Kinetics and Mechanism of Copper Elution from Protonated Dry Alginate Beads: Process Optimization and Stability Assessment
Abstract
1. Introduction
1.1. Importance of Reusing Ion Exchangers
1.2. The Use of PDABs for Copper Uptake
1.3. Chemistry and Elution Mechanism of Cu2+
2. Experimental Work
2.1. Copper Ion Removal
2.2. Copper Ion Elution
2.3. Chemical and Solids Analysis
3. Results and Discussion
3.1. PDAB Loading Assay
3.2. Effect of Agitation Rate
3.3. Effect of Temperature on Elution Rate
3.4. Effect of H2SO4 Concentration
3.5. Effect of Different Acid Reagents
3.6. Removal and Elution Cycle Study
3.7. Copper Elution Kinetics
3.8. Proposed Flowsheet for the Generation of Copper Sulfates
4. Conclusions
- Copper elution from alginate beads occurs through an ion exchange mechanism between copper ions and protons.
- Increasing the stirring rate of the solution enhances copper ion elution. Above 400 rev min−1, the amount of copper eluted is not significant.
- Higher temperatures and H2SO4 concentrations increase copper elution, reaching up to 98% at 80 °C and an H2SO4 concentration of 0.0056 M.
- Copper elution kinetics were analyzed using a pseudo-first-order kinetic model, which was employed to determine the dependence of the elution rate on temperature and H2SO4 concentration.
- The ion exchange reaction was found to be controlled by a surface chemical reaction, with an order of 0.4 regarding the H2SO4 concentration. The activation energy was calculated as 9.2 kJ mol−1 for the temperature range of 5–80 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, L.; Hu, Y.; Zhang, B. Kinetics and equilibrium adsorption of copper(II) and nickel(II) ions from aqueous solution using sawdust xanthate modified with ethanediamine. Trans. Nonferrous Met. Soc. China 2014, 24, 868–875. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Sulaiman, O.; Hakimi, M.; Yee, Y.; Mobin, B. Removal of Cu(II) and Pb(II) ions from aqueous solutions by adsorption on Sawdust of Meranti Wood. Desalination 2009, 247, 636–646. [Google Scholar] [CrossRef]
- Dursun, A.Y. A comparative study on determination of the equilibrium. kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ion sonto pretreated Aspergillus niger. Biochem. Eng. J. 2006, 28, 187–195. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Tang, C.X.; Brodie, P.; Li, Y.Z.; Grishkewich, N.J.; Brunsting, M.; Tam, K.C. Shape recoverable and mechanically robust cellulose aerogel beads for efficient removal of copper ions. Chem. Eng. J. 2020, 392, 124821. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye. Cu+2 and Ni+2 from water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Wang, J.; Lu, J.; Zhou, Y. Polydopamine modified cyclodextrin polymer as efficient adsorbent for removing cationic dyes and Cu2+. J. Hazard. Mater. 2020, 389, 121897. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6774. [Google Scholar] [CrossRef] [PubMed]
