Abstract
The study investigates the process of intensifying rare earth elements (REEs) group uncovering from the Kundybay deposit (Northern Kazakhstan). Currently, there is no interest in extracting REEs because the ore lying beneath is significantly richer in both rare elements and REEs. However, this type of raw material is a potential source of REEs. The total content of all REEs in the weathering crust is 372.7 ppm; of these, 82.6% are Sc, Y, La, Ce, and Nd, which are present in the form of complex salts and native minerals. In order to find the optimal REE group leaching mode, the influence of the L/S ratio, temperature, acid concentration in the leaching solution, leaching kinetics, and the influence of adding HF and Na2S2O5 on process efficiency were studied. The optimal conditions for REE leaching are as follows: L/S-20, T = 70 °C, t = 180 min. The maximum REE extraction yields, %: Sc-70.84; Y-90.2; La-99.8; Ce-99.24; Nd-97.98. The leaching process kinetic study results show that the process is managed by two steps. The activation energy differences for Sc, Y, La, Ce, and Nd, kJ/mol: 26.19, 23.83, 29.18, 11.15, and 15.13 allow to conclude that REEs in the Kundybay deposit weathering crust are in different forms.
1. Introduction
As it is known, 95% of global REY (group of metals including 15 lanthanides and yttrium) deposits belong to China. However, in recent years, large-scale use of rare earth resources with high concentration and growing demand for high-grade rare earth deposits have caused REY stocks to deplete in China. As a result, REY extraction from unconventional resources has high economic and strategic value since the demand for rare earth products continue to rise annually [1].
The continuous development of new advanced technologies caused an increased demand for rare earth elements (REEs) on the international market, and special attention is given to finding new resources to create an adequate supply for current and future use. Since the use of main commercially viable REE-rich sources containing up to 70% rare earth oxides (REO), such as carbonaceous bastnasite and phosphate monazite is becoming limited, research focus shifts toward ion-adsorption clays as an alternative source of lanthanides in ion-absorbed rare earth ore (IAREO) [2]. An increased importance is given to developing technologies for their extraction from rich deposit processing products, which are seen not as waste but as actual technogenic raw materials, such as coals [3], phosphoric slag [4], phosphogypsum [5,6], or clays [7,8]. Although argillaceous deposits containing adsorbed lanthanides have significantly lower REE content than other sources (0.05–0.5 REEs by mass), their abundance in surface layers, high specific surface area for adsorption, and relative extraction and processing simplicity make them economically important sources of rare earth elements.
The Kundybay deposit is located in Northern Kazakhstan and is one of the main and largest REE deposits in Central Asia [9,10]. The weathering core crust of the Kundybay deposit is a promising and relatively new industrial-genetic type of REE, featuring a complex mineralogical structure consisting of various minerals such as quartz (SiO2), churchite (YPO4×2H2O), bastnasite ((Ce,La,Y)CO3F), kaolinite, etc. REE content in this ore ranges from 0.048–0.064%, and according to some data, it reaches 0.320% [11,12]. It is known that REs and REEs can be interspersed within the matrix structure of argillaceous ores [2]. Consequently, enrichment methods used in metallurgy (flotation, magnetic concentration, etc.) are ineffective [13].
Acidic leaching is the main way of leaching for REEs. Normally, acids such as H2SO4, HNO3, and HCl, and their combinations are used [14,15]. However, there is no universally perfect acid that maximizes leaching efficiency for REE extraction from all sources. The efficiency of various acid types or their combinations largely depends on the specific mineralogy and elemental composition of processed materials. For example, in this study, silicate REE in the form of Ca2RE8(SiO4)6O2 was leached by HCl solution with 3 mol/L concentration, where the yield of La, Ce, and Nd reached 99.79%, 99.89%, and 99.73%, respectively [16]. However, the use of H2SO4 solution yielded only 88% of RE = La, Ce, Pr, Nd. This incomplete transition is associated with the formation of CaSO4, which in turn, co-sediments REEs [17]. Pan et al. [18] use a mixture of H2SO4 and HF to define the REE aluminosilicate fraction in coal and conclude that 69% of REMs are present in aluminosilicate form. The use of HF is related to a higher electronegativity of F− compared to other anions; due to this, it displaces the O2− ion from the Si–O bond because of its strong polarizing effect [19].
Water leaching is a simple and inexpensive process, depending on the initial material’s solubility. Rare earth minerals such as carbonates and phosphates have low water solubility. Thus, they are usually transformed into more soluble forms—chlorides or sulphates—before water leaching [20]. For example, after treating the Kundybay deposit ore with H2SO4 at 200 °C followed by water leaching at 90 °C, 84% of REEs were extracted [21]. In the case of mixed REE minerals, water leaching may dissolve calcinated products such as REE chlorides or sulphates, while slag components with low solubility are filtered out. Despite low costs, water leaching requires proper raw material preparation through preliminary annealing or similar treatment in order to turn REEs into soluble forms.
In recent years, ion sorption methods of REE extraction have gained more attention from the scientific community thanks to their efficiency, selectivity, and eco-friendliness. This type of ore is presented mainly by such minerals such as mica, potassium feldspar, caolinite, and quartz. Due to negative charge formation on clay particles and REE ions pulling to their surface, standard ion exchange technology takes place. Organic salts, sulphates, chlorides, and calcium/magnesium/ammonium/sodium nitrates are used as electrolytes [22,23,24]. According to hydration theory, leaching ability of electrolytes mainly depends potential of hydration ions’, as the force of cation gravitation toward clay minerals involves electrical interactions that depend on the ion charge and the distance between the hydration ion and the clay mineral surface. In case of identical concentration and ion valence, the smaller the hydration ion radius is, the higher the cation adsorption ability is; thus, the higher the leaching ability is; the higher the valence is, the higher the cation adsorption ability leading to stronger leaching ability. However, the REM leaching order found by Yang et al. and Yanfei et al. (Al3+ > NH4+ > Mg2+) suggests that there can be another interaction between ammonium ions and clay particle surfaces, for example, hydrogen bond formation [24,25]. The leaching yield salt solutions for ion sorption ores exceeds 95% [26,27,28].
