Study of Sulfide Ore from the Perspective of the Hierarchical Oxidation and Asymmetric Corrosion Mechanism of Minerals: (I) Chalcopyrite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dissolved Oxygen (DO) Experiments
2.3. Inductively Coupled Plasma Spectroscopy (ICP) Detection
2.4. X-Ray Photoelectron Spectroscopy (XPS) Measurements
2.5. Scanning Electron Microscopy (SEM) Experiments
3. Results and Discussion
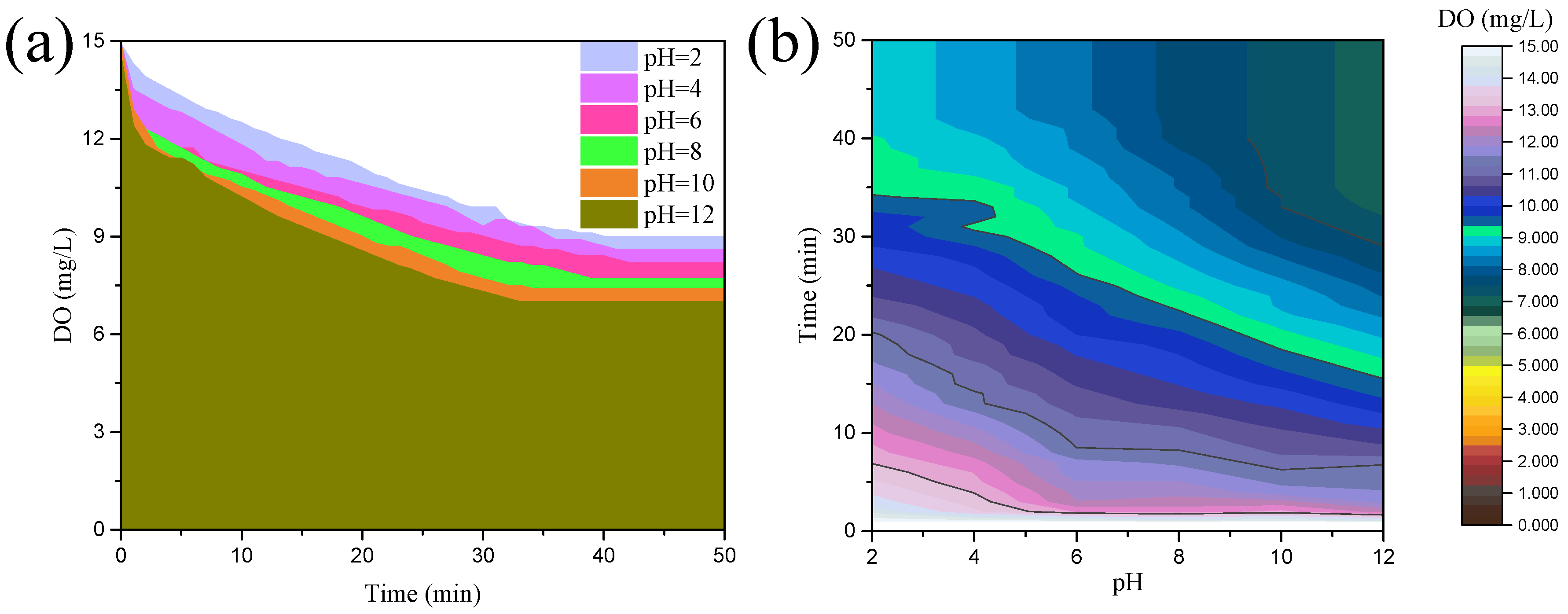
3.1. Characteristics of Oxygen Consumption of Oxidation–Corrosion
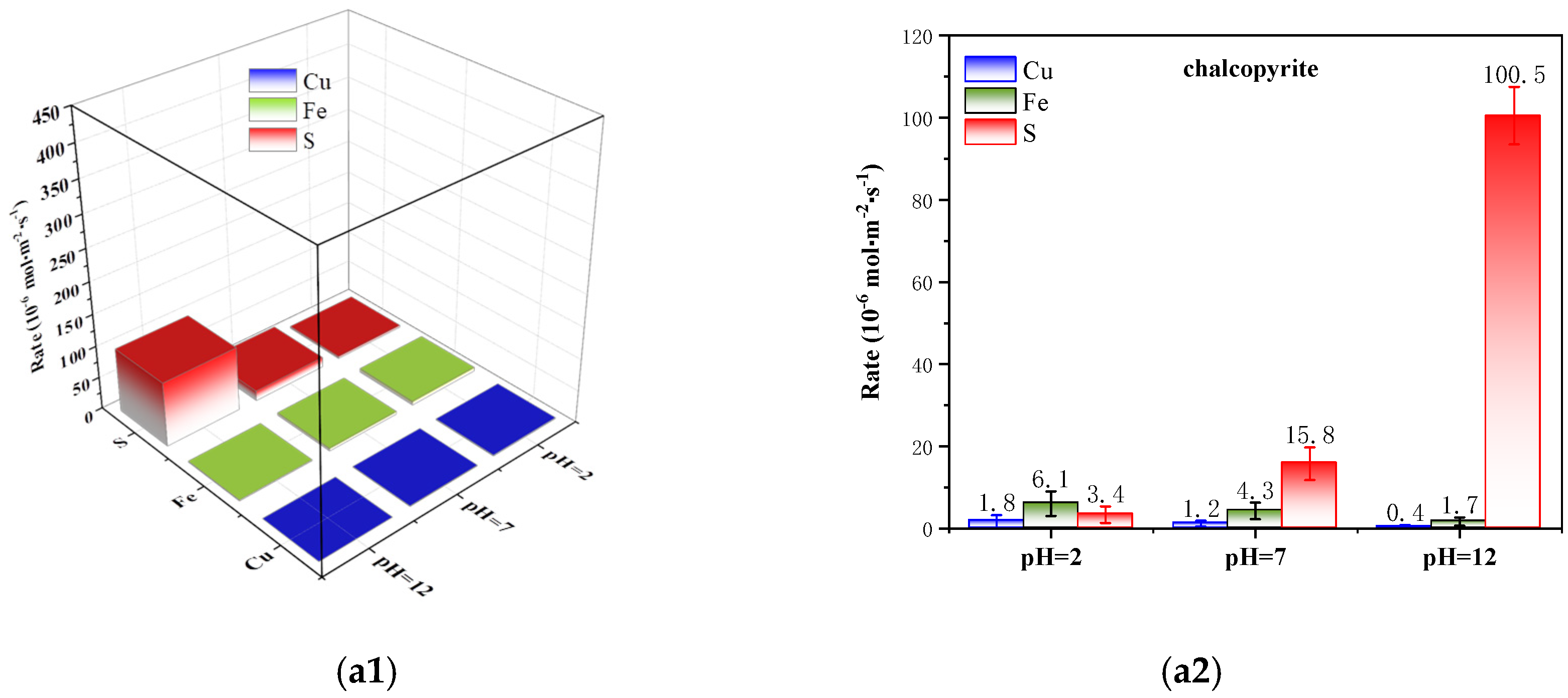
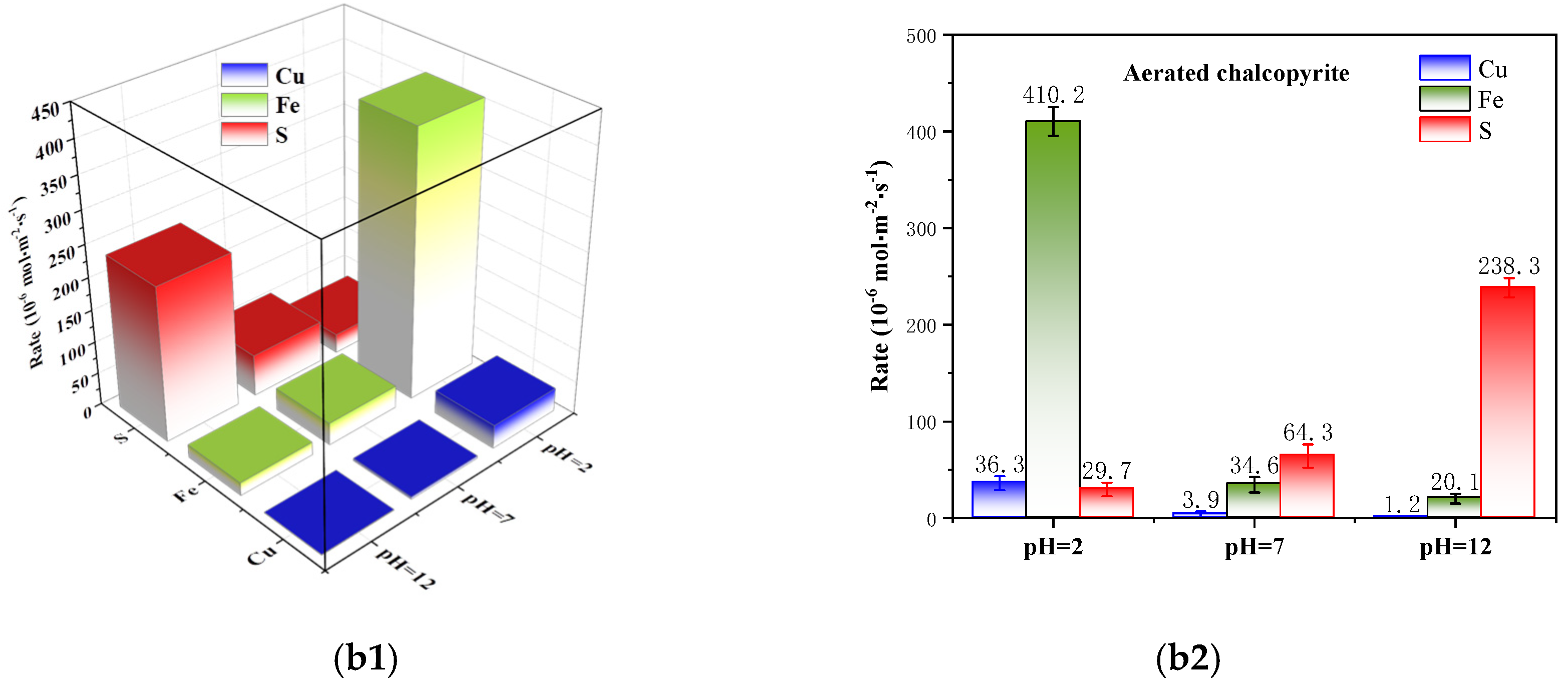
3.2. Kinetics Analysis of Corrosion
3.3. Effect of Oxidation Conditions on the Composition of Surface Components
