Siderite Decomposition Kinetics—Influence of Time, Temperature, and Isomorphous Impurities
Abstract
1. Introduction
2. Materials and Methods
2.1. X-Ray Fluorescence
2.2. X-Ray Powder Diffraction
2.3. Transmission Electron Microscopy
2.4. Mössbauer Spectroscopy
3. Results
3.1. X-Ray Fluorescence
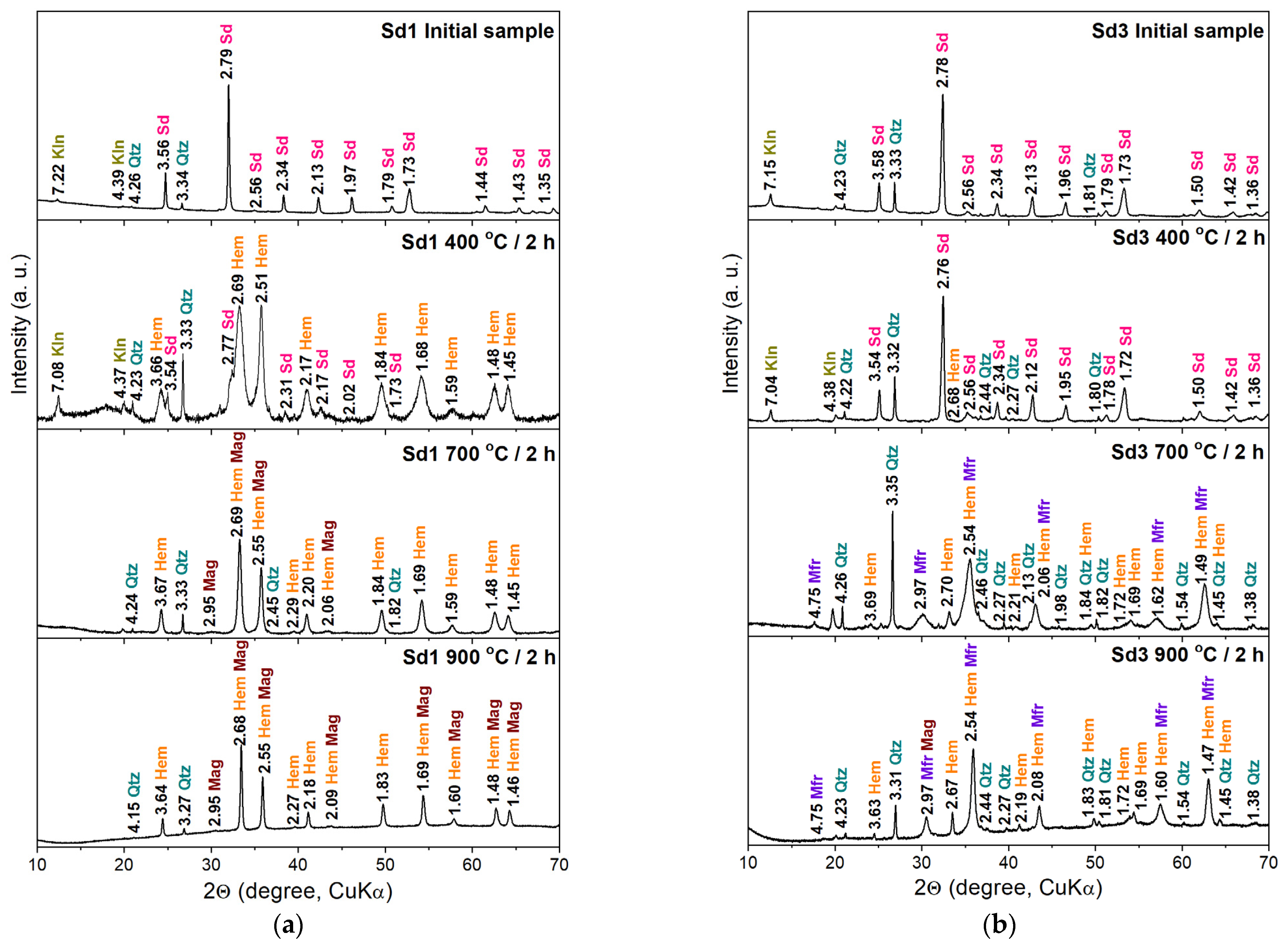
3.2. X-Ray Powder Diffraction
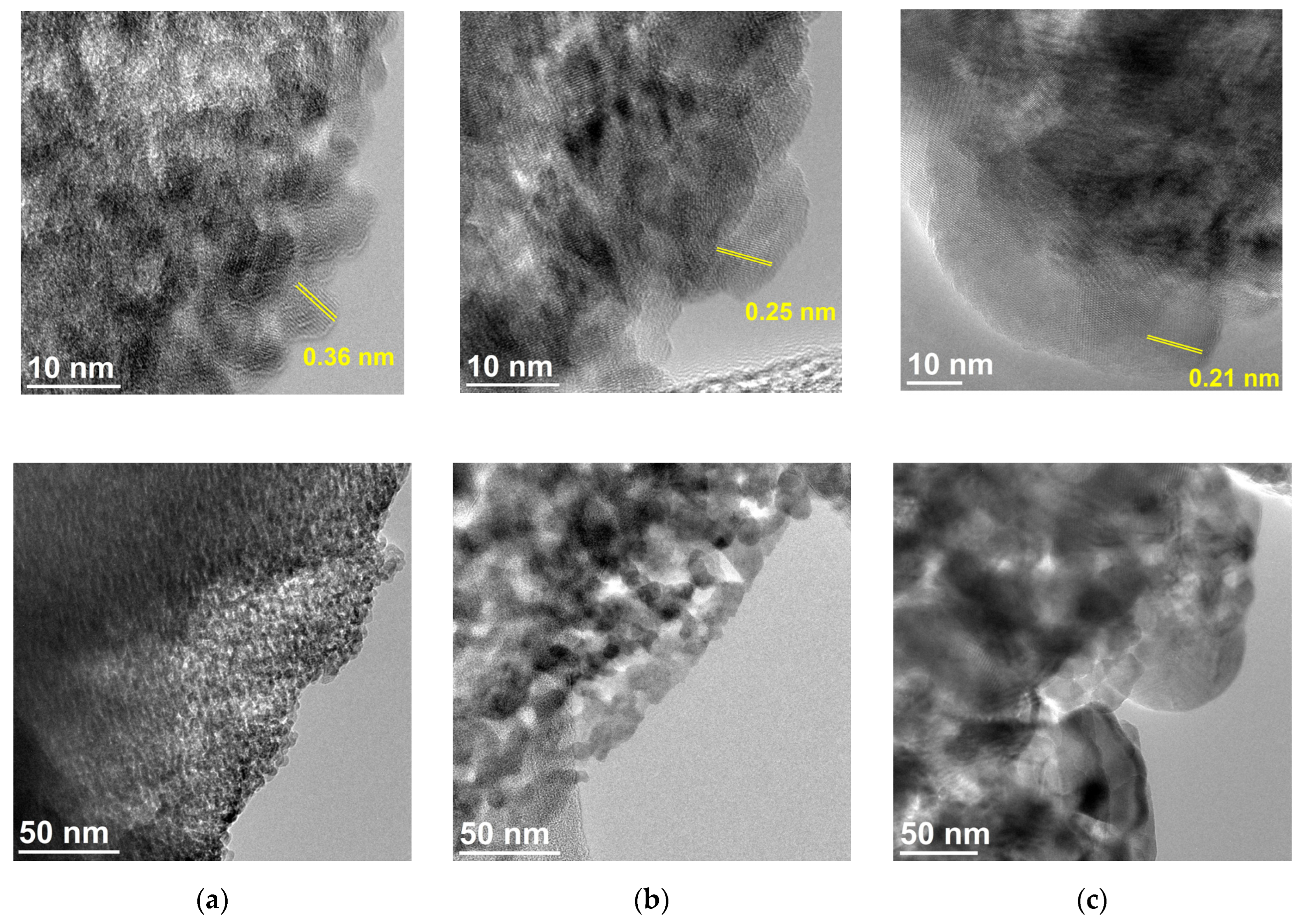
3.3. Transmission Electron Microscopy
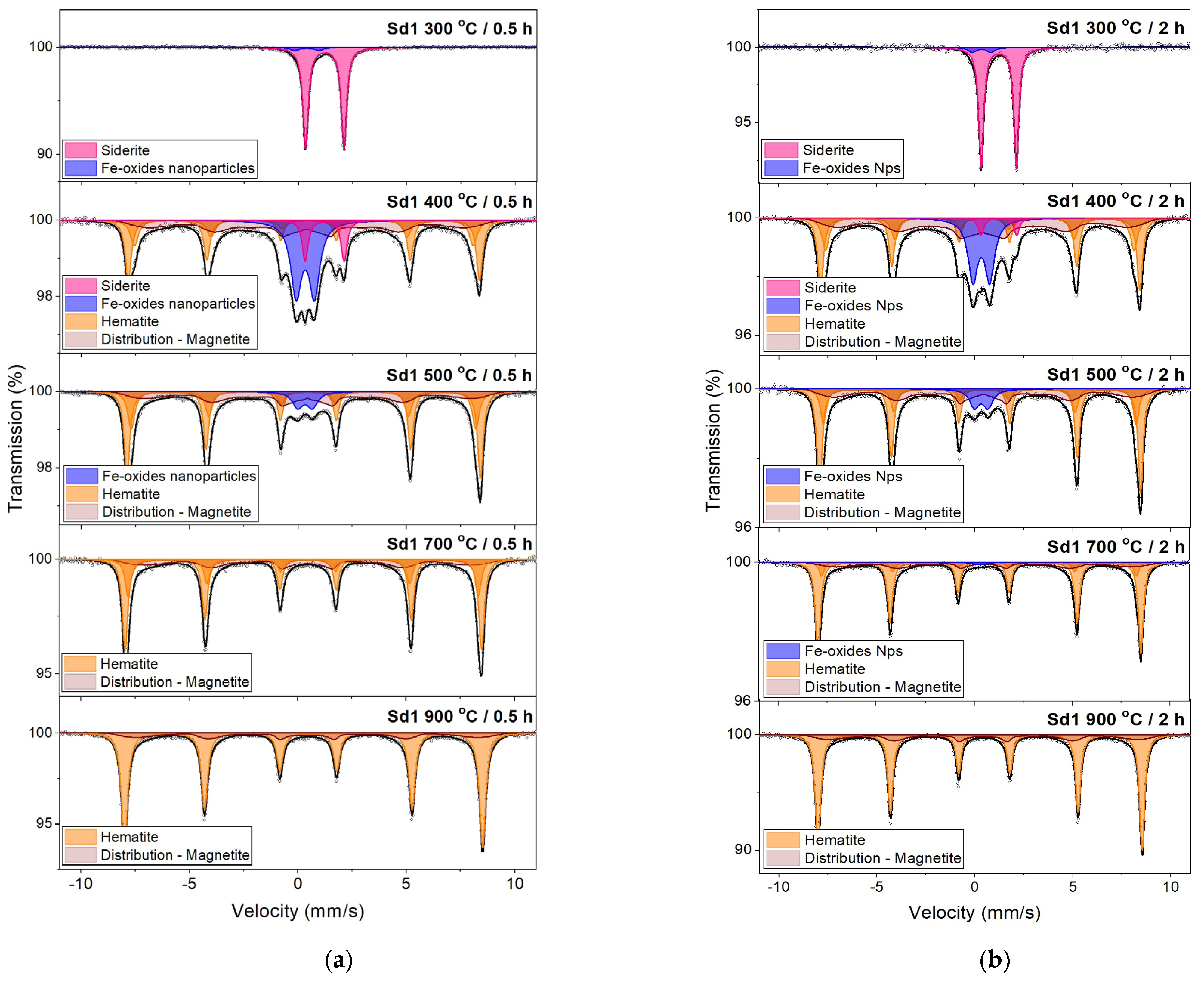
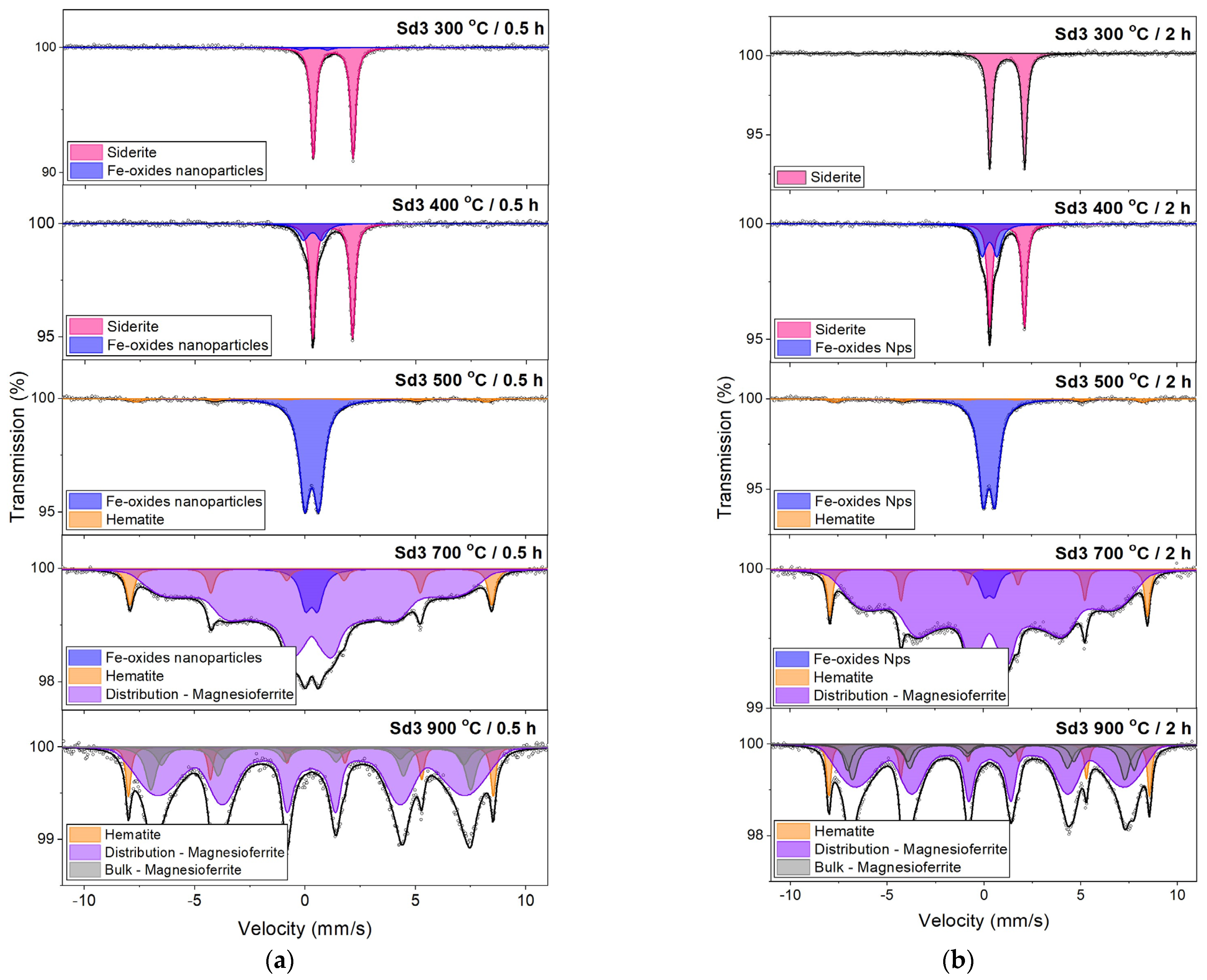
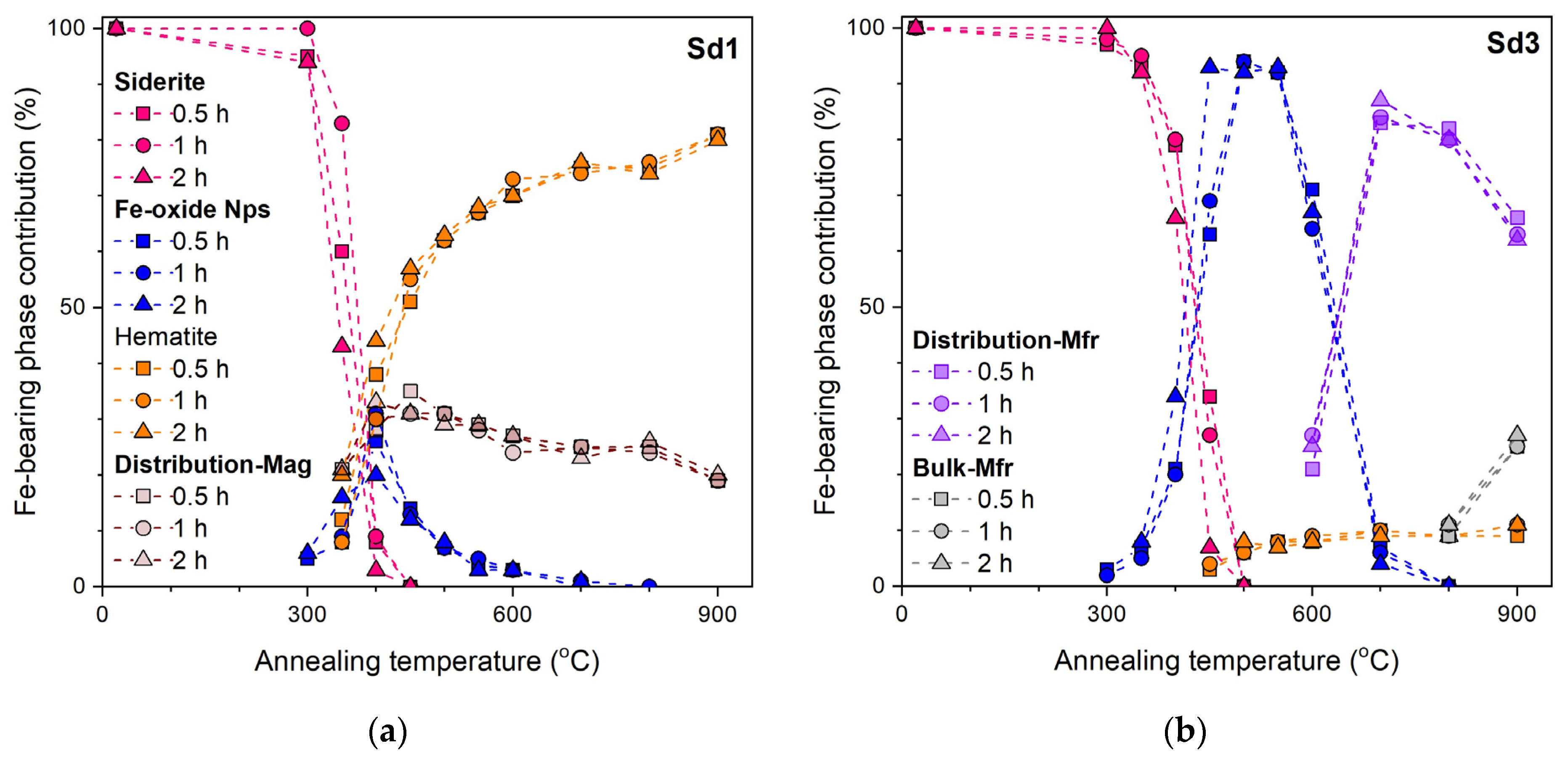
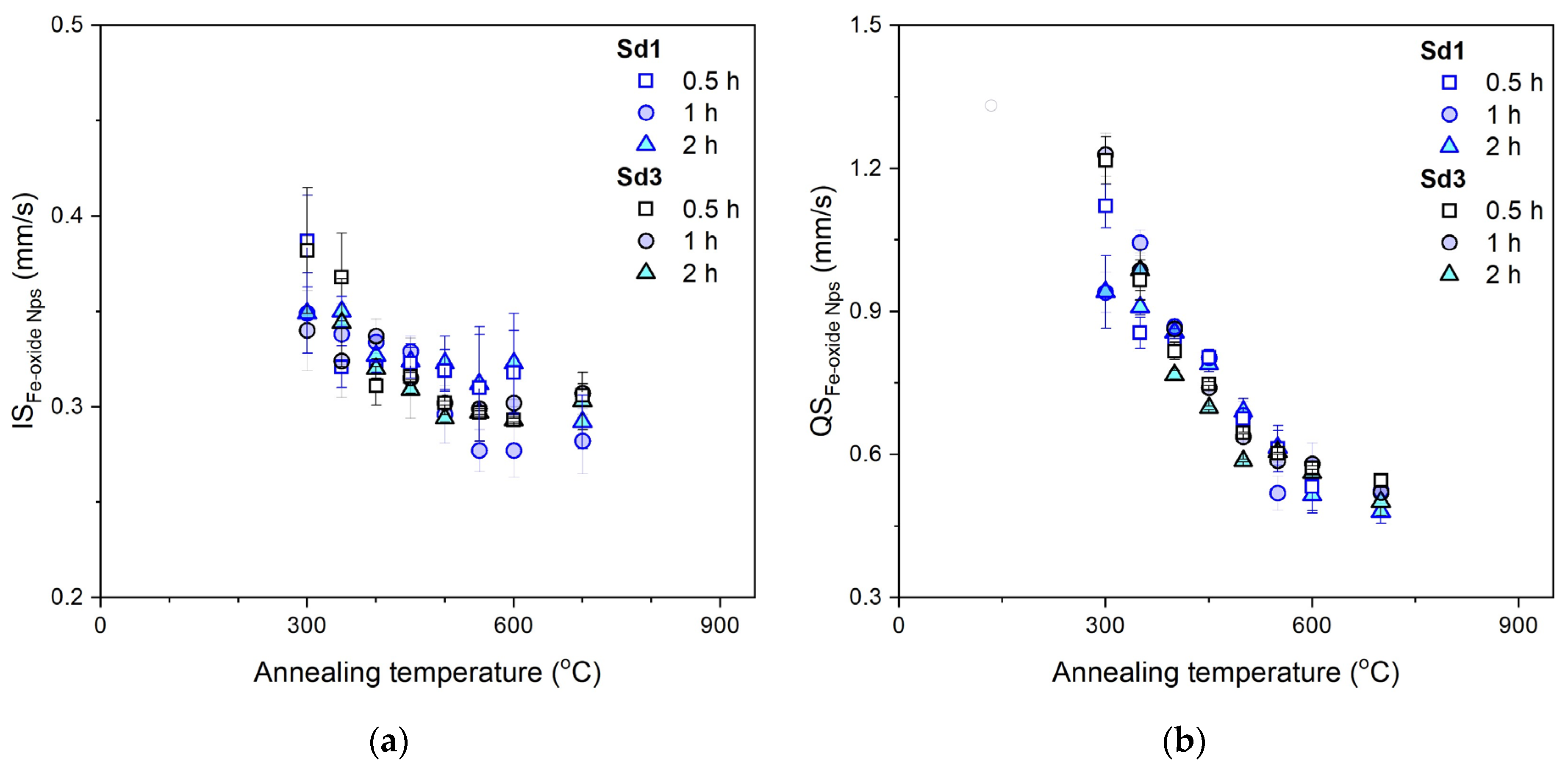
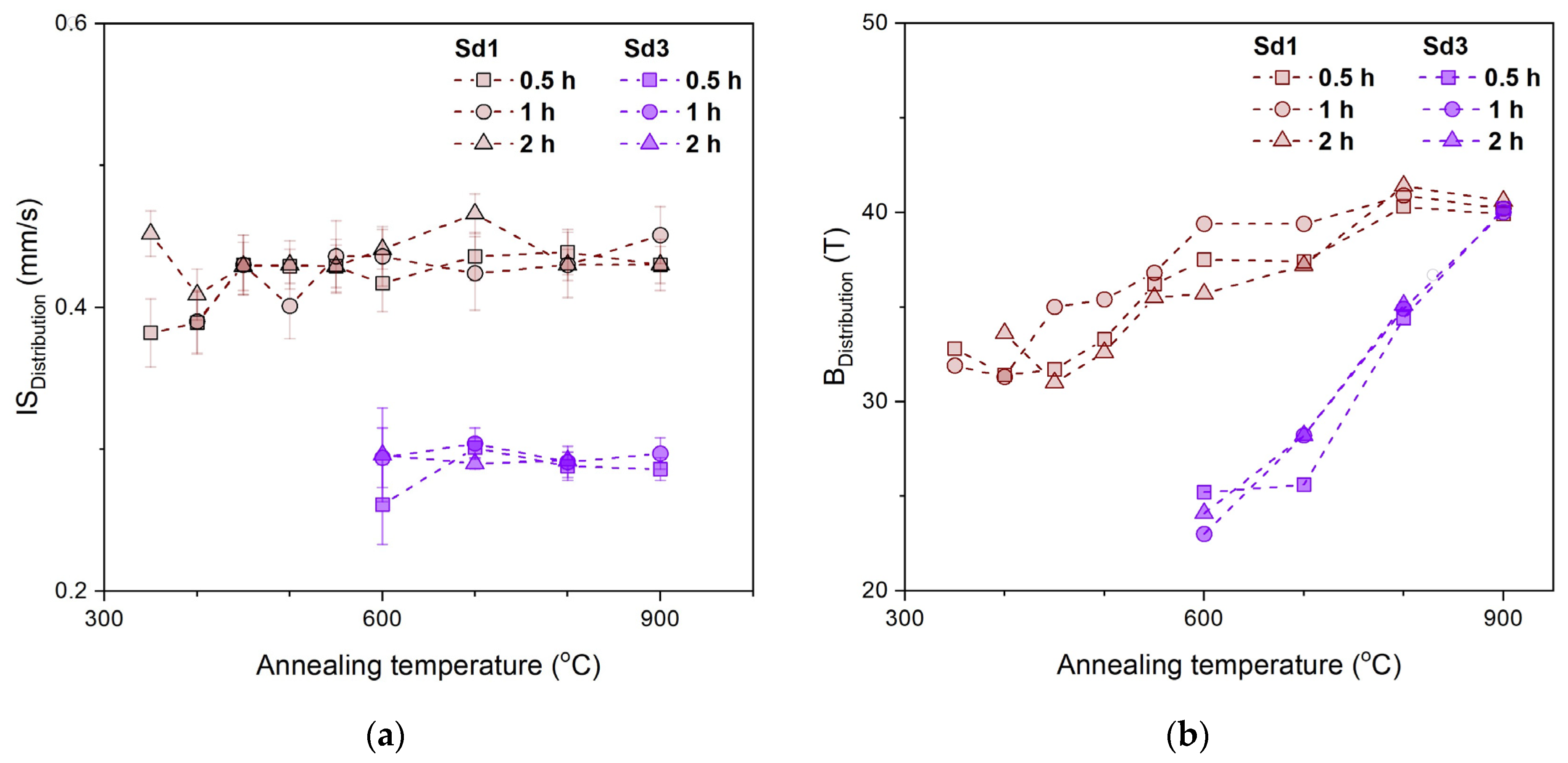
3.4. Mössbauer Spectroscopy
4. Discussion
5. Conclusions
- (1)
- The siderite decomposition process begins at a temperature of 300 °C. In the sample containing a small amount of magnesium (<1 wt.%), complete decomposition takes place at a temperature of 450 °C, and in a sample with a larger amount of this element (~4 wt.%) at a temperature of 500 °C.
- (2)
- The annealing time does not significantly affect the width of the temperature range where siderite decomposition occurs. Still, it does affect the degree of siderite decomposition: the longer the heating time, the greater the amount of siderite decomposition. The dynamics of the processes undergone by the sample annealed for half an hour and an hour are similar. However, in the sample annealed for two hours, the degree of siderite decomposition is much greater than shorter annealing times.
