Influences of Alkali-Carbonate Melt on the Electrical Conductivity of Dunite—Origin of the High Conductivity Anomaly Within the Tanzanian Cratonic Mantle
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Cause of High Conductivity in the Lithospheric Mantle of the Cratons
4.2. Cause of High Resistivity in the Lithospheric Mantle of Mozambique Mobile Belts/East African Rifts
5. Conclusions
- (1)
- The experiments revealed that the dunite samples containing Na2CO3 began to melt above 800 °C, leading to a rapid increase in electrical conductivity. A small amount of alkali-carbonate melt can increase the electrical conductivity of dunite by 1–2 orders of magnitude. Pure Na2CO3 started to melt above 1175 °C, reaching an electrical conductivity of 200–300 S/m. In partially molten Na2CO3-bearing peridotite samples, Na+ is the main charge carrier.
- (2)
- The carbon content of the lithospheric mantle of the Tanzanian craton is approximately 0.07 wt%, and the carbon content in the high conductivity layer is approximately 0.20 wt%. The carbon-rich phases at the bottom of the craton, such as alkali-rich carbonates, are more prone to melting due to the thermal influence of the deep mantle plume. Therefore, the presence of alkali-rich carbonate melt is the most likely and suitable mechanism to explain the high conductivity anomalies within the Archean Tanzanian craton.
- (3)
- The permeability barrier of the lithospheric mantle is the main reason why the electrical conductivity of the lithospheric mantle of the Tanzanian craton is 1–2 orders of magnitude higher than that of the lithospheric mantle of the Mozambique mobile belt, and it is also a prerequisite for the existence of a high conductivity layer within the Tanzanian craton.
- (4)
- The global average carbon content of the upper mantle is approximately 0.035 wt% [56]. In comparison, the Tanzanian craton is more carbon-rich. Carbon plays an important role in the evolution of cratons, as the presence of carbon-rich melts can disrupt the stability of cratons and cause thinning of the cratonic lithosphere. Our explanation for the causes of high conductivity in cratons and high resistivity in active zones also indirectly explains why the lithosphere of the Tanzanian craton is currently only about 135 km thick [31].
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MT | Magnetotelluric |
| En | Enstatite |
| Mag | Magnesite |
| Fo | Forsterite |
| Dol | Dolomite |
| Di | Diopside |
References
- Evans, R.L.; Jones, A.G.; Garcia, X.; Muller, M.; Hamilton, M.; Evans, S.; Fourie, C.J.S.; Spratt, J.; Webb, S.; Jelsma, H.; et al. Electrical lithosphere beneath the Kaapvaal craton, southern Africa. J. Geophys. Res.-Solid Earth 2011, 116. [Google Scholar] [CrossRef]
- Miensopust, M.P.; Jones, A.G.; Muller, M.R.; Garcia, X.; Evans, R.L. Lithospheric structures and Precambrian terrane boundaries in northeastern Botswana revealed through magnetotelluric profiling as part of the Southern African Magnetotelluric Experiment. J. Geophys. Res.-Solid Earth 2011, 116. [Google Scholar] [CrossRef]
- Bologna, M.S.; Dragone, G.N.; Muzio, R.; Peel, E.; Nuñez-Demarco, P.; Ussami, N. Electrical Structure of the Lithosphere From Rio de la Plata Craton to Parana Basin: Amalgamation of Cratonic and Refertilized Lithospheres in SW Gondwanaland. Tectonics 2019, 38, 77–94. [Google Scholar] [CrossRef]
