Petrological, Textural, Compositional, and Economic Potential of Carbonatites from the Peshawar Plain Alkaline Igneous Province, Northwestern Himalaya
Abstract
1. Introduction
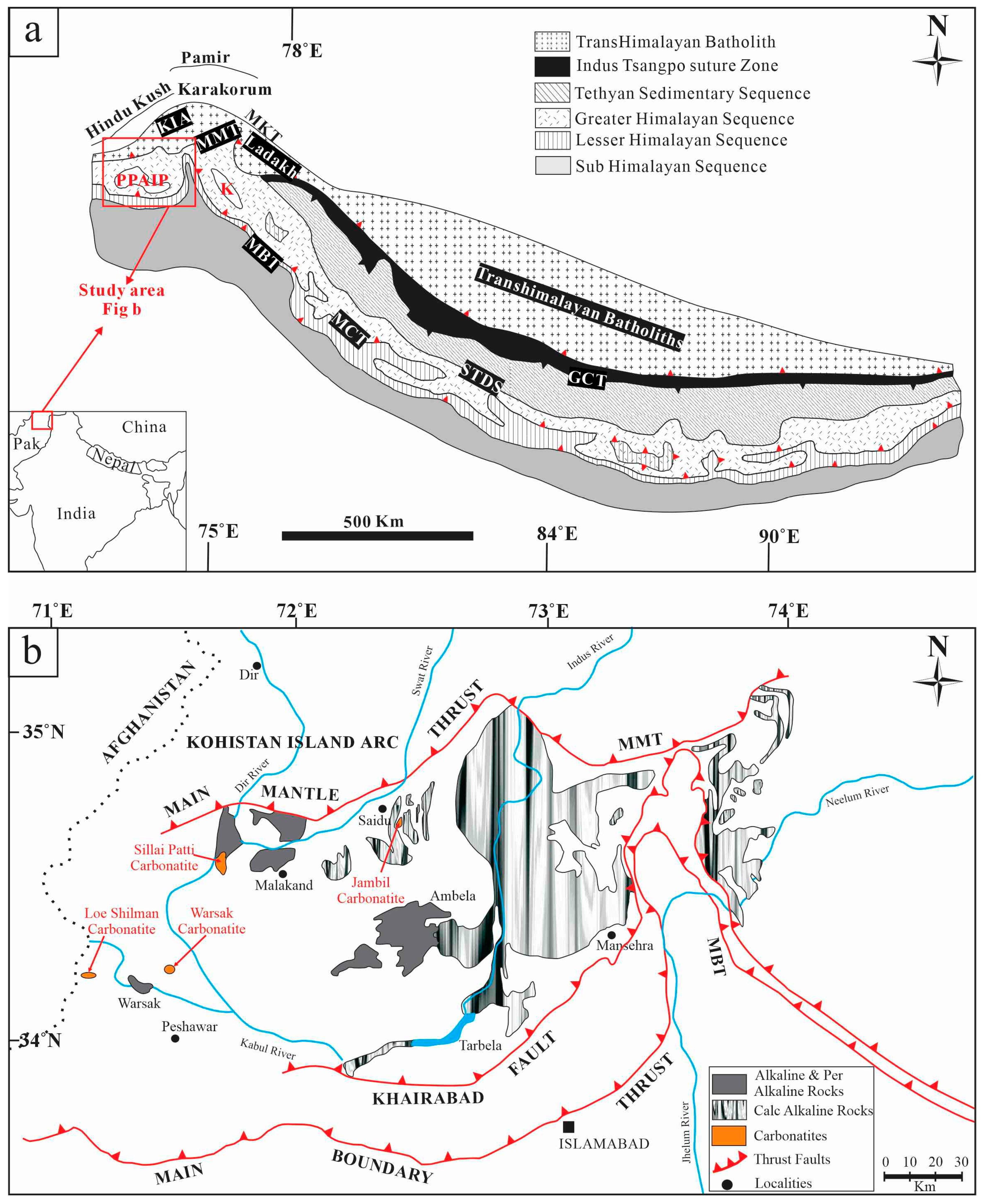
2. Geological Setting
3. Materials and Methods
4. Results
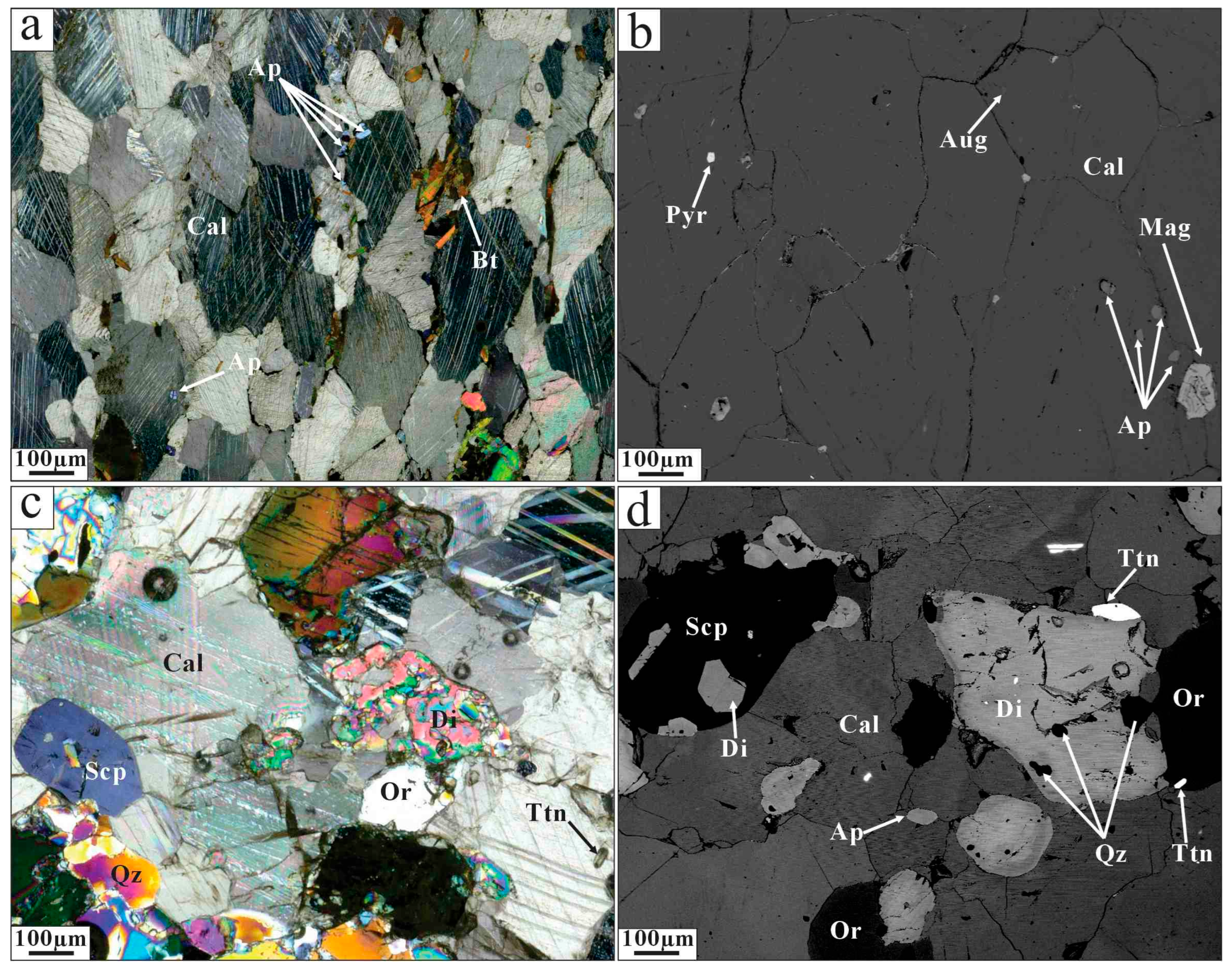
4.1. Petrography
4.1.1. Sillai Pati (SP) Carbonatites
4.1.2. Loe Shilman (LS) Carbonatites
4.1.3. Warsak Carbonatites (WC)
4.1.4. Jambil Carbonatites (JC)
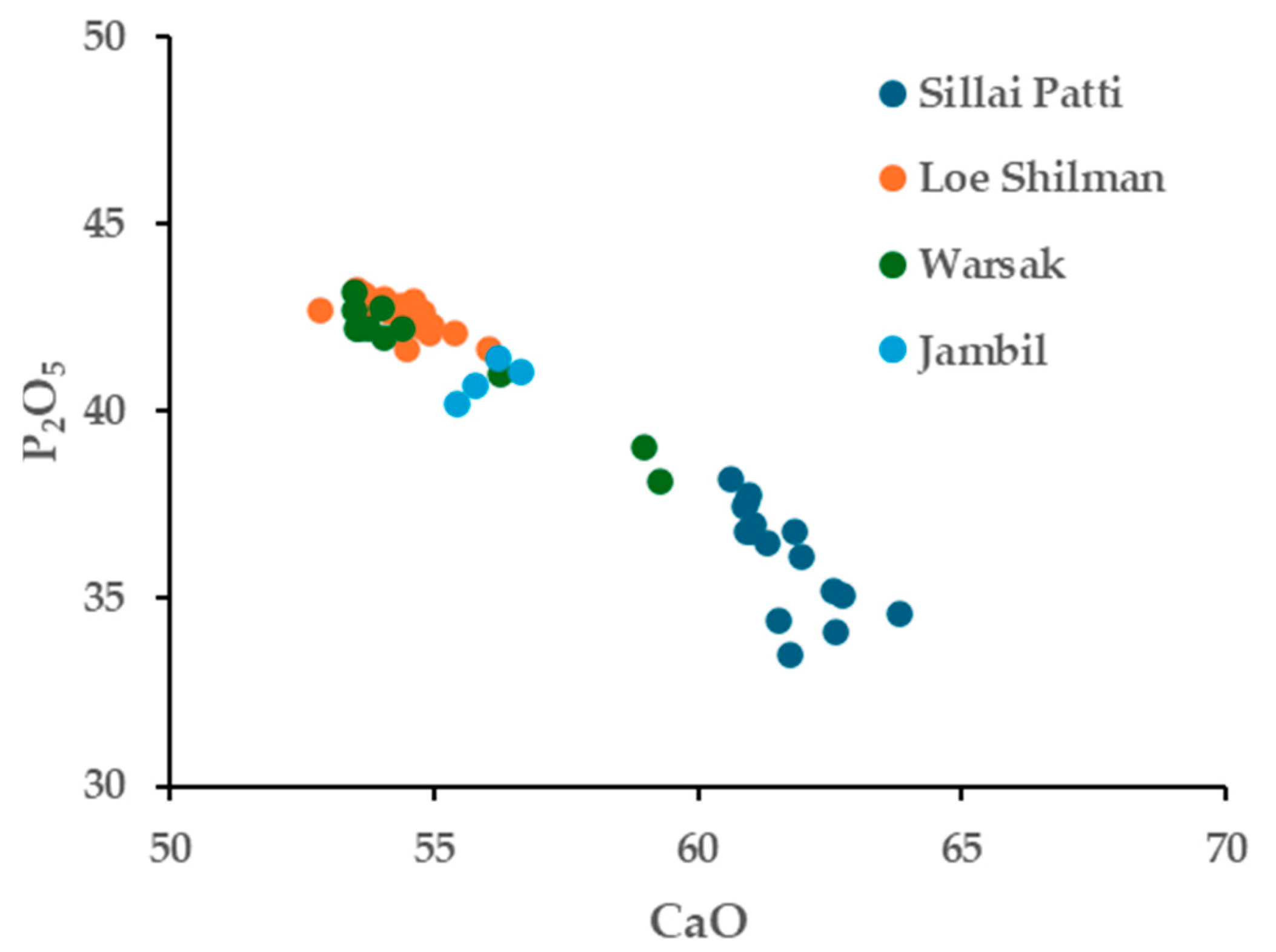
4.2. Whole-Rock Geochemistry
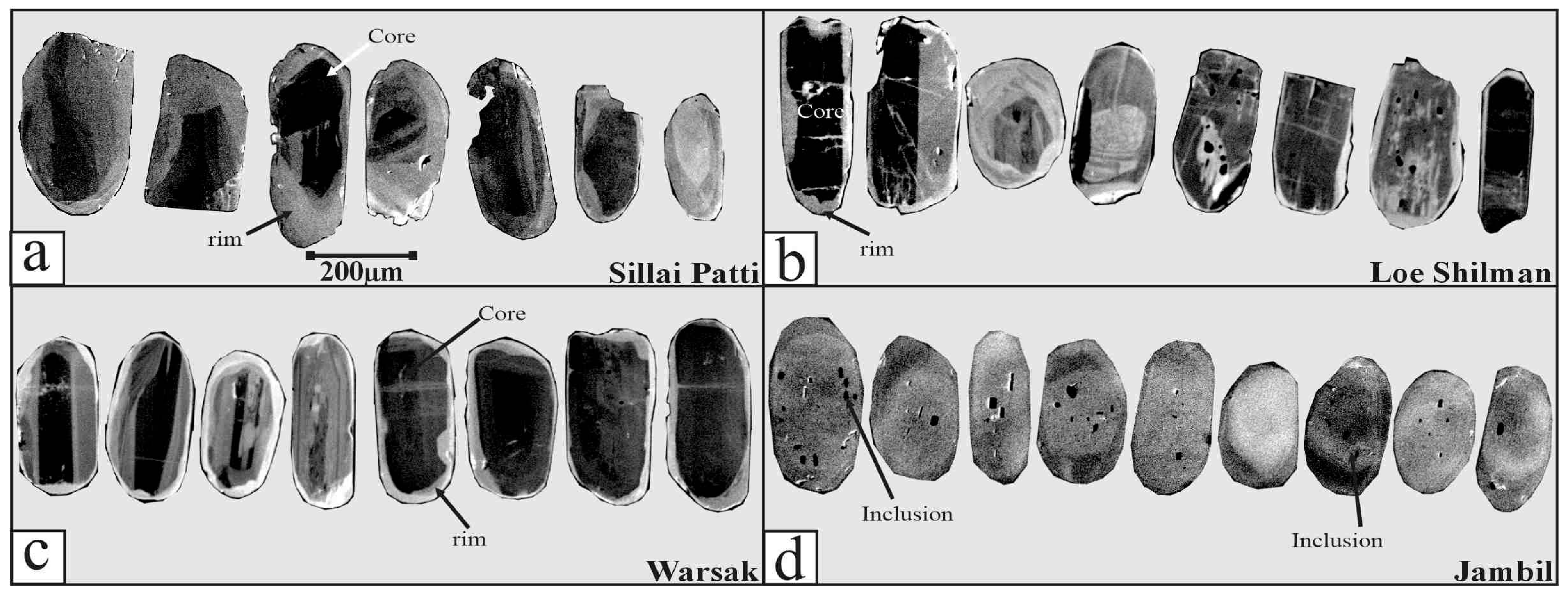
4.3. Apatite Textures
4.4. Mineral Chemistry
4.4.1. Apatite
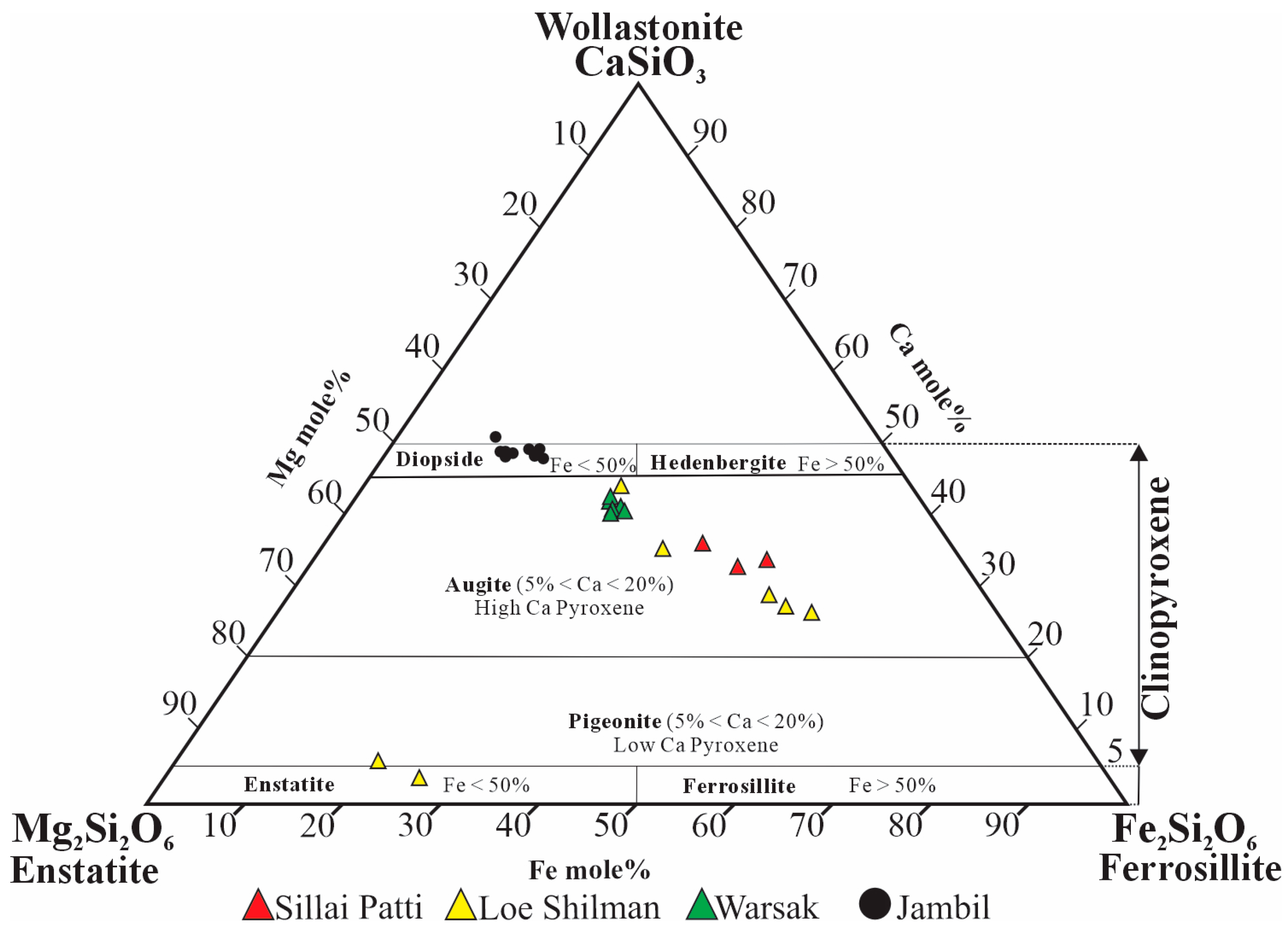
4.4.2. Pyroxene
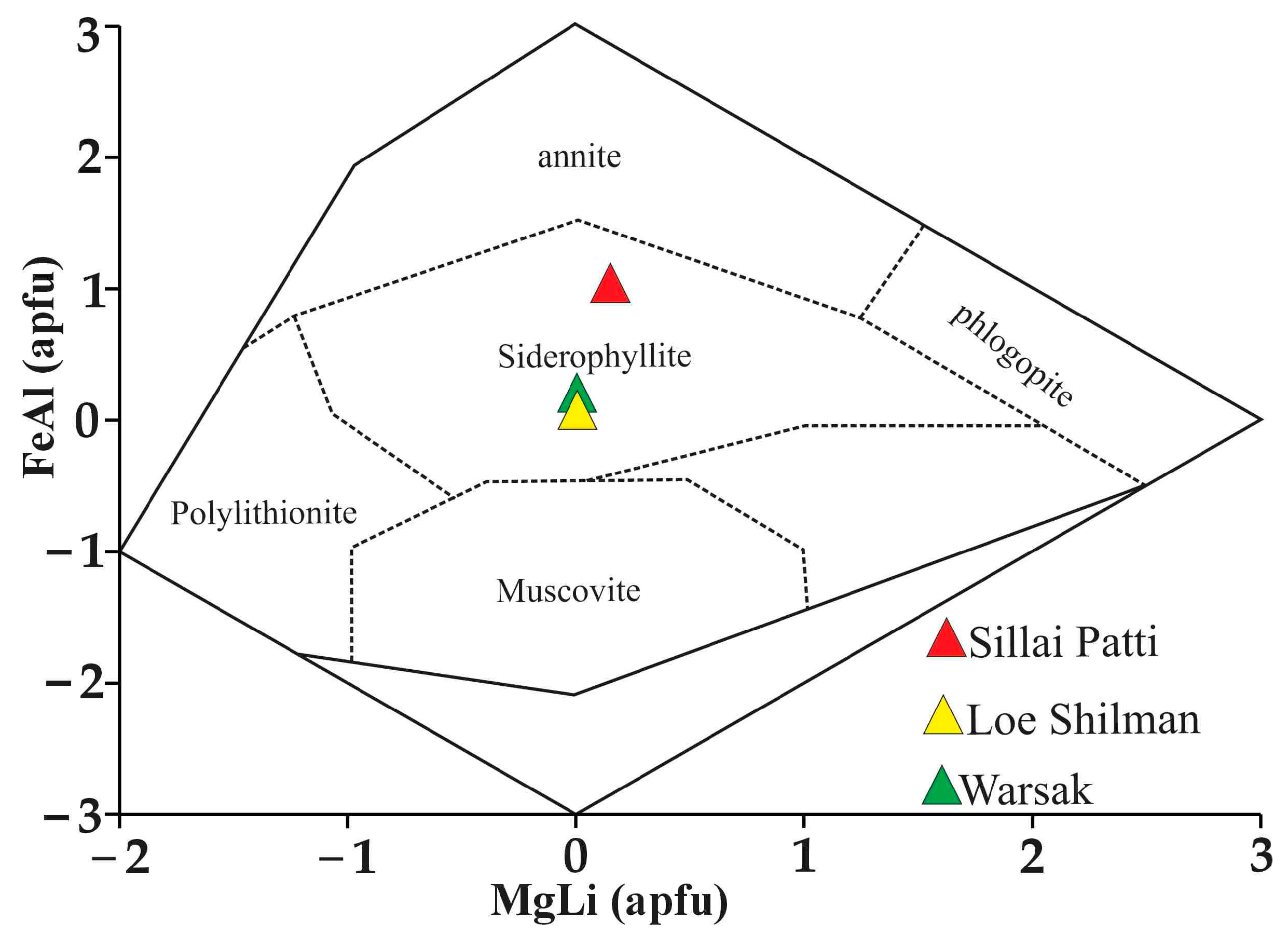
4.4.3. Biotite
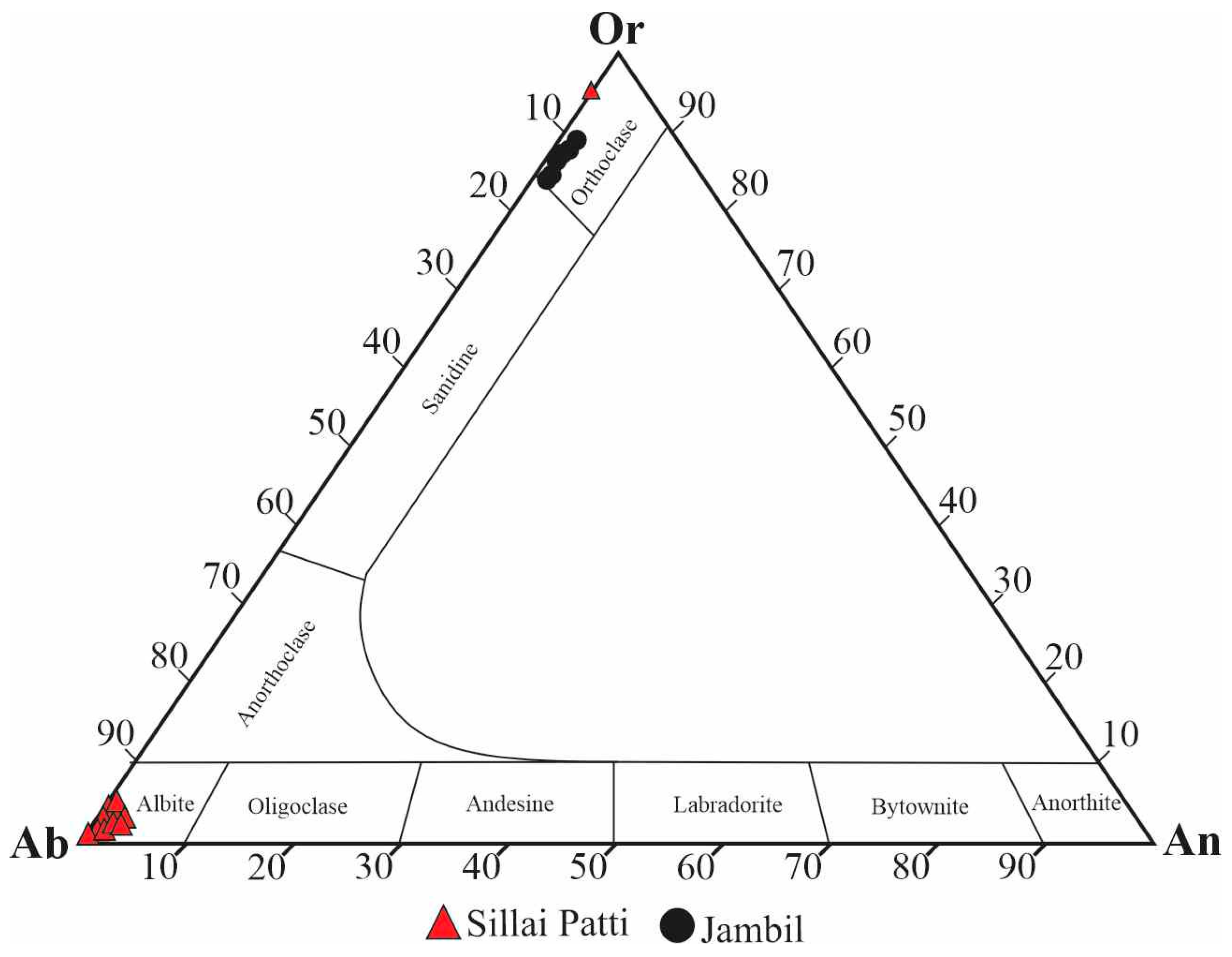
4.4.4. Feldspars
4.4.5. Opaques
4.4.6. Rare Earth Element-Bearing Minerals
4.4.7. Scapolite
5. Discussion
5.1. Interpretations Based on Petrography Regarding Magmatic Process
5.2. Interpretations Based on Whole-Rock Geochemistry
5.3. Interpretations from Mineral Chemistry
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Streckeisen, A. Classification and Nomenclature of Volcanic Rocks, Lamprophyres, Carbonatites, and Melilitic Rocks: Recommendations and Suggestions of the IUGS Subcommission on the Systematics of Igneous Rocks. Geology 1979, 7, 331–335. [Google Scholar] [CrossRef]
- Le Maitre, R.W. Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks. In Igneous Rocks: A Classification and Glossary of Terms; Cambridge University Press: Cambridge, UK, 2003; Volume 140, p. 367. [Google Scholar]
- Kjarsgaard, B.A.; Woolley, A.R. Paragenetic Types of Carbonatite as Indicated by the Diversity and Relative Abundances of Associated Silicate Rocks: Evidence from a Global Database. Geochim. Cosmochim. Acta Suppl. 2008, 72, A478. [Google Scholar]
- Woolley, A.R.; Kempe, D.R.C. Carbonatites: Nomenclature, Average Chemical Compositions and Element Distributions. In Carbonatites—Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 1–14. [Google Scholar]
- Aranha, M.; Porwal, A.; González-Álvarez, I. Indian Carbonatites in the Global Tectonic Context. Ore Energy Resour. Geol. 2023, 15, 100023. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Doroshkevich, A.G.; Elliott, H.A.L.; Zaitsev, A.N. Carbonatites: Contrasting, Complex, and Controversial. Elem. Int. Mag. Mineral. Geochem. Petrol. 2021, 17, 307–314. [Google Scholar] [CrossRef]
- Carnevale, G.; Caracausi, A.; Correale, A.; Italiano, L.; Rotolo, S.G. An Overview of the Geochemical Characteristics of Oceanic Carbonatites: New Insights from Fuerteventura Carbonatites (Canary Islands). Minerals 2021, 11, 203. [Google Scholar] [CrossRef]
- D’orazio, M.; Innocenti, F.; Tonarini, S.; Doglioni, C. Carbonatites in a Subduction System: The Pleistocene Alvikites from Mt. Vulture (Southern Italy). Lithos 2007, 98, 313–334. [Google Scholar] [CrossRef]