- (3)
- The decomposition products of siderite annealed in air are iron oxides. In the sample without Mg, the oxide was mainly hematite, which confirms the above-mentioned literature data, and magnesioferrite in the sample containing Mg. The consequence of substituting Mg in the Fe position is the stabilization of the structure of magnesioferrite, and its oxidation to hematite was not observed even at 900 °C.
- (4)
- Iron oxides formed directly in siderite decomposition are poorly crystalline, and we can treat them as nanoparticles. They maintain this form in a wide temperature range, especially in a magnesium-containing sample. The presence of this element significantly slows down the process of magnesioferrite crystallization. Mg2+ replaces Fe2+ in magnetite to form the magnesioferrite, whilst simultaneously stabilizing the crystal structure as a spinel. Due to the increase in the amount of magnesium or magnesioferrite in a sample, the transformation of hematite to magnetite would be reduced.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isambert, A.; Valet, J.-P.; Gloter, A.; Guyot, F. Stable Mn-magnetite derived from Mn-siderite by heating in air. J. Geophys. Res. 2003, 108, 2283. [Google Scholar] [CrossRef]
- Ponomar, V.P.; Dudchenko, N.O.; Brik, A.B. Phase transformations of siderite ore by the thermomagnetic analysis data. J. Magn. Magn. Mater. 2017, 423, 373–378. [Google Scholar] [CrossRef]