- Azeez, K.K.A.; Veeraswamy, K.; Gupta, A.K.; Babu, N.; Chandrapuri, S.; Harinarayana, T. The electrical resistivity structure of lithosphere across the Dharwar craton nucleus and Coorg block of South Indian shield: Evidence of collision and modified and preserved lithosphere. J. Geophys. Res.-Solid Earth 2015, 120, 6698–6721. [Google Scholar] [CrossRef]
- Bologna, M.S.; Padilha, A.L.; Vitorello, I.; Pádua, M.B. Signatures of continental collisions and magmatic activity in central Brazil as indicated by a magnetotelluric profile across distinct tectonic provinces. Precambrian Res. 2011, 185, 55–64. [Google Scholar] [CrossRef]
- Krueger, H.E.; Gama, I.; Fischer, K.M. Global Patterns in Cratonic Mid-Lithospheric Discontinuities From Sp Receiver Functions. Geochem. Geophys. Geosyst. 2021, 22, e2021GC009819. [Google Scholar] [CrossRef]
- Selway, K.; Ford, H.; Kelemen, P. The seismic mid-lithosphere discontinuity. Earth Planet. Sci. Lett. 2015, 414, 45–57. [Google Scholar] [CrossRef]
- Wölbern, I.; Rümpker, G.; Link, K.; Sodoudi, F. Melt infiltration of the lower lithosphere beneath the Tanzania craton and the Albertine rift inferred from S receiver functions. Geochem. Geophys. Geosyst. 2012, 13. [Google Scholar] [CrossRef]
- Bettac, S.P.; Unsworth, M.J.; Pearson, D.G.; Craven, J. New constraints on the structure and composition of the lithospheric mantle beneath the Slave craton, NW Canada from 3-D magnetotelluric data-Origin of the Central Slave Mantle Conductor and possible evidence for lithospheric scale fluid flow. Tectonophysics 2023, 851, 229760. [Google Scholar] [CrossRef]
- Jones, A.G.; Lezaeta, P.; Ferguson, I.J.; Chave, A.D.; Evans, R.L.; Garcia, X.; Spratt, J. The electrical structure of the Slave craton. Lithos 2003, 71, 505–527. [Google Scholar] [CrossRef]
- Özaydin, S.; Selway, K.; Foley, S.F.; Ezad, I.S.; Griffin, W.L.; Tarits, P.S.; Hautot, S. Role of Metasomatism in the Development of the East African Rift at the Northern Tanzanian Divergence: Insights From 3D Magnetotelluric Modeling. Geochem. Geophys. Geosyst. 2024, 25, e2023GC011191. [Google Scholar] [CrossRef]
- Selway, K. Negligible effect of hydrogen content on plate strength in East Africa. Nat. Geosci. 2015, 8, 543–546. [Google Scholar] [CrossRef]
- Thiel, S.; Heinson, G. Electrical conductors in Archean mantle—Result of plume interaction? Geophys. Res. Lett. 2013, 40, 2947–2952. [Google Scholar] [CrossRef]
- Wang, D.; Mookherjee, M.; Xu, Y.; Karato, S.-I. The effect of water on the electrical conductivity of olivine. Nature 2006, 443, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dai, L.; Li, H.; Sun, W.; Li, B. Effect of dehydrogenation on the electrical conductivity of Fe-bearing amphibole: Implications for high conductivity anomalies in subduction zones and continental crust. Earth Planet. Sci. Lett. 2018, 498, 27–37. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Yu, J.H.; Cai, Y.F. Unusually high electrical conductivity of phlogopite: The possible role of fluorine and geophysical implications. Contrib. Mineral. Petrol. 2016, 171, 37. [Google Scholar] [CrossRef]
- Zhang, B.; Yoshino, T. Effect of graphite on the electrical conductivity of the lithospheric mantle. Geochem. Geophys. Geosyst. 2017, 18, 23–40. [Google Scholar] [CrossRef]
- Watson, H.C.; Roberts, J.J.; Tyburczy, J.A. Effect of conductive impurities on electrical conductivity in polycrystalline olivine. Geophys. Res. Lett. 2010, 37, L02302. [Google Scholar] [CrossRef]