- Doucelance, R.; Hammouda, T.; Moreira, M.; Martins, J.C. Geochemical Constraints on Depth of Origin of Oceanic Carbonatites: The Cape Verde Case. Geochim. Cosmochim. Acta 2010, 74, 7261–7282. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Mostofa, K.M.G.; Wang, Y.; Yu, S.; Cai, Z.; Li, P.; Zhou, G.; Fu, C.; Mao, X. Petrogenesis of Carbonatites in the Luliangshan Region, North Qaidam, Northern Tibet, China: Evidence for Recycling of Sedimentary Carbonate and Mantle Metasomatism within a Subduction Zone. Lithos 2018, 322, 148–165. [Google Scholar] [CrossRef]
- Nasir, S.; Al-Khirbash, S.; Rollinson, H.; Al-Harthy, A.; Al-Sayigh, A.; Al-Lazki, A.; Theye, T.; Massonne, H.-J.; Belousova, E. Petrogenesis of Early Cretaceous Carbonatite and Ultramafic Lamprophyres in a Diatreme in the Batain Nappes, Eastern Oman Continental Margin. Contrib. Mineral. Petrol. 2011, 161, 47–74. [Google Scholar] [CrossRef]
- Vladykin, N.V.; Pirajno, F. Types of Carbonatites: Geochemistry, Genesis and Mantle Sources. Lithos 2021, 386, 105982. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Anenburg, M.; Tappe, S.; Decree, S.; Guzmics, T. Carbonatites: Classification, Sources, Evolution, and Emplacement. Annu. Rev. Earth Planet Sci. 2022, 50, 261–293. [Google Scholar] [CrossRef]
- Panina, L.I.; Motorina, I.V. Liquid Immiscibility in Deep-Seated Magmas and the Generation of Carbonatite Melts. Geochem. Int. 2008, 46, 448–464. [Google Scholar] [CrossRef]
- Ray, J.S.; Trivedi, J.R.; Dayal, A.M. Strontium Isotope Systematics of Amba Dongar and Sung Valley Carbonatite-Alkaline Complexes, India: Evidence for Liquid Immiscibility, Crustal Contamination and Long-Lived Rb/Sr Enriched Mantle Sources. J. Asian Earth Sci. 2000, 18, 585–594. [Google Scholar] [CrossRef]
- Nadeau, O.; Stevenson, R.; Jébrak, M. Evolution of Montviel Alkaline–Carbonatite Complex by Coupled Fractional Crystallization, Fluid Mixing and Metasomatism—Part I: Petrography and Geochemistry of Metasomatic Aegirine–Augite and Biotite: Implications for REE–Nb Mineralization. Ore Geol. Rev. 2016, 72, 1143–1162. [Google Scholar] [CrossRef]
- Su, B.-X.; Pan, Q.-Q.; Bai, Y.; Li, W.-J.; Cui, M.-M.; Pang, K.-N. Potassium Isotope Fractionation during Silicate-Carbonatite Melt Immiscibility and Phlogopite Fractional Crystallization. Am. Mineral. 2024, 109, 591–598. [Google Scholar] [CrossRef]
- Woolley, A.R.; Kjarsgaard, B.A. Paragenetic Types of Carbonatite as Indicated by the Diversity and Relative Abundances of Associated Silicate Rocks: Evidence from a Global Database. Can. Mineral. 2008, 46, 741–752. [Google Scholar] [CrossRef]
- Gittins, J.; Harmer, R.E. Myth and Reality in the Carbonatite–Silicate Rock “Association”. Period. Mineral. 2003, 72, 19–26. [Google Scholar]
- Jones, A.P.; Genge, M.; Carmody, L. Carbonate Melts and Carbonatites. Rev. Miner. Geochem. 2013, 75, 289–322. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Deady, E.A.; Beard, C.D.; Broom-Fendley, S.; Elliott, H.A.L.; van den Berg, F.; Öztürk, H. Carbonatites and Alkaline Igneous Rocks in Post-Collisional Settings: Storehouses of Rare Earth Elements. J. Earth Sci. 2021, 32, 1332–1358. [Google Scholar] [CrossRef]
- Yang, J.; Song, W.; Liu, Y.; Zhu, X.; Kynicky, J.; Chen, Q. Mineralogy and Element Geochemistry of the Bayan Obo (China) Carbonatite Dykes: Implications for REE Mineralization. Ore Geol. Rev. 2024, 165, 105873. [Google Scholar] [CrossRef]
