Development and Optimization of Bentonite-Based Slurry Sealing Material
Abstract
1. Introduction
2. Material and Experiment
2.1. Material Composition
- (1)
- Sodium Bentonite
- (2)
- Sodium dodecyl benzene sulfonate
- (3)
- Sodium carboxymethyl cellulose
2.2. Experimental Scheme of Slurry Sealing Material
3. Single-Factor Impact Analysis
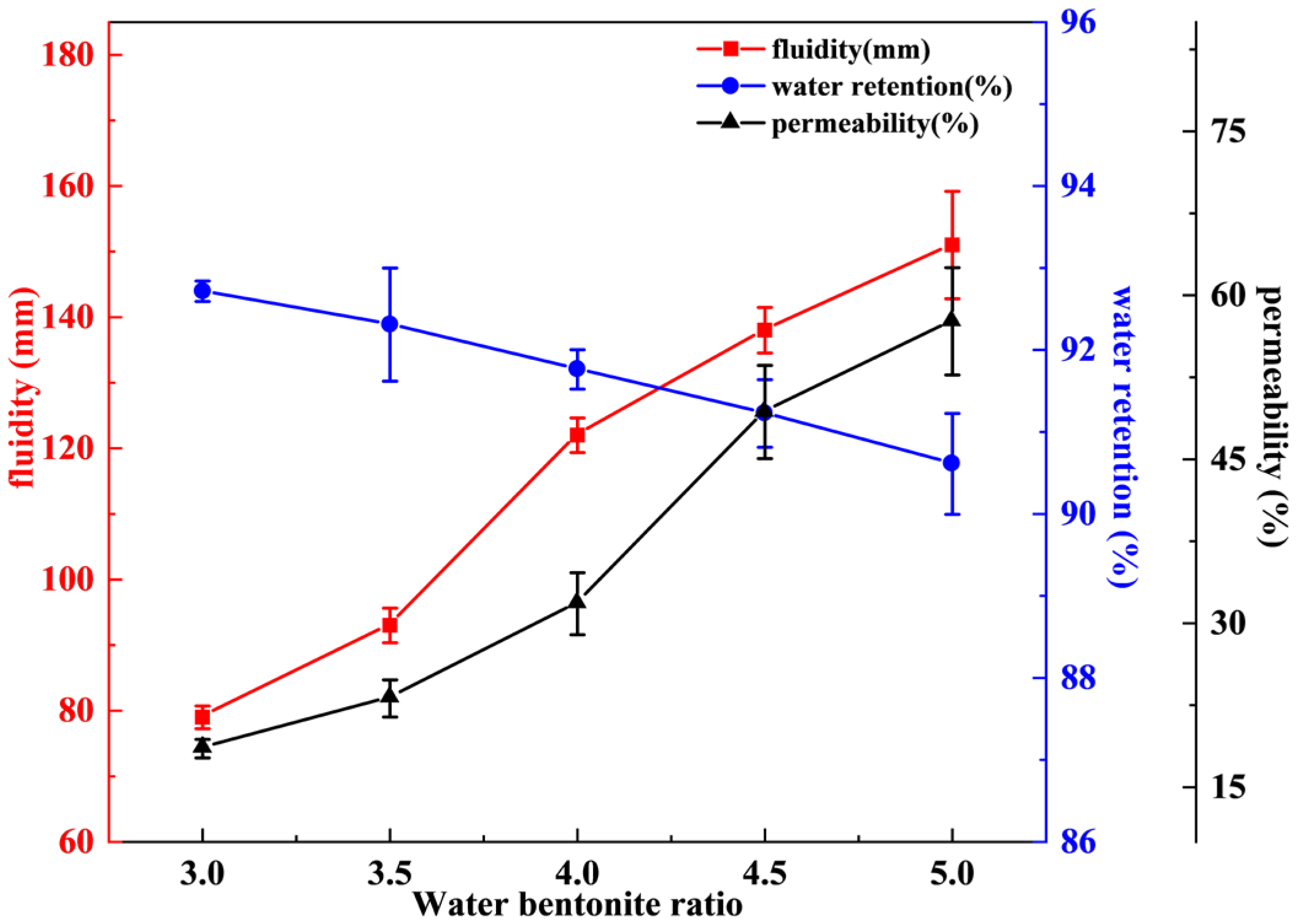
3.1. Effect of Water–Bentonite Ratio on Performance of Sealing Material
3.2. Effect of Dispersant Content on Performance of Sealing Material
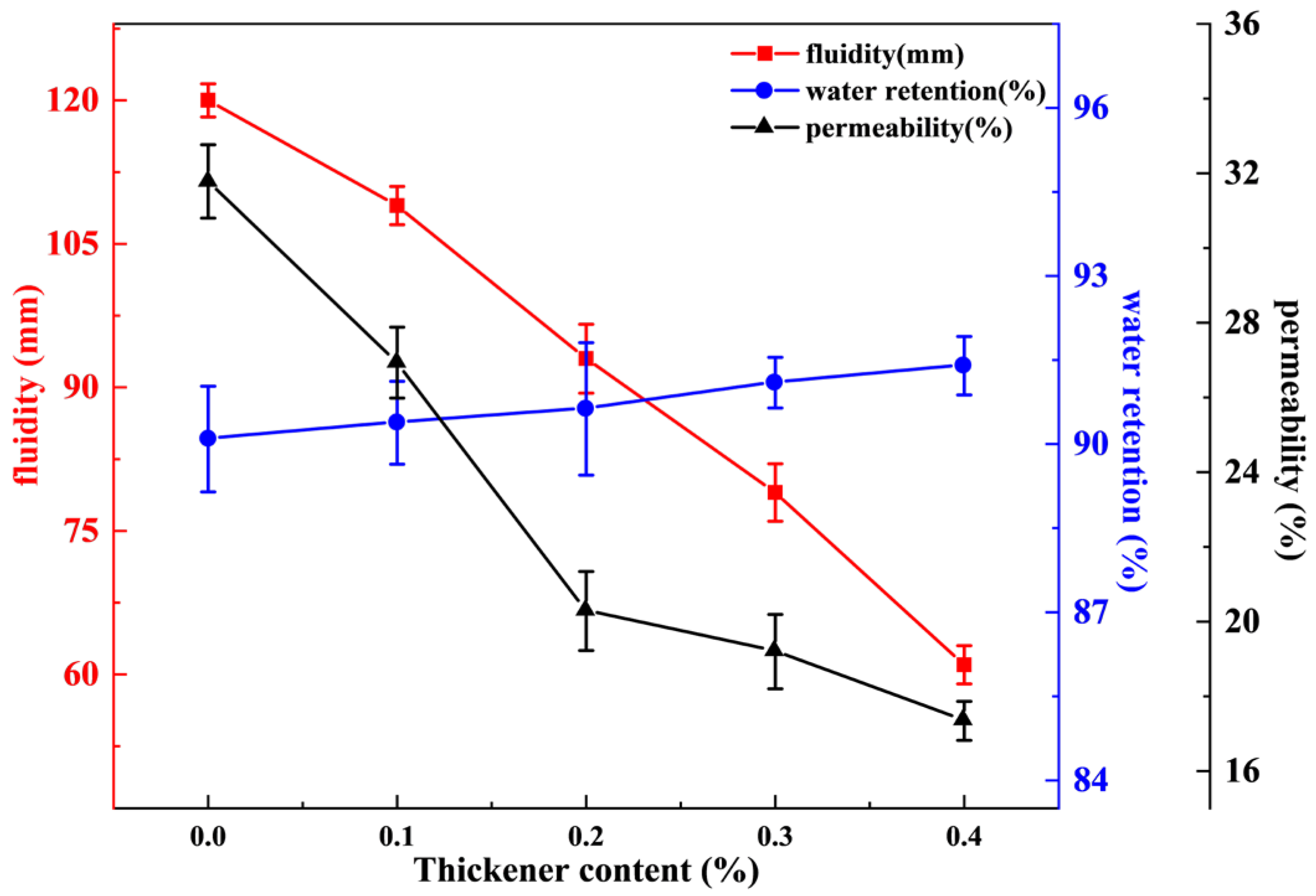
3.3. Effect of Thickener Content on Performance of Sealing Material
4. Slurry Sealing Material Ratio Optimization and Results
4.1. Experimental Design of Response Surface Analysis
- (1)
- According to the single-factor experimental results, the appropriate range of parameters was selected.
- (2)
- Using Design-Expert software, 17 groups of three-factor experiments were designed.
- (3)
- The model with the least deviation and best fitting effect was selected.
- (4)
- The response surface was drawn according to the model with the best fitting effect. This was used to predict and optimize the response value, analyze the interaction between any two factors, and obtain the interaction law.
- (5)
- For further analysis of the experimental results, use Design-Expert software to optimize the experimental program, select five groups of recommended experimental programs, and verify the recommended schemes in terms of the optimal group allocation ratio.
