Abstract
The calcareous nannoplankton comprises haptophyte eukaryotes known as coccolithophores, capable of calcifying elaborate external skeletons (coccoliths s.l.) which differ morphologically depending on the phase of the life cycle considered, and the locus (intra- or extracellular) of mineralization. No study is currently available that analyzes the impact of these differences on coccolith morphology. An analysis of the assembly of their crystals is conducted here in search of the following: (1) identical traits across life cycles; (2) fossil records diagnostic of extracellular calcification; and (3) influence of the geometry of biomineralization during the diploid phase on the long-term evolution of a clade. This study shows patterns such as correlation of characters and structural imprint that unify the haploid and diploid phases, indicating a strong cellular integrity and offering potent means to determine life cycles in living and fossil communities. It also shows that differences in diversity patterns and longevity among families and orders depend on coccolith geometry, concentric geometry being more favorable to stability, and superposition geometry facilitating morphological diversification. Extinction occurs when the potential for diversification is attained. Finally, I propose that the evolution of biomineralization in the calcareous nannoplankton may have been more complex than initially thought, with intra- and extracellular calcification evolving independently.
1. Introduction
The Rügen Kalk, the Dover and Austin Chalk, the Craie of the Reims region on which grape vines grow, many limestone deposits around the world, and the thick successions of soft and indurated calcareous oozes that have blanketed the sea floor since the Cretaceous contain, in abundance, minute skeletal remains of great diversity and delicacy. These remarkable sedimentary accumulations [1,2] are largely the result of a biologic process, called biomineralization, which is widespread among eukaryotes [3]. In the ocean, biomineralization is the trademark of a dominant quartet of unicellular planktons comprising foraminifera, calcareous nannoplankton, diatoms, and radiolarians, all of which play a fundamental role in the dynamics of the Earth system [4,5,6]. The micron-size skeletons of the oozes and chalks were calcified by the tiny, photosynthetic nannoplankton which appeared when the Tethys Ocean was forming, and have continued to evolve to this day. Their rock-forming remains are collectively known as calcareous nannofossils.
There are two facets to the study of biomineralization, and they have developed in parallel since the first description of mineralized tissues (bones, shells, teeth) by Leonardo da Vinci in the 15th and 16th centuries [7]. One facet relates to the process, which is how organisms produce a mineralized organic entity. The other facet concerns the structuring of that entity. For the calcareous nannoplankton these entities are the well-known coccoliths and nannoliths.
Numerous studies have been devoted to the description of the present and past diversity of the calcareous nannoplankton and its evolutionary history (lineages, trends), incorporating morphology, molecular biology, and processes of biomineralization as reviewed in [8]. I discuss here biomineralization in the calcareous nannoplankton from a different perspective, which is the biological context of their calcification. Recent advances in biology include the demonstration that all coccoliths are mineralized intracellularly following the same complex processes [9] and that the group known as ‘pentaliths’ [10] are probably calcified outside the cell membrane [11]. From the angle of a haploid–diploid life cycle, I explore the possible existence of morphological traits with broad biological significance in the living species. The question is whether there are, or are not, traits characteristic of life associations at taxonomic levels above the species and, from which, biological and paleobiologic inferences may be drawn. From the context of intracellular versus extracellular calcification, I investigate the impact of biomineralization on the long-term evolution of taxa. The question is whether the patterns of diversification and longevity are related to the locus of biomineralization. My findings lead to a reconsideration of the monophyletic origin of biomineralization in the nannoplankton, with a re-evaluation of its early fossil record.
2. Material and Methods
2.1. Documentation
Vast documentation is available for the bulk of the nannoplankton and their skeletons. Species have been named, described, and illustrated continuously since Wallich [12] formally described the first living species. Oceanographic cruises have recorded living species from all latitudes, while field programs on land and 58 years of deep-sea drilling have recovered nannofossil species worldwide. At the same time, increasingly performant technological and methodological advances have continuously deepened our scrutiny of them. A wealth of data from research in the Earth and Life sciences is thus available in the literature, some of which has been compiled in book chapters [4,13,14,15], monographs [16,17,18,19], and databases [20]. The data used below are centered on my monographs on Cenozoic Discoasterales [18], on the living haploid phases [19], and forthcoming monographs on Braarudosphaerales, Mesozoic Discoasterales, and fossil holococcoliths [21,22,23] which supersede out-of-print publications on their Cenozoic genera [24,25]. Much of the data on Pontosphaerales and Zygodiscales are based on an unpublished revision of Aubry [16].
2.2. Analytical Procedures
2.2.1. Description of Skeletal Structures
The methodology used here is an expansion of pioneering studies (see, for example, [26,27,28,29,30]). It involves consistent orientation of the skeletal pieces, followed by descriptions of their geometry and structure, including elements, cycles, structural units, fabrics, and segments, as required. Careful drawings are at the origin of this work, helping to decipher important features as well as commonality and differences between taxa (Figure 1). The iconography in this paper reflects this effort, with color codification that readily supports the discussion. Most drawings integrate information from multiple transmission and scanning electron micrographs. Occasionally, a specific unpublished photograph has served as a guide (with acknowledgements). Attention was also given to the coccospheres with an emphasis placed on the less known holococcolith-bearing coccospheres.
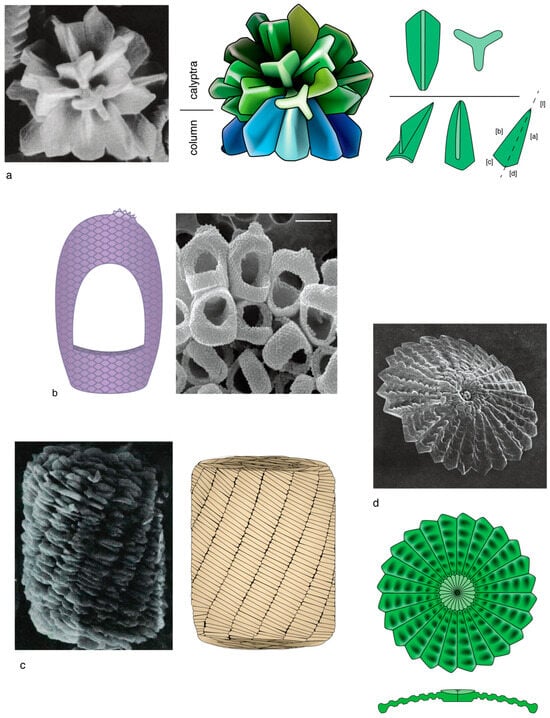
Figure 1.
Methodology followed for graphic representation of calcareous nannoplankton. (a) SEM illustration of a well preserved sphenolith (left) and graphic representation (right) with details of the symmetrical trihedral elements which form the proximal structural unit (see Section 3.1.1; blue, technical term is column), and the symmetrical and asymmetrical trihedral elements of the distal part (green, technical term is calyptra). (b) SEM illustration of the holococcoliths of Syracosphaera arethusae (right) and graphic representation (left). Attention is paid to the pavage fabric and ornamental details, such as the tiny distal crest. Holococcoliths are generally difficult to represent graphically. (c) SEM illustration of Nannoconus (left) and graphic representation (right) highlighting the organization in segments formed by twin lamellae. (d) SEM illustration of the distal face of a well preserved discoaster (above) and graphic illustration (below) highlighting the radially arranged wedge-shaped elements and the concentric patterns of shallow depressions; the cross-section clarifies the morphology and structure. Most illustrations in this work are carefully drawn graphic representations. SEM illustrations in this figure are from (a) [31], (pl. 19, Figure 11), as permitted by the Deep Sea Drilling Project; (b) [32] (p. 161, Figure 99B), with permission from the editors of Scientia Marina; (c) [33] (pl. 27, Figure 1), as available in [20]; (d) [34] (pl. 14, Figure 6), as permitted by the Deep Sea Drilling Project.
2.2.2. Verified and Inferred Life Cycles
The documentation of life cycles is dependent on observations in cultures when one generation replaces the other [35,36], and the fortuitous recovery in the wild of combination coccospheres, i.e., exceptional coccospheres consisting half of holococcoliths and half of heterococcoliths, which are thought to represent the critical moments of syngamy and/or meiosis [37,38].
Although twining is limited, it becomes possible to draw reliable inferences as to the likely taxonomic position of an isolated holococcolith when enough pairings are known for a given rank above the species level [19]. Therefore, once the holococcolith diversity has been described, categorized, and appropriately integrated into the formal heterococcolith-based taxonomy at the rank of genus or higher, important questions may be asked, such as whether the products of biomineralization in the two life phases are dissimilar in every way or whether commonality and even congruity can be found at some level.
2.2.3. Preservation and Species Concepts
The state of preservation is a constant concern in interpreting nannofossils. Dissolution is easily identified, but overgrowth can be deceiving. This problem is acute for Discoasterales heterococcoliths. These are strongly sensitive to overgrowth, with reshaping increasing with increasing age, often due to pressure from overlying sediments in most settings. This results in the loss of the delicate original features on individual elements, as can be seen by a comparison with exceptionally well-preserved specimens [18,39]. Parts can also be lost through dissolution, and sometimes they are merged. This has led to the characterization of the heterococcoliths in this order as heavily calcified coccoliths, when in fact they are lightweight structures with features as delicate as those seen in the present, living plankton. Although possibly not as acute, a similar problem concerns other skeletons, such as that of Nannoconus. It may be noted that the expression “heavily calcified coccolith” is ambiguous, as it can also mean large coccoliths among living species.
Biological and paleontological concepts of nannoplankton species are quite different. The paleontological concept is limited to coccolith morphotypes and ignores the complications found in living coccospheres (Appendix A) such as dimorphism and polymorphism (the differentiation of coccoliths around the cell, usually at the flagellar pole; Appendix A, Figure 4) and dithecatism (the double-layering of coccospheres; Appendix A, Figures 5 and 7). However, this study is mostly concerned with macroevolution, i.e., with taxonomic entities above the species level, and there are little differences between paleontological and biological concepts at the ranks of genus and order.
2.3. Chronostratigraphic Framework
The chronostratigraphic framework and the ages of the chronostratigraphic boundaries are those found in the International Chronostratigraphic Chart [40]. The chronostratigraphic ranges of the taxa discussed here are compiled from different sources [13,14,15].
3. Results
3.1. Background
3.1.1. Classification
In the Tree of Life, the calcareous nannoplankton belong to the Haptista Supergroup [41,42], and the class of Prymnesiophyceae Casper ex Hibberd 1976 [43] (or the class Coccolithophyceae Rothmaler 1951 [44] depending on authors’ preferences), but they are casually referred to as haptophytes for their special, flagella-like appendage (haptonema) (Figure 2). Ribosomal DNA phylogenies have indicated that the class of Prymnesiophyceae comprises several clades labelled A to E [45,46].
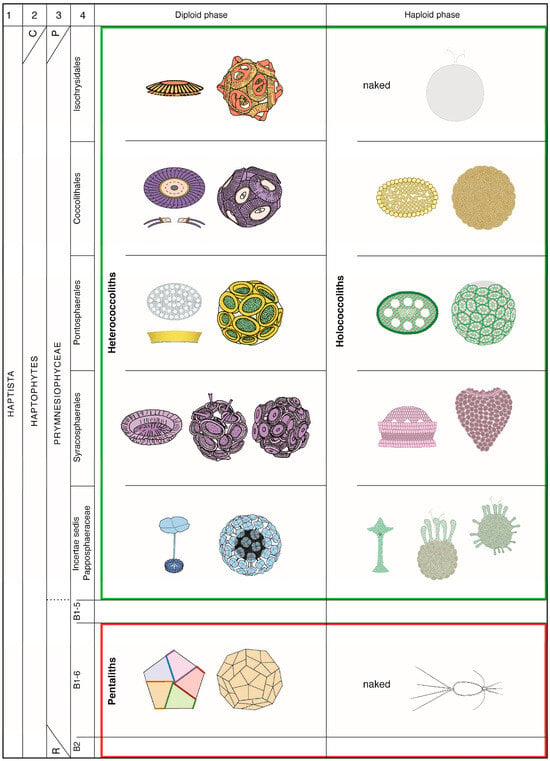
Figure 2.
Taxonomic classification and biomineralization types in the living nannoplankton in relation to the life cycles. Green box: orders forming the coccolithophores; red box: the order Braarudosphaerales. This order was introduced [24] to account for the special construction of the individual skeletal pieces in Braarudosphaera. It is shown here as belonging to clade B1-6 of Edvardsen et al. [46] to which clades B2 and B1-5 also belong. The concept of the order Braarudosphaeales applies well, also, to the fossil record. Cavalier-Smith et al., 2015 [41] include the haptophytes and the Centrohelida in the Haptista (C in column 2); the haptophytes include the Coccolithophyceae, Pavlovophyceae, and Rappephyceae [47] (P and R in column 3, respectively). Diversity among living coccolithophores is greater than that represented by the four currently accepted orders, but molecular data lack on several taxa, preventing an integral taxonomic classification. The family Papposphaeraceae Tangen 1972 [48] symbolizes in this figure the unclassified coccolithophores. The full names of the species cited in this work and authorship of the families and orders can be found online [20]. Coccoliths and coccospheres are modified from Figures 12 and 13 in [48], plates 5–7 in [49] and plate 9 in [50]. All other drawings are from [19,24]. The same color schemes characterize the taxonomic orders throughout this paper (see caption of Figure 3).
Living species of clade C are known as coccolithophores for the tiny calcitic platelets (coccoliths) with which they surround their cell to form a coccosphere (Appendix A). These are divided among four orders although not all coccolithophores have been sequenced. Molecular biology has helped clarify taxonomic relationships between living species, but it does not apply to the fossil record. The taxonomic classification of extinct species relies on the construction (or structure) of their coccoliths at the rank of genus and above, and on their morphology at the rank of species. Because coccoliths are inherent to species and their lineages, classifications of the living coccolithophores based on molecular biology, on the one hand, and skeletal construction, on the other, are congruous, validating a structural approach to their study as followed here.
Eight orders of Cenozoic Coccolithophores are confidently distinguished, all of which span the Cretaceous/Paleogene (K/P) boundary, having their roots deep in the Triassic, Jurassic, or Early Cretaceous (Figure 3). The distinctive criteria and taxonomic contents of each are given in [51]. This classification incorporates the recent discoveries that the orders Syracosphaerales and Isochrysidales secreted coccoliths during the Mesozoic Era [52,53,54], and the demonstration that the family Polycyclolithaceae Forchheimer, 1972 [55] belongs to the order Discoasterales [21].
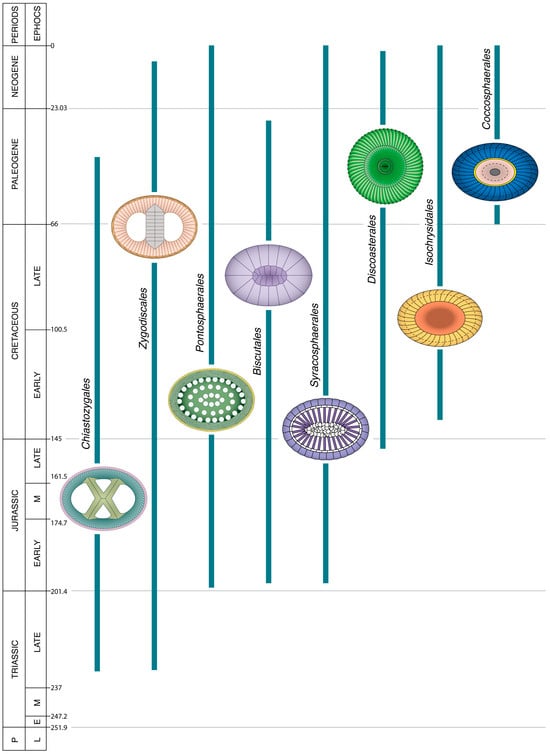
Figure 3.
Temporal range of the orders of coccolithophores that spanned the K/P boundary (66 Ma). A coccolith symbolizes each order; the number next to it is that of the genera in the order. The taxonomy of the genera restricted to the Mesozoic is insufficiently stable to be considered in this work. Unless given, the full names of the species and genera cited in this work can be found at [20]. Numerical ages (in Ma) of chronostratigraphic boundaries are given to the right of chronostratigraphic column. Coccoliths are not shown at scale. Each order is characterized by a set of colors which differentiate the structural components of its coccoliths.
Following Edvardsen et al. [46], it appeared that clade B1-6 also included organisms presently capable of secreting a skeleton of calcium carbonate. The Maximum Likelihood Phylogenetic trees (PhyML), based on18S rDNA and 18S rRNA gene sequences [56,57], respectively, showed the location of B. bigelowii in clade B of the Prymnesiales. This placement was very attractive on structural, biological, and physiological grounds. The structure of the Braarudosphaera liths differ profoundly from that of coccoliths, justifying their inclusion among nannoliths [20]. Unlike coccoliths, there is evidence that they are calcified outside the cell membrane [11], and the putative life cycle of Braarudosphaera is quite unlike that of a coccolithophore [56]. Their symbiosis with a diazotrophic bacteria [56,57] is also unknown among coccolithophores. A more recent PhyML tree based on 18S rRNA gene sequences has placed the family Braarudosphaeraceae in the Calcihaptophycidae, that is outside the Prymnesiales [58]. However, these authors recognize that “its placement in the Haptophyte tree is uncertain and changes depending on the analysis, and may also fall within Prymnesiales” (p. 86). This is where it is provisionally placed in this study (Figure 2).
Braarudosphaera bigelowii possesses an external, composite, mineralized skeleton but with an arrangement of the crystals not seen in the coccolithophores; the order Braarudosphaerales was introduced in recognition of this difference [24]. As for the coccolithophores, the skeleton structure is the direct means of identifying extinct taxa related to Braarudosphaera. The order contains three Cenozoic and 15 Mesozoic genera, four of which span the K/P boundary [22] (Figure 4). The Mesozoic genera have been previously allocated to different orders and families of the coccolithophores [59], although their mineralized skeletons are Braarudosphaera-like, not coccolith-like. In this study, to highlight this contrast for clarity, the vernacular name micalith (from L., grain) unites all skeletons secreted by species of the order Braarudosphaerales.