- Zhua, X.; Hana, Y.; Suna, Y.; Gaoa, P.; Li, Y. Thermal Decomposition of Siderite Ore in Different Flowing Atmospheres: Phase Transformation and Magnetism. Miner. Process. Extr. Metall. Rev. 2022, 44, 201–208. [Google Scholar] [CrossRef]
- Kelektsoglou, K. Carbon capture and storage: A review of mineral storage of CO2 in Greece. Sustainability 2018, 10, 4400. [Google Scholar] [CrossRef]
- Mendoza, M.; Santos, A.; López, E.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S. Iron oxides as efficient sorbents for CO2 capture. J. Mater. Res. Technol. 2019, 8, 2944–2956. [Google Scholar] [CrossRef]
- Fosbol, P.L.; Thomsen, K.; Stenby, E.H. Review and recommended thermodynamic properties of FeCO3. Corros. Eng. Sci. Technol. 2010, 45, 115–135. [Google Scholar] [CrossRef]
- Santoso, R.; Rahmawati, S.; Gadesa, A.; Wahyuningrum, D. Understanding Passive Layer Formation for Further Corrosion Management in Gas Production Pipes. J. Phys. Conf. Ser. 2017, 877, 012062. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Li, Y.; Gao, P. Reaction behavior and non-isothermal kinetics of suspension magnetization roasting of limonite and siderite. Int. J. Miner. Metall. Mater. 2023, 30, 824–833. [Google Scholar] [CrossRef]
- Zhu, D.; Luo, Y.; Pan, J.; Zhou, X. Reaction Mechanism of Siderite Lump in Coal-Based Direct Reduction. High Temp. Mater. Proc. 2016, 35, 185–194. [Google Scholar]
