Utilization of Natural Mineral Materials in Environmental Remediation: Processes and Applications
Abstract
1. Introduction
2. Mechanism of Environmental Purification Driven by NMMs
2.1. Photocatalytic Redox Reaction
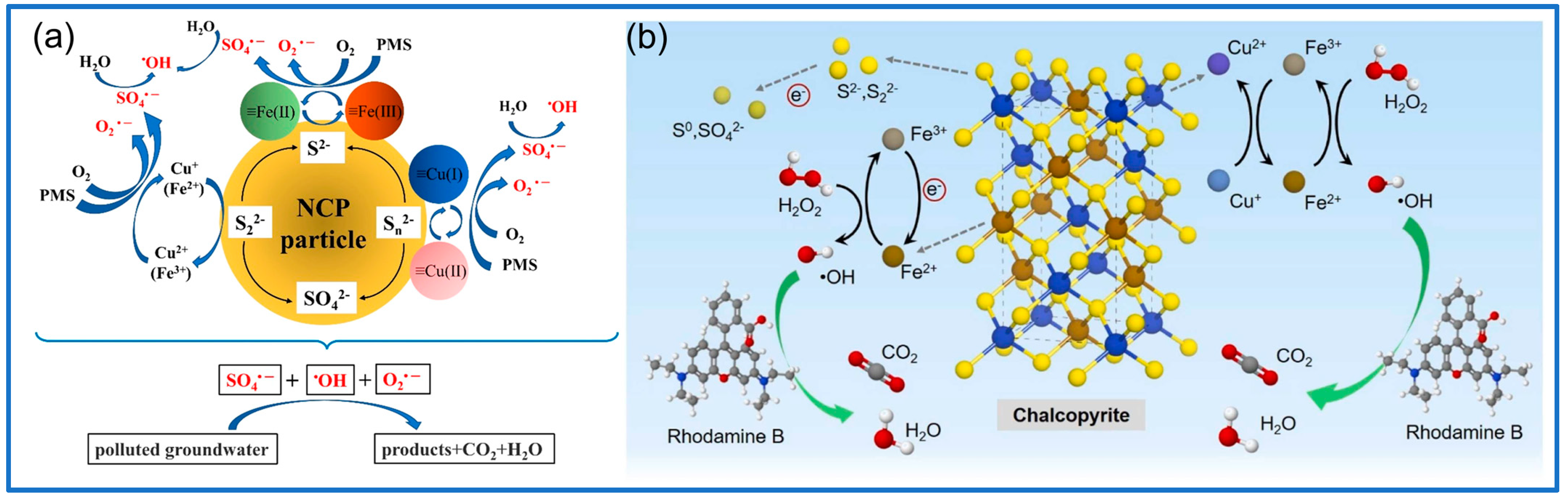
2.2. Fenton-like Process and Persulfate-Based Advanced Oxidation Process
2.3. Photocatalytic-Based Persulfate Activation
2.4. Other Remediation Mechanisms
3. NMMs for Sustainable Pollutants Degradation
3.1. Metal Oxide Materials
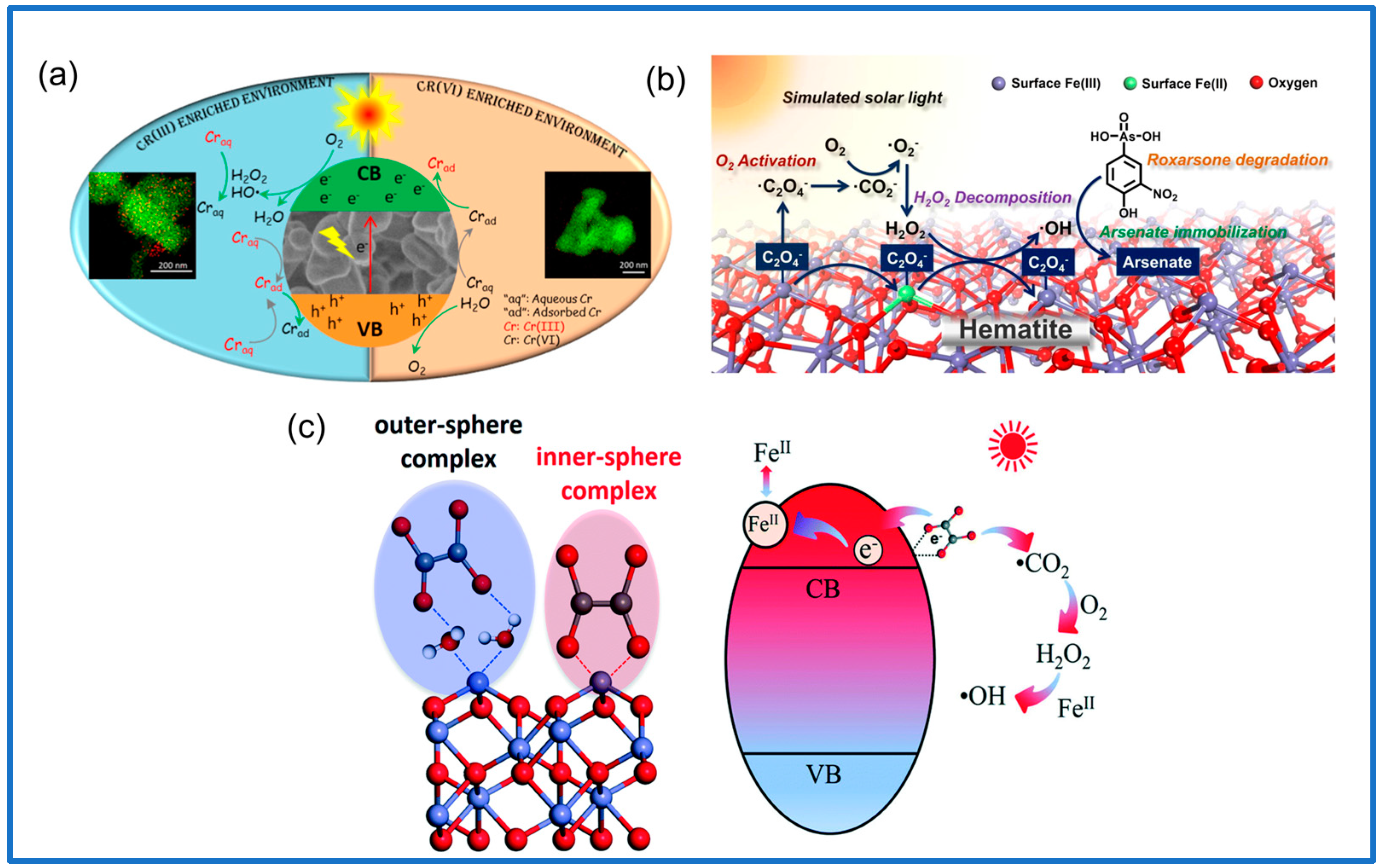
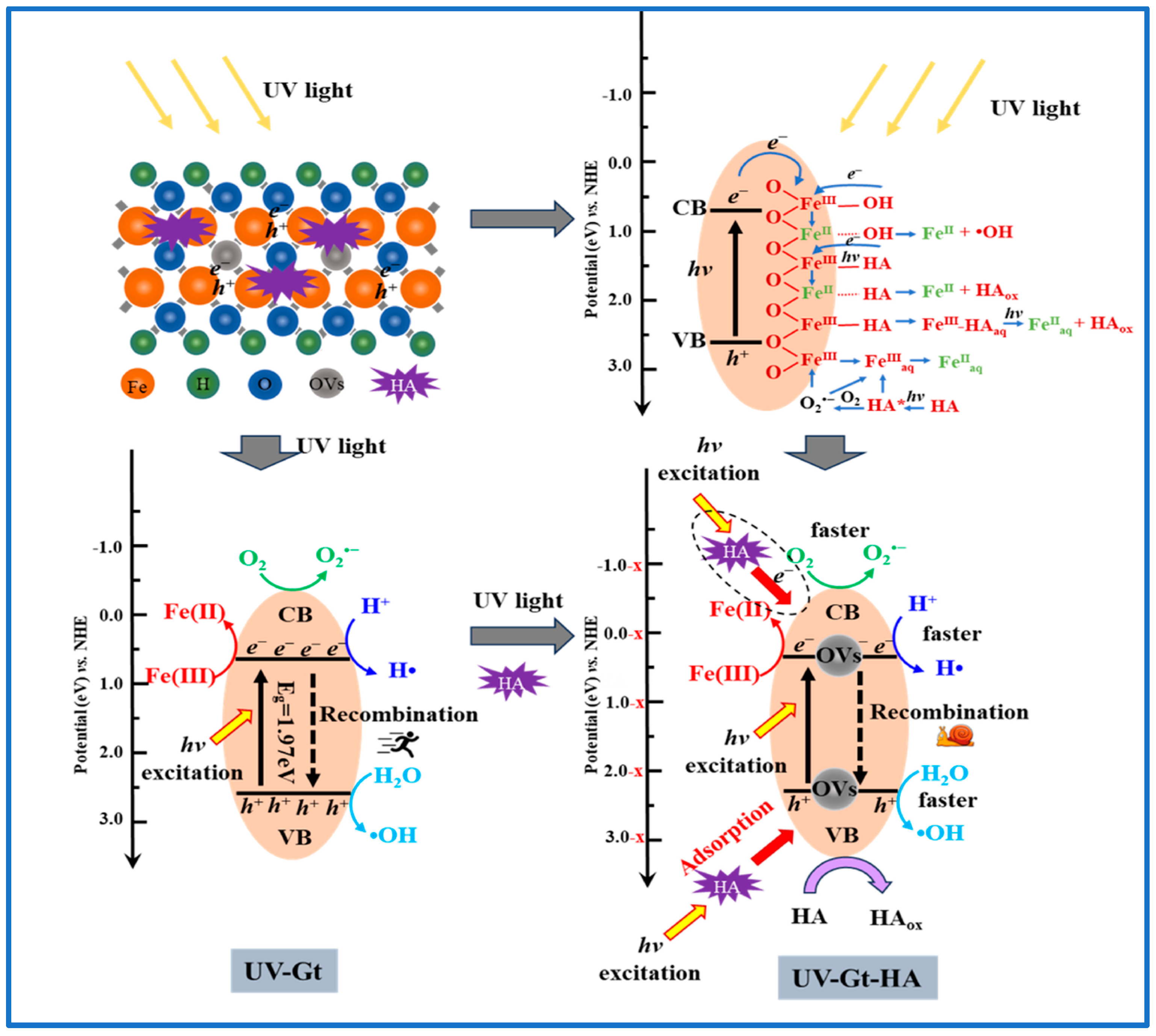
3.1.1. Iron Oxide Minerals
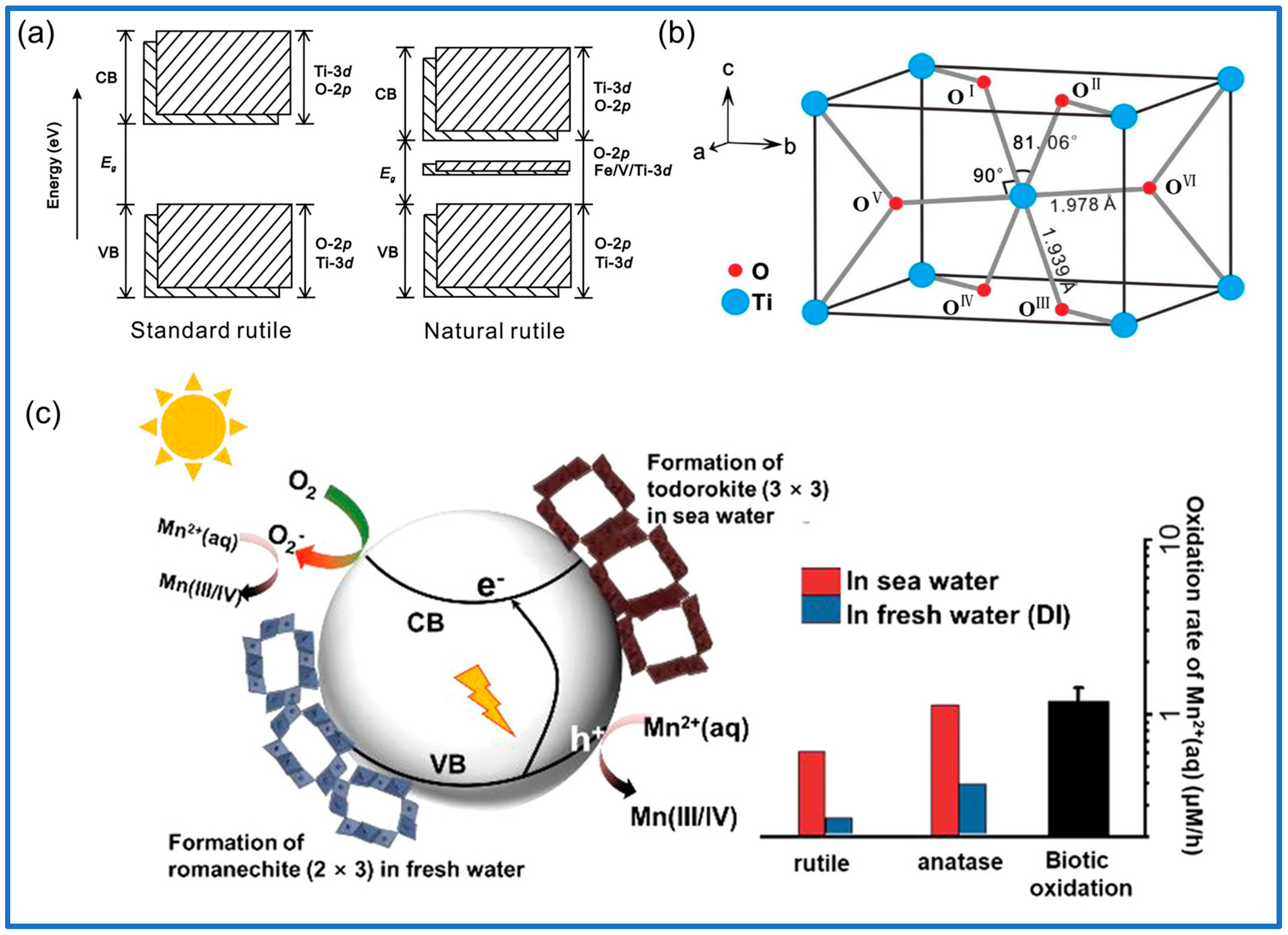
3.1.2. Titanium Oxide Mineral
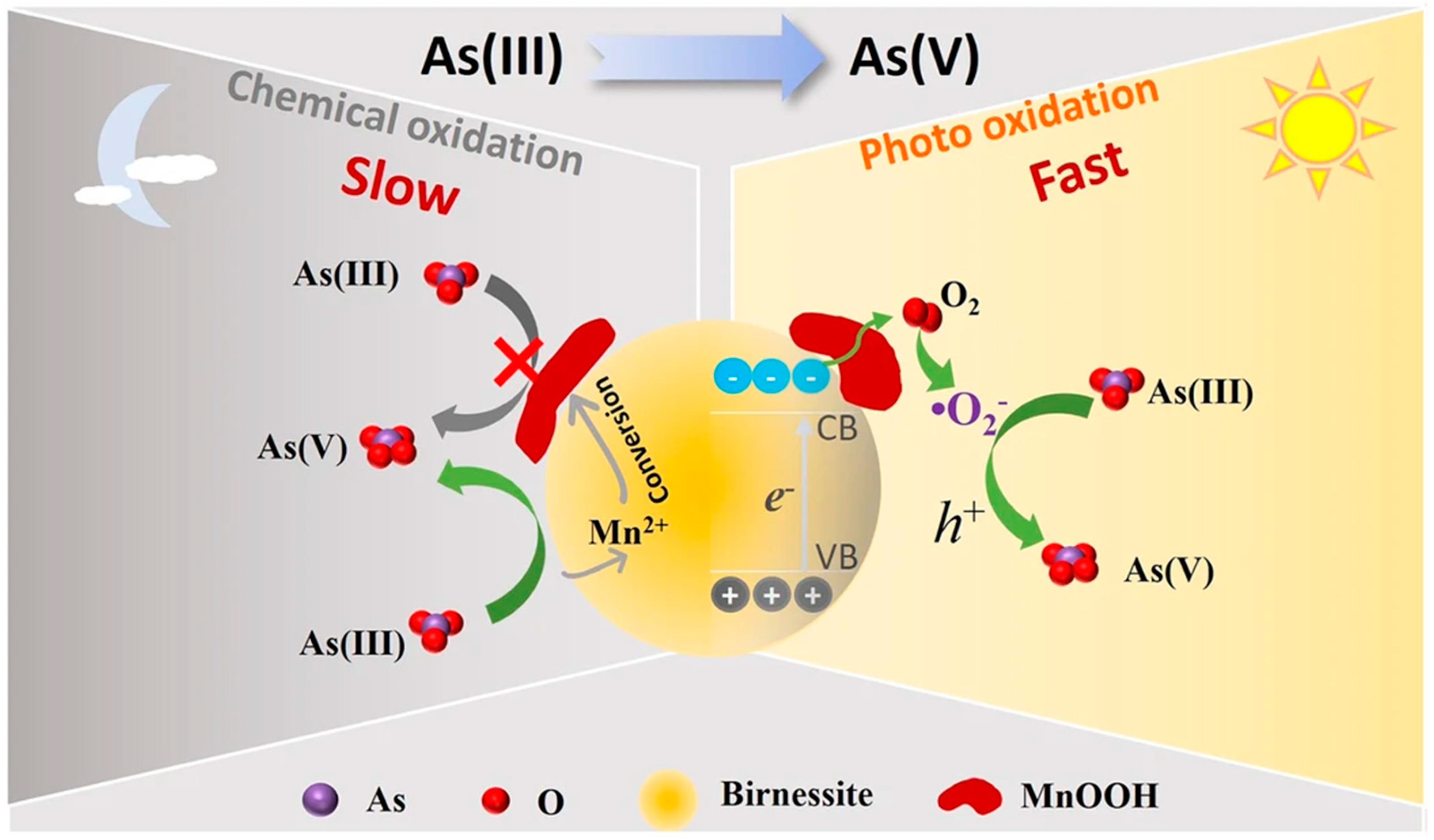
3.1.3. Manganese Oxide Mineral
3.2. Metal Sulfide Materials
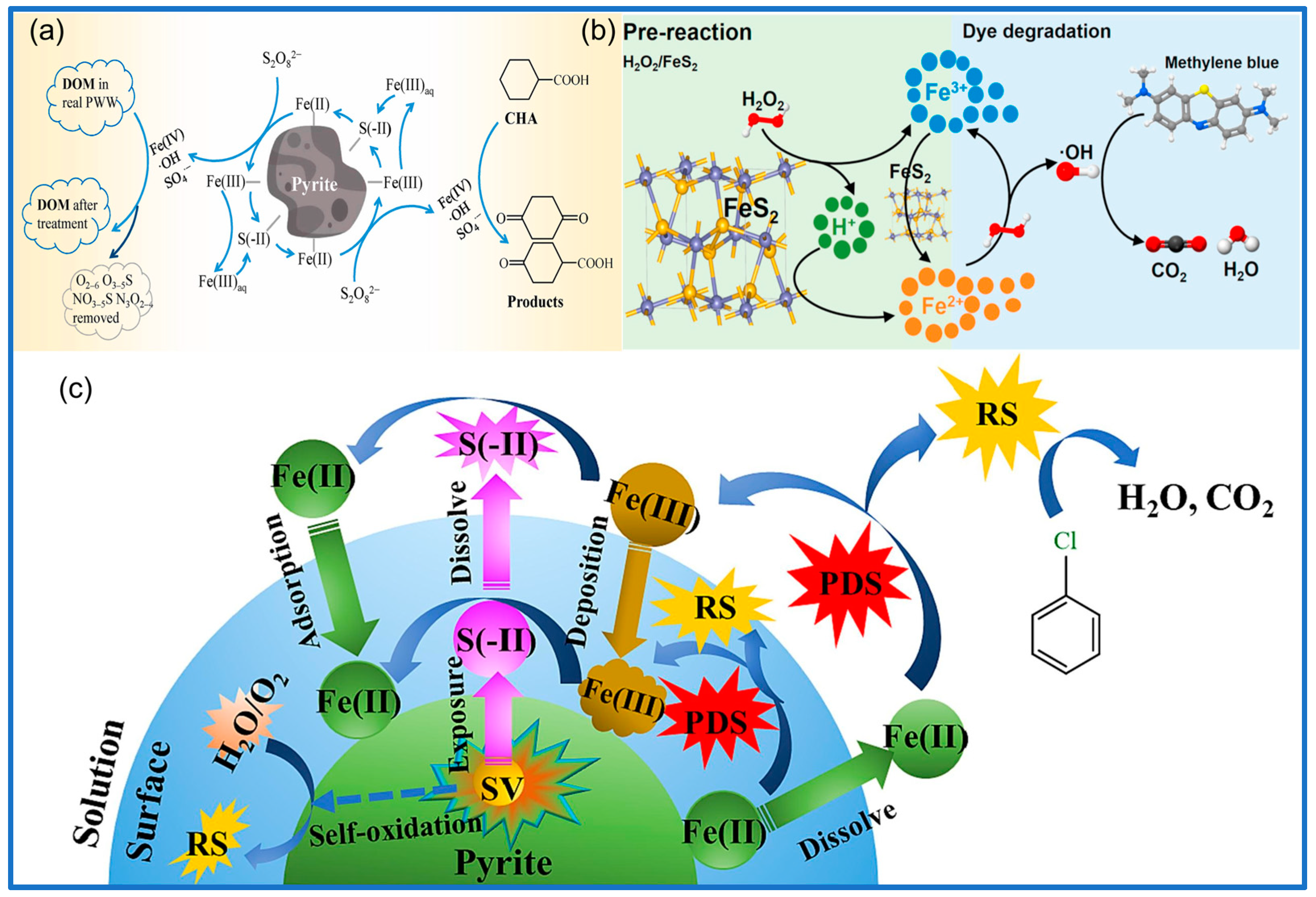
3.2.1. Pyrite
3.2.2. Chalcopyrite
3.2.3. Sphalerite
3.3. Silicates

4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Sam, K.; Coulon, F.; De Gisi, S.; Notarnicola, M.; Labianca, C. Recent Developments and Prospects of Sustainable Remediation Treatments for Major Contaminants in Soil: A Review. Sci. Total Environ. 2024, 912, 168769. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Carrera, P.; Wang, C.; Duc, V.N.; Heynderickx, P.M.; Feng, R.; Guo, S.; Wu, D. Assessment of Hydrogen Peroxide, Persulfate, and Peroxymonosulfate as Oxidizing Agents in Electrochemical Oxidation of Pyridine. Chem. Eng. J. 2025, 503, 158125. [Google Scholar] [CrossRef]
- Babu, S.G.; Ashokkumar, M.; Neppolian, B. The Role of Ultrasound on Advanced Oxidation Processes. Top. Curr. Chem. 2016, 374, 75. [Google Scholar] [CrossRef]
- Verma, P.; Samanta, S.K. Microwave-Enhanced Advanced Oxidation Processes for the Degradation of Dyes in Water. Environ. Chem. Lett. 2018, 16, 969–1007. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, P.; Yang, Y.; Hall, T.; Nie, G.; Yao, Y.; Duan, X.; Wang, S. Degradation of Microplastics by a Thermal Fenton Reaction. ACS EST Eng. 2022, 2, 110–120. [Google Scholar] [CrossRef]
- Chuang, Y.-H.; Chen, S.; Chinn, C.J.; Mitch, W.A. Comparing the UV/Monochloramine and UV/Free Chlorine Advanced Oxidation Processes (AOPs) to the UV/Hydrogen Peroxide AOP under Scenarios Relevant to Potable Reuse. Environ. Sci. Technol. 2017, 51, 13859–13868. [Google Scholar] [CrossRef]
- Liang, C.; Yin, S.; Huang, P.; Yang, S.; Wang, Z.; Zheng, S.; Li, C.; Sun, Z. The Critical Role of Minerals in Persulfate-Based Advanced Oxidation Process: Catalytic Properties, Mechanism, and Prospects. Chem. Eng. J. 2024, 482, 148969. [Google Scholar] [CrossRef]
- Elmaci, G.; Frey, C.E.; Kurz, P.; Zümreoğlu-Karan, B. Water Oxidation Catalysis by Birnessite@iron Oxide Core–Shell Nanocomposites. Inorg. Chem. 2015, 54, 2734–2741. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Walter, E.; Zong, M.; Wang, Y.; Zhang, X.; Qafoku, O.; Wang, Z.; Rosso, K.M. Facet-Specific Photocatalytic Degradation of Organics by Heterogeneous Fenton Chemistry on Hematite Nanoparticles. Environ. Sci. Technol. 2019, 53, 10197–10207. [Google Scholar] [CrossRef]
- Zhang, J.; Wageh, S.; Al-Ghamdi, A.; Yu, J. New Understanding on the Different Photocatalytic Activity of Wurtzite and Zinc-Blende CdS. Appl. Catal. B Environ. 2016, 192, 101–107. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; Fan, Z.; Sun, S.; Feng, Z.; Li, W.; Ding, H. Construction of R-TiO2/n-TiO2 Heterophase Photocatalysts for Efficient Degradation of Organic Pollutants. J. Alloys Compd. 2023, 968, 172127. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Xie, W.; Li, Z.; Zhou, Y.; Qin, R.; Wang, L.; Zhou, J.; Ren, G. One-Step-Modified Biochar by Natural Anatase for Eco-Friendly Cr(VI) Removal. Sustainability 2024, 16, 8056. [Google Scholar] [CrossRef]
- Gong, C.; Zhai, J.; Wang, X.; Zhu, W.; Yang, D.; Luo, Y.; Gao, X. Synergistic Improving Photo-Fenton and Photo-Catalytic Degradation of Carbamazepine over FeS2/Fe2O3/Organic Acid with H2O2 in-Situ Generation. Chemosphere 2022, 307, 136199. [Google Scholar] [CrossRef]
