Abstract
Red mud is a highly alkaline residue generated during the alumina extraction process, and its strong alkalinity significantly hinders its extensive and comprehensive utilization. The alkali in red mud mainly exists in two forms, namely, soluble alkali and insoluble chemically bound alkali. This study aims to provide a comprehensive overview of the dealkalization process progress for red mud, encompassing acid neutralization technology, salt (ion) precipitation or displacement technology, and metallurgical technology. The mechanisms, advantages, and disadvantages associated with each technology are discussed. To enhance readability for readers, mind maps are included for each dealkalization method of red mud. Based on the above analysis, it is advisable to choose the most suitable dealkalization method for red mud, considering its specific properties and the subsequent application strategy. Recommendations regarding future research on red mud dealkalization are proposed.
1. Introduction
Red mud is a strong alkaline solid waste produced by the alumina extraction process from bauxite [1]. The term “red mud” is derived from the fact that it usually contains a certain amount of iron trioxide, which presents as reddish-brown. According to different alumina production methods, red mud can be divided into three categories: Bayer red mud, sintering red mud, and combined red mud [2,3]. The Bayer process is the most economical method for the production of alumina, so alumina production is still dominated by the Bayer process, which accounts for more than 95% of the total global alumina production [4]. The annual global alumina production and red mud emissions from 2004 are illustrated in Figure 1. The global production of alumina has exhibited a consistent upward trend since 2004. Based on recent data, it can be roughly estimated that in 2024, the production of alumina increased by approximately two-fold compared to that in 2004, reaching a substantial volume of 134.36 million tons. The core driving force for the growth of alumina production is the expansion of global aluminum consumption, especially the industrialization and lightweight trend of emerging economies. Its application is highly concentrated in the transportation, construction, and packaging fields downstream of the aluminum industry chain, and it also plays an irreplaceable role in the high-tech industry. In the future, with the development of renewable energy (such as photovoltaic support) and the circular economy (aluminum recycling), the demand for alumina may continue to grow. Meanwhile, the quantity of red mud has experienced significant growth over the past two decades. The production of red mud in 2024 witnessed a remarkable increase of approximately 2.8 times compared to that in 2004, reaching an impressive volume of 238.84 million tons [5]. In addition, the global emission of red mud is growing at 120 million tons per year [4]. With the continuous increase in global alumina production and the gradual decrease in bauxite grade, the growth trend of red mud in the world is increasing year by year. However, the comprehensive utilization rate of red mud is meager, less than 10% in the world [6]. Red mud is difficult to comprehensively utilize due to its complex composition and strong alkalinity [7,8]. However, the stockpiling of red mud requires a large area of land and a lot of money to build and maintain [9]. The stacking of red mud in a red mud dam not only occupies a significant amount of land but also causes atmospheric pollution and poses radioactive hazards. Additionally, the solid alkaline components present in red mud directly contribute to land and groundwater contamination [10].
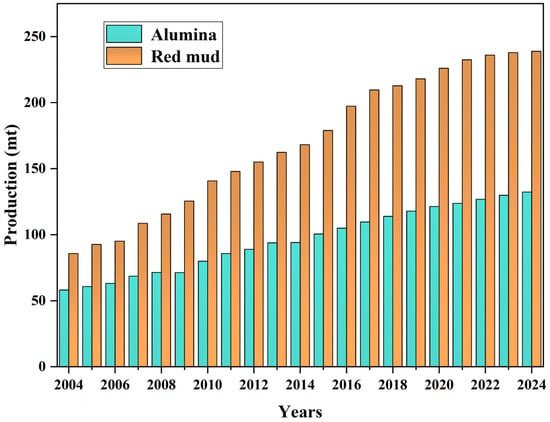
Figure 1.
Comparison of global alumina production and red mud emission from 2004 to 2024.
The alkalinity of red mud is attributed to its high content of potent alkaline substances, and the discharge of strong alkaline red mud introduces a lot of hazards to the environment. Figure 2 shows several common hazards of red mud. The pH value of the red mud-attached liquid is usually between 12 and 14, which is excessive wastewater according to the comprehensive discharge standard of sewage. The harm of alkali in red mud is not only manifested in the pollution of soil and water sources. At the same time, the alkali in red mud also enters the human body through the food chain, which harms human health [11]. The practice of stacking dams not only occupies a significant amount of land resources but also leads to the infiltration of heavy metals from red mud into soil and water, resulting in soil salinization, water pollution, and other related issues. In addition to the direct pollution of harmful components of red mud, the field dust after dehydration also causes pollution to the atmosphere [12]. At the same time, the alkaline substance present in red mud simultaneously corrodes the dam structure, posing a significant risk of dam failure.
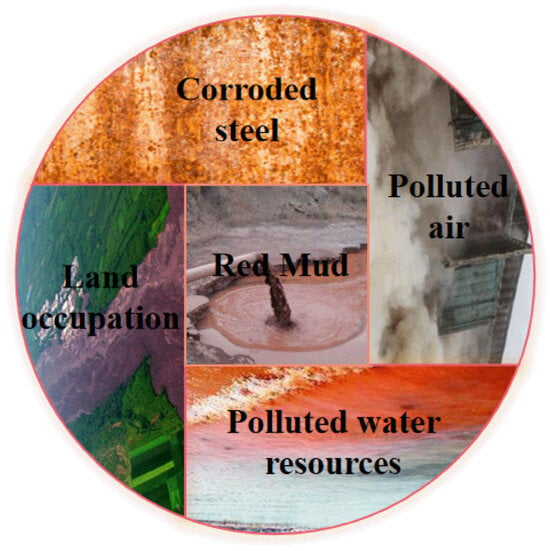
Figure 2.
Several common hazards caused by red mud.
The pH value of red mud is in the range of 10–12, but the anti-seepage measures of red mud accumulated in the open air are improper, which is leached by rainwater for a long time, and the pH value of the attached liquid reaches between 12 and 14. Therefore, red mud has strong alkalinity; when it penetrates into the ground, it causes an elevation of the groundwater pH value, water hardness, and fluorine content. It not only changes the soil’s physical structure and chemical composition but also disturbs the normal physiological activities of various vegetation and crops, making the red mud soil challenging to reuse. At the same time, it also affects the crops or other plants around the yard. More seriously, it causes the groundwater to be polluted by heavy metal elements and endangers human health [12]. With water body flow and continuous infiltration, red mud-attached liquid can pollute water sources at a depth of 700 m underground, causing irreversible harm to rivers and groundwater.
2. The Types and Sources of Alkali in Red Mud
The alumina production process primarily consists of Bayer, sintering, and combined processes. The Bayer process is the most economical method to extract alumina from bauxite. At present, the amount of aluminum produced by this method accounts for 95% of the world [13]. The Bayer process exhibits a straightforward procedural flow, low production costs, and yields high-quality products [14]. It is a production process widely used by alumina enterprises at home and abroad. The complex alkaline substances of red mud are closely related to the Bayer process (Figure 3). In the Bayer process, calcium hydroxide was added for pre-desilication treatment, and alumina in bauxite was dissolved by caustic alkali under high temperature and high pressure. The solid waste was red mud after slurry dilution and sedimentation separation [15].
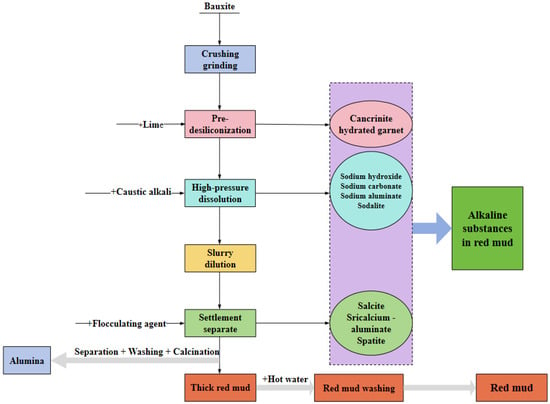
Figure 3.
Bayer method production process of red mud and alkaline substance formation diagram.
The alkali in red mud mainly exists in two forms, namely, soluble alkali and insoluble chemically bound alkali [16]. The soluble alkali is mainly present in the attached alkali solution brought in during the dissolution of bauxite, which accounts for about 20%–25% of the total alkali content in red mud. The desilication products in the dissolution process of alumina mainly exist in another state—that is, insoluble chemically bound alkali [16].