- Randive, K.; Meshram, T. An Overview of the Carbonatites from the Indian Subcontinent. Open Geosci. 2020, 12, 85–116. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Mian, I.; Rex, D.C. Age and Nature of Carbonatite Emplacement in North Pakistan. Geol. Rundsch. 1987, 76, 317–323. [Google Scholar] [CrossRef]
- Kempe, D.R.C.; Jan, M.Q. The Peshawar Plain Alkaline Igneous Province, NW Pakistan; University of Peshawar: Peshawar, Pakistan, 1980. [Google Scholar]
- Zhu, Y.-X.; Wang, L.-X.; Khattak, N.U.; Ma, C.-Q.; Luo, G.-M.; Ulrich, T. Petrogenesis of the Late Cretaceous Sillai Patti Carbonatites, NW Pakistan: Insights from CO-Sr-Nd Isotopes and Titanite UPb Dating. Lithos 2023, 442, 107087. [Google Scholar] [CrossRef]
- Khan, A.; Ullah, Z.; Li, H.; Faisal, S.; Rahim, Y. Apatite Texture, Trace Elements and SrNd Isotope Geochemistry of the Koga Carbonatite-Alkaline Complex, NW Pakistan: Implications for Petrogenesis and Mantle Source. Chem. Geol. 2025, 676, 122611. [Google Scholar] [CrossRef]
- Khan, A.; Faisal, S.; Ullah, Z.; Ali, L.; Ghaffari, A.; Nawab, J.; Rashid, M.U. Pyrochlore-Group Minerals from the Loe-Shilman Carbonatite Complex, NW Pakistan: Implications for Evolution of Carbonatite System. Period. Mineral. 2021, 90, 277–287. [Google Scholar]
- Hong, J.; Khan, T.; Li, W.; Khalil, Y.S.; Narejo, A.A.; Rashid, M.U.; Zeb, M.J. SHRIMP U–Pb Ages, Mineralogy, and Geochemistry of Carbonatite–Alkaline Complexes of the Sillai Patti and Koga Areas, NW Pakistan: Implications for Petrogenesis and REE Mineralization. Ore Geol. Rev. 2021, 139, 104547. [Google Scholar] [CrossRef]
- Khan, A.; Faisal, S.; Larson, K.P.; Robinson, D.M.; Li, H.; Ullah, Z.; Button, M.; Nawab, J.; Farhan, M.; Ali, L. Geochemistry and In-Situ U-Th/Pb Geochronology of the Jambil Meta-Carbonatites, Northern Pakistan: Implications on Petrogenesis and Tectonic Evolution. J. Earth Sci. 2023, 34, 70–85. [Google Scholar] [CrossRef]
- Aslam, M.; Hussain, A.; Ashraf, M.; Afridi, A.G.K. Geological Map of North West Frontier Province Pakistan; Geological Survey of Pakistan: Balochistan, Pakistan, 2006. [Google Scholar]
- Khan, S.R.; Khan, M.A.; Nawaz, R.; Karim, T. Stratigraphic Control for the Age of Peshawar-Plain Magmatism, Northern Pakistan. In Geological Bulletin; University of Peshawar: Peshawar, Pakistan, 1990; Volume 23, pp. 253–263. [Google Scholar]
- Pogue, K.R.; Wardlaw, B.R.; Harris, A.G.; Hussain, A. Paleozoic and Mesozoic Stratigraphy of the Peshawar Basin, Pakistan: Correlations and Implications. Geol. Soc. Am. Bull. 1992, 104, 915–927. [Google Scholar] [CrossRef]
- Kempe, D.R.C. A Note on the Ages of the Alkaline Rocks of the Peshawar Plain Alkaline Igneous Province, NW Pakistan. Geol. Bull. Univ. Peshawar 1986, 19, 113–119. [Google Scholar]
- Rafiq, M.; Ali, A.; Ali, S. Petrography of Alkaline-Igneous Complex from Michini, Mohmand Agency, NWFP, Pakistan. Geol Bull. Univ. Peshawar 2005, 38, 81–88. [Google Scholar]
- Rafiq, M.; Jan, M.Q. Geochemistry and Petrogenesis of Ambela Granitic Complex, NW Pakistan. Geological Bulletin, University of Peshawar 1989, 22, 159–179. [Google Scholar]
