The Role of Fluid Chemistry in the Diagenetic Transformation of Detrital Clay Minerals: Experimental Insights from Modern Estuarine Sediments
Abstract
1. Introduction
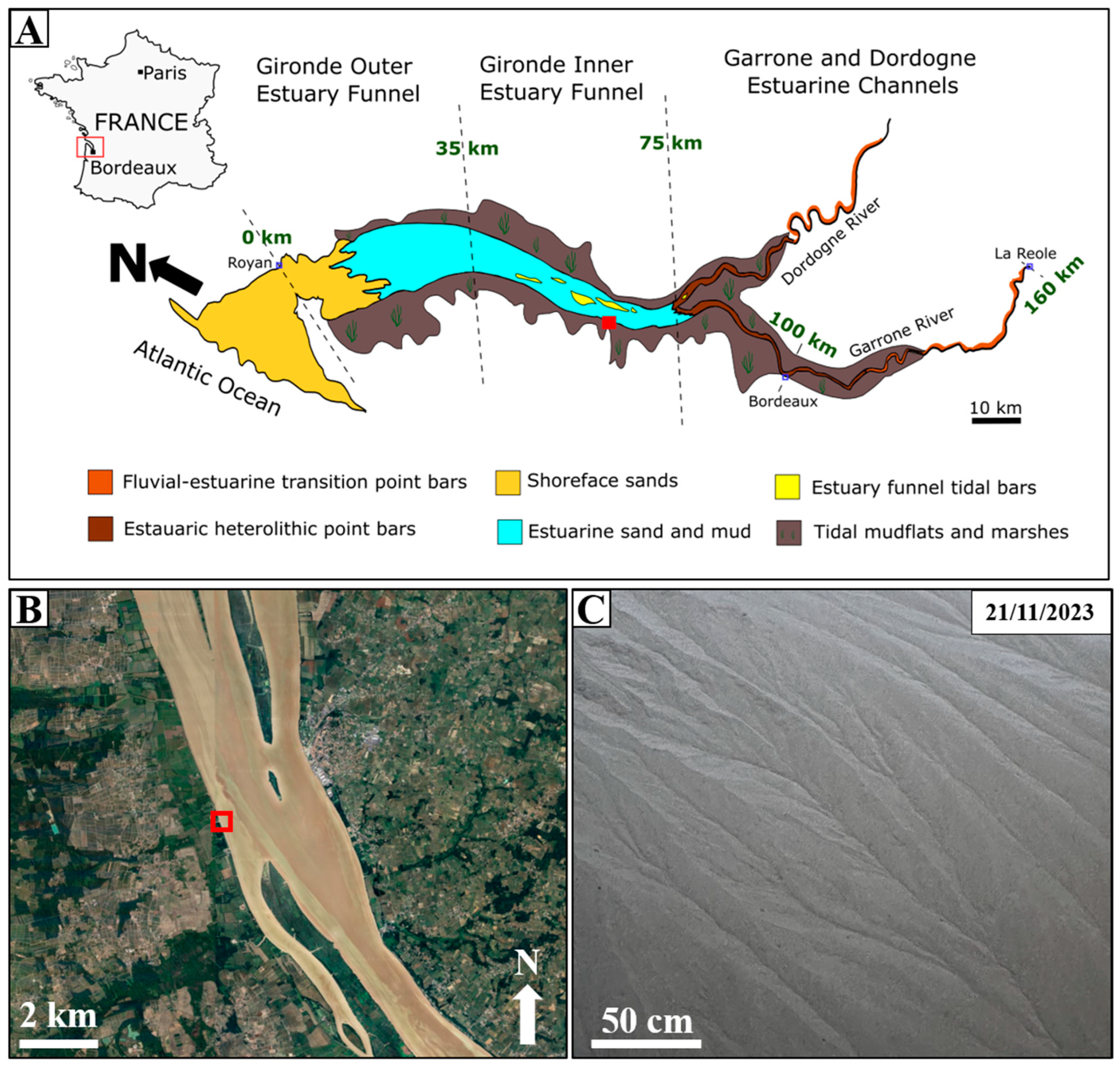
2. Study Area and Geologic Background of the Sample
3. Methods
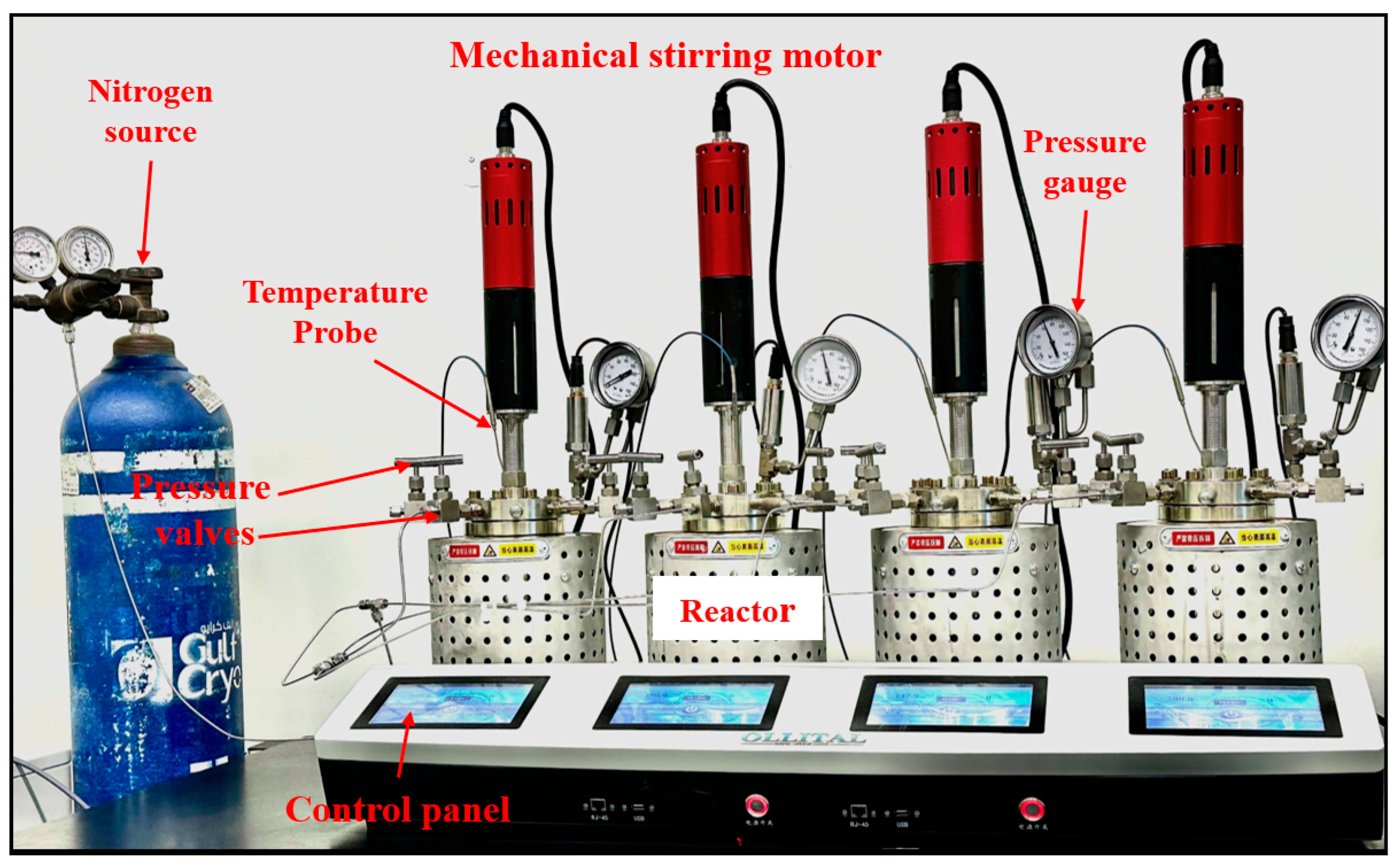
3.1. Hydrothermal Experiments
3.2. Analytical Techniques
3.2.1. SEM/SEM-EDS Analyses
3.2.2. XRF Analysis
3.2.3. Bulk and Clay Fraction XRD Analysis
3.2.4. STEM Analysis
3.2.5. ICP-OES Analysis
3.2.6. FIB-SEM Analysis
4. Results
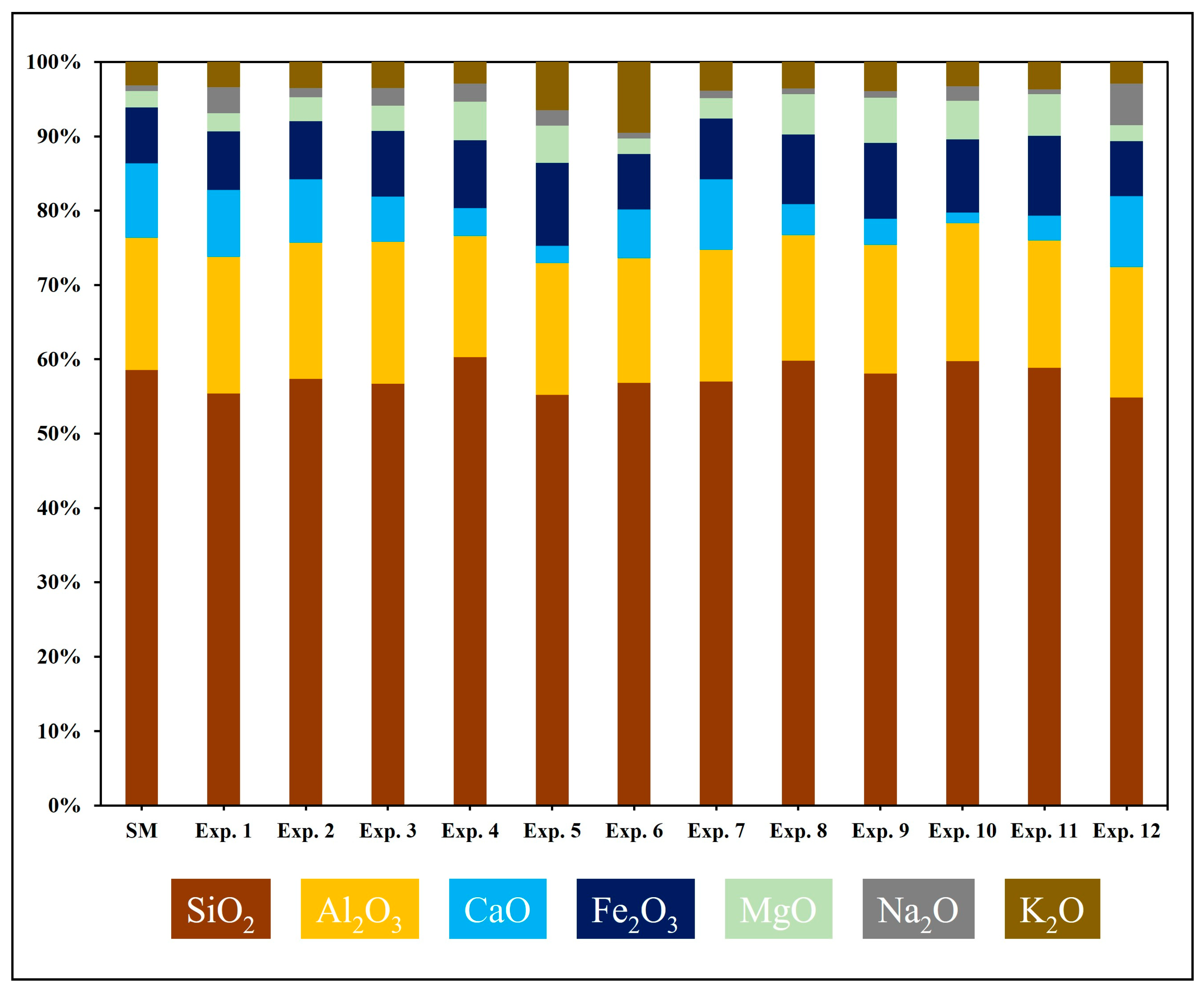
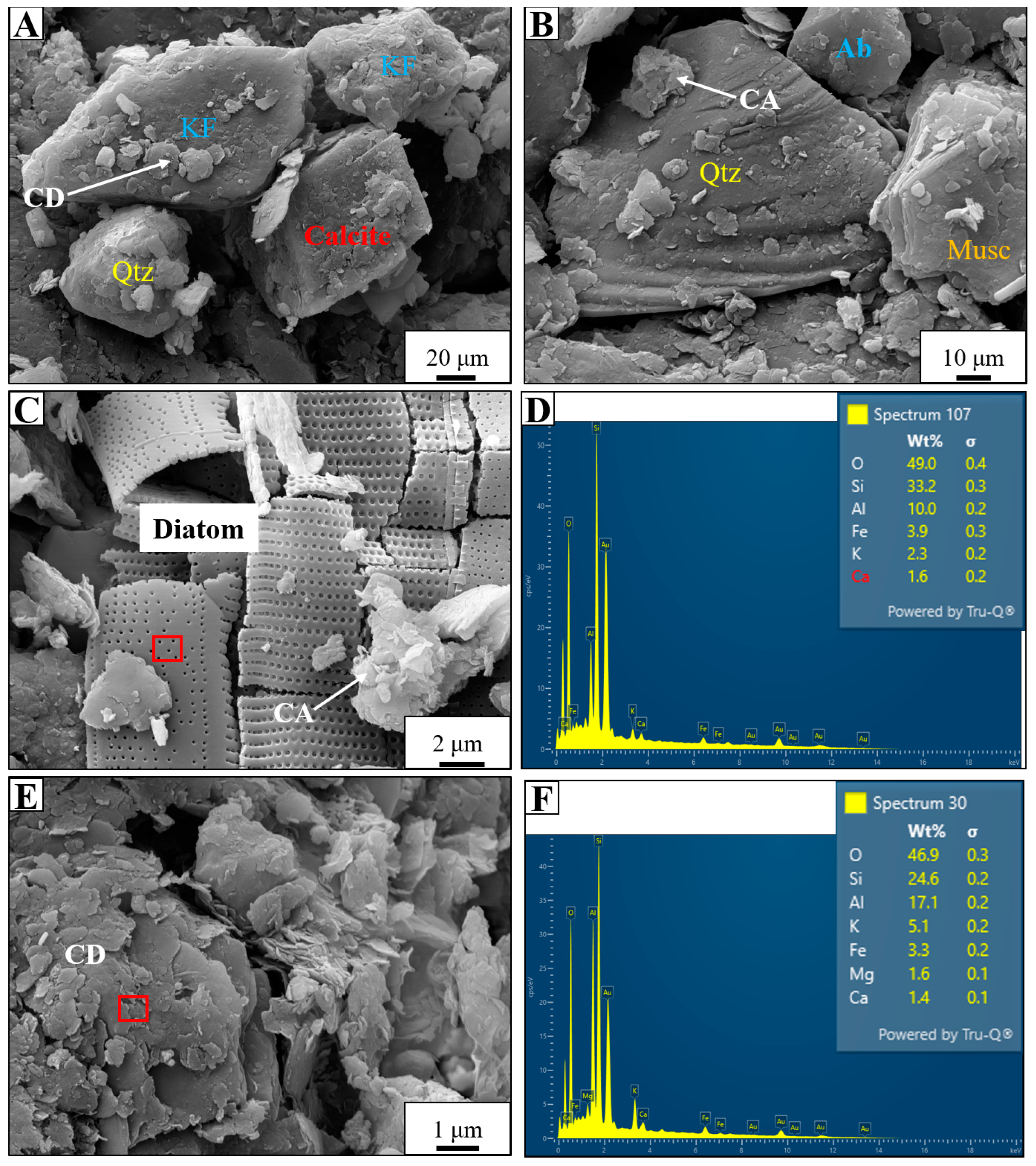
4.1. Compositional Evaluation of the Starting Material
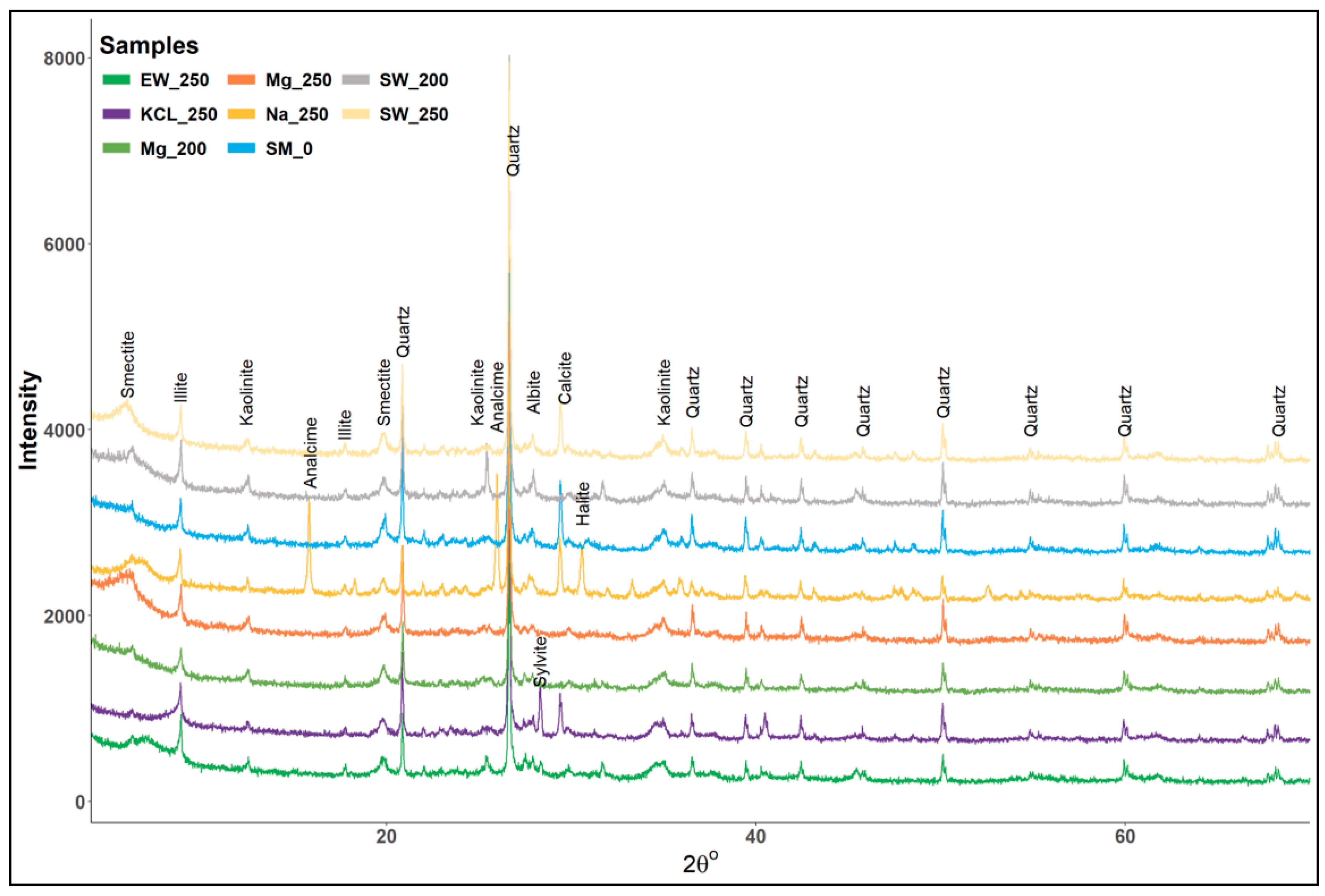
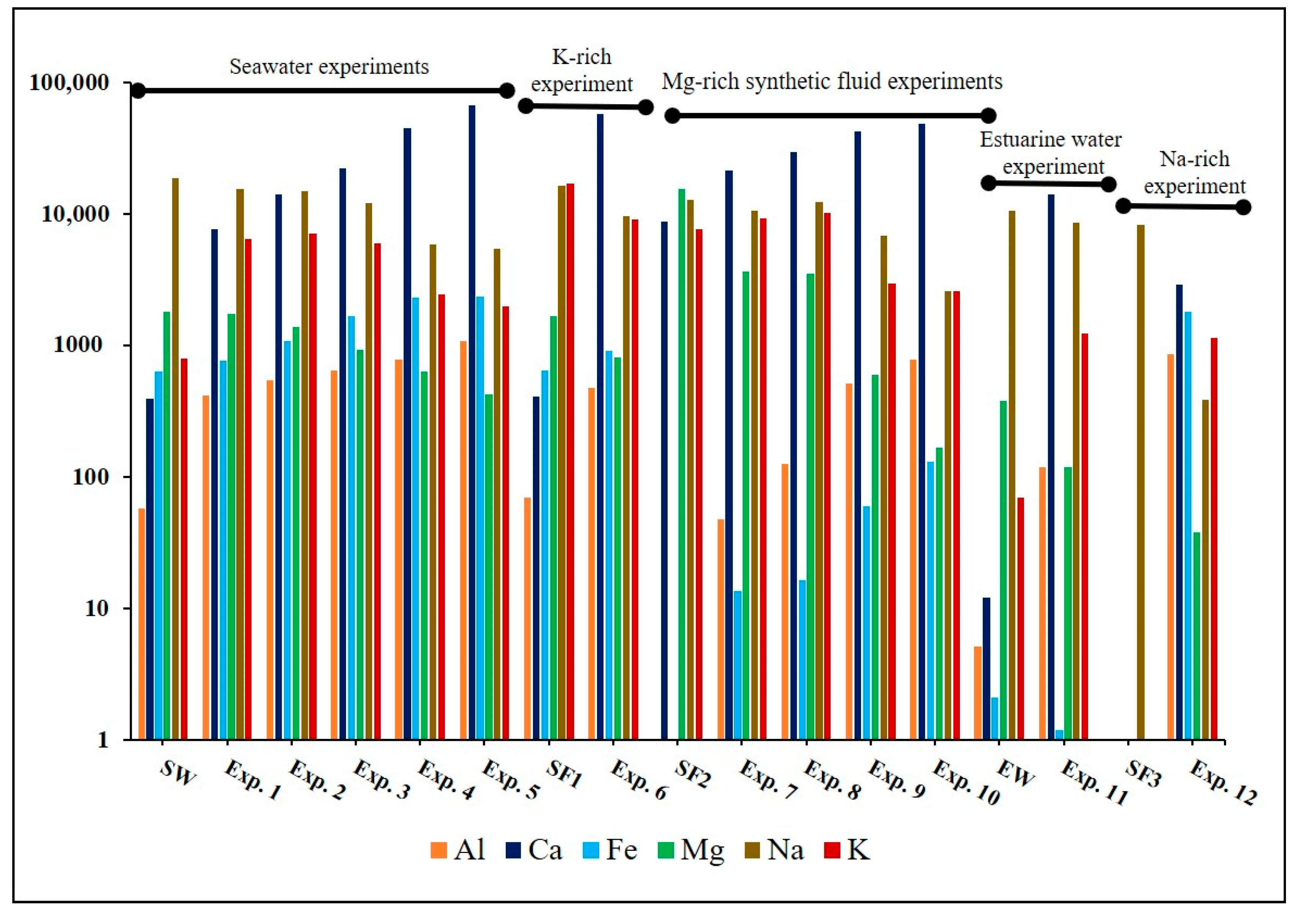
4.2. Geochemical and Mineralogical Evaluation of Post-Experiment Samples
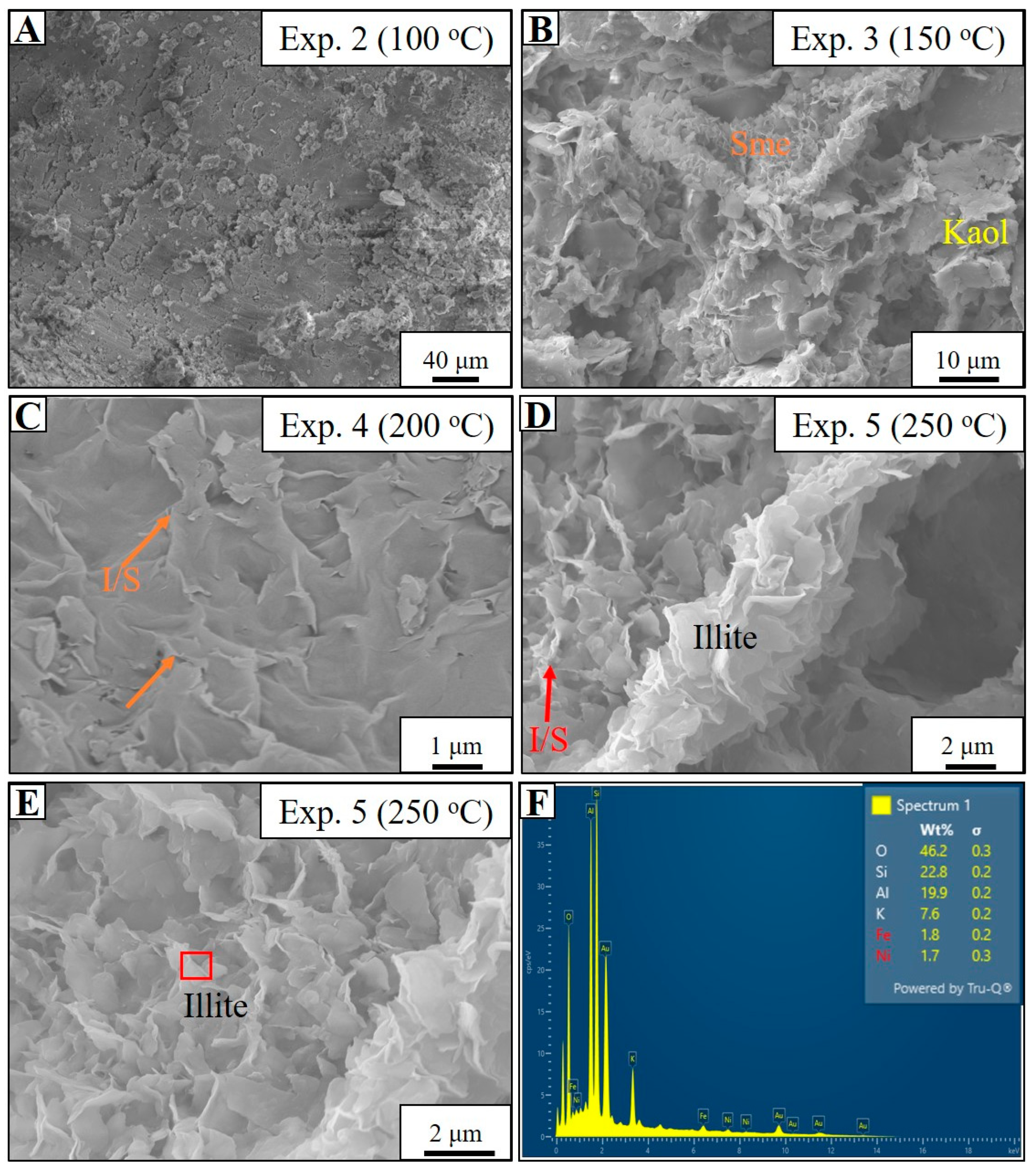
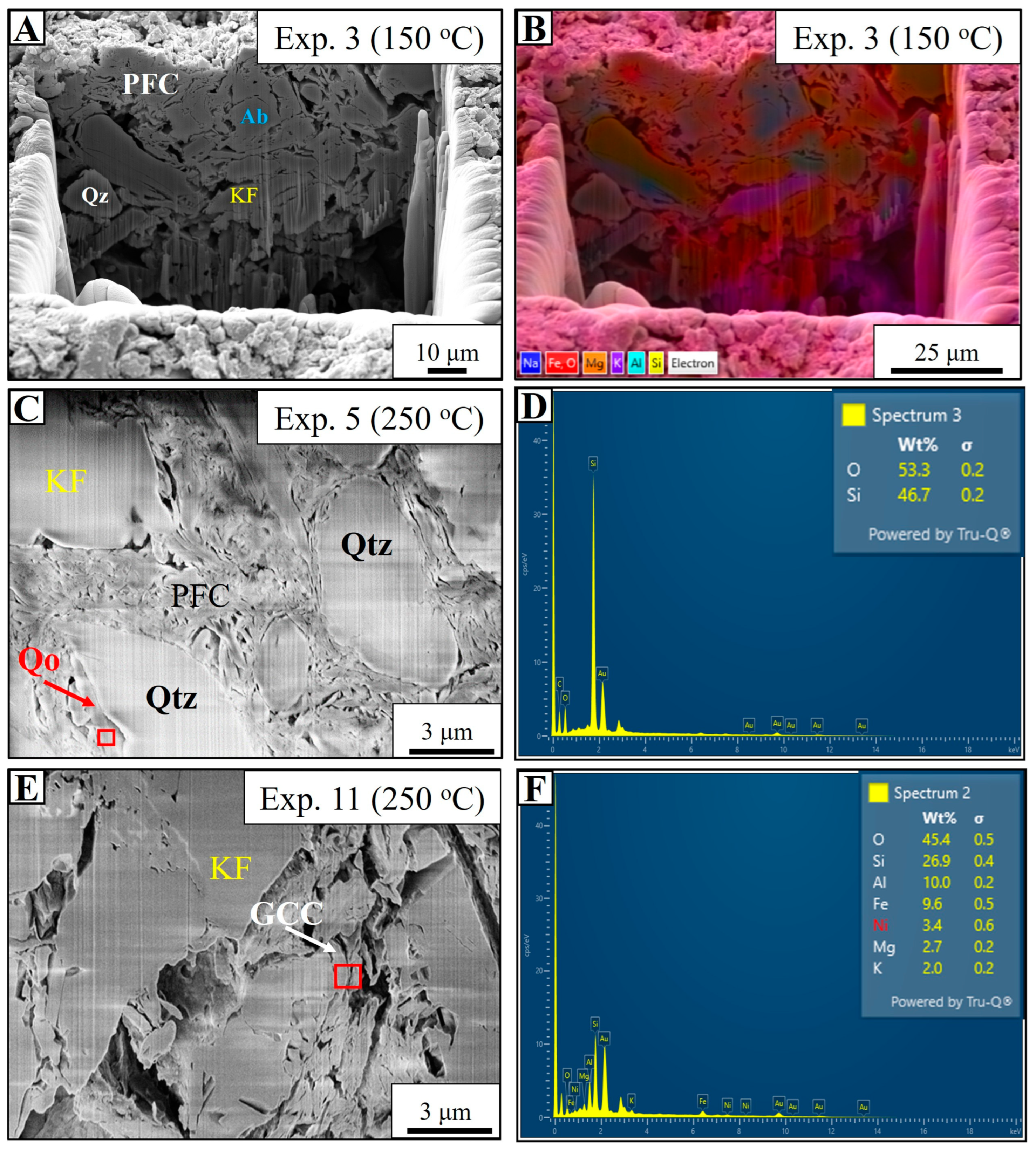
4.3. Seawater-Based Experiments (Experiments 1–6)
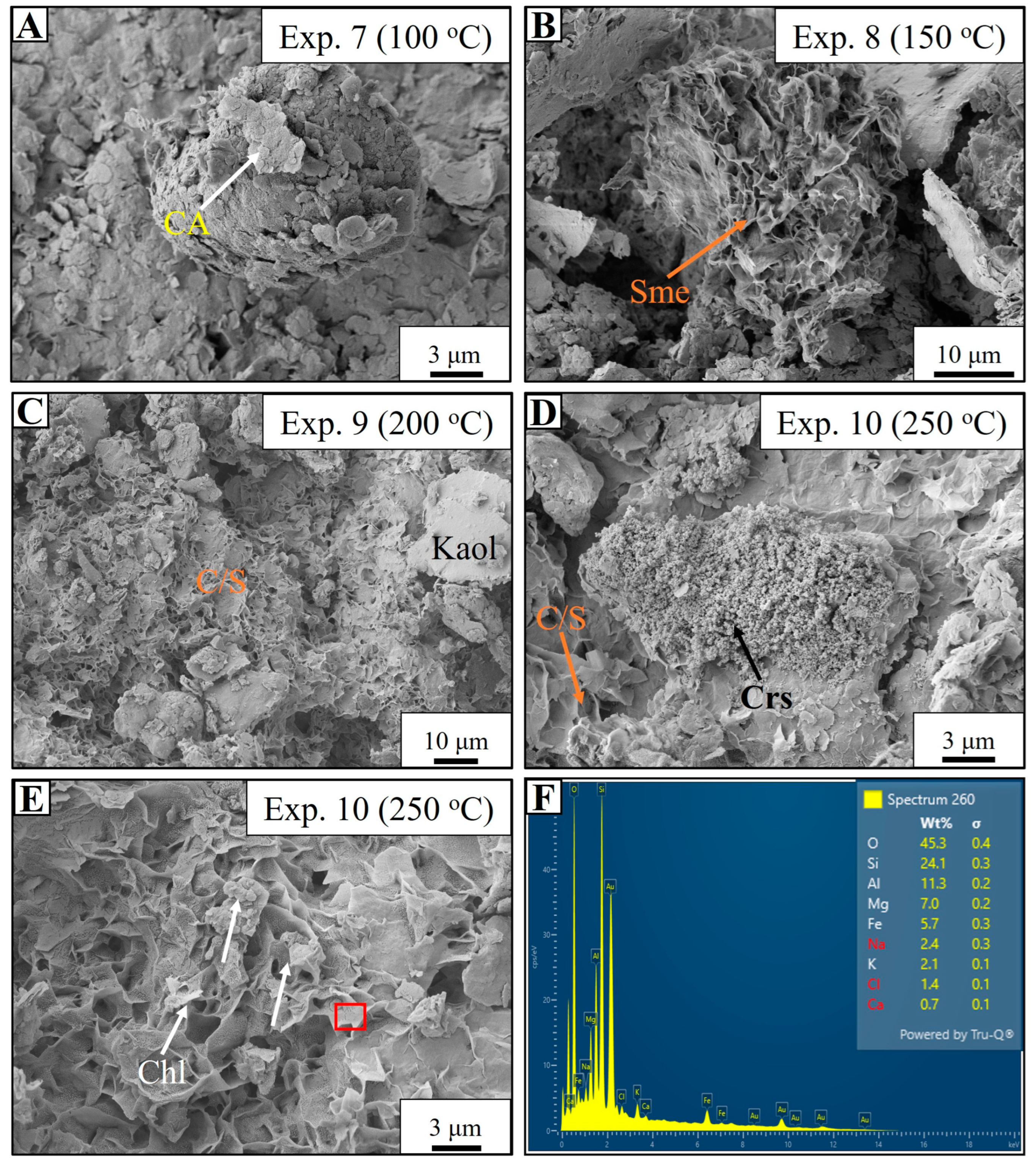
4.4. Mg-Rich Synthetic Fluid-Based Experiments (Experiments 7–10)
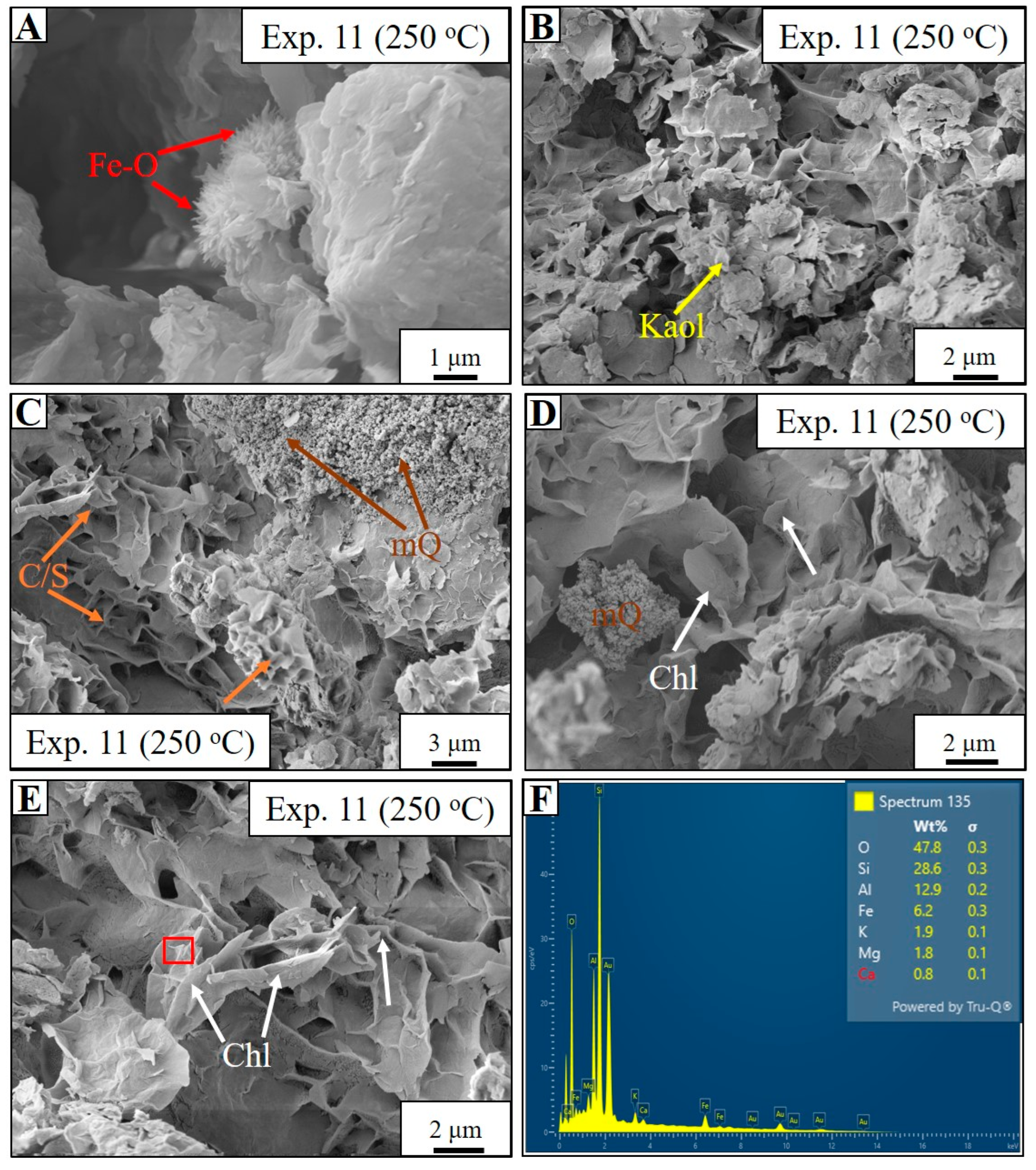
4.5. Estuarine Water-Based Experiment (Experiment 11)
4.6. Na-Rich Synthetic Fluid-Based Experiment (Experiment 12)