4.2. Orthogonal Experimental Results and Response Surface Analysis
4.2.1. Response Surface Analysis of Fluidity
- (1)
- Establishment of the model
- (2)
- Factor variance results
- (3)
- Response surface analysis
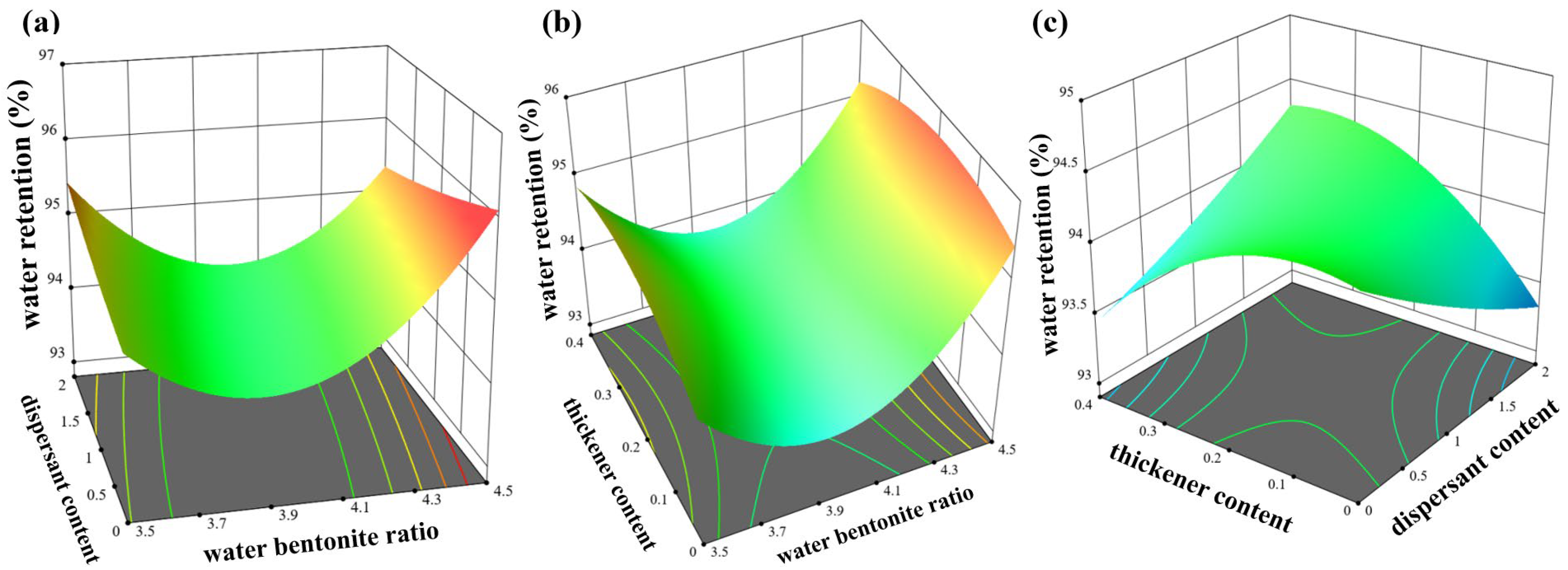
4.2.2. Response Surface Analysis of Water Retention
- (1)
- Establishment of the model
- (2)
- Factor variance results
- (3)
- Response surface analysis
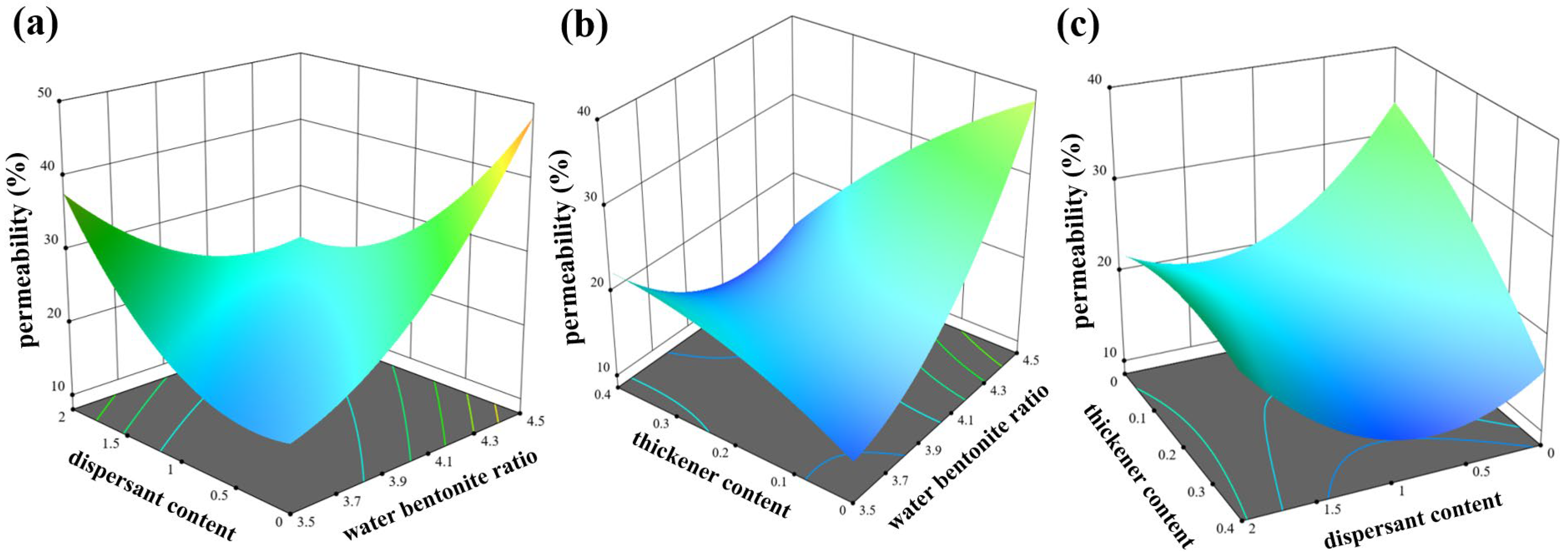
4.2.3. Response Surface Analysis of Permeability
- (1)
- Establishment of the model
- (2)
- Factor variance results
- (3)
- Response surface analysis
4.3. Optimization and Analysis of Experimental Results
5. Conclusions
- In the single-factor experiment, as the water–bentonite ratio increased from 3:1 to 5:0, the fluidity of the sealing material increased from 79 to 151 mm, the permeability increased from 18.5% to 57.6%, and the water retention decreased from 92.7% to 90.6%. With the increase in dispersant content, the water retention of the sealing material increased from 89.9% to 91.4% and the permeability increased from 31.8% to 41.8%, but the fluidity decreased from 120 mm to 101 mm. On the contrary, with the increase in thickener content, the water retention increased from 90.4% to 91.4%, the fluidity decreased from 109 mm to 61 mm, and the permeability decreased from 27% to 17.3%.
