Carbonaceous Shale Deposits as Potential Unconventional Sources for Rare Earth Elements at the Witbank Coalfield, Permian Vryheid Formation, South Africa
Abstract
1. Introduction
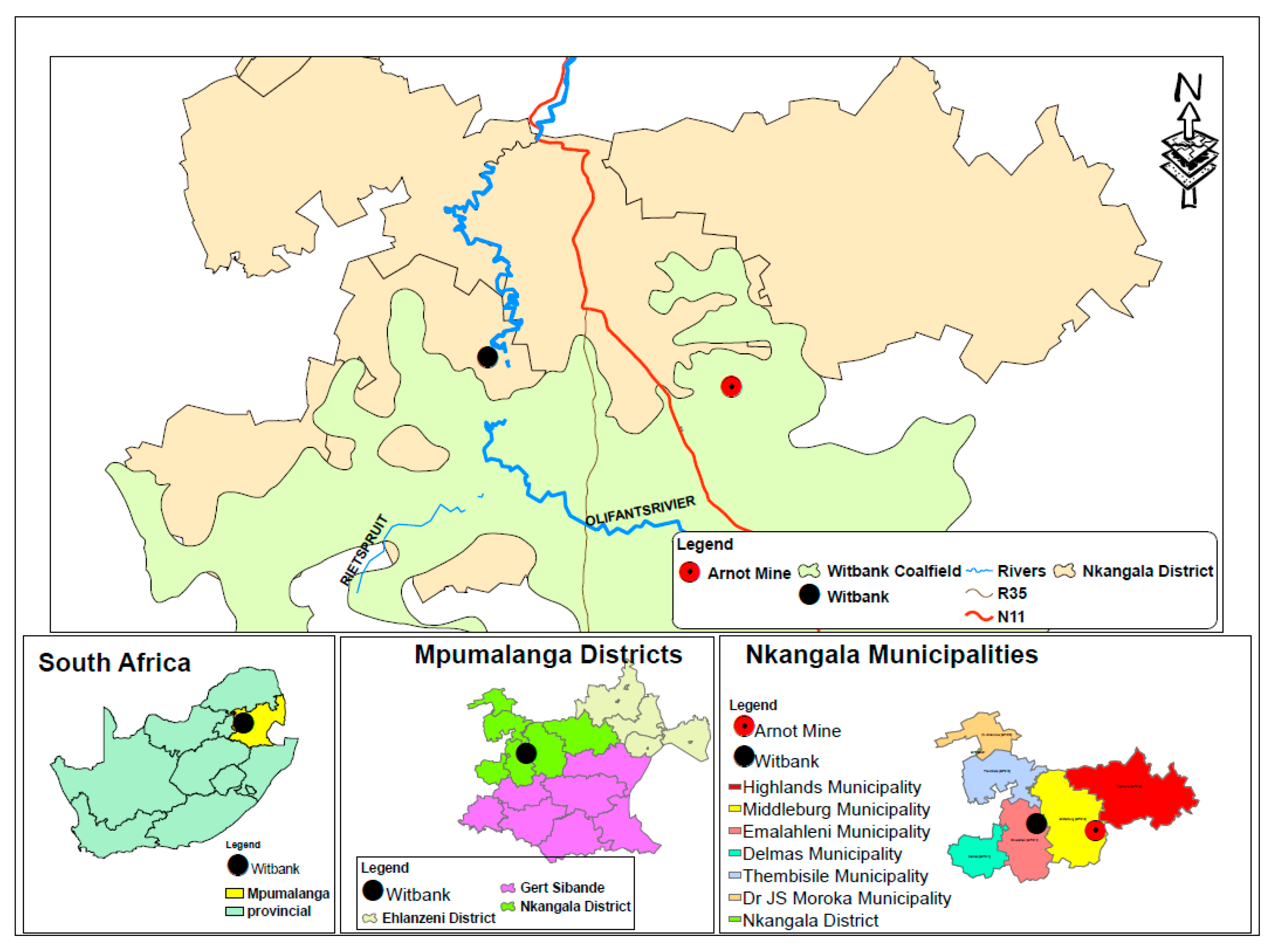
2. Geological Setting
3. Materials and Methods
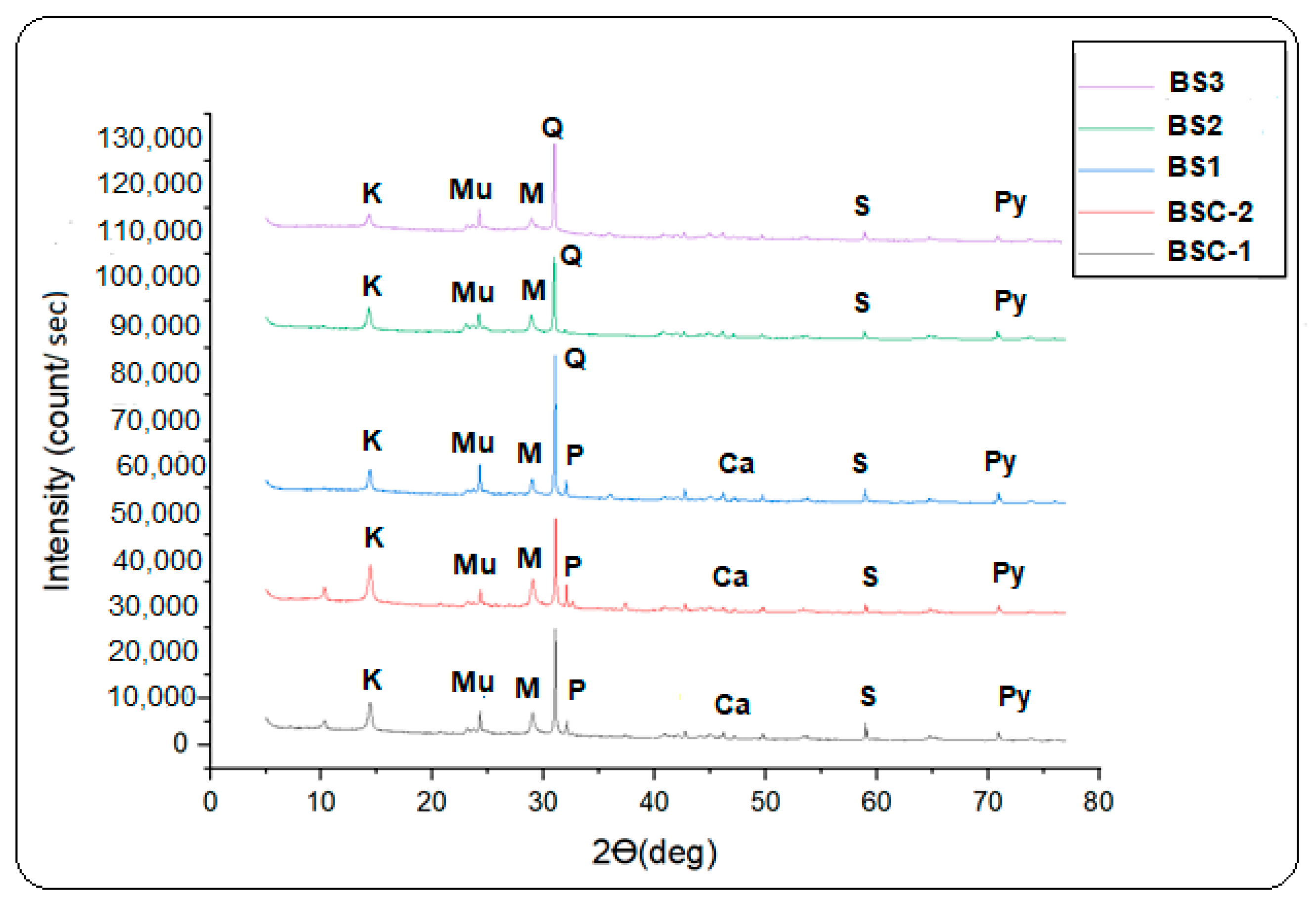
4. Results
5. Discussion
5.1. Evaluation of Rare Earth Elements in Carbonaceous Shale
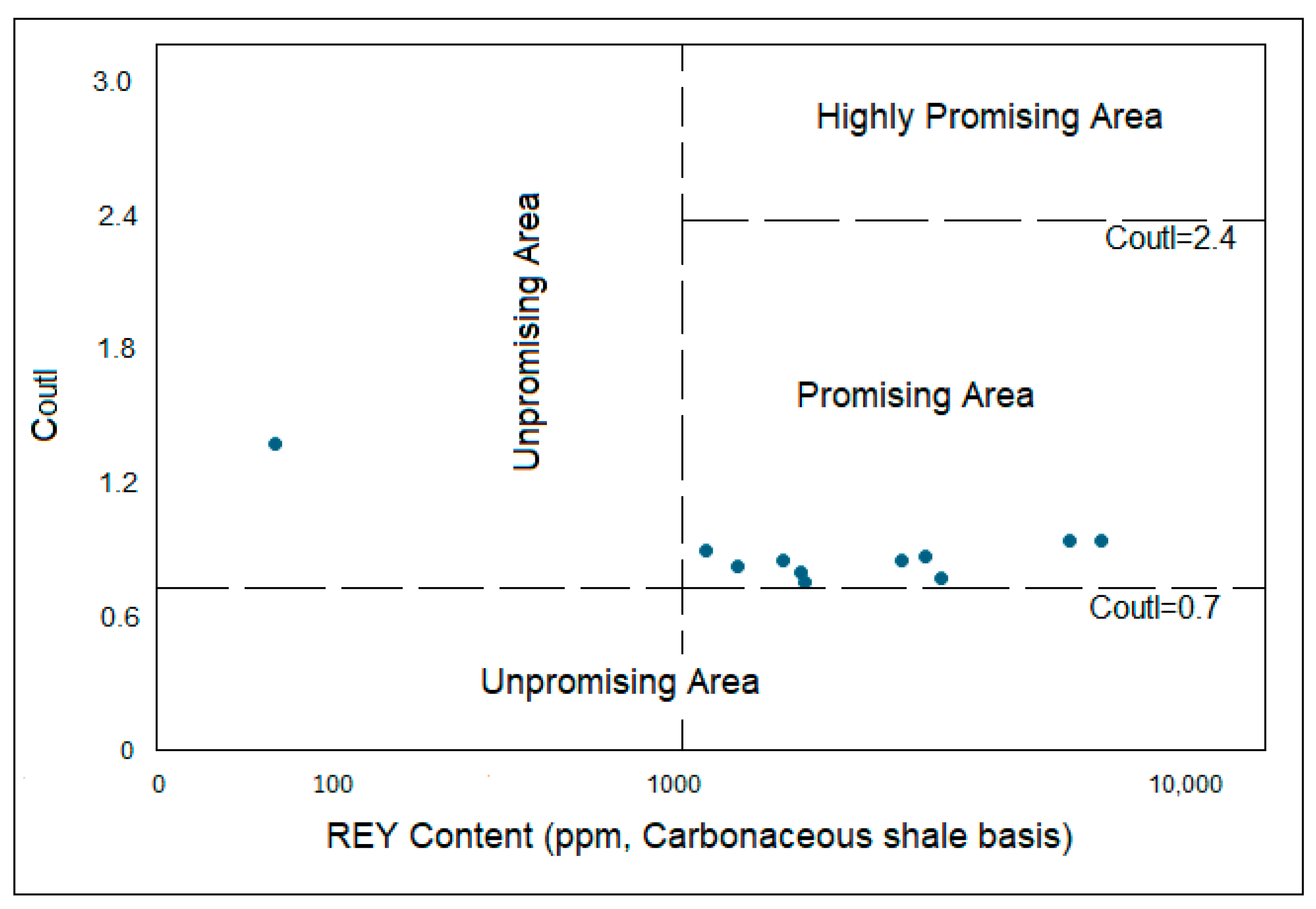
5.2. Enrichment Types and Anomalies of REY in Carbonaceous Shale
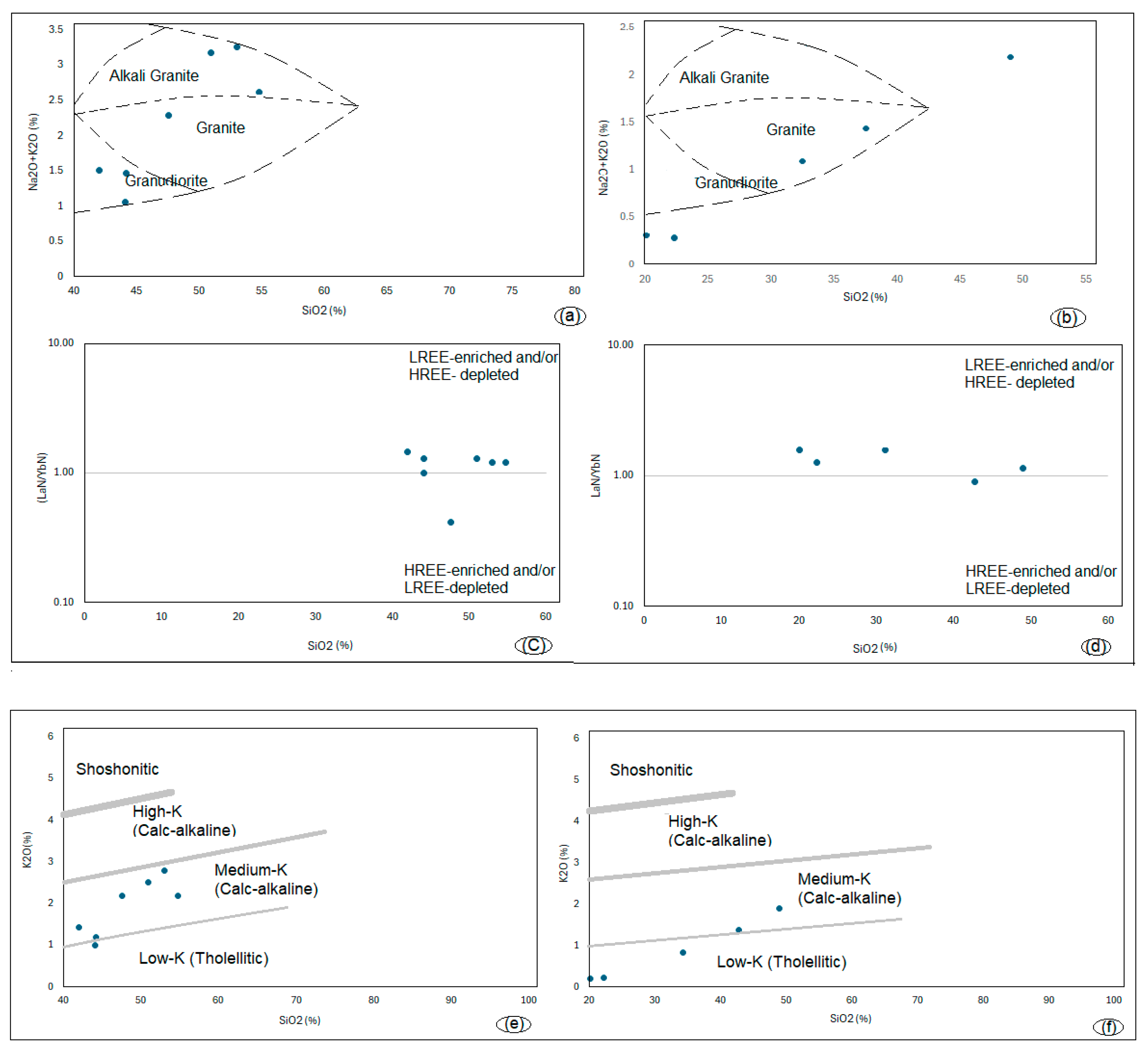
5.3. Sediment Provenance Region for REY
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J. Extraction and separation of heavy rare earth elements: A review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Smith, M.P.; Moore, K.; Kavecsánszki, D.; Finch, A.A.; Kynicky, J.; Wall, F. From mantle to critical zone: A review of large and giant sized deposits of the rare earth elements. Geosci. Front. 2016, 7, 315–334. [Google Scholar] [CrossRef]
- De Boer, M.; Lammertsma, K. Scarcity of rare earth elements. ChemSusChem 2013, 6, 2045–2055. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Gadea, O.C.A.; Khan, S.D.; Sisson, V.B. Estimating rare earth elements at various scales with bastnasite indices for Mountain Pass. Ore Geol. Rev. 2024, 173, 106254. [Google Scholar] [CrossRef]
- Hoshino, M.; Sanematsu, K.; Watanabe, Y. REE mineralogy and resources. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 129–291. [Google Scholar]
- Smith, M.; Campbell, L.; Kynicky, J. A review of the genesis of the world class Bayan Obo Fe–REE–Nb deposits, Inner Mongolia, China: Multistage processes and outstanding questions. Ore Geol. Rev. 2015, 64, 459–476. [Google Scholar] [CrossRef]
- Linnen, R.L.; Samson, I.M.; Williams-Jones, A.E.; Chakhmouradian, A.R. Geochemistry of the rare-earth element, Nb, Ta, Hf, and Zr deposits. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 543–568. [Google Scholar]
- Wall, F. Rare earth elements. In Critical Metals Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 312–339. [Google Scholar]