2.1. Soluble Alkalis
Soluble alkalis, as an important part of red mud, play a crucial role in providing alkalinity to red mud. The soluble alkali readily dissolves in water, generating a large amount of free alkaline anions and increasing the pH value within the red mud system. Therefore, soluble alkalis are also referred to as free alkalis [17,18]. Due to the different quality of bauxite raw materials and production processes used in the production process, the type and content of soluble alkalis are also different in different types of red mud. Compared with the sintering method and the combined method, the Bayer process yields a wider range and higher concentration of soluble alkaline substances. The soluble alkalis present in red mud primarily consist of NaOH, Na2CO3, NaHCO3, NaAl(OH)4, Na2SiO3, KOH, K2CO3, etc., with a relatively high concentration of NaOH, Na2CO3, NaHCO3, and NaAl(OH)4 [18]. The red mud slurry still contains residual alkali after undergoing multiple stages of washing. Furthermore, the residual alkali readily undergoes a reaction with atmospheric CO2 to generate sodium carbonate and sodium bicarbonate while also reacting with SiO2 to form soluble sodium silicate. The formation reactions of several typical free alkalis are shown in Equations (1)–(5) [19].
Free alkalis easily migrate to the surface of red mud under the action of the dissolution reaction and evaporation, resulting in efflorescence on the surface of red mud [20]. At the same time, the high free alkali content in red mud leads to the high pH value of the liquid phase of red mud, which has a significant impact on the vegetation and crops near the red mud yard, which is very unfavorable to the growth of plants [21]. Therefore, the directional regulation of free alkalis in red mud is crucial for achieving the safe storage and resource utilization of red mud [22].
2.2. Insoluble Chemically Bound Alkalis
In addition to the soluble alkali, the alkali in the red mud also exists within the insoluble phase structure. This part of the insoluble alkali is called the combined alkali or structural alkali. The process of pre-desilication, high-pressure dissolution, and sedimentation separation of some alkaline substances in red mud forms these insoluble chemically bound alkalis. These substances are stable in red mud after mineralization and precipitation reactions. Because of its low solubility and strong acid neutralization ability, it is also called chemically bound alkali [23]. The desilication products generated in alumina production mainly include nepheline, sodalite, tricalcium aluminate, hydro garnet, zeolite, calcite, and amorphous sodium aluminosilicate [7,8]. It is mainly sodium silicon slag (DSPs) and some insoluble mineral phases [24]. There is a dissolution equilibrium for these chemically bound alkalis, and the reaction equations for the dissolution of chemically bound alkalis are shown in Equations (6)–(10) [25].
Therefore, effectively regulating the alkalinity of red mud relies on inhibiting the dissolution of chemically bound alkali and stabilizing its occurrence state within the red mud. Additionally, it serves as an efficient approach to achieve in situ repair of the red mud yard [26].
3. Dealkalization Technology of Red Mud
According to the different occurrence states of alkali in red mud, the removal of alkali from red mud is mainly divided into two aspects: firstly, destroy the structure of chemically bound alkali in red mud, convert it into soluble alkali, and remove it via physical washing; secondly, convert soluble alkali into insoluble alkali through a series of methods to reduce its dissolution [27]. In practical applications, the components of red mud produced vary due to the diverse origins and production methods of alumina [28]. Consequently, a single method cannot effectively remove alkali from red mud. Extensive research conducted by scholars internationally has shown that combining two dealkalization principles is typically employed to develop a series of red mud dealkalization methods [29]. The more advanced dealkalization methods mainly include physical dealkalization, chemical dealkalization, and biological dealkalization [24,30]. The most essential physical method used for dealkalization is the water washing method. The chemical methods used for dealkalization include acid neutralization, salt (ion) precipitation or replacement technology, and metallurgical techniques [31,32]. The biological alkali adjustment method primarily employs microbial remediation to regulate the alkalinity of red mud. The main mechanisms of red mud dealkalization include neutralization, precipitation, and ion displacement reaction, as shown in Figure 4.
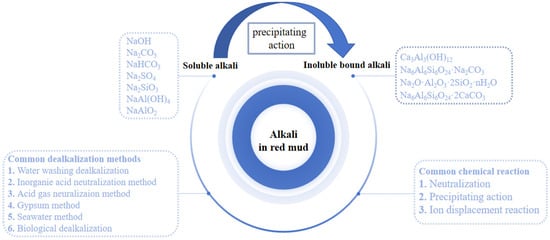
Figure 4.
The main mechanisms of red mud dealkalization.
3.1. Physical Dealkalization
The most essential method for dealkalization is water washing, whereby the red mud is directly washed with water to remove soluble alkali, as well as chemically bound alkali that has undergone structural damage. Water washing dealkalization is the simplest method of dealkalization, which is often used in alkali elution and alkali recovery before red mud discharge [30]. The mind map of dealkalization via water washing is shown in Figure 5.
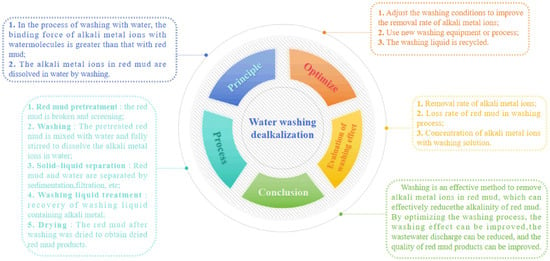
Figure 5.
The mind map of dealkalization via water washing method.
Kinnarinen et al. found that the increase in the liquid–solid ratio in the washing process of red mud significantly affects the leaching behavior of soluble Na, Al, and caustic alkali, thereby improving the alkaline leaching rate of red mud [33]. The water temperature has little effect on the dealkalization of red mud. When washing with water at different temperatures, the pH value of the obtained washing solution changes little. When the red mud is washed, there is a Na ion concentration difference between the surface of the red mud particles and the aqueous solution. The free alkali attached to the red mud can be dissolved in the red mud–water system. With the extension of time, the Na ion concentration in the red mud–water system continues to increase. The Na ion concentration difference also decreases. With repeated washing, the Na ion concentration difference can be increased, and the Na ion dissolution can be increased. Therefore, the removal of soluble Na in red mud is significantly influenced by the number of washing cycles. Due to the low efficiency of direct water use for alkalinity removal in red mud, numerous scholars have enhanced the elution efficiency by incorporating experimental steps. Li et al. [34] found that the multi-stage leaching test showed the optimal leaching rate of soluble alkaline anions at 86%. Wu et al. [35] observed that red mud subjected to five-stage countercurrent leaching with a flocculant (anionic polyacrylamide) and water achieved a dealkalization rate of 89.18%. Zhu et al. [18] investigated the dealkalization effect through water washing after the active roasting of red mud. During the calcination process at 700 °C, Na6CaAl6Si6 (CO3)O24·2H2O transformed into NaOH·H2O and Na2Ca(CO3)2. The dealkalization rate reached 82% after multi-stage leaching at 90 °C. Table 1 shows the specific technique of the water washing method and its advantages and disadvantages.

Table 1.
The analysis of water washing method.
The water washing method offers the advantage of a simple operation process and low equipment requirements. However, it requires higher dealkalization efficiency, necessitating the addition of specific experimental steps to enhance this aspect. Additionally, water washing dealkalization poses challenges in terms of significant water consumption and difficulty in recycling the washing liquid during later stages. Therefore, finding ways to improve the efficiency of water washing dealkalization and minimize water usage has become a crucial issue. In order to solve these two problems, some scholars [34] found a water-saving and efficient circulating red mud dealkalization system. In the dealkalization system, water was filtered after dealkalization of red mud, and the dealkalization solution was passed into the ion exchange system. The ion exchange resin in the exchange system was replaced with Na in the water, and the water, after alkaline removal, was recycled to the dealkalization system again. Due to the release of hydrogen ions from the resin during the cation exchange process, the dealkalization efficiency of water during the circulation process can be effectively improved. The whole process achieved multiple cycles of water, reducing the amount of water used, and the resin can also be recycled multiple times.