Various theoretical models are widely used in contemporary literature to study the kinetics of REE leaching from solid materials. One of the widely used models is the shrinking-core model, which includes the diffusion controlling model, the chemical reaction on the surface, and the mixed controlling model [29,30].
Chemical reaction controlling type:
Diffusion controlling type:
Mixed controlling type:
where x is the rare earth leaching efficiency, t is the leaching time, k1, k2, and k3 are apparent reaction rate constants, and β is the solid substance and film diffusion resistance ratio.
Yang and Honaker (2020) [31] studied kinetics of REE leaching from aluminosilicate ore. Leaching was performed using a 1M H2SO4 solution. It was found that process activation energy at the initial stage of reaction was 36 kJ/mol and fell to 27 kJ/mol in the following stages. The observed leaching mechanism changes from chemical reaction to diffusion control through product shell. Also, Kim et al. (2014) [32] studied lanthanum leaching from slag using H2SO4. The authors argue that this process kinetics is defined by two stages: initial chemical reaction and following diffusion through ashes layer. The activation energy for the chemical reaction stage is 10 kJ/mol, and for the diffusion stage—24.8 kJ/mol.
For ion adsorption, the activation energy varies between 8.48 and 8.59 kJ/mol; the process is controlled by internal diffusion [33,34].
The choice of opening method depends on multiple factors, the main one being the mineralogical composition of the layers. It should be noted that currently there is no data on the Kundybay deposit weathering crust ore processing targeted at REE concentrating. The abovementioned studies deal either with REE concentrate from the Kundybay deposit or with ores of similar composition, or aim at white soot obtaining technology development. Thus, it is important to perform complex kinetics research and systematically estimate the influence of reagents in order to find optimal REE extraction technology specifically for the ores from this deposit. The objective of this study is to research the kinetics and process intensification for REE extraction from the weathering crust ores of the Kundybay deposit. The obtained kinetic data will allow us to define limiting stages of the process and optimize technological parameters for the efficient extraction of rare earth elements’.
2. Materials and Methods
2.1. Initial Raw Material Sample Preparation
A gross sample of 5.00 kg of the Kundybay deposit weathering crust ore was mixed using the coning and quartering method and reduced through quartering, followed by sample collection using the chessboard method. The ore was crushed on a disk grinder ID-65 (Vibrotechnik LLC, St. Petersburg, Russia), to +0.04–0.063 mm grain size.
2.2. X-Ray Phase Analysis (XRD)
Initial raw material samples’ and post-leaching cakes’ structural characteristics were found using X-ray diffraction (XRD) on a MiniFlex 600 unit (Rigaku, Tokyo, Japan) using Cu Kα radiation at 40 kV and 15 mA. Measurements were taken within the range of 2θ between 3° and 100° at a speed of 2°/min and step 0.01°. The PDF-2 database was used for deciphering.
2.3. Morphological and Elemental Analysis
The morphology and the elemental distribution of the samples were conducted using the Scanning Electron Microscope–SEM Hitachi SU8230 (Hitachi, Tokyo, Japan), coupled with an Energy-Dispersive X-ray (EDX) detector, operated at 30 kV and cold-field emission (Oxford Instruments, Oxford, UK, AZtec Software, version 3.3). The samples were mounted on a double-sticky carbon band on top of Cu-Zn stubs. To prevent the alteration of the samples, no further processing was conducted (i.e., sputter coating with noble metals).
2.4. Analyzing Possibilities of REE Extraction by Salt Solutions
In order to estimate the efficiency of REE leaching from the Kundybay deposit weathering crust ore, 2% solutions of (NH4)2SO4, MgSO4, and Al2(SO4)3 were used. Leaching was performed for 1 h, T = 25 °C, L:S ratio = 10, mixing rate 180 rpm, and pH = 4. Leaching was performed through the method given in [2].
2.5. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
After leaching, samples were diluted 100-fold and quantitatively analyzed for REE content by the ICP-MS method Agilent 7500a (Agilent Technologies, Inc., Santa Clara, CA, USA). Solutions containing 5 to 1000 µg/L of REE amount were used for calibration by the External Standard Method.
2.6. Experimental Ore Properties and Composition
In order to find SiO2 content, the method of sintering with Na2CO3 followed by sintered mass treatment with HCl was used. Sintering conditions were 40 min at 800 °C.
The percentage of SiO2(X) was calculated according to the formula:
where:
is the mass of smelting pot with baked SiO2 sediment;
is the mass of baked smelting pot;
is the mass of ore aliquot, g.
For qualitative elemental analysis, preliminary sample decomposition in a microwave sample preparation system «Speedwave4» (Berghof GmbH, Eningen, Germany) was performed. An ore aliquot (0.2–0.4 g) and acid mixture (HCl, HNO3, and HF) were put in a Teflon autoclave and air-sealed. Decomposition was completed at 60 atm pressure, 2500 Hz microwave radiation, and 110 °C for 30 min. Sample uncovering was performed through the selection of decomposing mixture, acid concentration, temperature program, phase contact time, and L:S ratio. After decomposition, the autoclave content was transferred into a 50 mL flask and filled to the mark with distilled water. The obtained solutions were analyzed using ICP-MS. Subsequently, ore uncovering was completed through leaching in laboratory conditions by concentrated acid mixtures of HCl, H2SO4, and HNO3, followed by HF treatment for silicon removal.
