRef]
- Tejeogue, J.; Djakba, R.; Fotsop, C.; Dobe, N.; Mouhamadou, S.; Wangmene, B.; Harouna, M. Systematic metronidazole adsorption performance onto montmorillonite clay: Parametric study, process modelling and RSM optimisation. Results Chem. 2025, 14, 102153. [Google Scholar] [CrossRef]
- NP 143; Solos—Determinação dos Limites de Consistência. IGPAI: Lisboa, Portugal, 1969. (In Portuguese)
- Almeida, L.; Rocha, F.; Candeias, C. Portuguese lagoon clay sediments for pelotherapy applications: The study of technological properties. J. Appl. Sci. 2023, 23, 185–193. [Google Scholar] [CrossRef]
- Mendes, M.; Pedroti, L.; Carvalho, J.; Ferreira, F.; Mendes, B.; Xavier, L.; Fernandes, W.; Oliveira, N. Characterization of Yellow and Red Clays and Bauxite Residue: Analysis of Properties and Potential for Ceramic Products. In Characterization of Minerals, Metals, and Materials 2025; Peng, Z., Xie, K.Y., Zhang, M., Li, J., Li, B., Monteiro, S.N., Soman, R., Hwang, J.-Y., Kalay, Y.E., Escobedo-Diaz, J.P., et al., Eds.; TMS 2025; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Casagrande, A. Research on the Atterberg limits of soils. Public Roads 1932, 13, 121–136. [Google Scholar]
- Gippini, E. Contribution à l’étude des propriétés de moulage des argiles et des mélanges optimaux de matiéres premiéres. L’lndustrie Céramique 1969, 619, 423–435. [Google Scholar]
- Bain, J.; Highley, D. Regional appraisal of clay resources; a challenge to the clay mineralogist. In International Clay Conference 1978. Developments in Sedimentology; Mortland, M.M., Farmer, V.C., Eds.; Elsevier: Amsterdam, The Nederland, 1978. [Google Scholar]
- Sheppard, L. Fabrication of ceramics: The challenge continues. Am. Ceram. Soc. Bull. 1989, 68, 1815–1820. [Google Scholar]
- Reed, J. Principles of Ceramics Processing; John Wiley & Sons, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Ferraz, E. Caulino de Alvarães: Propriedades e Aplicações Cerâmicas. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 2004. Available online: http://hdl.handle.net/10773/16558 (accessed on 30 March 2025). (In Portuguese).
- King, A. Ceramic Technology and Processing: A Practical Working Guide; William Andrew: New York, NY, USA, 2001. [Google Scholar]
- Händle, F. The Art of Ceramic Extrusion; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- ISO/CIE 11664-4:2019; Colorimetry—Part 4: CIE 1976 L* a* b* Colour Space. ISSO: Geneva, Switzerland, 2019.
- ASTM C689-03a; Standard Test Method for Modulus of Rupture of Unfired Clays. American Society for Testing and Materials: West Conshohocken, PA, USA, 2004.
- ASTM C674-88; Standard Test Methods for Flexural Properties of Ceramic Whiteware Material. American Society for Testing Materials: West Conshohocken, PA, USA, 2006.
- ASTM C373-88; Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products. American Society for Testing and Materials: West Conshohocken, PA, USA, 2006.