4.7. FIB-SEM Observations
5. Discussion
5.1. Controls and Mechanisms of Authigenic Illite Formation
5.2. Authigenic Formation of Chlorite
5.3. Role of Fluid Chemistry in the Transformation Pathways of Modern Estuarine Sediments
5.4. Relevance of Hydrothermal Experiments to Natural Sandstone Diagenesis
6. Conclusions
- During the hydrothermal experiments, the dissolution of detrital feldspars and clay aggregates occurred at temperatures below 100 °C, followed by the authigenic formation of smectite at 150 °C.
- At higher temperatures (150 °C to 250 °C), K-rich fluids (SW, SF1) facilitated the illitization of smectite, replicating potassium-driven processes observed in natural reservoirs. This transformation predominantly occurred via dissolution-crystallization, with the required K+ supplied by the dissolution of K-feldspar and the experimental solutions.
- The chloritization of smectite occurred in both Mg-rich synthetic fluids (SF2) and natural Gironde estuary water (EW) at 250 °C, producing chlorites with distinct chemistries. SF2 yielded Mg-rich chlorites, while EW produced Fe-rich chlorites, consistent with diagenetic trends in marginal marine environments, where Fe-rich chlorites are associated with estuarine and deltaic systems.
- The formation of authigenic chlorites involved a combination of dissolution-crystallization and solid-state transformation. The transformation of smectite into chlorite released abundant silica into the experimental solutions, resulting in the precipitation of microcrystalline quartz and quartz overgrowths.
- Analcime (zeolite) was synthesized under highly alkaline (pH 11.32), Na-rich conditions (SF3), advancing the understanding of zeolite formation beyond its traditional association with volcanic glass precursors.
- The synthesized mineral phases exhibited chemical and morphological similarities to those found in natural sedimentary systems, highlighting the critical role of fluid chemistry in influencing diagenetic pathways and improving our understanding of detrital clay transformations during burial diagenesis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heald, M.T.; Larese, R.E. Influence of Coatings on Quartz Cementation. J. Sediment. Res. 1974, 44, 1269–1274. [Google Scholar] [CrossRef]
- Pittman, E.D.; Larese, R.E.; Heald, M.T. Clay Coats: Occurrence and Relevance to Preservation of Porosity in Sandstones. In Origin, Diagenesis, and Petrophysics of Clay Minerals in Sandstones; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1992; Volume 47. [Google Scholar]