- Özaydin, S.; Selway, K. MATE: An Analysis Tool for the Interpretation of Magnetotelluric Models of the Mantle. Geochem. Geophys. Geosyst. 2020, 21, e2020GC009126. [Google Scholar] [CrossRef]
- Hasterok, D.; Chapman, D.S. Heat production and geotherms for the continental lithosphere. Earth Planet. Sci. Lett. 2011, 307, 59–70. [Google Scholar] [CrossRef]
- Dawson, J.B.; Garson, M.S.; Roberts, B. Altered former alkalic carbonatite lava from Oldoinyo Lengai, Tanzania: Inferences for calcite carbonatite lavas. Geology 1987, 15, 765–768. [Google Scholar] [CrossRef]
- Dawson, J.B. Sodium carbonate lavas from Oldoinyo Lengai. Nature 1962, 195, 1075–1076. [Google Scholar] [CrossRef]
- Foley, S.F.; Link, K.; Tiberindwa, J.V.; Barifaijo, E. Patterns and origin of igneous activity around the Tanzanian craton. J. Afr. Earth Sci. 2012, 62, 1–18. [Google Scholar] [CrossRef]
- Foley, S.F.; Fischer, T.P. An essential role for continental rifts and lithosphere in the deep carbon cycle. Nat. Geosci. 2017, 10, 897–902. [Google Scholar] [CrossRef]
- Dawson, J.B. Quaternary kimberlitic volcanism on the Tanzania craton. Contrib. Mineral. Petrol. 1994, 116, 473–485. [Google Scholar] [CrossRef]
- Russell, J.K.; Porritt, L.A.; Lavallee, Y.; Dingwell, D.B. Kimberlite ascent by assimilation-fuelled buoyancy. Nature 2012, 481, 352–356. [Google Scholar] [CrossRef]
- Foley, S.F.; Yaxley, G.M.; Kjarsgaard, B.A. Kimberlites from Source to Surface: Insights from Experiments. Elements 2019, 15, 393–398. [Google Scholar] [CrossRef]
- Selway, K. Electrical Discontinuities in the Continental Lithosphere Imaged with Magnetotellurics. In Lithospheric Discontinuities; Yuan, H., Romanowicz, B., Eds.; Geophysical Monograph Book Series; AGU: Washington, DC, USA, 2019; Volume 239, pp. 89–109. [Google Scholar]
- O’Donnell, J.P.; Adams, A.; Nyblade, A.A.; Mulibo, G.D.; Tugume, F. The uppermost mantle shear wave velocity structure of eastern Africa from Rayleigh wave tomography: Constraints on rift evolution. Geophys. J. Int. 2013, 194, 961–978. [Google Scholar] [CrossRef]
- Celli, N.L.; Lebedev, S.; Schaeffer, A.J.; Gaina, C. African cratonic lithosphere carved by mantle plumes. Nat. Commun. 2020, 11, 92. [Google Scholar] [CrossRef]
- Boyce, A.; Bastow, I.D.; Cottaar, S.; Kounoudis, R.; De Courbeville, J.G.; Caunt, E.; Desai, S. AFRP20: New P-Wavespeed Model for the African Mantle Reveals Two Whole-Mantle Plumes Below East Africa and Neoproterozoic Modification of the Tanzania Craton. Geochem. Geophys. Geosyst. 2021, 22, e2020GC009302. [Google Scholar] [CrossRef]
- Afonso, J.C.; Ben-Mansour, W.; O’Reilly, S.Y.; Griffin, W.L.; Salajeghegh, F.; Foley, S.; Begg, G.; Selway, K.; Macdonald, A.; Januszczak, N.; et al. Thermochemical structure and evolution of cratonic lithosphere in central and southern Africa. Nat. Geosci. 2022, 15, 405–410. [Google Scholar] [CrossRef]
- Lee, C.-T.; Rudnick, R. Compositionally stratified cratonic lithosphere and Geochemistry of peridototic xenoliths from the Labait volcano Tanzania. In Proceedings of the 7th International Kimberlite Conference, Cape Town, South Africa, 11–17 April 1998; Gurney, J.J., Richardson, S.R., Eds.; 1999; pp. 503–521. Available online: https://www.researchgate.net/publication/290162967 (accessed on 27 April 2025).