- A nonlinear quadratic regression model for fluidity, water retention, and permeability concerning the three factors of water–bentonite ratio, dispersant, and thickener was developed using the response surface methodology of response surface class design methods. The interaction between the water–bentonite ratio and thickener had the most pronounced effect on fluidity, ranging from 75 mm to 160 mm. The interaction between dispersant and thickener had the most significant impact on water retention, ranging from 93.3% to 94.5%. The interaction between water–bentonite ratio and dispersant had the most substantial influence on permeability, ranging from 15% to 46%.
- The response surface analysis indicated that the deviation between the experimental results and the predicted values for the optimized ratio of the new slurry sealing material was less than 0.81%. The optimal composition is a water–bentonite ratio of 4.41:1, dispersant content of 0.38%, and thickener content of 0.108%. Under this composition, the material exhibits fluidity of 134 mm, water retention of 96.12%, and permeability of 41.45%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zhao, W.; Zhao, D.; Wang, K.; Fan, L.; Zhao, Z.; Dong, H.; Shu, L. Will greenhouse gas emissions increase with mining depth in coal mines? An analysis of gas occurrence under varying in-situ stress conditions. Sci. Total. Environ. 2024, 945, 173957. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X. Identification and Prediction of Thermodynamic Disasters During Deep Coal Mining. Int. J. Heat Technol. 2022, 40, 6. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, H.; Li, S.; Yang, E.; Ding, Y.; Bai, Y.; Zhou, Y. Accurate gas extraction (AGE) under the dual-carbon background: Green low-carbon development pathway and prospect. J. Clean. Prod. 2022, 377, 134372. [Google Scholar] [CrossRef]
- Gao, J.; Guan, C.; Zhang, B.; Li, K. Decreasing methane emissions from China’s coal mining with rebounded coal production. Environ. Res. Lett. 2021, 16, 124037. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, R.-H.; Liu, C.; Xue, J.-H.; Liu, H.; Jin, G.-H.; Chang, J.; Yan, J.; Zeng, X.-Z.; Wang, X.-L. Experimental study on strengthening and sealing materials and their application in coal mines. Adv. Mater. Sci. Eng. 2020, 2020, 6025452. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Yang, D.; Liu, T.; Li, X.; Tang, J.; Jiang, C. Study on the Interaction between Low-Viscosity High-Permeability Pregrouting Sealing Material and Coal and Its Application. Adv. Polym. Technol. 