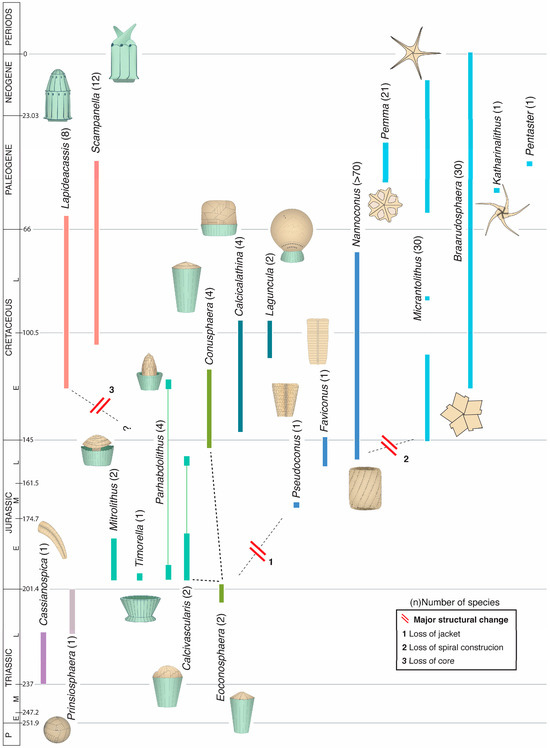
Figure 4.
Temporal range of the genera of the order Braarudosphaerales. Each genus is symbolized by a micalith; the number next to the genus name indicates the number of species in it. Dashed lines delineate tentative clades. The different colors used for stratigraphic ranges are indicative of as many phylogenetic pools. Cassianospica is tentatively included here for its resemblance with Favoconus. The term “homococcolith” was introduced to describe the structure of the skeletal pieces of the genus Braarudosphaera when the latter was still incorporated among the coccolithophores; the ability to secrete homococcoliths was the defining character of the order Braarudosphaerales. Recognition that this order may not belong to the coccolithophores makes “homococcolith” a misnomer and “micalith” is substituted for it. The description of the micalith applies not only to Braarudosphaera but to many Mesozoic nannofossils currently assigned to diverse Mesozoic families of coccolithophores (Appendix B). Numerical ages (in Ma) of chronostratigraphic boundaries are given to the right of the chronostratigraphic column. Micaliths are not shown at scale.
3.1.2. Biology
The most remarkable character of the coccolithophores is their extreme heteromorphic haploid–diploid life cycle (sensu [60]), marked by strikingly different coccoliths alternatively secreted by the haploid and diploid cells of the same species [37]. Although the two types are intracellular calcifications [9]. they differ in both shape and construction. In addition, the haploid cells, which are naked in the order Isochrysidales, are flagellate, whereas the diploid cells may be motile or non-motile depending on the taxonomic order to which they belong (Figure 5A,B). In many taxa, size differences between the smaller haploid and the larger diploid cells are matched by size differences between their coccoliths.
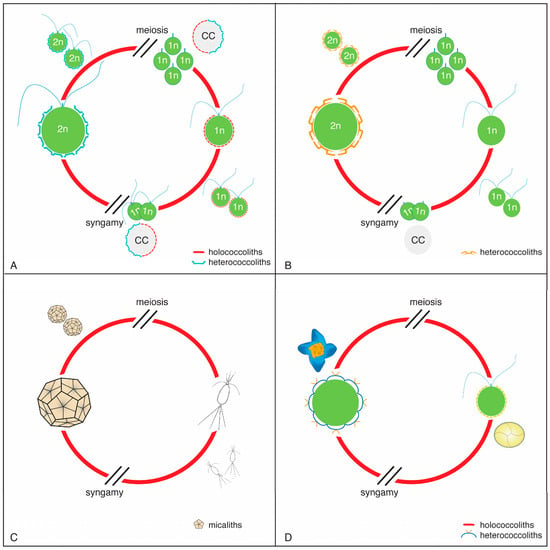
Figure 5.
Life cycles among the nannoplankton. (A) Coccolithophore with calcifying diploid and haploid phases; (B) Coccolithophore with calcifying diploid phase only; (C) Life cycle in Braarudosphaera as a model for all micalithophores; the alternative phase of Braarudosphaera (=Chrysochromulina parkeae) was identified through molecular biology [56]. (D) Example of an inferred life cycle in the order Discoasterales (see Section 4.1.2).
In living species, the diploid cells calcify heterococcoliths and the haploid cells calcify holococcoliths, and there is no reason to suspect that these associations would have been different in the past. The structure of a coccolith is thus a marker of a phase of the life cycle of the species it belongs to. The heterococcolith–holococcolith life associations are known for many living species, although not for all, with inequal representation among taxonomic entities.
Calcification in the coccolithophores is an intricate process which requires complex cellular structures and integrated chemical reactions prior to the formation of the coccoliths in a Golgi-derived compartment. It involves a complex of genes, large organic molecules, including CAPs (coccolith associated polysaccharides) and proteins that are specific of the haptophytes [9,61,62,63,64,65]. The CAPs also control the growth of the elements of the heterococcoliths, which is mediated by the presence of silica in some living species [9,65].
The living Braarudosphaera also exhibits an extreme heteromorphic life cycle (Figure 5C), from which it may be inferred that all Mesozoic and Cenozoic micalithophores had a heteromorphic life cycle. Calcification was probably restricted to the diploid phase alone, considering that no unusual nannofossils occur in micalith-rich sediments. Calcification in living Braarudosphaera bigelowii is likely external to the cell [11]. In it, each micalith (known as a pentalith) lies on an organic substrate and is coated by an organic layer. The five components of each pentalith are likewise coated. By inference, calcification would also likely have been extracellular in other micalithophores. This is supported by the Nannoconus micasphere being several folds larger than the cell in some species (Appendix C, Figure 5) and recent research on this genus [66].
3.1.3. Fossil Record
The coccolithophores have left a continuous stratigraphic record stretching back to the Late Triassic (Norian) [67] (Figure 3), with their first diversification spanning much of the Early Jurassic (~200 Ma to 180 Ma). They underwent a mass extinction at the K/P boundary but rebounded rapidly during the Early Paleocene. As a measure of their biological importance, their numbers are estimated at ~4000 Mesozoic species (~237 Ma to 66 Ma), and >1200 species in 180 genera for the Cenozoic (66 Ma to 0 Ma) [20]. More than 200 species are alive. By far, the diploid phase of the coccolithophores is better represented in the fossil record than the haploid phase. Their calcification being a complex, strictly controlled process, the heterococcoliths help restore the evolutionary history of the coccolithophores, not only from the standpoint of phylogenetic relationships but from that of macroevolutionary processes such as evolutionary trends [51,59,68,69].
The micalithophores also extend back to the Triassic (Figure 4). Unlike the coccolithophores which have held a rather consistent presence throughout the Mesozoic and Cenozoic eras, the micalithophores were most abundant and diversified during the Mesozoic, with only two of their fifteen genera spanning the K/P boundary and three more genera evolving thereafter (Figure 4). Diversity among the >200 species varies considerably, from a few species (≤4) in many genera to many species (>70) in Nannoconus. Their abundance in sediments also varies considerably from a few, geographically restricted specimens for some genera to massive, rock-forming occurrences for others, a capability that began as early as the Late Triassic (Rhaetian, ~208.7 Ma) [70].
3.2. Characterization of Heterococcoliths, Holococcoliths, and Micaliths
A discussion of biomineralization in the nannoplankton requires an analysis of three distinct categories of skeletons, each representing specific structural characters, and implying both a taxonomic specificity and a biological position. The category heterococcolith is associated with the diploid phase of coccolithophores; the category holococcolith is associated with their haploid phase; and the category micalith is associated with the diploid phase in the micalithophores (i.e., the order Braarudosphaerales). Each term refers to a specific construction, that is the organization of crystals into skeletal pieces. Although these have been amply illustrated, they have been objects of different interpretations. The terminology used here is described below.
3.2.1. Heterococcoliths
Heterococcoliths (Figure 6) typically exhibit a radial symmetry and possess a three-tiered organization, from elements to cycles to structural units.
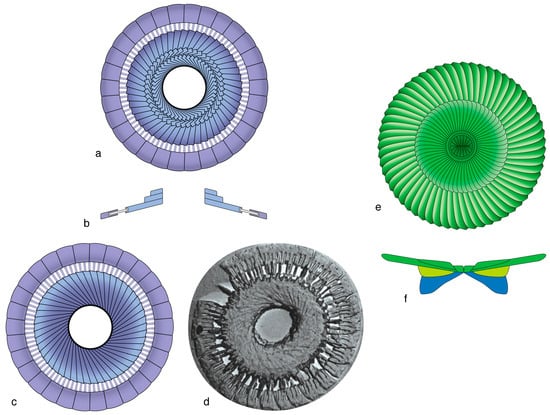
Figure 6.
Elements arranged in cycles and structural units in heterococcoliths. (a–d) Graphic representation of the base of a Blackites spinosus coccolith seen in distal (a), side (b), and proximal (c) view. (d) Electron micrograph of the proximal face. The coccolith consists of five cycles with elements of distinct size, shape, and orientation of the sutures. Note the interlocking of the elements of the outer two cycles in (d), each being an SU, and the imbrication of the elements of the three inner cycles which form a single SU. (e,f) Graphic representation of the distal face and cross-section of a coccolith in the order Discoasterales; the polycyclic distal SU (e) is underlain by two distinct SUs (f). The characteristic symmetry of these heterococcoliths is modified in extremely derived coccoliths, as, for instance, the polar appendages of the coccosphere in the genus Ophiaster [71]. (d) is from [72] (pl. 45, Figure 6), with permission from the Royal Danish Academy of Sciences and Letters and the author Katharina Perch-Nielsen Van Salis, coccoliths. Colors identify SUs.
All elements are strongly modified rhombohedrons of calcite. Their morphologies are remarkably diverse, from flat crystals with diverse shapes and sizes, to short or elongated triangular wedges, to short or long cylindrical rods (Figure 3 and Figure 6; Appendix A). The most elaborate elements may be those in the order Discoasterales, which sports trihedral (triades), fluted, and other intricate wedge-shaped elements, some with double bifurcations, others folded in various ways (Figure 7).
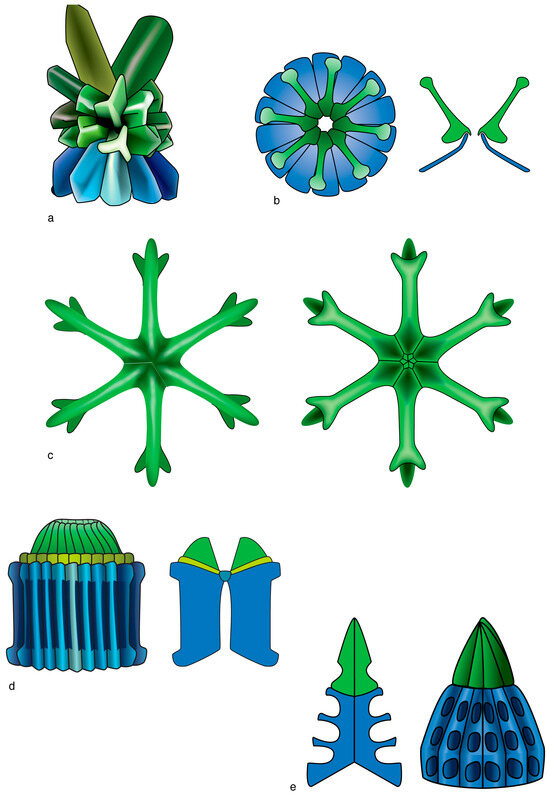
Figure 7.
Graphic representation of some of the morphological diversity of the heterococcoliths in the order Discoasterales. (a) Side view of a sphenolith (right) with details of the symmetrical trihedral elements which form the proximal end (SU technically named column [blue], see Figure 1), the symmetrical trihedral and asymmetrical trihedral elements which form the distal end (technically named calyptra [green]). (b) Distal face (above) and longitudinal section (below) of a coccolith with two thin, truncated conical SUs. (c) A discoaster. Note the differences between the proximal (right) and distal (left) faces concerning the shapes of the bifurcations, location of the sutures, and presence of knobs and depressions on the central disk. (d) Side view and longitudinal section of a fasciculith. Note the fluted elements of the column and the presence of an intermediate monocyclic SU (collaret [yellow]) between the column and the calyptra. (e) A fasciculith without collaret; note its conical outline, the short calyptra, the conical column with deep alveolar pattern; right image: side view; left image: longitudinal section across areas with depressions. Drawings from [18]. Colors identify homologous SUs: blue for the column; green for the calyptra (see Figure 1). (a) Sphenolithus multispinatus; (b) Ilselithina fusa; (c) Eudiscoaster surculus; (d) Lithoptychius ulii; (e) Fasciculithus alanii. Coccoliths are not shown at scale.
Cycles, which are elliptical, circular, or sometimes spiral, result from the repetitive arrangement of identical elements. Structural units (SU) consist of one or more cycles (Figure 6 and Figure 7). In a monocyclic SU, the sole cycle differs from those in adjacent SUs by one or more of the following characters: morphology of the elements, orientation of their sutures (straight, curved, radial, clockwise, anticlockwise), imbrication (dextral/sinistral) or a lack thereof, and crystallography (orientation of the c-axis to the major axes of the elements). In a polycyclic SU, all cycles are alike, albeit with centripetally decreasing size.
The SUs are the fundamental component of heterococcoliths, which support phylogenetic reconstructions in deep time, and for this reason it is practical to name them in the same way as anatomical features of multicellular eukaryotes are named (see Figure 1). The number of SUs in a heterococcolith commonly varies between one and four.
In most heterococcoliths, the primary construction is based on a concentric organization of the SUs, which creates a distinction between a marginal and a central area along a sharp contact across which a change in orientation of the elements occurs (Figure 8a–d). Such construction has been well-documented, and it has been at the origin of the R/V model [73,74,75,76]. With a few exceptions, heterococcoliths with concentric geometry underwent greater morphological (evolutionary) changes in the central area than in the margin, and their overall construction and appearance changed little through time, from the ancestral form to the last descendants. This applies to the heterococcolith-type called placolith (e.g., [30]) and also to the orders Zygodiscales and Chiastozygales.
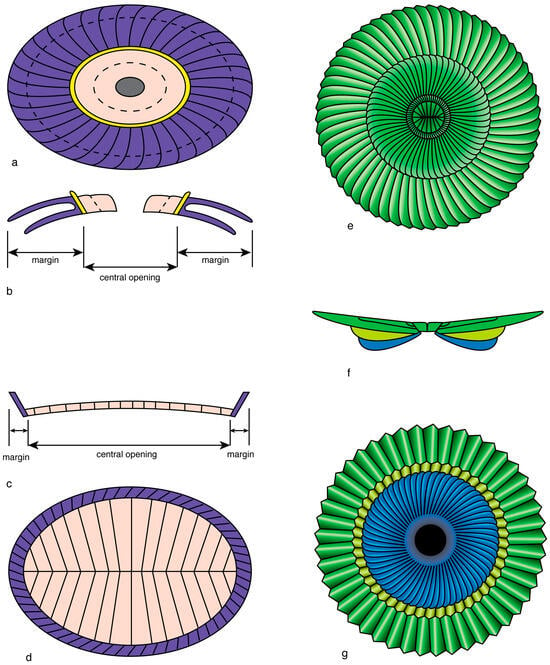
Figure 8.
Heterococcolith geometries. (a–d) Concentric configuration; note the arrangement of the SUs along a horizonal axis and the distinction of a margin (purple) and a central area (pink and yellow); (a,b) placolith; (c,d) representative of many other coccoliths; (e–g) superposition geometry as seen in a Discoasterales coccolith of Heliotrochus; blue is column, yellow green is intermediate cycle; green is calyptra. Note the vertical arrangement of the structural units. (a,e) Distal faces; (b,c,f) cross-sections; (d,g) proximal faces.
An alternative to primary concentric geometry in heterococcoliths is superposition geometry, which is developed in the order Discoasterales, resulting in a primary vertical arrangement of the SUs rather than a primary lateral configuration (Figure 8e–g). The SUs are superposed along a vertical axis, following a probable repositioning of the ancestral central SU above the ancestral marginal SU [18].
Primary concentric configuration does not preclude superposed geometry of specific SUs in some heterococcoliths (Section 3.4.1).
Heterococcolith-bearing coccospheres are as diverse as heterococcoliths are. Body and circum-flagellar coccoliths (that form a protective crown at the flagellar pole of the cell) differ generally little from one another and belong to the same layer (theca) around the cell. In dithecate coccospheres, the coccoliths of the endo- and exotheca can be very different (Appendix A, Figures 4 and 7). A single cell in some species is thus able to secrete three different types of coccoliths dedicated to specific locations while participating in the assembly of a coccosphere. The intracellular modalities that allow the diploid cell to switch from one coccolith type to another are currently unknown. Heterococcolith-bearing coccospheres have a weak fossil record, except those with interlocking coccoliths.
3.2.2. Holococcoliths
Most holococcoliths consist of plain rhombohedra of calcite (crystallites) that are linearly or concentrically arranged side-by-side or tip-to-tip to form specific patterns described as fabrics [19,77] (Figure 9). Fourteen fabrics can be differentiated among the living species, half of them being possibly secondary. Fabrics are rarely preserved in fossil holococcoliths due to strong overgrowth or recrystallization.