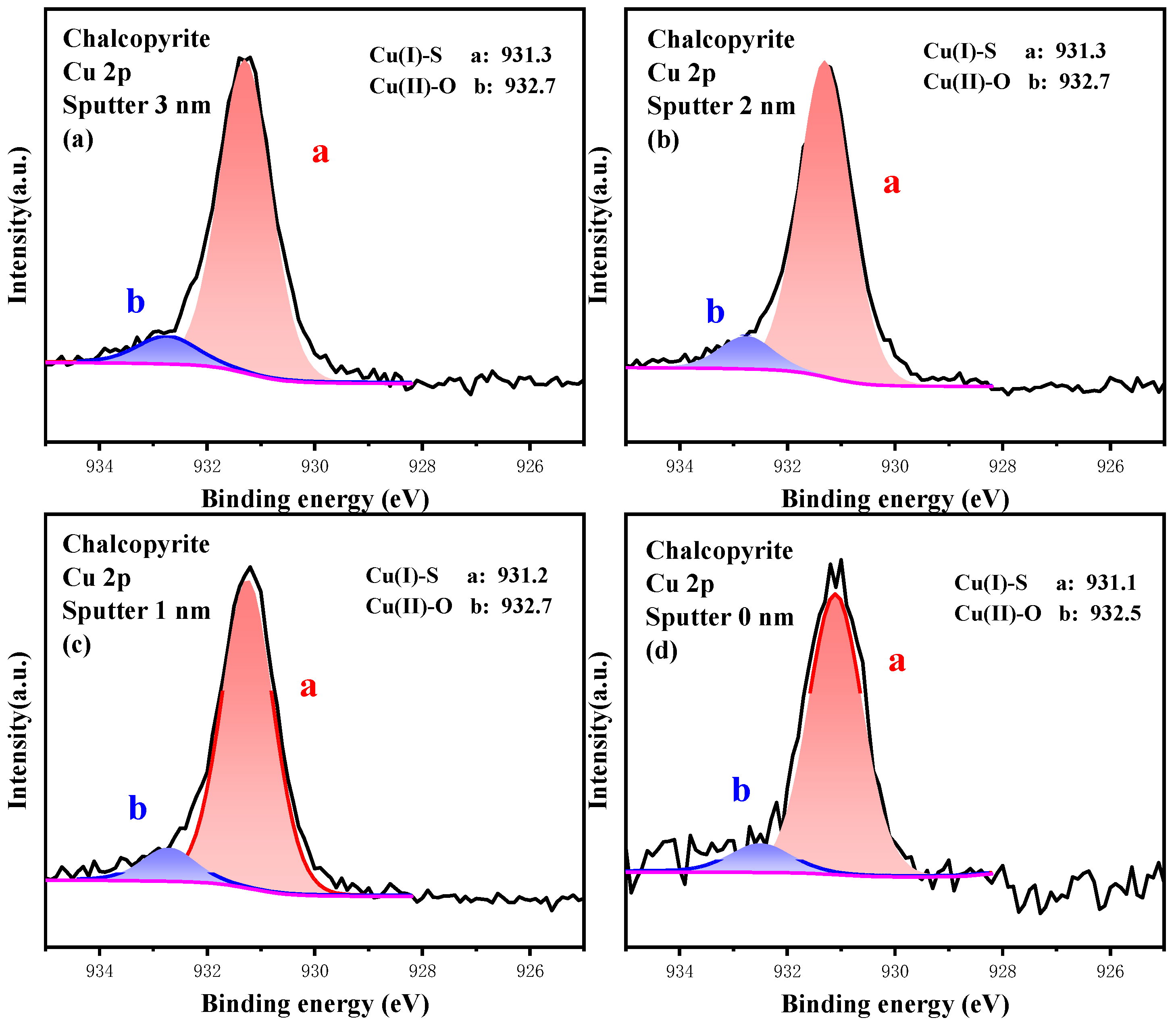
3.3.1. High-Resolution Spectrum Analysis of Cu 2p
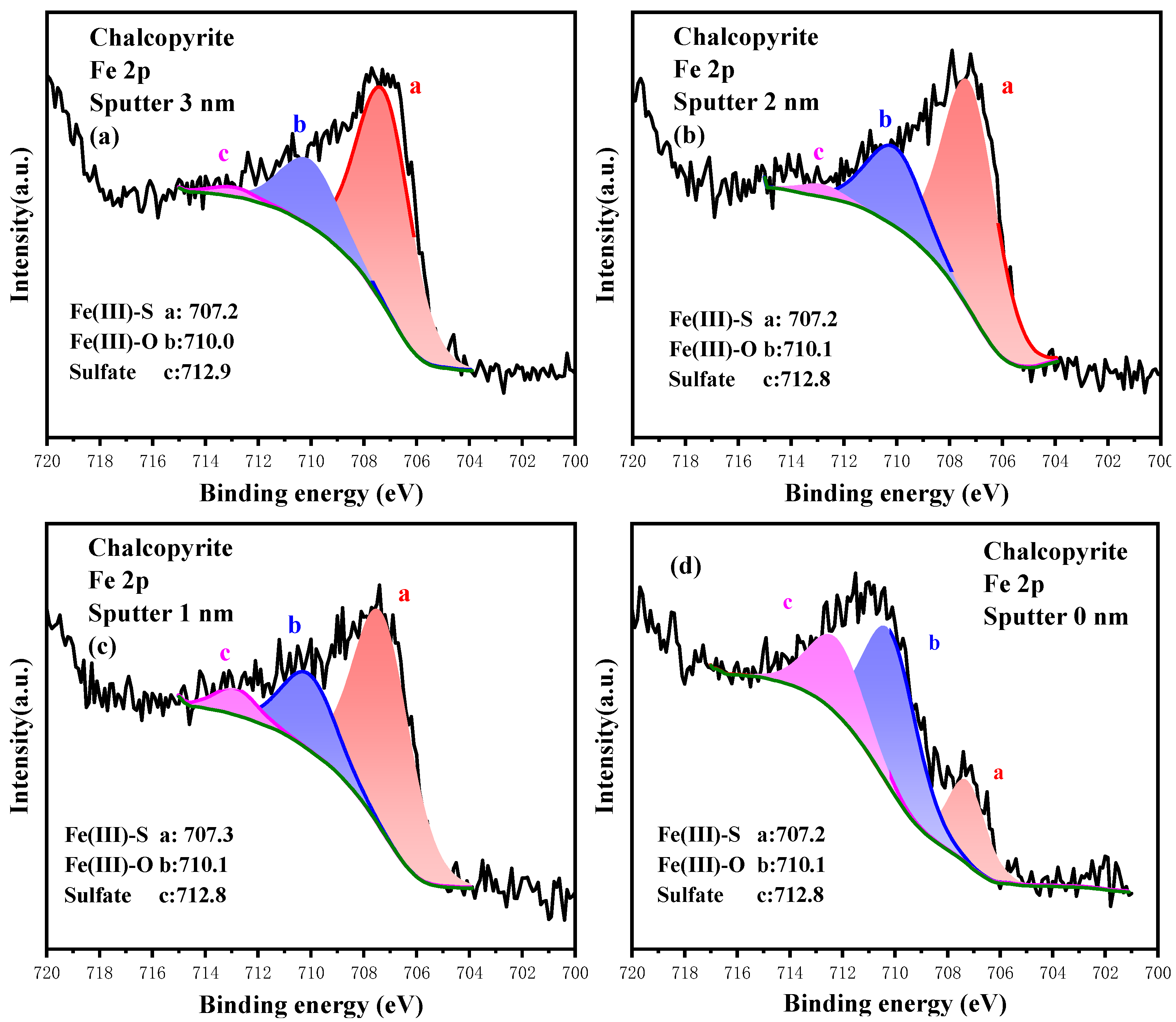
3.3.2. High-Resolution Spectrum Analysis of Fe 2p
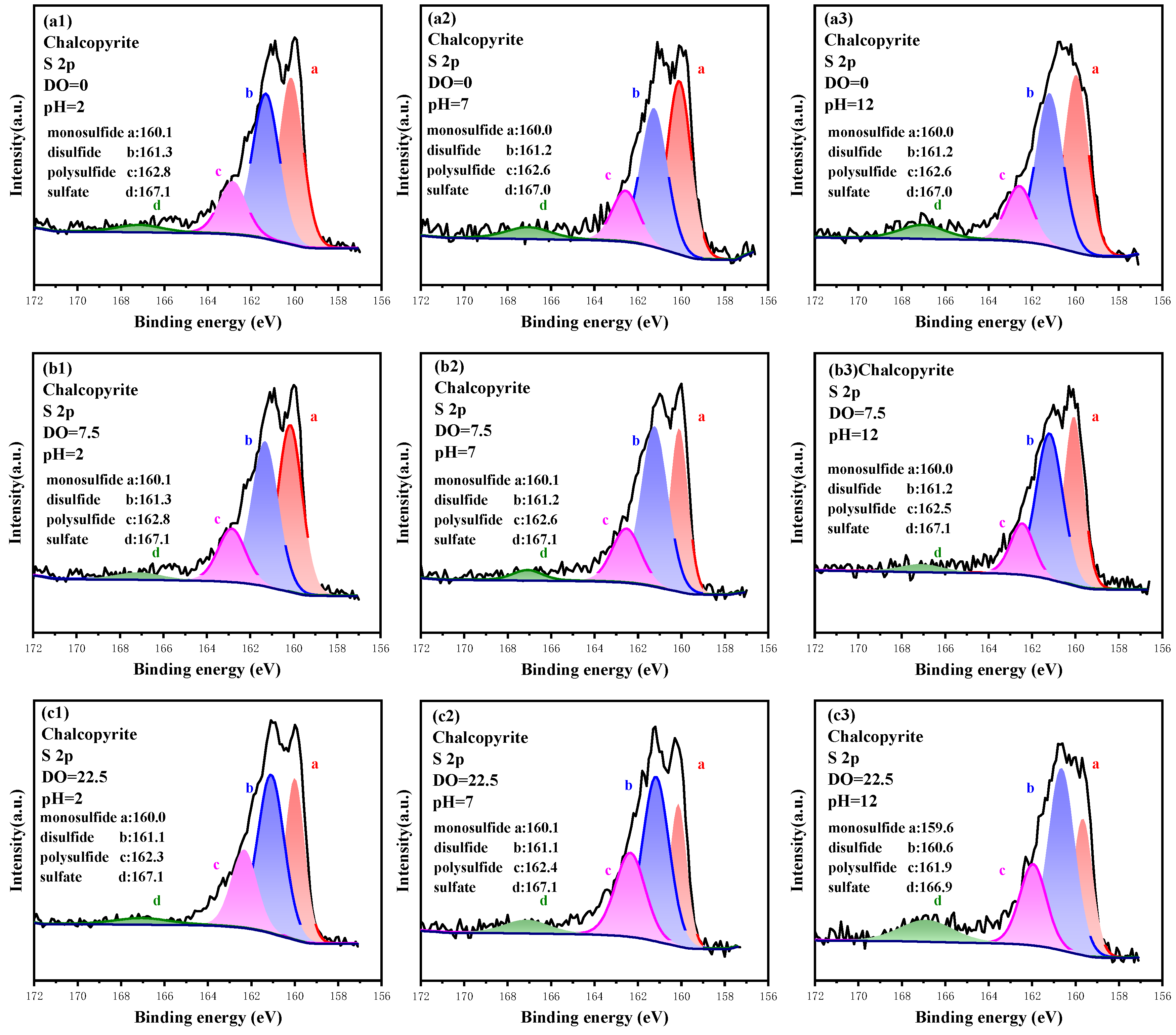
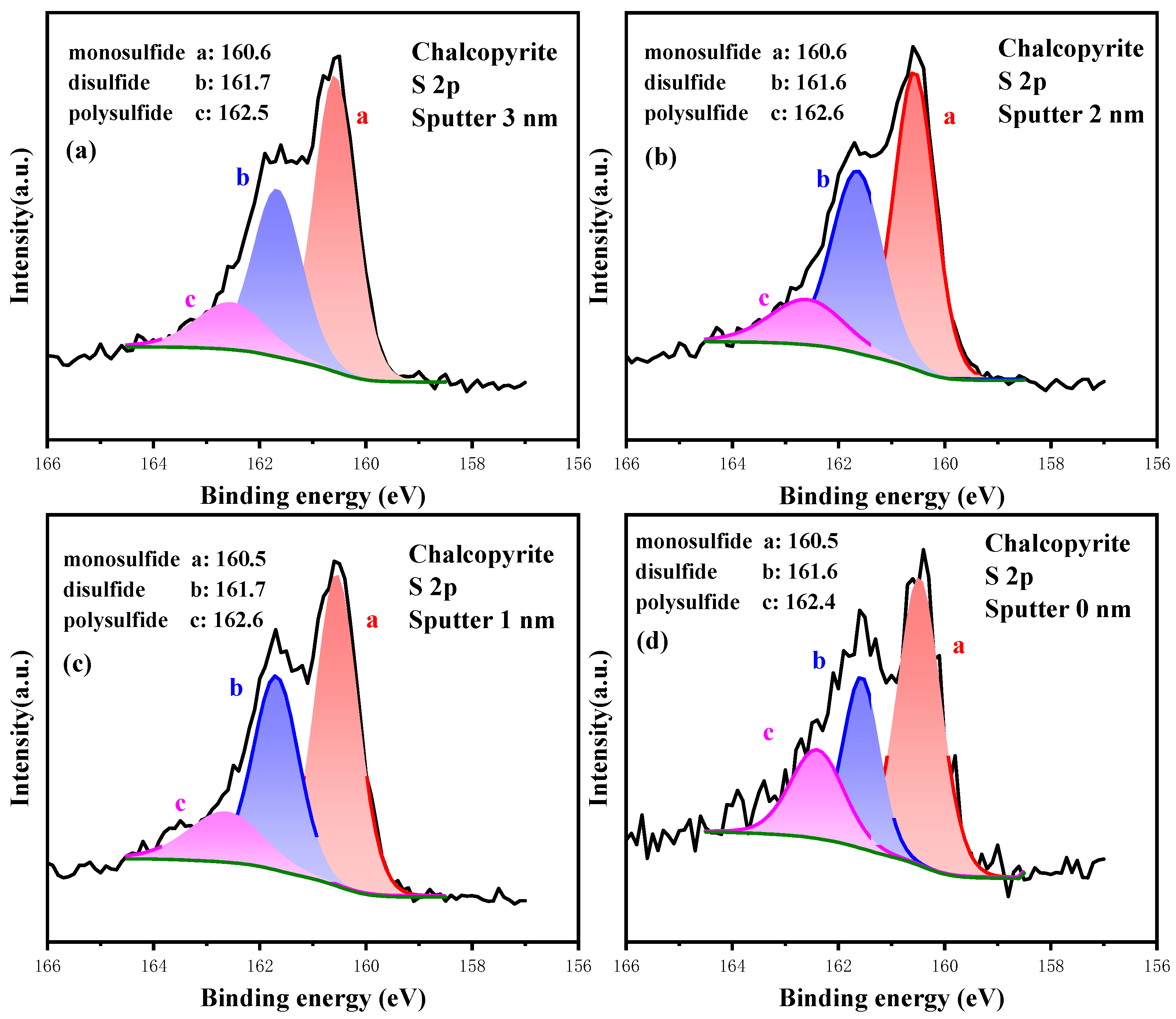
3.3.3. High-Resolution Spectrum Analysis of S 2p
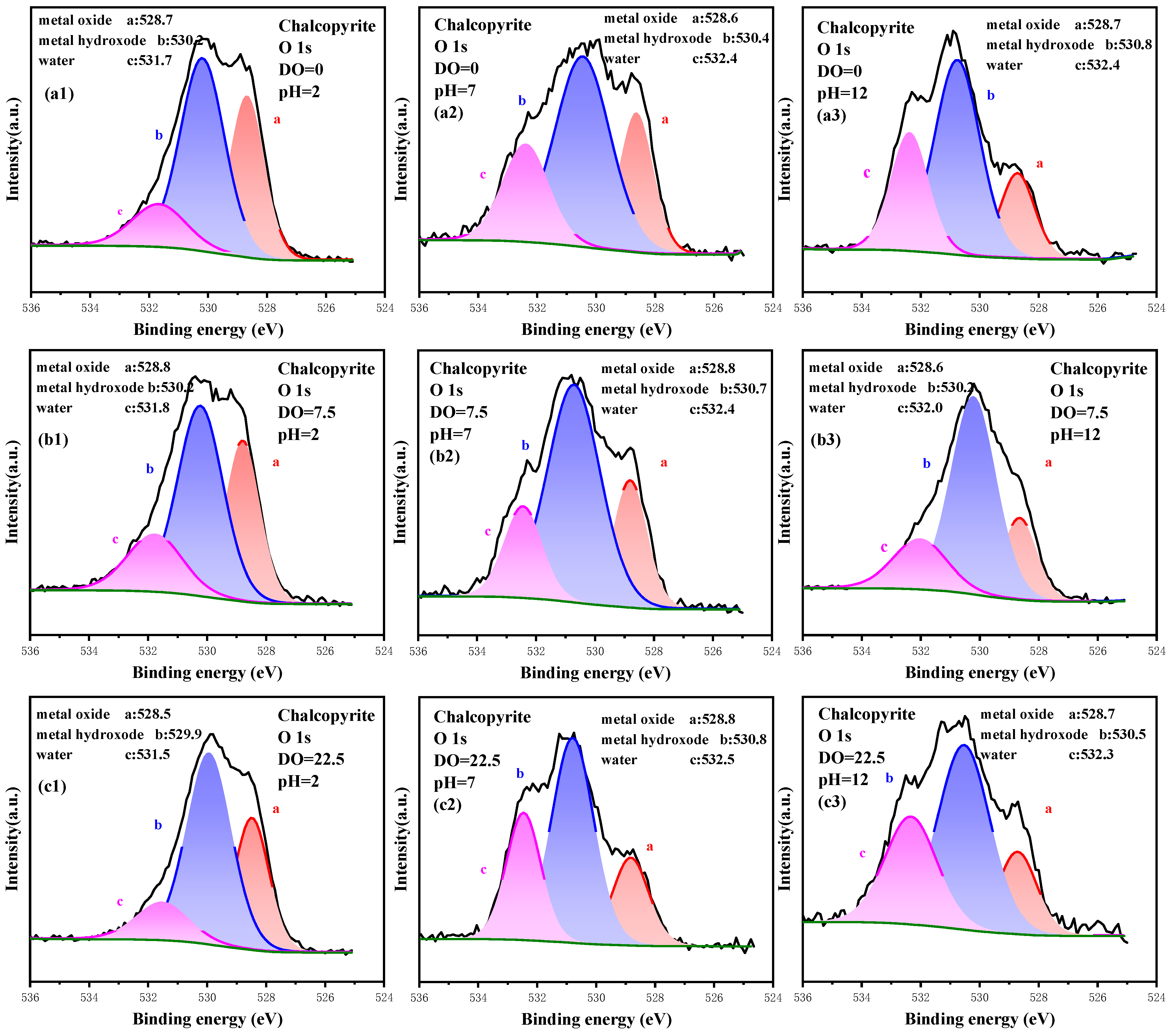
3.3.4. High-Resolution Spectrum Analysis of O 1s
3.4. Analysis of Hierarchical Oxidation Process of Mineral-Forming Elements
3.4.1. Analysis of the Oxidation Process of Cu Sites
3.4.2. Analysis of the Oxidation Process of Fe Sites
3.4.3. Analysis of the Oxidation Process of S Sites
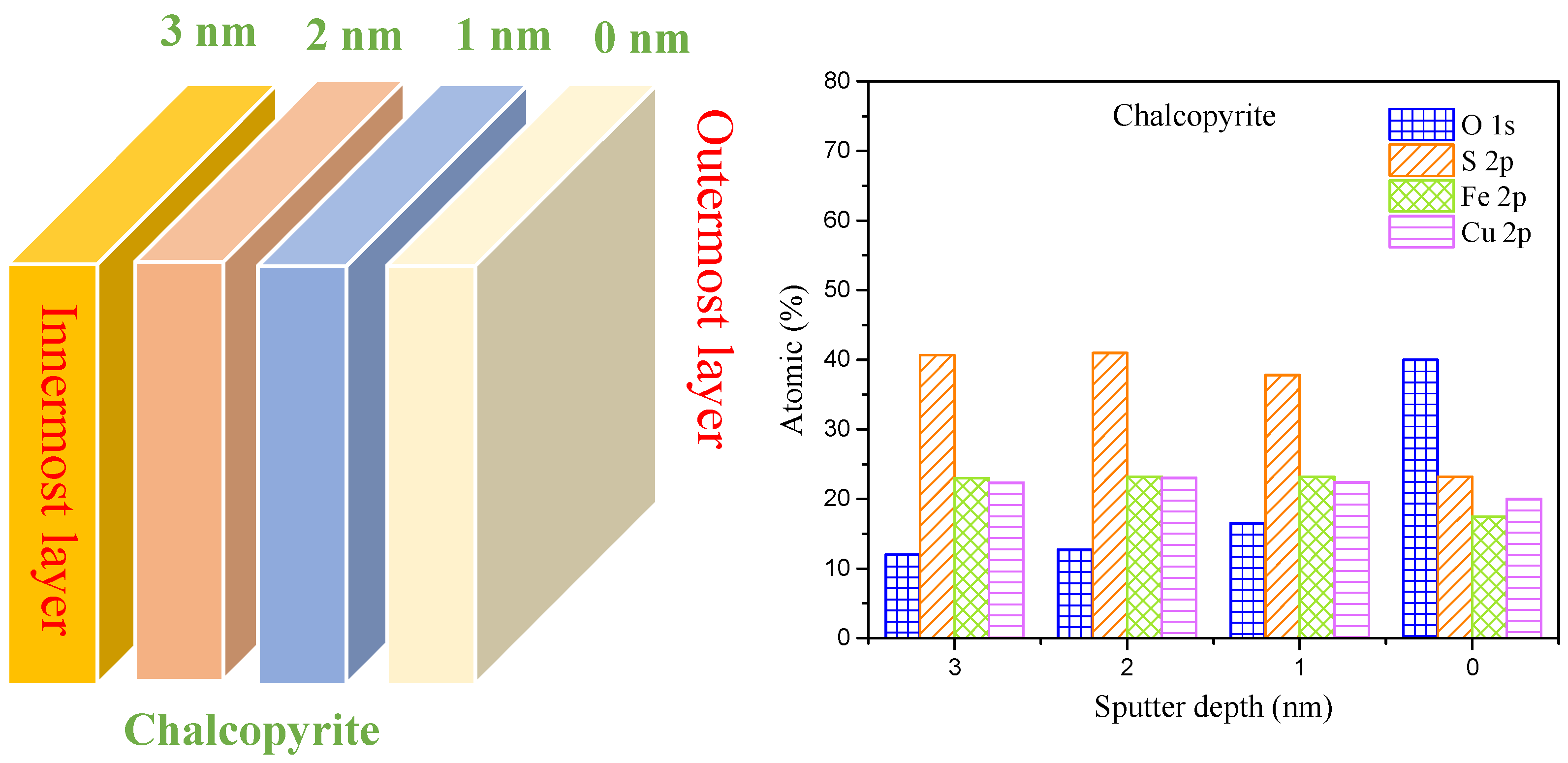