- ur Rashid, M.; Rehman, H.U.; Yamamoto, H.; Muhammad, J.Z. Petrography, Textural and Chemical Characteristics of Carbonatites from Northwest Himalayas Pakistan. Rep. Fac. Sci. Kagoshima Univ. 2023, 56, 9–18. [Google Scholar]
- Jan, M.Q.; Karim, A. Continental Magmatism Related to Late Paleozoic-Early Mesozoic Rifting in Northern Pakistan and Kashmir. J. Himal. Earth Sci. 1990, 23, 1–25. [Google Scholar]
- Tilton, G.R.; Bryce, J.G.; Mateen, A. Pb–Sr–Nd Isotope Data from 30 and 300 Ma Collision Zone Carbonatites in Northwest Pakistan. J. Petrol. 1998, 39, 1865–1874. [Google Scholar] [CrossRef]
- Khan, A.; Faisal, S.; Larson, K.P.; Robinson, D.M.; Ullah, Z.; Li, H.; Rehman, H.U. New Geochronological and Geochemical Constraints on Petrogenesis and Tectonic Setting of the Loe-Shilman Carbonatite Complex, Northwest Pakistan. Lithos 2021, 404, 106497. [Google Scholar] [CrossRef]
- Khan, M.; Li, H.; Algeo, T.J.; Khan, A.; Khan, A.; Xie, Y. Apatite as an Indicator of Tectono-Magmatic Evolution of Silica-Undersaturated to Silica-Oversaturated Rocks on the NW Indian Plate Margin. Chem. Geol. 2024, 672, 122516. [Google Scholar] [CrossRef]
- Mian, I. The Mineralogy and Geochemistry of the Carbonatites, Syenites and Fenites of North West Frontier Province Pakistan; University of Leicester: Leicester, UK, 1987; ISBN 1073242951. [Google Scholar]
- Khattak, N.U.; Khan, M.A.; Ali, N.; Abbas, S.M.; Tahirkheli, T.K. Recognition of the Time and Level of Emplacement of the Sillai Patti Carbonatite Complex, Malakand Division, Northwest Pakistan: Constraints from Fission-Track Dating. Russ. Geol. Geophys. 2012, 53, 736–744. [Google Scholar] [CrossRef]
- Khattak, N.U.; Akram, M.; Khan, M.A.; Khan, H.A. Emplacement Time of the Loe Shilman Carbonatite from NW Pakistan: Constraints from Fission-Track Dating. Radiat. Meas. 2008, 43, S313–S318. [Google Scholar] [CrossRef]
- Qasim, M.; Khan, S.D. Detection and Relative Quantification of Neodymium in Sillai Patti Carbonatite Using Decision Tree Classification of the Hyperspectral Data. Sensors 2022, 22, 7537. [Google Scholar] [CrossRef]
- Kempe, D.R.C. The Petrology of the Warsak Alkaline Granites, Pakistan, and Their Relationship to Other Alkaline Rocks of the Region. Geol. Mag. 1973, 110, 385–404. [Google Scholar] [CrossRef]
- Khan, A.; Aslam, M.; Khan, R.N. Regional Geological Map of the Jamrud Quadrangle Khyber Agency, NWFP; Geological Survey of Pakistan: Balochistan, Pakistan, 1995. [Google Scholar]
- Khan, R.N.; Iqbal, S.; Khan, S.; Aslam, M. Regional Geological Map of the Marghuzar Quadrangle Swat and Buner District NWFP; Geological Survey of Pakistan: Balochistan, Pakistan, 1995. [Google Scholar]
- Khattak, N.U.; Qureshi, A.A.; Akram, M.; Ullah, K.; Azhar, M.; Khan, M.A. Unroofing History of the Jambil and Jawar Carbonatite Complexes from NW Pakistan: Constraints from Fission-Track Dating of Apatite. J. Asian Earth Sci. 2005, 25, 643–652. [Google Scholar] [CrossRef]
