Strategic Recovery of Titanium from Low-Grade Titanium-Bearing Blast Furnace Slag via Hydrothermal-Crystallization Coupling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure
3. Results and Discussion
3.1. Hydrothermal Titanium Extraction from Low-Grade Titanium-Bearing Blast Furnace
3.1.1. Thermodynamic Behavior Analysis
3.1.2. Effect of Leaching Temperature
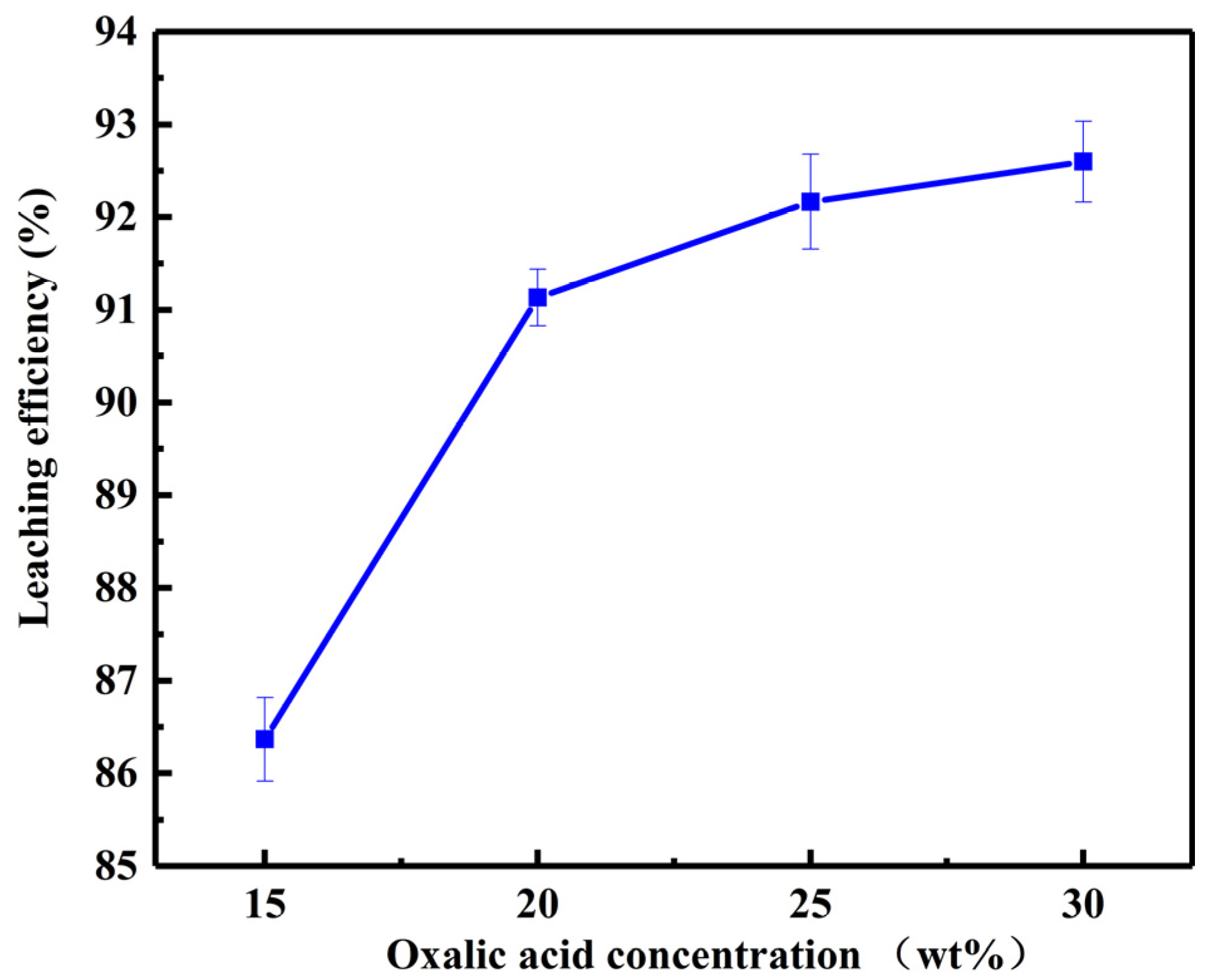
3.1.3. Effect of Oxalic Acid Concentration
3.2. Mineral Phase Evolution in Leaching Residues
3.3. Chemical Speciation of Key Elements
3.4. Hydrothermal Recovery of Titanium from Titanium-Rich Leachate
4. Conclusions
- (1)
- The hydrothermal leaching method enables efficient titanium extraction from low-grade titanium-bearing blast furnace slag. Under a hydrothermal temperature of 120 °C, a time of 90 min, an oxalic acid concentration of 25 wt%, and an agitation rate of 500 rpm, the leaching process achieved an optimal titanium extraction efficiency of 92.3%, with titanium predominantly existing as the [Ti(OH)2(C2O4)2]2− complex species.