- Liu, C.; Lin, S.; Liu, Y.; Li, M.; Shen, W.; Jiang, N.; Li, F.; Tian, J. Disclosing the Influence Mechanism of Facet-Dependent Pyrite Photo-Activation and Photo-Dissolution Processes on the Reduction of Cr(VI). Environ. Pollut. 2024, 359, 124578. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Fan, C.; Li, X.; Yang, Q.; Wang, D.; Duan, A.; Pan, S. Tourmaline/ZnAL-LDH Nanocomposite Based Photocatalytic System for Efficient Degradation of Mixed Pollutant Wastewater. Sep. Purif. Technol. 2024, 345, 127306. [Google Scholar] [CrossRef]
- Lu, A.H. Photocatalytic Properties of Inorganic Mineral Natural Self-Purification. Acta Petrol. Mineral. 2003, 22, 323–331. [Google Scholar]
- Toledo, A.G.R.; Bevilaqua, D.; Panda, S.; Akcil, A. Hydrometallurgical Processing of Sulfide Minerals from the Perspective of Semiconductor Electrochemistry: A Review. Miner. Eng. 2023, 204, 108409. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for Enhancing the Heterogeneous Fenton Catalytic Reactivity: A Review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Wang, L.; Luo, D.; Hamdaoui, O.; Vasseghian, Y.; Momotko, M.; Boczkaj, G.; Kyzas, G.Z.; Wang, C. Bibliometric Analysis and Literature Review of Ultrasound-Assisted Degradation of Organic Pollutants. Sci. Total Environ. 2023, 876, 162551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, J. Fe-Based Fenton-like Catalysts for Water Treatment: Preparation, Characterization and Modification. Chemosphere 2021, 276, 130177. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent Metal Catalysts in Fenton/Fenton-like Oxidation System: A Critical Review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Pan, S.; Cao, B.; Yuan, D.; Jiao, T.; Zhang, Q.; Tang, S. Complexes of Cupric Ion and Tartaric Acid Enhanced Calcium Peroxide Fenton-like Reaction for Metronidazole Degradation. Chin. Chem. Lett. 2024, 35, 109185. [Google Scholar] [CrossRef]
- Lee, H.; Seong, J.; Lee, K.-M.; Kim, H.-H.; Choi, J.; Kim, J.-H.; Lee, C. Chloride-Enhanced Oxidation of Organic Contaminants by Cu(II)-Catalyzed Fenton-like Reaction at Neutral pH. J. Hazard. Mater. 2018, 344, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hu, H.; Pan, Z.; Pei, F.; Qian, H.; Miao, K.; Guo, S.; Wang, W.; Feng, G. Efficient and Stable Catalysis of Hollow Cu9S5 Nanospheres in the Fenton-like Degradation of Organic Dyes. J. Hazard. Mater. 2020, 396, 122735. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Chen, Y.; Liang, J.; Zhou, L. Producing OH, SO4− and O2− in Heterogeneous Fenton Reaction Induced by Fe3O4-Modified Schwertmannite. Chem. Eng. J. 2020, 393, 124735. [Google Scholar] [CrossRef]
- Chen, F.; Huang, X.-T.; Bai, C.-W.; Zhang, Z.-Q.; Duan, P.-J.; Sun, Y.-J.; Chen, X.-J.; Zhang, B.-B.; Zhang, Y.-S. Advancements in Heterogeneous Activation of Persulfates: Exploring Mechanisms, Challenges in Organic Wastewater Treatment, and Innovative Solutions. Chem. Eng. J. 2024, 481, 148789. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J.; Zhou, Q. Heat-Activated Persulfate Oxidation of Atrazine: Implications for Remediation of Groundwater Contaminated by Herbicides. Chem. Eng. J. 2015, 263, 45–54. [Google Scholar] [CrossRef]
- Kang, D.W.; Kim, J.H.; Lim, J.H.; Kim, Y.; Kang, M.; Shin, J.; Son, S.; Yun, H.; Kim, H.; Park, S.; et al. Promoted Type I and II ROS Generation by a Covalent Organic Framework through Sonosensitization and PMS Activation. ACS Catal. 2022, 12, 9621–9628. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A Review of the Innovations in Metal- and Carbon-Based Catalysts Explored for Heterogeneous Peroxymonosulfate (PMS) Activation, with Focus on Radical vs. Non-Radical Degradation Pathways of Organic Contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Kim, J.; Du, P.; Liu, W.; Luo, C.; Zhao, H.; Huang, C.-H. Cobalt/Peracetic Acid: Advanced Oxidation of Aromatic Organic Compounds by Acetylperoxyl Radicals. Environ. Sci. Technol. 2020, 54, 5268–5278. [Google Scholar] [CrossRef]