- Worden, R.; Morad, S. Quartz Cementation in Oil Field Sandstones: A Review of the Key Controversies. Spec. Publ. Int. Assoc. Sed. 2000, 29, 1–20. [Google Scholar] [CrossRef]
- Taylor, T.R.; Giles, M.R.; Hathon, L.A.; Diggs, T.N.; Braunsdorf, N.R.; Birbiglia, G.V.; Kittridge, M.G.; MacAulay, C.I.; Espejo, I.S. Sandstone Diagenesis and Reservoir Quality Prediction: Models, Myths, and Reality. Am. Assoc. Pet. Geol. Bull. 2010, 94, 1093–1132. [Google Scholar] [CrossRef]
- Wooldridge, L.; Worden, R.; Griffiths, J.; Utley, J. Clay Coat Diversity in Marginal Marine Sediments. Sedimentology 2018, 66, 1118–1138. [Google Scholar] [CrossRef]
- Lander, R.H.; Bonnell, L.M.; Taylor, T.R.; Espejo, I. Chapter 13—Reservoir Quality and Diagenesis of Deepwater Sandstones. In Deepwater Sedimentary Systems; Rotzien, J.R., Yeilding, C.A., Sears, R.A., Hernández-Molina, F.J., Catuneanu, O.B.T.-D.S.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 471–514. ISBN 978-0-323-91918-0. [Google Scholar]
- Bello, A.; Amao, A.; Alqubalee, A.; Al-Hashem, M.; Albarri, H.; Almasrahy, M.; Al-Ramadan, K.; Babalola, L. Diagenetic Controls on Reservoir Porosity of Aeolian and Fluvial Deposits: A Case Study from Permo-Carboniferous Sandstones of Saudi Arabia. Arab. J. Sci. Eng. 2024, 49, 973–993. [Google Scholar] [CrossRef]