- Lloyd, F.E.; Bailey, D.K. Light element metasomatism of the continental mantle: The evidence and the consequences. Phys. Chem. Earth 1975, 9, 389–416. [Google Scholar] [CrossRef]
- Foley, S.F.; Jacob, D.E.; O’Neill, H.S.C. Trace element variations in olivine phenocrysts from Ugandan potassic rocks as clues to the chemical characteristics of parental magmas. Contrib. Mineral. Petrol. 2011, 162, 1–20. [Google Scholar] [CrossRef]
- Rosenthal, A.; Foley, S.F.; Pearson, D.G.; Nowell, G.M.; Tappe, S. Petrogenesis of strongly alkaline primitive volcanic rocks at the propagating tip of the western branch of the East African Rift. Earth Planet. Sci. Lett. 2009, 284, 236–248. [Google Scholar] [CrossRef]
- Giuliani, A.; Kamenetsky, V.S.; Phillips, D.; Kendrick, M.A.; Wyatt, B.A.; Goemann, K. Nature of alkali-carbonate fluids in the sub-continental lithospheric mantle. Geology 2012, 40, 967–970. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Tarasov, A.A.; Dymshits, A.M.; Kovaleva, E. Confocal Raman spectroscopic study of melt inclusions in olivine of mantle xenoliths from the Bultfontein kimberlite pipe (Kimberley cluster, South Africa): Evidence for alkali-rich carbonate melt in the mantle beneath Kaapvaal Craton. J. Raman Spectrosc. 2022, 53, 508–524. [Google Scholar] [CrossRef]
- Shen, K.; Wang, D.; Zhang, R.; Chen, G.; Han, K.; Chen, P.; Zhang, R.; Zhang, Z. Amphibole’s influence on mid-lithosphere discontinuity: Insights from electrical conductivity. Geophys. Res. Lett. 2025, 52, e2024GL113651. [Google Scholar] [CrossRef]
- Yoshino, T.; Laumonier, M.; McIsaac, E.; Katsura, T. Electrical conductivity of basaltic and carbonatite melt-bearing peridotites at high pressures: Implications for melt distribution and melt fraction in the upper mantle. Earth Planet. Sci. Lett. 2010, 295, 593–602. [Google Scholar] [CrossRef]
- Yoshino, T.; Gruber, B.; Reinier, C. Effects of pressure and water on electrical conductivity of carbonate melt with implications for conductivity anomaly in continental mantle lithosphere. Phys. Earth Planet. Inter. 2018, 281, 8–16. [Google Scholar] [CrossRef]
- Podborodnikov, I.V.; Shatskiy, A.; Arefiev, A.V.; Chanyshev, A.D.; Litasov, K.D. The system Na2CO3-MgCO3 at 3GPa. High Press. Res. 2018, 38, 281–292. [Google Scholar] [CrossRef]
- Bekhtenova, A.; Shatskiy, A.; Podborodnikov, I.V.; Are, A.V.; Litasov, K.D. Phase relations in carbonate component of carbonatized eclogite and peridotite along subduction and continental geotherms. Gondwana Res. 2021, 94, 186–200. [Google Scholar] [CrossRef]
- Holtzman, B.K. Questions on the existence, persistence, and mechanical effects of a very small melt fraction in the asthenosphere. Geochem. Geophys. Geosyst. 2016, 17, 470–484. [Google Scholar] [CrossRef]
- Xu, Y.; Shankland, T.J.; Duba, A.G. Pressure effect on electrical conductivity of mantle olivine. Phys. Earth Planet. Inter. 2000, 118, 149–161. [Google Scholar] [CrossRef]
- Sifré, D.; Hashim, L.; Gaillard, F. Effects of temperature, pressure and chemical compositions on the electrical conductivity of carbonated melts and its relationship with viscosity. Chem. Geol. 2015, 418, 189–197. [Google Scholar] [CrossRef]
- Yoshino, T.; McIsaac, E.; Laumonier, M.; Katsura, T. Electrical conductivity of partial molten carbonate peridotite. Phys. Earth Planet. Inter. 2012, 194–195, 1–9. [Google Scholar] [CrossRef]
- Rader, E.; Emry, E.; Schmerr, N.; Frost, D.; Cheng, C.; Menard, J.; Yu, C.Q.; Geist, D. Characterization and Petrological Constraints of the Midlithospheric Discontinuity. Geochem. Geophys. Geosyst. 2015, 16, 3484–3504. [Google Scholar] [CrossRef]
- Rudnick, R.; McDonough, W.; Orpin, A. Northern Tanzanian peridotite xenoliths: A comparison with Kaapvaal peridotites and inferences on metasomatic interactions. In Kimberlites, Related Rocks and Mantle Xenoliths. Meyer, H.O.A., Leonardos, O., Eds.; Proceeding of the Fifth International Kimberlite Conference, Brasilia. 1994, Volume 1, pp. 336–353. Available online: https://www.researchgate.net/publication/280948731 (accessed on 27 April 2025).