The metals percentage (X) was calculated using the following formula:
where:
- C is the metal concentration, mg/L;
- V is the solution volume, L;
- m is the mass of ore aliquot, g.
2.7. Reagents
ICP-MS multi-element calibration standard-1, 10 µg/mL Ce, Dy, Er, Eu, Gd, Ho, La, Lu, Nd, Pr, Sc, Sm, Tb, Th, Tm, Y, Yb in 5% HNO3, 100 mL, Agilent
sodium pyrosulfite, 98.9% (GOST 11683-76), CAS: 7681-57-4, EKOS-1
sulphuric acid 96.3% (GOST 2184-77), CAS: 7664-93-9, EKOS-1
hydrochloric acid 36% (GOST 3118-77), CAS: 7647-01-0, EKOS-1
fluohydric acid 45% (GOST 10484-78), CAS: 7664-39-3, EKOS-1
sodium hydroxide 99% (GOST 4328-77), CAS: 1310-73-2, EKOS-1
sodium carbonate 99.8% (GOST 83-79), CAS: 497-19-8, EKOS-1.
2.8. Finding Optimal Conditions for REE Leaching from Experimental Ore
The following were selected as controlled parameters in the optimal conditions finding process: L:S (5, 10, 20), temperature (20, 50, 70 °C), leaching mixture composition H2SO4 50 g/L, Na2S2O5 20 g/L, and HF 3 g/L; HCl 100 g/L; H2SO4 100 g/L. Leaching experiments were performed in thermal-resistant beakers of 100 mL volume on a heated electromagnetic mixer IKA RCT basic (IKA, Staufen, Germany), with a mixing rate of 150 rpm for 2 h.
2.9. Kinetic Modelling
The contracting core model, widely used to describe solid and liquid phase interaction processes, was applied to study ore leaching kinetics [35]. The experimental part used the Kundybay deposit weathering crust ore samples and a leaching solution containing HCl, HF, and Na2S2O5. It was supposed that ore particles have a spherical shape, which allows to the application of the contracting core model. The kinetic study of the leaching process was described through two main mechanisms: surface reaction and diffusion through forming product layer, which are expressed through Equations (1) and (2).
Kinetic constants obtained experimentally were used to calculate the leaching process activation energy via the Arrhenius equation:
where k is the reaction speed constant for chemical reaction (kc) and diffusion (kd), A is the pre-exponential factor (frequency factor), R is the gas constant, T is the thermodynamic temperature, Ea is the activation energy for chemical reaction process, and Ec is for diffusion Ed.
3. Results
Ore elemental composition was defined to identify ore type, its properties estimation, and further optimization of leaching processes (Table 1). The weathering crust is represented by elements such as O, Si, Al, Fe, and K; however, REEs were under the limit of detection of the EDX detector used. The total REE content found using the ICP-MS method is 372.7 ppm (Table 2). The main REE content in the ore is the following (ppm): La (55.2 ± 0.2), Ce (116.2 ± 0.5), Nd (61.2 ± 0.2), Y (52.2 ± 0.1), Sc (22.9 ± 0.1). According to previous studies, the prevailing elements in the weathering crust are light lanthanides with a dominant content of cerium [36]. Primary ore phases defined by the XRD method are quartz (SiO2)—76.49%, illite (KAl2(Si3Al)O10(OH)2×H2O)—18.58% and dickite (Al2Si2O5(OH)4)—4.93%. Intense peaks typical of quartz are seen at 2θ = 26.7° (d = 3.33 Å), 2θ = 20.92° (d = 4.24 Å), which prove its presence as the primary phase. Illite peaks are identified at 2θ = 8.95° (d = 9.86 Å), 2θ = 17.89° (d = 4.95 Å), 2θ = 26.94° (d = 3.306 Å), and dickite peaks at 2θ = 12.41° (d = 7.12 Å), 2θ = 24.93° (d = 3.56 Å), and 2θ = 45.64° (d = 1.986 Å). Dickite is found in the weathering crust of kaolinite deposits. This is explained by the fact that kaolinite found in the Kundybay deposit ore is exposed to geothermal activity, which causes the formation of dickite along with minerals [21,37]. In the weathering process, dickite and illite form microstructures in combination with secondary iron oxide compounds, which explains the yellow color of weathering crust (Figure 1) [38]. SEM analysis of the initial ore shows various distributions of the morphology, with bulk blocks with smooth and sharp edges as well as smaller particles (Figure 2). The REE mineral phases in the ore were under the limit of detection of the EDX detector. However, the elemental map reveals areas with La, Ce, and Ti concentrations (Figure 3).

Table 1.
Ore elemental composition found using EDS.

Table 2.
REE contents found using the ICP-MS method.
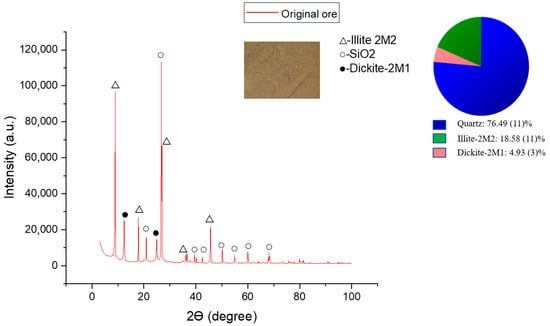
Figure 1.
Diffractogram of the weathering crust from the Kundybay deposit.

Figure 2.