- Brownell, W. Efflorescence resulting from sulfates in clay raw materials. J. Am. Ceram. Soc. 1958, 41, 310–314. [Google Scholar] [CrossRef]
- Ekosse, G.; Mulaba-Bafubiandi, A.; Nkoma, J. Physico-chemistry of continental bentonites and kaolin for ceramic applications. Afr. J. Sci. Technol. 2007, 8, 107–115. [Google Scholar]
- Murray, H. Applied Clay Mineralogy: Occurrences, Processing and Applications of Kaolins, Bentonites, Palygorskitesepiolite, and Common Clays; Elsevier: Amsterdam, The Nederland, 2006. [Google Scholar]
- Martínez-Martínez, S.; Pérez-Villarejo, L.; Garzón, E.; Sánchez-Soto, P. Influence of firing temperature on the ceramic properties of illite-chlorite-calcitic clays. Ceram. Int. 2023, 49, 24541–24557. [Google Scholar] [CrossRef]
- Aras, A.; Albayrak, M.; Arikan, M.; Sobolev, K. Evaluation of selected kaolins as raw materials for the Turkish cement and concrete industry. Clay Min. 2007, 42, 233–244. [Google Scholar] [CrossRef]
- Long, H.; Zhu, D.; Pan, J.; Li, S.; Yang, C.; Guo, Z. Advanced processing techniques and impurity management for high-purity quartz in diverse industrial applications. Minerals 2024, 14, 571. [Google Scholar] [CrossRef]
- Li, Z.; Ma, G.; Zheng, D.; Zhang, X. Effect of Fe2O3 on the crystallization behavior, microstructure and properties of glass-ceramics prepared from the modified tailings after reduction of copper slag. Mater Today Commun. 2022, 31, 103516. [Google Scholar] [CrossRef]
- Gikunju, E.; Bosire, G.; Onyari, J.; Nyongesa, F.; Beleta, D. Physico-chemical characterization of feldspars for application as fluxing agents: Experimental and ab initio computational approaches. Phys. Chem. Earth 2024, 135, 103651. [Google Scholar] [CrossRef]
- Tezza, V.; Scarpato, M.; Oliveira, L.; Bernardin, A. Effect of firing temperature on the photocatalytic activity of anatase ceramic glazes. Powder Technol. 2015, 276, 60–65. [Google Scholar] [CrossRef]
- Silva, T.; Resende, M.; Resende, D.; Junior, P.; Bezerra, A. Valorization of ceramic sludge waste as alternative flux: A way to clean production in the sanitary ware industry. Clean. Eng. Technol. 2022, 7, 100453. [Google Scholar] [CrossRef]
- Sun, H.; Yao, J.; Ma, B.; Knudsen, T.; Yuan, C. Siderite’s green revolution: From tailings to an eco-friendly material for the green economy. Sci. Total Environ. 2024, 914, 169922. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, K.; Dong, W.; Bao, Q.; Zhao, T.; Wang, Y. Effects of CaO-Li2O-K2O-Na2O fluxing agents on the properties of porcelain ceramic tiles. Key Eng. Mater. 2015, 655, 258–262. [Google Scholar] [CrossRef]
- Deer, W.; Howie, R.; Zussman, J. An Introduction to the Rock-Forming Minerals; Mineralogical Society of Great Britain and Ireland: Middlesex, UK, 2013. [Google Scholar] [CrossRef]
- Blundy, J.; Wood, B. Crystal-chemical controls on the partitioning of Sr and Ba between plagioclase feldspar, silicate melts, and hydrothermal solutions. Geochim. Cosmochim. Acta 1991, 55, 193–209. [Google Scholar] [CrossRef]
- Al-Ani, T.; Sarapää, O. Clay and clay mineralogy. Phys.-Chem. Prop. Indust. Uses 2008, 2008, 11–65. [Google Scholar]
- Kingery, W.; Bowen, H.; Uhlmann, D. Introduction to Ceramics; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Murray, H. Applied clay mineralogy today and tomorrow. Clay Min. 1999, 34, 39–49. [Google Scholar] [CrossRef]
- Lagaly, G. Chapter 5 Colloid Clay Science. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Ferreira, I. Estudo das Escombreiras do Antigo Couto Mineiro do Bessa (Montalegre) e o seu Potencial Reaproveitamento Para a Indústria Cerâmica. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 2020. Available online: http://hdl.handle.net/10773/29770 (accessed on 30 March 2025). (In Portuguese).