- Dasgupta, R.; Mallik, A.; Tsuno, K.; Withers, A.C.; Hirth, G.; Hirschmann, M.M. Carbon-dioxide-rich silicate melt in the Earth’s upper mantle. Nature 2013, 493, 211–215. [Google Scholar] [CrossRef]
- O’Leary, J.A.; Gaetani, G.A.; Hauri, E.H. The effect of tetrahedral Al3+ on the partitioning of water between clinopyroxene and silicate melt. Earth Planet. Sci. Lett. 2010, 297, 111–120. [Google Scholar] [CrossRef]
- Falloon, T.J.; Green, D.H. The solidus of carbonated, fertile peridotite. Earth Planet. Sci. Lett. 1989, 94, 364–370. [Google Scholar] [CrossRef]
- Selway, K.; Yi, J.; Karato, S.I. Water content of the Tanzanian lithosphere from magnetotelluric data: Implications for cratonic growth and stability. Earth Planet. Sci. Lett. 2014, 388, 175–186. [Google Scholar] [CrossRef]
- Minarik, W.G.; Watson, E.B. Interconnectivity of carbonate melt at low melt fraction. Earth Planet. Sci. Lett. 1995, 133, 423–437. [Google Scholar] [CrossRef]
- Heaman, L.M.; Kjarsgaard, B.A.; Creaser, R.A. The timing of kimberlite magmatism in North America: Implications for global kimberlite genesis and diamond exploration. Lithos 2003, 71, 153–184. [Google Scholar] [CrossRef]
- Aiuppa, A.; Casetta, F.; Coltorti, M.; Stagno, V.; Tamburello, G. Carbon concentration increases with depth of melting in Earth’s upper mantle. Nat. Geosci. 2021, 14, 697–703. [Google Scholar] [CrossRef]
