Leaching of Scheelite Concentrate for Tungsten Extraction
Abstract
1. Introduction
2. Scheelite Resources
3. Scheelite Hydrometallurgical Leaching Process
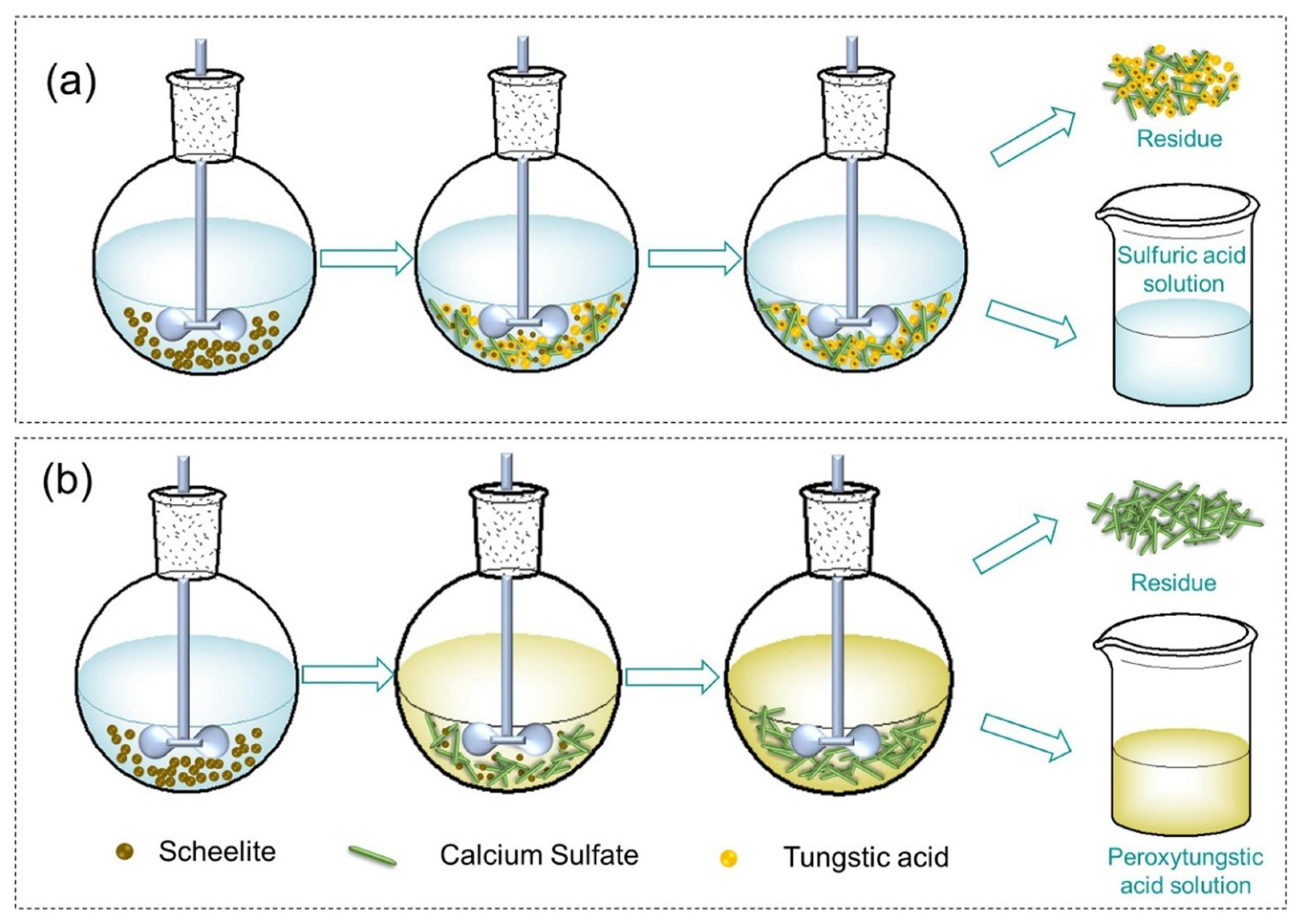
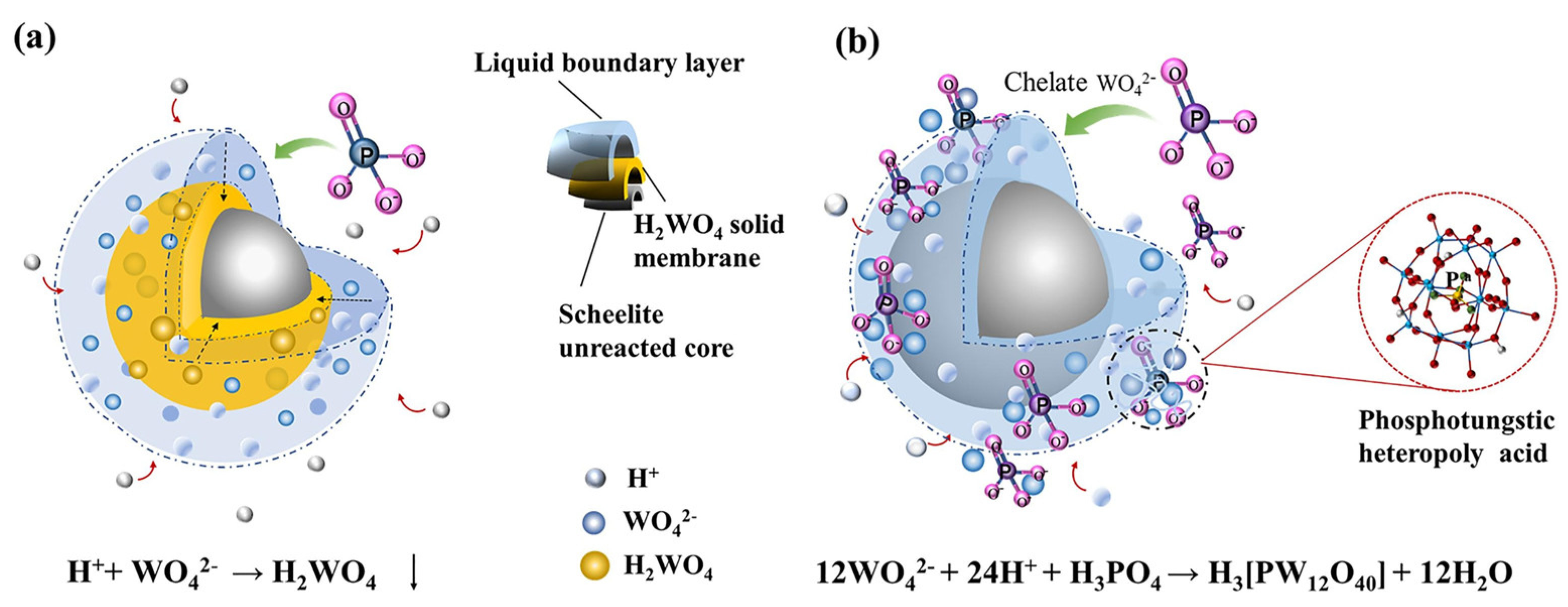
3.1. Acid Leaching Processes
3.1.1. HCl Leaching Process
3.1.2. H2SO4 Leaching Process
3.1.3. HNO3 Leaching Process
3.2. Alkaline Leaching Processes
3.2.1. NaOH High-Pressure Leaching Process
3.2.2. Na2CO3 High-Pressure Leaching Process
3.2.3. Phosphate High-Pressure Leaching Process
3.2.4. NaF High-Pressure Leaching Process
3.3. Other Leaching Processes
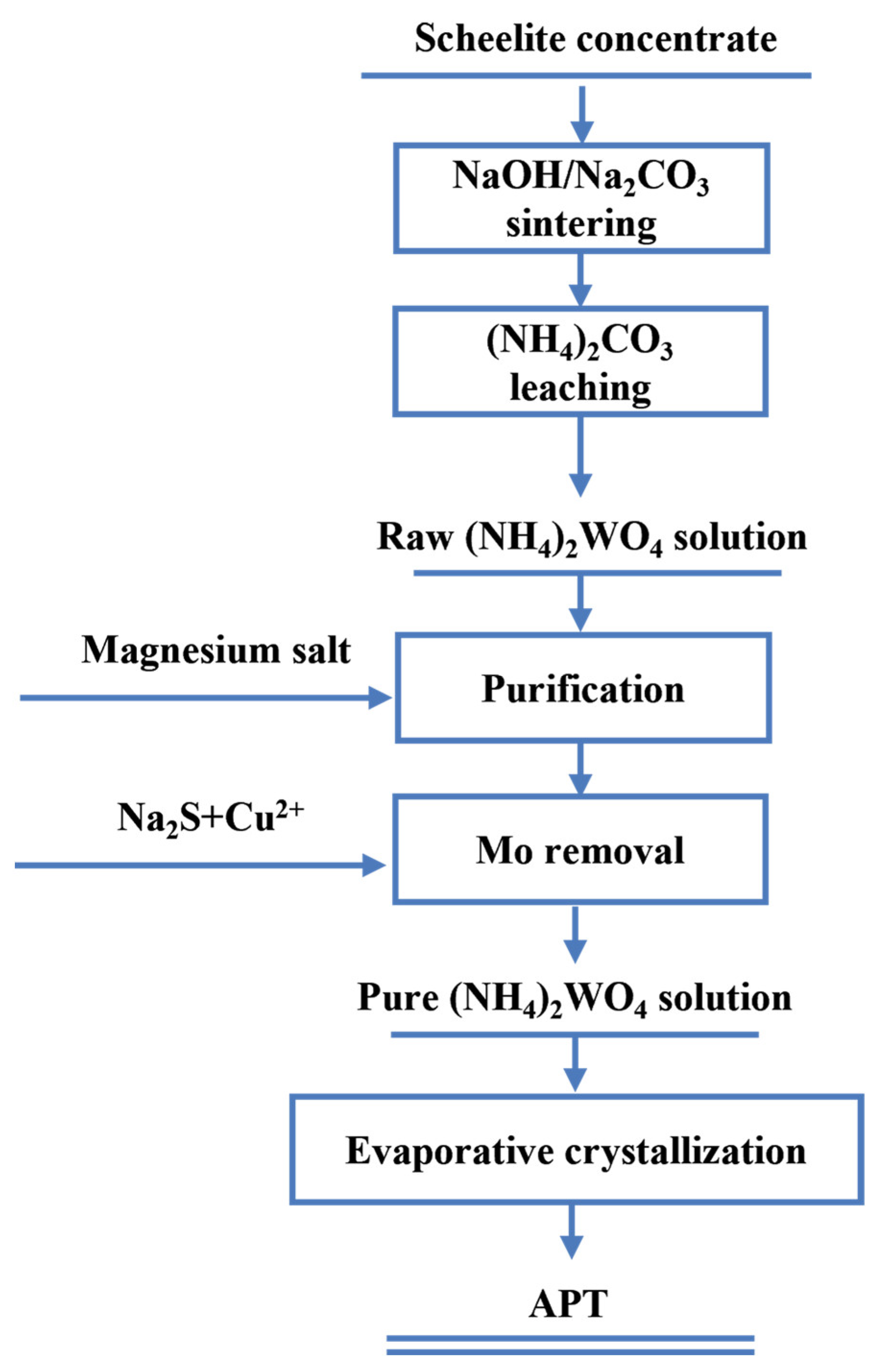
3.3.1. NaOH/Na2CO3 Sintering–Water Leaching Process
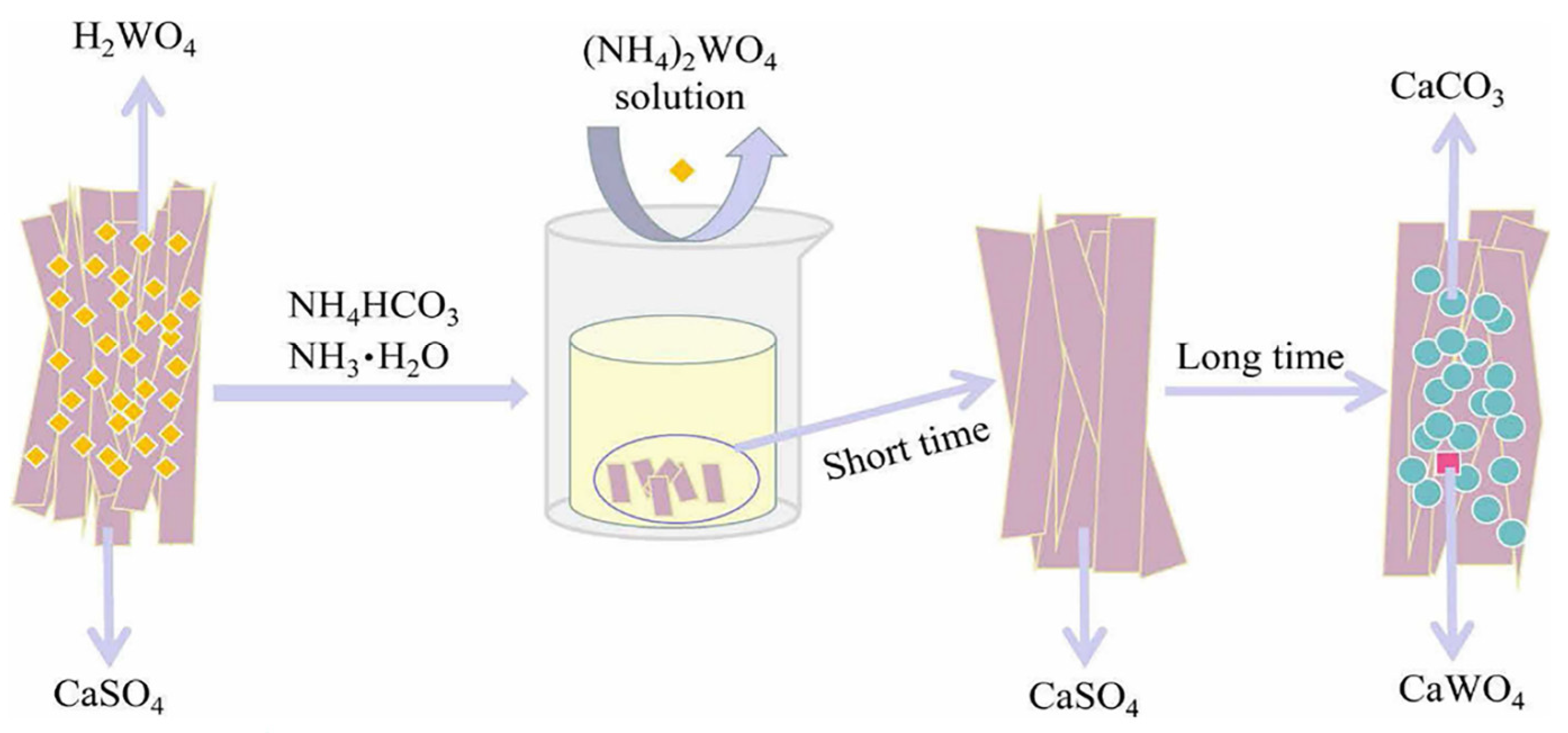
3.3.2. H2SO4 Conversion–NH4HCO3 Leaching Process
3.3.3. Combined (NH4)3PO4, NH4·H2O, and CaF2 Leaching Process
3.4. By-Product Composition and Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunk, H.J.; Hartl, H. Discovery, properties and applications of tungsten and its inorganic compounds. ChemTexts 2019, 5, 15. [Google Scholar] [CrossRef]
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability evaluation of essential critical raw materials: Cobalt, niobium, tungsten and rare earth elements. J. Phys. D Appl. Phys. 2018, 51, 203001. [Google Scholar] [CrossRef]
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, X.; Zhou, Q.; Peng, Z.; Liu, G.; Qi, T.; Taskinen, P. Kinetics of Scheelite Conversion in Sulfuric Acid. JOM 2018, 70, 2499–2504. [Google Scholar] [CrossRef]
- Gong, D.; Zhou, K.; Peng, C.; Li, J.; Chen, W. Sequential extraction of tungsten from scheelite through roasting and alkaline leaching. Miner. Eng. 2019, 132, 238–244. [Google Scholar] [CrossRef]
- Tejado, E.; Müller, A.v.; You, J.H.; Pastor, J.Y. The thermo-mechanical behaviour of W-Cu metal matrix composites for fusion heat sink applications: The influence of the Cu content. J. Nucl. Mater. 2018, 498, 468–475. [Google Scholar] [CrossRef]
- Ma, J.; Chen, W.; Li, R.; Elmarakbi, A.; Fu, Y.-Q. Cooling source strategy for synthesis of highly porous tungsten using freeze-drying technology. Mater. Today Commun. 2025, 44, 111974. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, X.; Zhao, Z. Leaching kinetics of scheelite with sodium phytate. Hydrometallurgy 2019, 186, 83–90. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Nie, C.; Zhang, J.; Steenari, B.-M.; Ekberg, C. Comprehensive treatments of tungsten slags in China: A critical review. J. Environ. Manag. 2020, 270, 110927. [Google Scholar] [CrossRef]
- Tang, L.; Wang, P.; Graedel, T.E.; Pauliuk, S.; Xiang, K.; Ren, Y.; Chen, W.-Q. Refining the understanding of China’s tungsten dominance with dynamic material cycle analysis. Resour. Conserv. Recycl. 2020, 158, 104829. [Google Scholar] [CrossRef]
- Liang, J.; Geng, Y.; Su, C.; Chen, W. An emergy-based comparison between primary and secondary tungsten production in China. Environ. Impact Assess. Rev. 2024, 107, 107548. [Google Scholar] [CrossRef]
- Lassner, E. From tungsten concentrates and scrap to highly pure ammonium paratungstate (APT). Int. J. Refract. Met. Hard Mater 1995, 13, 35–44. [Google Scholar] [CrossRef]
- Errandonea, D.; Manjón, F.J.; Somayazulu, M.; Häusermann, D. Effects of pressure on the local atomic structure of CaWO4 and YLiF4: Mechanism of the scheelite-to-wolframite and scheelite-to-fergusonite transitions. J. Solid State Chem. 2004, 177, 1087–1097. [Google Scholar] [CrossRef]
- Han, H.S.; Liu, W.L.; Hu, Y.H.; Sun, W.; Li, X.D. A novel flotation scheme: Selective flotation of tungsten minerals from calcium minerals using Pb–BHA complexes in Shizhuyuan. Rare Met. 2017, 36, 533–540. [Google Scholar] [CrossRef]