2020, 2020, 1217285. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, Q.; Guo, L. Air-leakage Model and sealing technique with sealing–isolation integration for gas-drainage boreholes in coal mines. Process Saf. Environ. Prot. 2020, 140, 258–272. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Wang, Y.; Zhang, J.; Sun, Z. A coupled model of air leakage in gas drainage and an active support sealing method for improving drainage performance. Fuel 2019, 237, 1217–1227. [Google Scholar] [CrossRef]
- Hong, Z.; Zuo, J.; Li, Z.; Xu, L. Characterization and field application of a novel dual-liquid gas leakage material: Mechanical properties and microscopic hydration mechanism. PLoS ONE 2023, 18, e0284140. [Google Scholar] [CrossRef]
- Yu, S.; Su, X.; Song, J.; Wang, Q.; You, Z. The hole sealing technology of solid–Liquid Materials with three pluggings and two injections for gas extraction hole in the coal mine. ACS Omega 2022, 7, 43847–43855. [Google Scholar] [CrossRef]
- Cheng, J.; Fu, Q.; Wang, Y.; Ma, Z.; Ran, D.; Sheng, S.; Ma, Y. Development of non-coagulable grout long-term sealing material for gas drainage. Inorg. Chem. Commun. 2023, 154, 110882. [Google Scholar] [CrossRef]
- He, Y.; Zhang, K.N.; Wu, D.Y. Experimental and modeling study of soil water retention curves of compacted bentonite considering salt solution effects. Geofluids 2019, 1, 4508603. [Google Scholar] [CrossRef]
- Khan, M.K.; Di Emidio, G.; Adam, B. Water retention curves of untreated and hyper clay geosynthetic clay liners. Environ. Geotech. 2022, 11, 90–101. [Google Scholar] [CrossRef]
- Albadri, W.M.; Alhani, I.J.; Shaia, H.A.; Sabri, M.M.S.; Lim, S.M.; Jabbar, M.S.; Mohammed, M.S. Investigating the hydro-mechanical behaviour of unsaturated sand-bentonite mixtures using suction-controlled triaxial test. Case Stud. Constr. Mater. 2023, 18, e01953. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, J. Research on interface modification and long-term sealing properties of bentonite-based sealing materials. Coal Eng. 2024, 56, 157–164. (In Chinese) [Google Scholar]
- Guo, X.; Xue, S.; Zheng, C.; Li, Y. Experimental research on performance of new gas drainage borehole sealing material with high fluidity. Adv. Mater. Sci. Eng. 2021, 2021, 6645425. [Google Scholar] [CrossRef]
- Guo, X.; Xue, S.; Li, Y.; Zheng, C.; Yang, G. Fluidity influencing factors analysis and ratio optimization of new sealing materials based on response surface method. Geofluids 2021, 2021, 5538302. [Google Scholar] [CrossRef]