- Zhang, X.; Han, Y.; Li, Y.; Sun, Y. Effect of Heating Rate on Pyrolysis Behavior and Kinetic Characteristics of Siderite. Minerals 2017, 7, 211. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Lu, P.; Chen, T.; Ma, W. Nanostructured α-Fe2O3 derived from siderite as an effective Hg(II) adsorbent: Performance and mechanism. Appl. Geochem. 2018, 96, 92–99. [Google Scholar] [CrossRef]
- Dubrawski, J.V. Thermal decomposition of some siderite-magnesite minerals using DSC. J. Therm. Anal. 1991, 37, 1213–1221. [Google Scholar] [CrossRef]
- Jagtap, S.B.; Pande, A.R.; Gokarn, A.N. Kinetics of thermal decomposition of siderite: Effect of particle size. Int. J. Miner. Process. 1992, 36, 113–124. [Google Scholar] [CrossRef]
- Goldin, D.M.; Kulikova, G.V. On the dissociation mechanism of carbonates and their isomorphous mixture. J. Therm. Anal. Calorim. 1984, 29, 139–145. [Google Scholar] [CrossRef]
- Kądziołka-Gaweł, M.; Nowak, J.; Szubka, M.; Klimontko, J.; Wojtyniak, M. Thermal Decomposition of Siderite and Characterization of the Decomposition Products under O2 and CO2 Atmospheres. Minerals 2023, 13, 1066. [Google Scholar] [CrossRef]
- Rancourt, D.; Ping, J. Voigt-based methods for arbitrary-shape static hyperfine parameter distributions in Mössbauer spectroscopy. Nucl. Instrum. Meth. B 1991, 58, 85–97. [Google Scholar] [CrossRef]
- Dhupe, A.P.; Gokarn, A.N. Studies in the thermal decomposition of natural siderites in the presence of air. Int. J. Miner. Process. 1990, 28, 209–220. [Google Scholar] [CrossRef]