3.4.4. Analysis of the Oxidation Process of O Sites
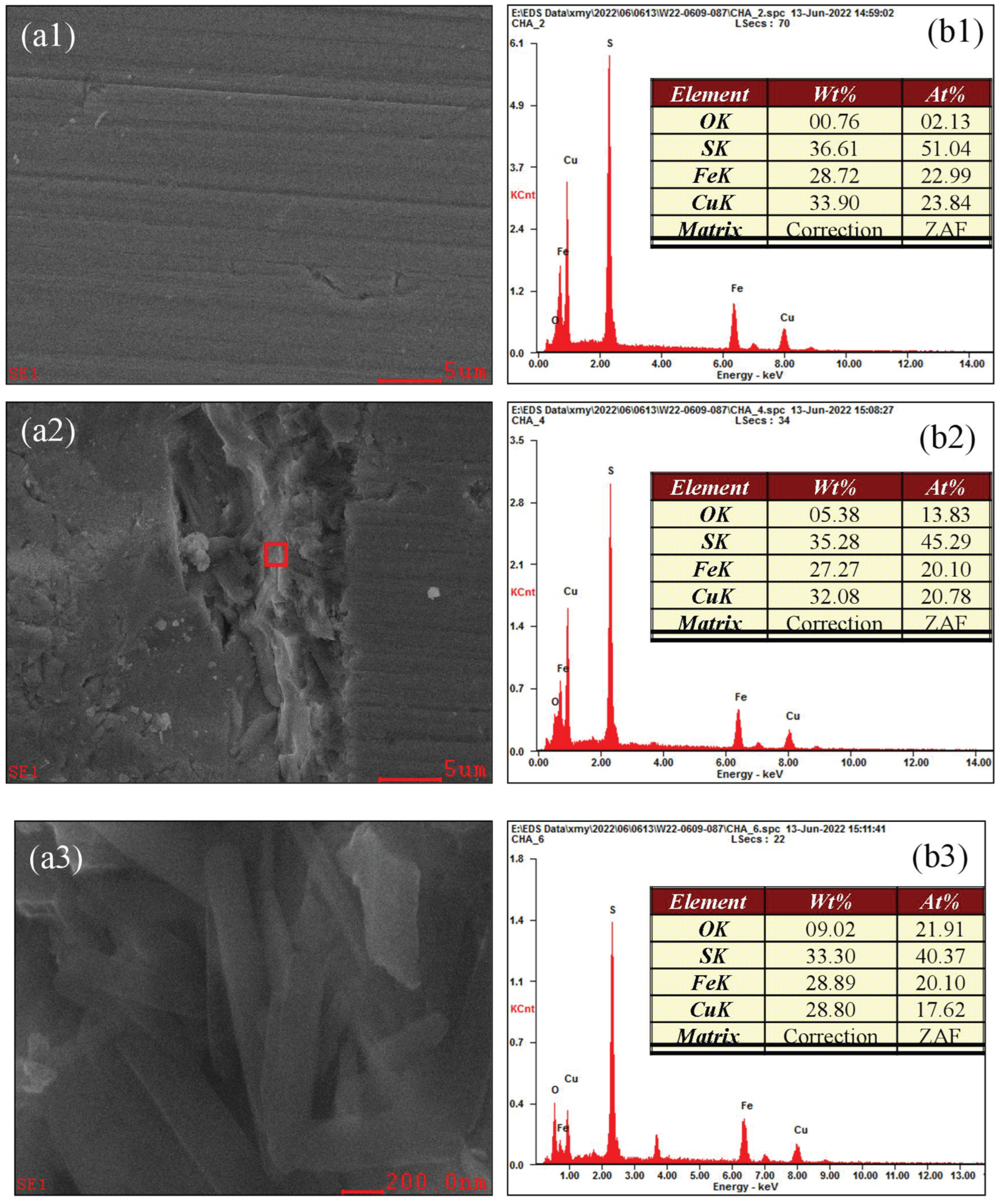
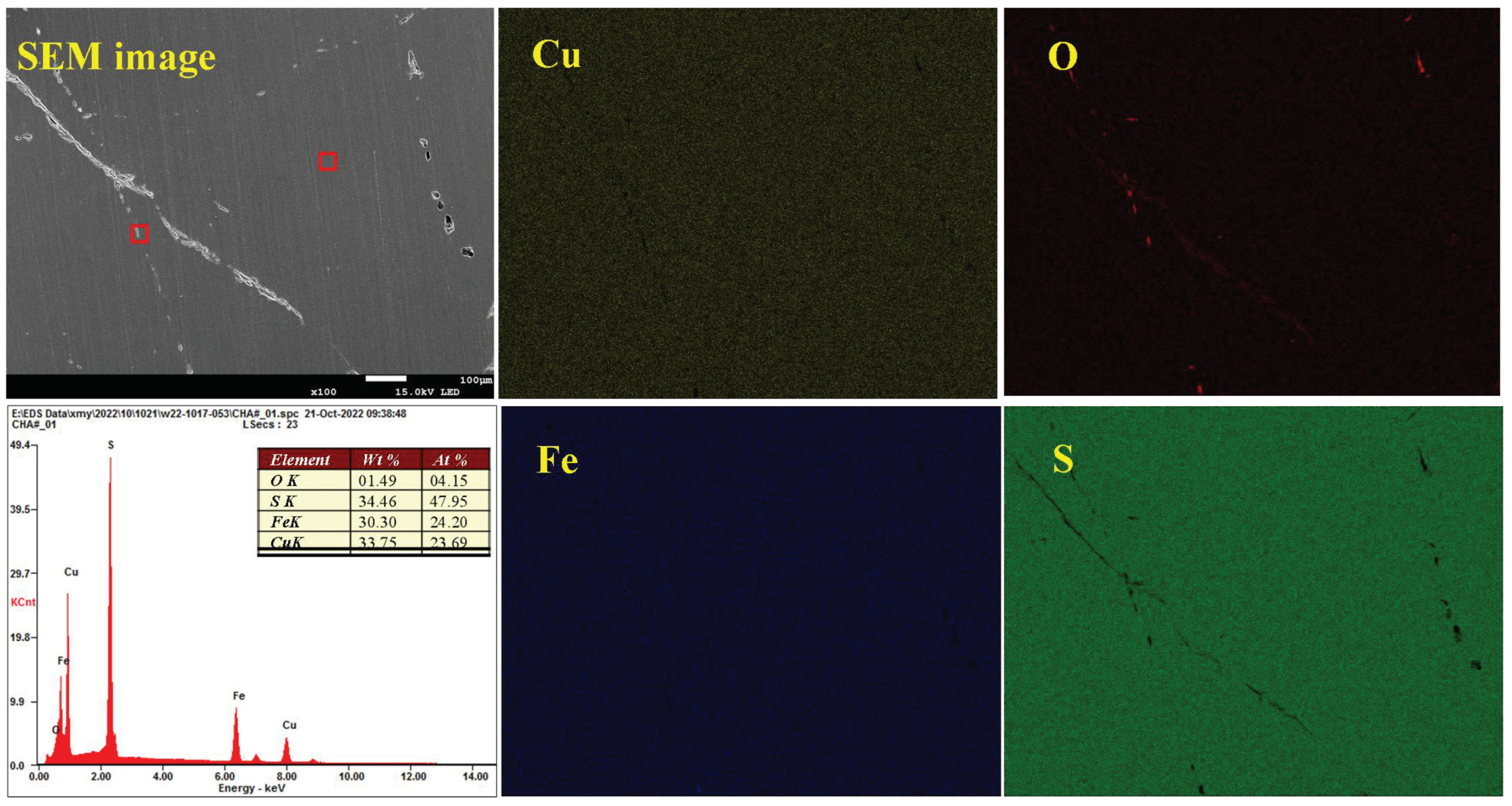
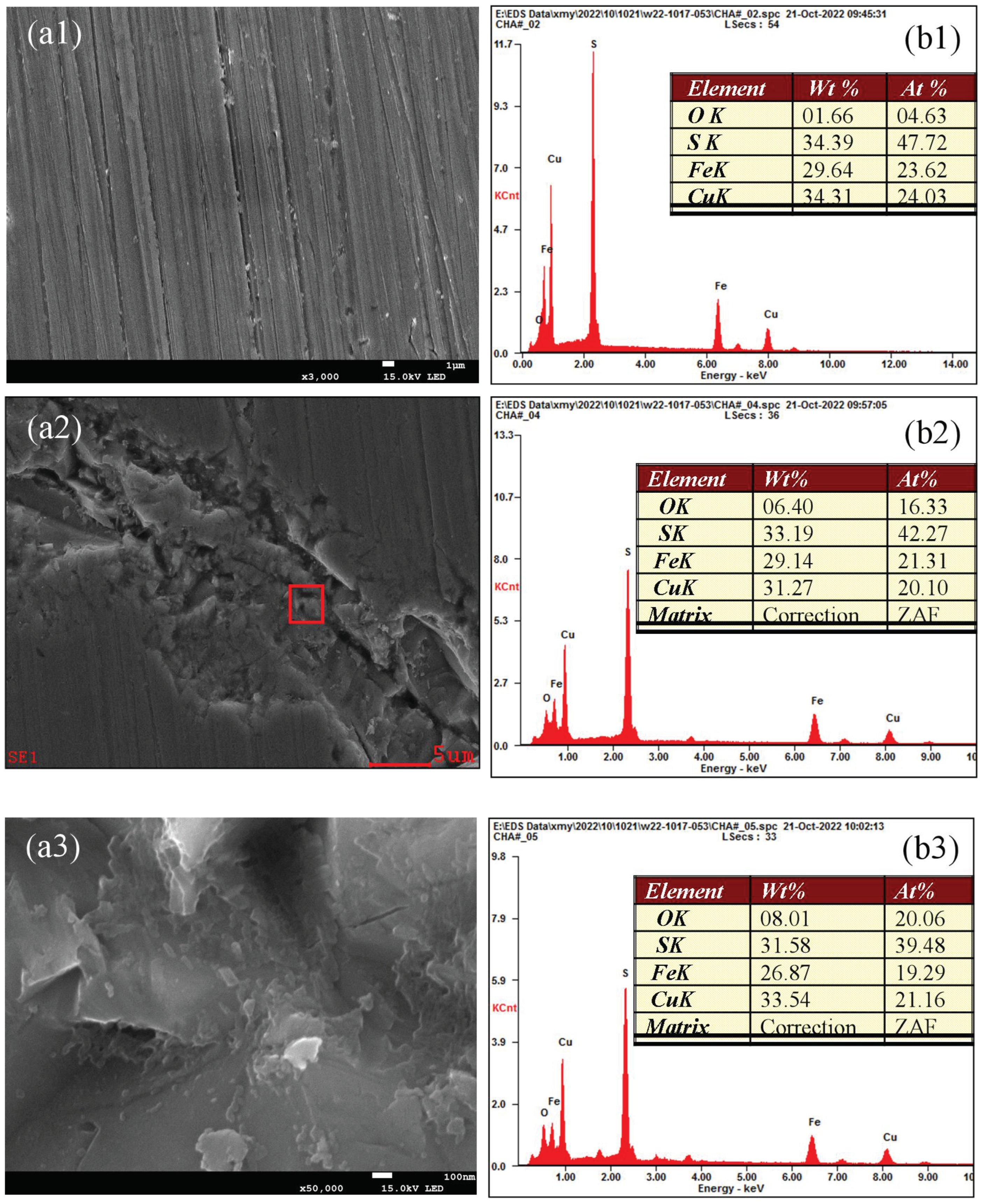
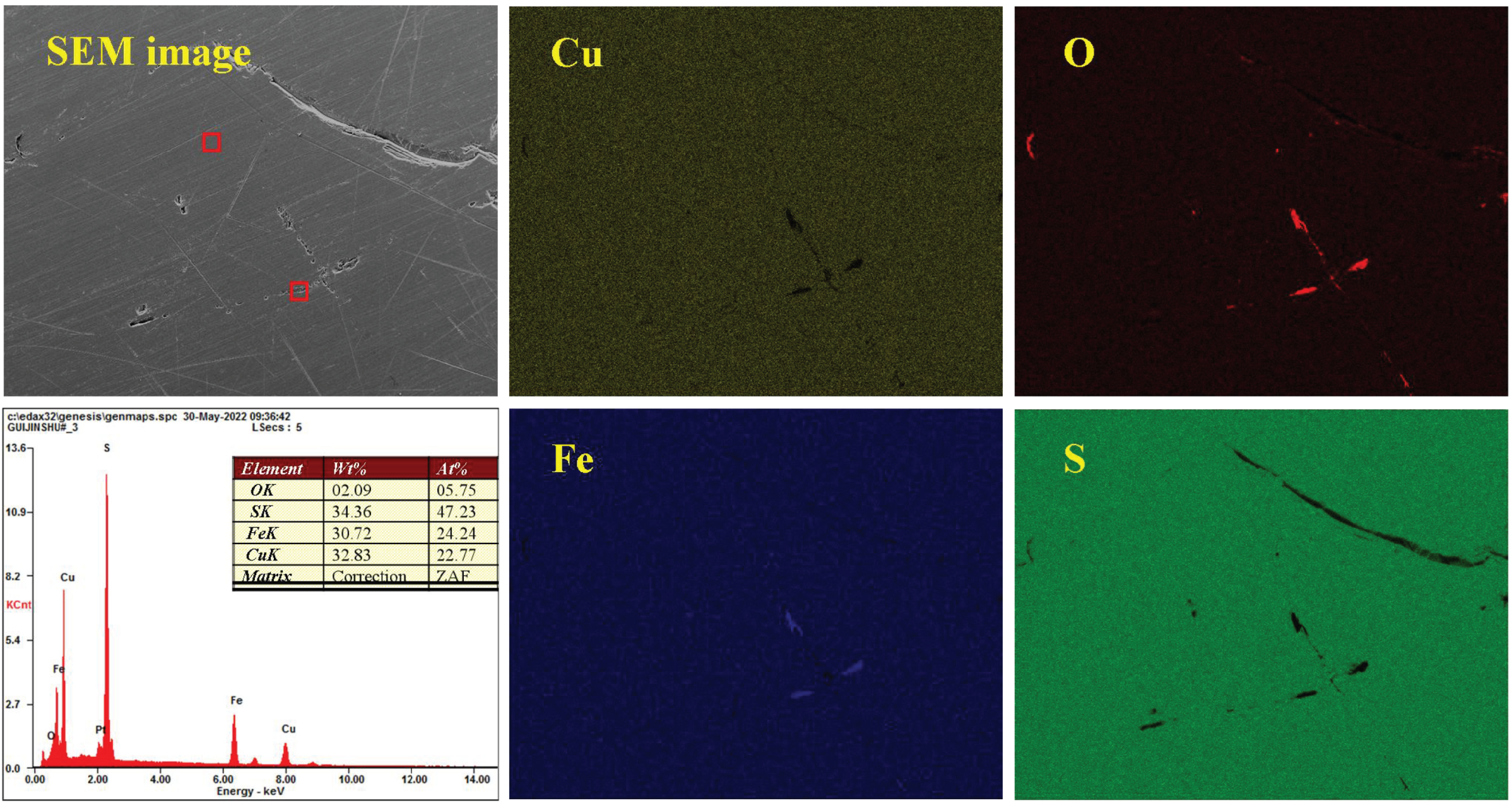
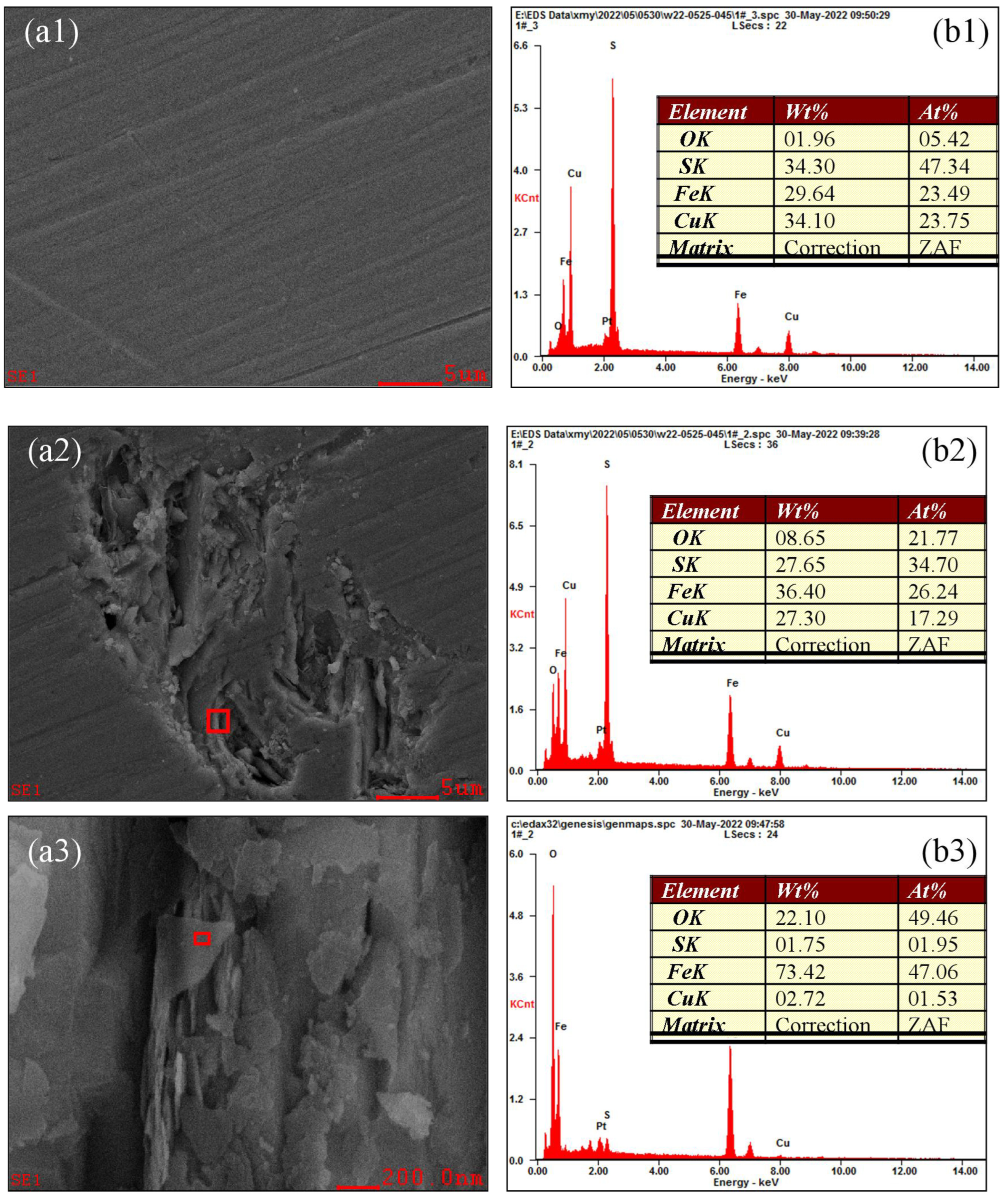
3.5. Analysis of the Oxidation–Corrosion Characteristics of Micro-Area
3.5.1. Micro-Area Oxidation–Corrosion Characteristics Under Acidic Condition
3.5.2. Micro-Area Oxidation–Corrosion Characteristics Under Moderate Condition
3.5.3. Micro-Area Oxidation–Corrosion Characteristics Under Alkaline Condition
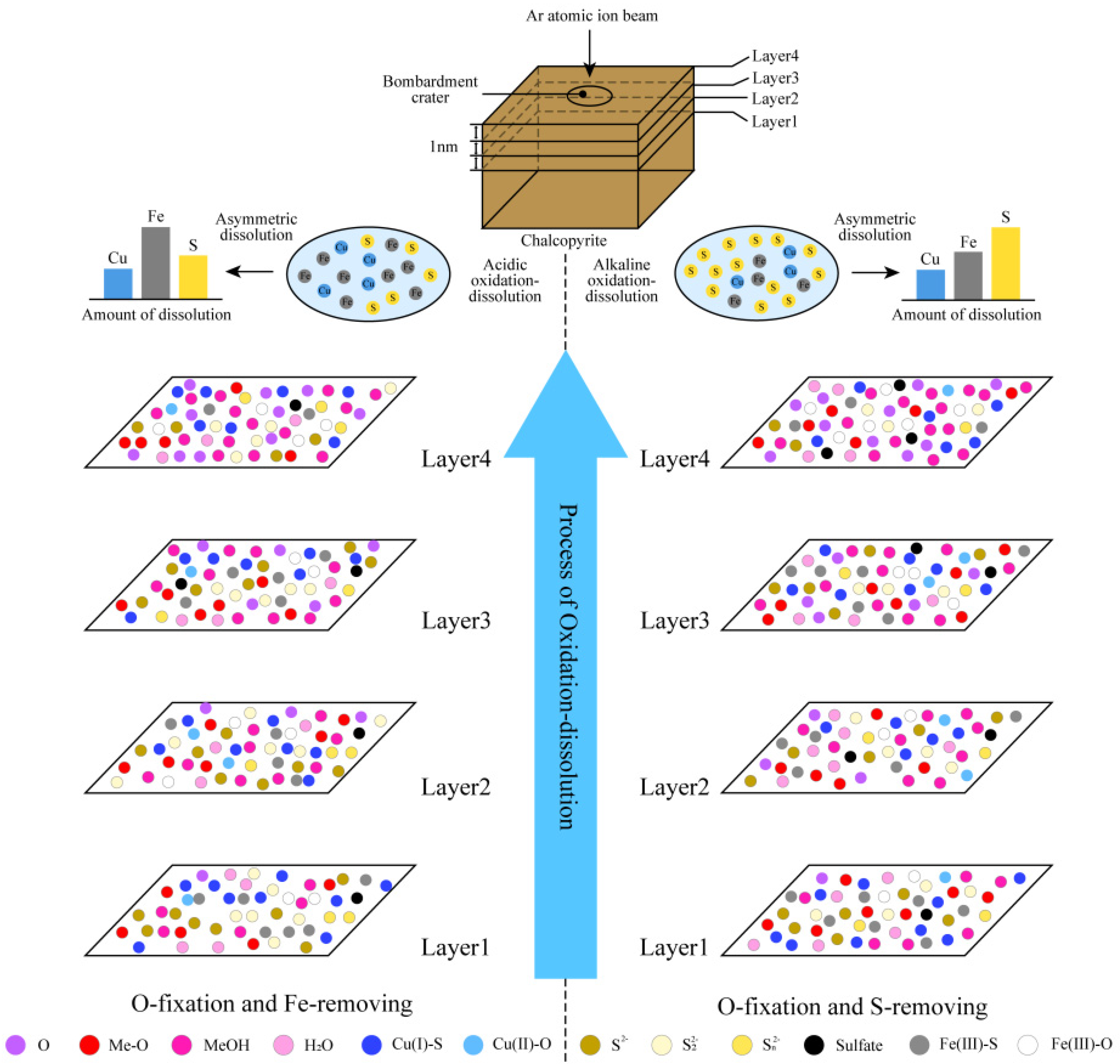
4. Hierarchical Oxidation–Asymmetric Corrosion Model of Chalcopyrite