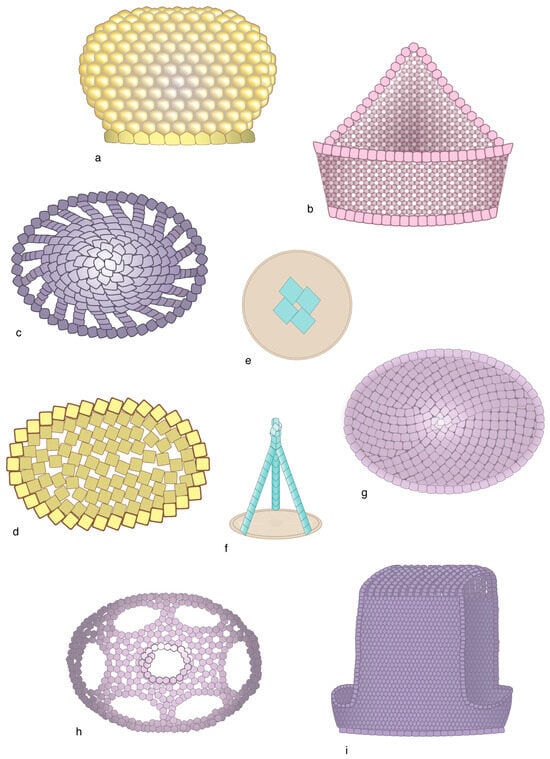
Figure 9.
Examples of fabrics created by the coccolithophores during the haploid phase. (a) Framboidal fabric; (b) Rosette; (c) cobblestone; (d) lacy; (e) tetrad of rhombohedra at the center of the basal plate; (f) Tipi-like construction with three struts; (g) dallage; (h) crochet; (i) cuspate pavage and filigrane (at top). Checkerboard (en damier), mosaic, and smooth pavage are illustrated in Figure 10 and Figure 13, respectively. Drawings from [19]. (a) Noelasphaera Aubry 2022 [19]; (b) Coronosphaera binodata, phase gracillima CFC; (c) Deutschlandia] sp. 2 Aubry 2022 [19], phase periperforata; (d) Coccolithus pelagicus, phase borealis; (e) Quaternariella obscura; (f) Wigwamma holococcolith; (g) Sphaerocalyptrina adenensis; (h) Cyrtosphaera aculeata, phase heimdaliae; (i) Poritectolithus maximus. Coccoliths are not shown at scale.
Holococcoliths vary in the degree of structural complexity [19]. While the simplest are no more than a few rhombohedra lying on an organic plate (Figure 9e), most comprise a rim surrounding a central field (Figure 10). The rim may consist of one or several superposed rings of crystallites, and the central field of one or several layers of fabric. The layer(s) may be continuous or interrupted by perforations, which, when large, are delineated by strands of fabric. Holococcoliths are often ornamented with single rows of crystallites forming tiny crests or ridges, or with a small heap of crystallites forming protuberances and pompoms (Figure 1). These ornamentations are taxon-specific, and they constitute a guide to regroup holococcoliths suspected to belong to a haploid–diploid unit of a given taxonomic rank [19]. No fossil holococcolith-bearing coccosphere has been illustrated to date.
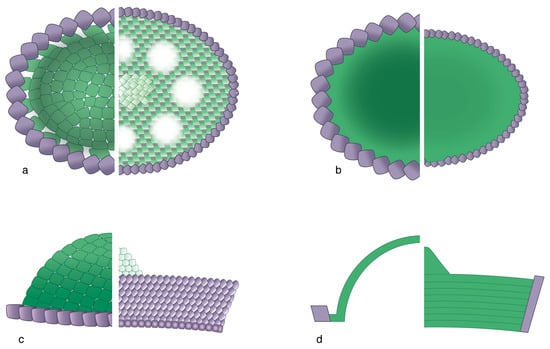
Figure 10.
Basic structure of holococcoliths. Each figure shows, on the left side, half of a body of a coccolith of Deutschlandia molischii, phase fragaria, and on the right ride, half of a body of a coccolith of Helicosphaera carteri, phase confusus. The holococcolith of the species molischii consists of a single, distally inflated sheet of crystallites stretched across a central field. Note the cobblestone fabric. The central field of the holococcolith of H. carteri is circumscribed by a tall rim and filled with superposed sheets of crystallites. Note the en damier fabric. Purple: rim; green: central field. (a) Distal face; (b) proximal face; (c) side view; (d) longitudinal section. For simplicity, the perforations in H. carteri, phase confusus in (a) are not shown in (b) and (d).
The different associations of shapes, structures, and fabrics result in an impressive morphological diversity among extant holococcoliths, which is amplified by the diversity of coccospheres, generally with numerous coccoliths [19]. The latter may be contiguous, leave interstitial spaces, or overlap; in rare cases, they are interlocking. Coccospheres with coccoliths arranged in a hexagonal pattern are tidy, whereas others appear disorderly. Dimorphism also occurs in some taxa, with circum-flagellar coccoliths differentiated from body coccoliths, often possessing different fabrics. On the other hand, dithecatism is unknown, suggesting that a haploid cell does not have the ability to secrete more than two coccolith types.
3.2.3. Micaliths
Micaliths exhibit a morphological diversity (Figure 4) that conceals a remarkable structural unity. They may be conical, spherical, plainly cylindrical with circular or pentagonal sections, or developed into large distal spines; they may flare distally and be almost cavate or filled with a sizeable protrusion. Regardless of this and the large size differences (3–30 µm), they all consist of identical adjoining segments that are stacks of lamellae of a similar shape [22] (Figure 11). In several micaliths, the segments are elongated and occupy equal sectors of the (sub)circular transversal section, which confers to them a rotational symmetry. In a few, the segments are arranged spirally, and in one genus they are arranged tangentially (Figure 12). The lamellae are mostly plain (e.g., triangular, quadrangular, trapezoidal). They may be stacked perpendicular to the vertical axis of the micalith (Braarudosphaera), oblique to it (e.g., Eoconusphaera), or inclined along the spirals (Nannoconus) (Figure 11 and Figure 12d–f). In Nannoconus, the lamellae are twinned [66] (Figure 12d).
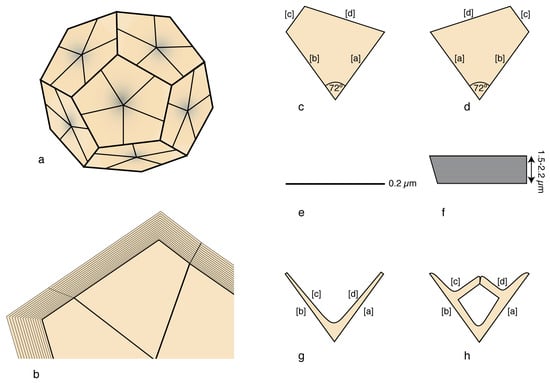
Figure 11.
Lamella are organized in segments in selected micaspheres; (a–f) Braarudosphaera bigelowii. The stacked laminae are very thin, trapezoidal, and with consistent peripheral truncations; (a) micasphere; (b) detail of the side of a micalith; (c–h): individual lamellae; note the consistent 72° angle between the internal sides [a] and [b] and the asymmetrical truncature between peripheral sides [c] and [d]. (a,c) distal face; (b,d) proximal face; (e) single lamella; (f) stack of lamellae; (g,h) distal face of laminae in the Micrantholithus and Pemma genera. Illustrations are modified from Aubry [24].
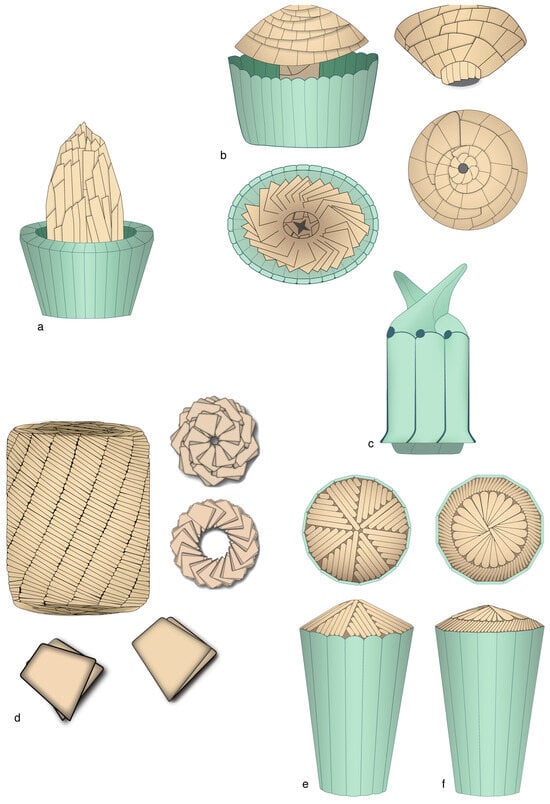
Figure 12.
Morphology and structure of selected micaliths. (a) Parhadolithus: side view. (b) Mitrolithus: upper left: side view with bulging core slightly detached from the jacket-made basket; upper right: detached core seen in side view; the proximal end of the core is not shown; low right: proximal face of the core; lower left: distal face of the bottom of the basket showing the basal segments of the core and the central point of attachment. Note the arrangement of the lamella. (c) Scampanella: side view. (d) Nannoconus: upper left: side view; note the spirally arranged segments; upper right: distal (above) and proximal (below) face; lower figure: twinned lamella of which the micaliths are formed (details redrawn from David Bord, prepared for INA14, Reston, Virginia). (e) Eoconusphaera: side view (below) and distal face (above). (f) Conusphaera: side view (below) and distal face (above); note the rotational symmetry in (e,f). As in other figures in this paper, the drawings attempt at synthesizing morphological and structural information from published SEM illustrations. The inner view of the Mitrolithus elegans basket (in (a)) was inspired by the well-preserved specimen labelled JRYSEPM-178-32.JPG [20]. The micaliths are not shown at scale.
Micaliths fall into three groups (Figure 4 and Figure 12; Appendix B). In one group, the segments form a central core surrounded by a characteristic layer of thin, vertical, adjoining laths forming a palisade-like structure, or jacket. In a second group, micaliths do not possess a jacket and the core is exposed. In a third group, the highly derived micaliths possess the jacket alone.
The micasphere of the extant Braarudosphaera is well known, and several fossil specimens have also been illustrated (Appendix C, Figures 1–3). Late Jurassic micaspheres of the genus Conusphaera consist of a thin layer of micaliths (3–8 µm) surrounding a large cell (15–25 µm) (Appendix C, Figure 4); by contrast, thick micaspheres enclose a tiny central cell in the Early Cretaceous species of Nannoconus (Appendix C, Figure 5); it may be inferred that their prominent central canal, whether thin or inflated into a cavity, was impregnated with organic matter, as in pentaliths. Micaspheres do not show evidence of dimorphism, and dithecatism is unexpected considering the shapes of the micaliths. They strongly suggest an external process of calcification (hence reinforcing their classification here).
3.2.4. Summary
Holococcoliths stand out by their construction and cannot be confused with either heterococcoliths or micaliths. They differ from heterococcoliths in two notable ways. First, they are collectively smaller. Among living species, their size is 2 µm on average, does not exceed 4 µm, and may be as small as 0.4 µm. Many heterococcoliths at present are more than 4 times larger. Among extinct species, the size ranges between 2 and 8 µm, although it may reach up to 17 and 18 µm in the Early Eocene and Late Cretaceous taxa, respectively, when heterococcoliths were also larger [23,69]. Second, and more importantly, holococcoliths do not have the three-tiered organized cyclic structure of heterococcoliths.
Micaliths and heterococcoliths share the same size range (~2 to 30 µm). Aside from this, there is little similarity between the two groups, except among the Early Jurassic taxa when morphological convergence is pervasive. The general morphologies are similar, but the individual crystalline components are different. On the one hand, the ubiquitous lamella of the micaliths show moderate morphological variation; on the other hand, there are the universal, highly diverse elements of the heterococcoliths. The outstanding difference, however, concerns the construction, which is two-tiered in micaliths (lamella–segment) but three-tiered in heterococcoliths (element–cycle–structural unit). Micaliths result from the consistent repetition of segments; heterococcoliths consist of the symmetrical arrangement of differentiated SUs. The former have a rotational or spiral symmetry, the latter a radial symmetry.
3.3. Biomineralization in Haploid Versus Diploid Phase of Coccolithophores
The overall morphological and structural differences between heterococcoliths and holococcoliths can be thought of as a measure of the gap in the capabilities of, respectively, the haploid and diploid coccolithophore cells in producing a mineralized skeleton. At face value, the evidence is that the doubling of the ploidy level results in far more complex coccoliths characterized by a cyclic, three-tiered organization of transformed rhombohedrons compared to the coccoliths produced by cells with half the ploidy level and most often consisting of a single layer of rhombohedra arranged in repetitive patterns across a central field delineated by a single ring. Additionally, during the diploid phase cells may form dithecate coccospheres, and the exococcoliths may be extremely derived [71].
It is not sufficient, however, to compare the bulk of heterococcoliths to the bulk of holococcoliths. A comprehensive comparison of biomineralization in haploid and diploid conditions begins with documentation of the holococcoliths and heterococcoliths produced by individual species during their life cycle, and the results contradict the sustained belief that morphological convergence is pervasive among holococcoliths [78,79].
3.3.1. Consistency Between the Two Phases
Contrary to the first impression of a muddled multitude among holococcoliths, there is a rational organization to their diversity, which matches in several ways that found in heterococcoliths.
The twinning of holococcolith and heterococcolith may concern, all at once, the morphology, structure, and fabric of the holococcoliths, and the characters of the coccospheres. This is observed in the order Pontosphaerales. The holococcoliths of its four genera consist of a compact rim of several superposed rings (each one crystal thick), and a central field with superposed layers of fabric interrupted (or not) by perforations, the shorter distal layers forming a rounded or digited central protuberance. The elaborate fabric, typically en damier or its variants, is reminiscent of the structure of the distal layer of the heterococcoliths. The coccospheres are monothecate and monomorphic, two characters also exhibited by the heterococcolith-bearing coccospheres (diploid phases) of the same four genera (Figure 13).
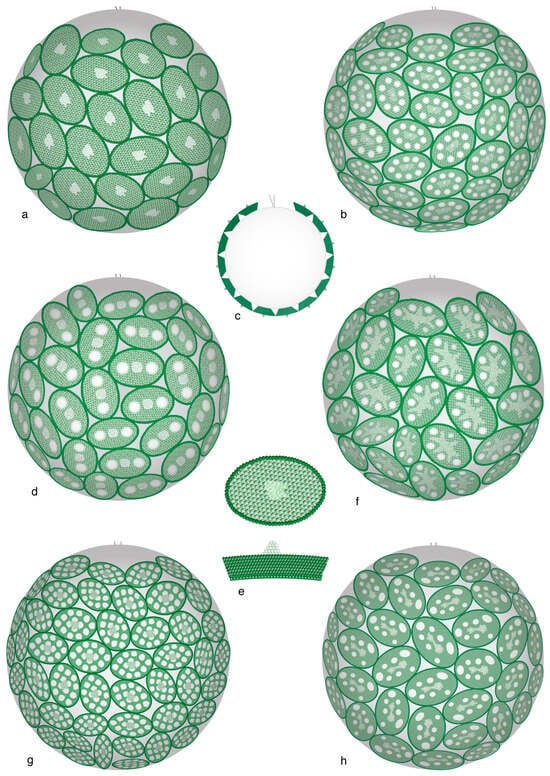
Figure 13.
Monothecate and monomorphic coccospheres of the haploid phase in the order Pontosphaerales. Note the close similarities between these coccospheres. (a–f) Genus Helicosphaera; (g) Genus Scyphosphaera; (h) Genus Pontosphaera. (c) cross-section of a coccosphere; (f,e) holococcolith: distal face (above), side view (below). (a,e) Helicosphaera carteri, phase catillifera; (b) H. carteri, phase confusa. (c,d) H. wallichii, phase ponticulifera. (f) H. pavimentum, phase dalmatica. (g) Scyphosphaera apsteinii, phase schilleri. (h) Pontosphaera japonica, phase japonica. Drawings are from [19].
Similarly, the lightly calcified holococcoliths in the two genera of the family Papposphaeraceae consist of en mosaic fabric, with minute triangular or rhombohedral crystallites depending on the genus, the crystallites being in point contacts at their apices. The coccospheres are dimorphic in the genus with dimorphic heterococcolith-bearing coccospheres, and conversely for the other genus (Figure 14).
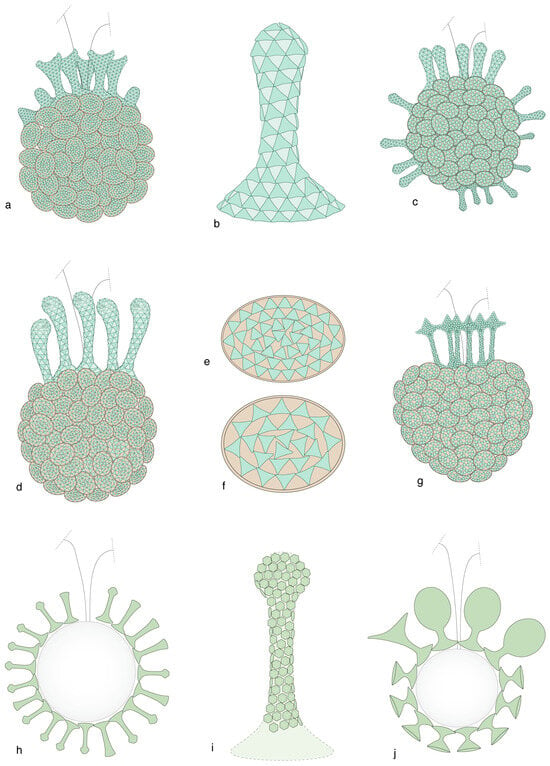
Figure 14.
