Carbon Capture Efficiency of Mechanically Activated Australian Halloysite-Rich Kaolin with Varying Iron Impurities and Its Potential Reuse for Removing Dyes from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Minerals and Their Geological Sources
2.2. Mechanical Activation by Ball Milling
2.3. Material Characterization
2.4. CO2 Sorption Experiments
2.5. Post-Sorption Analysis
2.6. Utilization of Laden Materials
3. Results and Discussion
3.1. Material Characterization
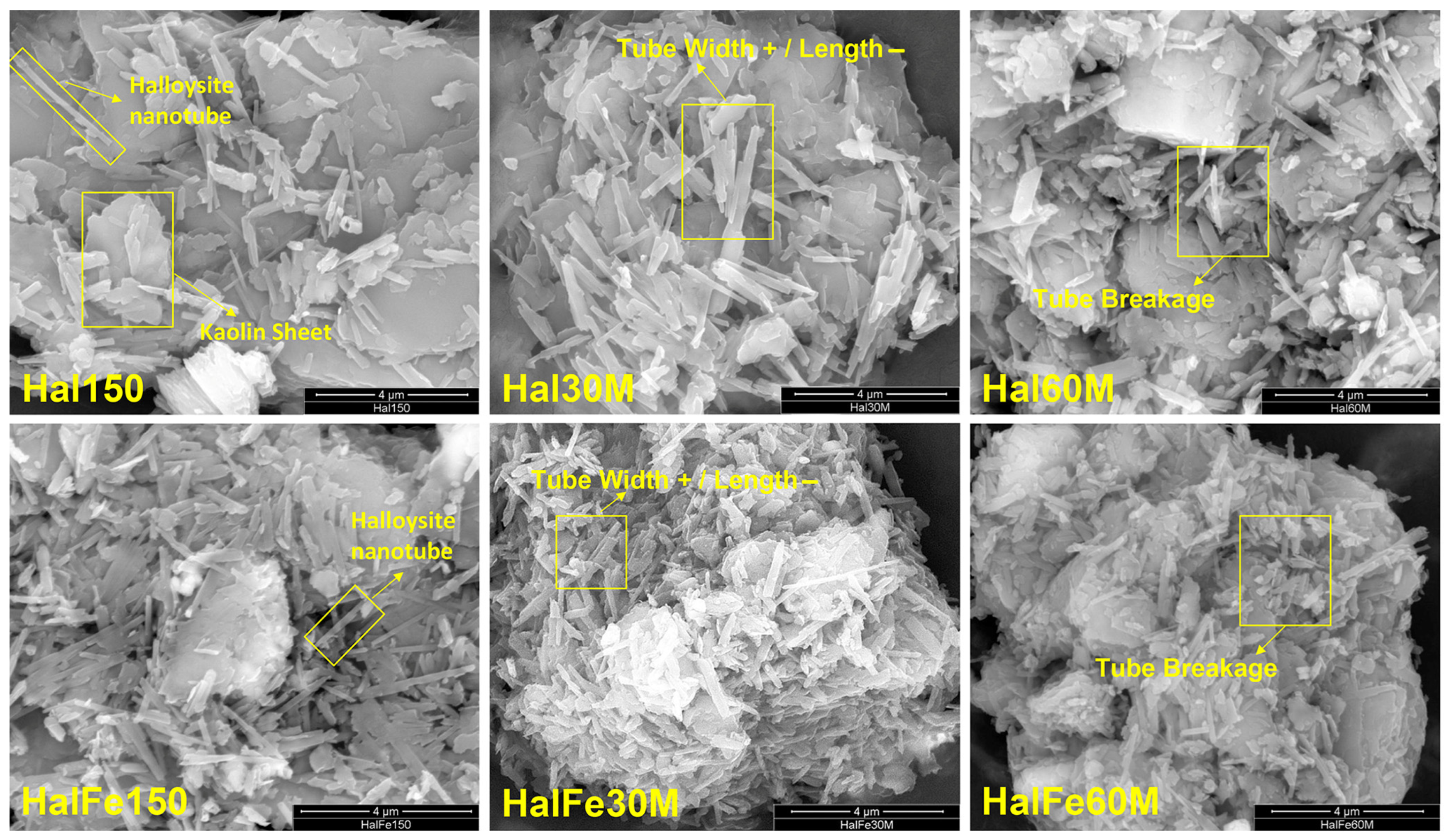
3.1.1. Morphological Analysis
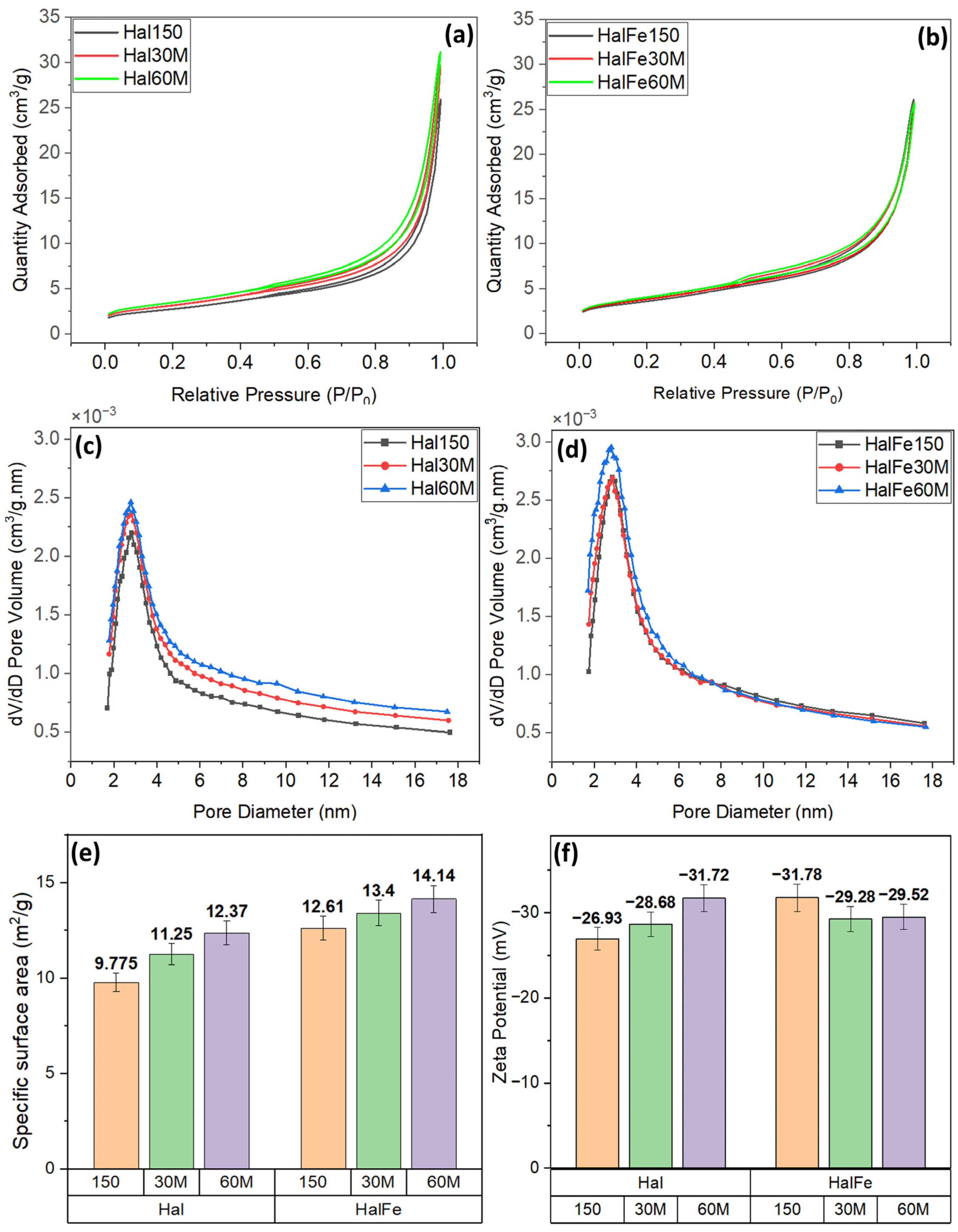
3.1.2. Surface Area and Pore Volume
3.1.3. Zeta Potential for Surface Charge Values
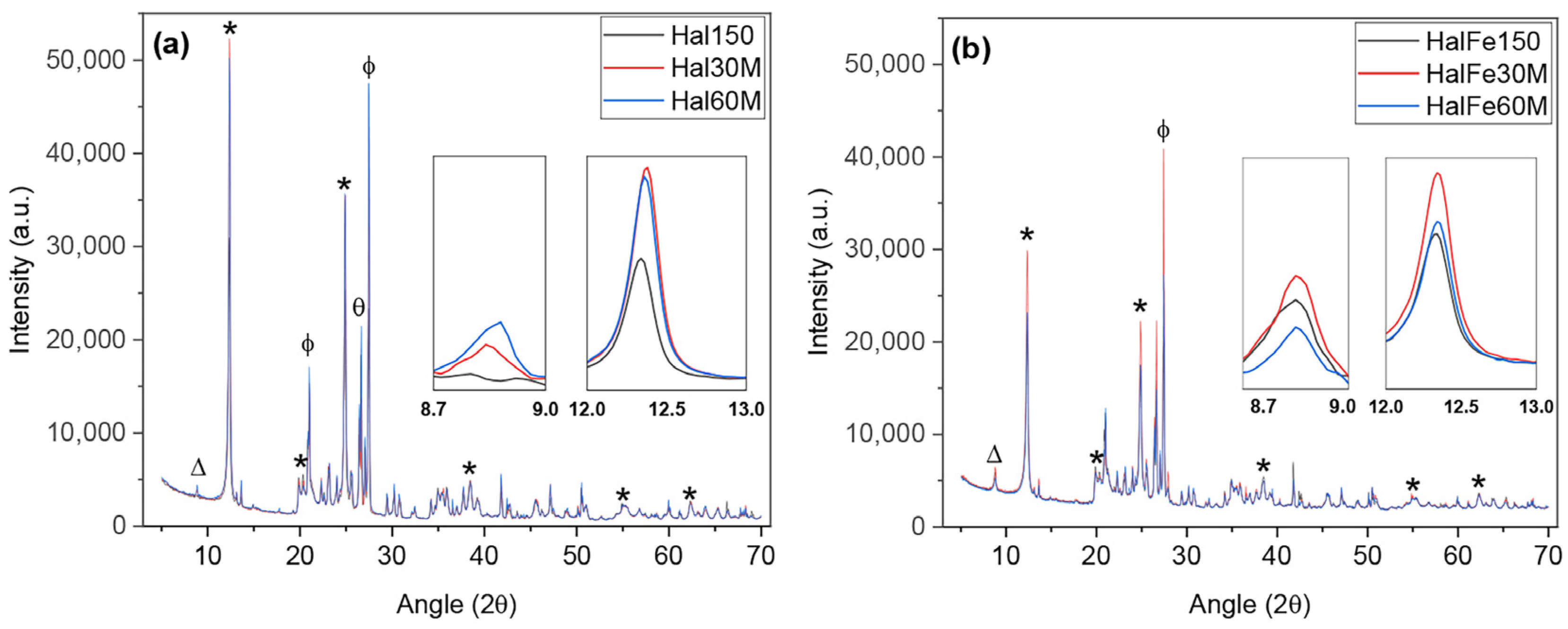
3.1.4. XRD Analysis
3.1.5. Overall Changes in Milled Samples and Implications
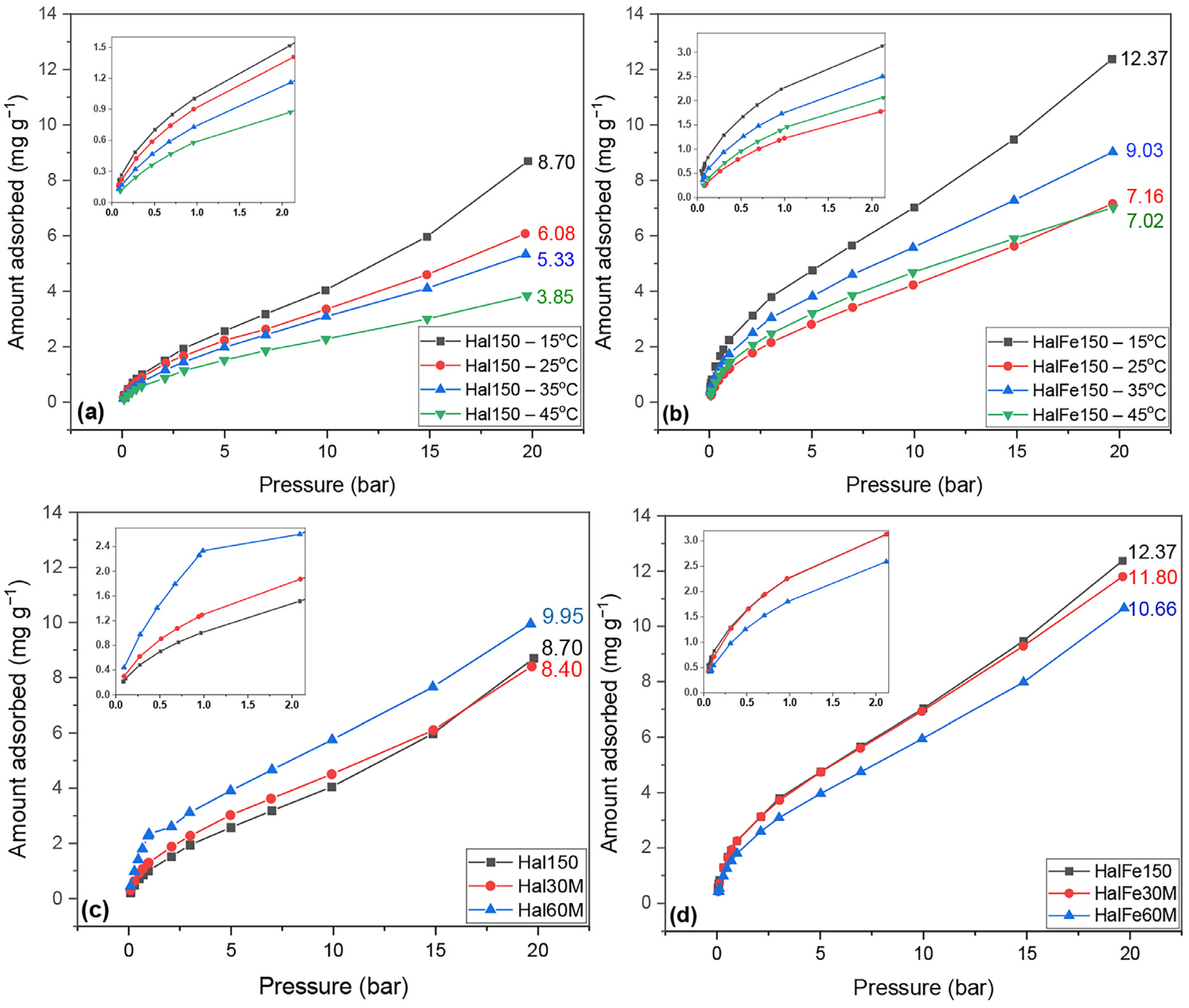