- Bello, A.M.; Butt, M.N.; Hussain, A.; Amao, A.O.; Olariu, C.; Koeshidayatullah, A.I.; Malik, M.H.; Al-Hashem, M.; Al-Ramadan, K. Impact of Depositional and Diagenetic Controls on Reservoir Quality of Syn-Rift Sedimentary Systems: An Example from Oligocene-Miocene Al Wajh Formation, Northwest Saudi Arabia. Sediment. Geol. 2023, 446, 106342. [Google Scholar] [CrossRef]
- Salisu, A.M.; Algheryafi, H.; Bello, A.M.; Amao, A.O.; Al-Otaibi, B.; Al-Ramadan, K. Depositional and Diagenetic Controls on the Reservoir Quality of Marginal Marine Sandstones: An Example from the Early Devonian Subbat Member, Jauf Formation, Northwest Saudi Arabia. Mar. Pet. Geol. 2024, 170, 107147. [Google Scholar] [CrossRef]
- Ehrenberg, S.N. Preservation of Anomalously High Porosity in Deeply Buried Sandstones by Grain-Coating Chlorite: Examples from the Norwegian Continental Shelf1. Am. Assoc. Pet. Geol. Bull. 1993, 77, 1260–1286. [Google Scholar] [CrossRef]
- Bloch, S.; Lander, R.H.; Bonnell, L. Anomalously High Porosity and Permeability in Deeply Buried Sandstone Reservoirs: Origin and Predictability. Am. Assoc. Pet. Geol. Bull. 2002, 86, 301–328. [Google Scholar] [CrossRef]