Coccospheres in the family Papposphaeraceae. The relation between haploid and diploid phases is so strong in this family that the haploid phase is assigned to the relevant genus even when the diploid phase has not been identified (as indicated by “sp.” in the species name). (a–g) Genus Pappomonas. (h–j) Genus Papposphaera. (a,c,d,g) Dimorphic to polymorphic coccospheres with circum-flagellar tubular coccoliths (b, i = pyrgoliths) and elliptical body coccoliths (e,f). Note the triangular en mosaic fabric. (h,j) Cross-sections of coccospheres with tubular coccoliths only. (i) Tubular coccolith with rhomboidal mosaic fabric. (a,e) Pappomonas borealis, phase diskoensis. (b,c) Pappomonas garrisonii, phase Trigonapsis sp. A. (d,f) Pappomonas sp., phase minutissima. (g) Pappomonas sp. phase melvillea. (h,i) Papposphaera sarion, phase sarion. (j) Papposphaera sp., phase polybotrys. Coccospheres are not shown at scale.
3.3.2. Correlation of Characters
The complementarity between holococcoliths and heterococcoliths is not always as narrow as in the above two cases. However, although with a few exceptions, some relations are constant across taxa. The character, monomorphic, dimorphic, or varimorphic, of coccospheres is generally the same for both phases in any taxon. Other characters are fleeting. En rosette, and en pavage fabrics are not taxonomically restricted, and seem to be randomly distributed among species assigned here to the family Deutschlandiaceae of the order Syracosphaerales, although more broadly placed in the family Syracosphaeraceae following [80]. However, in this family, a very specific correlation of characters occurs between the circum-flagellar holococcoliths of the haploid phases and the exothecal heterococcoliths of the diploid phases, and this occurs regardless of the morphology/structure of the body coccoliths (Figure 15). These correlations involve highly distinctive coccoliths in both phases, which are located at different loci on the coccospheres, and with the holococcoliths obligatory (always present on the coccosphere) but the exothecal heterococcoliths optional (not always present on the coccosphere). The central field of the elliptical holococcoliths is spanned by a transversal, leaf-like, double-blade distal expansion aligned with the transversal axis of the coccolith and perpendicular to its distal surface [19]. The thin, circular, or elliptical heterococcoliths consist of three cycles of elements [71]. The two coccolith types are so characteristic that two names, dorolith and planolith, single them out. The dorolith–planolith correlation is strong, predictable (Figure 16), and independent of any pre-existing taxonomic framework. Similar correlations of characters occur in other genera of the order Syracosphaerales.
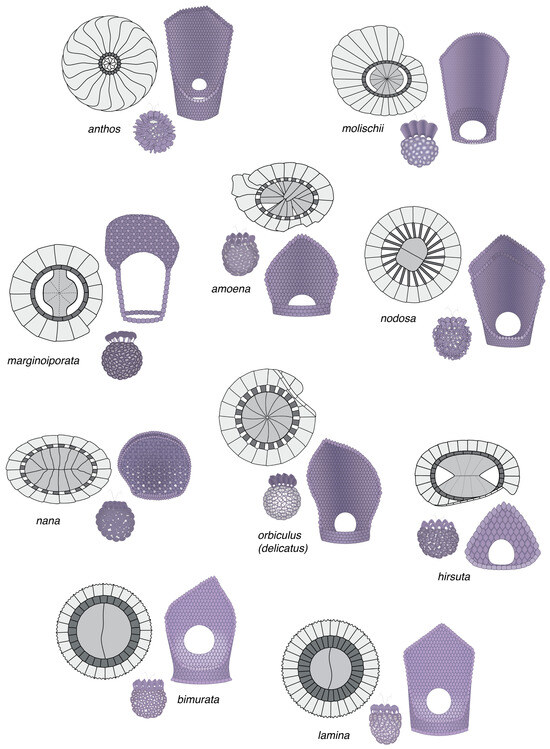
Figure 15.
Correlation between the circum-flagellar holococcoliths (doroliths) and the heterococcoliths (planoliths) of the exotheca in the family Deutchlandiaceae. These are documented associations through the recovery of combination coccospheres in the ocean. For each species, the side view of the holococcolith is shown to the right, the distal view of the heterococcolith to the left, and the holococcolith-bearing coccosphere (side view) in between. The species may be assigned to the genus Deutschlandia (species anthos through hirsuta) and Alveosphaera (incorrectly assigned to the family Calciosoleniaceae). Coccoliths and coccospheres are not shown at scale.

Figure 16.
Unpaired hetero- and holococcoliths (above and below, respectively) inferred to belong to the same family. Their simultaneous documentation here is intended to invite scrutiny for combination of coccospheres in communities rich in one of these coccoliths. Coccoliths and coccospheres are not shown at scale.
3.3.3. Structural Imprint
Intimate relationships between haploid and diploid phases in the same taxonomic unit occur in a form indicative of a structural imprint.
A remarkable example of imprint is found among coccoliths of the families Rhabdosphaeraceae and Deutschlandiaceae (order Syracosphaerales). The crystallites in the central field of the holococcoliths and the elements in the central area of the heterococcoliths of both families are organized along curved lines, but the curvature is dextral in the family Rhabdosphaeraceae and sinistral in the family Deutschlandiaceae (Figure 17). Because of this, morphologically similar holococcoliths that have not been paired with a heterococcolith, may be confidently assigned to one family or the other, depending on the orientation of its crystallites.
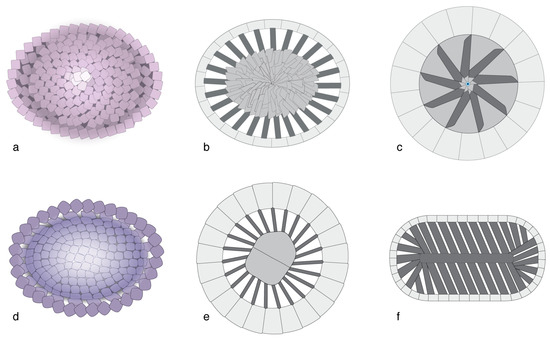
Figure 17.
Structural imprint in haploid and diploid phases in the order Syracosphaerales. (a,d) holococcoliths; (b,c,e,f) heterococcoliths. (a–c) Family Rhabdosphaeraceae. (d–f) Family Deutschlandiaceae. Note the dextral orientation of the elements in the former family, and their sinistral orientation in the latter family. (a,b) Body coccoliths of the haploid (a) and diploid (b) phases of Acanthoica quattrospina. (c) Body coccolith, diploid phase of Rhabdosphaera xiphos. (d) Body coccoliths of Deutschlandia molischii, phase fragaria. (e) Exococcoliths of D. nodosa. (f) Endococcoliths of Calciopappus caudatus. Coccoliths are not shown at scale.
Structural imprint can also be seen in coccospheres of the family Alisphaeraceae. The coccoliths of both phases are arranged in the same short spiral arrangement of the Fibonacci sequence (Figure 18).
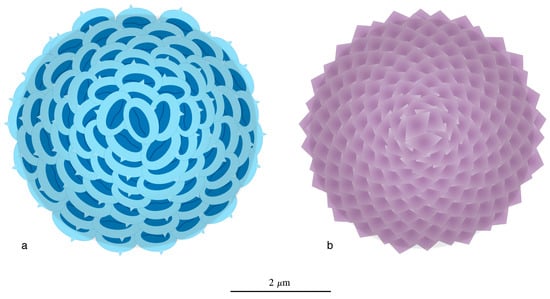
Figure 18.
(a,b) Coccospheres of the diploid and haploid phases in the species Alisphaera exhibiting the short spiral arrangement of the Fibonacci sequence. The haploid cells of Alisphaera secrete aragonitic coccospheres [81,82].
3.4. Intracellular Versus Extracellular Biomineralization: Geometric Constraints
While heterococcoliths and micaliths are both produced by diploid cells, the locus of mineralization is intracellular in the former and (likely) extracellular in the latter (Section 3.1.2). Thus, the marked difference between these two calcitic entities most likely reflects different degrees of genetic control on biomineralization by cells which are otherwise of a similar size and share the same planktonic habitat. Intracellular genetic control on biomineralization leads to complex, highly differentiated skeletons with radial symmetry and with interlocking cycles; extracellular biomineralization results in simpler skeletons, mostly with rotational or spiral symmetry, consisting of lamella inferred to have been held by organic matter. The process of calcification in the micalithophores has not yet been elucidated and the intensity of the genetic control is unknown [11]; it is notable that the basic lamella of the micaliths is more akin to the platy crystals of calcium carbonate in the shells of mollusks than to the elements of heterococcoliths.
Regardless of these profound differences, micaliths and heterococcoliths exhibit diversification patterns that are strikingly similar with respect to construction, whether at the generic level in multiple orders of coccolithophores, or in terms of species richness in the micaliths.
3.4.1. Constraint of Geometry on Diversification
Heterococcoliths
As explained above (Section 3.2.1), the heterococcoliths of most orders (extinct and alive) exhibit a concentric geometry. An exception concerns the order Discoasterales in which the heterococcoliths have a superposition geometry.
The array of morphological diversity (or disparity) in the order Discoasterales is considerably larger than in other orders, to the point that it can be difficult to relate the crown taxa of the Cenozoic to the stem taxa of the Mesozoic. The order includes 15 Mesozoic and 16 Cenozoic genera, which is twice as much as the generic diversity in orders with concentric geometry (Figure 3), and their Cenozoic species richness has been nearly half the species richness of Cenozoic nannoplankton communities at low latitude [51].
The greater generic diversity among the Discoasterales compared to other orders is readily explained by the difference in primary geometry. Concentric geometry (Figure 8a,b) places many constraints on the shape of each SU of a heterococcolith, whether the SU belongs to the margin or the central area; this considerably limits the potential for morphological innovation during diversification, and most taxonomic orders are restricted to a single or a few closely related morphologies. For instance, the Coccolithales, Biscutales, and Isochrysidales orders comprise placoliths only. In contrast to concentric geometry, superposition geometry (Figure 8e–g) places few constraints on the morphological evolution of any individual SU; whether proximal and distal, all SUs can expand vertically or laterally independently from one another; new cycles may be added; others may disappear; and an SU may disappear entirely (Figure 19). While concentric geometry has produced a single morphology—placolith—for three main orders, superposition geometry has produced at least six main morphologies in the order Discoasterales (Figure 19).
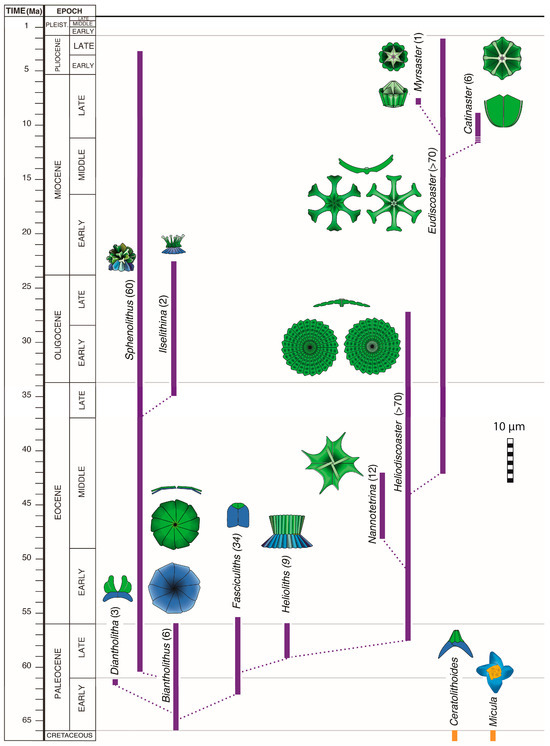
Figure 19.
Simplified Cenozoic history of morphological diversification in the order Discoasterales during the Cenozoic. A simple form (Biantholithus) comprising two superposed disc-shaped SUs (proximal column and distal calyptra) is at the origin of the remarkable morphological diversity in the order, with cylindrical, diabolo-, rosette-, spine-, and star-shaped coccoliths, all resulting from differentiation of the same two SUs. The temporal range of each morphology as symbolized by a coccolith is shown, with either the name of the corresponding genus or of the morphological group. Colors highlight the homologous SUs, with the column (blue) and the calyptra (green). Note the loss of the column in the earliest Eocene, and the decrease in surface area of the calyptra that began in the Miocene. The sequential Pliocene occurrences of delicate hexa-, penta-, tetra-, and triradial morphologies of Eudiscoaster are illustrated in Figure 3 of [83]. The Mesozoic history of the order is quite different and described elsewhere; it is alluded to by the inclusion of two Late Cretaceous genera.
A combination of primary concentric and secondary superposition geometry increases the potential for diversification. In the order Pontosphaerales, the heterococcoliths exhibit a primary concentric geometry and a secondary superposition geometry. A marginal cycle (flange) surrounds a central area occupied proximally by a basal plate. Both SUs are covered by a thin distal layer (blanket) of concentrically arranged elements. Helicosphaera differs from Pontosphaera by the spiral flange, and this is at the origin of some diversity. However, much greater morphological diversity is achieved in the genus Scyphosphaera by the unconstrained flaring of the blanket to produce bowl, amphora-, and other vase-shaped coccoliths (Figure 20).
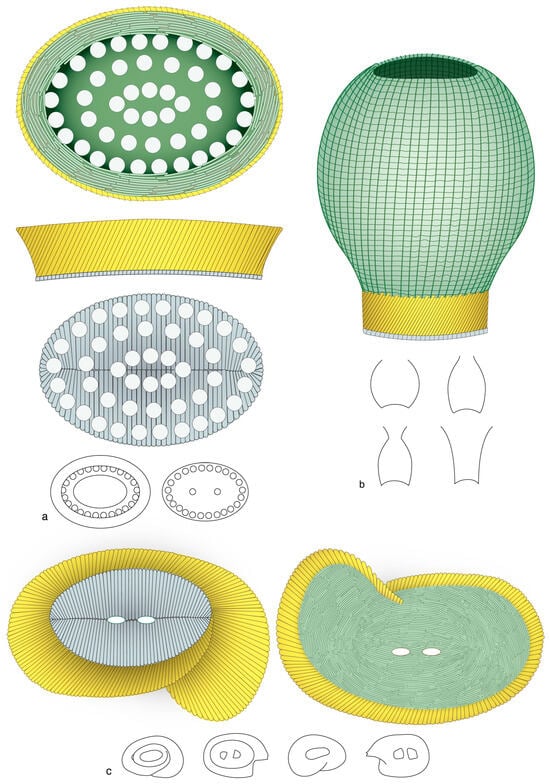
Figure 20.
Present generic diversity in the order Pontosphaerales. The coccoliths in this order exhibit a primary concentric configuration with a well-defined margin and central area, as seen on the proximal side; the former is formed by the “flange” (gold), the latter is occupied by the “basal plate” (grayish blue). Flat elements in rows form the distal “blanket” (green). The flange is elliptical, low and/or slightly flaring in Pontosphaera (a) and Scyphosphaera (b), and helicoidal in Helicosphaera (c). The greatest morphological diversity (silhouettes) is achieved in Scyphosphaera in which the blanket flares distally.
Micaliths
Differences in the species richness among micaliths is also a function of construction (Table 1). The eight genera with both core and jacket have very low diversity, none with more than four species, and their micaliths are short and narrow (≤6 µm). The two genera with a jacket have a slightly higher diversity (up to 12 species), but their micaliths are substantially larger (10–12 µm high on average, although up to 30 µm, and 5–8 µm wide). Diversity in the ten genera without jacket can be very high, with a record of >70 species in Nannoconus. To interpret these data, it is important to note that diversity is not correlated with longevity. Also, possible disagreements on species discrimination and synonymies cannot account for a >15-fold difference in the species richness between micaliths with core alone and micaliths with core and jacket or jacket alone.

Table 1.
Relation between diversity (# spp.) and structure among the micalithophore genera. The estimated maximum life span (LS) in millions of years (Myr) of each genus is given for comparison. Cassianospica and Prinsiosphaera are included here for information on longevity. The structure of Cassianospica is not well documented, and there is a question whether Prinsiosphaera possessed an external envelope (possibly homologous to the jacket [84]). Species number and life spans are from [20]. Bold highlights the lack of correlation between species richness and longevity.
Diversity among micaliths appears to have been determined by the space available for morphological differentiation. The presence of a jacket was limitative, allowing only for small changes to the core, such as modification of the shape of the lamellae and their organization; in one group, the lengths of the core and jacket appeared to be correlated to one another so that the jacket did not expand distally beyond the core. The loss of the core permitted the jacket to expand, resulting in larger micaliths with two or three tiers of laths and the addition of distal spines. However, a much greater diversity resulted from the loss of the jacket, a loss that allowed vertical and lateral expansion of the segments, either radially (Micrantholithus, Braarudosphaera) or spirally (Nannoconus). For instance, the pentaradial segments attained diameters of 30 µm, five times larger than a maximum of 6 µm in the micaliths with core and jacket.
3.4.2. Diversification and Extinction
Order Discoasterales
Half of the eight orders that span the K/P boundary have become extinct (Figure 3). The orders Chiastozygales, Zygodiscales, and Biscutales have remained little diversified during the Cenozoic, but the order Discoasterales became the most diversified of all Cenozoic coccolithophores. The order appeared in the Late Jurassic to Early Cretaceous [21,28]. In maintaining itself for ~130 Myr, it survived the K/P boundary mass extinction event (66 Ma) and re-diversified for another 64 Myr, despite the global warming at the Paleocene/Eocene boundary (56 Ma), and the global cooling at the Eocene/Oligocene boundary (33.7 Ma). It did not survive the initiation of glaciation in the northern hemisphere (2.6 Ma), becoming extinct at ~2.2 Ma (Figure 19). What caused its extinction?