- Tischendorf, G.; Förster, H.-J.; Gottesmann, B. The Correlation between Lithium and Magnesium in Trioctahedral Micas: Improved Equations for Li2O Estimation from MgO Data. Miner. Mag. 1999, 63, 57–74. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Behera, K.K.; Swain, S.P. Apatite-Hosted REE Mineralization in the Eastern Ghats Mobile Belt: A Future Prospect for REE. Geosystems Geoenvironment 2024, 3, 100290. [Google Scholar] [CrossRef]
- Benson, E.K.; Watts, K.E. Apatite and Monazite Geochemistry Record Magmatic and Metasomatic Processes in Rare Earth Element Mineralization at Mountain Pass, California. Econ. Geol. 2024, 119, 1611–1642. [Google Scholar] [CrossRef]
- Walter, B.F.; Giebel, R.J.; Steele-MacInnis, M.; Marks, M.A.W.; Kolb, J.; Markl, G. Fluids Associated with Carbonatitic Magmatism: A Critical Review and Implications for Carbonatite Magma Ascent. Earth Sci. Rev. 2021, 215, 103509. [Google Scholar] [CrossRef]
- Aranha, M.; Porwal, A.; Sundaralingam, M.; González-Álvarez, I.; Markan, A.; Rao, K. Rare Earth Elements Associated with Carbonatite–Alkaline Complexes in Western Rajasthan, India: Exploration Targeting at Regional Scale. Solid Earth 2022, 13, 497–518. [Google Scholar] [CrossRef]
- Hamisi, J.; Etschmann, B.; Tomkins, A.; Pitcairn, I.; Pintér, Z.; Wlodek, A.; Morrissey, L.; Micklethwaite, S.; Trcera, N.; Mills, S. Complex Sulfur Speciation in Scapolite–Implications for the Role of Scapolite as a Redox and Fluid Chemistry Buffer in Crustal Fluids. Gondwana Res. 2023, 121, 418–435. [Google Scholar] [CrossRef]
- Li, H.; Han, J.; Li, F.; Li, H.; Zhao, Z.; Liu, Y.; Chen, J.; Yin, Y.; Han, Y. Apatite Textural and Geochemical Insights into the Petrogenesis of Intrusive Rocks. Sci. Rep. 2024, 14, 31985. [Google Scholar] [CrossRef]
- Lu, J.; Chen, W.; Ying, Y.; Jiang, S.; Zhao, K. Apatite Texture and Trace Element Chemistry of Carbonatite-Related REE Deposits in China: Implications for Petrogenesis. Lithos 2021, 398, 106276. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E. Compositions of the Apatite-Group Minerals: Substitution Mechanisms and Controlling Factors. Rev. Miner. Geochem. 2002, 48, 13–49. [Google Scholar] [CrossRef]
- Belousova, E.A.; Griffin, W.L.; O’Reilly, S.Y.; Fisher, N.I. Apatite as an Indicator Mineral for Mineral Exploration: Trace-Element Compositions and Their Relationship to Host Rock Type. J. Geochem. Explor. 2002, 76, 45–69. [Google Scholar] [CrossRef]
- Viladkar, S.G. Pyroxene-Sövite in Amba Dongar Carbonatite-Alkalic Complex, Gujarat. J. Geol. Soc. India 2017, 90, 591–594. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.-X.; Malaviarachchi, S.P.K.; Sakyi, P.A.; Dharmapriya, P.L. Transformation of Olivine to Diopside via Interaction with Carbonatite Melts. Sci. China Earth Sci. 2023, 66, 2332–2339. [Google Scholar] [CrossRef]
- Anenburg, M.; Walters, J.B. Metasomatic Ijolite, Glimmerite, Silicocarbonatite, and Antiskarn Formation: Carbonatite and Silicate Phase Equilibria in the System Na2O–CaO–K2O–FeO–MgO–Al2O3–SiO2–H2O–O2–CO2. Contrib. Mineral. Petrol. 2024, 179, 40. [Google Scholar] [CrossRef]
- Prokopyev, I.; Kozlov, E.; Fomina, E.; Doroshkevich, A.; Dyomkin, M. Mineralogy and Fluid Regime of Formation of the REE-Late-Stage Hydrothermal Mineralization of Petyayan-Vara Carbonatites (Vuoriyarvi, Kola Region, NW Russia). Minerals 2020, 10, 405. [Google Scholar] [CrossRef]