- Richter, L.; Diamond, L.W.; Atanasova, P.; Banks, D.A.; Gutzmer, J. Hydrothermal formation of heavy rare earth element (HREE)–xenotime deposits at 100 C in a sedimentary basin. Geology 2018, 46, 263–266. [Google Scholar] [CrossRef]
- Yan, S.; Liu, W. Rare earth elements in the iron-oxide apatite (IOA) deposit: Insights from apatite. Int. Geol. Rev. 2022, 64, 3230–3247. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Y.; Wang, Y.; Xu, Y.; Lin, Z.; Liang, X.; Cheng, H. Review of rare earth element (REE) adsorption on and desorption from clay minerals: Application to formation and mining of ion-adsorption REE deposits. Ore Geol. Rev. 2023, 157, 105446. [Google Scholar] [CrossRef]
- Castrillón, A.; Lartaud, F.; Delgado-Huertas, A.; Núñez-Useche, F. Mineralogical, petrographic, and geochemical analyzes which confirm the hydrothermal origin of the sediments that overlie the peridotites of Cerro Matoso, Colombia. Bol. Geol. 2023, 45, 53–86. [Google Scholar] [CrossRef]
- Veerasamy, N.; Sahoo, S.K.; Murugan, R.; Kasar, S.; Inoue, K.; Fukushi, M.; Natarajan, T. ICP-MS measurement of trace and rare earth elements in beach placer-deposit soils of Odisha, East Coast of India, to estimate natural enhancement of elements in the environment. Molecules 2021, 26, 7510. [Google Scholar] [CrossRef]
- Andreoli MA, G.; Smith, C.B.; Watkeys, M.; Moore, J.M.; Ashwal, L.D.; Hart, R.J. The geology of the Steenkampskraal monazite deposit, South Africa; implications for REE-Th-Cu mineralization in charnockite-granulite terranes. Econ. Geol. 1994, 89, 994–1016. [Google Scholar] [CrossRef]
- Batapola, N.M.; Dushyantha, N.P.; Premasiri, H.M.R.; Abeysinghe, A.M.K.B.; Rohitha, L.P.S.; Ratnayake, N.P.; Dissanayake, D.M.D.O.K.; Ilankoon, I.M.S.K.; Dharmaratne, P.G.R. A comparison of global rare earth element (REE) resources and their mineralogy with REE prospects in Sri Lanka. J. Asian Earth Sci. 2020, 200, 104475. [Google Scholar] [CrossRef]
- Bagdonas, D.A.; Enriquez, A.J.; Coddington, K.A.; Finnoff, D.C.; McLaughlin, J.F.; Bazilian, M.D.; Phillips, E.H.; McLing, T.L. Rare earth element resource evaluation of coal byproducts: A case study from the Powder River Basin, Wyoming. Renew. Sustain. Energy Rev. 2022, 158, 112148. [Google Scholar] [CrossRef]