- Xing, D.; Shao, S.; Yang, Y.; Zhou, Z.; Jing, G.; Zhao, X. Mechanistic Insights into the Efficient Activation of Peracetic Acid by Pyrite for the Tetracycline Abatement. Water Res. 2022, 222, 118930. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Zhou, H.; Zhang, H.; Zhou, P.; You, J.; Yao, G.; Pan, Z.; Liu, Y.; Lai, B. Heterogeneous Photocatalyst-Driven Persulfate Activation Process under Visible Light Irradiation: From Basic Catalyst Design Principles to Novel Enhancement Strategies. Chem. Eng. J. 2022, 428, 131166. [Google Scholar] [CrossRef]
- Sundaram, I.M.; Kalimuthu, S.; Ponniah, G. Highly Active ZnO Modified g-C3N4 Nanocomposite for Dye Degradation under UV and Visible Light with Enhanced Stability and Antimicrobial Activity. Compos. Commun. 2017, 5, 64–71. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, S.; Zhong, H.; Yan, M.; Yang, X. Activation of Persulfates by Carbonaceous Materials: A Review. Chem. Eng. J. 2021, 418, 129297. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.; Ma, J.; Lu, X.; Qi, J.; Song, S. Strong Promoted Catalytic Ozonation of Atrazine at Low Temperature Using Tourmaline as Catalyst: Influencing Factors, Reaction Mechanisms and Pathways. Chem. Eng. J. 2018, 354, 113–125. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Wang, Y.; Xu, X.; Qin, L.; Wu, Z. Natural Piezoelectric Tourmaline Mineral for Piezocatalytic Decomposition of Organic Dyes under Vibration. J. Am. Ceram. Soc. 2024, 107, 1682–1690. [Google Scholar] [CrossRef]
- Abazari, R.; Heshmatpour, F.; Balalaie, S. Pt/Pd/Fe Trimetallic Nanoparticle Produced via Reverse Micelle Technique: Synthesis, Characterization, and Its Use as an Efficient Catalyst for Reductive Hydrodehalogenation of Aryl and Aliphatic Halides under Mild Conditions. ACS Catal. 2013, 3, 139–149. [Google Scholar] [CrossRef]
- Belaidi, S.; Mammeri, L.; Mechakra, H.; Remache, W.; Benhamouda, K.; Larouk, S.; Kribeche, M.A.; Sehili, T. UV and Solar Light Induced Natural Iron Oxide Activation: Characterization and Photocatalytic Degradation of Organic Compounds. Int. J. Chem. React. Eng. 2019, 17, 20180027. [Google Scholar] [CrossRef]
- Huang, H.H.; Lu, M.C.; Chen, J.-N. Catalytic Decomposition of Hydrogen Peroxide and 2-Chlorophenol with Iron Oxides. Water Res. 2001, 35, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramírez, E.G.; Theng, B.K.G.; Mora, M.L. Clays and Oxide Minerals as Catalysts and Nanocatalysts in Fenton-like Reactions—A Review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, R.; Chen, Q.; Zhou, H.; Liang, X.; Ma, L.; Parker, S.C. The Significant Effect of Photo-Catalyzed Redox Reactions on the Immobilization of Chromium by Hematite. Chem. Geol. 2019, 524, 228–236. [Google Scholar] [CrossRef]
- Choi, J.; Choi, W.; Hwang, H.; Tang, Y.; Jung, H. Natural Sunlight-Driven Oxidation of Mn2+(Aq) and Heterogeneous Formation of Mn Oxides on Hematite. Chemosphere 2024, 348, 140734. [Google Scholar] [CrossRef]
- Chen, N.; Wan, Y.; Zhan, G.; Wang, X.; Li, M.; Zhang, L. Simulated Solar Light Driven Roxarsone Degradation and Arsenic Immobilization with Hematite and Oxalate. Chem. Eng. J. 2020, 384, 123254. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Q.; Young, R.P.; Zhang, X.; Walter, E.D.; Chen, Y.; Nakouzi, E.; Taylor, S.D.; Loring, J.S.; Wang, Z.; et al. Photo-Production of Reactive Oxygen Species and Degradation of Dissolved Organic Matter by Hematite Nanoplates Functionalized by Adsorbed Oxalate. Environ. Sci. Nano 2020, 7, 2278–2292. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Jin, L.; Wang, H.; Huang, Y.; Huang, D.; Liu, X. Peracetic Acid Activation by Modified Hematite for Water Purification: Performance, Degradation Pathways, and Mechanism. Langmuir 2024, 40, 15301–15309. [Google Scholar] [CrossRef]
- Bel Hadjltaief, H.; Sdiri, A.; Gálvez, M.E.; Zidi, H.; Da Costa, P.; Ben Zina, M. Natural Hematite and Siderite as Heterogeneous Catalysts for an Effective Degradation of 4-Chlorophenol via Photo-Fenton Process. ChemEngineering 2018, 2, 29. [Google Scholar] [CrossRef]
- Dai, M.; Dong, X.; Yang, Y.; Wu, Y.; Chen, L.; Jiang, C.; Guo, Z.; Yang, T. Mechanistic Insight into the Impact of Interaction between Goethite and Humic Acid on the Photooxidation and Photoreduction of Bifenthrin. Environ. Res. 2024, 252, 118779. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Zong, M.; Chen, M.; He, H.; Wang, R.; Lu, X. Mn Substitution and Distribution in Goethite and Influences on Its Photocatalytic Properties: A Combined Study Using First-Principles Calculations and Photocatalytic Experiments. Am. Mineral. 2023, 108, 968–977. [Google Scholar] [CrossRef]