The typical Late Cretaceous Discoasterales species became extinct at 66 Ma, but a form (Biantholithus sparsus) with heterococcoliths simply consisting of a monocyclic proximal SU overlain by a monocyclic distal SU (column and calyptra, respectively) appeared in the earliest Paleocene (Figure 19). The column was directly inherited from the Mesozoic ancestors, and the calyptra most likely evolved from their “diaphragm” [18,28]. Biantholithus soon evolved into a flurry of elaborate forms with prominent columns and subdued calyptra, a diversification event known as the radiation of the fasciculiths [30,39,85]. Further diversification led to a greater development of the calyptra coincident with a reduction of the column until its loss ~10 Myr after the appearance of Biantholithus. Discoasterales coccoliths thereafter consisted of the calyptra (=distal unit) alone (Figure 19). Its subsequent modifications led to a wholesale diversification marked by the rise of five genera, three of which were short-lived. However, the diversification process in the two long ranging and prolific genera (Heliodiscoaster and Eudiscoaster) was accompanied by a thinning of the rays and a general trend towards a reduction of their number from more than sixty in the Late Paleocene to three in the Late Pliocene, when the order became extinct).
This record may be interpreted as an evolutionary failure of the Discoasterales resulting from their inability to ultimately respond morphologically to environmental forcing due to extreme stretching of their adaptive possibilities. Reduction to a single monocyclic structural unit, however broad its potential for diversification originally was, and a trend towards a decreasing number of participating elements, placed an upper limit on longevity. There was no more avenue for diversification of a radial structure beyond the triradial symmetry.
Nannoconus
Nannoconus spans the Tithonian to Campanian interval (>75 Myr; ~149 Ma to 72 Ma) but the bulk of the species spanned a much shorter interval (36 Myr) of the Early Cretaceous. The morphological diversity of its micaliths is enormous. They differ by size (5–30 µm), shape (e.g., cylindrical, hemispherical, piriform, conical, distally flaring, bulbous at one end, constricted at mid height, etc.), diameter of the proximal and distal openings, size of the axial canal or cavity, and the variable arrangement of the coupled lamella that spiral around it.
Although the number of described species has almost doubled, the description of the diversification patterns in Nannoconus by Perch-Nielsen [13] (Figure 21) holds. The Tithonian appearance of the genus involved elongated micaliths with a narrow axial canal and, in some, a basal cavity. The Berriasian–Hauterivian interval was marked by the appearance of forms of diverse shape with a large central cavity. These were replaced in the middle of the Aptian by much smaller, short-lived forms, also with a large central cavity. By the end of the Aptian, diversity was suddenly reduced to cylindrical forms with a broad, parallel-sided canal. Only seven long-ranging species reached the end of the Campanian without new innovations, except for constrictions in the species multicadus. By the end of the Aptian (113 Ma), Nannoconus had reached its diversity potential.
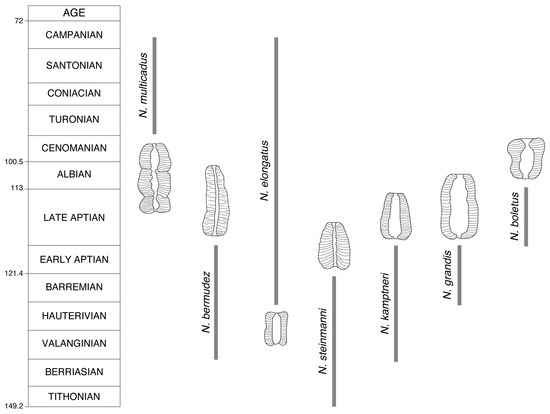
Figure 21.
Cretaceous diversification in Nannoconus. This simplified sketch outlines the main morphological changes that Nannoconus micaliths underwent through the Cretaceous, as summarized in Figure 44 in Perch-Nielsen [13]. Each evolutionary step is symbolized by a micalith redrawn from that figure (with permission from the author).
4. Discussion
The objective of this paper is to compare the products of biomineralization in the nannoplankton under three different circumstances relative to (1) the ploidy (haploid/diploid) level of cells, and (2) the loci (intra/extracellular) of mineralization. The data presented above may not appear, at first glance, to overlap because I have turned to living organisms to address the first topic, and to the fossil record to engage in the second one. In fact, this approach allows for a discussion at the intersection between biology and paleobiology, where one discipline informs the other. I discuss below some of the implications of the findings above. I begin with those that affect the living nannoplankton in the short term, and extend the discussion to those with long-term impact on macroevolutionary patterns. I then offer insights on the evolution of biomineralization in the calcareous nannoplankton.
4.1. Patterns in Life Cycle of Coccolithophores
4.1.1. Holococcoliths and Heterococcoliths Are Homologous
Although morphology and structure are much simpler in holococcoliths than in heterococcoliths, the transgenerational correlation of characters implies that biomineralization in both phases are under the same genetic control. This is conformed with the fact that mineralization in both coccolithophore phases is intracellular and follows the same two-step process up to the time of nucleation of an initial rhomb. In the haploid phase, the initial rhombs become the typical crystallites of which holococcoliths are formed, whereas in the diploid phase the initial rhombs grow into the elements characteristic of heterococcoliths [9].
This implies that the rim and margin, on the one hand, and the central field and central area, on the other hand, of a holococcolith and a heterococcolith are homologous characters, respectively, although the rim does not develop into the well differentiated margin of the heterococcolith. As a first approximation, this may be taken as an indication of the different biomineralization potential of a haploid versus a diploid phase. Two copies of the same genes produce much more complex coccoliths than a single copy. However, is it possible that ecological pressure has stabilized the evolutive potential of the haploid phase? Coccolithophores favor shallow oligotrophic waters during the haploid phase, and deeper waters during the diploid phase (e.g., [86]), and the simpler holococcolith structure may be an adaptive response to ever changing hydrographic conditions (light intensity, temperature, turbulence, low nutrient conditions).
4.1.2. Inferring Life Cycles Among Extinct Taxa from Structural Imprint
The chances of encountering combination coccospheres to determine the life cycles of extinct taxa are next to nil. However, it would be well worth attempting to determine the life cycles of extinct species, if only to reconstitute the dynamics of past communities. Matching stratigraphic ranges of holococcoliths and heterococcoliths would seem a logical approach to this end, but for unknown reasons (mostly preservation?), such attempts have not been successful.
Structural imprint as described above among living coccolithophores constitute another potential pathway to unite extinct phases. Structural imprint would not lead to the identification of the life cycles of individual extinct species, but it would allow us to associate holococcoliths and heterococcoliths in the same genus or family. Determining the taxonomic order to which a holococcolith belongs would be a notable improvement to the current situation in which the two phases remain isolated from one another.
Based on structural imprint, it is likely that the Late Cretaceous holococcoliths of the Calculites, Orastrum, and Oweina genera belonged to the order Discoasterales. The central field of these elliptical coccoliths with a narrow rim comprising crystallites segregated into four, six, or more blocks, with sutures oriented clockwise on the concave (i.e., proximal) side of the coccoliths, and anticlockwise on their convex (i.e., distal) side (Figure 22). Such a structure is unknown in living coccolithophores, which excludes assignment of these holococcoliths to one of the extant orders. One of the main diagnostic characters of the Discoasterales heterococcoliths is the orientation of the sutures between elements, anticlockwise on the distal face, clockwise on the proximal face (Figure 22) [18,39]. Structural imprint in the form of the orientation of sutures thus allows us to recreate quite confidently what a life cycle among the Late Cretaceous Discoasterales might have been (Figure 5D).
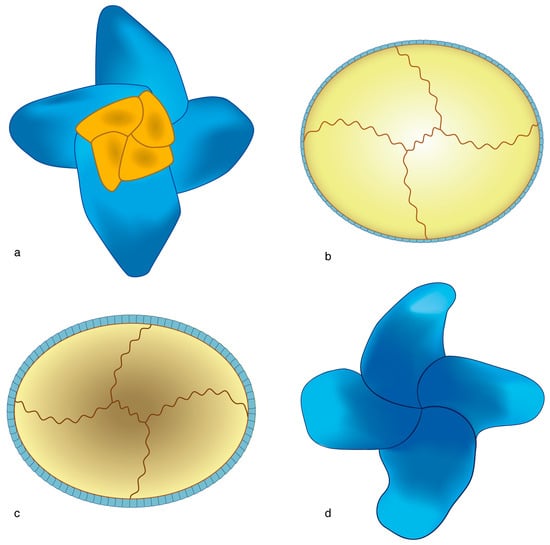
Figure 22.
Life cycles in extinct taxa as inferred from structural imprint. (a,d) Micula murus (heterococcolith); (b,c) Calculites or Orastrum (holococcoliths); (a,b) distal face; (c,d) proximal face. Note the matching orientation of the sutures on the respective faces of the hetero- and holococcoliths. A reconstruction of the life cycle is proposed in Figure 5D. The coccoliths are not shown at scale.
4.1.3. Correlation of Characters
The correlation of characters described here between the haploid and diploid phases of the family Deutschlandiaceae was unexpected and it may generate interest as a biological problem. Regardless, it has important implications for taxonomic discrimination within the currently broadly conceived family Syracosphaeraceae. Four consistent circum-flagellar holococcolith and exococcolith associations may be distinguished in the latter [19], justifying my use of the genus Deutschlandia [20] in this work. This complex problem is beyond the objectives of this paper.
4.2. Patterns in Deep Time
4.2.1. Longevity, Success, Evolvability, and Extinction
The differences in longevity and species richness in the fossil record have been central to paleobiology since the early 1970s [87,88]. It affects all eukaryotes, at all taxonomic levels through geological history [89].
Diversity patterns of Cenozoic coccolithophores are strong and reliable (1) because of their abundant and continuous stratigraphic records documented through many different regions of the world (Section 2.1), and (2) because their taxonomy at the genus level and above is essentially stable. Thus, the most striking feature is arguably that diversity and longevity at the family and ordinal levels are not correlated in any group [51].
I have shown above that there is greater morphological diversity in the order Discoasterales than in any other order of the coccolithophores, and in the genus Nannoconus than in any other genus of the order Braarudosphaerales, which are related to geometric configuration, i.e., superposition geometry instead of concentric geometry in the first group, spiral geometry instead of rotative or sprial geometry in the second. I have also shown that, while both geometry types promoted diversification by weakening the constraints on morphology, they also hastened the extinction of taxa by leading to evolutionary dead ends, as seen in the order Discoasterales. The acquisition of superposition geometry leading to a successful clade occurred only once in the history of the coccolithophores from a yet indeterminate ancestor. This suggests a strong genetic control for maintaining concentric geometry in coccoliths, and there is no better example of a successful and stable coccolith-type than placolith. In this type, the margin consists of two superposed shields which together constitute the most stable SU in all coccolithophores, and it has been shown that the positioning of the elements in Gephyrocapsa is achieved with great precision [90]. For example, the genus Watnaueria dominated Mesozoic communities for over 100 Myr [91], the order Biscutales maintained itself for >150 Myr, and the order Coccolithales existed through the 66 Myr of the Cenozoic, while the family Noelarhabdaceae dominated Cenozoic communities at mid latitudes from the Early Eocene (~52 Ma [92]).
An understanding of the processes at play in controlling shape/configuration at the cellular/genetic level will require broad scientific expertise. As a first approximation, morphology at the deep level (=concentric versus superposition geometry) may be at the interplay between structural and regulatory genes. A unique set of mutations may have allowed a successful new geometric configuration, at the same time possibly weakening the role of regulatory genes on morphology. The new configuration would have conferred an enhanced diversification potential, or evolvability, to the stem species endowed with it [93].
Evolvability is a determinant factor in the ability of a taxon to survive major events in Earth’s history, such as great mass extinctions; for the nannoplankton this means the capability to survive, for example, the K/P boundary events. The current view is that most of the Mesozoic species became extinct at 66 Ma, with new Cenozoic lineages evolving from a few, tiny, r-selected surviving species [94,95,96], and most Mesozoic families of coccolithophores became extinct [59], prompting a profound turnover. However, the measurement of extinction is highly dependent on high order classification, and there has not been agreement among specialists on classification of the nannoplankton since the first comprehensive framework [97]. Notably, the classifications published before the discovery of a bolide impact at the K/P, and its dramatic consequences for life [98], encompass both the Mesozoic and Cenozoic taxa, whereas subsequent classifications [14,15,99] treat them separately, and find no Mesozoic ancestors for an order as prominent as the order Discoasterales, although Prins [28] soundly illustrated the deep ancestry of this order. As I have discussed in Section 4.2.1, the order had reached a diversification dead end by ~2.2 Ma, when its phenotypic evolvability had been exhausted. This example suggests that the vulnerability of taxa to mass extinction depends on their evolvability, which in turn may explain the taxonomic selectivity associated with these events. The role of biology in micropaleontological research tends to be ignored to the sole benefit of abiotic factors controlling evolutionary patterns [91,96,100,101,102], although the possibility of intervening biotic factors is advanced or affirmed [103,104].
4.2.2. Discontinuous Occurrences
The stratigraphic ranges of many micalithophore genera are discontinuous, although phylogenetically related taxa have coeval records (Figure 4). An intriguing situation concerns the occurrences of Eoconusphaera zlambachensis from the Rhaetian to the mid-Sinemurian (~218.4 Ma to 195 Ma; based on [15]) and Conusphaera mexicana from the Tithonian (~147 Ma to 144 Ma [15]). Both can be very abundant where they occur. Morphological and structural evidence indicate a close filiation (Figure 12e,f). They are both in the shape of elongated cones; the inner cores are similarly constructed, although with double the number of segments in the older species, and the addition of an outer core of obliquely arranged lamella in the younger species. The structural evidence indicates that these two taxa and Calcivascularis jansae (Figure 3) from the upper Sinemurian to the lower Toarcian (~197 Ma to 182 Ma [15,105] most likely belonged to the same genetic pool. Eoconusphaera zlambachensis and Calcivascularis jansae overlapped in time, but there are no comparable forms in the 45 Myr that separate Conusphaera mexicana from them.
There is no fossil record of the Eoconusphaera–Conusphaera transition, although there is strong evidence of (linear or otherwise) phenotypic evolution over >45 Myr, leading to the possibility that the genetic make-up of these taxa continued to evolve in the absence of mineralization. It is unclear when Conusphaera appeared; and the mechanism that triggered mineralization to resume massively after a several million year interruption needs determination.
4.3. Origin of Biomineralization in the Haptophytes
The origin of calcification in the haptophytes has been discussed from the understanding that the coccolithophores are a monophyletic group [5,73,106]. The removal of the order Braarudosphaerales from them brings a new perspective on the origin of calcification in the haptophytes. The taxonomic position of Braarudosphaera in the molecular tree is currently unresolved (Section 3.1.1); regardless, the biology, physiology, and skeletal structure (compare Figure 3 and Figure 4) profoundly distinguishes this organism from the coccolithophores. It is thus possible that calcification in the haptophytes evolved independently in the two groups.
The molecular and fossil evidence for the origin of calcification in the haptophytes yields incongruent data (Figure 23). The broadly accepted molecular estimate is the Late Carboniferous, between 329 and 291 Ma [106], revised to between 323.2 and 298.9 Ma, based on [40]. There is currently no direct molecular evidence on the timing of the ability of micalithophores to calcify, because the taxonomic sample in [106] did not include Braarudosphaera bigelowii. The much younger fossil evidence is limited by poor preservation, and also by the difficulty to interpret tiny, isolated fossils as possible stem taxa in the light of more derived forms.
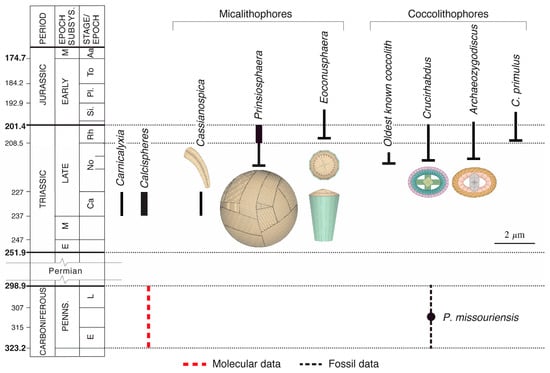
Figure 23.
Origin of biomineralization in the coccolithophores. Data from [67,70,106,107,108,109,110]. Note the abundance of Prinsiosphaera in the Rhaetian and calcispheres in the Carnian (as indicated by thicker black lines symbolizing the ranges of the taxa). Dotted lines indicate uncertain age in selected temporal interval.
4.3.1. Paleozoic Records
Citations of nannofossils in Paleozoic rocks have been dismissed on the grounds of poor preservation and contamination [59]. However, is it possible that a nannofossil, described from the Pennsylvanian (Upper Carboniferous; 323–299 Ma) of Kansas enlightens the origin of calcification in the haptophytes, thereby reconciling molecular and fossil records? Two specimens of Paleococcolithus missouriensis have been described as “anomalous coccolith-like objects, 3–3.5 µm in diameter and constructed of radially arranged, lath-like elements” [109] (Appendix E). Their chemical composition is unknown, although their occurrence in a CaCO3 only limestone suggests calcite. Preservation is not perfect, but the regular shape of most laths and their flattened terminations indicate a biological origin and minimal overgrowth. These fossils are very different from, for instance, the recrystallized calcispheres of the Tethyan Triassic [110]. A possible interpretation is that, rather than isolated skeletal components, these are whole calcified coccosphere-like skeletons. Their tiny size and that of individual components (0.7 µm wide by 0.1 µm long) are compatible with a nannoplankton affiliation. Whether P. missouriensis was an ancestral calcifying haptophyte is not possible to say, but this finding should encourage a systematic search for similar nannofossils in late Paleozoic limestone.