- (2)
- The resulting leaching residue was primarily composed of calcium oxalate and amorphous silica, indicating an effective separation of titanium from the Ca-Si-Al-Mg matrix. The Ti, Fe, Ca, Mg, Mn, and Al species in the leachate mainly existed in the forms of Ti(OH)2(C2O4)22−, Fe2+, Mg2+, Mn2+, Ca2+, Al(C2O4)2−, AlHC2O42+, and AlC2O4+, respectively.
- (3)
- The hydrothermal decomposition method enables complete titanium separation from leachates through thermally driven crystallization. Under a hydrothermal temperature of 170 °C for 90 min, the [Ti(OH)2(C2O4)2]2− complex underwent decomposition, achieving 99.4% titanium precipitation as mixed-phase composites of TiO2, Ti3O5, and Ti7O13. Finally, the grade of TiO2 in the product was enhanced to 90.5% through alkaline washing.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rao, K.N.; Gururaja, U.V. Manufacture of titanium and titanium alloys at Midhani: An overview. Trans. Indian Inst. Met. 2008, 61, 349–354. [Google Scholar]
- Tang, L.; Du, Y.T. Experimental study on green electrical discharge machining in tap water of Ti–6AL–4V and parameters optimization. Int. J. Adv. Manuf. Technol. 2014, 70, 469–475. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Li, K.R. Comparison and analysis of main effect elements of machining distortion for aluminum alloy and titanium alloy aircraft monolithic component. Int. J. Adv. Manuf. Technol. 2014, 70, 1803–1811. [Google Scholar] [CrossRef]
- Ge, M.Z.; Tang, Y.; Zhang, Y.K.; Wang, Y. Enhancement in fatigue property of Ti-6Al-4V alloy remanufactured by combined laser cladding and laser shock peening processes. Surf. Coat. Technol. 2022, 444, 128671. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Wei, H.; Cai, J.; Luo, K.; Xu, X.; Lu, J. Microstructural evolution and tensile property enhancement of remanufactured Ti6Al4V using hybrid manufacturing of laser directed energy deposition with laser shock peening. Addit. Manuf. 2022, 55, 102877. [Google Scholar] [CrossRef]
- Ding, R.; Tang, D.; Zhao, A.; Guo, H.; He, J.; Zhi, C. Effect of ultragrain refinement on quenching and partitioning steels manufactured by a novel method. Mater. Des. 2015, 87, 640–649. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Z.; Wu, G.; Mao, X. Titanium microalloying of steel: A review of its effects on processing, microstructure and mechanical properties. Int. J. Miner. Metall. Mater. 2022, 29, 645–661. [Google Scholar] [CrossRef]
- Froschl, T.; Hormann, U.; Kubiak, P. High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chem. Soc. Rev. 2012, 41, 5313–5360. [Google Scholar] [CrossRef]
- Tee, S.Y.; Kong, J.; Koh, J.J.; Teng, C.P.; Zu Wang, X.; Wang, X.; Seh, Z.W. Structurally and surficially activated TiO2 nanomaterials for photochemical reactions. Nanoscale 2024, 16, 18165–18212. [Google Scholar] [CrossRef]