3.2. Chemical Dealkalization
Currently, the chemical dealkalization methods commonly employed in research primarily encompass acid neutralization dealkalization, salt (ion) precipitation or displacement technology, and metallurgical dealkalization. The acid neutralization method predominantly comprises inorganic and acid gas neutralization techniques. Salt (ion) precipitation or displacement technology encompasses seawater neutralization, brine neutralization, and gypsum treatment.
3.2.1. Acid Neutralization Dealkalization
When it comes to the removal of alkali, the most commonly used method is acid neutralization. An acid–base neutralization reaction is the most straightforward and direct reaction in chemistry, so acid neutralization can effectively reduce the alkalinity of red mud. The acid neutralization method mainly includes the inorganic and acid gas neutralization methods.
- (1)
- Inorganic acid neutralization
The effect of inorganic acid on regulating the alkalinity of red mud is remarkable, enabling profound adjustment and enhanced efficiency in alkaline regulation. Inorganic acid not only significantly reduces the content of free alkali in red mud but also effectively regulates the alkalinity of chemically bound alkali [36]. The principle behind the inorganic acid neutralization method lies in utilizing hydrogen ions from various acids to react with hydroxide ions, carbonate ions, aluminate ions, and silicate ions in red mud [37]. The main reactions of this method are shown in Equations (11)–(15) [38], while Figure 6 presents a mind map illustrating the selection process and principle involved in the use of inorganic acid for neutralization.
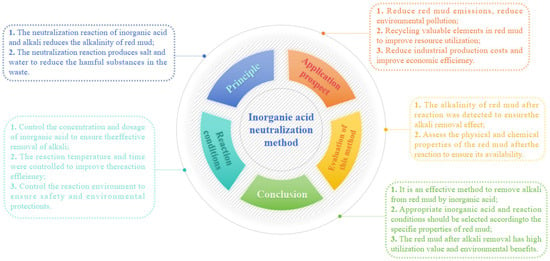
Figure 6.
The mind map of inorganic acid neutralization method.
The hydrochloric acid and sulfuric acid not only completely neutralize the free alkali in red mud but also react with chemically bound alkali, such as grossular, calcite, and nepheline to expedite phase transformation and reduce the overall alkalinity and pH value of red mud. Simultaneously, inorganic acids can also facilitate the formation of large aggregates within red mud and enhance its physical structure [32]. Khaitan et al. used hydrochloric acid to neutralize red mud to reduce its pH value from 12.5 to 4.6–8.0, and the pH value of the supernatant of red mud treated with sulfuric acid decreased from 10.26 to 8.22, respectively. The pH value was maintained by acid neutralization titration. The red mud system took about 20~50 days to reach the reaction equilibrium, and the red mud alkalinity was effectively regulated [39]. Acid leaching can significantly reduce the alkalinity of red mud. However, after a period of time, the pH value of red mud rebounds, which indicates that acid may have other reactions with red mud. However, the alkalinity of red mud was effectively regulated [40].
- (2)
- Acid gas neutralization
The acid gas neutralization method operates on the principle of utilizing carbon dioxide and sulfur dioxide to generate carbonic acid or sulfurous acid within the solution, thereby continuously releasing hydrogen ions that react with hydroxide, aluminate, and silicate compounds present in the red mud. CO2 is an acidic gas mixed with water to form H2CO3, so CO2 in the atmosphere or industrial waste gas is another potentially important acid source for neutralizing red mud. Dealkalization via the CO2 method is a three-phase reaction of gas, liquid, and solid. When CO2 is introduced into the red mud system, it first dissolves in the solution and reacts with the alkaline substances, such as Na salt, attached to the red mud to make them soluble ions, gradually leaving the red mud in the solution. The main reactions of this method are shown in Equations (18)–(27) [41]. The acid gas neutralization method is visually represented in Figure 7 as a mind map, providing a more comprehensive understanding of the various steps and processes involved.
Li and Han [30] conducted a CO2 absorption experiment of red mud, and the characteristics of red mud in the process of CO2 capture were studied. By employing the sintering technique, red mud demonstrates a remarkable capacity for CO2 absorption, with a capture rate of 0.11~0.12 g CO2 per gram. Li et al. [42] found that the red mud was dealkalized using the air flotation method, and CO2 was introduced into the red mud. It was found that the increase in the liquid–solid ratio was beneficial in expanding the reaction area of solid and liquid, and it easily dissolved CO2. When the liquid–solid ratio is excessively large, the solid phase system per unit volume is diminished, thereby reducing the efficiency of dealkalization. Elevated temperatures that are too high also decrease the solubility of CO2. Therefore, when the liquid–solid ratio is 7:1, the reaction temperature is 50 °C, the reaction time is 2 h, and the dealkalization efficiency is about 49.3%. Khaitan et al. [43] placed red mud in a 1.01 × 105 Pa atmospheric pressure CO2 environment and carbonized it for one day. The pH value of red mud decreased to 7.7, and then the pH value of red mud system slowly rose to 9.9. It was found that only when the tricalcium aluminate (TCA) in red mud was dissolved entirely (i.e., the pH value of discharged red mud was less than 9.9), CO2 was stably present in the solid phase of red mud in the form of calcium carbonate. The effect of long-term CO2 storage of red mud could be effectively reflected. The American Aluminium Company in Western Australia has applied CO2 dealkalization on a large scale. The company added gaseous and liquid CO2 to the thickened red mud slurry, and CO2 reacted with the alkaline components in the slurry to achieve dealkalization [43]. The degree of neutralization of red mud by CO2 mainly depends on the pressure and exposure time of CO2. Enick et al. [44] found that at 295 K and 6.7 MPa, the liquid CO2 was contacted with the red mud for 10~15 min. After a few weeks, the pH value of the red mud was reduced to 9.5~10.0 when the pressure was balanced with the atmospheric pressure.
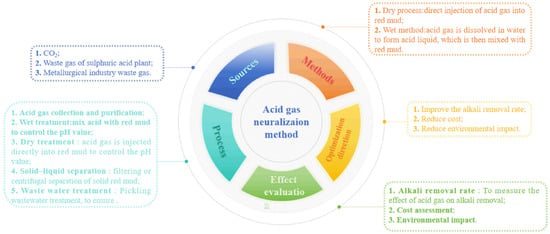
Figure 7.
The mind map of acid gas neutralizaion method.
There are few studies on the regulation of red mud alkalinity via SO2. Some researchers use red mud for tail gas desulfurization, which can achieve red mud dealkalization. Red mud can effectively absorb SO2. The desulfurization rate of industrial waste gas in power plants is as high as 93%, and the pH value of red mud decreases from 10.3 to about 4.0 and tends to be stable [45]. Fois et al. [46] found that red mud is added to the bubbling reactor, and a certain proportion of water is added to form a red mud suspension state, which can efficiently absorb SO2 gas. The reaction of red mud and SO2 can not only effectively regulate the alkalinity of red mud but also effectively prepare a small amount of polymer. Table 2 shows the specific technique of the acid neutralization methods and their advantages and disadvantages.

Table 2.
An analysis of acid neutralization methods.
Regulating the alkalinity of red mud using the acid gas neutralizaion method not only helps with the management of red mud but can also effectively capture carbon dioxide. The “treating waste with waste” scheme is conducive to the industry’s sustainable development. At the same time, using red mud to capture carbon dioxide can effectively respond to “carbon neutralization and carbon peak”, which is helpful for establishing a green environmental protection economic system and a low-carbon and clean, modern energy production and consumption system. The reaction of red mud and acid gas can not only effectively regulate the alkalinity of red mud but also effectively prepare a small amount of polymer. Currently, there are significant regional limitations on the utilization of acid gas for alkali removal due to the lack of large-scale transportation capabilities for both acid gas and red mud. This method is only suitable for facilities in close proximity to limestone calcination kilns, cement plants, thermal power plants, and other factories that generate substantial amounts of acid gas. Additionally, the time-consuming nature of using carbon dioxide to treat red mud further hinders its widespread application.
3.2.2. Salt (Ion) Precipitation or Replacement Technology
Dealkalization via the ion precipitation or replacement method is primarily based on the principle that the solubility of calcium, magnesium, and other hydroxides and salts is negligible to reduce the number of free alkaline anions in red mud and decrease its overall alkalinity. Unlike acid neutralization, this method converts soluble and alkaline solid substances into insoluble and weakly alkaline substances instead of removing hydroxides or carbonates from red mud.