SEM micrograph of the initial weathering crust from the Kundybay deposit showing block materials with smooth (white arrows) and sharp (black arrows) edges, as well as smaller particles (circles) with distributed randomly.
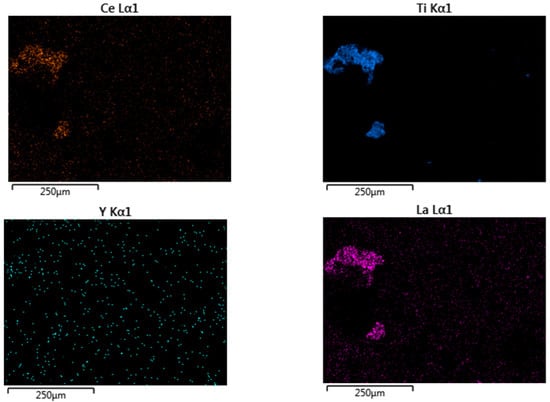
Figure 3.
Elemental map of the weathering crust from the Kundybay deposit.
Despite the presence of clay minerals such as illite (KAl2(Si3Al)O10(OH)2×H2O) and dickite (Al2Si2O5(OH)4), whose structure may include adsorbed forms of REEs, the results of leaching using salt solutions show extremely low total extraction not exceeding 1.2% (Table 3). This suggests that REEs are mostly present in the ore in the form of their own phosphate and silicate minerals or isomorphically included in silicate minerals composition. Primary REE silicate minerals are allanite ((Ce,Ca,Y)2(Al,FE2+,Fe3+)3(SiO4)3(OH)), gadolinite (Y2Fe2+Be2Si2O10), thortveitite (Sc,Y)2Si2O7), cascandite (Ca(Sc,Fe3+)HSi3O9), and gerwustite ((Na,Ca,Fe2+)(Sc,Mg,Fe2+)Si2O6) [39]. Considering this, the acid leaching method was selected for further studies. This method allows for the destruction of the structure of clay minerals containing REE and transfers them into soluble form. HCl/H2SO4 acids were selected as leaching solutions (LS because they dissolve REE well [40]. HF is an efficient reagent for the destruction of silicate minerals [41], which are matrices for REE. Na2S2O5 is a reducing agent that reduces iron for its further separation from REE [42].

Table 3.
Results of REE leaching with salt solutions.
3.1. Investigation of the L/S Effect
For all leaching solutions, an increase in the L/S ratio positively influences REE extraction (Figure 4). At higher L/S, a more efficient REE solution is reached as acid ions go deeper into ore pores [43]. In the case of using an HCl 100 g/L solution, the total REE extraction rate is higher than in the case of leaching using H2SO4 100 g/L. At L/S = 5 and with the use of HCl, Sc extraction is 10.6%, and in the case of using H2SO4, it is 7.5%. The extraction rate using HCl is 25.9%, whereas using H2SO4 is 22.3%. As L/S increases, REE extraction rises in both cases. This way, the La extraction rate using HCl rises from 43.4% to 74.6%, and using H2SO4, it rises from 34.7% to 54.5%. A similar trend is typical for Ce and Nd. This behavior can be explained by the fact that HCI destroys the mineralogical matrix more easily than H2SO4. There is a study on REE leaching from phosphate ores, where La leaching activation energy with HCl (10.3 kJ/M) is lower than with H2SO4 (16.7 kJ/M) [44].
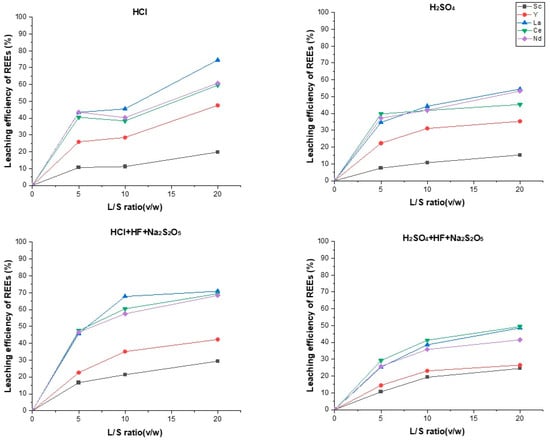
Figure 4.
L/S ratio optimization.
In the case of using a leaching solution consisting of HCl, HF, and Na2S2O5, the highest REE extraction rates are achieved. For example, for La and Ce at L/S = 20, extraction rates are over 70%; similarly, Nd is extracted by 68.4%.
In the case of using a leaching solution consisting of H2SO4, HF, and Na2S2O5, the Sc extraction rate is 10% higher than with a solution consisting of H2SO4 (L/S = 20); however, absolute values are generally lower than with HCl (70.9%) under the same conditions.
3.2. Temperature Optimization Results
At increased temperatures, there is a tendency of REE yield to rise (Figure 5) as temperature rise speeds up chemical reactions and diffusion [45]. For single component leaching solutions, raising the temperature to 70 °C helps increase the extraction rates of Y, La, and Ce, while Nd is extracted less efficiently at the same temperature. For leaching solutions consisting of HCl/H2SO4, HF, and Na2S2O5, temperature rise consistently increases the yield of all REEs. Compared to a leaching solution consisting of H2SO4, HF, and Na2S2O5, the best results are achieved with a combination of HCl, HF, and Na2S2O5 at 70 °C, where the extraction of La, Ce, and Nd are 91.3%, 92.0%, and 94.1%, respectively.