3.2. CO2 Sorption Experiments
3.3. Mechanism of CO2 Sorption on Raw and Milled Materials
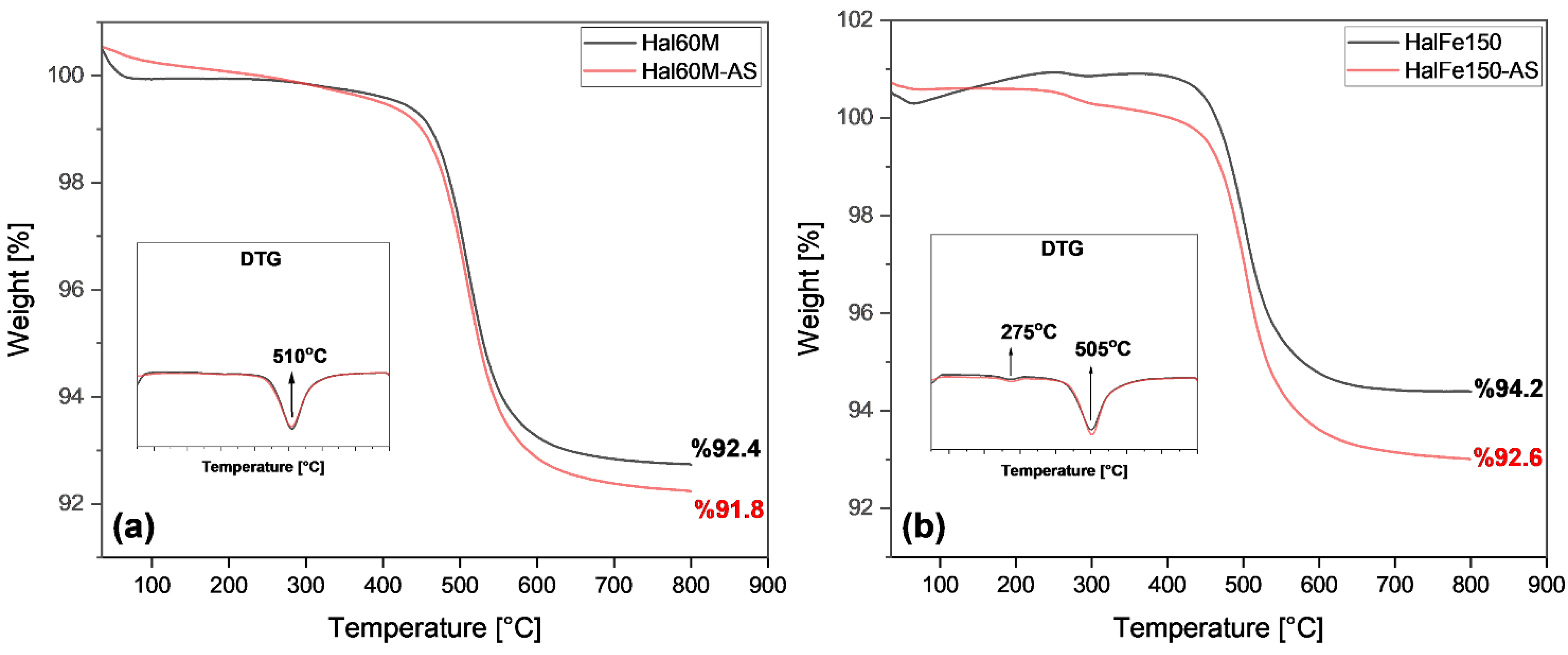
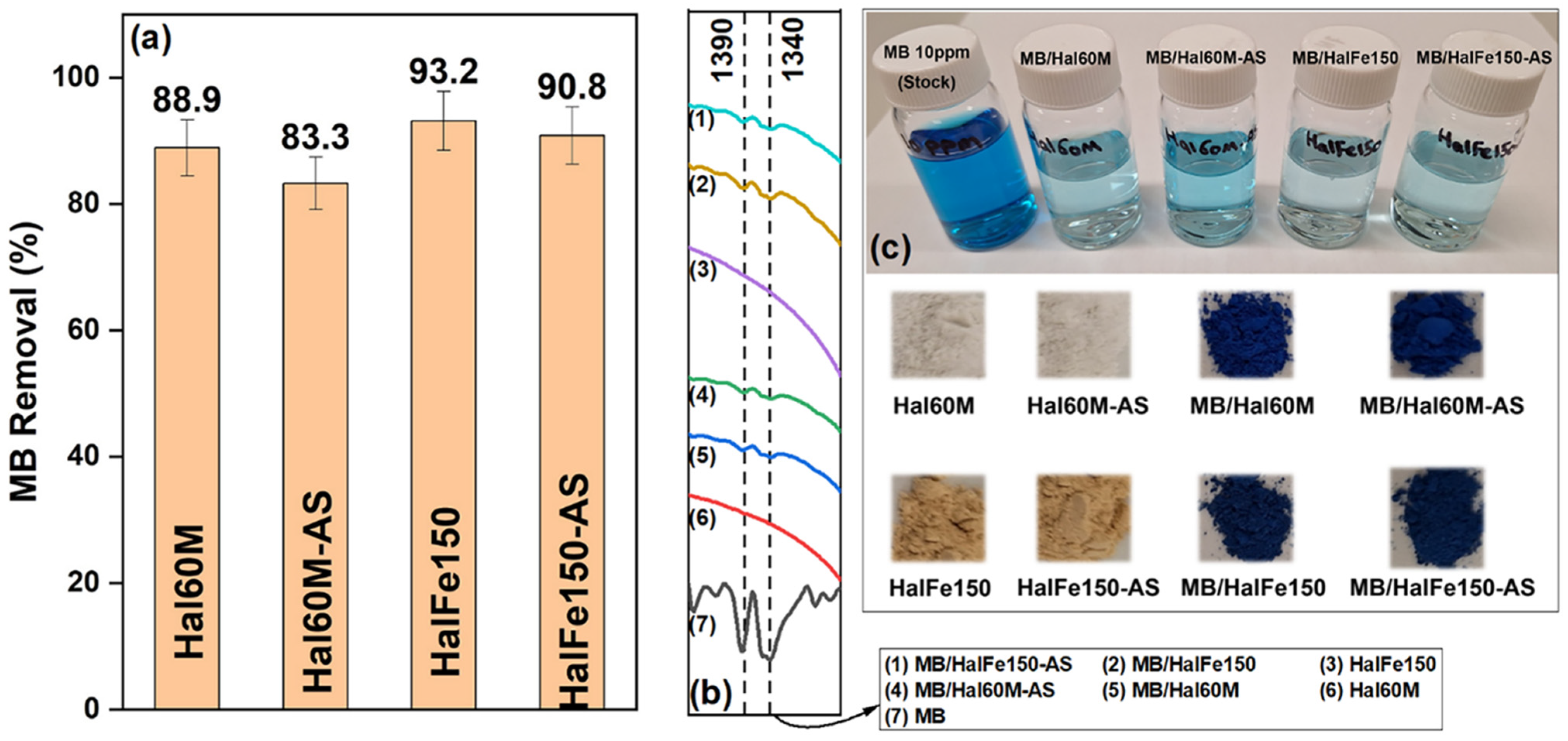
3.4. Utilization of Milled and CO2-Laden Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Liu, L.; Liu, H.; Tian, G.; Peng, G.; Zhuo, L.; Wang, Z. Zeolite-X synthesized from halloysite nanotubes and its application in CO2 capture. J. Taiwan Inst. Chem. Eng. 2022, 133, 104281. [Google Scholar] [CrossRef]
- Ramadass, K.; Singh, G.; Lakhi, K.S.; Benzigar, M.R.; Yang, J.H.; Kim, S.; Almajid, A.M.; Belperio, T.; Vinu, A. Halloysite nanotubes: Novel and eco-friendly adsorbents for high-pressure CO2 capture. Microporous Mesoporous Mater. 2019, 277, 229–236. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Point of Departure and Key Concepts. In Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Sánchez-Rodríguez, R., Sutherland, M., Eds.; Cambridge University Press: Cambridge, UK, 2023; pp. 121–196. [Google Scholar]
- NOAA Research. No Sign of Greenhouse Gases Increases Slowing in 2023. Available online: https://research.noaa.gov/2024/04/05/no-sign-of-greenhouse-gases-increases-slowing-in-2023/ (accessed on 15 October 2024).