- Ristić, M.; Krehula, S.; Reissner, M.; Musić, S. 57Fe Mössbauer, XRD, FT-IR, FE SEM Analyses of Natural Goethite, Hematite and Siderite. Croat. Chem. Acta 2017, 90, 499–507. Available online: https://hrcak.srce.hr/190802 (accessed on 17 December 2017). [CrossRef]
- Bodker, F.; Hansen, M.F.; Bender Koch, C.; Lefmann, K.; Morup, S. Magnetic properties of hematite nanoparticles. Phys. Rev. B Condens. Matter 2000, 61, 6826–6838. [Google Scholar] [CrossRef]
- De Grave, E.; Bowen, L.; Amarasiriwardena, D.; Vandenberghe, R. 57Fe Mössbauer effect study of highly substituted aluminum hematites: Determination of the magnetic hyperfine field distributions. J. Magn. Magn. Mater. 1988, 72, 129–140. [Google Scholar] [CrossRef]
- Chen, C.; Han, Y.; Zhang, Y.; Liu, Y.; Liu, Y. Efficient Utilization of Siderite- and Hematite-Mixed Ore by Suspension Magnetization Roasting: A Pilot-Scale Study. Sustainability 2022, 14, 10353. [Google Scholar] [CrossRef]
- Koziol, A. Experimental determination of siderite stability and application to Martian Meteorite ALH84001. Am. Min. 2004, 89, 294–300. [Google Scholar] [CrossRef]
- Halmar, N.; Hammer, M.; Netland, H.; Perkins, N.; Streyle, T.; Thomasson, C.; Vanhoever, L. Characterization of Iron Oxide Samples Using Mössbauer Spectroscopy and Hysteresis Loops. J. Undergrad. Rep. Phys. 2023, 33, 100010. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kim, C. Superexchange Interactions in MgFe2O4. J. Korean Phys. Soc. 2006, 48, 583–588. [Google Scholar]