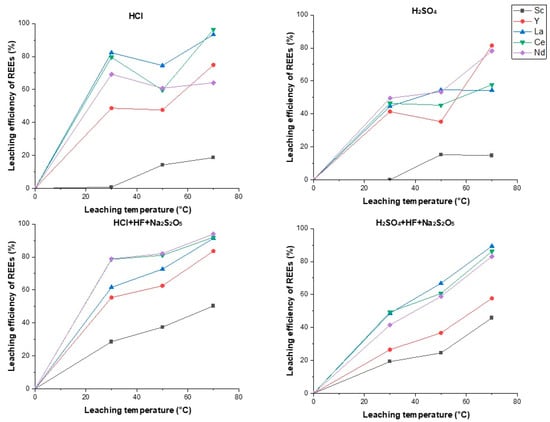
Figure 5.
Temperature optimization.
3.3. Investigation of the Leaching Solution Concentration
Single component leaching solutions extract La, Ce, Nd, and Y to 75%–80% and Sc to 20%. Leaching with single component solutions gives unsatisfactory results due to the fact that REEs are most likely to be present in hard-opened silicate matrices [40]. After the HCl concentration increased from 50 to 150 g/L, Sc leaching rose from 12.3% to 18.3%, while the H2SO4 concentration change did not bring significant changes (Table 4). Y, La, Ce, and Nd leaching decreased with acid concentration rise. Leaching efficiency decline can be explained by the concept of solvation. As electrolyte concentration rises, the share of water in the solution falls, which causes a decrease in the number of water molecules available for the leaching reaction as cations and anions hold them strongly in the solution [46].

Table 4.
Investigation of the effect of concentration for HCl and H2SO4.
In the case of using a leaching solution consisting of HCl, HF, and Na2S2O5, with HF addition, the total REE extraction rate reaches 90%–95% (Table 5). In addition, an HF concentration increase from 3 to 12 g/L causes the Sc extraction rate to rise from 50.3% to 91.2%.

Table 5.
Investigation of the effect of concentration for HCl + HF + Na2S2O5 and H2SO4 + HF + Na2S2O5.
Sc’s ionic radius is smaller than those of other REEs (Sc-0.745, Y-0.9, La-1.032, Ce-1.01, Nd-0.983 nm) [47]. Due to this, Sc3+ can displace Ti, Fe, and Al in silicate structures or be included in quartz minerals [48]. Additionally, an increase in dickite dissolution and REE extraction rates are seen, which most likely suggests REE inclusion in dickite composition (Figure 6). Dickite is similar to other kaolinite group minerals and can dissolve in acidic environments, especially in the presence of HF. For example, at HF treatment, dickite dissolves faster than some other kaolinite polymorphic forms [49]. The reaction between dickite and HF is described by the Equation (7):
Al2Si2O5(OH)4 + 12HF → 2H2[SiF6] +2AlF3 + 5H2O
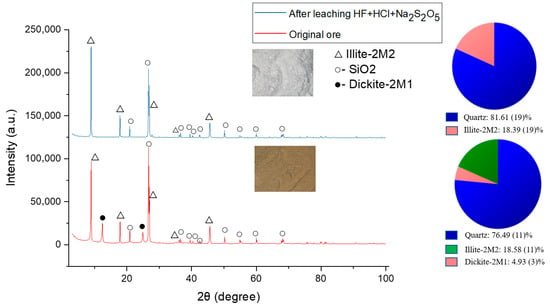
Figure 6.
Diffractogram of the Kundybay deposit ore before and after leaching.
3.4. Process Kinetics Study
The study of any chemical reaction is an important part of understanding chemical processes. Most leaching processes are reactions between liquid and solid phases. Our leaching system consists of the Kundybay ore weathering crust and a leaching solution consisting of HCl, HF, and Na2S2O5. Based on the fact that ore particles have a spherical shape, a kinetic study of leaching can be performed using the shrinking core model.
Both mechanisms show relatively good correlation with experimental data, proving that the kinetic model can describe the process in two steps. In the beginning of the process, the limiting stage is the chemical reaction of REE minerals interacting with leaching solution components. It should be noted that some REEs (La, Ce, Nd) are leached faster than Sc, Y (Figure 7). This may be related to REE distribution in different minerals, which have different chemical natures and stabilities. Then the leaching process is managed by a diffusion model through the product layer as silicic acid formed during silicate minerals dissolving turns into a silicic acid product [41]. The formed silicic acid creates resistance, blocking the ore particles surface, thus reducing the diffusion of the leaching solution.
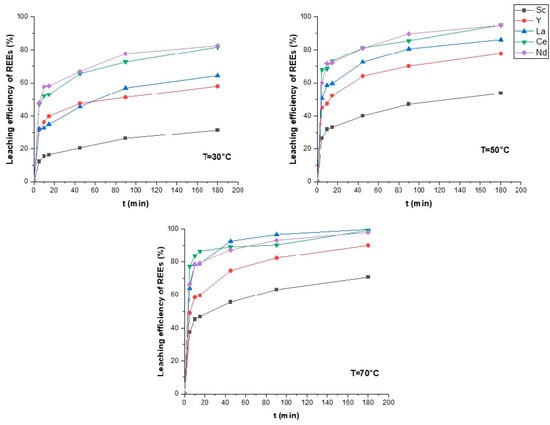
Figure 7.
Leaching kinetics at different temperatures.
Kinetic constants given in Table 6 and Table 7 are used to calculate process activation energy using Equation (4), also known as the Arrhenius equation (Table 8).

Table 6.
Reaction speed constant and linear correlation factor.

Table 7.
Diffusion constant and linear correlation factor.

Table 8.
Activation energy of chemical reaction process and diffusion.