- Weiss, Y.; Czas, J.; Navon, O. Fluid Inclusions in Fibrous Diamonds. Rev. Mineral. Geochem. 2022, 88, 475–532. [Google Scholar] [CrossRef]
- Aulbach, S. Cratonic Lithosphere Discontinuities: Dynamics of Small-Volume Melting, Metacratonization, and a Possible Role for Brines. In Lithospheric Discontinuities; Yuan, H., Romanowicz, B., Eds.; Geophysical Monograph Book Series; AGU: Washington, DC, USA, 2019; Volume 239, pp. 177–203. [Google Scholar]
- Guo, H.H.; Keppler, H. Electrical Conductivity of NaCl-Bearing Aqueous Fluids to 900 °C and 5 GPa. J. Geophys. Res.-Solid Earth 2019, 124, 1397–1411. [Google Scholar] [CrossRef]
- Glover, P.W.J. A generalized Archie’s law for n phases. Geophysics 2010, 75, E247–E265. [Google Scholar] [CrossRef]
- Newton, R.C.; Sharp, W.E. Stability of forsterite + CO2 and its bearing on the role of CO2 in the mantle. Earth Planet. Sci. Lett. 1975, 26, 239–244. [Google Scholar] [CrossRef]
- Hirschmann, M.M. Mantle solidus: Experimental constraints and the effects of peridotite composition. Geochem. Geophys. Geosyst. 2000, 1. [Google Scholar] [CrossRef]
- Stamm, N.; Schmidt, M.W. Asthenospheric kimberlites: Volatile contents and bulk compositions at 7 GPa. Earth Planet. Sci. Lett. 2017, 474, 309–321. [Google Scholar] [CrossRef]