- Hu, S.; Li, H.; Wang, P.; Liu, C.; Shi, Z.; Li, F.; Liu, T. Interfacial Photoreactions of Cr(VI) and Oxalate on Lepidocrocite Surface under Oxic and Acidic Conditions: Reaction Mechanism and Potential Implications for Contaminant Degradation in Surface Waters. Chem. Geol. 2021, 583, 120481. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Li, X.; Cao, Y.; Jia, K. Degradation of Organic Pollutants in Natural Magnetite-Activated Peroxymonosulfate System Enhanced by Hydroxylamine: Catalytic Performance, Reaction Pathway, and Enhancement Mechanism. J. Water Process Eng. 2024, 66, 106000. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Zhang, T.; Xu, Z.; Li, Y.; Li, B.; Tian, S. UV Facilitated Synergistic Effects of Polymetals in Ore Catalyst on Peroxymonosulfate Activation: Implication for the Degradation of Bisphenol S. Chem. Eng. J. 2022, 431, 133989. [Google Scholar] [CrossRef]
- Xia, D.; He, H.; Liu, H.; Wang, Y.; Zhang, Q.; Li, Y.; Lu, A.; He, C.; Wong, P.K. Persulfate-Mediated Catalytic and Photocatalytic Bacterial Inactivation by Magnetic Natural Ilmenite. Appl. Catal. B Environ. 2018, 238, 70–81. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ding, Z.; Wang, W.; Song, J.; Li, P.; Liang, J.; Fan, Q. Light Promotes the Immobilization of U(VI) by Ferrihydrite. Molecules 2022, 27, 1859. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Li, Y.; Ding, C.; Wu, J.; Lu, A.; Ding, H.; Qin, S.; Wang, C. Absolute Band Structure Determination on Naturally Occurring Rutile with Complex Chemistry: Implications for Mineral Photocatalysis on Both Earth and Mars. Appl. Surf. Sci. 2018, 439, 660–671. [Google Scholar] [CrossRef]
- Lu, A.; Li, Y.; Lv, M.; Wang, C.; Yang, L.; Liu, J.; Wang, Y.; Wong, K.-H.; Wong, P.-K. Photocatalytic Oxidation of Methyl Orange by Natural V-Bearing Rutile under Visible Light. Sol. Energy Mater. Sol. Cells 2007, 91, 1849–1855. [Google Scholar] [CrossRef]
- Jung, H.; Snyder, C.; Xu, W.; Wen, K.; Zhu, M.; Li, Y.; Lu, A.; Tang, Y. Photocatalytic Oxidation of Dissolved Mn2+ by TiO2 and the Formation of Tunnel Structured Manganese Oxides. ACS Earth Space Chem. 2021, 5, 2105–2114. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Y.; Ding, H.; Li, Y.; Lu, A. The Fine Characterization and Potential Photocatalytic Effect of Semiconducting Metal Minerals in Danxia Landforms. Minerals 2018, 8, 554. [Google Scholar] [CrossRef]
- Zhu, S.; Ho, S.-H.; Jin, C.; Duan, X.; Wang, S. Nanostructured Manganese Oxides: Natural/Artificial Formation and Their Induced Catalysis for Wastewater Remediation. Environ. Sci. Nano 2020, 7, 368–396. [Google Scholar] [CrossRef]
- Crowther, D.L.; Dillard, J.G.; Murray, J.W. The Mechanisms of Co(II) Oxidation on Synthetic Birnessite. Geochim. Cosmochim. Acta 1983, 47, 1399–1403. [Google Scholar] [CrossRef]
- Scott, M.J.; Morgan, J.J. Reactions at Oxide Surfaces. 2. Oxidation of Se(IV) by Synthetic Birnessite. Environ. Sci. Technol. 1996, 30, 1990–1996. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Ok, Y.S.; Oze, C. Cr(VI) Formation Related to Cr(III)-Muscovite and Birnessite Interactions in Ultramafic Environments. Environ. Sci. Technol. 2013, 47, 9722–9729. [Google Scholar] [CrossRef]
- Abernathy, M.J.; Schaefer, M.V.; Vessey, C.J.; Liu, H.; Ying, S.C. Oxidation of V(IV) by Birnessite: Kinetics and Surface Complexation. Environ. Sci. Technol. 2021, 55, 11703–11712. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.; Wang, J.; Wang, W.; Ding, Z.; Liang, J.; Fan, Q. The Photocatalytic Oxidation of As(III) on Birnessite. npj Clean Water 2024, 7, 19. [Google Scholar] [CrossRef]
- Ghanbari, S.; Fatehizadeh, A.; Ebrahimi, A.; Bina, B.; Taheri, E.; Iqbal, H.M.N. Hydrothermally Improved Natural Manganese-Containing Catalytic Materials to Degrade 4-Chlorophenol. Environ. Res. 2023, 226, 115641. [Google Scholar] [CrossRef]