- Zaccaron, A.; Nandi, V.; Bernardin, A. Fast drying for the manufacturing of clay ceramics using natural clays. J. Build. Eng. 2021, 33, 101877. [Google Scholar] [CrossRef]
- Noor-E-Khuda, A.; Albermani, F. Mechanical properties of clay masonry units: Destructive and ultrasonic testing. Construc. Build. Mater. 2019, 219, 111–120. [Google Scholar] [CrossRef]
- Hennetier, L.; Almeida, J.; Correia, A.; Ferreira, V. Efflorescence and its quantification in ceramic building materials. Br. Ceram. Trans. 2001, 100, 72–76. [Google Scholar] [CrossRef]


| Mineral | B1 | B2 | B3 | B4 |
|---|---|---|---|---|
| ∑ phyllosilicates | 76 | 72 | 70 | 69 |
| quartz | 14 | 21 | 20 | 20 |
| K-feldspar | 2 | 1 | 2 | 5 |
| plagioclase | 0 | 0.5 | 1 | 0 |
| siderite | 0 | 0 | 0 | 1 |
| bassanite | tr | tr | tr | tr |
| anatase | 0.5 | 0.5 | 1 | 1 |
| m-m | 7.5 | 5 | 6 | 4 |
| Mineral | B1 | B2 | B3 | B4 |
|---|---|---|---|---|
| smectite | 16 | 22 | 81 | 61 |
| illite | 73 | 53 | 14 | 21 |
| kaolinite | 11 | 25 | 5 | 18 |
| Var | B1 | B2 | B3 | B4 |
|---|---|---|---|---|
| Al2O3 | 21.28 | 21.18 | 20.46 | 21.03 |
| CaO | 1.11 | 0.58 | 0.55 | 0.68 |
| Cl | 0.05 | 0.04 | 0.05 | 0.08 |
| Fe2O3 | 9.16 | 5.79 | 6.54 | 5.77 |
| K2O | 4.45 | 4.65 | 3.51 | 3.78 |
| MgO | 2.91 | 2.07 | 2.05 | 1.85 |
| MnO | 0.03 | 0.02 | 0.03 | 0.02 |
| Na2O | 0.27 | 0.24 | 0.30 | 0.25 |
| P2O5 | 0.06 | 0.05 | 0.03 | 0.05 |
| SiO2 | 52.39 | 58.16 | 60.57 | 59.21 |
| SO3 | 0.02 | 0.02 | 0.07 | 0.03 |
| TiO2 | 0.88 | 0.88 | 0.96 | 0.92 |
| LOI | 6.91 | 5.98 | 4.73 | 6.12 |
| Var | B1 | B2 | B3 | B4 |
|---|---|---|---|---|
| Ba | 200 | 300 | 300 | 200 |
| Ce | 68 | 200 | 55 | 65 |
| Co | 11.7 | 9.2 | 8.3 | 8.6 |
| Cr | 72 | 51 | 70 | 54 |
| Cs | 23 | 28 | 17 | 23 |
| Cu | 23 | 17 | 18 | 15 |
| Ga | 24 | 22 | 15 | 20 |
| La | 40 | 62 | 30 | 44 |
| Nd | 33 | 58 | 24 | 29 |
| Ni | 37 | 18 | 16 | 15 |
| Pb | 28 | 31 | 22 | 26 |
| Rb | 300 | 300 | 200 | 200 |
| Sn | 15 | 18 | 13 | 16 |
| Sr | 86 | 95 | 53 | 87 |
| Th | 14 | 19 | 12 | 17 |
| V | 86 | 100 | 71 | 100 |
| Y | 20 | 34 | 18 | 24 |
| Zn | 20 | 24 | 16 | 21 |
| Zr | 200 | 300 | 300 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candeias, C.; Santos, I.; Rocha, F. Characterization and Suitability for Ceramics Production of Clays from Bustos, Portugal. Minerals 2025, 15, 503. https://doi.org/10.3390/min15050503
Candeias C, Santos I, Rocha F. Characterization and Suitability for Ceramics Production of Clays from Bustos, Portugal. Minerals. 2025; 15(5):503. https://doi.org/10.3390/min15050503
Chicago/Turabian StyleCandeias, Carla, Isaac Santos, and Fernando Rocha. 2025. "Characterization and Suitability for Ceramics Production of Clays from Bustos, Portugal" Minerals 15, no. 5: 503. https://doi.org/10.3390/min15050503
APA StyleCandeias, C., Santos, I., & Rocha, F. (2025). Characterization and Suitability for Ceramics Production of Clays from Bustos, Portugal. Minerals, 15(5), 503. https://doi.org/10.3390/min15050503