- Sparks, D.W.; Parmentier, E.M. Melt extraction from the mantle beneath spreading centers. Earth Planet. Sci. Lett. 1991, 105, 368–377. [Google Scholar] [CrossRef]
- Hammouda, T.; Laporte, D. Ultrafast mantle impregnation by carbonatite melts. Geology 2000, 28, 283–285. [Google Scholar] [CrossRef]
- Muirhead, J.D.; Fischer, T.P.; Oliva, S.J.; Laizer, A.; van Wijk, J.; Currie, C.A.; Lee, H.; Judd, E.J.; Kazimoto, E.; Sano, Y.; et al. Displaced cratonic mantle concentrates deep carbon during continental rifting. Nature 2020, 582, 67–72. [Google Scholar] [CrossRef] [PubMed]
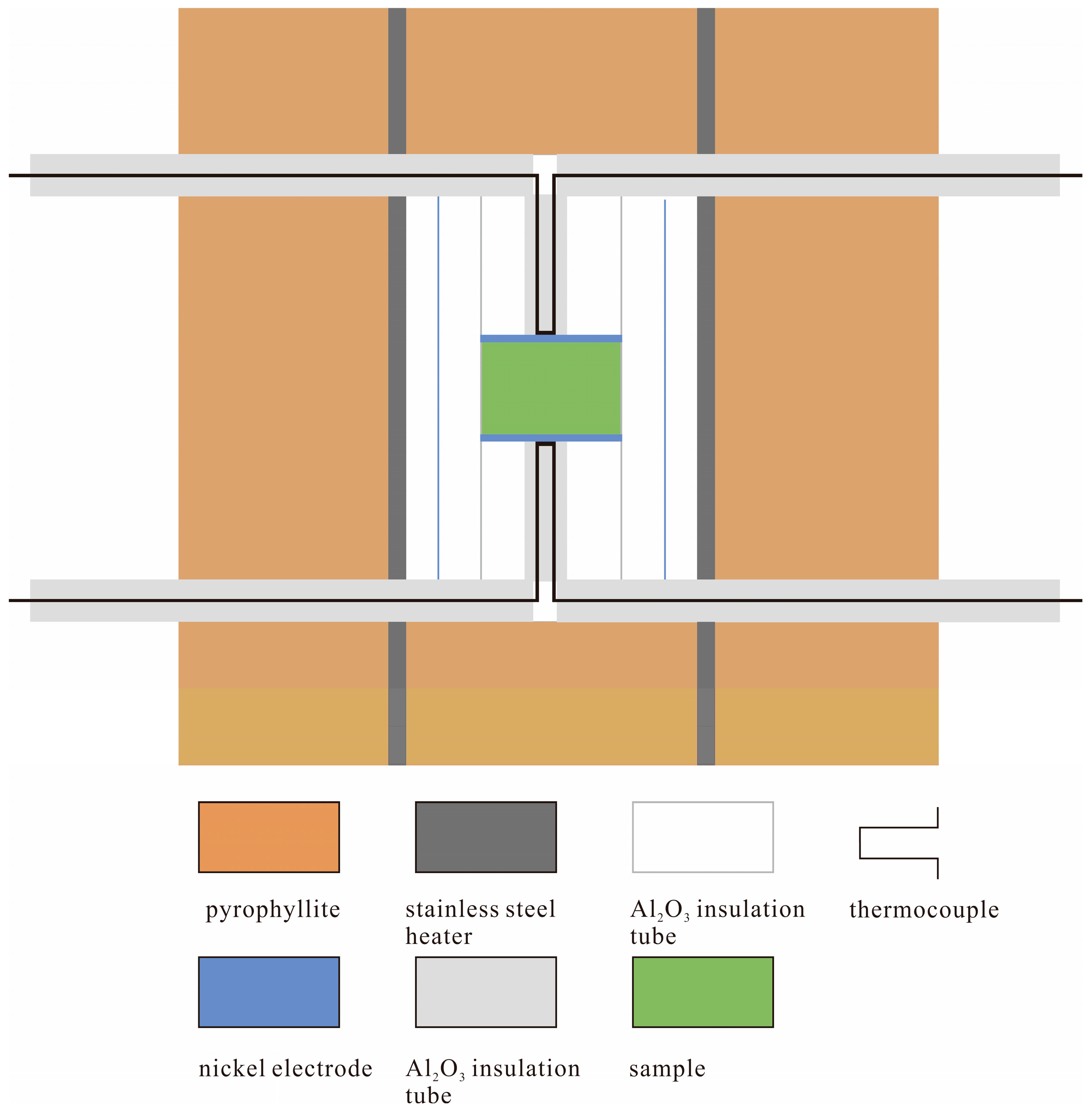


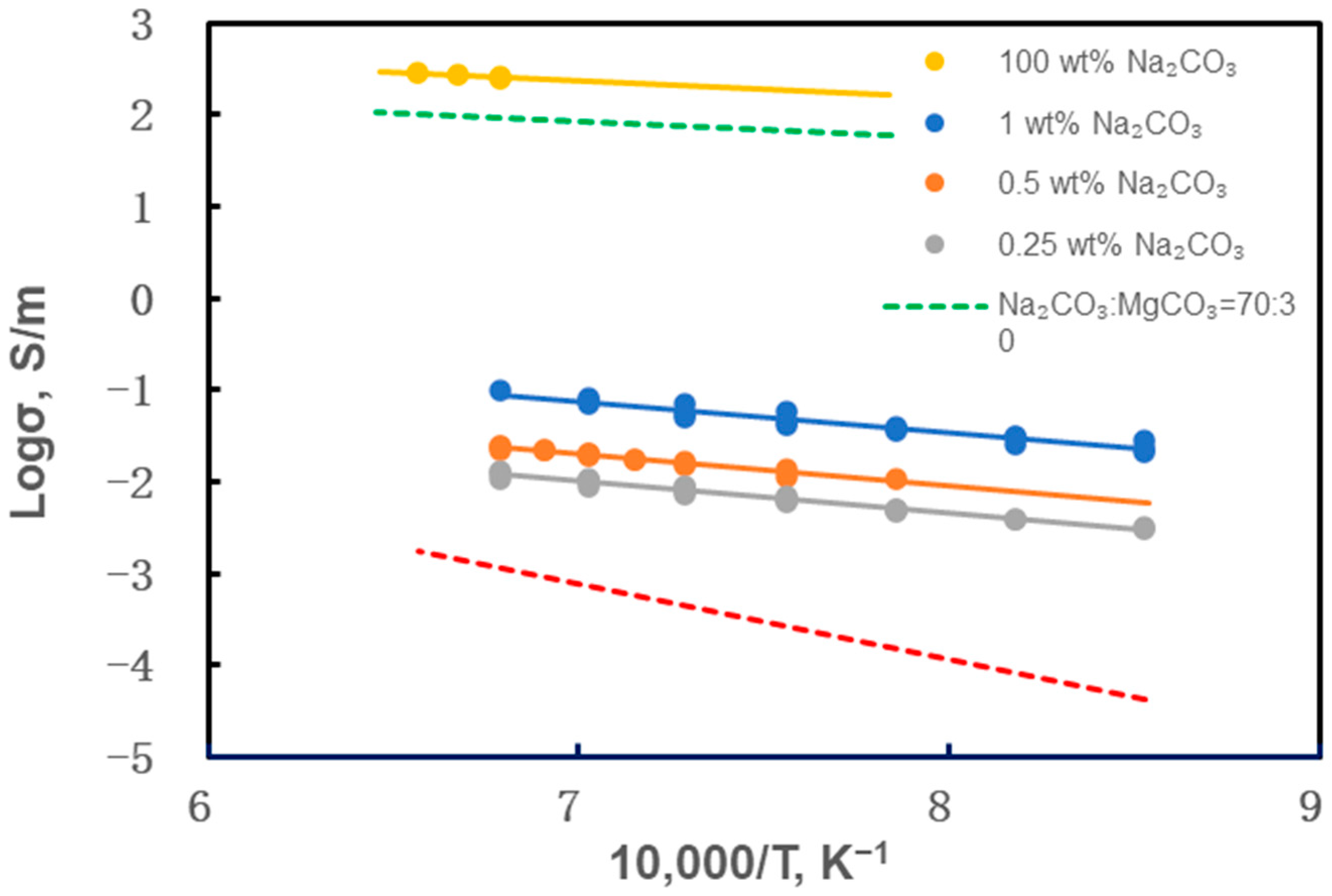



| Sample | P (GPa) | T (°C) | logσ0 (S/m) | H (kJ/mol) | R2 | Ref. |
|---|---|---|---|---|---|---|
| 0.25 wt% Na2CO3 | 3 | 900–1200 | 0.46 | 67.05 | 0.97 | This study |
| 0.5 wt% Na2CO3 | 3 | 1000–1200 | 0.70 | 65.64 | 0.95 | This study |
| 1 wt% Na2CO3 | 3 | 900–1200 | 1.26 | 65.22 | 0.94 | This study |
| 100 wt% Na2CO3 | 3 | 1200–1250 | 3.71 | 36.48 | 0.81 | This study |
| Na2CO3:MgCO3 = 70:30 | 3.4 | 1000–1427 | 3.20 | 34.73 | [41] | |
| Na2CO3:MgCO3 = 50:50 | 3 | 1050–1350 | 3.33 | 33.55 | [46] | |
| Dolomite | 3 | 1327–1527 | 3.13 | 38 | [47] | |