- (1)
- Seawater neutralization
When the alkaline control of red mud was carried out using the seawater method, anions, such as hydroxide, carbonate, and aluminate, in red mud were precipitated by calcium and magnesium ions in seawater. In this process, insoluble calcium carbonate and magnesium carbonate, brucite, hydrotalcite, hydrocalumite, aluminum hydrogen calcite, and magnesioferrite are formed, thereby reducing the alkalinity of red mud [47]. The main reactions of this method are shown in Equations (28)–(31) [48]. Figure 8 depicts the mind map of the seawater method, enabling a more comprehensive understanding of this approach.
Menzies et al. discussed the effect of different liquid–solid ratios on the alkalinity of red mud in seawater treatment. The study found that in the red mud suspension with a liquid–solid ratio of 50, the pH value decreased from 12.0 to 9.0 after seawater treatment for 1 min. After 20 days of seawater treatment, the pH value was finally stabilized at about 8.5 [49]. Rai et al. screened the suitable conditions for regulating the alkalinity of red mud via the seawater method using the Taguchi experimental optimization method. The effects of the liquid–solid ratio, stirring time, and temperature on the pH value and related chemical properties of red mud were investigated. It was found that the amount of red mud and seawater was an essential factor in the regulation of red mud alkalinity, and the degree of influence was 53.59% and 44.92%, respectively. Under the optimized conditions (a liquid–solid ratio of 1:6, stirring time of 30 min, and temperature of 30 °C), the pH value can be reduced to 8.0, which meets the requirements for the safe disposal of red mud [50].
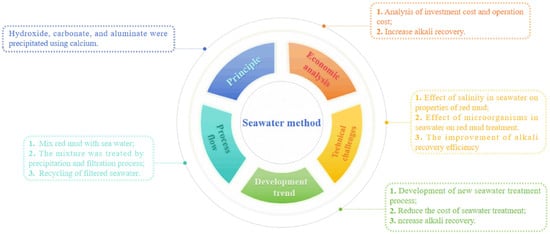
Figure 8.
The mind map of the seawater method.
The utilization of seawater for regulating the alkalinity of red mud results in the conversion of hydroxide and carbonate present in red mud into insoluble solid precipitates without their removal. Consequently, this approach significantly decreases the pH value of red mud while preserving its acid-neutralization capacity. The precipitated calcium in the red mud treated using seawater can also promote the soil occurrence of red mud and the growth of plants, which is of great significance to the restoration of red mud yards [26]. This is mainly because the adsorption capacity of red mud treated using seawater for phosphate is significantly increased, and phosphorus is one of the elements necessary for plant growth. Therefore, red mud treated using seawater can be used as a substrate for plant growth. However, many studies at home and abroad have found that in the use of seawater to treat red mud, compared with the stirring time, temperature, and other conditions that can be controlled in the laboratory, the relative use of red mud and seawater is the most critical factor in determining the alkalinity of red mud. Because only the amount of seawater is enough to provide sufficient amounts of calcium ions and magnesium ions, in this process, the amount of seawater used is considerable, usually up to 20 times the amount of red mud used. Therefore, the method of alkalinity regulation in red mud through seawater is commonly employed in alumina plants located near coastal areas. If there is an increase in the concentration of calcium and magnesium ions in seawater, it significantly reduces the required amount of seawater. In inland regions, this approach can also be utilized for alkalinity regulation in red mud, thus giving rise to the brine method.
- (2)
- Brine neutralization
The principle of improving red mud using brine neutralization is analogous to that of the seawater method. If the salinity of seawater is increased to about 1.5 times its original level, the volume of seawater required to regulate the same amount of red mud is reduced to about 58% of the original. At present, the ratio of calcium and magnesium in brine can be adjusted using an artificial ratio, making the concentration of calcium and magnesium more than 20 times higher than the corresponding ion concentration in seawater. This method uses calcium ions and magnesium ions to precipitate with the alkaline anion in red mud to reduce the pH value of red mud [51]. Clark used brine to regulate the alkalinity of red mud. It was found that when the liquid–solid ratio was 5, the pH value of the red mud liquid phase decreased from 13.1 to 7.5, mainly due to the precipitation reaction of a large number of hydroxide, carbonate, and aluminate with calcium and magnesium ions in the brine, resulting in a decrease in the pH value and a significant decrease in the total alkalinity of red mud [28]. Paradis et al. pointed out that adding brine to red mud can effectively precipitate the alkaline anions produced by the dissolution of free alkali in red mud, reduce the pH value of red mud, and improve the acid neutralization ability of red mud. Moreover, alkalinity has long-term stability characteristics, which allow for the storage and comprehensive utilization of red mud [23].
- (3)
- Gypsum treatment
Soluble calcium ions and magnesium ions in seawater or brine play a key role in regulating the alkalinity of red mud when using the seawater method and brine method. Gypsum can continuously release calcium ions to regulate red mud as a slightly soluble calcium salt. At the same time, the fluidity of gypsum is poor, so it is often used to repair red mud yards. Barrow added gypsum to red mud and found that calcium ions preferentially precipitated with alkaline carbonates in red mud and then replaced calcium and sodium with chemically bound alkali [52]. Gypsum can also react with free hydroxide, carbonate, and aluminate ions in the liquid phase of red mud to form calcium hydroxide, calcium carbonate, tricalcium aluminate, and hydrocalumite. The main reactions of this method are shown in Equations (32)–(35) [53], while Figure 9 depicts the mind map of the gypsum treatment.
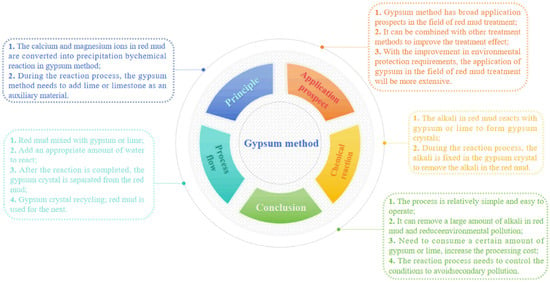
Figure 9.
The mind map of gypsum method.
Gypsum can not only react with carbonate ions to form calcium carbonate and reduce the pH value of the red mud system but also remove 86% of Al and 81% of As in the red mud liquid phase [54]. Courtney and Timpson found that when the amount of sand added was 25%, the amount of gypsum was 3%, the pH value of the red mud sand system could be reduced to 8.0, and the biomass of clover was high. Gypsum can not only effectively regulate the alkalinity of red mud and reduce the pH value of the system but also increase the content of Ca and Mg in red mud and promote plant growth in the process of red mud in situ repair [55]. Gypsum can not only reduce the alkalinity of red mud but also significantly improve the physical and chemical properties of red mud. In addition, many studies have shown that gypsum and organic matter have a synergistic effect and can also change the physical and chemical properties. Tian et al. found that the mixing of phosphogypsum, PVC powder, and red mud stimulated the stability of aggregates in red mud [56]. Wang et al. found that in the soil culture experiment, gypsum, earthworm manure, and red mud were used for soil culture experiments to study the effect on agglomeration [57]. The biological activity of earthworms increased the carbon content of red mud, and the combination of gypsum and vermicompost could significantly increase the formation of water-stable aggregates of red mud. Although the addition of gypsum and organic matter helps plants obtain the nutrients necessary for growth in red mud, plant growth is a very long process, which is only possible to maintain with long-term investment, which is why this method is currently challenging to promote. Table 3 shows the specific techniques of the seawater method, brine method, and gypsum method and their advantages and disadvantages.

Table 3.
The analysis of the seawater method, brine method, and gypsum method.
In addition to the use of the seawater method, brine method, and gypsum method to regulate the alkalinity of red mud, some scholars use CaCl2 waste liquid to eliminate the alkalinity of red mud. The principle is to use the CaCl2 and MgCl2 in the waste liquid to react with the alkali in the red mud so that the Na+ in the red mud enters the solution and is removed. Under certain experimental conditions, the removal rate of Na+ can reach 7% [58]. Some other scholars have used the principle that a certain amount of H+ produced by the hydrolysis of NH4Cl in the solution is neutralized with free alkali, and the ammonium ion is ion-exchanged with the Na+ adsorbed on the surface of red mud to eliminate the alkalinity of red mud. The amount of NH4Cl is 1.5 times the theoretical amount, the reaction temperature is 160 °C, the reaction time is 4 h, and the red mud particle size is 94~150 μm. Under these conditions, the Na2O content in the red mud residue can be reduced to less than 1% (mass fraction).