- Soo, X.Y.D.; Lee, J.J.C.; Wu, W.-Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 capture by absorption and adsorption: A comprehensive review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon dioxide removal through physical adsorption using carbonaceous and non-carbonaceous adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104142. [Google Scholar] [CrossRef]
- Cui, H.; Xu, J.; Shi, J.; You, S.; Zhang, C.; Yan, N.; Liu, Y.; Chen, G. Evaluation of different potassium salts as activators for hierarchically porous carbons and their applications in CO2 adsorption. J. Colloid Interface Sci. 2021, 583, 40–49. [Google Scholar] [CrossRef]
- Shi, J.; Cui, H.; Xu, J.; Yan, N. Carbon spheres synthesized from KHCO3 activation of glucose derived hydrochar with excellent CO2 capture capabilities at both low and high pressures. Sep. Purif. Technol. 2022, 294, 121193. [Google Scholar] [CrossRef]
- Shi, J.; Cui, H.; Xu, J.; Yan, N.; Liu, Y. Design and fabrication of hierarchically porous carbon frameworks with Fe2O3 cubes as hard template for CO2 adsorption. Chem. Eng. J. 2020, 389, 124459. [Google Scholar] [CrossRef]
- Pajdak, A.; Skoczylas, N.; Szymanek, A.; Lutyński, M.; Sakiewicz, P. Sorption of CO2 and CH4 on Raw and Calcined Halloysite—Structural and Pore Characterization Study. Materials 2020, 13, 917. [Google Scholar] [CrossRef]
- Jana, S.; Das, S.; Ghosh, C.; Maity, A.; Pradhan, M. Halloysite Nanotubes Capturing Isotope Selective Atmospheric CO2. Sci. Rep. 2015, 5, 8711. [Google Scholar] [CrossRef]
- Wang, Z.; Pang, Y.; Guo, H.; Wang, H.; Liu, L.; Wang, X.; Zhang, S.; Cui, W. Increased CO2 capture capacity via amino-bifunctionalized halloysite nanotubes adsorbents. Fuel 2024, 364, 131036. [Google Scholar] [CrossRef]
- Tao, H.; Qian, X.; Zhou, Y.; Cheng, H. Research progress of clay minerals in carbon dioxide capture. Renew. Sustain. Energy Rev. 2022, 164, 112536. [Google Scholar] [CrossRef]
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-García, E.; Besghaier, S.; Chlendi, M.; Franco Duro, F.I.; Rodriguez Castellon, E.; Bagane, M. CO2 Adsorption of Materials Synthesized from Clay Minerals: A Review. Minerals 2019, 9, 514. [Google Scholar] [CrossRef]
- Deb, A.K.; Biswas, B.; Goswami, N.; Hilder, E.F.; Naidu, R.; Rahman, M.M. Synthesis of environmentally benign ultra-small copper nanoclusters-halloysite composites and their catalytic performance on contrasting azo dyes. Appl. Surf. Sci. 2021, 546, 149122. [Google Scholar] [CrossRef]
- Biswas, B.; Islam, M.R.; Deb, A.K.; Greenaway, A.; Warr, L.N.; Naidu, R. Understanding Iron Impurities in Australian Kaolin and Their Effect on Acid and Heat Activation Processes of Clay. ACS Omega 2023, 8, 5533–5544. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, S.; Liu, J.; Sun, X. Recent Advances of Halloysite Nanotubes in Biomedical Applications. Small 2024, 20, 2306169. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.D. Clay minerals. In Encyclopedia of Soil Science; Lal, R., Ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 187–192. [Google Scholar]
- Abdul, F.; Rahman, R.F.; Purwanto, K.A.; Ma’ruf, F.I.; Setiyorini, Y.; Setyowati, V.A.; Pintowantoro, S. Application of iron-rich slag to capture carbon dioxide gas through direct gas-solid carbonation. Glob. J. Environ. Sci. Manag. 2024, 10, 1675–1686. [Google Scholar] [CrossRef]
- Fabozzi, A.; Cerciello, F.; Senneca, O. Reduction of Iron Oxides for CO2 Capture Materials. Energies 2024, 17, 1673. [Google Scholar] [CrossRef]
- Mora Mendoza, E.Y.; Sarmiento Santos, A.; Vera López, E.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Iron oxides as efficient sorbents for CO2 capture. J. Mater. Res. Technol. 2019, 8, 2944–2956. [Google Scholar] [CrossRef]
- Hakim, A.; Marliza, T.S.; Abu Tahari, M.N.; Yusop, M.R.; Mohamed Hisham, M.W.; Yarmo, M.A. Development of α-Fe2O3 as Adsorbent and its Effect on CO2 Capture. Mater. Sci. Forum 2016, 840, 421–426. [Google Scholar] [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-Impregnated Mesoporous Silica Nanotube as an Emerging Nanocomposite for CO2 Capture. ACS Appl. Mater. Interfaces 2016, 8, 17312–17320. [Google Scholar] [CrossRef]
- Taheri, F.S.; Ghaemi, A.; Maleki, A. High Efficiency and Eco-Friendly TEPA-Functionalized Adsorbent with Enhanced Porosity for CO2 Capture. Energy Fuels 2019, 33, 11465–11476. [Google Scholar] [CrossRef]
- Taheri, F.S.; Ghaemi, A.; Maleki, A.; Shahhosseini, S. High CO2 Adsorption on Amine-Functionalized Improved Mesoporous Silica Nanotube as an Eco-Friendly Nanocomposite. Energy Fuels 2019, 33, 5384–5397. [Google Scholar] [CrossRef]
- Khoshraftar, Z.; Taheri, F.S.; Nezami, S.; Ghaemi, A. Using halloysite nanotubes modified by tetraethylenepentamine for advanced carbon capture: Experimental and modeling via RSM and ANNs. Chem. Eng. J. Adv. 2023, 16, 100543. [Google Scholar] [CrossRef]
- Park, S.; Ryu, J.; Cho, H.Y.; Sohn, D. Halloysite nanotubes loaded with HKUST-1 for CO2 adsorption. Colloids Surf. A 2022, 651, 129750. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Chen, H.; Fang, Y. Removal of Methylene Blue from Aqueous Solutions by Surface Modified Talc. Materials 2023, 16, 3597. [Google Scholar] [CrossRef] [PubMed]
- Harindintwali, J.D.; He, C.; Xiang, L.; Dou, Q.; Liu, Y.; Wang, M.; Wen, X.; Fu, Y.; Islam, M.U.; Chang, S.X.; et al. Effects of ball milling on biochar adsorption of contaminants in water: A meta-analysis. Sci. Total Environ. 2023, 882, 163643. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Stolle, A.; Stelter, M. Sustainable Synthesis of High-Surface-Area Graphite Oxide via Dry Ball Milling. ACS Sustain. Chem. Eng. 2018, 6, 6358–6369. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, Y.; Xue, B.; Han, M.; Ren, G.; Liu, Y.; Ling, M.; Li, F. Effects of ball milling and ultrasonic treatment on the UV shielding performance of illite micro flakes. Colloids Surf. A 2018, 556, 316–325. [Google Scholar] [CrossRef]
- Tole, I.; Habermehl-Cwirzen, K.; Cwirzen, A. Mechanochemical activation of natural clay minerals: An alternative to produce sustainable cementitious binders—Review. Miner. Pet. 2019, 113, 449–462. [Google Scholar] [CrossRef]
- Gao, P.; Fan, X.; Sun, D.; Zeng, G.; Wang, Q.; Wang, Q. Recent Advances in Ball-Milled Materials and Their Applications for Adsorptive Removal of Aqueous Pollutants. Water 2024, 16, 1639. [Google Scholar] [CrossRef]
- ESG Minerals. Cloud 9 Halloysite & Kaolinite Project. Available online: https://esgminerals.com.au/cloud-9-halloysite-kaolinite-project/ (accessed on 12 March 2025).