4.3.2. Triassic Records
Norian
The oldest, undoubtful coccolith known to date is from the Late Triassic (middle Norian, Alaunian 3 [67]; ~217.5 Ma to 214 Ma, based on [111]). It is poorly preserved and the number of cycles in it is unclear. However, the slightly younger coccoliths of Archaeozygodiscus koessenensis and Crucirhabdus minutus recorded in the same stratigraphic succession have a well differentiated margin and central area (see Section 3.1.1. for terminology). The transverse bar is divided by a longitudinal suture and forms a bevel in the basal plate of A. koessenensis, which characterizes the order Zygodiscales. With its crossbar spanning the central opening, Crucirhabdus minutus represents a different order and is possibly related to the order Chiastozygales. Classification of these two species differ in [20] but there is agreement that they belong to different orders. In other words, two orders were already differentiated when the first heterococcoliths appeared in the Norian stratigraphic record. This strongly suggests that calcification in the coccolithophores was older than the Norian.
The first coccoliths would have been holococcoliths calcified by both the haploid and diploid cells, and their preservation is unlikely [9,19]. The ability of the diploid cells to calcify heterococcoliths would have required specialized organic baseplate scales and CAPs [9]. The time needed for these developments, that led to extreme heteromorphy, is unknown.
Prinsiosphaera, the oldest micalith, has also been recovered from the middle Norian [67,70], which firmly establishes the occurrence of the micaliths as slightly older than the first known heterococcolith. A report from the Carnian [107] has not been verified. If the tentative taxonomic assignment of Cassianospica is correct, micaliths were present in the Carnian. Nevertheless, there is a >80 Myr gap between the molecular estimate of haptophyte calcification and their oldest continuous sedimentary record.
Rhaetian
It was not until the Rhaetian (208.5–201.4 Ma), that the coccolithophores became more common, increasing in size, and beginning to diversify [15,59]. The micalith Eoconusphaera also diversified [67,107,112]. The striking event of the Rhaetian concerns Prinsiosphaera. Present in low numbers in the middle Norian, it reached rock-forming abundances in the Rhaetian [70]. This was the first haptophyte to exhibit a strategy that would be repeated over time by evolving micalithophores. Their dominance in oceanic and epicontinental communities [113,114,115,116,117,118,119] would be the trademark of the group. Whereas the evidence is that coccolithophores and micalithophores evolved in parallel, single species of micalithophores contributed enormously to marine sedimentation much earlier than the coccolithophores, which did not produce monospecific oozes until the Kimmeridgian [120,121]. The ‘ecological success’ of the micalithophores as early as the Rhaetian may have been due to their symbiotic association with UCYN-A, the unicellular diazotroph cyanobacterium discovered in Braarudosphaera [56,57]. This symbiosis is thought to have been established in the Early Cretaceous when Braarudosphaera evolved [122]. However, it was possibly inherited from the ancestral lineages back to the Triassic (Section 3.1.3). The micalithophores would have considerably contributed to the marine food chain and the progressive enrichment of seawater in accessible sources of nitrogen [56,122], indirectly contributing to the success of the coccolithophores.
The evolution of these two groups was part of a broader diversification of planktonic calcifyers, including the (possibly) dinoflagellate Carnicalyxia [108] and the incertae sedis calcispheres that radiated in the earliest Late Carnian immediately after the Middle Carnian Pluvial Event [110]. Even if Paleococcolithus missouriensis should be among the first haptophytes to have calcified, there is a >80 Myr gap between the molecular estimate of the origin of haptophyte calcification and their oldest continuous sedimentary record, implying that the early history of biocalcification in the plankton may not be documented, although unfolding during a time of major commotion on the planet, including the closure of Pangea [123], the Permian mass extinction [124], the Carnian pluvial event [125], and the large variations in the Mg/Ca ratio induced by changes in the sea floor spreading rates [83].
5. Conclusions
The first objective of this study was to assess the effect of biomineralization in the context of an extreme heteromorphic life cycle. Marked differences between the coccoliths of each phase conceal patterns such as correlative characters and structural imprints that demonstrate the integrity of the coccolithophore cell; this is in accord with the unity of the biomineralization processes in the two phases of the life cycle. The description of the structure of holococcoliths in different families and orders, together with a careful comparison with the structure of the life cycle-associated heterococcoliths, constitute a step forward towards a better understanding of what a haploid–diploid cycle represents for coccolithophores. The second objective of this study was to assess the long-term effect of the locus of biomineralization on evolutionary patterns. It turns out that the geometry of biomineralization plays a determinant role on the species richness and longevity of a clade/order, whether biomineralization occurs inside or outside cells. Phylogenetic studies may consider the potential for modification of the ancestral structure. The history of biomineralization in the calcareous nannoplankton will require, among others, an understanding of the evolvability of individual taxonomic orders. Part of this study relies on the distinction of a specific group of nannoliths, which I have named ‘micaliths’ for the purpose of this project. Represented today by Braarudosphaera, they are seen here as representing a taxonomic entity distinct from the coccolithophores, raising the possibility that calcification in the Haptophytes evolved at least twice.
At the core of this study are remarkable, tiny, single-celled organisms whose complex processes of biomineralization are progressively unraveled, and whose skeletons, embedded in thousand meters-thick sedimentary successions worldwide, can be described crystal-by-crystal for a comprehensive history of calcification. No other organism offers this opportunity, which possibly makes coccolithophores a model for the study of the phylogeny of biomineralization.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
I would like to express my sincere thanks to Y. Dauphin, Muséum national d’Histoire naturelle, Paris, France, for her invitation to contribute the present paper. This paper represents a synthesis of research conducted for the Handbook on Cenozoic calcareous nannoplankton (Micropaleontology Press) and more recently for Coccolithophores (SEPM). My gratitude goes to those with whom I have discussed these works, colleagues, students and reviewers, and in particular Luc Beaufort, David Bord, Rajkumar Chowdhury, and Fabienne Giraud on specific topics addressed here. I thank Luc Beaufort for sharing SEM photographs of living coccolithophores, and the editors who have permitted me to reproduce selected photographs of nannofossils. I acknowledge the generous help of the Rutgers Librarian, Kayo Denda, at Rutgers University. Kyle Liu at the Minerals Editorial Office has been most kind and efficient throughout the preparation of this manuscript. This work would not have been possible without the talent, commitment, and patience of the Graphic Artist, Beatriz Inglessis, who I thank most warmly. I am indebted to Annalisa Eisen and Erik Berggren for their kind support during preparation of this manuscript. I express my deepest gratitude to William A. Berggren. I warmly thank four anonymous reviewers for their thoughtful comments and discussion. This work benefitted from a six-month sabbatical from Rutgers University.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PhyML | Maximum Likelihood Phylogenetic trees |
| SU | Structural unit |
Appendix A
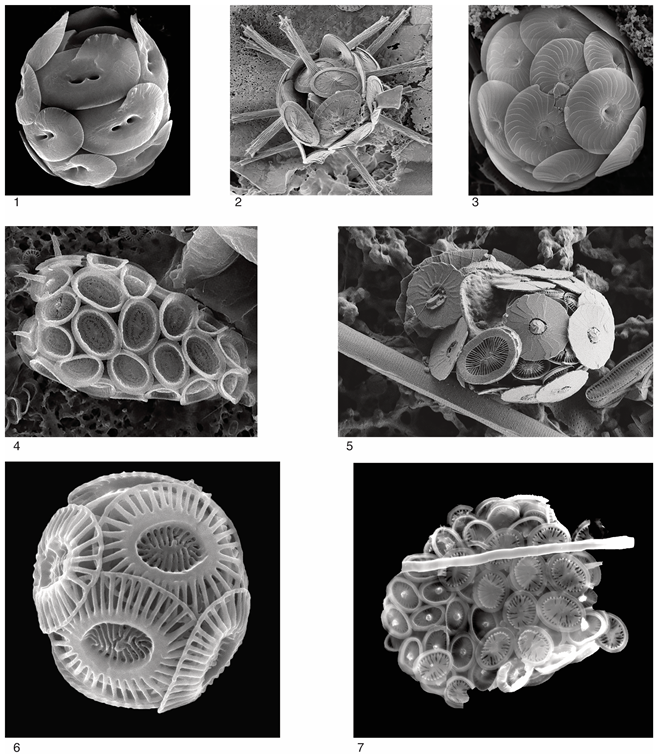
- (1)
- Helicosphaera carteri (Wallich 1877) Kamptner, 1954 [11,126]. See Figure 20. Note the shape of the margin and the flagellar opening
- (2)
- Rhabdosphaera clavigera Murray & Blackman, 1898 [127]. Note the dimorphism, with flat and stem-bearing coccoliths
- (3)
- Calcidiscus leptoporus (Murray & Blackman 1898) Loeblich & Tappan, 1978. [127,128] Coccoliths with a broad margin and a tiny central area
- (4)
- Deutschlandia anthos Lohmann 1912 [129]. Coccosphere partly collapsed with exococcoliths concealing the endococcospheres. A coccolith of Syracosphaera pulchra rests on the bottom left side of the coccosphere.
- (5)
- Syracosphaera prolongata Gran 1912 ex Lohmann, 1913. [130,131] Note the crown of the stem coccoliths at the circum-flagellar pole
- (6)
- Emiliania huxleyi (Lohmann 1902) Hay & Mohler in Hay et al. 1967 [132,133]. Placoliths (see top coccolith is side view) with large margin
- (7)
- Syracosphaera histrica Kamptner, 1941 [134]. Note the dithecatism with an external layer of exococcoliths
All photographs from Luc Beaufort, CEREGE
Appendix B
The micaliths, as a natural group, have not been clearly recognized hitherto, and the taxonomic assignments overlap. The micalith-bearing genera discussed here, as members of a single taxonomic order, are placed by other authors in three different taxonomic orders or are unclassified (Appendix D). However, successive authors have noted characteristics that unify some of them or differentiate them from heterococcoliths. Assignment of Nannoconus to the order Braarudosphaerales Aubry 2013 followed from the revision of the genus Braarudosphaera and related Cenozoic genera [22]. Based on very poor specimens, Grun and Alleman [29] determined a radial arrangement for the laminae of the core of Conusphaera micaliths, an interpretation that is still generally accepted. However, the first correct interpretation of the structure of the closely related Eoconusphaera and Calcivascularis micaliths are from [135], who showed that the lamella are not radially arranged, but organized in equal sectors, as recently confirmed by [112]. Kristan-Tollmann [136] further proposed that Calcivascularis, Mitrolithus, and Parhabdolithus evolved from Eoconusphaera. In her study of Nannoconus, Van Niel [137] commented on the similarities between Conusphaera, Eoconusphaera, Faviconus, Mitrolithus, Nannoconus, and Pseudoconus, which is in support of the taxonomic framework herein. More recently, Abdi et al. [138] have pointed to the homeomorphy between Nannoconus and the spine of Mitrolithus. The descriptions below follow from Aubry [22] in which supporting references are given.
The oldest micalith (genus Prinsiosphaera; Late Triassic, Norian–Rhaetian) is spherical. It consists of segments, in the shape of small parallelepipeds, and is about 1 µm wide, oriented randomly with their long axis tangential to the surface of the spheres to form a continuous layer, leaving no gaps between sutures despite the disorderly arrangement. Each segment comprises 6 to 12 parallel lamellae, lying perpendicular to the long axis of the segment. Inner segments occupy the center of the micasphere. It is unclear whether a thin, smooth external layer overlaid the outer segments.
Another Rhaetian micalith (genus Eoconusphaera) is in the shape of an elongated cone, 2–6 µm high and truncated at both ends, with a gently concave proximal face and a low cone distal face. It comprises a core and a surrounding jacket. The core consists of eight, radially arranged segments, each of them with six parallel, wedge-shaped lamellae. The longest lamella lies along the radius of the core and the others are against the longest lamella of the next segment. This creates a characteristic clockwise pattern in cross-section with the segments forming equal sectors of the cone. The core is enclosed in the characteristic jacket with its thin, tall, trapezoidal laths.
Similarly constructed micaliths occur in the Jurassic and Cretaceous but with some structural differences. The core in Calcivascularis (Sinemurian–early Toarcian) and Conusphaera are the closest to Eoconusphaera, but with six and four radially arranged segments, respectively, together with a vertical differentiation of the lamellae of the segments in Calcivascularis, and differentiation of an inner and outer core in Conusphaera.
The Mitrolithus, Parhabdolithus, and Timorella micaliths are clearly related to one another and to Eoconusphaera, and the beginning of each segment is marked by larger lamellae. In the singular Mitrolithus (Sinemurian–early Toarcian) the low, knob-like distal protuberance is formed by short crescent-like segments superposed in very low, broken spirals. The bottom of the basket formed by the jacket is lined with intersecting segments. In the extended distal protuberance of Parhabdolithus (Sinemurian–lower Toarcian) the segments are arranged in tall broken spirals. Timorella (Pliensbachian) essentially consists of a basket-shaped jacket lined proximally with a few quadrangular lamellae of the core.
The jacket is low in the elliptical Calcicalathina micaliths (lower Aptian–lower Cenomanian), and the core may be tall or low. The tall cores appear to consist of segments roughly in the shape of small rectangular prisms that are disorderly superposed; the low cores comprise curved segments which are readily identified in light microscopy and consist of short, spirally organized lamellae. The core of the Laguncula micalith (late Aptian–Cenomanian) balloons out of the narrow jacket, probably consisting of horizontally superposed segments.
Micaliths without jacket occur for a brief time in the Middle Jurassic (Bathonian) and then again in the Late Jurassic. In one group (Pseudoconus, Bathonian; Faviconus, Kimmeridgian–Berriasian) the lamellae are arranged in columnar segments reminiscent of the Triassic Cassianospica. In a second group (Nannoconus, Tithonian–Campanian), the lamella are organized in duos and spirally arranged in segments. In the third group (Micrantholithus, Berriasian-upper Aptian; Braarudosphaera, Aptian–Present), tall, triangular, and trapezoidal segments are radially arranged to form pentaliths.
Micaliths without a core appear in the Aptian. Their jacket is multi-tiered (Lapideacassis, Aptian–Priabonian; Scampanella, Coniacian–Lutetian).
Appendix C

- (1)
- Broken micasphere of Braarudosphaera bigelowii preserved in an upper Miocene Braarudosphaera chalk. SEM; note scale at bottom right. Originally published in [117] (pl. 1, Figure 2); with permission from Dr. Martin Zuschin, Museum of Natural History, Vienna, Austria.
- (2)
- Fossil micasphere of Braarudosphaera orthia from the Rupelian calcareous sands of the Paris Basin.
- (3)
- Micasphere of Conusphaera mexicana in an upper Tithonian limestone from Mexico. Thin section of rock using polarized light; ~×1000. Originally published in [113] (pl. 3, Figure 1); with permission from Dr. Jaime S. Valente, Mexican Petroleum Institute, 07730 Mexico City, Mexico.
- (4)
- Micasphere of Nannoconus steinmannii in an upper Hauterivian limestone from Mexico. Thin section of rock using polarized light; ×800. Note the tiny cell, at least three times smaller than a micalith. Originally published in [113] (pl. 5, Figure 9); with permission from Dr. Jaime S. Valente.
Appendix D
The taxonomic classification of micalith-bearing genera in [20] and, herein, the taxonomic classification in [20] follows from [15,99]. The taxonomic classification herein follows from [22]. A comprehensive description of the micaliths in each genus is given in the latter monograph while a brief overview can be found in Appendix A. Note that the genera of the Mesozoic family Polycyclolithaceae Forchheimer [55] are not assigned to the order Braarudosphaerales Aubry [24] as in [20], but to the order Discoasterales Hay [97] emend. Aubry [18]. The Polycyclolithaceae family comprise heterococcoliths with radial symmetry and superposition geometry, consisting of wedge-shaped elements organized in cycles forming structural units, and not of lamella forming segments.
| Genus | Ordinal Classification | |
| [20] | Herein | |
| Eiffelithales | ||
| Calcicalathina | ||
| Stephanolithiales | ||
| Calcivascularis | ||
| Mitrolithus | ||
| Parhabdolithus | ||
| Timorella | ||
| Braarudosphaerales | ||
| Braarudosphaera | ||
| Favoconus | ||
| Micrantholithus | ||
| Nannoconus | Braarudosphaerales | |
| Pemma | ||
| Pentaster | ||
| Informal Group | ||
| Heterococcolith incertae sedis | ||
| Laguncula | ||
| Nannoliths incertae sedis | ||
| Conusphaera | ||
| Eoconusphaera | ||
| Lapideacassis | ||
| Scampanella | ||
| Holococcolith | ||
| Pseudoconus | ||
| Mesozoic non-coccoliths | ||
| Cassianospica | ||
| Prinsiosphaera | ||
Appendix E
Paleococcolithus missouriensis Gartner & Gentile 1973 [109]
- “parts of three specimens on the cleavage surface of a piece of the Pennsylvanian Tecumseh Shale of Missouri. ×7250”.
- “Enlargement of the holotype. ×20.000”. Reproduced with permission from Dr. M. Kaminski, Editor, Micropaleontology Press.