- Yao, Z.; Chen, R.; Han, N.; Sun, H.; Wong, N.H.; Ernawati, L.; Wang, S.; Sunarso, J.; Liu, S. Natural Manganese Ores for Efficient Removal of Organic Pollutants via Catalytic Peroxymonosulfate-Based Advanced Oxidation Processes. Asia-Pac. J. Chem. Eng. 2023, 18, e2907. [Google Scholar] [CrossRef]
- Zhong, C.; Jiang, Y.; Liu, Q.; Sun, X.; Yu, J. Natural Siderite Derivatives Activated Peroxydisulfate toward Oxidation of Organic Contaminant: A Green Soil Remediation Strategy. J. Environ. Sci. 2023, 127, 615–627. [Google Scholar] [CrossRef]
- Ding, J.; Shen, L.; Yan, R.; Lu, S.; Zhang, Y.; Zhang, X.; Zhang, H. Heterogeneously Activation of H2O2 and Persulfate with Goethite for Bisphenol a Degradation: A Mechanistic Study. Chemosphere 2020, 261, 127715. [Google Scholar] [CrossRef]
- Tian, L.; Wang, L.; Wei, S.; Zhang, L.; Dong, D.; Guo, Z. Enhanced Degradation of Enoxacin Using Ferrihydrite-Catalyzed Heterogeneous Photo-Fenton Process. Environ. Res. 2024, 251, 118650. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ding, Z.; Gong, X.; Yu, A.; Wen, S.; Bai, S. Degradation of Residual Xanthate in Flotation Tailing Wastewater by Activated H2O2 with Natural Ilmenite: A Heterogeneous Fenton-like Reaction Catalyzed at Natural pH. Sep. Purif. Technol. 2024, 332, 125819. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Z.; Yu, M.; Wang, Q.; Chen, C.; Ma, J. Efficient Removal of Naphthenic Acids from Real Petroleum Wastewater by Natural Pyrite Activated Persulfate System. J. Environ. Manag. 2023, 348, 119239. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Ye, X.; Ma, J.; Lv, M.; Lin, Z. Removal of Microcystis Aeruginosa by Natural Pyrite-Activated Persulfate: Performance and the Significance of Iron Species. Chem. Eng. J. 2022, 428, 132565. [Google Scholar] [CrossRef]
- Han, Y.; Sun, S.; Zhang, B.; Du, J.; Duan, X. Activation of Peroxymonosulfate by Natural Pyrite for Efficient Degradation of V(IV)-Citrate Complex in Groundwater. J. Colloid Interface Sci. 2022, 617, 683–693. [Google Scholar] [CrossRef]
- Mashayekh-Salehi, A.; Akbarmojeni, K.; Roudbari, A.; van der Hoek, J.P.; Nabizadeh, R.; Dehghani, M.H.; Yaghmaeian, K. Use of Mine Waste for H2O2-Assisted Heterogeneous Fenton-like Degradation of Tetracycline by Natural Pyrite Nanoparticles: Catalyst Characterization, Degradation Mechanism, Operational Parameters and Cytotoxicity Assessment. J. Clean. Prod. 2021, 291, 125235. [Google Scholar] [CrossRef]
- Peng, S.; Feng, Y.; Liu, Y.; Wu, D. Applicability Study on the Degradation of Acetaminophen via an H2O2/PDS-Based Advanced Oxidation Process Using Pyrite. Chemosphere 2018, 212, 438–446. [Google Scholar] [CrossRef]
- Li, C.; Yuan, D.; Yang, K.; Wang, H.; Wang, Z.; Zhang, Q.; Tang, S. Reutilization of Pyrite Tailings in Peracetic Acid-Based Advanced Oxidation Process for Water Purification. Sep. Purif. Technol. 2025, 354, 129155. [Google Scholar] [CrossRef]
- Nie, W.; Mao, Q.; Ding, Y.; Hu, Y.; Tang, H. Highly Efficient Catalysis of Chalcopyrite with Surface Bonded Ferrous Species for Activation of Peroxymonosulfate toward Degradation of Bisphenol a: A Mechanism Study. J. Hazard. Mater. 2019, 364, 59–68. [Google Scholar] [CrossRef]
- Yang, J.; Jia, K.; Lu, S.; Cao, Y.; Boczkaj, G.; Wang, C. Thermally Activated Natural Chalcopyrite for Fenton-like Degradation of Rhodamine B: Catalyst Characterization, Performance Evaluation, and Catalytic Mechanism. J. Environ. Chem. Eng. 2024, 12, 111469. [Google Scholar] [CrossRef]
- Yang, K.; Zhai, Z.; Liu, H.; Zhao, T.; Yuan, D.; Jiao, T.; Zhang, Q.; Tang, S. Peracetic Acid Activation by Natural Chalcopyrite for Metronidazole Degradation: Unveiling the Effects of Cu-Fe Bimetallic Sites and Sulfur Species. Sep. Purif. Technol. 2023, 305, 122500. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, X.; Zhao, X.; Liu, T.; Zhang, H.; Lv, Y.; Wang, L. Efficient Degradation of Minocycline by Natural Bornite-Activated Hydrogen Peroxide and Persulfate: Kinetics and Mechanisms. Environ. Sci. Pollut. Res. 2021, 28, 69314–69328. [Google Scholar] [CrossRef]