- Al-Ramadan, K.; Morad, S.; Proust, J.-N.; Al-Aasm, I. Distribution of Diagenetic Alterations within the Sequence Stratigraphic Framework of Shoreface Siliciclastic Deposits: Evidence from the Jurassic Deposits of NE France. J. Sediment. Res. 2005, 75, 10–38. [Google Scholar] [CrossRef]
- Walderhaug, O.; Porten, K.W. How Do Grain Coats Prevent Formation of Quartz Overgrowths? J. Sediment. Res. 2022, 92, 988–1002. [Google Scholar] [CrossRef]
- McKinley, J.M.; Worden, R.H.; Ruffell, A.H. Smectite in Sandstones: A Review of the Controls on Occurrence and Behaviour during Diagenesis. In Clay Mineral Cements in Sandstones; Wiley: Hoboken, NJ, USA, 1999; pp. 109–128. [Google Scholar]
- Khan, M.A.; Khan, T.; Ali, A.; Bello, A.M.; Radwan, A.E. Role of Depositional and Diagenetic Controls on Reservoir Quality of Complex Heterogenous Tidal Sandstone Reservoirs: An Example from the Lower Goru Formation, Middle Indus Basin, Southwest Pakistan. Mar. Pet. Geol. 2023, 154, 106337. [Google Scholar] [CrossRef]
- Franks, S.G.; Zwingmann, H. Origin and Timing of Late Diagenetic Illite in the Permian–Carboniferous Unayzah Sandstone Reservoirs of Saudi Arabia. Am. Assoc. Pet. Geol. Bull. 2010, 94, 1133–1159. [Google Scholar] [CrossRef]