- Xue, S.; Guo, X.; Zheng, C.; Li, Y.; Zheng, X. Response Surface Method for Strength Analysis and Proportion Optimization of New Type Sealing Materials. Geofluids 2021, 2021, 8769809. [Google Scholar] [CrossRef]
- Mao, J.; Guanhua, N.; Yuhang, X.; Hui, W.; Zhao, L.; Zhenyang, W. Modeling and optimization of mechanical properties of drilling sealing materials based on response surface method. J. Clean. Prod. 2022, 377, 134452. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Q. Sodium alginate/modified bentonite composite bead adsorptive removal of norfloxacin: Static and dynamic adsorption. Polymers 2022, 14, 3984. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, Z.; Wang, Y.; Fu, Q.; Ran, D.; Ma, Y.; Yang, C.; Hu, X.; Chu, T. Performance and application of new inorganic retarding sealing material suitable for multi-scale fractures. Heliyon 2023, 9, e16603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, W. Analysis of the main sealing methods of gas drainage borehole in coal mine. Coal Sci. Technol. 2014, 42, 31–34. (In Chinese) [Google Scholar] [CrossRef]
- Goncharuk, V.V.; Dubrvina, L.V.; Makarova, E.V. Conservation of Water in Gel and Dispersed Structurally Stable Composites. J. Water Chem. Technol. 2018, 40, 46–50. [Google Scholar] [CrossRef]
- Boylu, F.; Ateşok, G.; Dincer, H. The effect of carboxymethyl cellulose (CMC) on the stability of coal-water slurries. Fuel 2015, 84, 315–319. [Google Scholar] [CrossRef]
- JC/T 1023-2007; Gypsum Based Self-Leveling Floor Compound. Building Material Net: Beijing, China, 2007.
- Cheng, J.; Ran, D.; Fu, Q.; Lu, W.; Sheng, S.; Ma, Z.; Fediuk, R.; Liu, J. Solid-liquid particle flow sealing mucus (PFSM) in enhancing coalbed methane (CBM) recovery: Multiple-perspectives analysis and mechanism insights. Constr. Build. Mater. 2024, 435, 136670. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Yao, B.; Mian, W. Time-dependent viscosity diffusion model of cement mortar grouting for gas drainage borehole sealing. Coal Sci. Technol. 2020, 48, 52–59. (In Chinese) [Google Scholar] [CrossRef]
- Gao, Z. Application of Secondary Grouting Sealing Technology to Improve Gas Extraction Efficiency. Coal Technol. 2017, 36, 141–143. (In Chinese) [Google Scholar] [CrossRef]
- Hamza, H.; Eterigho-Ikelegbe, O.; Jibril, A.; Bada, S.O. Application of the response surface methodology to optimise the leaching process and recovery of rare earth elements from discard and run of mine coal. Minerals 2022, 12, 938. [Google Scholar] [CrossRef]
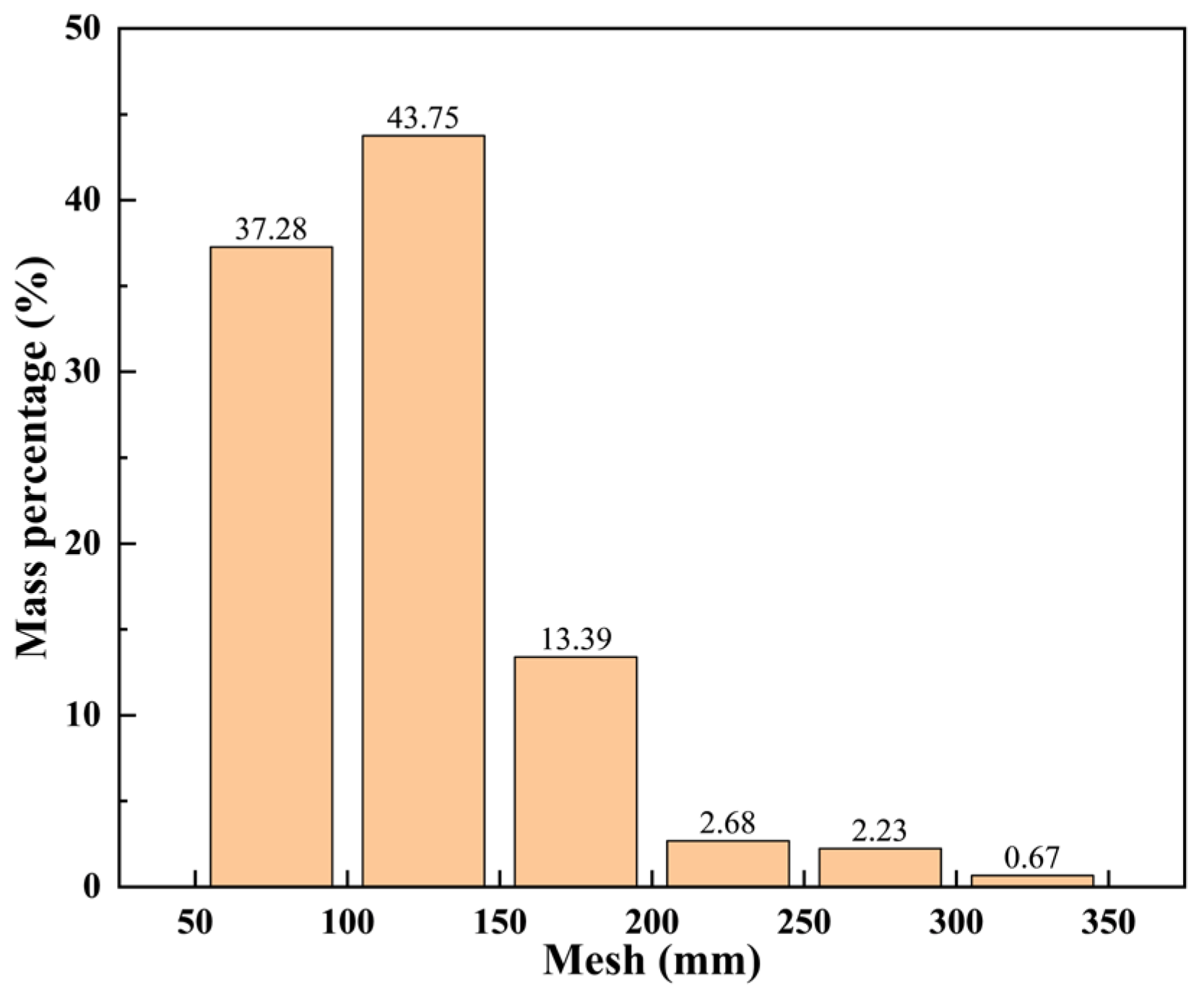


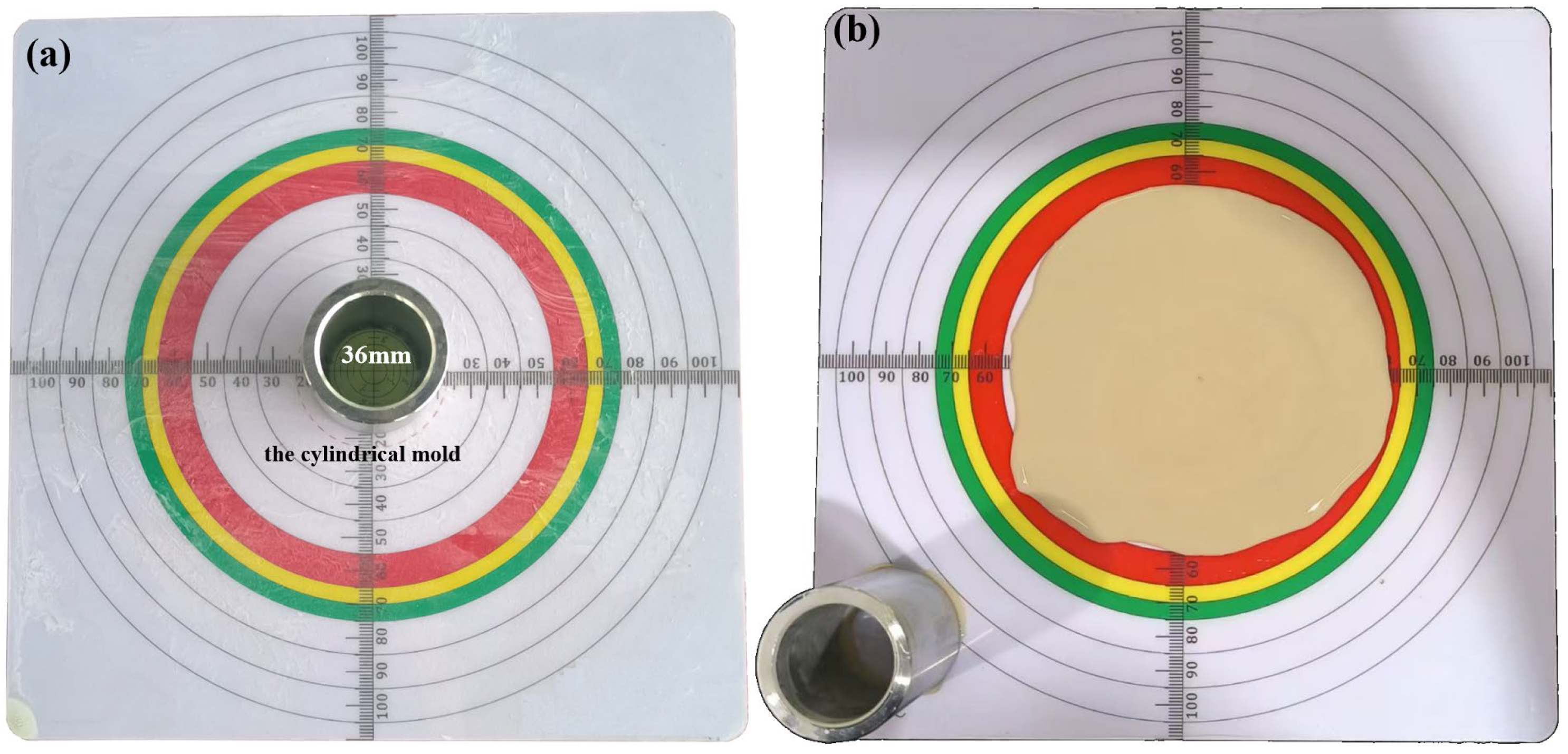