4.1. Hierarchical Oxidation–Asymmetric Corrosion Model Under Alkaline Condition
4.2. Hierarchical Oxidation–Asymmetric Corrosion Model Under Acidic Condition
5. Conclusions
- (1)
- The DO detection results demonstrate that chalcopyrite possessed various DO consumption characteristics under various pH conditions; more specifically, its oxygen consumption rate and degree are enhanced with the increased pulp pH. Therefore, chalcopyrite is more prone to oxidation–corrosion under alkaline conditions.
- (2)
- The ICP measurements indicated that the specific corrosion rates of Cu, Fe and S are asymmetric, and they are affected by pulp pH and aeration conditions. The elements of Cu and Fe are prone to corrosion under acidic conditions while S is prior to corrosion under alkaline conditions. Moreover, pulp aeration can selectively accelerate the specific corrosion rate of Fe and S under acidic and alkaline conditions, respectively, thus further promoting the asymmetric corrosion of mineral-forming elements.
- (3)
- The XPS measurements confirmed that the asymmetric corrosion of Cu, Fe and S contributed to the generation of non-equilibrium layers, meaning the selective corrosion of Fe promoted the formation of the “metal-deficient and S-rich” layer, while the selective corrosion of S promoted the formation of the “S-deficient and metal-rich” layer. Otherwise, surface oxidation could be strengthened via increasing pH or pulp aeration intensity, but the strengthening effect of the latter is smaller than that of the former.
- (4)
- Surface etching results indicate that the oxidation of Cu, Fe and S sites is a multi-component and multi-phase transition reaction, and the hierarchical oxidation process of Cu, Fe and S sites are Cu(I)-S → Cu(II)-O, Fe(III)-S → Fe(III)-O → sulfate and S2−→ S22−→ Sn2−→ SO42−.
- (5)
- The SEM-EDS results further prove that the surface oxidation–corrosion of chalcopyrite is uneven, while significant corrosion occurs in strongly oxidized areas, demonstrating that surface oxidation occurs accompanied with corrosion and they promote each other.