- Choi, I.H.; Moon, G.; Lee, J.Y.; Jyothi, R.K. Hydrometallurgical processing of spent selective catalytic reduction (SCR) catalyst for recovery of tungsten. Hydrometallurgy 2018, 178, 137–145. [Google Scholar] [CrossRef]
- Mishra, D.; Sinha, S.; Sahu, K.K.; Agrawal, A.; Kumar, R. Recycling of Secondary Tungsten Resources. Trans. Indian Inst. Met. 2017, 70, 479–485. [Google Scholar] [CrossRef]
- Xiao, L.; Ji, L.; Yin, C.; Chen, A.; Chen, X.; Liu, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Tungsten extraction from scheelite hydrochloric acid decomposition residue by hydrogen peroxide. Miner. Eng. 2022, 179, 107461. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, X.; Zhao, Z.; Liu, X.; Sun, F. Pressure leaching of wolframite using a sulfuric-phosphoric acid mixture. Miner. Eng. 2021, 169, 106941. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Chen, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Acidic decomposition of scheelite by organic sodium phytate at atmospheric pressure. Miner. Eng. 2021, 172, 107125. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel depressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Dai, L.; Feng, B.; Zhang, W.; Guo, W.; Liu, R. Study of the effect and mechanism of environmentally friendly depressant tragacanth gum on scheelite and apatite separation. Process Saf. Environ. Prot. 2025, 196, 106913. [Google Scholar] [CrossRef]
- Gong, D.; Zhou, K.; Li, J.; Peng, C.; Chen, W. Rapid Leaching of Synthetic Scheelite by a Resin-in-Pulp Process. JOM 2018, 70, 2846–2855. [Google Scholar] [CrossRef]
- Longgang, Y.E.; Ouyang, Z.; Liu, S. Removal of Iron from Polluted Tungsten Powder by Acid Leaching for Recovery of Tungsten. JOM 2020, 72, 933–938. [Google Scholar] [CrossRef]
- Zeiler, B.; Bartl, A.; Schubert, W.-D. Recycling of tungsten: Current share, economic limitations, technologies and future potential. Int. J. Refract. Met. Hard Mater. 2021, 98, 105546. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Zhao, Z.; Chen, X.; Liu, X.; He, L.; Sun, F. Efficient extraction of tungsten, calcium, and phosphorus from low-grade scheelite concentrate. Miner. Eng. 2022, 181, 107462. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Ding, A.; Xiao, C. A review on the extraction and recovery of critical metals using molten salt electrolysis. J. Environ. Chem. Eng. 2023, 11, 109746. [Google Scholar] [CrossRef]
- Chen, X.; Guo, F.; Chen, Q.; Liu, X.; Zhao, Z. Leaching tungsten and rare earth elements from scheelite through H2SO4–H3PO4 mixed acid decomposition. Miner. Eng. 2020, 156, 106526. [Google Scholar] [CrossRef]
- Liu, X.; Deng, L.; Chen, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Recovery of tungsten from acidic solutions rich in calcium and iron. Hydrometallurgy 2021, 204, 105719. [Google Scholar] [CrossRef]
- Wang, X.; Qin, W.-Q.; Jiao, F.; Dong, L.-Y.; Guo, J.-G.; Zhang, J.; Yang, C.-R. Review of tungsten resource reserves, tungsten concentrate production and tungsten beneficiation technology in China. Trans. Nonferrous Met. Soc. China 2022, 32, 2318–2338. [Google Scholar] [CrossRef]
- Das, S.K.; Nagesh, C.H.R.V.; Sreenivas, T.; Kundu, T.; Angadi, S.I. A treatise on occurrence, beneficiation and plant practices of tungsten-bearing ores. Powder Technol. 2023, 429, 118938. [Google Scholar] [CrossRef]
- Chen, Y.; Huo, G.; Guo, X.; Chen, J. A review of flowsheets for tungsten recovery from scheelite, wolframite and secondary resources and challenges for sustainable production. Hydrometallurgy 2025, 234, 106455. [Google Scholar] [CrossRef]