References
- Sorby, H.C. On the organic origin of the so-called “crystalloids” of the Chalk. Ann. Mag. Nat. Hist. 1861, 8, 193–200. [Google Scholar] [CrossRef]
- Bramlette, M.N. Significance of coccolithophorids in calcium-carbonate deposition. Bull. Gcol. Soc. Am. 1958, 69, 121–126. [Google Scholar] [CrossRef]
- Knoll, A. Biomineralization and Evolutionary History. Rev. Min. Geochem. 2003, 54, 329–356. [Google Scholar] [CrossRef]
- Thierstein, H.R.; Young, J.R. (Eds.) Coccolithophores. From Molecular Processes to Global Impact; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- De Vargas, C.; Aubry, M.-P.; Probert, Y.; Young, J. Origin and evolution of Coccolithophores: A paleonto-genomic approach. In Evolution of Primary Producers in the Sea; Falkowski, P., Knoll, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 251–285. [Google Scholar]
- Mayers, K.M.J.; Poulton, A.J.; Daniels, C.J.; Wells, S.R.; Woodward, E.M.S.; Tarran, G.A.; Widdicombe, C.E.; Mayor, D.J.; Atkinson, A.; Giering, S.L.C. Growth and mortality of coccolithophores during spring in a temperate Shelf Sea (Celtic Sea, April 2015). Progr. Oceanogr. 2015, 177, 101928. [Google Scholar] [CrossRef]
- Dauphin, Y. A Brief History of Biomineralization Studies. ACS Biomater. Sci. Eng. 2023, 9, 1774–1790. [Google Scholar] [CrossRef] [PubMed]
- Henderiks, J.; Daniela Sturm, D.; Šupraha, L.; Langer, G. Evolutionary Rates in the Haptophyta: Exploring Molecular and Phenotypic Diversity. J. Mar. Sci. Eng. 2022, 10, 798. [Google Scholar] [CrossRef]
- Langer, G.; Taylor, A.R.; Walker, C.E.; Meyer, E.M.; Joseph, O.B.; Gal, A.; Harper, G.M.; Probert, I.; Brownlee, C.; Wheeler, G.L. Role of silicon in the development of complex crystal shapes in coccolithophores. New Phytol. 2021, 231, 1845–1857. [Google Scholar] [CrossRef]
- Braarud, T.; Deflandre, G.; Halldal, P.; Kamptner, E. Terminology, nomenclature, and systematics of the Coccolithophoridae. Micropaleont 1955, 1, 157–159. [Google Scholar] [CrossRef]
- Hagino, K.; Tomioka, N.; Young, J.; Takano, Y.; Onuma, R.; Horiguchi, T. Extracellular calcification of Braarudosphaera bigelowii deduced from electron microscopic observations of cell surface structure and elemental composition of pentaliths. Mar. Micropal. 2016, 125, 85–94. [Google Scholar] [CrossRef]
- Wallich, G.C. Observations on the coccosphere. Ann. Mag. Nat. Hist. 1877, 19, 342–350. [Google Scholar] [CrossRef]
- Perch–Nielsen, K. Mesozoic calcareous nannofossils. In Plankton Stratigraphy; Bolli, H.M., Saunders, J.B., Perch–Nielsen, K., Eds.; Cambridge University Press: Cambridge, UK, 1985; pp. 329–426. [Google Scholar]
- Perch–Nielsen, K. Cenozoic calcareous nannofossils. In Plankton Stratigraphy; Bolli, H.M., Saunders, J.B., Perch–Nielsen, K., Eds.; Cambridge University Press: Cambridge, UK, 1985; pp. 427–554. [Google Scholar]
- Bown, P.R. (Ed.) Calcareous Nannofossil Biostratigraphy, British Micropaleontological Society Publications Series; Kluver Academic Publishers: London, UK, 1998; pp. 1–324. [Google Scholar]
- Aubry, M.-P. Handbook of Cenozoic Calcareous Nannoplankton; Micropaleontology Press: New York, NY, USA, 1990; Volume 4, pp. 1–381. [Google Scholar]
- Aubry, M.-P. Handbook of Cenozoic Calcareous Nannofossils; Micropaleontology Press: New York, NY, USA, 1999; Volume 5, pp. 1–368. [Google Scholar]
- Aubry, M.-P. Coccolithophores: Cenozoic Discoasterales—Biology, Taxonomy, Stratigraphy; SEPM: Tulsa, OK, USA, 2021; CSP14; pp. 1–452. [Google Scholar]
- Aubry, M.-P. Coccolithophores: The Haploid Phase of Living Coccolithophores—Biology, Taxonomy, Stratigraphy; SEPM: Tulsa, OK, USA, 2022; CSP15; pp. 1–402. [Google Scholar]
- Young, J.R.; Bown, P.R.; Lees, J.A. Nannotax 3 (Online Resource and Guide to the Biodiversity and Taxonomy of Coccolithophores). 2024. Available online: https://www.mikrotax.org/Nannotax3/ (accessed on 1 October 2024).
- Aubry, M.-P. Coccolithophores: Mesozoic Discoasterales—Taxonomy, Stratigraphy, Biogeography; SEPM: Tulsa, OK, USA, 2025; in progress. [Google Scholar]
- Aubry, M.-P. Coccolithophores: Braarudosphaerales—Taxonomy, Stratigraphy, Biogeography; SEPM: Tulsa, OK, USA, 2025; in progress. [Google Scholar]
- Aubry, M.-P. Coccolithophores: The Haploid Phase of Extinct Coccolithophores—Taxonomy, Stratigraphy, Biogeography; SEPM: Tulsa, OK, USA, 2025; in progress. [Google Scholar]
- Aubry, M.-P. Cenozoic Coccolithophores: Braarudosphaerales; Micropaleontology Press: New York, NY, USA, 2013; pp. 1–336. [Google Scholar]
- Aubry, M.-P. Cenozoic Coccolithophores: Extinct Holococcoliths; Micropaleontology Press: New York, NY, USA, 2017; pp. 1–366. [Google Scholar]
- Stradner, H.; Edwards, A.R. Electron microscope studies on Upper Eocene coccoliths from the Oamaru diatomite, New Zealand. Jahrb. Geol. Bund. Sond. 1968, 13, 1–66. [Google Scholar]
- Prins, B. Evolution and stratigraphy of coccolithinids from the Lower and Middle Lias. In Proceedings of the First International Conference on Planktonic Microfossils, Geneva 1967; Bronnimann, P., Renz, H.H., Eds.; E.J. Brill: Leiden, The Netherlands, 1969; Volume 2, pp. 547–558. [Google Scholar]
- Prins, B. Speculations on relations, evolution, and stratigraphic distribution of discoasters. In Proceedings of the II Planktonic Conference, Roma, 1970; Farinacci, A., Ed.; Ediz. Tecnoscienza: Roma, Italy, 1971; Volume 1, pp. 1017–1037. [Google Scholar]
- Grun, W.; Allemann, F. The Lower Cretaceous of Caravaca (Spain). Berriasian calcareous nannoplankton of the Miravetes section (Subbetic Zone, Prov. of Murcia). Eclog. Geol. Helv. 1975, 68, 147–211. [Google Scholar]
- Romein, A.J.T. Lineages in Early Paleogene calcareous nannoplankton. Utrecht Micropal. Bull. 1979, 22, 1–231. [Google Scholar]
- Edwards, A.R.; Perch-Nielsen, K. Calcareous nannofossils from the southern southwest Pacific, Deep Sea Drilling Project, Leg 29. In Initial Reports of the Deep Sea Drilling Project; Kennett, J.P., Houtz, R.E., et al., Eds.; U.S. Government Printing Office: Washington, DC, USA, 1975; Volume 29, pp. 469–539. [Google Scholar]
- Cros, L.; Fortuño, J.-M. Atlas of Northwestern Mediterranean Coccolithophores. Scientia Marina 2002, 66 (Suppl. 1), 7–182. [Google Scholar] [CrossRef]
- Hattner, J.G.; Wise, S.W., Jr. Upper Cretaceous Calcareous Nannofossil Biostratigraphy of South Carolina. South Carolina Geology 1980, 24, 41–117. [Google Scholar]
- Perch-Nielsen, K. Remarks on Late Cretaceous to Pleistocene coccoliths from the North Atlantic. In Initial Reports of the Deep Sea Drilling Project; Laughton, A.S., Berggren, W.A., et al., Eds.; U.S. Government Printing Office: Washington, DC, USA, 1972; Volume 12, pp. 1003–1069. [Google Scholar]
- Parke, M.; Adams, I. The motile (Crystallolithus hyalinus Gaarder & Markali) and non-motile phases in the life history of Coccolithus pelagicus (Wallich) Schiller. J. Mar. Biol. Assoc. UK 1960, 39, 263–274. [Google Scholar]
- Houdan, A.; Billard, C.; Marie, D.; Not, F.; Sáez, A.G.; Young, J.; Probert, I. Holococcolithophore-heterococcolithophore (Haptophyta) life cycles: Flow cytometric analysis of relative ploidy levels. Syst. Biodiv. 2004, 1, 453–465. [Google Scholar] [CrossRef]
- Cros, L.; Kleijne, A.; Zeltner, A.; Billard, C.; Young, J.R. New examples of holococcolith-heterococcolith combination coccospheres and their implications for coccolithophorid biology. Mar. Micropal. 2000, 39, 1–34. [Google Scholar] [CrossRef]
- Geisen, M.; Billard, C.; Broerse, A.T.C.; Cros, L.; Probert, J.; Young, J.R. Life-cycle associations involving pairs of holococcolithophorid species: Intraspecific variation or cryptic speciation? Eur. J. Phys. 2002, 37, 531–550. [Google Scholar] [CrossRef]
- Aubry, M.-P. Peering into the biology of Extinct Coccolithophores: The Order Discoasterales. In Geologic Problem Solving with Microfossils IV; Denne, R., Kahn, A., Eds.; SEPM: Tulsa, OK, USA, 2019; Volume 111. [Google Scholar] [CrossRef]
- Cohen, K.M.; Finney, S.C.; Gibbard, P.L.; Fan, J.-X. The ICS International Chronostratigraphic Chart. Episodes 2013, 36, 199–204. [Google Scholar] [CrossRef]
- Cavalier-Smith, T.; Chao, E.E.; Lewis, R. Multiple origins of Heliozoa from flagellate ancestors: New cryptist subphylum Corbihelia, superclass Corbistoma, and monophyly of Haptista, Cryptista, Hacrobia and Chromista. Mol. Phyl. Evol. 2015, 93, 331–362. [Google Scholar] [CrossRef]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G.B. The new tree of Eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, D.J. The ultrastructure and taxonomy of the Chrysophyceae and Prymnesiophyceae (Prymnesiophyceae): A survey with some new observations on the ultrastructure of the Chrysophyceae. Bot. J. Linnean Soc. 1976, 72, 55–80. [Google Scholar] [CrossRef]
- Rothmaler, W. Die Abteilungen und Klassen der Pflanzen. Feddes Repert. Specierum Nov. Regni Veg. 1951, 54, 256–266. [Google Scholar] [CrossRef]
- Edvardsen, B.; Eikrem, W.; Green, J.C.; Andersen, R.A.; Stayy, S.Y.M.; Medlin, L.K. Phylogenetic reconstructions of the Haptophyta inferred from 18S ribosomal DNA sequences and available morphological data. Phycologia 2000, 39, 19–35. [Google Scholar] [CrossRef]
- Edvardsen, B.; Eikrem, W.; Throndsen, J.; Sáez, A.G.; Probert, I.; Medlin, L.K. Ribosomal DNA phylogenies and a morphological revision provide the basis for a revised taxonomy of the Prymnesiales (Haptophyta). Eur. J. Phycol. 2011, 46, 202–228. [Google Scholar] [CrossRef]
- Kawachi, M.; Nakayama, T.; Kayama, M.; Nomura, M.; Miyashita, H.; Bojo, O.; Rhodes, L.; Sym, S.; Pienaar, R.N.; Probert, I.; et al. Rappemonads are haptophyte phytoplankton. Curr. Biol. 2021, 31, 2395–2403. [Google Scholar] [CrossRef]
- Tangen, K. Papposphaera lepida, gen. nov., n. sp. a new marine coccolithophorid from Norwegian coastal waters. Norw. J. Bot. 1972, 19, 171–178. [Google Scholar]
- Heimdal, B.R. Modern coccolithophorids. In Marine Phytoplankton: A Guide to Nakes Flagellates and Coccolithophorids; Thomas, C.R., Ed.; Academy Press: San Diego, CA, USA, 1980; pp. 147–247. [Google Scholar]
- Throndsen, J. The planktonic marine flagellates. In Marine Phytoplankton: A Guide to Nakes Flagellates and Coccolithophorids; Thomas, C.R., Ed.; Academy Press: San Diego, CA, USA, 1980; pp. 7–145. [Google Scholar]
- Aubry, M.-P.; Bord, D. Reshuffling the cards in the photic zone at the Eocene/Oligocene boundary. In The Late Eocene Earth—Hothouse, Icehouse, and Impacts; Koeberl, C., Montanari, A., Eds.; GSA: Tulsa, OK, USA, 2009; SPEA 452; pp. 281–301. [Google Scholar] [CrossRef]
- Bown, P.R.; Young, J.R.; Lees, J.A. On the Cretaceous origin of the Order Syracosphaerales and the genus Syracosphaera. J. Micropal. 2017, 36, 153–165. [Google Scholar] [CrossRef]
- Si, W.; Novak, J.B.; Richter, N.; Polissar, P.; Mai, R.; Santos, E.; Nirenberg, J.; Herbert, T.D.; Aubry, M.-P. Alkenone-derived estimates of Cretaceous pCO2. Geology 2024, 52, 555–559. [Google Scholar] [CrossRef]
- Aubry, M.-P.; Si, W.; Beaufort, L.; Giraud, F.; Ma, R.; Shcherbinina, E.; Novak, J.B.; Richter, N.; Polissr, P.; Herbert, T. From Alkenones to Cretaceous Marine Isochrysidales to Coccoliths. 2024. Available online: https://ina.tmsoc.org/meetings/INA19Conwy/INA19timetable.html (accessed on 1 February 2025).