- Wu, W.; Lin, X.; Duan, H.; Wang, J. Mössbauer And High-Temperature Magnetic Properties of Different Size Fe3O4 Nanostructure. Int. J. Mod. Phys. B 2012, 26, 1250073. [Google Scholar] [CrossRef]
- Gervits, N.; Gippius, A.; Tkachev, A.; Demikhov, E.; Starchikov, S.; Lyubutin, I.; Vasiliev, A.; Chekhonin, V.; Abakumov, M.; Semkina, M.; et al. Magnetic properties of biofunctionalized iron oxide nanoparticles as magnetic resonance imaging contrast agents. Beilstein J. Nanotechnol. 2019, 10, 1964–1972. [Google Scholar] [CrossRef]
- Klekotka, U.; Winska, E.; Satuła, D.; Kalska-Szostko, B. Mössbauer Studies of Surface Modified Magnetite Particles. Acta Phys. Pol. A 2018, 134, 1003–1006. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Du, Y.; Guo, Y.-F.; Guo, X.-M. Quantitative Investigation of MgO, Al2O3 and SiO2 Effects on Solid-State Formation of Secondary Hematite in Sintering Process of Iron Ore Fines. Minerals 2022, 12, 282. [Google Scholar] [CrossRef]
- Satuła, D.; Kalska-Szostko, B.; Szymańskia, K.; Dobrzyńskia, L.; Kozubowski, J. Microstructure and Magnetic Properties of Iron Oxide Nanoparticles Prepared by Wet Chemical Method. Acta Phys. Pol. A 2008, 114, 1615–1621. [Google Scholar] [CrossRef]
- Gallagher, P.; Warne, S. Thermomagnetometry and thermal decomposition of siderite. Thermochim. Acta 1981, 43, 253–267. [Google Scholar] [CrossRef]
- Musić, S.; Popović, S. Mössbauer spectroscopic and X-ray diffraction study of the thermal decomposition of natural siderite and goethite. J. Radioanal. Nucl. Chem. 1987, 111, 27–41. [Google Scholar] [CrossRef]