- Lee, M.S.; Sohn, S.H.; Lee, M.H. Ionic Equilibria and Ion Exchange of Molybdenum(VI) from Strong Acid Solution. Bull.—Korean Chem. Soc. 2011, 32, 3687–3691. [Google Scholar] [CrossRef][Green Version]
- Nguyen, T.H.; Lee, M.S. Separation of molybdenum and vanadium from acid solutions by ion exchange. Hydrometallurgy 2013, 136, 65–70. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, Y.; Sun, W.; Drelich, J.W. Surface-Charge Anisotropy of Scheelite Crystals. Langmuir 2016, 32, 6282–6288. [Google Scholar] [CrossRef]
- Shen, L.; Li, X.; Lindberg, D.; Taskinen, P. Tungsten extractive metallurgy: A review of processes and their challenges for sustainability. Miner. Eng. 2019, 142, 105934. [Google Scholar] [CrossRef]
- Dimitrijevi, S.B.; Velikovi, S.R.; Ivanovi, A.T.; Veljkovi, F.M.; Jovanovi, M.M.; Kamberovi, E.; Dimitrijevi, S.P. Recovery of tungsten trioxide from waste diamond core drilling crowns by nitric acid leaching. Int. J. Refract. Met. Hard Mater. 2021, 101, 105695. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, F.; Liu, X.H.; Zhao, Z. Understanding the wet decomposition processes of tungsten ore: Phase, thermodynamics and kinetics. Hydrometallurgy 2022, 213, 105928. [Google Scholar] [CrossRef]
- Martins, J.P.; Martins, F. Soda ash leaching of scheelite concentrates: The effect of high concentration of sodium carbonate. Hydrometallurgy 1997, 46, 191–203. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Wang, S.; Li, H.; Liu, M.; Sun, P.; Li, Y. Extracting tungsten from scheelite concentrate with caustic soda by autoclaving process. Hydrometallurgy 2011, 108, 152–156. [Google Scholar] [CrossRef]
- Lassner, E.; Schubert, W.D. Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds; Springer: Berlin, Germany, 1999. [Google Scholar]
- Yang, L.; Zhang, X.; Cao, C.; Huang, X.; Wan, L. Sustainable and efficient leaching of tungsten from scheelite using the mixture of ammonium phosphate, ammonia and calcium fluoride. Hydrometallurgy 2022, 210, 105846. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Cao, C.; Xue, X.; Wan, L. A new process for the efficient decomposition of low-grade scheelite based on the formation of insoluble calcium fluorophosphate. Miner. Eng. 2022, 177, 107372. [Google Scholar] [CrossRef]
- Gong, D.; Zhang, Y.; Wan, L.; Qiu, T.; Chen, Y.; Ren, S. Efficient extraction of tungsten from scheelite with phosphate and fluoride. Process Saf. Environ. Prot. 2022, 159, 708–715. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Yin, C.; Chen, X.; Liu, X.; Chen, A.; Li, J.; He, L.; Sun, F.; Zhao, Z. Selectively extracting H2WO4 of high purity from the H2SO4 decomposition product of scheelite through hydrogen peroxide coordination followed by purification using ion-exchange and solvent extraction and thermal decomposition. Hydrometallurgy 2023, 221, 106134. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, P.; Xia, L.; Chen, J.; Che, J.; Wang, C.; Ma, B. Understanding the role of hydrogen peroxide in sulfuric acid system for leaching low-grade scheelite from the perspective of phase transformation and kinetics. Sep. Purif. Technol. 2021, 277, 119407. [Google Scholar] [CrossRef]
- He, G.; Zhao, Z.; Wang, X.; Li, J.; Chen, X.; He, L.; Liu, X. Leaching kinetics of scheelite in hydrochloric acid solution containing hydrogen peroxide as complexing agent. Hydrometallurgy 2014, 144–145, 140–147. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z. Kinetics of scheelite concentrate digestion with sulfuric acid in the presence of phosphoric acid. Hydrometallurgy 2016, 163, 55–60. [Google Scholar] [CrossRef]