| San Carlos olivine | 4 | 1000–1400 | 2.69 | 159 | [45] |
| Conductivities | 75 km/760 °C | 100 km/938 °C | 130 km/1152 °C |
|---|---|---|---|
| σ max = 0.10 | 7.03 | 3.00 | 1.43 |
| σ median = 0.03 | 2.84 | 1.21 | 0.58 |
| σ min = 0.01 | 1.24 | 0.53 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Dai, W. Influences of Alkali-Carbonate Melt on the Electrical Conductivity of Dunite—Origin of the High Conductivity Anomaly Within the Tanzanian Cratonic Mantle. Minerals 2025, 15, 466. https://doi.org/10.3390/min15050466
Huang X, Dai W. Influences of Alkali-Carbonate Melt on the Electrical Conductivity of Dunite—Origin of the High Conductivity Anomaly Within the Tanzanian Cratonic Mantle. Minerals. 2025; 15(5):466. https://doi.org/10.3390/min15050466
Chicago/Turabian StyleHuang, Xiaoge, and Weiqi Dai. 2025. "Influences of Alkali-Carbonate Melt on the Electrical Conductivity of Dunite—Origin of the High Conductivity Anomaly Within the Tanzanian Cratonic Mantle" Minerals 15, no. 5: 466. https://doi.org/10.3390/min15050466
APA StyleHuang, X., & Dai, W. (2025). Influences of Alkali-Carbonate Melt on the Electrical Conductivity of Dunite—Origin of the High Conductivity Anomaly Within the Tanzanian Cratonic Mantle. Minerals, 15(5), 466. https://doi.org/10.3390/min15050466