- Dai, S.; Graham, I.T.; Ward, C.R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- Modiba, D.; Wagner, N. An assessment of Rare Earth Elements in borehole cores from the Ermelo, Witbank and Waterberg Coalfields, South Africa: Focus on mode of occurrence. J. South. Afr. Inst. Min. Metall. 2024, 124, 559–566. [Google Scholar] [CrossRef]
- Chitlango, F.Z. Rare Earth Elements in South African Coals: Concentration and Mode of Occurrence in Density Fractionated Samples from the Waterberg Coalfield; University of Johannesburg: Johannesburg, South Africa, 2023. [Google Scholar]
- Harrar, H.; Eterigho-Ikelegbe, O.; Modiga, A.; Bada, S. Mineralogy and distribution of rare earth elements in the Waterberg coalfield high ash coals. Miner. Eng. 2022, 183, 107611. [Google Scholar] [CrossRef]
- Hutton, A.; Mandile, A. Quantitative XRD measurement of mineral matter in Gondwana coals using the Rietveld method. J. Afr. Earth Sci. 1996, 23, 61–72. [Google Scholar] [CrossRef]
- Akintola, G.O.; Amponsah-Dacosta, F.; Rupprecht, S.; Mhlongo, S.E. Stable isotopic, micro-FTIR, and geochemical characteristics of the Permian Madzaringwe shale of Tuli Basin, South Africa: Implications for organic-rich shale provenance. Minerals 2022, 12, 1160. [Google Scholar] [CrossRef]
- Wagner, J.; Malumbazo, N.; Falcon, R.M. Southern African Coals and Carbons: Definitions and Applications of Organic Petrology; Struik Nature: Cape Town, South Africa, 2018. [Google Scholar]
- Donahue, C.J.; Rais, E.A. Proximate analysis of coal. J. Chem. Educ. 2009, 86, 222. [Google Scholar]
- Thompson, R.L.; Bank, T.; Montross, S.; Roth, E.; Howard, B.; Verba, C.; Granite, E. Analysis of rare earth elements in coal fly ash using laser ablation inductively coupled plasma mass spectrometry and scanning electron microscopy. Spectrochim. Acta Part B At. Spectrosc. 2018, 143, 1–11. [Google Scholar]
- Thomas, G.; Sheridan, C.; Holm, P.E. Arsenic contamination and rare earth element composition of acid mine drainage impacted soils from South Africa. Miner. Eng. 2023, 203, 108288. [Google Scholar] [CrossRef]