- Aymen, M.; Zaoui, F.; El Houda, R.; Elhadj, B.; Zohra, F.; Choukchou-Braham, E.; Bounaceur, B.; Ma, Y.; Bhardwaj, M.; Ma, H. Ultrasonic-assisted adsorption of heavy copper and lead metal ions by g-C3N4, application of g-C3N4@MNPs (M: Pb, Cu) in the catalytic photoreduction of organic pollutants. J. Water Process Eng. 2024, 58, 104724. [Google Scholar]
- Ibáñez, J.P.; Aracena, A. Uptake of Zn2+ from dilute aqueous solutions using protonated dry alginate beads. Can. Metall. Q. 2014, 53, 82–87. [Google Scholar] [CrossRef]
- Ibáñez, J.P.; Umetsu, Y. Uptake of Cd2+ from aqueous solutions using protonated dry alginate beads. Can. Metall. Q. 2008, 47, 45–50. [Google Scholar] [CrossRef]
- Aracena, A.; Guajardo, N.; Ibáñez, J.P.; Jerez, O.; Carlesi, C. Uptake of nickel ions from aqueous solutions using protonated dry alginate beads. Can. Metall. Q. 2015, 54, 58–65. [Google Scholar] [CrossRef]
- Chen, M.; Long, A.; Zhang, W.; Wang, Z.; Xiao, X.; Gao, Y.; Zhou, L.; Li, Y.; Wang, J.; Sun, S.; et al. Recent advances in alginate-based hydrogels for the adsorption-desorption of heavy metal ions from water: A review. Sep. Purif. Technol. 2025, 353, 128265. [Google Scholar] [CrossRef]
- Khormaei, M.; Nasernejad, B.; Edrisi, M.; Eslamzadeh, T. Copper biosorption from aqueous solutions by sour orange residue. J. Hazard. Mater. 2007, 149, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.H.; Wu, X.M. Adsorption of copper using macroporous phosphonic acid resin. Trans. Nonferrous Met. Soc. China 2003, 13, 1446–1450. [Google Scholar]
- Jiao, F.; Gao, H.W. On-site solid-phase extraction and application to in situ preconcentration of heavy metals in surface water. Environ. Monit. Assess. 2013, 185, 39–44. [Google Scholar] [CrossRef]
- Fadel, D.A.; El-Bahy, S.M.; Abdelaziz, Y.A. Heavy metals removal using iminodiacetate chelating resin by batch and column techniques. Desalination Water Treat. 2016, 57, 25718–25728. [Google Scholar] [CrossRef]
- Xiong, C.H.; Wang, Y.J.; Shi, L.M. Studies on adsorption behavior and mechanism of copper(II) onto amino methylene phosphonic acid resin. Chem. Res. Chin. Univ. 2003, 19, 366–369. [Google Scholar]
- Yargiç, A.S.; Yarbay Şahun, R.Z.; Özbay, N.; Önal, E. Assessment of toxic copper(II) biosorption from aqueous solution by chemically-treated tomato waste. J. Clean. Prod. 2015, 88, 152–159. [Google Scholar] [CrossRef]
- Di Caprio, F.; Altimari, P.; Uccelletti, D.; Pagnanelli, F. Mechanistic modelling of copper biosorption by wild type and engineered Saccharomyces cerevisiase biomasses. Chem. Eng. J. 2014, 244, 561–568. [Google Scholar] [CrossRef]
- Benaïssa, H.; Elouchdi, M.A. Biosorption of copper (II) ions from synthetic aqueous solutions by drying bed activated sludge. J. Hazard. Mater. 2011, 194, 69–78. [Google Scholar] [CrossRef]