- Wagner, T.; Boyce, A.J.; Erzinger, J. Fluid-Rock Interaction during Formation of Metamorphic Quartz Veins: A REE and Stable Isotope Study from the Rhenish Massif, Germany. Am. J. Sci. 2010, 310, 645–682. [Google Scholar] [CrossRef]
- Bühn, B.; Trumbull, R.B. Comparison of Petrogenetic Signatures between Mantle-Derived Alkali Silicate Intrusives with and without Associated Carbonatite, Namibia. Lithos 2003, 66, 201–221. [Google Scholar] [CrossRef]
- Baudouin, C.; Parat, F.; Michel, T. CO2-Rich Phonolitic Melt and Carbonatite Immiscibility in Early Stage of Rifting: Melt Inclusions from Hanang Volcano (Tanzania). J. Volcanol. Geotherm. Res. 2018, 358, 261–272. [Google Scholar] [CrossRef]
- Brod, J.A.; Gaspar, J.C.; De Araújo, D.P.; Gibson, S.A.; Thompson, R.N.; Junqueira-Brod, T.C. Phlogopite and Tetra-Ferriphlogopite from Brazilian Carbonatite Complexes: Petrogenetic Constraints and Implications for Mineral-Chemistry Systematics. J. Asian Earth Sci. 2001, 19, 265–296. [Google Scholar] [CrossRef]
- Lee, M.J.; Garcia, D.; Moutte, J.; Lee, J.I. Phlogopite and Tetraferriphlogopite from Phoscorite and Carbonatite Associations in the Sokli Massif, Northern Finland. Geosci. J. 2003, 7, 9–20. [Google Scholar] [CrossRef]
- Gajdošová, M.; Huraiová, M.; Hurai, V.; Slobodník, M.; Siegfried, P.R. Two Types of Scapolite in Evate Carbonatite Deposit (Mozambique): Implications for Magmatic versus Metamorphic Origins. Acta Geol. Slovaca 2019, 11, 63–74. [Google Scholar]
- Le Bas, M.J. Fenites Associated with Carbonatites. Can. Mineral. 2008, 46, 915–932. [Google Scholar] [CrossRef]
- Anenburg, M.; Mavrogenes, J.A.; Frigo, C.; Wall, F. Rare Earth Element Mobility in and around Carbonatites Controlled by Sodium, Potassium, and Silica. Sci. Adv. 2020, 6, eabb6570. [Google Scholar] [CrossRef]
- Anenburg, M.; Broom-Fendley, S.; Chen, W. Formation of Rare Earth Deposits in Carbonatites. Elem. Int. Mag. Mineral. Geochem. Petrol. 2021, 17, 327–332. [Google Scholar] [CrossRef]
- Carnevale, G.; Arroyo Rey, X.; Correale, A.; Rotolo, S.G. Procesos Hidrotermales Con Enriquecimiento En REE En Las Carbonatitas de Fuerteventura: Evidencias En Minerales Accesorios. Geogaceta 2021, 69, 23–26. [Google Scholar] [CrossRef]


| Mineral Phase | Sillai Patti | Loe Shilman | Warsak | Jambil | |
|---|---|---|---|---|---|
| Calcitic | Biotite | ||||
| Calcite |  |  |  |  |  |
| Apatite |  |  |  |  |  |
| Pyroxene |  |  |  |  |  |
| Biotite |  |  |  |  | |
| Titanite |  |  |  | ||
| Amphibole |  | ||||
| Alkali feldspar |  |  | |||
| Opaque |  |  |  |  |  |
| Quartz |  |  | |||
| Scapolite |  | ||||
| Monazite |  |  | |||
| Pyrochlore |  | ||||
| Britholite |  | ||||
| Zircon |  |  | |||
| Locality | Spot | CaO | Al2O3 | SiO2 | P2O5 | TiO2 | FeO | La2O3 | CeO2 | Nb2O5 | Nd2O3 | Total | Minerals | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sillai Patti | i4 | 4.3 | 0.08 | 0.09 | 30.45 | 0.37 | 0.05 | 25.44 | 39.21 | b.d.l | b.d.l | 99.99 | Mnz | |
| m4 | b.d.l | b.d.l | b.d.l | 34.65 | b.d.l | b.d.l | 26.27 | 39.08 | b.d.l | b.d.l | 100 | Mnz | ||