- (6)
- The hierarchical oxidation-asymmetric corrosion model of chalcopyrite is firstly proposed and established in this study.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, L.; Cui, Y.; Qiao, L.; Lan, S.; Zheng, Q.; Shen, P.; Liu, D. A critical review on the flotation of calcium-containing minerals. Sep. Purif. Technol. 2025, 360, 131082. [Google Scholar] [CrossRef]
- Mweene, L.; Chipili, C.; Munganyinka, J.P.; Khanal, G.P.; Sankaran, S.; Kim, H.; Filippov, L. Experimental, benchmarks and theoretical investigations into the complexation of chalcopyrite and silica with guar gum and beneficiation of a siliceous copper ore using polyethylene oxide as a silica depressant. Miner. Eng. 2024, 216, 108874. [Google Scholar] [CrossRef]
- Zheng, Q.; Dong, L.; Shen, P.; Liu, D. A novel oxidation reagent scheme to realize efficient flotation separation of chalcopyrite and pyrrhotite. Appl. Surf. Sci. 2025, 693, 162794. [Google Scholar] [CrossRef]
- Sun, X.; Huang, L.; Wu, D.; Tong, X.; Yang, S.; Hu, B. The selective depression effect of dextrin on pyrite during the Zn–Fe sulfides flotation under low alkaline conditions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129573. [Google Scholar] [CrossRef]
- Abraitis, P.K.; Pattrick, R.A.D.; Vaughan, D.J. Variations in the compositional, textural and electrical properties of natural pyrite: A review. Int. J. Miner. Process. 2004, 74, 41–59. [Google Scholar] [CrossRef]
- Owusu, C.; Addai-Mensah, J.; Fornasiero, D.; Zanin, M. Estimating the electrochemical reactivity of pyrite ores-their impact on pulp chemistry and chalcopyrite flotation behaviour. Adv. Powder Technol. 2013, 24, 801–809. [Google Scholar] [CrossRef]
- Chimonyo, W.; Corin, K.C.; Wiese, J.G.; O’Connor, C.T. Redox potential control during flotation of a sulphide mineral ore. Miner. Eng. 2017, 110, 57–64. [Google Scholar] [CrossRef]
- Ran, J.; Qiu, X.; Hu, Z.; Liu, Q.; Song, B.; Yao, Y. Enhance flotation separation of arsenopyrite and pyrite by low-temperature oxygen plasma surface modification. Appl. Surf. Sci. 2019, 480, 1136–1146. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, W.; Wang, W.; Cao, Y.; Qi, M.; Huang, Y. A green method for selective separation of molybdenite and pyrite via electrochemical oxidation pretreatment-flotation and its mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133508. [Google Scholar] [CrossRef]
- Forson, P.; Zanin, M.; Skinner, W.; Asamoah, R. Differential flotation of pyrite and Arsenopyrite: Effect of pulp aeration and the critical importance of collector concentration. Miner. Eng. 2022, 178, 107421. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Qin, W.; Tian, Z.; Wu, C.; Wei, Q.; Jiao, F. In situ Raman investigation of dissolved constituent and its evolution in pulp during Zn-S selective flotation with two-step pulp regulation. Miner. Eng. 2023, 192, 107994. [Google Scholar]
- Lei, D.; Wu, Z.; Zhang, Y.; Zhang, Y.; Zhang, J.; Xue, J.; Peng, X.; Wang, Y. New insight into the inevitable oxidation-dissolution behavior of MoS2 at humid environments: Unexpected persistent generation of hydroxyl radicals. Sep. Purif. Technol. 2024, 332, 125800. [Google Scholar] [CrossRef]
- Kuang, J.; Zou, Z.; Huang, Z.; Liu, P.; Yuan, W.; Zhu, L. Surface dissolution of scheelite under different regulators and its effect on flotation behavior. Miner. Eng. 2021, 164, 106811. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Cao, Q.B.; Yan, Y.; Zou, H.; Huang, X.G.; Liu, D.W. Flotation separation of galena and chalcopyrite by using hydroxyl radicals from an Fe2+/NaClO system as depressants. Appl. Surf. Sci. 2025, 686, 162128. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Ke, B.L.; Lei, L.; Wan, J.H.; Feng, H.Y.; Yao, L.Y. Insight into the effect of ultrasonic treatment on surface properties and flotation behavior of chalcopyrite after galvanic corrosion of grinding media and chalcopyrite. Mater. Today Commun. 2024, 41, 110745. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, G.; Li, C.; Li, B.; Ye, G. The surface dissolution process of smithsonite and its effect on flotation behaviour. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132118. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Zhai, Q.; Dong, W.; Xie, Z.; Sun, W.; Hu, W. Prospects of pulp aeration for the cleaner production of pyrrhotite-rich type copper sulfide ore: Mechanism and application. J. Clean. Prod. 2023, 406, 136921. [Google Scholar] [CrossRef]
- Pratt, A.; Muir, I.; Nesbitt, H. X-ray photoelectron and Auger electron spectroscopic studies of pyrrhotite and mechanism of air oxidation. Geochim. Cosmochim. Acta 1994, 58, 827–841. [Google Scholar]
- Moses, C.O.; Nordstrom, D.K.; Herman, J.S.; Mills, A.L. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar]
- Jiang, K.; Liu, J.; Wang, Y.; Zhang, D.J.; Han, Y.X. Surface properties and flotation inhibition mechanism of air oxidation on pyrite and arsenopyrite. Appl. Surf. Sci. 2023, 610, 155476. [Google Scholar] [CrossRef]
- Goryachev, B.E.; Nikolaev, A.A.; Lyakisheva, L.N. Electrochemistry of galena oxidation as the basis for optimization of agent modes in flotation of polymetallic ores. J. Mining Sci. 2010, 46, 681–689. [Google Scholar] [CrossRef]
- Peng, W.J.; Liu, S.G.; Cao, Y.J.; Wang, W.; Lv, S.; Huang, Y.K. A novel approach for selective flotation separation of chalcopyrite and molybdenite—Electrocatalytic oxidation pretreatment and its mechanism. Appl. Surf. Sci. 2022, 597, 153753. [Google Scholar] [CrossRef]
- Tolley, W.; Kotlyar, D.; Van Wagoner, R. Fundamental electrochemical studies of sulfide mineral flotation. Miner. Eng. 1996, 9, 603–637. [Google Scholar] [CrossRef]
- Pashkevich, D.; Li, R.H.; Kökkiliç, O.; Waters, K.E. Investigations of Monomineralic Flotation of Galena, Sphalerite, and Pyrite at Different Temperatures. Minerals 2023, 13, 615. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, M.; Liu, R.; Sun, W. A review on the electrochemistry of galena flotation. Miner. Eng. 2020, 150, 106272. [Google Scholar] [CrossRef]
- Hayes, R.A.; Ralston, J. The collectorless flotation and separation of sulphide minerals by Eh control. Int. J. Miner. Process. 1988, 23, 55–84. [Google Scholar] [CrossRef]
- Owusu, C.; Fornasiero, D.; Addai-Mensah, J.; Zanin, M. Influence of pulp aeration on the flotation of chalcopyrite with xanthate in chalcopyrite/pyrite mixtures. Int. J. Miner. Process. 2015, 134, 50–57. [Google Scholar] [CrossRef]
- Lin, S.; Liu, R.; Bu, Y.; Wang, C.; Wang, L.; Sun, W.; Hu, Y. Oxidative depression of arsenopyrite by using calcium hypochlorite and sodium humate. Minerals 2018, 8, 463. [Google Scholar] [CrossRef]
- Kim, R.; Ghahreman, A. The effect of ore mineralogy on the electrochemical gold dissolution behavior in various cyanide and oxygen concentrations; Effect of sulfidic ores containing heavy metals. Hydrometallurgy 2019, 184, 75–87. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Antonijevic, M.M.; Dimitrijevic, V. Investigation of the kinetics of pyrite oxidation by hydrogen peroxide in hydrochloric acid solutions. Miner. Eng. 1999, 12, 165–174. [Google Scholar] [CrossRef]
- Luo, Q.; Shi, Q.; Liu, D.; Li, B.; Jin, S. Effect of deep oxidation of chalcopyrite on surface properties and flotation performance. Int. J. Mining Sci. Technol. 2022, 32, 907–914. [Google Scholar] [CrossRef]
- Goh, S.W.; Buckley, A.N.; Lamb, R.N.; Rosenberg, R.A.; Moran, D. The oxidation states of copper and iron in mineral sulfides, and the oxides formed on initial exposure of chalcopyrite and bornite to air. Geochim. Cosmochim. Acta 2006, 70, 2210–2228. [Google Scholar]
- Pearce, C.; Pattrick, R.; Vaughan, D.; Henderson, C.; Van der Laan, G. Copper oxidation state in chalcopyrite: Mixed Cu d9 and d10 characteristics. Geochim. Cosmochim. Acta 2006, 70, 4635–4642. [Google Scholar]
- Kalegowda, Y.; Chan, Y.-L.; Wei, D.-H.; Harmer, S.L. X-PEEM, XPS and ToF-SIMS characterisation of xanthate induced chalcopyrite flotation: Effect of pulp potential. Surf. Sci. 2015, 635, 70–77. [Google Scholar] [CrossRef]
- Smart, R.S.C. Surface layers in base metal sulphide flotation. Miner. Eng. 1991, 4, 891–909. [Google Scholar] [CrossRef]