- Yang, R.; Zeng, G.; Zhou, Z.; Xu, Z.; Lyu, S. Naphthalene Degradation Dominated by Homogeneous Reaction in Fenton-like Process Catalyzed by Pyrite: Mechanism and Application. Sep. Purif. Technol. 2023, 310, 123150. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Cao, Y. A Novel Strategy for Enhancing Heterogeneous Fenton Degradation of Dye Wastewater Using Natural Pyrite: Kinetics and Mechanism. Chemosphere 2021, 272, 129883. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Z.; Wang, C.; Hu, Y.; Xu, X.; Zhao, F.; Pi, Y.; Qian, J.; Hu, F.; Peng, X. Activation of PAA by Pyrite for the Degradation of Tetracycline with Adjusted pH Conditions: The Overlooked Role of Sulfur species. Sep. Purif. Technol. 2025, 361, 131225. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, P.; Zhang, Z.; Wang, C.; Wang, Q.; Rončević, S.D.; Sun, H. The Interface Mechanism of Ball-Milled Natural Pyrite Activating Persulfate to Degrade Monochlorobenzene in Soil: Intrinsic Synergism of S and Fe Species. Sep. Purif. Technol. 2024, 341, 126946. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Xiang, W.; Liu, X.; Wu, X.; Zhou, T. Catalytic Activation of Persulfate by Mechanically-Treated Natural Pyrite: Revisit on the Dominant Role of Sulfur Vacancies and Size Effect. Chem. Eng. J. 2022, 450, 138269. [Google Scholar] [CrossRef]
- Wu, C.; Su, L.; Zhang, B.; Wang, X.; Wang, M.; Zhang, J.; Wang, Q. Ball Milling of Pyrite in Air to Significantly Promote the Fenton Reaction: A Mechanistic Study. J. Hazard. Mater. 2024, 480, 136081. [Google Scholar] [CrossRef]
- Khosravi, A.A.; Kundu, M.; Jatwa, L.; Deshpande, S.K.; Bhagwat, U.A.; Sastry, M.; Kulkarni, S.K. Green Luminescence from Copper Doped Zinc Sulphide Quantum Particles. Appl. Phys. Lett. 1995, 67, 2702–2704. [Google Scholar] [CrossRef]
- Wang, H.; Liao, B.; Hu, M.; Ai, Y.; Wen, L.; Yang, S.; Ye, Z.; Qin, J.; Liu, G. Heterogeneous Activation of Peroxymonosulfate by Natural Chalcopyrite for Efficient Remediation of Groundwater Polluted by Aged Landfill Leachate. Appl. Catal. B Environ. 2022, 300, 120744. [Google Scholar] [CrossRef]
- Zhou, B.X.; Zhou, Z.G.; Huang, G.F.; Xiong, D.N.; Yan, Q.; Huang, W.Q.; Wang, Q.L. Mass Production of ZnxCd1−xS Nanoparticles with Enhanced Visible Light Photocatalytic Activity. Mater. Lett. 2015, 158, 432–435. [Google Scholar] [CrossRef]
- Lee, G.-J.; Anandan, S.; Masten, S.J.; Wu, J.J. Photocatalytic Hydrogen Evolution from Water Splitting Using Cu Doped ZnS Microspheres under Visible Light Irradiation. Renew. Energy 2016, 89, 18–26. [Google Scholar] [CrossRef]
- Li, Y.; Lu, A.; Wang, C.; Wu, X. Characterization of Natural Sphalerite as a Novel Visible Light-Driven Photocatalyst. Sol. Energy Mater. Sol. Cells 2008, 92, 953–959. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Lu, A.; Yan, Y.; Wang, C.; Wong, P.-K. Photocatalytic Reduction of Carbon Tetrachloride by Natural Sphalerite under Visible Light Irradiation. Sol. Energy Mater. Sol. Cells 2011, 95, 1915–1921. [Google Scholar] [CrossRef]
- Wu, M.; Song, S.; Wang, T.; Sun, W.; Xu, S.; Yang, Y. Natural Sphalerite Photocatalyst for Treatment of Oily Wastewater Produced by Solvent Extraction from Spent Lithium-Ion Battery Recycling. Appl. Catal. B Environ. 2022, 313, 121460. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Li, G.; Liu, Z.; Wong, P.K.; An, T. Mechanism Insights into Bacterial Sporulation at Natural Sphalerite Interface with and without Light Irradiation: The Suppressing Role in Bacterial Sporulation by Photocatalysis. Environ. Int. 2022, 168, 107460. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, A.; Li, Y.; Zhang, L.; Yip, H.Y.; Zhao, H.; An, T.; Wong, P.-K. Naturally Occurring Sphalerite as a Novel Cost-Effective Photocatalyst for Bacterial Disinfection under Visible Light. Environ. Sci. Technol. 2011, 45, 5689–5695. [Google Scholar] [CrossRef]