3.2.3. Metallurgical Method
There is a variety of valuable metal elements, such as Fe, Al, and Ti, in red mud, so it can also be considered as a resource. In recent decades, extensive research has been conducted on different approaches for the retrieval of precious metals using metallurgical methods. One traditional method commonly employed is pyrometallurgy, which involves the recovery of sodium alongside aluminum through a soda–lime roasting process. However, many studies on pyrometallurgy are mainly based on the recovery of valuable metals in red mud. Little attention has been paid to the change in pH value before and after treatment, but this process can generally reduce sodium oxide content in the residue to less than 1% [59].
In recent years, some scholars have used roasting to remove sodium from red mud alone. This method does not need additional additives, and the roasting temperature is lower than soda lime sintering. The main principle is to roast and activate the red mud and then use the water-leaching process to treat the roasted red mud, thereby removing the alkali in the red mud [18]. Zhu et al. found that under the optimum conditions of calcination at 700 °C for 30 min, water immersion at 90 °C for 60 min, and liquid–solid ratio of 7 mL/g, the final dealkalization rate could reach 82%, and the sodium content in the residue was reduced to 0.95%. The process of high-temperature roasting, however, demands a significant amount of energy and poses economic infeasibility. Therefore, there are few studies, and most of these are in the laboratory stage, which cannot be applied on a large scale. In addition to using the high-temperature roasting method, in recent years, some scholars have begun to use hydrothermal methods to remove alkali from red mud and recover aluminum. A two-step hydrothermal reaction generally achieves this process. First, the red mud, sodium hydroxide, and calcium oxide mixture slurry are reacted under high-temperature and high-pressure conditions, and the aluminum-containing components, such as sodalite, are decomposed into sodium aluminate for aluminum recovery. Then, the residue mixed with the alkali solution in the previous step is reacted under a high temperature and high pressure to convert sodium into sodium silicate for removal. Under appropriate process conditions, the extraction rate of aluminum can reach 87.8%, and the removal rate of sodium can reach 96.4% [60]. Table 4 shows the specific technique of the metallurgical method and its advantages and disadvantages. These studies are still conducted in laboratory settings and have not yet been extensively implemented in industrial applications.

Table 4.
The analysis of metallurgical method.
3.3. Biological Dealkalization
Microbial dealkalization primarily employs microbial metabolites (mainly organic acids and carbon dioxide) to neutralize alkali in red mud. The dealkalization reactions are analogous to acid neutralization methods; the main reactions of this method are shown in Equations (36)–(41) [61].
However, red mud exhibits high salinity, strong alkalinity, and a low organic matter content, resulting in a limited number of viable bacteria. Therefore, the current focus of research on the biological alkali adjustment method lies in screening suitable strains, establishing an environment conducive to microbial growth, and enhancing the efficiency of microbial metabolism and acid production to regulate the alkalinity of red mud [62], Figure 10 presents a mind map illustrating the principles and implementation steps involved in biological dealkalization.
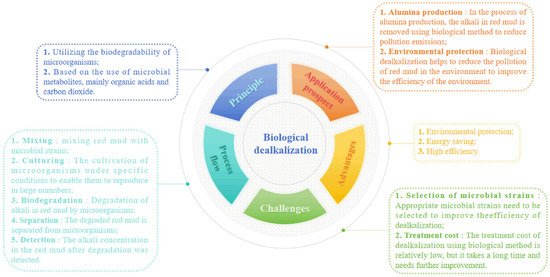
Figure 10.
The mind map of biological dealkalization.
Some scholars adjust the alkalinity of red mud by adding biomass to red mud and adding microbial strains. Liao et al. found that by adding acid waste residue of wood cellulose into red mud and adding microbial strains to eliminate alkalinity from red mud, the pH value of red mud could be reduced to below 9, the organic matter content could be increased from 6.13 g/kg to 24.92 g/kg, and the water capacity was greatly improved, which could effectively avoid the occurrence of the red mud anti-alkali phenomenon and meet the growth requirements of alkali-resistant plants [63].
Plant reconstruction is a promising method for the ecological treatment of red mud. The tolerant plants Lathyrus quinquenervius, Chloris virgata, Nicacia nilotica, Pongamia pinnata, Bauhinia variegata, and Albizia lebbeck have high tolerance to saline–alkali soil. They can be used as selective plants for the biological dealkalization of red mud [64,65]. In addition to their use in plant reconstruction, some microorganisms have good alkali resistance, and the use of such microorganisms in red mud to produce acid can achieve alkaline regulation. Some strains, such as Acidobacteriaceae, Nitrosomonadaceae, and Caulobacteraceae, have potential advantages in adapting to the robust alkaline environment of red mud and alkaline regulation, which can be used as essential indicators for ecological restoration of the yard [66]. Krishna et al. found that Aspergillus tubingensis can grow in a high-salt and a highly alkaline environment and regulate the alkalinity of red mud via metabolic acid production to reduce the pH value of red mud [67]. Based on the adequately improved red mud matrix, the joint regulation of alkaline via tolerant plants and functional microorganisms is also the critical direction of ecological restoration research in red mud yards. Plants can not only improve the physical structure of red mud but also provide an excellent metabolic environment for microorganisms, which is more conducive to microbial alkali adjustment [12,68]. Table 5 shows the specific technique of biological dealkalization and its advantages and disadvantages.

Table 5.
The analysis of biological dealkalization.
The effect of using biological methods to remove alkali from red mud is very good. The cost is not high, the cycle of using biological methods to remove alkali is very long, and it is not easy to achieve large-scale application.
3.4. Other Methods
There are “three wastes” of the dealkalization method: it uses waste gas, wastewater, and waste residue produced in industrial production, such as acidic waste gas containing CO2, SO2, and other gases; wastewater containing HNO3, H2SO4, and other substances; and acidic waste residue, etc., to treat red mud [69]. The “Three wastes” are mostly acidic substances, which can be used to neutralize red mud to reduce its alkalinity [45]. The principle of using waste gas containing CO2, SO2, and wastewater containing HNO3, H2SO4, and other substances to remove alkali from red mud has been mentioned previously [70]. Based on biomass’s unique dealkalization function, some research teams have researched the dealkalization of red mud using lignocellulosic waste combined with composite microbial bacteria [71]. When the mass ratio of red mud to lignocellulosic waste residue was 7:3, the pH value of red mud decreased from 11.08 to 9.02. The pH value of red mud can be reduced from 9.02 to 8.35 by adding compound microbial agents, and the treated red mud can be used to support the average growth of saline–alkali tolerant plants [31,32]. The "three wastes" neutralization method can achieve the coordinated disposal of wastes, reducing the emissions of waste gas, wastewater, and waste residue. This approach effectively addresses the critical issue of a high alkali content in red mud.
Membrane sodium removal technology separates diluted red mud from the pure dispersant utilizing a semi-permeable membrane. The sodium and other alkali metal ions in the red mud can pass through the semi-permeable membrane, enter the dispersant, and then be separated [43]. If vacuum filtration is added, the penetration rate of sodium is accelerated. Selective flocculation technology uses a high-efficiency flocculant to selectively adsorb on the surface of red mud particles. At the same time, sodium and other alkaline earth metal ion compounds still maintain a stable dispersion state, and dealkalization is achieved after solid–liquid separation.
In view of the low dealkalization rate of the single dealkalization method, some researchers have combined two or more dealkalization methods to remove alkali from red mud. Clark et al. carbonized red mud with CO2 and neutralized it with seawater. After treatment, the pH value of red mud reached 8.0~8.5, and the acid neutralization capacity (ANC) of red mud increased [28]. Wang et al. used carbide slag (CaO) and flue gas (SO2) to remove alkali from red mud. Under the optimal conditions, the residual Na2O in red mud after dealkalization using carbide slag was found to be below 3% (mass fraction), while the residual Na2O in red mud after dealkalization using flue gas was observed to be less than 2% (mass fraction) [72].