| Experiment | Water–Bentonite Ratio | Dispersant Content (%) | Thickener Content (%) |
|---|---|---|---|
| 1 | 3.0 | 0 | 0 |
| 2 | 3.5 | 0 | 0 |
| 3 | 4.0 | 0 | 0 |
| 4 | 4.5 | 0 | 0 |
| 5 | 5.0 | 0 | 0 |
| 6 | 4.0 | 0 | 0 |
| 7 | 4.0 | 0.5 | 0 |
| 8 | 4.0 | 1.0 | 0 |
| 9 | 4.0 | 1.5 | 0 |
| 10 | 4.0 | 2 | 0 |
| 11 | 4.0 | 0 | 0 |
| 12 | 4.0 | 0 | 0.1 |
| 13 | 4.0 | 0 | 0.2 |
| 14 | 4.0 | 0 | 0.3 |
| 15 | 4.0 | 0 | 0.4 |
| Experimental Group | Value Range of Each Factor (%) | ||
|---|---|---|---|
| Water–Bentonite Ratio | Dispersant Content | Thickener Content | |
| 1–17 | 3.5–4.5 | 0–2 | 0–0.4 |
| Experiment | Influencing Factor | |||
|---|---|---|---|---|
| Experimental Ratio (Water–Bentonite Ratio: Dispersant Content: Thickener Content) | Fluidity (mm) | Water Retention (%) | Permeability (%) | |
| 1 | 3.5:0:0.2 | 75 ± 2.65 | 94.64 ± 0.33 | 17.71 ± 0.58 |
| 2 | 4.5:0:0.2 | 129 ± 2.00 | 95.76 ± 0.21 | 53.03 ± 0.99 |
| 3 | 3.5:2:0.2 | 80 ± 1.73 | 95.82 ± 0.54 | 33.07 ± 0.33 |
| 4 | 4.5:2:0.2 | 115 ± 2.65 | 95.56 ± 0.63 | 21.84 ± 0.92 |
| 5 | 3.5:1:0 | 91 ± 2.65 | 94.65 ± 0.3 | 11.64 ± 0.52 |
| 6 | 4.5:1:0 | 163 ± 2.65 | 95.7 ± 0.49 | 33.12 ± 1.07 |
| 7 | 3.5:1:0.4 | 88 ± 1.00 | 94.66 ± 0.09 | 28.59 ± 0.58 |
| 8 | 4.5:1:0.4 | 110 ± 1.73 | 95.16 ± 0.51 | 15.38 ± 0.12 |
| 9 | 4.0:0:0 | 124 ± 2.00 | 94.44 ± 0.90 | 34.82 ± 0.73 |
| 10 | 4.0:2:0 | 99 ± 1.73 | 92.91 ± 0.27 | 28.8 ± 1.06 |
| 11 | 4.0:0:0.4 | 80 ± 1.73 | 93.88 ± 1.45 | 9.98 ± 0.94 |
| 12 | 4.0:2:0.4 | 94 ± 2.00 | 94.21 ± 0.21 | 22.81 ± 0.72 |
| 13 | 4.0:1:0.2 | 93 ± 2.65 | 93.47 ± 0.79 | 16.61 ± 0.78 |
| 14 | 4.0:1:0.2 | 97 ± 2.65 | 93.82 ± 0.89 | 20.35 ± 0.78 |
| 15 | 4.0:1:0.2 | 100 ± 2.00 | 94.39 ± 0.93 | 18.78 ± 1.14 |
| 16 | 4.0:1:0.2 | 95 ± 1.00 | 94.51 ± 0.64 | 18.1 ± 0.91 |
| 17 | 4.0:1:0.2 | 99 ± 2.00 | 94.55 ± 1.56 | 20.02 ± 0.84 |
| Sequential p-Value | Adjusted R2 | Predicted R2 | Suggested |
|---|---|---|---|
| Linear | 0.7816 | 0.7312 | No |
| 2FI | 0.9200 | 0.8719 | No |
| Quadratic | 0.9940 | 0.9864 | Yes |
| Cubic | 0.9648 | 0.9792 | No |
| Source | Sum of Squares | Mean Square | F | p |
|---|---|---|---|---|
| Model | 6255 | 695.1 | 129.6 | <0.0001 |
| A | 3741 | 3741 | 697.4 | <0.0001 |
| B | 50 | 50 | 9.320 | 0.0185 |
| C | 1128 | 1128 | 210.3 | <0.0001 |
| AB | 90.25 | 90.25 | 16.82 | 0.0046 |
| AC | 400 | 400 | 74.57 | <0.0001 |
| BC | 380.3 | 380.3 | 70.89 | <0.0001 |
| Sequential p-Value | Adjusted R2 | Predicted R2 | Suggested |
|---|---|---|---|
| Linear | 0.0684 | −0.1470 | No |
| 2FI | 0.1999 | −0.2800 | No |
| Quadratic | 0.9354 | 0.8240 | Yes |