- Nesbitt, H.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Bao, Z.; Zhao, Z. Geochemistry of mineralization with exchangeable REY in the weathering crusts of granitic rocks in South China. Ore Geol. Rev. 2008, 33, 519–535. [Google Scholar]
- Ferris, A.; Jepson, W. The exchange capacities of kaolinite and the preparation of homoionic clays. J. Colloid Interface Sci. 1975, 51, 245–259. [Google Scholar] [CrossRef]
- Ma, C.; Eggleton, R.A. Cation exchange capacity of kaolinite. Clays Clay Miner. 1999, 47, 174–180. [Google Scholar]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B.J.C.M. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar]
- Cheshire, M.C.; Bish, D.L.; Cahill, J.F.; Kertesz, V.; Stack, A.G. Geochemical evidence for rare-earth element mobilization during kaolin diagenesis. ACS Earth Space Chem. 2018, 2, 506–520. [Google Scholar]
- Shen, M.; Dai, S.; French, D.; Graham, I.T.; Spiro, B.F.; Wang, N.; Tian, X. Geochemical and mineralogical evidence for the formation of siderite in Late Permian coal-bearing strata from western Guizhou, SW China. Chem. Geol. 2023, 637, 121675. [Google Scholar]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Sutcu, E.C.; Şentürk, S.; Kapıcı, K.; Gökçe, N. Mineral and rare earth element distribution in the Tunçbilek coal seam, Kütahya, Turkey. Int. J. Coal Geol. 2021, 245, 103820. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B.; French, D.; Hower, J.C.; Graham, I.T.; Zhao, F. Modes of occurrence of elements in coal: A critical evaluation. Earth-Sci. Rev. 2021, 222, 103815. [Google Scholar] [CrossRef]
- Chou, C.-L. Sulfur in coals: A review of geochemistry and origins. Int. J. Coal Geol. 2012, 100, 1–13. [Google Scholar] [CrossRef]
- Mahooana, P.E.; Moroeng, O.M.; Wagner, N.J. Petrology of the A and B Seams, Ermelo Coalfield (South Africa): Indications for changing palaeoenvironmental and sedimentary conditions. Int. J. Coal Geol. 2022, 263, 104135. [Google Scholar] [CrossRef]
- DeCuir, M.J.; Gupta, R.B.; Sastri, B. Beneficiation of coal using supercritical water and carbon dioxide extraction: Sulfur removal. Int. J. Coal Sci. Technol. 2021, 8, 717–726. [Google Scholar] [CrossRef]