4. Discussion
There are numerous techniques used for red mud dealkalization. However, these dealkalization methods have different application methods and application scenarios. The water washing method and the seawater method are often used in the red mud washing process. The inorganic acid neutralization method is often used in waste acid synergistic treatment. The acid gas neutralization method is often used in red mud discharge pretreatment. The gypsum method and biological method are often used in red mud yard improvement. Each method possesses its own set of advantages and disadvantages, which are comprehensively analyzed and compared in Table 6.

Table 6.
The analysis of dealkalization methods.
The water washing method is simple and has no reagent consumption, but it requires many iterations of alkali removal, greater water consumption, and a long time [73]. Only the free alkali in red mud can be removed, so its alkali removal effect is limited, which is generally used as the choice of preliminary alkali removal [34].
It is reported that the research on inorganic acid in red mud dealkalization is mostly valuable, but the inorganic acid consumption is significant [74]. Therefore, considering the source and cost of acid, achieving large-scale application in the short term takes time and effort. Subsequent research should, therefore, prioritize the utilization of waste acid for neutralizing the alkali present in red mud [71]. This approach not only effectively eliminates alkali from red mud but also facilitates the consumption and reduction of waste acid pollution.
The utilization of acid gas for alkali removal from red mud effectively converts the basic anions and some bound alkalis, thereby reducing the pH value of the red mud [75]. Many alumina enterprises employ CO2 to regulate slurry alkalinity prior to red mud discharge, enabling effective control over red mud alkalinity. However, specific equipment requirements must be maintained to ensure proper CO2 partial pressure, posing challenges in treating uncarbonized discharged red mud [76].
The alkali removal mechanism of each salt (ion) precipitation or replacement technology (the seawater method, brine method, and gypsum method) is basically similar: when the brine or seawater rich in Ca2+ and Mg2+ is mixed with red mud, the precipitation reaction and replacement reaction occur, and the alkalinity (mainly NaOH) and other soluble alkalis are transformed into low-solubility hydroxides, carbonates, and hydroxyl carbonate minerals, thereby reducing the pH value of red mud [25,54]. The alkali removal rate of salt (ion) precipitation or replacement technology is high, but red mud has strong acidity and poor filtration after alkali removal, which greatly limits its industrial application and development [77].
Biological dealkalization mainly uses microbial metabolism to produce acid to regulate the alkalinity of red mud and stimulates microbial metabolism by adding nutrients to reduce the pH value of red mud effectively [68]. This method shows promising potential for the application of alkaline regulation in red mud yards. If suitable acid-producing bacteria can be screened out, and the efficiency of alkaline regulation treatment can be improved [78], reducing the cost of alkali regulation and expanding the scale of alkali regulation will be beneficial, but this has always been a hot topic and involves challenging research [62].
The problems of economy, periodicity, and secondary pollution of dealkalization hinder the large-scale application of the red mud dealkalization method, and the dealkalization of red mud is still a difficult problem in current research.
5. Conclusions and Prospective
The issue of red mud has been a persistent problem worldwide since the emergence of the alumina industry. Over time, the accumulation of large quantities of red mud poses significant environmental safety risks. The safe disposal of red mud remains a global challenge, with dealkalization being key to its proper management.
Free alkali and chemically combined alkali are two kinds of alkaline forms existing in red mud. The dissolution of these alkaline components increases the pH value of red mud. In order to effectively regulate the alkalinity of red mud, research has been conducted based on the characteristics and mechanisms of alkaline dissolution and alkali adjustment. However, current methods for adjusting the alkali content in red mud have not thoroughly explored the occurrence state, dissolution characteristics, and mechanism of critical alkaline components. The key aspect of regulating the alkalinity of red mud lies in achieving targeted regulation and stable transformation of free alkali and chemically combined alkali within it. Acid neutralization, seawater, brine, gypsum, and biological methods are commonly used for dealkalization; however, efficiently utilizing these methods to remove alkali from red mud remains an urgent issue. Given certain challenges in dealkalizing red mud, the following suggestions are proposed:
- (1)
- An in-depth study of the formation mechanism of various alkalis in red mud should be conducted.
- (2)
- The exploration of efficient and cost-effective dealkalization techniques for red mud should be pursued.
- (3)
- Research on the combined dealkalization method deserves greater attention. It is important to identify appropriate combined dealkalization technologies for red mud with varying properties.
All in all, the problems of the economy, periodicity, and secondary pollution of dealkalization hinder the large-scale application of the red mud dealkalization method. Looking forward to the future, finding an economical and efficient method of dealkalization for red mud will be the primary objective; effective alkali treatment is of great significance to the realization of large-scale resource utilization of red mud.
Author Contributions
Conceptualization, X.L. and J.Y.; methodology, X.L.; software, K.C. and J.Y.; validation, X.L., J.Y. and J.Q.; formal analysis, J.L.; investigation, J.Y.; resources, X.L.; data curation, H.L.; writing—original draft preparation, J.Y.; writing—review and editing, K.C. and X.L.; visualization, H.L.; supervision, J.L.; project administration, X.L.; funding acquisition, X.L. and J.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Open Foundation of State Key Laboratory of Mineral Processing, grant number (BGRIMM-KJSKL-2023-22; BGRIMM-KJSKL-2025-03); the Natural Science Foundation of Shandong Province, grant number ZR2024ME082; and the Start Up Fund for Talent Introduction and Scientific Research of Shandong University of Science and Technology, grant number skr21-3-C-108.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, X.B.; Li, W.; Guan, X.W. Research status and analysis of comprehensive utilization of red mud. Multipurp. Util. Miner. Resour. 2016, 7–10. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Klauber, M.; Klauber, C. Bauxite residue issues: IV. Old obstacles and new pathways for in situ residue bioremediation. Hydrometallurgy 2011, 108, 46–59. [Google Scholar]
- Chen, X.; Guo, Y.G.; Ding, S.; Zhang, H.Y.; Xia, F.Y.; Wang, J.; Zhou, M.K. Utilization of red mud in geopolymer-based pervious concrete with function of adsorption of heavy metal ions. J. Clean. Prod. 2019, 207, 789–800. [Google Scholar]