- Cao, C.; Qiu, X.; Li, Y.; Yang, L.; Pang, Z.; Yuan, Z. Study on leaching behaviour of tungstates in acid solution containing phosphoric acid. Hydrometallurgy 2020, 197, 105392. [Google Scholar] [CrossRef]
- Gürmen, S.; Tımur, S.; Arslan, C.; Duman, I. Acidic leaching of scheelite concentrate and production of hetero-poly-tungstate salt. Hydrometallurgy 1999, 51, 227–238. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, Z.; Ma, R.; Wang, P.; Shu, Y.; He, J. A new pathway for preparing ultrafine tungsten powder via non-contact carbon reduction followed by hydrogen reduction. Mater. Lett. 2024, 368, 136682. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, B.; Zheng, S.; Wu, H.; Chen, N.; Wang, D. Emerging activated tungsten dust: Source, environmental behaviors, and health effects. Environ. Int. 2024, 188, 108774. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huo, G.; Guo, X.; Wang, Q. Sustainable extraction of tungsten from the acid digestion product of tungsten concentrate by leaching-solvent extraction together with raffinate recycling. J. Clean. Prod. 2022, 375, 133924. [Google Scholar] [CrossRef]
- Martins, J.I.; Moreira, A.; Costa, S.C. Leaching of synthetic scheelite by hydrochloric acid without the formation of tungstic acid. Hydrometallurgy 2003, 70, 131–141. [Google Scholar] [CrossRef]
- Kahruman, C.; Yusufoglu, I. Leaching kinetics of synthetic CaWO4 in HCl solutions containing H3PO4 as chelating agent. Hydrometallurgy 2006, 81, 182–189. [Google Scholar] [CrossRef]
- Jin, H.; Fanghao, X.; Haijian, X.; Youming, Y. Coordination Leaching of Scheelite Ore by HCl-H3PO4. Chin. J. Rare Met. 2014, 38, 703–710. [Google Scholar] [CrossRef]
- Xuin, G.H.; Yu, D.Y.; Su, Y.F. Leaching of scheelite by hydrochloric acid in the presence of phosphate. Hydrometallurgy 1986, 16, 27–40. [Google Scholar] [CrossRef]
- Chen, Y.; Huo, G.; Guo, X.; Wang, Q. A sustainable process for tungsten extraction from wolframite concentrate. Int. J. Refract. Met. Hard Mater. 2022, 107, 105903. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Che, J.; Wang, C.; Ma, B. Green leaching of tungsten from synthetic scheelite with sulfuric acid-hydrogen peroxide solution to prepare tungstic acid. Sep. Purif. Technol. 2020, 241, 116752. [Google Scholar] [CrossRef]
- Gong, C.; Chen, X.; Liu, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. The dissolution behavior of different forms of tungstic acid in hydrogen peroxide. Hydrometallurgy 2021, 202, 105598. [Google Scholar] [CrossRef]
- Yin, C.; Ji, L.; Chen, X.; Liu, X.; Zhao, Z. Efficient leaching of scheelite in sulfuric acid and hydrogen peroxide solution. Hydrometallurgy 2020, 192, 105292. [Google Scholar] [CrossRef]
- Wang, H.; Liu, P.; Chen, X.; Liu, X.; Chen, A.; Li, J.; He, L.; Sun, F.; Zhao, Z. Efficient dissolution of tungstic acid by isopolytungstate solution based on the polymerization theory of tungsten. Hydrometallurgy 2022, 209, 105835. [Google Scholar] [CrossRef]
- Ting-Ting, L.I.; Shen, Y.B.; Zhao, S.K.; Yin, Y.Y.; Wei, D.Z. Leaching kinetics of scheelite concentrate with sodium hydroxide in the presence of phosphate. Trans. Nonferrous Met. Soc. China 2019, 29, 634–640. [Google Scholar] [CrossRef]
- Potashnikov, Y.M.; Gamol’Skii, A.M.; Mokhosoev, M.V.; Kozlova, F.M. Kinetics of the dissolution of calcium tungstate in oxalate acid solution. Zh. Neorg. Khim 1970, 15, 502–508. [Google Scholar]
- Kalpakli, A.O.; Ilhan, S.; Kahruman, C.; Yusufoglu, I. Dissolution behavior of calcium tungstate in oxalic acid solutions. Hydrometallurgy 2012, 121–124, 7–15. [Google Scholar] [CrossRef]