- Ruan, X.; Li, S.; Huang, C.; Zheng, W.; Cui, X.; Ravi, S.K. Catalyzing artificial photosynthesis with TiO2 heterostructures and hybrids: Emerging trends in a classical yet contemporary photocatalyst. Adv. Funct. Mater. 2024, 36, 2305285. [Google Scholar] [CrossRef]
- Fu, W.G.; Xie, H.E. Progress in technologies of vanadium-bearing titanomagnetite smelting in pangang. Steel Res. Int. 2011, 82, 501–504. [Google Scholar] [CrossRef]
- Li, L.; Jiang, T.; Chen, B.; Wen, J. Recycling of Ti-extraction blast furnace slag: Preparation of calcium silicate board with high slag content by steam pressure curing. Process Saf. Environ. Prot. 2011, 158, 432–444. [Google Scholar] [CrossRef]
- Valighazvini, F.; Rashchi, F.; Nekouei, R.K. Recovery of titanium from blast furnace slag. Ind. Eng. Chem. Res. 2013, 52, 1723–1730. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, F.; Guo, Y.; Jiang, T.; Travyanov, A.Y.; Qiu, G. Kinetics of hydrochloric acid leaching of titanium from titanium-bearing electric furnace slag. JOM 2016, 68, 1476–1484. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Liu, W.; Zhang, G.; Tang, S. Recovery of titanium, aluminum, magnesium and separating silicon from titanium-bearing blast furnace slag by sulfuric acid curing—Leaching. Int. J. Miner. Metall. Mater. 2022, 29, 1705–1714. [Google Scholar] [CrossRef]
- Qu, Y.; Gao, M.; Zhao, S.; Ren, Q.; Li, L.; Long, Y. Progress and Prospects for Titanium Extraction from Titanium-Bearing Blast Furnace Slag. Materials 2024, 17, 6291. [Google Scholar] [CrossRef]
- Ju, J.; Feng, Y.; Li, H.; Wu, R.; Wang, B. An approach towards utilization of water-quenched blast furnace slag for recovery of titanium, magnesium, and aluminum. J. Environ. Chem. Eng. 2022, 10, 108153. [Google Scholar] [CrossRef]
- Peng He, S.Q.; Sun, H.J.; Tan, D.Y.; Peng, T.J. Recovery of titanium compounds from Ti-enriched product of alkali melting Ti-bearing blast furnace slag by dilute sulfuric acid leaching. Procedia Environ. Sci. 2016, 31, 977–984. [Google Scholar]
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Li, G.Q.; Sui, Z.T. Recovery of titanium compounds from molten Ti-bearing blast furnace slag under the dynamic oxidation condition. Miner. Eng. 2007, 20, 684–693. [Google Scholar] [CrossRef]
- Dong, Z.H.; Zhang, j.; Yan, B.J. Co-extraction of vanadium titanium and chromium from vanadium slag by oxalic acid hydrothermal leaching with synergy of Fe powder. Metall. Mater. Trans. B 2021, 52, 3961–3969. [Google Scholar] [CrossRef]
- Dong, Z.H.; Zhang, J.; Yan, B.J. A new approach for the comprehensive utilization of vanadium slag. Metall. Mater. Trans. B 2022, 53, 2198–2208. [Google Scholar] [CrossRef]
- Chen, X.X.; Yan, B.J. A novel method to extract V3+ from iron vanadate spinel minerals by one leaching step. Hydrometallurgy 2020, 198, 105517. [Google Scholar] [CrossRef]