- Li, C.; Jing, H.; Lv, B.; Zhou, D.; Fu, S.; Tang, X.; Wang, Z.; Wu, W.; Jiang, D. Interfacial Chemical Bonds Enhanced Photocatalytic Performance of Kaolinite/NiFe Layered Double Oxide Heterostructure for Photocatalytic Activated Peroxymonosulfate. Appl. Clay Sci. 2024, 260, 107520. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Dong, X.; Sun, Z.; Duan, X.; Ren, B.; Zheng, S.; Dionysiou, D.D. Highly Efficient Activation of Peroxymonosulfate by Natural Negatively-Charged Kaolinite with Abundant Hydroxyl Groups for the Degradation of Atrazine. Appl. Catal. B Environ. 2019, 247, 10–23. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wang, X.; Yang, S.; Tan, Y.; Yuan, F.; Zheng, S.; Dionysiou, D.D.; Sun, Z. Defect Engineering Modulated Iron Single Atoms with Assist of Layered Clay for Enhanced Advanced Oxidation Processes. Small 2022, 18, 2204793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.; Jiang, L.; Dai, H.; Zhao, Y.; Guan, X.; Bai, J.; Wang, H. Manipulation of the Halloysite Clay Nanotube Lumen for Environmental Remediation: A Review. Environ. Sci. Nano 2022, 9, 841–866. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite Nanotubes as Support for Metal-Based Catalysts. J. Mater. Chem. A Mater. Energy Sustain. 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Bi, H.; Zhou, S.; Chen, J.; Zhang, S.; Huang, Y.; Chang, F.; Zhang, H.; Wågberg, T.; et al. Nanomanganese Cobaltate-Decorated Halloysite Nanotubes for the Complete Degradation of Ornidazole via Peroxymonosulfate Activation. J. Colloid Interface Sci. 2023, 630, 855–866. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, G.; Yang, Y.; Niu, Y.; Gao, W.; Li, Z. Enhanced Ornidazole Degradation via Peroxymonosulfate Activation Using Nano CoFe2O4-Decorated Halloysite Nanotubes: A High-Efficiency and Stable Catalyst Approach. Adv. Sustain. Syst. 2024, 9, 2400677. [Google Scholar] [CrossRef]
- Zhao, X.; Duan, L.; Chen, M.; Yang, P.; Liu, Q.; Liu, Y.; Zhang, H.; He, Z.; Hu, G.; Zhou, Y. Regulating the Microenvironment of Atomically Dispersed Cobalt to Achieve the Record-Breaking Mineralization and 100. Chem. Eng. J. 2024, 485, 149928. [Google Scholar] [CrossRef]
- Al Kausor, M.; Sen Gupta, S.; Bhattacharyya, K.G.; Chakrabortty, D. Montmorillonite and Modified Montmorillonite as Adsorbents for Removal of Water Soluble Organic Dyes: A Review on Current Status of the Art. Inorg. Chem. Commun. 2022, 143, 109686. [Google Scholar] [CrossRef]
- Gao, C.; Zhao, G.; Zuo, X.; Yang, H. Molten Salt Induced Strong Interaction between Co3O4 and Montmorillonite for the Promoted Peroxymonosulfate Activation toward Tetracycline Degradation. Appl. Clay Sci. 2024, 255, 107414. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Zhong, S.; Chen, W.; Feng, C.; Yang, S. Nano Zero-Valent Iron/Montmorillonite Composite for the Removal of Cr(VI) from Aqueous Solutions: Characterization, Performance, and Mechanistic Insights. Appl. Clay Sci. 2024, 253, 107345. [Google Scholar] [CrossRef]
- Rehman, S.; Sun, H.; Hanif, M.; Peng, T.; Tang, X.; Satti, O.T.; Bilal, M. Environmentally Friendly Nitrogen Doped Paired Mineral-Carbon for Catalytic Degradation of Diethyl Phthalate and Crop Damage Mitigation. Chem. Eng. J. 2025, 508, 160321. [Google Scholar] [CrossRef]
- Chen, G.; Nengzi, L.; Li, B.; Gao, Y.; Zhu, G.; Cheng, X. Octadecylamine Degradation through Catalytic Activation of Peroxymonosulfate by FeMn Layered Double Hydroxide. Sci. Total Environ. 2019, 695, 133963. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, X.; Yang, Q.; Chen, F.; Wang, S.; Ma, Y.; Wu, Y.; Zhu, X.; Huang, X.; Wang, D. Heterogeneous Activation of Peroxymonosulfate Using Mn-Fe Layered Double Hydroxide: Performance and Mechanism for Organic Pollutant Degradation. Sci. Total Environ. 2019, 663, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, N.; Sadiq, M.; Abdennouri, M.; Qourzal, S.; Khataee, A.; Sillanpää, M.; Barka, N. Recent Advances in the Synthesis and Environmental Catalytic Applications of Layered Double Hydroxides-Based Materials for Degradation of Emerging Pollutants through Advanced Oxidation Processes. Mater. Res. Bull. 2022, 154, 111924. [Google Scholar] [CrossRef]
- Li, D.; Yao, Z.; Lin, J.; Tian, W.; Zhang, H.; Duan, X.; Wang, S. Nano-Sized FeVO4·1.1H2O and FeVO4 for Peroxymonosulfate Activation towards Enhanced Photocatalytic Activity. J. Environ. Chem. Eng. 2022, 10, 107199. [Google Scholar] [CrossRef]