- Liu, Q.S.; Liu, Q.S.; Tao, T.U.; Tao, T.U.; Guo, H.; Guo, H.; Cheng, H.J.; Cheng, H.J.; Wang, X.Z.; Wang, X.Z. Complexation extraction of scheelite and transformation behaviour of tungsten-containing phase using H2SO4 solution with H2C2O4 as complexing agent. Trans. Nonferrous Met. Soc. China 2021, 31, 3150–3161. [Google Scholar] [CrossRef]
- Li, J.T.; Gao, L.L.; Zhao, Z.W.; Liu, X.H.; Chen, X.Y. Efficient recovery of tungsten from scheelite concentrates using a sulfur-phosphorus mixed acid leaching system. Tungsten 2024, 6, 821–832. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Q.; Guo, F.; Liu, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Extraction and separation of W and rare earth from H2SO4-H3PO4 mixed acid leaching liquor of scheelite by primary amine N1923. Sep. Purif. Technol. 2021, 257, 117908. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Wang, X.; Li, H.; Chen, Y.; Wu, W. Recovery of tungsten and titanium from spent SCR catalyst by sulfuric acid leaching process. Waste Manag. 2023, 155, 338–347. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Zhao, Z. Leaching kinetics of scheelite with nitric acid and phosphoric acid. Int. J. Refract. Met. Hard Mater. 2015, 52, 78–84. [Google Scholar] [CrossRef]
- Zhang, W.J.; Yang, J.H.; Zhao, Z.W.; Wang, W.Q.; Li, J.T. Coordination leaching of tungsten from scheelite concentrate with phosphorus in nitric acid. J. Cent. South Univ. 2016, 23, 1312–1317. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, L.; Wu, S.; Qing, J.; Li, Q.; Cao, Z.; Wang, M.; Zhang, G.; Guan, W. Eco-friendly extraction of arsenic and tungsten from hazardous tungsten residue waste by pressure oxidation leaching in alkaline solutions: Mechanism and kinetic model. J. Environ. Manag. 2023, 325, 116586. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.I.; Lima, J.L.F.C.; Moreira, A.; Costa, S.C. Tungsten recovery from alkaline leach solutions as synthetic scheelite. Hydrometallurgy 2007, 85, 110–115. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, Y.; Li, H. Kinetics of sodium hydroxide leaching of scheelite. Int. J. Refract. Met. Hard Mater. 2011, 29, 289–292. [Google Scholar] [CrossRef]
- Liu, L.; Xue, J.; Liu, K.; Zhu, J.; Wang, Z. Complex Leaching Process of Scheelite in Hydrochloric and Phosphoric Solutions. JOM 2016, 68, 2455–2462. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, Y.; Liu, X.; Chen, A.; Li, H. Sodium hydroxide digestion of scheelite by reactive extrusion. Int. J. Refract. Met. Hard Mater. 2011, 29, 739–742. [Google Scholar] [CrossRef]
- Luo, L.; Miyazaki, T.; Shibayama, A.; Yen, W.; Fujita, T. A novel process for recovery of tungsten and vanadium from a leach solution of tungsten alloy scrap. Miner. Eng. 2003, 16, 665–670. [Google Scholar] [CrossRef]
- Tingting, L.; Xiangxi, Z.; Wei, Z.; Jin, Z.; Yanbai, S.; Dezhou, W. Technology Status and Development Trend of Scheelite Leaching Process. Met. Mine 2017, 10, 128–134. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Liu, X.; Chen, X.; Zhao, Z. Sustainable and Efficient Recovery of Tungsten from Wolframite in a Sulfuric Acid and Phosphoric Acid Mixed System. ACS Sustain. Chem. Eng. 2020, 8, 13583–13592. [Google Scholar] [CrossRef]
- Spooren, J.; Wouters, W.; Michielsen, B.; Seftel, E.M.; Koelewijn, S.-F. Microwave-assisted alkaline fusion followed by water-leaching for the selective extraction of the refractory metals tungsten, niobium and tantalum from low-grade ores and tailings. Miner. Eng. 2024, 217, 108963. [Google Scholar] [CrossRef]
- Yang, J.-H.; He, L.-H.; Liu, X.-H.; Ding, W.-T.; Song, Y.-F.; Zhao, Z.-W. Comparative kinetic analysis of conventional and ultrasound-assisted leaching of scheelite by sodium carbonate. Trans. Nonferrous Met. Soc. China 2018, 28, 775–782. [Google Scholar] [CrossRef]