- Mielczarski, J.; Cases, J.; Alnot, M.; Ehrhardt, J. XPS characterization of chalcopyrite, tetrahedrite, and tennantite surface products after different conditioning. 2. Amyl xanthate solution at pH 10. Langmuir 1996, 12, 2531–2543. [Google Scholar]
- Mycroft, J.R.; Nesbitt, H.W.; Pratt, A.R. X-ray photoelectron and Auger electron spectroscopy of air-oxidized pyrrhotite: Distribution of oxidized species with depth. Geochim. Cosmochim. Acta 1995, 59, 721–733. [Google Scholar]
- Sun, W.; Dai, S.; Zhang, H.; Chen, Y.; Yu, X.; Li, P.; Liu, W. Selective flotation of chalcopyrite from galena using a novel collector benzoic diethylcarbamothioic thioanhydride: An experimental and theoretical investigation. J. Mol. Liq. 2022, 365, 120027. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Semoto, Y.; Miki, H.; Hirajima, T.; Sasaki, K.; Ochi, D.; Aoki, Y.; Berdakh, D.; Ura, K. Effect of sodium metabisulfite and slaked lime on the floatability and surface properties of chalcopyrite. Powder Technol. 2022, 408, 117750. [Google Scholar] [CrossRef]
- Capece, F.; Di Castro, V.; Furlani, C.; Mattogno, G.; Fragale, C.; Gargano, M.; Rossi, M. “Copper chromite” catalysts: XPS structure elucidation and correlation with catalytic activity. J. Electron Spectrosc. Relat. Phenom. 1982, 27, 119–128. [Google Scholar]
- Nesbitt, H.W.; Muir, I.J. X-ray photoelectron spectroscopic study of a pristine pyrite surface reacted with water vapour and air. Geochim. Cosmochim. Acta 1994, 58, 4667–4679. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. An X-ray photoelectron spectroscopic study of the oxidation of chalcopyrite. Aust. J. Chem. 1984, 37, 2403–2413. [Google Scholar]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Yamane, M.; Takida, E.; Kuroiwa, S.; Imaizumi, Y. Selective flotation of chalcopyrite and molybdenite using H2O2 oxidation method with the addition of ferrous sulfate. Miner. Eng. 2018, 122, 312–326. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, W.; Zhang, L.; Guo, Y.; Wang, T.; Wang, H. The role of sodium alginate in the flotation separation of apatite and dolomite. Powder Technol. 2020, 373, 620–626. [Google Scholar]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Adsorption characteristics and mechanisms of O-Carboxymethyl chitosan on chalcopyrite and molybdenite. J. Colloid Interface Sci. 2019, 552, 659–670. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Kuroiwa, S.; Aoki, Y. Effect of H2O2 and potassium amyl xanthate on separation of enargite and tennantite from chalcopyrite and bornite using flotation. Miner. Eng. 2020, 152, 106371. [Google Scholar] [CrossRef]
- Farquhar, M.L.; Wincott, P.L.; Wogelius, R.A.; Vaughan, D.J. Electrochemical oxidation of the chalcopyrite surface: An XPS and AFM study in solution at pH 4. Appl. Surf. Sci. 2003, 218, 34–43. [Google Scholar]
- Mielczarski, J.; Cases, J.; Alnot, M.; Ehrhardt, J. XPS characterization of chalcopyrite, tetrahedrite, and tennantite surface products after different conditioning. 1. Aqueous solution at pH 10. Langmuir 1996, 12, 2519–2530. [Google Scholar]
- Goldhaber, M.B. Experimental study of metastable sulfur oxyanion formation during pyrite oxidation at pH 6-9 and 30 degrees C. Am. J. Sci. 1983, 283, 193–217. [Google Scholar]
- Tu, Z.; Guo, C.; Zhang, T.; Lu, G.; Wan, J.; Liao, C.; Dang, Z. Investigation of intermediate sulfur species during pyrite oxidation in the presence and absence of Acidithiobacillus ferrooxidans. Hydrometallurgy 2017, 167, 58–65. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The stability of thiosulfate in the presence of pyrite in low-temperature aqueous solutions. Geochim. Cosmochim. Acta 1995, 59, 4605–4622. [Google Scholar] [CrossRef]





| Aeration Flow (mL/min) | DO (mg/L) | pH | Atom Content/% | Fe/S Ratio | Cu/S Ratio | |||
|---|---|---|---|---|---|---|---|---|
| S | O | Fe | Cu | |||||
| 0.00 | 0.00 | 2.0 | 29.50 | 36.10 | 16.60 | 17.80 | 0.56 | 0.60 |
| 7.0 | 29.80 | 36.20 | 16.90 | 17.10 | 0.57 | 0.57 | ||
| 12.0 | 22.50 | 40.70 | 17.90 | 18.90 | 0.80 | 0.84 | ||
| 20.00 | 7.50 | 2.0 | 28.00 | 37.70 | 17.50 | 16.80 | 0.63 | 0.60 |
| 7.0 | 27.10 | 37.40 | 18.50 | 17.00 | 0.68 | 0.63 | ||
| 12.0 | 19.50 | 41.86 | 20.50 | 18.14 | 1.05 | 0.93 | ||
| 100.00 | 22.50 | 2.0 | 31.13 | 34.15 | 13.95 | 20.76 | 0.45 | 0.67 |
| 7.0 | 26.30 | 38.34 | 18.20 | 17.16 | 0.69 | 0.65 | ||
| 12.0 | 12.30 | 49.43 | 23.90 | 14.37 | 1.94 | 1.17 | ||
| Aeration Flow (mL/min) | pH | Content/% | |
|---|---|---|---|
| Cu(I)-S | Cu(II)-O | ||
| 0.00 | 2.0 | 83.33 | 16.67 |
| 7.0 | 81.30 | 18.70 | |
| 12.0 | 75.76 | 24.24 | |
| 20.00 | 2.0 | 84.03 | 15.97 |
| 7.0 | 81.97 | 18.03 | |
| 12.0 | 75.22 | 24.78 | |
| 100.00 | 2.0 | 84.75 | 15.25 |
| 7.0 | 78.74 | 21.26 | |
| 12.0 | 76.34 | 23.66 | |
| DO mg/L | pH | Content/% | ||
|---|---|---|---|---|
| Fe(III)-S | Fe(III)-O | Sulfate | ||
| 7.50 | 2.0 | 20.21 | 53.19 | 26.60 |
| 7.50 | 7.0 | 16.40 | 54.91 | 28.69 |
| 7.50 | 12.0 | 13.87 | 55.80 | 30.32 |
| 0.00 | 7.0 | 21.76 | 58.82 | 19.41 |
| 7.50 | 7.0 | 16.40 | 54.91 | 28.69 |
| 22.50 | 7.0 | 14.66 | 52.36 | 32.98 |
| DO mg/L | pH | Content/% | |||
|---|---|---|---|---|---|
| Monosulfide (S2−) | Disulfide (S22−) | Polysulfide (Sn2−) | Sulfate (SO42−) | ||
| 0.00 | 2.0 | 41.15 | 40.09 | 14.99 | 3.76 |
| 7.0 | 42.55 | 38.30 | 14.04 | 5.11 | |
| 12.0 | 41.18 | 39.09 | 13.99 | 5.76 | |
| 7.50 | 2.0 | 41.84 | 40.17 | 15.06 | 2.93 |
| 7.0 | 38.42 | 41.66 | 16.72 | 3.20 | |
| 12.0 | 37.95 | 39.64 | 18.84 | 3.57 | |
| 22.50 | 2.0 | 32.47 | 43.29 | 20.78 | 3.46 |
| 7.0 | 24.44 | 44.44 | 25.33 | 5.78 | |
| 12.0 | 20.07 | 47.39 | 23.85 | 8.69 | |
| DO mg/L | pH | Content/% | |||
|---|---|---|---|---|---|
| Me-O | Me-OH | H2O | Me-OH/Me-O | ||
| 0.00 | 2.0 | 34.03 | 52.36 | 13.61 | 1.54 |
| 7.0 | 23.94 | 53.19 | 22.87 | 2.22 | |
| 12.0 | 17.93 | 54.35 | 27.72 | 3.03 | |
| 7.50 | 2.0 | 32.00 | 50.00 | 18.00 | 1.56 |
| 7.0 | 22.16 | 59.88 | 17.96 | 2.70 | |
| 12.0 | 17.28 | 52.36 | 30.37 | 3.03 | |
| 22.50 | 2.0 | 29.71 | 57.14 | 13.14 | 1.92 |
| 7.0 | 19.25 | 59.48 | 21.27 | 3.08 | |
| 12.0 | 19.38 | 64.50 | 18.13 | 3.71 | |
| Sputter Depth (nm) | Content/% | |
|---|---|---|
| Cu(I)-S | Cu(II)-O | |
| 3.0 | 92.50 | 7.50 |
| 2.0 | 92.00 | 8.00 |
| 1.0 | 92.00 | 8.00 |
| 0.0 | 92.00 | 8.00 |
| Sputter Depth (nm) | Content/% | ||
|---|---|---|---|
| Fe(III)-S | Fe(III)-O | Sulfate | |
| 3.0 | 73.63 | 24.26 | 2.21 |
| 2.0 | 71.43 | 25.71 | 2.86 |
| 1.0 | 63.99 | 30.66 | 5.35 |
| 0.0 | 22.44 | 51.37 | 26.19 |
| Sputter Depth (nm) | Content/% | ||
|---|---|---|---|
| Monosulfide (S2−) | Disulfide (S22−) | Polysulfide (Sn2−) | |
| 3.0 | 51.81 | 34.72 | 13.47 |
| 2.0 | 51.33 | 35.12 | 13.55 |
| 1.0 | 51.23 | 34.34 | 14.43 |
| 0.0 | 37.00 | 41.68 | 21.32 |
| Sputter Depth (nm) | Content/% | ||
|---|---|---|---|
| Me-O | Me-OH | H2O | |
| 3.0 | 39.50 | 33.50 | 27.00 |
| 2.0 | 36.42 | 38.53 | 25.05 |
| 1.0 | 33.74 | 47.14 | 19.12 |
| 0.0 | 23.35 | 61.28 | 15.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Wu, Y.; Wang, C.; Hu, Y.; Xie, X.; Sun, W.; Tong, X. Study of Sulfide Ore from the Perspective of the Hierarchical Oxidation and Asymmetric Corrosion Mechanism of Minerals: (I) Chalcopyrite. Minerals 2025, 15, 384. https://doi.org/10.3390/min15040384
Liu R, Wu Y, Wang C, Hu Y, Xie X, Sun W, Tong X. Study of Sulfide Ore from the Perspective of the Hierarchical Oxidation and Asymmetric Corrosion Mechanism of Minerals: (I) Chalcopyrite. Minerals. 2025; 15(4):384. https://doi.org/10.3390/min15040384
Chicago/Turabian StyleLiu, Runqing, Yacong Wu, Changtao Wang, Yuehua Hu, Xian Xie, Wei Sun, and Xiong Tong. 2025. "Study of Sulfide Ore from the Perspective of the Hierarchical Oxidation and Asymmetric Corrosion Mechanism of Minerals: (I) Chalcopyrite" Minerals 15, no. 4: 384. https://doi.org/10.3390/min15040384
APA StyleLiu, R., Wu, Y., Wang, C., Hu, Y., Xie, X., Sun, W., & Tong, X. (2025). Study of Sulfide Ore from the Perspective of the Hierarchical Oxidation and Asymmetric Corrosion Mechanism of Minerals: (I) Chalcopyrite. Minerals, 15(4), 384. https://doi.org/10.3390/min15040384