- Hareendran, A.; Dais, E.; Shinoy, D.; Srikripa, S.; Shibu, G.M.; Kurian, M. Nitrogen- and Sulfur-Doped Zinc Ferrite Nanoparticles as Efficient Heterogeneous Catalysts in Advanced Oxidation Processes. J. Phys. Chem. Solids 2022, 161, 110398. [Google Scholar] [CrossRef]
- Mao, L.B.; Meng, Y.F.; Meng, X.S.; Yang, B.; Yang, Y.L.; Lu, Y.J.; Yang, Z.Y.; Shang, L.M.; Yu, S.-H. Matrix-Directed Mineralization for Bulk Structural Materials. J. Am. Chem. Soc. 2022, 144, 18175–18194. [Google Scholar] [CrossRef]
| Mineral Materials | Band Gap Eg/eV | Wavelength/nm |
|---|---|---|
| Hematite (Fe2O3) | 2.20 | 565 |
| Goethite (FeOOH) | 2.60 | 478 |
| Anatase (TiO2) | 3.20 | 388 |
| Pyrolusite (MnO2) | 0.25 | 4972 |
| Ilmenite (FeTiO3) | 2.80 | 444 |
| Pyrite (FeS2) | 0.95 | 1309 |
| Alabandite (MnS) | 3.00 | 414 |
| Molybdenite (MoS) | 1.17 | 1062 |
| Greenokite (CdS) | 2.40 | 518 |
| Chalcopyrite (CuFeS2) | 0.35 | 3552 |
| Reaction | E (V) vs. NHE (pH = 0) |
|---|---|
| H2O + h+ → OH + H+ | 2.38 |
| 2H2O + 2h+ → H2O2 + 2H+ | 1.763 |
| H2O2 + h+ → •O2− + 2H+ | 1.72 |
| O2 + e− → •O2− | −0.33 |
| O2 + H2O + e− → H2O2 + OH− | −0.134 |
| H2O2 + e− → •OH + OH− | 0.93 |
| Mineral | Target Pollutant | Oxidant Concentration | Catalyst Dosage | Time | pH | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Hematite | Roxarsone | Oxalate, 2.0 mM | 0.5 g L−1 | 6 h | 4.5 | 85.10% | [46] |
| Hematite | Cefazolin | PAA, 0.4 mM | 0.3 g L−1 | 80 min | 7 | 98.10% | [49] |
| Siderite | 2-chlorophenol | PDS, 0.5 mM | 0.05 g⋅L−1 | 180 min | 8.1 | 82.50% | [70] |
| Goethite | Bisphenol A | H2O2, 1 mM | 0.5 g L−1 | 240 min | 3.5 | 87.60% | [71] |
| Magnetite | Rhodamine B | PMS, 1 mM | 0.2 g L−1 | 60 min | 7 | 94.78% | [54] |
| Natural manganese-containing minerals | 4-Chlorophenol | O3, 0.6 mg min−1 | 1.0 g L−1 | 15 min | 7 | 84.11% | [68] |
| Ferrihydrite | Enoxacin | H2O2, 10 mM | 0.4 g L−1 | 120 min | 3 | 89.70% | [72] |
| Ilmenite | Sodium butyl xanthate | H2O2, 2 mM | 4.0 g L−1 | 80 min | 8.7 | 92.52% | [73] |
| Minerals | Target Pollutants | Oxidant Concentration | Catalyst Dosage | Time | pH | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Pyrite | Cyclohexanoic acid | PS, 4.0 mM | 2.0 g L−1 | 120 min | 3–11 | 85.10% | [74] |
| Pyrite | Tetracycline | H2O2, 5 mM | 1.0 g L−1 | 60 min | 4.1 | 85% | [77] |
| Pyrite | V(IV)-citrate | PMS, 5 mM | 8.0 g L−1 | 180 min | N.A. | 99.40% | [76] |
| Pyrite | Acetaminophen | PDS, 5 mM | 2.0 g L−1 | 300 min | 4 | 100% | [78] |
| Pyrite | sulfamethoxazole | PAA, 460 μM | 0.3 g L−1 | 50 min | 5.8 | 93.67% | [79] |
| Chalcopyrite | Bisphenol A | PMS, 0.3 mM | 0.1 g L−1 | 20 min | 6 | 99.70% | [80] |
| Chalcopyrite | Rhodamine B | H2O2, 43 mM | 0.75 g L−1 | 50 min | 5.1 | 97.20% | [81] |
| Chalcopyrite | metronidazole | PAA, 460 μM | 4.0 g L−1 | 30 min | 3 | 83.92% | [82] |
| Bornite | Tetracycline | PDS, 11.1 mM | 3.5 g⋅L−1 | 180 min | 4.5 | 87.50% | [83] |
| Catalyst | Binding Energy (eV) | Atom Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| Zn 2p | S 2p | O 1s | Fe 2p | Cu 2p | Zn/S | Fe/S | Cu/S | |
| Natural sphalerite | 1022.15 | 161.68, 162.86, 169.14 | 532.11 | 712.48 | 932.26 | 0.694 | 0.329 | 0.033 |
| Pure sphalerite | 1021.58 | 161.7 | 531.64 | - | - | 1.06 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Yang, Y.; Gan, L. Utilization of Natural Mineral Materials in Environmental Remediation: Processes and Applications. Minerals 2025, 15, 318. https://doi.org/10.3390/min15030318
Xu D, Yang Y, Gan L. Utilization of Natural Mineral Materials in Environmental Remediation: Processes and Applications. Minerals. 2025; 15(3):318. https://doi.org/10.3390/min15030318
Chicago/Turabian StyleXu, Di, Yongkui Yang, and Lingqun Gan. 2025. "Utilization of Natural Mineral Materials in Environmental Remediation: Processes and Applications" Minerals 15, no. 3: 318. https://doi.org/10.3390/min15030318
APA StyleXu, D., Yang, Y., & Gan, L. (2025). Utilization of Natural Mineral Materials in Environmental Remediation: Processes and Applications. Minerals, 15(3), 318. https://doi.org/10.3390/min15030318