- Boisvert, J.P.; To, T.C.; Berrak, A.; Jolicoeur, C. Phosphate adsorption in flocculation processes of aluminium sulphate and poly-aluminium-silicate-sulphate. Water Res. 1997, 31, 1939–1946. [Google Scholar] [CrossRef]
- Galarneau, E.; Gehr, R. Phosphorus removal from wastewaters: Experimental and theoretical support for alternative mechanisms. Water Res. 1997, 31, 328–338. [Google Scholar] [CrossRef]
- Srinivas, K.; Sreenivas, T.; Natarajan, R.; Padmanabhan, N.P.H. Studies on the recovery of tungsten from a composite wolframite–scheelite concentrate. Hydrometallurgy 2000, 58, 43–50. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Z.; Chen, X. The behaviour of phosphorus impurities in the novel selective precipitation process. Hydrometallurgy 2013, 139, 111–115. [Google Scholar] [CrossRef]
- He, G.X.; He, L.H.; Zhao, Z.W.; Chen, X.Y.; Gao, L.L.; Liu, X.H. Thermodynamic study on phosphorus removal from tungstate solution via magnesium salt precipitation method. Trans. Nonferrous Met. Soc. China 2013, 23, 3440–3447. [Google Scholar] [CrossRef]
- Osseo-Asare, K. Solution chemistry of tungsten leaching systems. Metall. Trans. B 1982, 13, 555–564. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Guo, X.-Y.; Wang, Q.-M.; Tian, Q.-H.; Zhang, J.-X.; Huang, S.-B. Physicochemical and environmental characteristics of alkali leaching residue of wolframite and process for valuable metals recovery. Trans. Nonferrous Met. Soc. China 2022, 32, 1638–1649. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Zhao, Z. Recovery of Tungsten and Molybdenum from Low-Grade Scheelite. JOM 2017, 69, 1958–1962. [Google Scholar] [CrossRef]
- Byun, S.Y.; Park, J.S.; Kang, J.H.; Seo, S.; Kim, M.J. Recovery of tungsten and cobalt from cemented tungsten carbide wastes using carbonate roasting and water leaching. J. Air Waste Manag. Assoc. 2021, 71, 711–720. [Google Scholar] [CrossRef]
- Zhang, G.; Guan, W.; Xiao, L.; Zhang, Q. A novel process for tungsten hydrometallurgy based on direct solvent extraction in alkaline medium. Hydrometallurgy 2016, 165, 233–237. [Google Scholar] [CrossRef]
- Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Luo, L.; Kejun, L.; Shibayama, A.; Yen, W.; Fujita, T.; Shindo, O.; Katai, A. Recovery of tungsten and vanadium from tungsten alloy scrap. Hydrometallurgy 2004, 72, 1–8. [Google Scholar] [CrossRef]
- Chen, W.; Huang, L.; Li, M.; Huang, Z.; Wang, H.; Liu, C.; Luo, X. From waste to wealth: Valuable metals recovered from waste tungsten leaching residue by a smelting reduction process using CaO-Fe capture agent. J. Clean. Prod. 2023, 422, 138629. [Google Scholar] [CrossRef]
- Li, M.; Huang, L.; Chen, W.; Huang, Z.; Wang, H.; Liu, C.; Luo, X.; Barati, M. Synergistic utilization of industrial solid wastes: Extraction of valuable metals from tungsten leaching residue by photovoltaic sawing waste. Waste Manag. 2024, 184, 10–19. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, B.; Wang, R.; Xu, S.; Wang, J.; Xu, Z. Extraction and separation of tungsten from acidic high-phosphorus solution. Hydrometallurgy 2016, 164, 97–102. [Google Scholar] [CrossRef]
- Wang, X.; Ge, Q. Separation and recovery of NaF from fluorine containing solution by the common ion effect of Na+. Heliyon 2018, 4, e01029. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Cao, C.-F.; Chen, X.-Y. Separation of macro amounts of tungsten and molybdenum by precipitation with ferrous salt. Trans. Nonferrous Met. Soc. China 2011, 21, 2758–2763. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z.; Cui, Y.; Li, J. Ca3WO6 prepared by roasting tungsten-containing materials and its leaching performance. Int. J. Refract. Met. Hard Mater. 2015, 52, 151–158. [Google Scholar] [CrossRef]