- Speight, J.G. Handbook of Coal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Wagner, N.J.; Matiane, A. Rare earth elements in select Main Karoo Basin (South Africa) coal and coal ash samples. Int. J. Coal Geol. 2018, 196, 82–92. [Google Scholar] [CrossRef]
- Fathy, D.; Wagreich, M.; Ntaflos, T.; Sami, M. Provenance characterization of campanian lacustrine organic-rich mudstones on the southern tethyan margin, egypt. ACS Earth Space Chem. 2021, 5, 197–209. [Google Scholar] [CrossRef]
- Hayashi, K.-I.; Fujisawa, H.; Holland, H.D.; Ohmoto, H. Geochemistry of∼ 1.9 Ga sedimentary rocks from northeastern Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 4115–4137. [Google Scholar] [CrossRef]
- Xia, B.; Zhu, J.-M.; Wang, X.; Zhang, L.; Wu, G. Chromium isotope fractionation during black shale weathering and its environmental implications. Sci. Total Environ. 2021, 783, 147126. [Google Scholar] [PubMed]
- Catuneanu, O. Sequence stratigraphy. In Regional Geology and Tectonics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 605–686. [Google Scholar]



| Reaction Phases | Experimental Method | |
|---|---|---|
| 1 | Water soluble | 8 g Carbonaceous shale and Coal samples + 60 mL water, 25 °C, 24 h |
| 2 | Ion exchangeable | Residue of Phase 1 + 60 mL NH4Ac, 25 °C, 24 h |
| 3 | Organic bonded | Residue of Phase 2 + 1.47 g/cm3 the floating dried at 40 °C, ashed at 650 °C, +3 mL HClO4, 200 °C 60 h |
| 4 | Carbonate | The sinking of Residue of Phase 2, washed by alcohol and dried at 40° C, +20 mL 0.5% HCl |
| 5 | Silicate | Residue of Phase 3 + 2.89 g/cm3, CHBr3, the floating dried at 40° C, ashed at 650° C, +3 mL HNO3 and 3 mL HF, 200 °C, 60 h |
| 6 | Sulfide | The sinking of Residue of Phase 3, washed by water and dried at 40 °C, +HNO3, 5 h |
| Samples Name | Samples Type | Al2O3 (%) | CaO (%) | Cr2O3 (%) | Fe2O3 (%) | K2O (%) | MgO (%) | MnO (%) | Na2O (%) | P2O5 (%) | SiO2 (%) | TiO2 (%) | Al2O3/TiO2 (%) | L.O.I. | CIA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS-1 | CS | 22.05 | 0.27 | 0.01 | 3.54 | 2.18 | 0.92 | 0.05 | 0.44 | 0.06 | 54.81 | 0.92 | 23.97 | 14.70 | 88.41 |
| BS-2 | 22.06 | 0.35 | 0.01 | 6.14 | 2.50 | 1.10 | 0.11 | 0.67 | 0.08 | 50.96 | 0.80 | 27.58 | 15.29 | 86.24 | |