- Zhu, Y.; Banerjee, S.; Gill, J.; Williams, Q. Rock magnetic properties related to thermal treatment of siderite: Behavior and interpretation. J. Geophys. Res. 2000, 105, 783–794. [Google Scholar] [CrossRef]
- Garcia, S.; Rosenbauer, R.J.; Palandri, J.; Maroto-Valer, M.M. Sequestration of non-pure carbon dioxide streams in iron oxyhydroxide-containing saline repositories. Int. J. Greenh. Gas Control 2012, 7, 89–97. [Google Scholar] [CrossRef]
| Sample | Element Concentration (wt.%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Al | Si | P | K | Ca | Ti | Mn | Fe | Sum | |
| Sd1 | 6.17 | 48.60 | 0.80 | 2.63 | 4.58 | 0.17 | 0.32 | 0.86 | 0.09 | 0.60 | 34.96 | 99.78 |
| Sd3 | 6.26 | 50.80 | 3.93 | 4.06 | 6.93 | 0.07 | 0.52 | 0.92 | 0.13 | 0.37 | 25.53 | 99.52 |
| Temperature | Initial Sd1 | 400 °C | 700 °C | 900 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Component | 0.5 h | 1 h | 2 h | 0.5 h | 1 h | 2 h | 0.5 h | 1 h | 2 h | |
| Siderite | 94 | 28 | 27 | 20 | - | - | - | - | - | - |
| Quartz | Trace | 11 | 9 | 6 | 2 | 3 | 2 | 1 | 1 | 1 |
| Illite | 1 | - | - | - | - | - | - | - | - | - |
| Kaolinite | 5 | 4 | 1 | 4 | - | - | - | - | - | - |
| Hematite | - | 57 | 63 | 70 | 93 | 95 | 97 | 90 | 81 | 56 |
| Magnetite | - | - | - | - | 5 | 2 | 1 | 8 | 18 | 43 |
| Temperature | Initial Sd3 | 400 °C | 700 °C | 900 °C | ||||||
| Component | 0.5 h | 1 h | 2 h | 0.5 h | 1 h | 2 h | 0.5 h | 1 h | 2 h | |