- Paulino, J.F.; Afonso, J.C.; Mantovano, J.L.; Vianna, C.A.; Cunha, J.W.S.D.d. Recovery of tungsten by liquid–liquid extraction from a wolframite concentrate after fusion with sodium hydroxide. Hydrometallurgy 2012, 127–128, 121–124. [Google Scholar] [CrossRef]
- Shen, L.-T.; Liu, Y.; Guo, J.-L.; Zhou, Q.-S.; Qi, T.-G.; Peng, Z.-H.; Liu, G.-H.; Li, X.-B. Leaching of WO3 from sulfuric acid converted product of scheelite in NH3·H2O−NH4HCO3 solution. Trans. Nonferrous Met. Soc. China 2025, 35, 326–337. [Google Scholar] [CrossRef]
- Kholmogorov, A.G.; Kononova, O.N.; Panchenko, O.N. A review of the use of ion exchange for molybdenum recovery in Russia. Can. Metall. Q. 2004, 43, 297–304. [Google Scholar] [CrossRef]
- Dodbiba, G.; Fujita, T.; Kikuchi, T.; Manjanna, J.; Matsuo, S.; Takahashi, H.; Tohji, K. Synthesis of iron-based adsorbents and their application in the adsorption of molybdenum ions in nitric acid solution. Chem. Eng. J. 2011, 166, 496–503. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, F.; Hu, Y.; Wang, S.; Sun, P.; Huo, G.; Li, H. Adsorption behaviour of WO42− onto 201 × 7 resin in highly concentrated tungstate solutions. Int. J. Refract. Met. Hard Mater. 2010, 28, 633–637. [Google Scholar] [CrossRef]
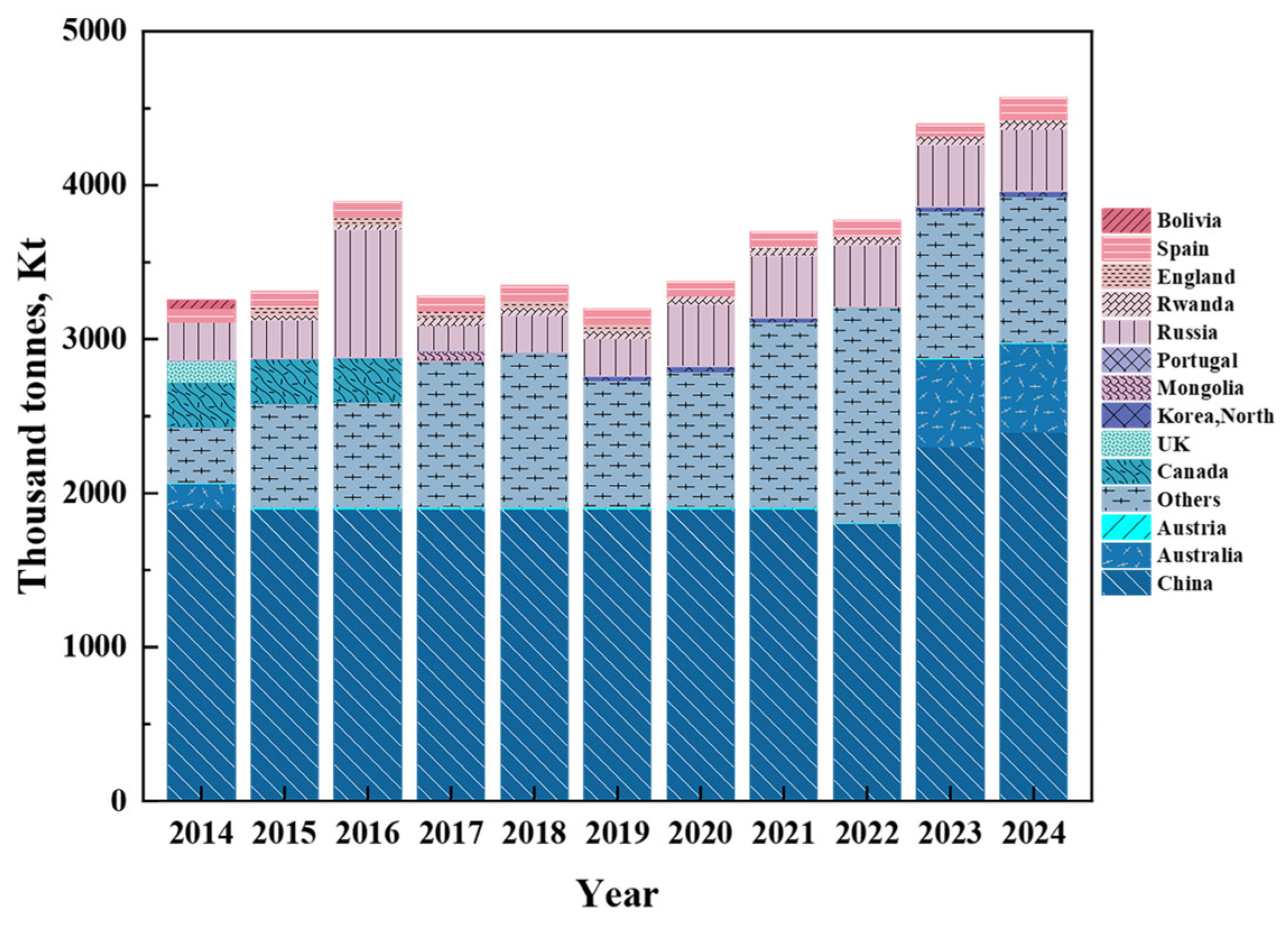
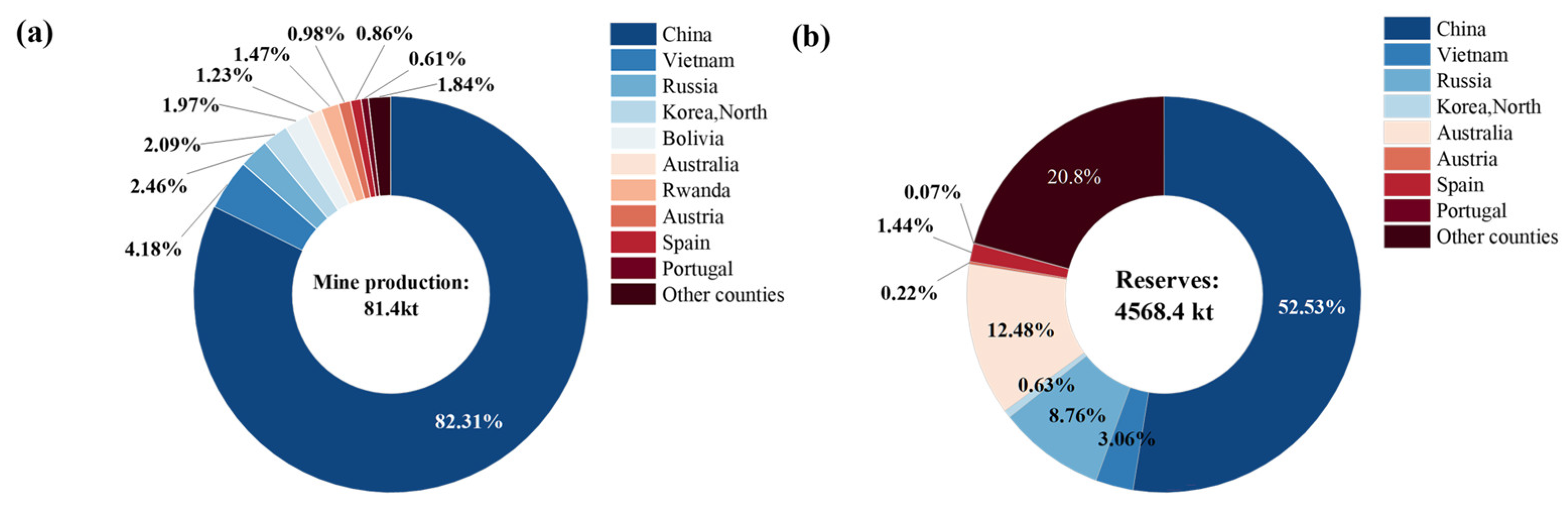




| Deposit Type | Associated Gangue Minerals | Associated Metallic Minerals | Deposit | Region | Resource Quantity (104 t) | Average Grade of WO3 (%) |
|---|---|---|---|---|---|---|
| Skarn | Garnet, diopside, fushanite, epidote, etc. | Shalcopyrite, pyrrhotite, molybdenite, galena, sphalerite, and other sulfides, as well as tin, wolframite, and so on. | Zhuxi tungsten mine | Jiangxi, China | >100 | 0.64 |
| Malipo tungsten mine | Yunnan, China | 53 | 0.43 | |||
| Sandaozhuang molybdenum–tungsten | Henan, China | 42 | 0.12 | |||
| Mactung | Canada | 59 | 1.08 | |||
| Mittersill tungsten mine | Austria | 610 | 0.5 | |||
| Tmyauz | Russia | 504 | / | |||
| Quartz vein | Quartz, followed by feldspar, muscovite, biotite, fluorite, etc. | Molybdenum, pyrite, arsenopyrite, cassiterite, and other metal minerals symbiosis; some deposits contain natural bismuth and niobium tantalum minerals. | Shizhuyuan polymetallic mine | Hunan, China | 71 | 0.34 |
| Hemerdon | United Kingdom | 401 | 0.19 | |||
| Verkhne-Kayrakt | Russia | 62 | / | |||
| Mt. Carbine | Australia | 223 | 0.12 | |||
| Porphyry | Quartz, potassium feldspar, sericite, chlorite, fluorite, etc. | Molybdenite, chalcopyrite, pyrite, galena, sphalerite, etc.; some deposits contain cassiterite or rare metal minerals. | Xintianling tungsten–molybdenum–mismuth mine | Hunan, China | 32 | 0.37 |
| Xinluokeng tungsten mine | Fujian, China | 30 | 0.23 | |||
| Pegmatite | Quartz, potassium feldspar, albite, muscovite, spodumene, beryl, niobium tantalite, tourmaline and so on. | Cassiterite and molybdenite metal minerals; some deposits contain natural bismuth, bismuth sulfide minerals, or rare earth minerals. | Dahuting tungsten mine | Jiangxi, China | 106 | 0.2 |
| Other type | / | / | Chuangkou tungsten mine | Hunan, China | 33 | / |
| Jerseyemerald | Canada | 504 | / | |||
| Logtung | Canada | 401 | / | |||
| Sisson | Canada | 334 | / | |||
| Northern Dancer | Canada | 223 | 0.1 | |||
| Laparilla | Spain | 334 | / | |||
| Dolphin | Australia | 87 | 0.55 | |||
| Nuiphao | Vietnam | 87 | / |
| Method | Main Process Parameters | Leaching Recovery | References | |
|---|---|---|---|---|