- Kiran, B.; Thanasekaran, K. Copper biosorption on Lyngbya putealis: Application of response surface methodology (RSM). Int. Biodeterior. Biodegrad. 2011, 65, 840–845. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Xu, M.; Zheng, F.; Zhao, M. Study of the mechanism of Cu2+ biosorption by ethanol/caustic-pretreated baker’s yeast biomass. J. Hazard. Mater. 2010, 178, 1085–1093. [Google Scholar] [CrossRef]
- Yahaya, Y.A.; Don, M.M.; Bhatia, S. Biosorption of copper (II) onto immobilized cells of Pycnoporus sanguineus from aqueous solution: Equilibrium and kinetic studies. J. Hazard. Mater. 2009, 161, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Al-Rub, F.A.; El-Naas, M.H.; Ashour, I.; Al-Marzouqi, M. Biosorption of copper on Chlorella vulgaris from single, binary and ternary metal aqueous solutions. Process Biochem. 2006, 41, 457–464. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Lu, K.; Tian, Y. Characterization of Acidosasa edulis shoot shell and its biosorption of copper ions from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 357–364. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.; Chen, J.P.; Hong, L. Sorption of lead. copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanism. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Sorption of dyes and copper ions onto biosorbents. Process Biochem. 2003, 38, 1047–1061. [Google Scholar] [CrossRef]
- Chiron, N.; Guilet, R.; Deydier, E. Adsorption of Cu(II) and Pb(II) onto a grafted silica: Isotherms and kinetic models. Water Res. 2003, 37, 3079–3086. [Google Scholar] [CrossRef]
- Alluri, H.K.; Ronda, S.R.; Settalluri, V.S.; Bondili, J.S.; Suryanarayana, V.; Venkateshwar, P. Biosorption: An eco-friendly alternative for heavy metal removal. Afr. J. Biotechnol. 2007, 6, 2924–2931. [Google Scholar]
- Vijayaraghavana, K.; Yun, Y.-S. Bacterial Biosorbents and Biosorption. In Biotechnology Advances; Elsevier: Amsterdam, The Netherlands, 2008; Volume 26, pp. 266–291. [Google Scholar]
- Aracena, A.; Álvarez, C.; Jerez, O.; Guajardo, N. Uptake of copper ion using protonated dry alginate beads from dilute aqueous solutions. Physicochem. Probl. Miner. Process. 2019, 55, 732–744. [Google Scholar]
- Roine, A. HSC Chemistry 6.0.; OutoKumpu Research Py: Pori, Finland, 1999. [Google Scholar]
- Ibáñez, J.P.; Umetsu, Y. Potential of protonated alginate beads for heavy metals uptake. Hydrometallurgy 2002, 64, 89–99. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]













| Resin Type | Adsorption Capacity, mg/g Resin | Acid Class and Concentration Used in Elution | Percentage Eluted, % | Decrease in Eluted Capacity | Reference |
|---|---|---|---|---|---|
| Sour orange residue | 21.7 | HCl, 0.1 M | 99% | After the first cycle, the biosorption capacity decreased by 14% | [14] |
| Chelate resin | 168.0 | HAc-NaAc, 1.0–3.0 mol/L | 100 | NR | [15] |
| D401 resin | Only the percentage recovered was reported | HNO3, 0.5–2.5 mol/L | 60 | NR | [16] |
| Iminodiacetate chelating | 1.79 | HNO3 7.2 mmol/L | NR | Five sorption–desorption cycles with a small loss of adsorption capacity | [17] |