| m5 | b.d.l | b.d.l | b.d.l | 32.65 | b.d.l | b.d.l | 31.22 | 35.69 | b.d.l | b.d.l | 99.56 | Mnz | ||
| n6 | b.d.l | b.d.l | b.d.l | 33.2 | b.d.l | b.d.l | 29.31 | 37.49 | b.d.l | b.d.l | 99.99 | Mnz | ||
| Loe Shilman | LS—Biotite variety | a5 | 10.15 | 10.82 | 33 | b.d.l | b.d.l | 21.33 | 10.27 | 14.44 | b.d.l | b.d.l | 100.01 | Brt |
| a6 | 9.9 | 11.93 | 32.15 | 0.01 | 1.72 | 19.81 | 9.82 | 13.64 | b.d.l | b.d.l | 98.98 | Brt | ||
| c5 | 10.52 | 11.77 | 33.56 | b.d.l | b.d.l | 21.06 | 10.42 | 12.68 | b.d.l | b.d.l | 100.01 | Brt | ||
| c6 | 11.94 | 11.88 | 33.68 | b.d.l | b.d.l | 20.86 | b.d.l | 12.87 | b.d.l | b.d.l | 91.23 | Brt | ||
| c7 | 11.24 | 11.29 | 33.17 | b.d.l | b.d.l | 20.39 | 10.81 | 13.1 | b.d.l | b.d.l | 100 | Brt | ||
| c8 | 10.39 | 11.96 | 34.07 | b.d.l | b.d.l | 20.37 | 9.34 | 13.86 | b.d.l | b.d.l | 99.99 | Brt | ||
| d7 | 11.01 | 9.93 | 32.78 | b.d.l | b.d.l | 21.35 | 9.79 | 14.1 | b.d.l | b.d.l | 98.96 | Brt | ||
| LS—Calcitic Variety | a1 | 14.17 | b.d.l | b.d.l | b.d.l | 2.44 | b.d.l | b.d.l | 2.55 | 80.84 | b.d.l | 100 | Pcl | |
| a2 | 14.42 | b.d.l | b.d.l | b.d.l | 1.61 | b.d.l | b.d.l | 2.72 | 81.25 | b.d.l | 100 | Pcl | ||
| a3 | 14.78 | b.d.l | b.d.l | b.d.l | 3.25 | b.d.l | b.d.l | b.d.l | 81.97 | b.d.l | 100 | Pcl | ||
| a4 | 15.17 | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | 84.83 | b.d.l | 100 | Pcl | ||
| b1 | b.d.l | b.d.l | b.d.l | 32.69 | b.d.l | b.d.l | 28.04 | 39.27 | b.d.l | b.d.l | 100 | Mnz | ||
| b2 | 56.16 | b.d.l | b.d.l | 11.39 | b.d.l | b.d.l | 13.1 | 17.76 | b.d.l | b.d.l | 98.41 | Mnz | ||
| b3 | b.d.l | b.d.l | b.d.l | 32.09 | b.d.l | b.d.l | 29.48 | 38.43 | b.d.l | b.d.l | 100 | Mnz | ||
| b4 | b.d.l | b.d.l | b.d.l | 32.01 | b.d.l | b.d.l | 28.85 | 39.15 | b.d.l | b.d.l | 100.01 | Mnz | ||
| b5 | b.d.l | b.d.l | b.d.l | 31.95 | b.d.l | b.d.l | 30.8 | 37.25 | b.d.l | b.d.l | 100 | Mnz | ||
| b6 | b.d.l | b.d.l | b.d.l | 32.73 | b.d.l | b.d.l | 29.44 | 37.84 | b.d.l | b.d.l | 100.01 | Mnz | ||
| b7 | 4.71 | b.d.l | b.d.l | 30.93 | b.d.l | b.d.l | 27.46 | 36.91 | b.d.l | b.d.l | 100.01 | Mnz | ||
| b8 | 0.86 | b.d.l | b.d.l | 31.84 | b.d.l | b.d.l | 28.48 | 38.82 | b.d.l | b.d.l | 100 | Mnz | ||
| c1 | 13.23 | b.d.l | b.d.l | b.d.l | 4.37 | b.d.l | b.d.l | 2.78 | 76.89 | 2.72 | 100 | Pcl | ||
| c2 | 15.47 | b.d.l | b.d.l | b.d.l | 2.16 | b.d.l | b.d.l | b.d.l | 82.37 | b.d.l | 100 | Pcl | ||
| c3 | 17.27 | b.d.l | b.d.l | b.d.l | 5.07 | b.d.l | b.d.l | b.d.l | 77.66 | b.d.l | 100 | Pcl | ||
| d1 | b.d.l | b.d.l | b.d.l | 32.98 | b.d.l | b.d.l | 28.12 | 38.9 | b.d.l | b.d.l | 100 | Mnz | ||
| d2 | b.d.l | b.d.l | b.d.l | 33.17 | b.d.l | b.d.l | 25.45 | 41.38 | b.d.l | b.d.l | 100 | Mnz | ||
| d5 | 18.21 | b.d.l | b.d.l | 26.35 | b.d.l | b.d.l | 23.41 | 32.04 | b.d.l | b.d.l | 100.01 | Mnz | ||
| d6 | 8.27 | 0.86 | b.d.l | 27.65 | b.d.l | b.d.l | 28.35 | 34.88 | b.d.l | b.d.l | 100.01 | Mnz | ||
| d7 | b.d.l | b.d.l | b.d.l | 33.33 | b.d.l | b.d.l | 25.81 | 40.87 | b.d.l | b.d.l | 100.01 | Mnz | ||
| d8 | b.d.l | b.d.l | b.d.l | 31.96 | b.d.l | b.d.l | 29.38 | 38.66 | b.d.l | b.d.l | 100 | Mnz | ||