- Forchheimer, S. Scanning electron microscope studies of Cretaceous coccoliths from the Köpingsberg borehole no.1, SE Sweden. Sver. Geol. Unders. 1972, 14, 1–141. [Google Scholar]
- Thompson, A.W.; Foster, R.A.; Krupke, A.; Brandon, J.; Carter, B.J.; Musat, N.; Vaulot, D.; Kuypers, M.M.M.; Zehr, J.P. Unicellular Cyanobacterium Symbiotic with a Single-Celled Eukaryotic Alga. Science 2012, 337, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Hagino, K.; Onuma, R.; Kawachi, M.; Horiguchi, T. Discovery of an Endosymbiotic Nitrogen-Fixing Cyanobacterium UCYN-A in Braarudosphaera bigelowii (Prymnesiophyceae). PLoS ONE 2013, 8, e81749. [Google Scholar] [CrossRef]
- Edvardsen, B.; Egge, E.S.; Vaulot, D. Diversity and distribution of haptophytes revealed by environmental sequencing and metabarcoding—A review. Perspect. Phycol. 2016, 3, 77–91. [Google Scholar] [CrossRef]
- Bown, P.; Lees, J.A.; Young, J.R. Calcareous nannoplankton evolution and diversity through time. In Coccolithophores. From Molecular Processes to Global Impact; Thierstein, H.R., Young, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 481–508. [Google Scholar]
- Mable, B.K.; Otto, S.P. The evolution of life cycles with haploid and diploid phases. BioEssays 1998, 20, 453–462. [Google Scholar] [CrossRef]
- Taylor, A.R.; Brownlee, C.; Wheeler, G. Coccolithophore cell biology: Chalking up progress. Ann. Rev. Mar. Sci. 2017, 9, 283–310. [Google Scholar] [CrossRef]
- Brownlee, C.; Langer, G.; Wheeler, G.L. Coccolithophore calcification: Changing paradigms in changing oceans. Acta Biomater. 2021, 120, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Skeffington, A.; Fischer, A.; Sviben, A.; Brzezinka, M.; Górka, M.; Bertinetti, L.; Woehle, C.; Huettel, B.; Graf, A.; Scheffel, A. A joint proteomic and genomic investigation provides insights into the mechanism of calcification in coccolithophores. Nat. Commun. 2023, 14, 3749. [Google Scholar] [CrossRef]
- Walker, C.E.; Alison, R.; Taylor, A.R.; Langer, G.; Durak, G.M.; Heath, S.; Probert, I.; Tyrrell, T.; Brownlee, C.; Glen, L.; et al. The requirement for calcification differs between ecologically important coccolithophore species. New Phytol. 2018, 220, 147–162. [Google Scholar] [CrossRef]
- Henriksen, K.; Stipp, S.L.S.; Young, J.R.; Marsh, M.E. Biological control on calcite crystallization: AFM investigation of coccolith polysaccharide function. Am. Mineral. 2004, 89, 1709–1716. [Google Scholar] [CrossRef]
- Chowdhury, R.; Boudjehem, R.; Suchéras-Marx, B.; Dupraz, M.; Kulow, A.; da Silva, J.C.; Hazemann, J.L.; Aubry, M.-P.; Javier Perez, J.; Fernandez-Martinez, A.; et al. Micro-structural reconstruction of Nannoconus from synchrotron-radiation-based ptychographic X-ray Computed Tomography. Int. J. Earth Planet. Mater. Res. 2025. in progress. [Google Scholar]
- Demangel, I.; Kovács, Z.; Richoz, S.; Gardin, S.; Krystyn, L.; Baldermanne, A.; Piller, W.E. Development of early calcareous nannoplankton in the late Triassic (Northern Calcareous Alps, Austria). Glob. Planet Change 2020, 193, 103254. [Google Scholar] [CrossRef]
- Aubry, M.-P. Early Paleogene calcareous nannoplankton evolution: A tale of climatic amelioration. In Late Paleocene–Early Eocene Climatic and Biotic Events in the Marine and Terrestrial Records; Aubry, M.-P., Lucas, S., Berggren, W.A., Eds.; Columbia University Press: New York, NY, USA, 1998; pp. 158–203. [Google Scholar]
- Aubry, M.-P. A major mid-Pliocene calcareous nannoplankton turnover: Change in life strategy in the photic zone. In Large Ecosystem Perturbations: Causes and Consequences; Monechi, S., Rampino, M., Coccioni, R., Eds.; GSA: Tulsa, OK, USA, 2007; Special Paper; pp. 25–51. [Google Scholar]
- Preto, N.; Agnini, C.; Rigo, M.; Sprovieri, M.; Westphal, H. The calcareous nannofossil Prinsiosphaera achieved rock-forming abundances in the latest Triassic of western Tethys: Consequences for the 13C of bulk carbonate. Biogeosciences 2013, 10, 6053–6068. [Google Scholar] [CrossRef]
- Aubry, M.-P. A sea of lilliputians. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 284, 88–113. [Google Scholar] [CrossRef]
- Perch–Nielsen, K. Elektronenmikrokopische Untersuchungen an Coccolithen und verwandten Formen aus dem Eozän von Dänemark. Kongelige Danske Videnskabernes Selskab Biologiske Skrifter 1971, 18, 1–76. [Google Scholar]
- Young, J.R.; Didymus, J.M.; Bown, P.R.; Prins, B.; Mann, S. Crystal assembly and phylogenetic evolution in heterococcoliths. Nature 1992, 356, 1–3. [Google Scholar] [CrossRef]
- Young, J.R.; Davis, S.A.; Bown, P.R.; Mann, S. Coccolith ultrastructure and biomineralisation. J. Struct. Biol. 1999, 126, 195–215. [Google Scholar] [CrossRef]
- Young, J.R.; Henriksen, K. Biomineralization Within Vesicles: The Calcite of Coccoliths. Rev. Min. Geochem. 2003, 54, 189–215. [Google Scholar] [CrossRef]
- Young, J.R.; Henriksen, K.; Proberts, J. Structure and morphogenesis of the coccoliths of the CODENET species. In Coccolithophores: From Molecular Processes to Global Impact; Thierstein, H.R., Young, J.R., Eds.; Springer: Berlin, Germany, 2004; pp. 191–216. [Google Scholar] [CrossRef]
- Young, J.R.; Geisen, M.; Cros, L.; Kleijne, A.; Sprengel, C.; Probert, I.; Østergaard, J. A guide to extant coccolithophore taxonomy. JNR Spec. Issue 2003, 1, 1–125. [Google Scholar] [CrossRef]
- Frada, M.; Percopo, I.; Young, J.; Zingone, A.; De Vargas, C.; Probert, I. First observations of heterococcolithophore–holococcolithophore life cycle combinations in the family Pontosphaeraceae (Calcihaptophycideae, Haptophyta). Mar. Micropal. 2009, 71, 20–27. [Google Scholar] [CrossRef]
- Keuter, S.; Young, J.R.; Koplovitz, G.; Zingone, A.; Frada, M.J. Novel heterococcolithophores, holococcolithophores and life cycle combinations from the families Syracosphaeraceae and Papposphaeraceae and the genus Florisphaera. J. Micropal. 2021, 40, 75–99. [Google Scholar] [CrossRef]
- Jordan, R.W.; Young, J.R. Proposed changes to the classification system of living coccolithophorids. Int. Nannoplankton Assoc. (INA) Newsl. 1990, 12, 15–18. [Google Scholar] [CrossRef]
- Manton, I.; Oates, K. Polycrater galapagensis gen. et sp. nov., a putative coccolithophorid from the Galapagos Islands with an unusual aragonitic periplast. Brit. Phycol. J. 1980, 15, 95–103. [Google Scholar] [CrossRef]
- Kleijne, A.; Jordan, R.W.; Heimdal, B.R.; Samtleben, C.; Chamberlain, A.H.L.; Cros, L. Five new species of the coccolithophorid genus Alisphaera (Haptophyta) with notes on their distribution, coccolith structure and taxonomy. Phycologia 2001, 40, 583–601. [Google Scholar] [CrossRef]
- Stanley, S.M.; Hardie, L.A. Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry. Palaeogeogr. Palaeoclim. Palaeoecol. 1998, 144, 3–19. [Google Scholar] [CrossRef]
- Bralower, T.J.; Bown, P.R.; Siesser, W.G. Upper triassic calcareous nannoplankton biostratigraphy, wombat plateau, northwest Australia. In Proceedings of the Ocean Drilling Program, Scientific Results; von Rad, U., Haq, B.U., et al., Eds.; 1992; Volume 122, pp. 437–451. [Google Scholar]
- Monechi, S.; Reale, V.; Bernaola, G.; Balestra, B. The Danian/Selandian boundary at Site 1262 (South Atlantic) and in the Tethyan region: Biomagnetostratigraphy, evolutionary trends in fasciculiths and environmental effects of the latest Danian event. Mar. Micropal. 2013, 98, 28–40. [Google Scholar] [CrossRef]
- De Vries, J.; Monteiro, F.; Wheeler, G.; Poulton, A.; Godrijan, J.; Cerino, F.; Malinverno, E.; Langer, G.; Brownlee, C. Haplo-diplontic life cycle expands coccolithophore niche. Biogeosciences 2021, 18, 1161–1184. [Google Scholar] [CrossRef]
- Foote, M. The evolution of morphological diversity. Ann. Rev. Ecol. Syst. 1997, 28, 129–152. [Google Scholar] [CrossRef]
- Hughes, M.; Gerber, S.; Wills, M.A. Clades reach highest morphological disparity early in their evolution. Proc. Nat. Acad. Sci. USA 2013, 110, 13875–13879. [Google Scholar] [CrossRef]
- Benton, M.J.; Harper, D.A.T. Introduction to Paleobiology and the Fossil Record; Wiley Blackwell: London, UK, 2009; ISBN 9781405141574. [Google Scholar]
- Triccas, A.; Laidlaw, F.; Songleton, M.R.; Nudelman, F. Control of crystal growth during coccolith formation by the coccolithophore Gephyrocapsa oceanica. J. Struct. Biol. 2024, 216, 108066. [Google Scholar] [CrossRef]
- Suchéras-Marx, B.; Mattioli, E.; Giraud, F.; Escarguel, G. Paleoenvironmental and paleobiological origins of coccolithophorid genus Watznaueria emergence during the late Aalenian–early Bajocian. Paleobiology 2015, 41, 415–435. [Google Scholar] [CrossRef]
- Agnini, C.; Muttoni, G.; Kent, D.V.; Rio, D. Eocene biostratigraphy and magnetic stratigraphy from Possagno, Italy: The calcareous nannofossil response to climate variability. Earth Planet. Sci. Lett. 2006, 241, 815–830. [Google Scholar] [CrossRef]
- Love, A.C.; Grabowski, M.; Houle, D.; Hsiang Liow, L.; Porto, A.; Tsuboi, M.; Voje, K.L.; Hunt, G. Evolvability in the fossil record. Paleobiology 2022, 48, 186–209. [Google Scholar] [CrossRef]
- Perch-Nielsen, K. Les coccolithes du Paléocène près de El Kef, Tunisie, et leurs ancêtres. Cah. Micropal. 1981, 3, 7–23. [Google Scholar]
- Bown, P. Selective calcareous nannoplankton survivorship at the Cretaceous-Tertiary boundary. Geology 2005, 33, 653–656. [Google Scholar] [CrossRef]
- Bralower, T.J.; Cosmidis, J.; Fantle, M.S.; Lowery, C.M.; Passey, B.H.; Gulick, S.P.S.; Morgan, J.V.; Vajda, V.; Whalen, M.T.; Wittmannet, A.; et al. The habitat of the nascent Chicxulub crater. AGU Adv. 2020, 1, e2020AV000208. [Google Scholar] [CrossRef]
- Hay, W.W. Calcareous nannofossils. In Oceanic Micropaleontology; Ramsay, A.T.S., Ed.; Academic Press: London, UK, 1977; Volume 2, pp. 1055–1200. [Google Scholar]
- Alvarez, L.W.; Alvarez, W.; Asaro, F.; Michel, H.V. Extraterrestrial Cause for the Cretaceous-Tertiary Extinction. Science 1980, 208, 1095–1108. [Google Scholar] [CrossRef]
- Bown, P.R.; Young, J.R. Proposals for a revised classification system for calcareous nannoplankton. JNR 1997, 19, 15–47. [Google Scholar]
- Aubry, M.-P. Late Paleogene calcareous nannoplankton evolution: A tale of climatic deterioration. In The Eocene-Oligocene Climatic and Biotic Changes; Prothero, D., Berggren, W.A., Eds.; Princeton University Press: Princeton, NJ, USA, 1992; pp. 272–309. [Google Scholar] [CrossRef]
- Erba, E. The first 150 million years history of calcareous nannoplankton: Biosphere–geosphere interactions. Palaeogeogr. Palaeoclim. Palaeoecol. 2006, 232, 237–250. [Google Scholar] [CrossRef]
- Ma, R.; Aubry, M.-P.; Bord, D.; Jin, X.; Liu, C. Inferred nutrient forcing on the late middle Eocene to early Oligocene (~40–31 Ma) evolution of the coccolithophore Reticulofenestra (order Isochrysidales). Paleobiology 2024, 50, 29–42. [Google Scholar] [CrossRef]
- Gollain, B.; Mattioli, E.; Kenjo, S.; Bartolini, A.; Reboulet, S. Size patterns of the coccolith Watznaueria barnesiae in the lower Cretaceous: Biotic versus abiotic forcing. Mar. Micropal. 2019, 152, 101740. [Google Scholar] [CrossRef]
- Bendif, E.M.; Nevado, B.; Wong, E.L.Y.; Hagino, K.; Probert, I.; Young, J.R.; Rickaby, R.E.M.; Filatov, D.A. Repeated species radiations in the recent evolution of the key marine phytoplankton lineage Gephyrocapsa. Nat. Commun. 2019, 10, 4234. [Google Scholar] [CrossRef]
- Mattioli, E.; Erba, E. Synthesis of calcareous nannofossil events in Tethyan Lower and Middle Jurassic successions. Riv. Ital. Paleont. Stratigr. 1999, 105, 343–376. [Google Scholar] [CrossRef]
- Liu, H.; Aris-Brosou, S.; Probert, I.; de Vargas, C. A Time line of the Environmental Genetics of the Haptophytes. Mol. Biol. Evol. 2010, 27, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Jafar, S.A. Significance of Late Triassic calcareous nannoplankton from Austria and southern Germany. Neues Jahrb. Geol. Paläontol. Abh. 1983, 166, 218–259. [Google Scholar] [CrossRef]
- Janofske, D. Kalkiges Nannoplankton, insbesondere Kalkige Dinoflagellaten-Zysten der alpinen Ober-Trias: Taxonomie, Biostratigraphie und Bedeutung für die Phylogenie der Peridiniales. Berliner Gcowiss. Abh. 1992, E4, 33. [Google Scholar]
- Gartner, S.; Gentile, R. Problematic Pennsylvanian coccoliths from Missouri. Micropaleontology 1972, 18, 401–404. [Google Scholar] [CrossRef]
- Preto, N.; Willems, H.; Guaiumi, C.; Westphal, H. Onset of significant pelagic carbonate accumulation after the Carnian Pluvial Event (CPE) in the western Tethys. Facies 2013, 59, 891–914. [Google Scholar] [CrossRef]
- Ogg, J.G.; Chen, Z.-Q. The Triassic Period, with contributions by M.J. Orchard and H.S. Jiang. Chapter 25, p. 903=953. In Geological Time Sale 2020; Gradstein, F.M., Ogg, J.G., Schmitz, M.D., Ogg, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, ISBN 978-0-12-824363-3. [Google Scholar]
- Demangel, I.; Howe, R.; Gardin, S.; Richoz, S. Eoconusphaera hallstattensis sp. nov., a review of the Rhaetian genus Eoconusphaera. J. Nannoplankton Res. 2021, 39, 77–87. [Google Scholar] [CrossRef]
- Trejo, M. Conusphaera mexicana, un nuevo cocolitoforido del Jurasico superior de Mexico. Rev. Instît. Mexic. L. Petr. 1969, 1, 5–15. [Google Scholar]
- Mutterlose, J.; Bottini, C. Early Cretaceous chalks from the North Sea giving evidence for global change. Nat. Commun. 2013, 4, 1686. [Google Scholar] [CrossRef]
- Kelly, D.C.; Norris, R.D.; Zachos, J.C. Deciphering the paleoceanographic significance of Early Oligocene Braarudosphaera chalks in the South Atlantic. Mar. Micropaleontol. 2003, 49, 49–63. [Google Scholar] [CrossRef]
- Liebrand, D.; Raffi, I.; Fraguas, Á.; Laxenaire, R.; Bosmans, J.H.C.; Hilgen, F.J.; Wilson, P.A.; Batenburg, S.J.; Beddow, H.M.; Bohaty, S.M.; et al. Orbitally forced hyper-stratification of the Oligocene South Atlantic Ocean. Paleoceanogr. Paleoclim. 2018, 33, 511–529. [Google Scholar] [CrossRef]
- Stradner, H.; Fuchs, R. Über Nannoplanktonvorkommen im Sarmatian (Ober-Miozan) der Zentralen Paratethys in Niederösterreich und im Burgenland. Beiträge Paläontolgie Osterr. 1980, 7, 251–279. [Google Scholar]
- Bartol, M.; Pavsic, J.; Dobnikar, M.; Bernasconi, S.M. Unusual Braarudosphaera bigelowii and Micrantholithus vesper enrichment in the Early Miocene sediments from the Slovenian Corridor, a seaway linking the central Paratethys and the Mediterranean. Palaeogeog. Palaeoclimat. Palaeoecol. 2008, 267, 77–88. [Google Scholar] [CrossRef]
- Auer, G.; Piller, W.E.; Harzhauser, M. High-resolution calcareous nannoplankton palaeoecology as a proxy for small-scale environmental changes in the Early Miocene. Mar. Micropaleontol. 2014, 111, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Gallois, R.W. Coccolith bloom in the Kimmeridge Clay and the origin of the North Sea oil. Nature 1976, 259, 473–475. [Google Scholar] [CrossRef]
- Gallois, R.W.; Medd, A.W. Coccolith-rich marker bands in the English Kimmeridge Clay. Geol. Mag. 1979, 116, 247–260. [Google Scholar] [CrossRef]
- Coale, T.H.; Valentina Loconte, V.; Turk-Kubo, K.A.; Vanslembrouck, B.; Mak, W.K.E.; Cheung, S.; Ekman, A.; Jian-Hua Chen, J.H.; Kyoko Hagino, K.; Takano, Y.; et al. Nitrogen-fixing organelle in a marine alga. Science 2024, 384, 217–222. [Google Scholar] [CrossRef]
- Rogers, J.J.W.; Santosh, M. Continents and Supercontinents; Oxford University Press: Oxford, UK, 2004; p. 146. ISBN 978-0-19-516589-0. [Google Scholar]
- Erwin, D.H. The End-Permian Mass Extinction. Ann. Rev. Ecol. Evol. Syst. 1990, 21, 69–91. [Google Scholar] [CrossRef]
- Simms, M.J.; Ruffell, A.H. Synchroneity of climatic change and extinctions in the Late Triassic. Geology 1989, 17, 265–268. [Google Scholar] [CrossRef]
- Kamptner, E. Untersuchungen über den Feinbau der Coccolithen. Arch. Protist. 1954, 100, 2–90. [Google Scholar]
- Murray, J.; Blackman, V.H. On the nature of the coccospheres and rhabdospheres. Phil. Trans. Roy. Soc. Lond. Ser. B 1898, 190, 427–441. [Google Scholar]
- Loeblich, A.R.; Tappan, H. The coccolithophorid genus Calcidiscus Kamptner and its synonyms. J. Paleontol. 1978, 52, 1390–1392. [Google Scholar]
- Lohmann, H. Untersuchungen über das Pflanzen- und Tierleben der Hochsee, zugleich ein Bericht über die biologischen Arbeiten auf der Fahrt der “Deutschland” von Bremerhaven nach Buenos-Aires in der Zeit vom 7. Mai bis 7. September 1911. Veröffentlichungen Inst. Meereskd. Univ. Berl. 1912, 1, 1–92. [Google Scholar]
- Gran, H.H.; Braarud, T. Pelagic plant life. In The Depth of the Ocean; Murray, J., Hjort, J., Eds.; MacMillan and Co.: London, UK, 1912; pp. 307–386. [Google Scholar]
- Lohmann, H. Über Coccolithophoriden. Verh. Deuts. Zool. Ges. 1913, 23, 143–164. [Google Scholar]
- Lohmann, H. Die Coccolithophoridae, eine Monographie der Coccolithen bildenden Flagellaten, zugleich ein Beitrag zur Kenntnis des Mittelmeerauftriebs. Arch. Protistenkd. 1902, 1, 89–165. [Google Scholar]
- Hay, W.W.; Mohler, H.P.; Roth, P.H.; Schmidt, R.R.; Boudreaux, J.E. Calcareous nannoplankton zonation of the Cenozoic of the Gulf Coast and Caribbean–Antillean area, and transoceanic correlation. Trans. Gulf Coast Assoc. Geol. Soc. 1967, 17, 428–480. [Google Scholar]
- Kamptner, E. Die Coccolithineen der Südwestküste von Istrien. Ann. Naturhist. Mus. Wien 1941, 51, 54–149. [Google Scholar]
- Kristan-Tollmann, E. I. Coccolithen aus den älteren Allgäuschichten (alpiner lias, sinemur) von Timor, Indonesien. Geol. Paläont. Mitt. Innsbruck 1988, 15, 71–83. [Google Scholar]
- Kristan-Tollmann, E. II. Coccolithen aus dem Pliensbach (Ältere Allgäuschichten Alpiner Lias) von Timor, Inonesien. Geol. Paläontol. Mitt. Innsbr. 1988, 15, 109–133. [Google Scholar]
- Van Niel, B. Unusual twin specimens of Nannoconus abundans (calcareous nannofossil, incertae sedis). J. Micropal. 1995, 14, 159–164. [Google Scholar] [CrossRef]
- Abdi, A.; Mattioli, E.; Bádenas, B. A New Calcareous Nannofossil Record from the Lower Jurassic of Kermanshah, Western Iran: Implications for Biostratigraphy and Evolutionary Reconstructions. Geosciences 2022, 12, 59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).