- Liu, W.C.; Chen, X.Q.; Li, W.X.; Yu, Y.F.; Yan, K. Environmental assessment, management and utilization of red mud in China. J. Clean. Prod. 2014, 84, 606–610. [Google Scholar] [CrossRef]
- Carneiro, J.; Tobaldi, D.; Capela, M.N.; Novais, R.; Seabra, M.; Labrincha, J.A. Synthesis of ceramic pigments from industrial wastes: Red mud and electroplating sludge. Waste Manag. 2018, 80, 371–378. [Google Scholar]
- Xu, B.; Smith, P. The effect of iron sources on caustic and alumina recovery from synthetic bayer DSP (sodalite). Hydrometallurgy 2012, 129, 26–29. [Google Scholar]
- Si, C.H.; Ma, Y.Q.; Lin, C.X. Red mud as a carbon sink: Variability, affecting factors and environmental significance. J. Hazard. Mater. 2013, 244, 54–59. [Google Scholar]
- Xu, G.; Ding, X.H.; Kuruppu, M.; Zhou, W.; Biswas, W. Research and application of non-traditional chemical stabilizers on bauxite residue (red sand) dust control, a review. Sci. Total Environ. 2018, 616, 1552–1565. [Google Scholar]
- Solovey, V.V.; Shevchenko, A.A.; Zipunnikov, M.M.; Kotenko, A.L.; Khiem, N.T.; Tri, B.D.; Hai, T.T. Development of high pressure membraneless alkaline electrolyzer. Int. J. Hydrog. Energy 2022, 47, 6975–6985. [Google Scholar] [CrossRef]
- Zhu, F.; Zhou, J.Y.; Xue, S.G.; Hartley, W.; Wu, C.; Guo, Y. Aging of bauxite residue in association of regeneration: A comparison of methods to determine aggregate stability & erosion resistance. Ecol. Eng. 2016, 92, 47–54. [Google Scholar]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Man, K.; Zhu, Q.; Li, L.; Liu, C.; Xing, Z. Preparation and performance of ceramic filter material by recovered silicon dioxide as major leached component from red mud. Ceram. Int. 2017, 43, 7565–7572. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Xue, S.G.; Li, X.F.; Kong, X.F.; Wu, C.; Li, Y.W.; Li, M.; Li, C.X. Alkaline regulation of bauxite residue: A comprehensive review. Acta Sci. Circumstantiae 2017, 37, 2815–2828. [Google Scholar]
- Xue, S.G.; Kong, X.F.; Zhu, F.; Hartley, W.; Li, X.F.; Li, Y.W. Proposal for management and alkalinity transformation of bauxite residue in China. Environ. Sci. Pollut. Res. 2016, 23, 12822–12834. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Guan, X. An active dealkalization of red mud with roasting and water leaching. J. Hazard. Mater. 2015, 286, 85–91. [Google Scholar] [CrossRef]
- Pan, X.L.; Yu, H.Y.; Tu, G.F. Reduction of alkalinity in bauxite residue during Bayer digestion in high-ferrite diasporic bauxite. Hydrometallurgy 2015, 151, 98–106. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.W.; Xue, S.G.; Zhu, F.; Wu, C.; Wang, Q.L. Salt composition changes in different stacking ages of bauxite residue. Chin. J. Nonferrous Met. 2016, 26, 2433–2439. [Google Scholar]
- Jones, B.E.H.; Haynes, R.J.; Phillips, I.R. Influence of organic waste and residue mud additions on chemical, physical and microbial properties of bauxite residue sand. Environ. Sci. Pollut. Res. 2011, 18, 199–211. [Google Scholar]
- Jones, B.E.H.; Haynes, R.J. Bauxite Processing Residue: A Critical Review of Its Formation, Properties, Storage, and Revegetation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 271–315. [Google Scholar] [CrossRef]
- Paradis, M.; Duchesne, J.; Lamontagne, A.; Isabel, D. Long-term neutralisation potential of red mud bauxite with brine amendment for the neutralisation of acidic mine tailings. Appl. Geochem. 2007, 22, 2326–2333. [Google Scholar]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar]
- Lyu, F.; Hu, Y.H.; Wang, L.; Sun, W. Dealkalization processes of bauxite residue: A comprehensive review. J. Hazard. Mater. 2021, 403, 17. [Google Scholar]
- Zhu, F.; Han, F.S.; Xue, S.G.; Guo, Y.; LI, M.; Liao, J.X. Fractal characteristics of bauxite residue aggregates in red mud yard. Chin. J. Nonferrous Met. 2016, 26, 1316–1323. [Google Scholar]
- Palmer, S.J.; Reddy, B.J.; Frost, R.L. Characterisation of red mud by UV-vis-NIR spectroscopy. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2009, 71, 1814–1818. [Google Scholar]
- Clark, M.W.; Johnston, M.; Reichelt-Brushett, A.J. Comparison of several different neutralisations to a bauxite refinery residue: Potential effectiveness environmental ameliorants. Appl. Geochem. 2015, 56, 1–10. [Google Scholar]
- Johnston, M.; Clark, M.W.; McMahon, P.; Ward, N. Alkalinity conversion of bauxite refinery residues by neutralization. J. Hazard. Mater. 2010, 182, 710–715. [Google Scholar]
- Kinnarinen, T.; Lubieniecki, B.; Holliday, L.; Helsto, J.J.; Häkkinen, A. Recovery of sodium from bauxite residue by pressure filtration and cake washing. Int. J. Min. Process. 2015, 141, 20–26. [Google Scholar]
- Wang, X.K.; Zhang, Y.H.; Lv, F.Z.; An, Q.; Lu, R.R.; Hu, P.; Jiang, S.B. Removal of Alkali in the Red Mud by SO2 and Simulated Flue Gas Under Mild Conditions. Environ. Prog. Sustain. Energy 2015, 34, 81–87. [Google Scholar]
- Kong, X.F.; Li, M.; Xue, S.G.; Hartley, W.; Chen, C.R.; Wu, C.; Li, X.F.; Li, Y.W. Acid transformation of bauxite residue: Conversion of its alkaline characteristics. J. Hazard. Mater. 2017, 324, 382–390. [Google Scholar] [PubMed]
- Kinnarinen, T.; Holliday, L.; Hökkinen, A. Dissolution of sodium, aluminum and caustic compounds from bauxite residues. Miner. Eng. 2015, 79, 143–151. [Google Scholar]
- Li, X.F.; Ye, Y.Z.; Xue, S.G.; Jiang, J.; Wu, C.; Kong, X.F.; Hartley, W.; Li, Y.W. Leaching optimization and dissolution behavior of alkaline anions in bauxite residue. Trans. Nonferrous Met. Soc. China 2018, 28, 1248–1255. [Google Scholar]
- Wu, S.B.; Nie, D.P.; Wang, Z.J.; Liu, A.R.; Xue, A. Recycling alkali from red mud by countercurrent leaching. Chem. Ind. Eng. Prog. 2014, 33, 1607–1609. [Google Scholar]
- Liang, W.T.; Couperthwaite, S.J.; Kaur, G.; Yan, C.; Johnstone, D.W.; Millar, G.J. Effect of strong acids on red mud structural and fluoride adsorption properties. J. Colloid Interf. Sci. 2014, 423, 158–165. [Google Scholar]
- Yang, Y.; Wang, X.W.; Wang, M.Y.; Wang, H.G.; Xian, P.F. Iron recovery from the leached solution of red mud through the application of oxalic acid. Int. J. Min. Process. 2016, 157, 145–151. [Google Scholar]
- Couperthwaite, S.J.; Johnstone, D.W.; Millar, G.J.; Frost, R.L. Neutralization of Acid Sulfate Solutions Using Bauxite Refinery Residues and Its Derivatives. Ind. Eng. Chem. Res. 2013, 52, 1388–1395. [Google Scholar]
- Silva, P.M.P.; do Carmo, A.L.V.; Holanda, R.B.; Gomes, F.G.; Nogueira, E.; da Costa, R.V.; de Melo, C.C.A.; Lucheta, A.R.; Montini, M. Brazilian Bauxite Residue Physical-Chemical Characterization and Acidic Neutralization Potential. In Proceedings of the Light Metals Symposia at the 149th The-Minerals-Metals-and-Materials-Society (TMS) Annual Meeting and Exhibition, San Diego, CA, USA, 23–27 February 2020; pp. 115–123. [Google Scholar]
- Kishida, M.; Harato, T.; Tokoro, C.; Owada, S. In situ remediation of bauxite residue by sulfuric acid leaching and bipolar-membrane electrodialysis. Hydrometallurgy 2017, 170, 58–67. [Google Scholar]
- Liu, Z.B.; Li, H.X. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar]
- Li, A.M.; Wang, A.M.; He, Y.M.; Guo, Y.; Liu, J.L.; Hao, S.; Yang, F.S. Experimental Study on the Dealkalization of Red Mud Waste from Bayer Process by CO2 Gas Floatation. Guangdong Chem. Ind. 2018, 45, 94–95. [Google Scholar]