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple-Point Temperature to 1100 K at Pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S. Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: Adsorption kinetic, isotherm and mechanism study. Surf. Interfaces 2020, 18, 100422. [Google Scholar] [CrossRef]
- Tan, C.; Zhao, P.; Zhou, Y.; Liu, M. Hydrophobic Halloysite Nanotubes via Ball Milling for Stable Pickering Emulsions: Implications for Food Preservation. ACS Appl. Nano Mater. 2022, 5, 11289–11301. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F.; Yan, W.; Fan, M.; Liu, D.; He, H. Changes in Structure, Morphology, Porosity, and Surface Activity of Mesoporous Halloysite Nanotubes Under Heating. Clays Clay Miner. 2012, 60, 561–573. [Google Scholar] [CrossRef]
- Bekri-Abbes, I.; Srasra, E. Effect of mechanochemical treatment on structure and electrical properties of montmorillonite. J. Alloys Compd. 2016, 671, 34–42. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, H.; Fang, X.; Qiu, Z.; Du, T. CO2 and water vapor adsorption properties of framework hybrid W-ZSM-5/silicalite-1 prepared from RHA. RSC Adv. 2020, 10, 24642–24652. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.; Marliza, T.S.; Abu Tahari, N.M.; Wan Isahak, R.W.N.; Yusop, R.M.; Mohamed Hisham, W.M.; Yarmo, A.M. Studies on CO2 Adsorption and Desorption Properties from Various Types of Iron Oxides (FeO, Fe2O3, and Fe3O4). Ind. Eng. Chem. Res. 2016, 55, 7888–7897. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Zakutevskyy, O.; Sydorchuk, V.; Diyuk, O.; Lakhnik, A. The Effect of High-Energy Ball Milling of Montmorillonite for Adsorptive Removal of Cesium, Strontium, and Uranium Ions from Aqueous Solution. Eng 2023, 4, 2812–2825. [Google Scholar] [CrossRef]
- Maleki, S.; Karimi-Jashni, A. Effect of ball milling process on the structure of local clay and its adsorption performance for Ni(II) removal. Appl. Clay Sci. 2017, 137, 213–224. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; D’Amico, F.; Tammaro, L.; Gorrasi, G. Fabrication and Characterization of Bio-Nanocomposites Based on Halloysite-Encapsulating Grapefruit Seed Oil in a Pectin Matrix as a Novel Bio-Coating for Strawberry Protection. Nanomaterials 2022, 12, 1265. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes filled with MgO for paper reinforcement and deacidification. Appl. Clay Sci. 2021, 213, 106231. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lisuzzo, L.; Lazzara, G.; Milioto, S. Printable Hydrogels Based on Alginate and Halloysite Nanotubes. Int. J. Mol. Sci. 2022, 23, 3294. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, R.; Li, H.; Su, M.; Jin, X.; Qin, D. Loading and Sustained Release of Benzyl Ammonium Chloride (BAC) in Nano-Clays. Materials 2019, 12, 3780. [Google Scholar] [CrossRef]
- Konyukhov, Y.V.; Nguyen, V.M.; Ryzhonkov, D.I. Kinetics of Reduction of α-Fe2O3 Nanopowder with Hydrogen under Power Mechanical Treatment in an Electromagnetic Field. Inorg. Mater. Appl. Res. 2019, 10, 706–712. [Google Scholar] [CrossRef]
- Sgarlata, C.; Formia, A.; Siligardi, C.; Ferrari, F.; Leonelli, C. Mine Clay Washing Residues as a Source for Alkali-Activated Binders. Materials 2022, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Raganati, F.; Chirone, R.; Ammendola, P. CO2 Capture by Temperature Swing Adsorption: Working Capacity As Affected by Temperature and CO2 Partial Pressure. Ind. Eng. Chem. Res. 2020, 59, 3593–3605. [Google Scholar] [CrossRef]
- Wei, M.; Yu, Q.; Xie, H.; Zuo, Z.; Hou, L.; Yang, F. Kinetics studies of CO2 adsorption and desorption on waste ion-exchange resin-based activated carbon. Int. J. Hydrogen Energy 2017, 42, 27122–27129. [Google Scholar] [CrossRef]