| Amino methylene phosphonic acid resin | 181.0 | HCl, 1.0–3.0 mol/L | NR | NR | [18] |
| Tomato waste | 46.0 | NR | NR | NR | [19] |
| Saccharomyces cerevisiae biomass | 28.8 | NR | NR | NR | [20] |
| Dried activated sludge | 62.5 | NR | NR | NR | [21] |
| Lyngbya putealis | 7.8 | NR | NR | NR | [22] |
| Caustic baker’s yeast | 5.7 | NR | NR | NR | [23] |
| Ethanol baker’s yeast | 3.3 | NR | NR | NR | [23] |
| Pristine baker’s yeast | 2.4 | NR | NR | NR | [23] |
| Pycnoporus sanguineus | 2.8 | NR | NR | NR | [24] |
| Chlorella vulgaris | 58.8 | NR | NR | NR | [25] |
| Acidosasa edulis shoot shell | 2.51 | NR | NR | NR | [26] |
| Padina sp. | 72.4 | NR | NR | NR | [27] |
| Sargassum sp. | 62.9 | NR | NR | NR | [27] |
| Ulva sp. | 47.7 | NR | NR | NR | [27] |
| Gracillaria sp. | 37.5 | NR | NR | NR | [27] |
| Peat | 14.3 | NR | NR | NR | [28] |
| Grafted silica | 16.6 | NR | NR | NR | [29] |
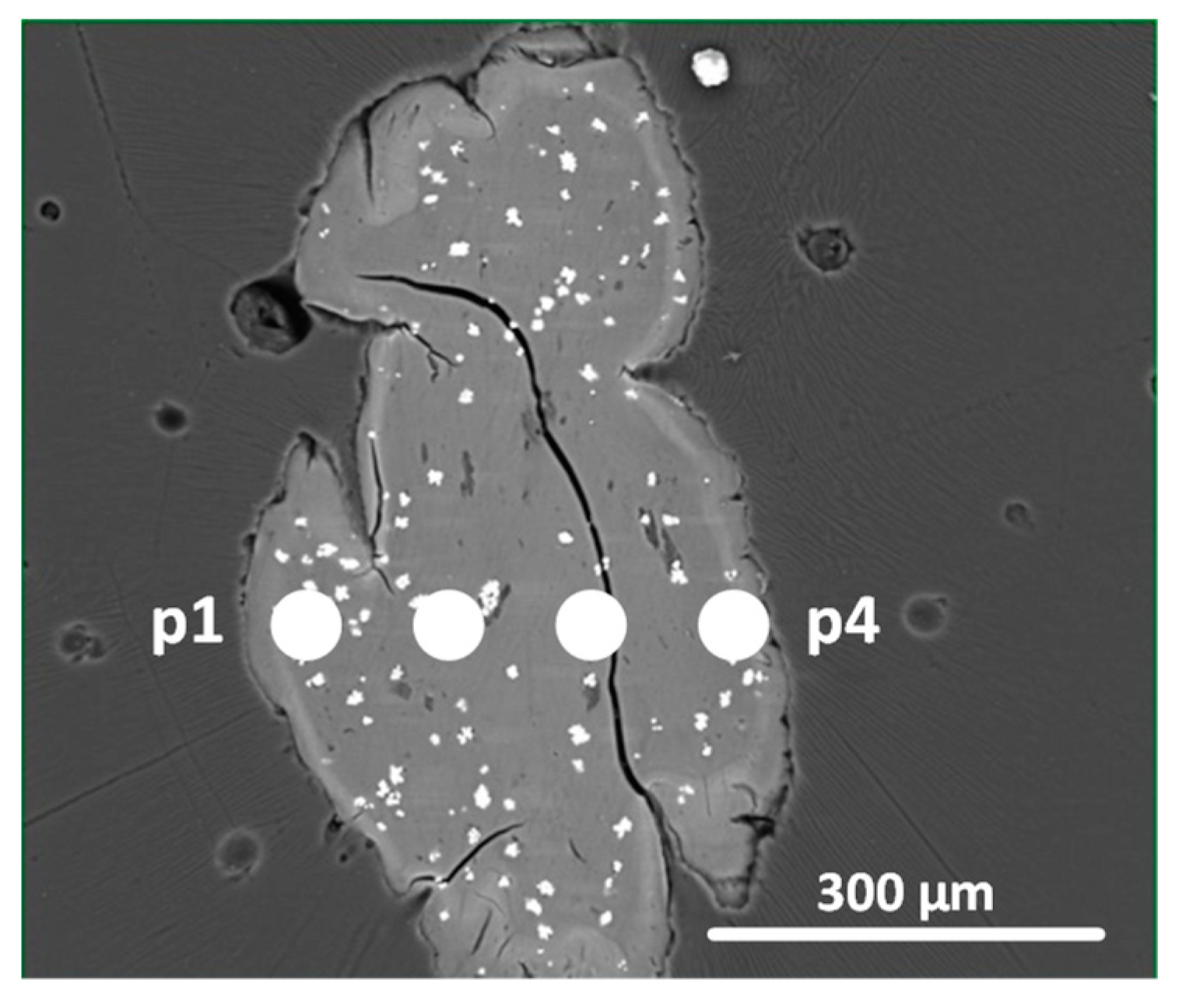
| Point | Cu Concentration, % |
|---|---|
| pH = 6.0 | |
| 1 | 26.86 |
| 2 | 23.89 |
| 3 | 21.85 |
| 4 | 23.18 |
| Point | Cu Concentration, % | ||
|---|---|---|---|
| Original Sample | 0.0010 M | 0.0056 M | |
| 1 | 26.86 | 12.08 | 4.90 |
| 2 | 23.89 | 19.32 | 8.17 |
| 3 | 21.85 | 14.39 | 9.01 |
| 4 | 23.18 | 13.63 | 9.04 |
| Average | 23.95 | 14.86 | 7.78 |
| T [°C (K)] | 1000/T (1/K) | kapp × 103 | −ln kapp |
|---|---|---|---|
| 5 (278) | 3.5971 | 18.2 | 4.007 |
| 10 (283) | 3.5336 | 23.9 | 3.732 |
| 17 (290) | 3.4483 | 26.0 | 3.649 |
| 40 (313) | 3.1949 | 34.3 | 3.372 |
| 60 (333) | 3.0030 | 35.9 | 3.326 |
| 80 (353) | 2.8329 | 49.5 | 3.005 |
| T, °C | kapp, min−1 | k1, min−1 (H2SO4)−0.4 |
|---|---|---|
| 5 | 0.00181 | 0.14476 |
| 10 | 0.0239 | 0.19057 |
| 17 | 0.0260 | 0.20706 |
| 40 | 0.0343 | 0.27303 |
| 60 | 0.0359 | 0.28585 |
| 80 | 0.0495 | 0.39396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aracena, A.; Fuenzalida, P.; Álvarez, C.; Jerez, O. Kinetics and Mechanism of Copper Elution from Protonated Dry Alginate Beads: Process Optimization and Stability Assessment. Minerals 2025, 15, 465. https://doi.org/10.3390/min15050465
Aracena A, Fuenzalida P, Álvarez C, Jerez O. Kinetics and Mechanism of Copper Elution from Protonated Dry Alginate Beads: Process Optimization and Stability Assessment. Minerals. 2025; 15(5):465. https://doi.org/10.3390/min15050465
Chicago/Turabian StyleAracena, Alvaro, Paz Fuenzalida, César Álvarez, and Oscar Jerez. 2025. "Kinetics and Mechanism of Copper Elution from Protonated Dry Alginate Beads: Process Optimization and Stability Assessment" Minerals 15, no. 5: 465. https://doi.org/10.3390/min15050465
APA StyleAracena, A., Fuenzalida, P., Álvarez, C., & Jerez, O. (2025). Kinetics and Mechanism of Copper Elution from Protonated Dry Alginate Beads: Process Optimization and Stability Assessment. Minerals, 15(5), 465. https://doi.org/10.3390/min15050465