- Salisu, A.M.; Alqubalee, A.; Bello, A.M.; Al-Hussaini, A.; Adebayo, A.R.; Amao, A.O.; Al-Ramadan, K. Impact of Kaolinite and Iron Oxide Cements on Resistivity and Quality of Low Resistivity Pay Sandstones. Mar. Pet. Geol. 2023, 158, 106568. [Google Scholar] [CrossRef]
- Dowey, P.J.; Hodgson, D.M.; Worden, R.H. Pre-Requisites, Processes, and Prediction of Chlorite Grain Coatings in Petroleum Reservoirs: A Review of Subsurface Examples. Mar. Pet. Geol. 2012, 32, 63–75. [Google Scholar] [CrossRef]
- Small, J.S.; Hamilton, D.L.; Habesch, S. Experimental Simulation of Clay Precipitation within Reservoir Sandstones; 1, Techniques and Examples. J. Sediment. Res. 1992, 62, 508–519. [Google Scholar] [CrossRef]
- Haile, B.G.; Hellevang, H.; Aagaard, P.; Jahren, J. Experimental Nucleation and Growth of Smectite and Chlorite Coatings on Clean Feldspar and Quartz Grain Surfaces. Mar. Pet. Geol. 2015, 68, 664–674. [Google Scholar] [CrossRef]
- Almon, W.R.; Fullerton, L.B.; Davies, D.K. Pore Space Reduction in Cretaceous Sandstones through Chemical Precipitation of Clay Minerals. J. Sediment. Res. 1976, 46, 89–96. [Google Scholar] [CrossRef]