| BS-3 | 17.72 | 0.95 | 0.01 | 8.45 | 2.79 | 1.60 | 0.10 | 0.46 | 0.11 | 53.04 | 0.83 | 21.35 | 14.00 | 80.84 | |
| BS2-1 | 21.54 | 0.37 | 0.01 | 4.79 | 2.19 | 1.19 | 0.15 | 0.10 | 0.10 | 47.57 | 0.86 | 25.05 | 20.82 | 89.01 | |
| BS2-4 | 13.91 | 1.33 | 0.07 | 14.37 | 1.19 | 4.69 | 0.19 | 0.28 | 0.06 | 44.18 | 0.33 | 42.15 | 19.02 | 83.24 | |
| BS2-2 | 10.22 | 0.93 | 0.02 | 1.67 | 0.99 | 0.57 | 0.02 | 0.07 | 0.01 | 44.12 | 0.81 | 12.62 | 39.87 | 83.70 | |
| BS2-3 | 18.99 | 0.68 | 0.01 | 6.37 | 1.43 | 0.95 | 0.18 | 0.08 | 0.06 | 42.04 | 0.87 | 21.83 | 28.29 | 89.66 | |
| BSC-1 | Coal | 20.01 | 0.59 | 0.02 | 3.86 | 1.89 | 0.71 | 0.08 | 0.30 | 0.12 | 48.98 | 0.79 | 25.33 | 22.21 | 87.80 |
| BSC-2 | 5.18 | 1.00 | 0.01 | 3.18 | 0.22 | 0.31 | 0.01 | 0.06 | 0.01 | 22.30 | 0.29 | 17.86 | 66.59 | 80.19 | |
| BSC-3 | 7.55 | 1.38 | bdl | 0.79 | 0.21 | 0.48 | 0.02 | 0.10 | 0.02 | 20.15 | 0.46 | 16.41 | 67.85 | 81.71 | |
| BSC2-1 | 17.00 | 0.20 | 0.01 | 1.74 | 1.37 | 0.57 | 0.02 | 0.08 | 0.04 | 42.76 | 0.69 | 24.64 | 35.50 | 91.15 | |
| Ave | 11.79 | 0.84 | 0.01 | 2.75 | 0.84 | 0.58 | 0.05 | 0.08 | 0.03 | 34.27 | 0.62 | 19.02 | 47.62 | 85.68 |
| Samples | S/Type | Qtz | Kaol | Pyr | Mus | Anat | Rut | Micro | Sid | Plag | Organic C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BS-1 | CS | 26.1 | 44.2 | 0 | 7.7 | 0.6 | 0.4 | 7.4 | 1.2 | 0.7 | 11.7 |
| BS-2 | 18.5 | 45.1 | 0 | 9 | 0.7 | 0.6 | 7.2 | 2.8 | 3 | 13.2 | |
| BS2-1 | 21.8 | 42.3 | 0 | 8.5 | 0.6 | 0.5 | 5 | 3.4 | 0.1 | 17.7 | |
| BS2-4 | 30.6 | 15.7 | 0.6 | 6.5 | 0.3 | 0 | 5.3 | 23.5 | 0.6 | 17 | |
| BS-3 | 36.3 | 26.8 | 0.4 | 4.9 | 0.4 | 0.1 | 10.2 | 6.9 | 0.8 | 13.2 | |
| BS2-2 | 26.3 | 27.1 | 0 | 3.5 | 0.1 | 0.7 | 4.7 | 0 | 0 | 37.6 | |
| BS2-3 | 16 | 40.1 | 0 | 5.8 | 0.4 | 0.8 | 5.9 | 4.9 | 0.1 | 26 | |
| BSC-1 | Coal | 22.2 | 41.6 | 0 | 5.2 | 0.4 | 0.8 | 7.5 | 1.3 | 0.8 | 20.3 |
| BSC-2 | 16.2 | 13.3 | 1.4 | 1.3 | 0 | 0.1 | 3.3 | 0 | 0 | 64.3 | |
| BSC2-1 | 17.8 | 36.8 | 0 | 6.1 | 0.2 | 0.4 | 5.5 | 0.1 | 0.1 | 33 | |
| BSC-3 | 12 | 19.6 | 0 | 1.3 | 0.1 | 0.1 | 1.5 | 0 | 0 | 65.4 |
| Samples | CS BS1 | CS BS2 | CS BS3 | CS BS2-1 | CS BS2-4 | CS BS2-2 | CS BS2-3 | Coal BSC-1 | Coal BSC-2 | Coal BSC-3 | Coal BSC-4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % inherent moisture content | 1.3 | 1.4 | 0.6 | 2.0 | 4.0 | 1.7 | 1.6 | 1.3 | 2.3 | 2.5 | 2.0 |