| Cubic | 0.8148 | 0.6592 | No |
| Source | Sum of Squares | Mean Square | F | p |
|---|---|---|---|---|
| Model | 9.01 × 10−4 | 1.00 × 10−4 | 3.950 | 0.0419 |
| A | 7.26 × 10−5 | 7.26 × 10−5 | 2.870 | 0.1343 |
| B | 6.05 × 10−7 | 6.05 × 10−7 | 0.024 | 0.8816 |
| C | 5.51 × 10−7 | 5.51 × 10−7 | 0.022 | 0.8869 |
| AB | 4.76 × 10−5 | 4.76 × 10−5 | 1.880 | 0.2128 |
| AC | 7.56 × 10−6 | 7.56 × 10−6 | 0.300 | 0.6018 |
| BC | 8.65 × 10−5 | 8.65 × 10−5 | 3.410 | 0.1072 |
| Sequential p-Value | Adjusted R2 | Predicted R2 | Suggested |
|---|---|---|---|
| Linear | 0.1492 | −0.0471 | No |
| 2FI | 0.6713 | 0.4741 | No |
| Quadratic | 0.9881 | 0.8715 | Yes |
| Cubic | 0.9849 | 0.7295 | No |
| Source | Sum of Squares | Mean Square | F | p |
|---|---|---|---|---|
| Model | 0.1600 | 0.0170 | 5.61 | 0.0166 |
| A | 0.0130 | 0.0130 | 4.22 | 0.0791 |
| B | 0.0102 | 0.0102 | 0.33 | 0.5845 |
| C | 0.0120 | 0.0120 | 4.02 | 0.0848 |
| AB | 0.0540 | 0.0540 | 17.44 | 0.0042 |
| AC | 0.0300 | 0.0300 | 9.69 | 0.017 |
| BC | 0.0088 | 0.0888 | 2.86 | 0.1347 |
| Number | Experimental Optimization Ratio | Fluidity (mm) | Water Retention (%) | Permeability (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Water–Bentonite Ratio | Dispersant Content (%) | Thickener Content (%) | Predicted | Experimental | Predicted | Experimental | Predicted | Experimental | |
| 1# | 4.15 | 0.29 | 0.039 | 126.31 | 122 | 94.52 | 94.13 | 34.07 | 34.24 |
| 2# | 4.38 | 1.04 | 0.19 | 117.85 | 115 | 95.08 | 94.51 | 24.97 | 25.12 |
| 3# | 4.47 | 0.65 | 0.16 | 131.46 | 132 | 95.72 | 94.77 | 35.61 | 35.62 |
| 4# | 4.39 | 0.13 | 0.25 | 113.67 | 109 | 95.29 | 94.09 | 35.91 | 36.45 |
| 5# | 4.41 | 0.38 | 0.108 | 135.09 | 134 | 95.54 | 96.12 | 41.13 | 41.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Xiao, C.; Su, Z.; Zhuo, H.; Qiang, T. Development and Optimization of Bentonite-Based Slurry Sealing Material. Minerals 2025, 15, 385. https://doi.org/10.3390/min15040385
Zhang Q, Xiao C, Su Z, Zhuo H, Qiang T. Development and Optimization of Bentonite-Based Slurry Sealing Material. Minerals. 2025; 15(4):385. https://doi.org/10.3390/min15040385
Chicago/Turabian StyleZhang, Qingsong, Changyuan Xiao, Zhaoyang Su, Hui Zhuo, and Tuo Qiang. 2025. "Development and Optimization of Bentonite-Based Slurry Sealing Material" Minerals 15, no. 4: 385. https://doi.org/10.3390/min15040385
APA StyleZhang, Q., Xiao, C., Su, Z., Zhuo, H., & Qiang, T. (2025). Development and Optimization of Bentonite-Based Slurry Sealing Material. Minerals, 15(4), 385. https://doi.org/10.3390/min15040385