| e6 | b.d.l | b.d.l | b.d.l | 33.11 | b.d.l | b.d.l | 25.03 | 41.86 | b.d.l | b.d.l | 100 | Mnz | ||
| e7 | b.d.l | b.d.l | b.d.l | 33.16 | b.d.l | b.d.l | 26.92 | 39.93 | b.d.l | b.d.l | 100.01 | Mnz | ||
| Locality | Spot | CaO | Na2O | MgO | Al2O3 | SiO2 | P2O5 | K2O | TiO2 | Mno | FeO | F | Cl | SO3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jambil Carbonatite | a1 | 10.99 | 7.22 | b.d.l | 23.52 | 50.41 | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | 1.32 | 2.07 | 95.53 |
| a2 | 11.05 | 7.13 | b.d.l | 22.93 | 50.67 | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | 1.28 | 2.10 | 95.16 | |
| a3 | 10.70 | 7.55 | b.d.l | 23.07 | 50.39 | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | 1.32 | 2.09 | 95.12 | |
| c5 | 12.14 | 7.41 | b.d.l | 24.79 | 53.33 | 0.19 | 0.53 | 0.03 | b.d.l | 0.13 | 0.08 | 1.36 | b.d.l | 99.99 | |
| c6 | 12.69 | 6.49 | b.d.l | 24.92 | 51.93 | 0.08 | 0.33 | 0.02 | 0.07 | 0.02 | 0.21 | 1.02 | 2.20 | 99.98 | |
| c7 | 12.75 | 7.00 | b.d.l | 25.33 | 53.07 | 0.10 | 0.50 | 0.05 | 0.05 | 0.02 | b.d.l | 1.13 | b.d.l | 100.00 | |
| d1 | 24.71 | b.d.l | 0.03 | 27.34 | 39.46 | 0.01 | b.d.l | 0.19 | 0.09 | 8.14 | 0.02 | 0.01 | b.d.l | 100.00 | |
| d2 | 24.14 | 0.03 | 0.05 | 28.36 | 39.71 | 0.05 | 0.04 | 0.26 | 0.02 | 7.34 | b.d.l | b.d.l | b.d.l | 100.00 | |
| d3 | 24.52 | 0.01 | 0.08 | 28.53 | 39.47 | 0.14 | b.d.l | 0.13 | 0.23 | 6.90 | b.d.l | b.d.l | b.d.l | 100.01 | |
| e1 | 23.92 | 0.08 | 0.05 | 28.24 | 39.78 | 0.14 | 0.01 | b.d.l | 0.18 | 7.54 | b.d.l | 0.04 | b.d.l | 99.98 | |
| e2 | 24.75 | 0.05 | 0.05 | 28.56 | 39.60 | b.d.l | b.d.l | 0.22 | b.d.l | 6.74 | b.d.l | 0.04 | b.d.l | 100.01 | |
| e3 | 10.99 | 7.22 | b.d.l | 23.52 | 50.41 | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | 1.32 | 2.07 | 95.53 | |
| e4 | 11.05 | 7.13 | b.d.l | 22.93 | 50.67 | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | 1.28 | 2.10 | 95.16 | |
| e5 | 10.70 | 7.55 | b.d.l | 23.07 | 50.39 | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | b.d.l | 1.32 | 2.09 | 95.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.u.; Rehman, H.U. Petrological, Textural, Compositional, and Economic Potential of Carbonatites from the Peshawar Plain Alkaline Igneous Province, Northwestern Himalaya. Minerals 2025, 15, 439. https://doi.org/10.3390/min15050439
Rashid Mu, Rehman HU. Petrological, Textural, Compositional, and Economic Potential of Carbonatites from the Peshawar Plain Alkaline Igneous Province, Northwestern Himalaya. Minerals. 2025; 15(5):439. https://doi.org/10.3390/min15050439
Chicago/Turabian StyleRashid, Mehboob ur, and Hafiz U. Rehman. 2025. "Petrological, Textural, Compositional, and Economic Potential of Carbonatites from the Peshawar Plain Alkaline Igneous Province, Northwestern Himalaya" Minerals 15, no. 5: 439. https://doi.org/10.3390/min15050439
APA StyleRashid, M. u., & Rehman, H. U. (2025). Petrological, Textural, Compositional, and Economic Potential of Carbonatites from the Peshawar Plain Alkaline Igneous Province, Northwestern Himalaya. Minerals, 15(5), 439. https://doi.org/10.3390/min15050439