- Lee, D.; Jin, Y.; Jung, N.; Lee, J.; Lee, J.; Jeong, Y.S.; Jeon, S. Gravimetric Analysis of the Adsorption and Desorption of CO2 on Amine-Functionalized Mesoporous Silica Mounted on a Microcantilever Array. Environ. Sci. Technol. 2011, 45, 5704–5709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lu, D.-L. CO2 capture by kaolinite and its adsorption mechanism. Appl. Clay Sci. 2015, 104, 221–228. [Google Scholar] [CrossRef]
- Veerasingam, S.; Venkatachalapathy, R. Estimation of carbonate concentration and characterization of marine sediments by Fourier Transform Infrared Spectroscopy. Infrared Phys. Technol. 2014, 66, 136–140. [Google Scholar] [CrossRef]
- Djellali, S.; Touati, A.; Semmeq, A.; Kebaili, M.; Badawi, M.; Bonilla-Petriciolet, A. Unravelling the Methylene Blue Adsorption Mechanism on Doped and Nondoped Polyaniline: A Combined Molecular Modeling and Experimental Investigation. Int. J. Chem. Eng. 2022, 2022, 3181963. [Google Scholar] [CrossRef]
- Aaddouz, M.; Azzaoui, K.; Akartasse, N.; Mejdoubi, E.; Hammouti, B.; Taleb, M.; Sabbahi, R.; Alshahateet, S.F. Removal of methylene blue from aqueous solution by adsorption onto hydroxyapatite nanoparticles. J. Mol. Struct. 2023, 1288, 135807. [Google Scholar] [CrossRef]
- Czuma, N.; Casanova, I.; Baran, P.; Szczurowski, J.; Zarębska, K. CO2 sorption and regeneration properties of fly ash zeolites synthesized with the use of differentiated methods. Sci. Rep. 2020, 10, 1825. [Google Scholar] [CrossRef]
- Islam, M.R.; Biswas, B.; Naidu, R. CO2 Capture Using Zeolite Synthesized from Coal Fly Ash and Its Subsequent Utilization for Fire Retardation and Dye Removal. ACS Sustain. Resour. Manag. 2024, 1, 799–809. [Google Scholar] [CrossRef]


| Halloysite (%) | Kaolinite (%) | Iron Oxide (%) | Major Kaolin Clay Species | |
|---|---|---|---|---|
| Hal | 58 | 20 | 0.32 | Halloysite |
| HalFe | 42 | 31 | 5.4 | Halloysite |
| Hal | HalFe | ||||
|---|---|---|---|---|---|
| 30M | 60M | 30M | 60M | ||
| Morphology | Tube length | ~21% ↓ | ~41% ↓ | ~54% ↓ | ~58% ↓ |
| Tube width | - | ~56% ↑ | - | ~50% ↑ | |
| Tubular structure | Mostly intact, with some tube breakage and flattening | More damaged, with flattened and compromised tubular structures | |||
| Zeta potential | ↓ | ↓ | ↑ | ↑ | |
| Surface area | 15% ↑ | 26% ↑ | 6.5% ↑ | 12% ↑ | |
| Pore characteristics | Improved mesoporosity | Slightly improved mesoporosity | |||
| Crystallinity | Retained √ | Retained √ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davoodi, S.; Biswas, B.; Naidu, R. Carbon Capture Efficiency of Mechanically Activated Australian Halloysite-Rich Kaolin with Varying Iron Impurities and Its Potential Reuse for Removing Dyes from Water. Minerals 2025, 15, 399. https://doi.org/10.3390/min15040399
Davoodi S, Biswas B, Naidu R. Carbon Capture Efficiency of Mechanically Activated Australian Halloysite-Rich Kaolin with Varying Iron Impurities and Its Potential Reuse for Removing Dyes from Water. Minerals. 2025; 15(4):399. https://doi.org/10.3390/min15040399
Chicago/Turabian StyleDavoodi, Siavash, Bhabananda Biswas, and Ravi Naidu. 2025. "Carbon Capture Efficiency of Mechanically Activated Australian Halloysite-Rich Kaolin with Varying Iron Impurities and Its Potential Reuse for Removing Dyes from Water" Minerals 15, no. 4: 399. https://doi.org/10.3390/min15040399
APA StyleDavoodi, S., Biswas, B., & Naidu, R. (2025). Carbon Capture Efficiency of Mechanically Activated Australian Halloysite-Rich Kaolin with Varying Iron Impurities and Its Potential Reuse for Removing Dyes from Water. Minerals, 15(4), 399. https://doi.org/10.3390/min15040399