| Alkaline leaching process | Na2CO3 high-pressure leaching | Temperature: 200–230 °C, L/S: 5 mL/g, 2–3 times NaCO3 dosage, reaction: 5h. | 95%–98% | [40] |
| NaOH high-pressure leaching | Temperature: 180 °C, L/S: 0.8 mL/g, 2.5 times NaOH, stirring speed: 400 rpm, reaction: 2 h. | 98% | [41] | |
| Acid leaching process | HCl leaching | Temperature: 28–100 °C, pH: 1.5–3. | 99% | [42] |
| H2SO4 leaching | Temperature: 100 °C, mineral particle size range: 32–44 μm, stirring speed: 400 rpm, L/S: 0.8 mL/g, sulfuric acid concentration: 3 mol/L. | 90% | [27] | |
| Sintering–leaching process | Na2CO3 sintering–H2O leaching | Sintering temperature: 800 °C, 3 times Na2CO3, 2 h; Leaching temperature: 90 °C, 4 mol NaOH. | 98% | [5] |
| Na3PO4-CaF2 mixed leaching process | Temperature: 180 °C, 1.6 times of the theoretical dosage of Na3PO4, 2 times of the theoretical dosage of CaF2, initial NH3·H2O concentration 30 g/L, L/S: 2 mL/g, reaction: 3h. | 98% | [43] | |
| Temperature: 180 °C, 2 times the theoretical amount of Na3PO4, 1.5 times the theoretical amount of CaF2, 14% NaOH, L/S: 4 mL/g. | 98% | [44] | ||
| Temperature: 16 °C, L/S: 2 mL/g, stirring speed: 350 rpm, ore: CaF2 = 21.25, 1 mol/L Na3PO4, 1.67 h. | 98% | [45] | ||
| New leaching process | H2SO4–H2O2 leaching | Temperature: 45–50 °C, H2SO4 concentration: 2–3 mol/L, H2O2 concentration: 1.5–2.5 mol/L, L/S: 6–10 mL/g, reaction: 60–90 min; Temperature: 90 °C, reaction: 4 h. | 80%–99% | [46,47,48] |
| H2SO4–H3PO4 leaching | Temperature: 90 °C, 1.0–2.5mol/L H2SO4, 0.5–1mol/L H3PO4, L/S: 5–10 mL/g. | 99% | [27] | |
| HCl–H2O2 leaching | Temperature: 30 °C, 1.6 mol/L H2O2 and 2 mol/L HCl, stirring speed: 400 rpm, reaction: 3 h. | 79% | [48] | |
| Method | By-Products | Toxicity | Treatment |
|---|---|---|---|
| Na2CO3 high-pressure leaching | CaCO3 | Low toxicity | Used as building material, in soil improvement, and in the paper industry. |
| NaOH high-pressure leaching | Ca(OH)2 | Corrosive | Used in neutralization treatment, as building material, and in soil improvement. |
| Phosphate high-pressure leaching | Ca3(PO4)2 Ca5(PO4)3(OH) | Low toxicity | Used as fertilizer and in the production of H3PO4. |
| NaF high-pressure leaching | CaF2 | Low toxicity | Used in the production of hydrogen fluoride (HF), fluoride products, cement retarder, etc. |
| Na2CO3 sintering–H2O leaching | CaCO3 | Low toxicity | Used as building material, in soil improvement, and in the paper industry. |
| HCl leaching | CaCl2 | Low toxicity | At low concentrations, dilution may be considered for discharge. Alternatively, calcium chloride may be recovered by evaporation, concentration, and crystallization. |
| H2SO4 leaching | CaSO4 | Low toxicity | Used as gypsum building materials, in soil improvement, and in landfill disposal. |
| H2SO4-H2O2 leaching | CaSO4 | Low toxicity | |
| H2SO4-H3PO4 leaching | CaSO4 | Low toxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jiang, H.; Peng, Z.; Duan, A.; Zhang, T.; Gong, Z. Leaching of Scheelite Concentrate for Tungsten Extraction. Minerals 2025, 15, 475. https://doi.org/10.3390/min15050475
Li X, Jiang H, Peng Z, Duan A, Zhang T, Gong Z. Leaching of Scheelite Concentrate for Tungsten Extraction. Minerals. 2025; 15(5):475. https://doi.org/10.3390/min15050475
Chicago/Turabian StyleLi, Xinran, Hao Jiang, Zhiwei Peng, Anan Duan, Tong Zhang, and Zexi Gong. 2025. "Leaching of Scheelite Concentrate for Tungsten Extraction" Minerals 15, no. 5: 475. https://doi.org/10.3390/min15050475
APA StyleLi, X., Jiang, H., Peng, Z., Duan, A., Zhang, T., & Gong, Z. (2025). Leaching of Scheelite Concentrate for Tungsten Extraction. Minerals, 15(5), 475. https://doi.org/10.3390/min15050475