According to the Arrhenius equation, the slope of the line equals −Ea/R (Figure 8). Thus, activation energy can be calculated using a straight-line equation. The activation energy of the chemical reaction process and diffusion is given in Table 8. The diffusion process’s activation energy is higher than chemical reaction activation energy for all elements. This is explained by the fact that diffusion through forming a silicic acid layer requires more energy than the chemical reaction of matrix destruction and ions complexing themselves. Relatively high activation energies for Sc, Y, and La suggest that they are mostly present in silicate phases, which require overcoming energy barriers for their dissolving: 1—silicon backbone destruction; 2—leaching solution ions diffusion through silicic acid layer toward ore particles. On the other hand, activation energies of the chemical reaction process and diffusion for Ce and Nd have similar values. Cerium and neodymium are likely present in an easily opened form, forming reaction products, and do not create an energy barrier for further leaching solution ions diffusing onto the ore surface. Considering this, it can be suggested that REEs in the Kundybay deposit weathering crust have different mineralogical distributions.
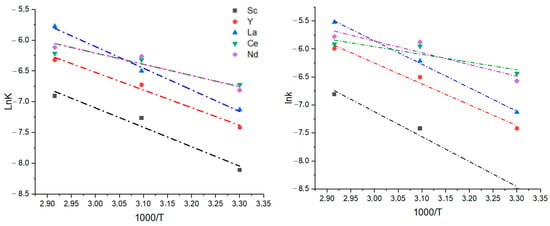
Figure 8.
Dependency of chemical reaction (left) and diffusion (right) constants on reciprocal temperature value.
4. Conclusions
This study presents the results of optimizing REE leaching from weathering crust and looks into the kinetics of the process. Dickite has been identified in the weathering crust instead of kaolinite, suggesting mineralogical changes occurring in the weathering process and that influence REE distribution. It has been found that this type of ore is not an ion-adsorptive one, as REE extraction rates do not exceed 2%. An optimal leaching solution consisting of HF 3 g/L, HCl 70 g/L, and Na2S2O5 10 g/L has been found; it provides efficient REE extraction. The optimal conditions for REE leaching are as follows: L/S-20, T = 70 °C, t = 180 min. Maximum REE extraction yields, %: Sc-70.84; Y-90.2; La-99.8; Ce-99.24; Nd-97.98. The kinetic study results of the leaching process indicate that the process is managed by two steps. The differences in activation energy for Sc, Y, La, Ce, and Nd, kJ/mol: 26.19, 23.83, 29.18, 11.15, and 15.13 allow us to conclude that REEs in the Kundybay deposit weathering crust exists in different forms. Future research prospects may involve REE selective separation from leaching solutions and the development of methods to obtain their individual concentrates.
Author Contributions
Conceptualization, R.T., T.K., Z.I., B.Z., E.Z. and M.N.; methodology, T.K., Z.I., B.Z., A.C. and E.Z.; validation, Z.I., B.Z. and E.Z.; formal analysis, R.T., T.K., Z.I., B.Z. and E.Z.; investigation, T.K., Z.I., B.Z., A.C., K.K. and E.Z., resources, R.T., K.K. and M.N.; data curation, R.T.; writing—original draft preparation, T.K., Z.I., B.Z., A.C. and K.K.; writing—review and editing, R.T., T.K., Z.I., K.K. and M.N.; supervision, R.T. and M.N.; project administration, R.T. and M.N.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been performed in the Center of Physico-Chemical Methods of Research and Analysis of al-Farabi Kazakh National University as a part of the scientific research program-targeted financing for 2022–2024, funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan, for the program BR18574219 “Development of environmentally safe technologies for obtaining innovative products from natural and man-made raw materials of Kazakhstan”.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ferhaoui, S.; Kechiched, R.; Bruguier, O.; Sinisi, R.; Kocsis, L.; Mongelli, G.; Bosch, D.; Ameur-Zaimeche, O.; Laouar, R. Rare earth elements plus yttrium (REY) in phosphorites from the Tébessa region (Eastern Algeria): Abundance, geochemical distribution through grain size fractions, and economic significance. J. Geochem. Explor. 2022, 241, 107058. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Y.; Wang, Y.; Xu, Y.; Lin, Z.; Liang, X.; Cheng, H. Review of rare earth element (REE) adsorption on and desorption from clay minerals: Application to formation and mining of ion-adsorption REE deposits. Ore Geol. Rev. 2023, 157, 105446. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Pan, J.; Long, X.; He, X.; Shi, S.; Yang, Y.; Zhang, H.; Zhou, C. Study on the leaching behavior differences of rare earth elements from coal gangue through calcination-acid leaching. Sep. Purif. Technol. 2024, 344, 127222. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhang, L. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes—A critical review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar] [CrossRef]
- Tokpayev, R.; Khavaza, T.; Ibraimov, Z.; Kishibayev, K.; Atchabarova, A.; Abdimomyn, S.; Abduakhytova, D.; Nauryzbayev, M. Phosphogypsum conversion under conditions of SC-CO2. J. CO2 Util. 2022, 63, 102–120. [Google Scholar] [CrossRef]