- Dong, Z.H.; Zhang, J.; Yan, B.J. Hydrothermal separation of titanium vanadium and chromium from a pregnant oxalic acid leachate. Materials 2022, 15, 1538. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Hashimoto, H. FT-IR-ATR study of depth profile of SiO2 ultra-thin films. Appl. Surf. Sci. 2001, 172, 307–311. [Google Scholar] [CrossRef]
- Song, S.; Cho, H.B.; Kim, H.T. Surfactant-free synthesis of high surface area silica nanoparticles derived from rice husks by employing the Taguchi approach. J. Ind. Eng. Chem. 2018, 61, 281–287. [Google Scholar] [CrossRef]
- Valido, I.H.; Rius-Bartra, J.M.; Boada, R.; Resina-Gallego, M.; Valiente, M.; López-Mesas, M. Characterization of calcium oxalate hydrates and the transformation process. ChemPhysChem 2020, 21, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Krulic, D.; Larabi, N.; Fatouros, N. Voltamperometric study of the titanium IV–oxalate complexes. J. Electroanal. Chem. 2005, 579, 239–242. [Google Scholar] [CrossRef]
- Van de Velde, G.M.H. The oxalato complexes of titanium (IV)—I: Mononuclear Ti(OH)2 (C2O4)22− in solution. J. Inorg. Nucl. Chem. 1977, 39, 1357–1362. [Google Scholar] [CrossRef]














| Compound | TiO2 | CaO | SiO2 | Al2O3 | MgO | Fe2O3 |
|---|---|---|---|---|---|---|
| Wt Pct | 8.61 | 34.89 | 30.52 | 11.13 | 8.58 | 2.44 |
| Chemical Composition | CaO | SiO2 | Fe2O3 | Al2O3 | TiO2 | MgO | Na2O | P2O5 |
|---|---|---|---|---|---|---|---|---|
| Mass fraction (wt%) | 69.81 | 26.92 | 0.20 | 1.29 | 0.31 | 0.94 | 0.15 | 0.01 |
| Concentration | Ti | Ca | Mg | Al | Mn | Fe | C2O42− |
|---|---|---|---|---|---|---|---|
| g/L | 3.73 | 0.46 | 4.63 | 9.17 | 0.11 | 0.34 | 130.7 |
| Chemical Composition | TiO2 | SiO2 | Fe2O3 | Al2O3 | K2O | CaO | V2O5 | P2O5 |
|---|---|---|---|---|---|---|---|---|
| Mass fraction (wt%) | 52.44 | 44.03 | 0.84 | 0.71 | 0.36 | 0.23 | 0.18 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Yang, R.; Wang, S.; Chen, C.; Zhao, M.; Zhou, N.; Zhang, P.; Wang, Y. Strategic Recovery of Titanium from Low-Grade Titanium-Bearing Blast Furnace Slag via Hydrothermal-Crystallization Coupling. Minerals 2025, 15, 445. https://doi.org/10.3390/min15050445
Dong Z, Yang R, Wang S, Chen C, Zhao M, Zhou N, Zhang P, Wang Y. Strategic Recovery of Titanium from Low-Grade Titanium-Bearing Blast Furnace Slag via Hydrothermal-Crystallization Coupling. Minerals. 2025; 15(5):445. https://doi.org/10.3390/min15050445
Chicago/Turabian StyleDong, Zihui, Ruichen Yang, Shuokang Wang, Changyong Chen, Mingming Zhao, Nannan Zhou, Peipei Zhang, and Yingxin Wang. 2025. "Strategic Recovery of Titanium from Low-Grade Titanium-Bearing Blast Furnace Slag via Hydrothermal-Crystallization Coupling" Minerals 15, no. 5: 445. https://doi.org/10.3390/min15050445
APA StyleDong, Z., Yang, R., Wang, S., Chen, C., Zhao, M., Zhou, N., Zhang, P., & Wang, Y. (2025). Strategic Recovery of Titanium from Low-Grade Titanium-Bearing Blast Furnace Slag via Hydrothermal-Crystallization Coupling. Minerals, 15(5), 445. https://doi.org/10.3390/min15050445