| %Ash content | 86.0 | 85.5 | 86.5 | 81.2 | 83.3 | 62.3 | 73.2 | 78.5 | 33.1 | 32.1 | 66.4 |
| %volatile Matter | 9.1 | 9.9 | 10.7 | 10.1 | 13.4 | 14.1 | 13.7 | 11.0 | 18.6 | 18.9 | 11.5 |
| %Fixed carbon (by Cal) | 3.6 | 3.2 | 2.2 | 6.7 | 0.7 | 11.9 | 11.5 | 29.2 | 46 | 46.5 | 20.1 |
| %Total carbon | 5.41 | 5.37 | 5.23 | 8.68 | 2.76 | 26.14 | 14.84 | 11.42 | 50.28 | 51.06 | 22.61 |
| %Organic carbon | 4.69 | 4.6 | 4.9 | 6.95 | 2.77 | 24.04 | 14.36 | 11.32 | 48.69 | 49.32 | 21.11 |
| %Elemental Carbon | 0.72 | 0.77 | 0.39 | 1.78 | <0.05 | 2.06 | 0.55 | 0.18 | 1.58 | 1.59 | 1.49 |
| Sample | Sc | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | ΣREE | ΣREY | ΣREY + Sc | Coutl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS-1 | 19.11 | 67.51 | 134.84 | 14.7 | 55.26 | 10.14 | 1.99 | 8.65 | 1.33 | 8.6 | 1.67 | 4.59 | 0.63 | 4.13 | 0.68 | 44.79 | 314.72 | 359.51 | 378.62 | 0.86 |
| BS-2 | 17.74 | 67.29 | 149.33 | 15.51 | 58.87 | 11.67 | 1.97 | 8.67 | 1.25 | 7.7 | 1.54 | 4.32 | 0.61 | 3.8 | 0.58 | 38.84 | 333.11 | 371.95 | 389.69 | 0.76 |
| BS2-1 | 21.16 | 82.51 | 179.1 | 19.02 | 68 | 12.75 | 2.26 | 11.69 | 1.66 | 9.49 | 1.9 | 5.3 | 0.74 | 5.04 | 0.71 | 50.52 | 400.17 | 450.69 | 471.85 | 0.77 |
| BS2-4 | 27.87 | 9.86 | 19.21 | 2.37 | 9.15 | 1.72 | 0.65 | 1.9 | 0.35 | 2.28 | 0.53 | 1.51 | 0.23 | 1.73 | 0.25 | 15.46 | 51.74 | 67.2 | 95.07 | 1.38 |
| BS-3 | 16.44 | 60.55 | 113.89 | 14.14 | 50.68 | 9.74 | 1.62 | 8.14 | 1.3 | 6.79 | 1.33 | 3.75 | 0.62 | 3.42 | 0.58 | 38.56 | 276.55 | 315.11 | 331.55 | 0.90 |
| BS2-2 | 28.15 | 94.24 | 152.17 | 16.32 | 52.98 | 15.2 | 1.51 | 10.65 | 0.25 | 10.26 | 1.5 | 4.09 | 0.48 | 6.93 | 0.62 | 60.75 | 367.2 | 427.95 | 456.1 | 0.86 |
| BS2-3 | 39.52 | 115.42 | 173.77 | 27.41 | 63.73 | 26.2 | 2.02 | 21.54 | 1.14 | 9.15 | 0.41 | 5.18 | 0.72 | 5.82 | 0.54 | 71 | 453.05 | 524.05 | 563.57 | 0.94 |
| BSC-1 | 18.33 | 67.26 | 143.84 | 15.23 | 56.91 | 11.22 | 1.87 | 9.37 | 1.38 | 8.26 | 1.66 | 4.29 | 0.63 | 4.34 | 0.62 | 42.67 | 326.88 | 369.55 | 387.88 | 0.80 |