- Khavaza, T.; Tokpayev, R.; Ibraimov, Z.; Atchabarova, A.; Kishibayev, K.; Nauryzbayev, M. Study of cerium and neodymium leaching from Kazakhstan phosphogypsum. Int. J. Biol. Chem. 2021, 14, 93–100. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. Recovery of rare earth elements adsorbed on clay minerals: II. Leaching with ammonium sulfate. Hydrometallurgy 2013, 131–132, 158–166. [Google Scholar] [CrossRef]
- Meng, X.; Zhao, H.; Zhao, Y.; Shen, L.; Gu, G.; Qiu, G. Heap leaching of ion adsorption rare earth ores and REEs recovery from leachate with lixiviant regeneration. Sci. Total Environ. 2023, 898, 165417. [Google Scholar] [CrossRef]
- Abisheva, Z.S.; Bochevskaya, Y.G.; Karshigina, Z.B.; Sargelova, E.A. The extraction of rare earths from refractory ores of a deposit in Kazakhstan. In Proceedings of the IMPC 2016–28th International Mineral Processing Congress, Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Bochevskaya, Y.G.; Abisheva, Z.S.; Karshigina, Z.B.; Sargelova, E.A.; Kvyatkovskaya, M.N.; Akchulakova, S.T. Effect of the Temperature Conditions of Sulfation Process on Extraction of Rare-Earth Metals from Refractory Ore. Metallurgist 2018, 62, 574–586. [Google Scholar] [CrossRef]
- Omirserikov, M. Forms of Occurrence of Rare Earth Elements in the Weathering Crust of Kundybay Deposit (North Kazakhstan). In Proceedings of the 15th International Multidisciplinary Scientific GeoConference SGEM2015, Albena, Bulgaria, 16–25 June 2015. [Google Scholar]
- Stepanenko, N.I.; Dyusembaeva, K.S.; Isaeva, L.D. Ore-bearing weathering mantle of Kundybay rare earth deposit (North Kazakhstan). Gorn. Zhurnal 2017, 2, 33–38. [Google Scholar] [CrossRef]
- Cheng, S.; Li, W.; Han, Y.; Sun, Y.; Gao, P.; Zhang, X. Recent process developments in beneficiation and metallurgy of rare earths: A review. J. Rare Earths 2024, 42, 629–642. [Google Scholar] [CrossRef]
- Costis, S.; Mueller, K.K.; Coudert, L.; Neculita, C.M.; Reynier, N.; Blais, J.-F. Recovery potential of rare earth elements from mining and industrial residues: A review and cases studies. J. Geochem. Explor. 2021, 221, 106699. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements, resources, applications, extraction technologies, chemical characterization, and global trade—A comprehensive review. In Treatise on Geochemistry, 3rd ed.; Anbar, A., Weis, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 193–233. [Google Scholar] [CrossRef]
- Zhou, Y.; Xue, X.; Yang, H.; Song, S.; Huang, X. Novel Harmless Utilization of Bayan Obo Tailings: Separation and Recovery of Iron and Rare Earth. Ind. Eng. Chem. Res. 2020, 59, 13682–13695. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Cheng, G.; Xue, X.; Yang, H. Carbothermal reduction followed by sulfuric acid leaching of Bayan Obo tailings for selective concentration of iron and rare earth metals. Sep. Purif. Technol. 2021, 271, 118742. [Google Scholar] [CrossRef]
- Pan, J.; Nie, T.; Hassas, B.V.; Rezaee, M.; Wen, Z.; Zhou, C. Recovery of rare earth elements from coal fly ash by integrated physical separation and acid leaching. Chemosphere 2020, 248, 126112. [Google Scholar] [CrossRef]
- Mitra, A.; Rimstidt, J.D. Solubility and dissolution rate of silica in acid fluoride solutions. Geochim. Cosmochim. Acta 2009, 73, 7045–7059. [Google Scholar] [CrossRef]
- Chi, R.; Zhang, X.; Zhu, G.; Zhou, Z.; Wu, Y.; Wang, C.; Yu, F. Recovery of rare earth from bastnasite by ammonium chloride roasting with fluorine deactivation. Miner. Eng. 2004, 17, 1037–1043. [Google Scholar] [CrossRef]
- Karshigina, Z.; Abisheva, Z.; Bochevskaya, Y.; Akcil, A.; Sargelova, E.; Sukurov, B.; Silachyov, I. Recovery of rare earth metals (REMs) from primary raw material: Sulphatization-leaching-precipitation-extraction. Miner. Process. Extr. Met. Rev. 2018, 39, 319–338. [Google Scholar] [CrossRef]
- Shen, L.; Chen, J.; Chen, L.; Liu, C.; Zhang, D.; Zhang, Y.; Su, W.; Deng, Y. Extraction of mid-heavy rare earth metal ions from sulphuric acid media by ionic liquid [A336][P507]. Hydrometallurgy 2016, 161, 152–159. [Google Scholar] [CrossRef]
- Lai, F.; Gao, G.; Huang, L.; Xiao, Y.; Yang, R.; Li, K. Compound leaching of rare earth from the ion-adsorption type rare earth ore with magnesium sulfate and ascorbic acid. Hydrometallurgy 2018, 179, 25–35. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Z.; Huang, X.; Huang, L.; Chen, Y.; Liu, X.; Wang, L.; Long, Z. Recovery of rare earth from the ion-adsorption type rare earths ore: II. Compound leaching. Hydrometallurgy 2016, 163, 83–90. [Google Scholar] [CrossRef]
- Yang, L.; Li, C.; Wang, D.; Li, F.; Liu, Y.; Zhou, X.; Liu, M.; Wang, X.; Li, Y. Leaching ion adsorption rare earth by aluminum sulfate for increasing efficiency and lowering the environmental impact. J. Rare Earths 2019, 37, 429–436. [Google Scholar] [CrossRef]