- Chen, S.; Chen, Y.J.; Xie, X.; Dong, Z.J.; Zhang, Q.; Fu, J.L.; Huang, J.H. Research Progress on Dealkaliation Methods and Mechanism of Red Mud. Bull. Chin. Ceram. Soc. 2021, 40, 3414–3426. [Google Scholar]
- Enick, R.; Beckman, E.; Shi, C.; Xu, J. Remediation of Metal-Bearing Aqueous Waste Streams via Direct Carbonation. Energy Fuels 2001, 15, 256–262. [Google Scholar]
- Jia, S.D.; Dong, J.Y.; Wang, B. Comprehensive Utilization Technology Advances of Mixed C4 Fraction. Technol. Dev. Chem. Ind. 2013, 42, 67–69. [Google Scholar]
- Fois, E.; Lallai, A.; Mura, G. Sulfur Dioxide Absorption in a Bubbling Reactor with Suspensions of Bayer Red Mud. Ind. Eng. Chem. Res. 2007, 46, 6770–6776. [Google Scholar]
- Kannan, P.; Banat, F.; Hasan, S.W.; Abu Haija, M. Neutralization of Bayer bauxite residue (red mud) by various brines: A review of chemistry and engineering processes. Hydrometallurgy 2021, 206, 13. [Google Scholar]
- Palmer, S.; Frost, R.; Nguyen, T. Hydrotalcites and their role in coordination of anions in Bayer liquors: Anion binding in layered double hydroxides. Coordin. Chem. Rev. 2009, 253, 250–267. [Google Scholar]
- Menzies, N.W.; Fulton, I.M.; Morrell, W.J. Seawater neutralization of alkaline bauxite residue and implications for revegetation. J. Environ. Qual. 2004, 33, 1877–1884. [Google Scholar]
- Rai, S.; Wasewar, K.L.; Lataye, D.H.; Mukhopadhyay, J.; Yoo, C.K. Feasibility of red mud neutralization with seawater using Taguchi’s methodology. Int. J. Environ. Sci. Technol. 2013, 10, 305–314. [Google Scholar]
- Despland, L.M.; Clark, M.W.; Aragno, M.; Vancov, T. Minimising Alkalinity and pH Spikes from Portland Cement-Bound Bauxsol (Seawater-Neutralized Red Mud) Pellets for pH Circum-Neutral Waters. Environ. Sci. Technol. 2010, 44, 2119–2125. [Google Scholar]
- Barrow, N.J.J.C.; Science, P. Possibility of using caustic residue from bauxite for improving the chemical and physical properties of sandy soils. Aust. J. Agric. Res. 1982, 33, 275–285. [Google Scholar]
- Kirwan, L.; Hartshorn, A.; McMonagle, J.; Fleming, L.; Funnell, D. Chemistry of bauxite residue neutralisation and aspects to implementation. Int. J. Miner. Process. 2013, 119, 40–50. [Google Scholar]
- Burke, I.T.; Peacock, C.L.; Lockwood, C.L.; Stewart, D.I.; Mortimer, R.J.G.; Ward, M.B.; Renforth, P.; Gruiz, K.; Mayes, W.M. Behavior of Aluminum, Arsenic, and Vanadium during the Neutralization of Red Mud Leachate by HCl, Gypsum, or Seawater. Environ. Sci. Technol. 2013, 47, 6527–6535. [Google Scholar] [PubMed]
- Courtney, R.G.; Timpson, J.P. Reclamation of Fine Fraction Bauxite Processing Residue (Red Mud) Amended with Coarse Fraction Residue and Gypsum. Water Air Soil Poll. 2005, 164, 91–102. [Google Scholar]
- Tian, T.; Wu, Y.J.; Xue, S.G.; Huang, L.; Jiang, J.; Zhu, F. Effects of gypsum amendment on salinity ion migration in bauxite residue. J. Univ. Chin. Acad. Sci. 2019, 36, 521–529. [Google Scholar]
- Wang, Q.L.; Ye, Y.Z.; Xue, S.G.; Jiang, J.; Zhu, F.; Tian, T. Effects of ameliorants on aggregate stability of bauxite residue. J. Univ. Chin. Acad. Sci. 2019, 36, 530–536. [Google Scholar]
- Cui, S.S.; Wang, N.; Gu, H.N. Application of CaCl2 waste liqior in dealkalizition of red mud. Environ. Prot. Chem. Ind. 2016, 36, 553–556. [Google Scholar]
- Mishra, B.; Staley, A.; Kirkpatrick, D. Recovery of value-added products from red mud. Min. Metall. Explor. 2002, 19, 87–94. [Google Scholar]
- Zhong, L.; Zhang, Y.; Zhang, Y. Extraction of alumina and sodium oxide from red mud by a mild hydro-chemical process. J. Hazard. Mater. 2009, 172, 1629–1634. [Google Scholar]
- Santini, T.C.; Kerr, J.L.; Warren, L.A. Microbially-driven strategies for bioremediation of bauxite residue. J. Hazard. Mater. 2015, 293, 131–157. [Google Scholar]
- Banning, N.C.; Phillips, I.R.; Jones, D.L.; Murphy, D.V. Development of Microbial Diversity and Functional Potential in Bauxite Residue Sand under Rehabilitation. Restor. Ecol. 2011, 19, 78–87. [Google Scholar] [CrossRef]
- Liao, J.X.; Tang, Q.; Zhou, L.W.; Zhu, S.X.; Huang, K.Z.; Zeng, D.J. Studies of Lignocellulose Waste Residue on Dealkalization and Amendment of Red Mud. J. Environ. Sci. Technol. 2019, 42, 31–36. [Google Scholar]
- Zhang, J.T.; Mu, C.S. Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus quinquenervius. Soil Sci. Plant Nutr. 2009, 55, 685–697. [Google Scholar] [CrossRef]
- Alshaal, T.; Domokos-Szabolcsy, É.; Márton, L.; Czakó, M.; Kátai, J.; Balogh, P.; Elhawat, N.; El-Ramady, H.; Fári, M. Phytoremediation of bauxite-derived red mud by giant reed. Environ. Chem. Lett. 2013, 11, 295–302. [Google Scholar] [CrossRef]
- Zhu, F.; Liao, J.; Xue, S.; Hartley, W.; Zou, Q.; Wu, H. Evaluation of aggregate microstructures following natural regeneration in bauxite residue as characterized by synchrotron-based X-ray micro-computed tomography. Sci. Total Environ. 2016, 573, 155–163. [Google Scholar]
- Krishna, P.; Babu, A.G.; Reddy, M.S. Bacterial diversity of extremely alkaline bauxite residue site of alumina industrial plant using culturable bacteria and residue 16S rRNA gene clones. Extremophiles 2014, 18, 665–676. [Google Scholar]
- Zhu, F.; Li, X.; Xue, S.; Hartley, W.; Wu, C.; Han, F. Natural plant colonization improves the physical condition of bauxite residue over time. Environ. Sci. Pollut. Res. 2016, 23, 22897–22905. [Google Scholar] [CrossRef]
- Chen, Y.N.; Nie, J.X. Adsorption of SO2 from Flue Gas with Wastewater in Red Mud. J. Nonferrous Met. 2007, 59, 153–155. [Google Scholar]
- Nan, X.L.; Zhang, T.A.; Wu, Y.Q.; Dou, Z. A study on absorption of low-concentration SO2 by Bayer red mud. Dongbei Daxue Xuebao/J. Northeast. Univ. 2010, 31, 986–989. [Google Scholar]
- Tao, L.; Wu, H.; Wang, J.; Li, B.; Wang, X.Q.; Ning, P. Removal of SO2 from flue gas using Bayer red mud: Influence factors and mechanism. J. Cent. South Univ. 2019, 26, 467–478. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liu, J.; Hu, P.; Meng, K.; Lv, F.; Tong, W.; Chu, P. Dealkalization of Red Mud by Carbide Slag and Flue Gas. Clean Soil Air Water 2017, 46, 1700634. [Google Scholar]
- Kong, X.F.; Jiang, X.X.; Xue, S.G.; Huang, L.; Hartley, W.; Wu, C.; Li, X.F. Migration and distribution of saline ions in bauxite residue during water leaching. Trans. Nonferrous Met. Soc. China 2018, 28, 534–541. [Google Scholar] [CrossRef]
- Zhu, X.B.; Li, W.; Guan, X.M. Kinetics of titanium leaching with citric acid in sulfuric acid from red mud. Trans. Nonferrous Met. Soc. China 2015, 25, 3139–3145. [Google Scholar]
- Santini, T.C.; Hinz, C.; Rate, A.W.; Carter, C.M.; Gilkes, R.J. In situ neutralisation of uncarbonated bauxite residue mud by cross layer leaching with carbonated bauxite residue mud. J. Hazard. Mater. 2011, 194, 119–127. [Google Scholar] [CrossRef]
- Ilahi, K.; Debbarma, S.; Mathew, G.; Inyang, H.I. Carbon capture and mineralisation using red mud: A systematic review of its principles and applications. J. Clean. Prod. 2024, 473, 21. [Google Scholar]
- Shi, B.; Qu, Y.; Li, H. Gypsum alleviated hydroxyl radical-mediated oxidative damages caused by alkaline bauxite residue in leaves of Atriplex canescens. Ecol. Eng. 2017, 98, 166–171. [Google Scholar]
- Hamdy, M.K.; Williams, F.S. Bacterial amelioration of bauxite residue waste of industrial alumina plants. J. Ind. Microbiol. Biotechnol. 2001, 27, 228–233. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).