| BSC-2 | 40.25 | 90.46 | 185.65 | 37.45 | 65.73 | 34.35 | 1.74 | 20.48 | 1.96 | 9.67 | 1.12 | 5.61 | 1.25 | 5.28 | 0.41 | 80.98 | 461.16 | 542.14 | 582.39 | 0.94 |
| BSC2-1 | 16.63 | 56.17 | 138.11 | 14.4 | 47.76 | 10.56 | 0.85 | 7.46 | 0.14 | 6.51 | 0.42 | 3.21 | 0.33 | 2.6 | 0.53 | 27.73 | 289.05 | 316.78 | 333.41 | 0.65 |
| BSC-3 | 39.12 | 78.37 | 154.65 | 26.14 | 65.73 | 23.14 | 2.56 | 10.28 | 2.14 | 10.17 | 2.54 | 6.18 | 0.54 | 6.45 | 0.51 | 51.75 | 389.4 | 441.15 | 480.27 | 0.88 |
| Samples | LaN/LuN | LaN/SmN | GdN/LuN | EuN/EuN* | CeN/CeN* | GdN/GdN* |
|---|---|---|---|---|---|---|
| BS-1 | 1.06 | 1.00 | 1.07 | 1.01 | 0.98 | 1.07 |
| BS-2 | 1.24 | 0.86 | 1.26 | 0.92 | 1.05 | 1.05 |
| BS2-1 | 1.24 | 0.97 | 1.39 | 0.91 | 1.03 | 1.15 |
| BS2-4 | 0.42 | 0.86 | 0.64 | 1.65 | 0.91 | 1.02 |
| BS-3 | 1.11 | 0.93 | 1.18 | 0.85 | 0.89 | 1.03 |
| BS2-2 | 1.62 | 0.93 | 1.45 | 0.70 | 0.87 | 2.04 |
| BS2-3 | 2.28 | 0.66 | 3.36 | 0.50 | 0.70 | 1.82 |
| BSC-1 | 1.16 | 0.90 | 1.27 | 0.87 | 1.02 | 1.09 |
| BSC-2 | 2.35 | 0.40 | 4.21 | 0.32 | 0.70 | 1.18 |
| BSC2-1 | 1.13 | 0.80 | 1.19 | 0.57 | 1.11 | 2.13 |
| BSC-3 | 1.64 | 0.51 | 1.70 | 0.63 | 0.77 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akintola, G.O. Carbonaceous Shale Deposits as Potential Unconventional Sources for Rare Earth Elements at the Witbank Coalfield, Permian Vryheid Formation, South Africa. Minerals 2025, 15, 388. https://doi.org/10.3390/min15040388
Akintola GO. Carbonaceous Shale Deposits as Potential Unconventional Sources for Rare Earth Elements at the Witbank Coalfield, Permian Vryheid Formation, South Africa. Minerals. 2025; 15(4):388. https://doi.org/10.3390/min15040388
Chicago/Turabian StyleAkintola, George Oluwole. 2025. "Carbonaceous Shale Deposits as Potential Unconventional Sources for Rare Earth Elements at the Witbank Coalfield, Permian Vryheid Formation, South Africa" Minerals 15, no. 4: 388. https://doi.org/10.3390/min15040388
APA StyleAkintola, G. O. (2025). Carbonaceous Shale Deposits as Potential Unconventional Sources for Rare Earth Elements at the Witbank Coalfield, Permian Vryheid Formation, South Africa. Minerals, 15(4), 388. https://doi.org/10.3390/min15040388