- Xiao, Y.-F.; Chen, Y.-Y.; Feng, Z.-Y.; Huang, X.-W.; Huang, L.; Long, Z.-Q.; Cui, D.-L. Leaching characteristics of ion-adsorption type rare earths ore with magnesium sulfate. Trans. Nonferrous Met. Soc. China 2015, 25, 3784–3790. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Z.; Huang, X.; Huang, L.; Chen, Y.; Wang, L.; Long, Z. Recovery of rare earths from weathered crust elution-deposited rare earth ore without ammonia-nitrogen pollution: I. leaching with magnesium sulfate. Hydrometallurgy 2015, 153, 58–65. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Li, J.; Huang, L.; Xu, J.; Xiao, Y. Strengthening rare earth and inhibiting aluminum leaching in magnesium salt-acetic acid compound system from ion-adsorption type rare earth ore. Sep. Purif. Technol. 2024, 334, 126070. [Google Scholar] [CrossRef]
- Faraji, F.; Alizadeh, A.; Rashchi, F.; Mostoufi, N. Kinetics of leaching: A review. Rev. Chem. Eng. 2020, 38, 113–148. [Google Scholar] [CrossRef]
- Mubarak, Y. Kinetics of Hydrochloric Acid Leaching of Copper from its Ore. Int. J. Emerg. Trends Eng. Res. 2020, 8, 5006–5015. [Google Scholar] [CrossRef]
- Yang, X.; Honaker, R.Q. Leaching Kinetics of Rare Earth Elements from Fire Clay Seam Coal. Minerals 2020, 10, 491. [Google Scholar] [CrossRef]
- Kim, C.-J.; Yoon, H.-S.; Chung, K.W.; Lee, J.-Y.; Kim, S.-D.; Shin, S.M.; Lee, S.-J.; Joe, A.-R.; Lee, S.-I.; Yoo, S.-J.; et al. Leaching kinetics of lanthanum in sulfuric acid from rare earth element (REE) slag. Hydrometallurgy 2016, 146, 133–137. [Google Scholar] [CrossRef]
- Xiao, Y.-F.; Feng, Z.-Y.; Hu, G.-H.; Huang, L.; Huang, X.-W.; Chen, Y.-Y.; Li, M.-L. Leaching and mass transfer characteristics of elements from ion-adsorption type rare earth ore. Rare Met. 2015, 34, 357–365. [Google Scholar] [CrossRef]
- Yan, H.; Liang, T.; Liu, Q.; Qiu, T.; Ai, G. Compound leaching behavior and regularity of ionic rare earth ore. Powder Technol. 2018, 333, 106–114. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Wadsworth, M.E. Rate Processes of Extractive Metallurgy; Springer: New York, NY, USA, 1979. [Google Scholar]
- Shautenov, M.R.; Begalinov, A.; Akkazina, N.T. Processing of rare earth ore of weathering crust. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2024, 35–41. [Google Scholar] [CrossRef]
- Gaudin, A.; Ansan, V.; Lorand, J.-P.; Pont, S. Genesis of a florencite-bearing kaolin deposit on ordovician schists at Saint-Aubin-des-Châteaux, Armorican Massif, France. Ore Geol. Rev. 2020, 120, 103445. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, T.; Han, W.; Xu, X.; Yan, X.; Yan, J. The Color Formation of “Lumu Stone” in the Weathering Processes: The Role of Secondary Hematite and Goethite. Minerals 2023, 13, 860. [Google Scholar] [CrossRef]
- Qi, D. Hydrometallurgy of Rare Earths: Extraction and Separation; Elsevier Science: Amsterdam, The Netherlands, 2018; p. 814. [Google Scholar]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Van Gerven, T. Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Kumar, M.; Babu, M.N.; Mankhand, T.; Pandey, B. Precipitation of sodium silicofluoride (Na2SiF6) and cryolite (Na3AlF6) from HF/HCl leach liquors of alumino-silicates. Hydrometallurgy 2010, 104, 304–307. [Google Scholar] [CrossRef]
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.; Durão, F.O.; Margarido, F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018, 71, 350–361. [Google Scholar] [CrossRef]
- Zhao, Z.; Qiu, Z.; Yang, J.; Lu, S.; Cao, L.; Zhang, W.; Xu, Y. Recovery of rare earth elements from spent fluid catalytic cracking catalysts using leaching and solvent extraction techniques. Hydrometallurgy 2017, 167, 183–188. [Google Scholar] [CrossRef]
- Kandil, A.; Aly, M.; Moussa, E.; Kamel, A.; Gouda, M.; Kouraim, M. Column leaching of lanthanides from Abu Tartur phosphate ore with kinetic study. J. Rare Earths 2010, 28, 576–580. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, F.; Chi, R.; Liu, X.; Xu, Y.; Liu, Q. Effect of a novel compound on leaching process of weathered crust elution-deposited rare earth ore. Miner. Eng. 2018, 129, 63–70. [Google Scholar] [CrossRef]
- Junior, A.B.B.; Espinosa, D.C.R.; Vaughan, J.; Tenório, J.A.S. Extraction of Rare-Earth Elements from Silicate-Based Ore through Hydrometallurgical Route. Metals 2022, 12, 1133. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Vasyukova, O.V. The Economic Geology of Scandium, the Runt of the Rare Earth Element Litter. Econ. Geol. 2018, 113, 973–988. [Google Scholar] [CrossRef]
- Liu, Z.; Zong, Y.; Li, H.; Zhao, Z. Characterization of scandium and gallium in red mud with Time of Flight-Secondary Ion Mass Spectrometry (ToF-SIMS) and Electron Probe Micro-Analysis (EPMA). Miner. Eng. 2018, 119, 263–273. [Google Scholar] [CrossRef]
- Fraser, A.R.; Wilson, M.J.; Roe, M.J.; Shen, Z.Y. Use of hydrofluoric acid dissolution for the concentration of dickite and nacrite from kaolin deposits: An FTIR study. Clay Miner. 2002, 37, 559–570. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).