| Siderite | 84 | 81 | 80 | 75 | - | - | - | - | - | - |
| Quartz | 7 | 8 | 9 | 10 | 12 | 13 | 12 | 13 | 13 | 13 |
| Illite | 3 | - | - | - | - | - | - | - | - | - |
| Kaolinite | 6 | 9 | 9 | 11 | - | - | - | - | - | - |
| Hematite | - | 1 | 1 | 2 | 11 | 6 | 5 | 13 | 10 | 11 |
| Magnetite | - | - | - | - | 15 | 10 | 9 | 10 | 20 | 22 |
| Magnesioferrite | - | 1 | 1 | 2 | 62 | 71 | 74 | 64 | 57 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kądziołka-Gaweł, M.; Adamczyk, Z.; Łukowiec, D.; Klimontko, J.; Wojtyniak, M.; Nowak, J. Siderite Decomposition Kinetics—Influence of Time, Temperature, and Isomorphous Impurities. Minerals 2025, 15, 428. https://doi.org/10.3390/min15040428
Kądziołka-Gaweł M, Adamczyk Z, Łukowiec D, Klimontko J, Wojtyniak M, Nowak J. Siderite Decomposition Kinetics—Influence of Time, Temperature, and Isomorphous Impurities. Minerals. 2025; 15(4):428. https://doi.org/10.3390/min15040428
Chicago/Turabian StyleKądziołka-Gaweł, Mariola, Zdzisław Adamczyk, Dariusz Łukowiec, Joanna Klimontko, Marcin Wojtyniak, and Jacek Nowak. 2025. "Siderite Decomposition Kinetics—Influence of Time, Temperature, and Isomorphous Impurities" Minerals 15, no. 4: 428. https://doi.org/10.3390/min15040428
APA StyleKądziołka-Gaweł, M., Adamczyk, Z., Łukowiec, D., Klimontko, J., Wojtyniak, M., & Nowak, J. (2025). Siderite Decomposition Kinetics—Influence of Time, Temperature, and Isomorphous Impurities. Minerals, 15(4), 428. https://doi.org/10.3390/min15040428