- Worden, R.; Morad, S. Clay Minerals in Sandstones: Controls on Formation, Distribution and Evolution. In Clay Mineral Cements in Sandstones; Wiley: Hoboken, NJ, USA, 2009; Volume 34, pp. 1–41. ISBN 9781444304336. [Google Scholar]
- Ajdukiewicz, J.M.; Larese, R.E. How Clay Grain Coats Inhibit Quartz Cement and Preserve Porosity in Deeply Buried Sandstones: Observations and Experiments. Am. Assoc. Pet. Geol. Bull. 2012, 96, 2091–2119. [Google Scholar] [CrossRef]
- Bello, A.M.; Usman, M.B.; Amao, A.O.; Salisu, A.M.; Al-Ramadan, K.; Abubakar, U.; Mukkafa, S.; Kwami, I.A.; Chiroma, L.U.; Al-Hashem, M.; et al. Linking Provenance and Diagenesis to Reservoir Quality Evolution of Sandstones: The Paleocene-Eocene Kerri-Kerri Formation, Northeastern Nigeria. Mar. Pet. Geol. 2025, 172, 107227. [Google Scholar] [CrossRef]
- Worden, R.H.; Armitage, P.J.; Butcher, A.R.; Churchill, J.M.; Csoma, A.E.; Hollis, C.; Lander, R.H.; Omma, J.E. Petroleum Reservoir Quality Prediction: Overview and Contrasting Approaches from Sandstone and Carbonate Communities. Geol. Soc. Spec. Publ. 2018, 435, 1–31. [Google Scholar] [CrossRef]
- Wooldridge, L.J.; Worden, R.H.; Griffiths, J.; Utley, J.E.P. Clay-Coated Sand Grains in Petroleum Reservoirs: Understanding Theirdistribution via a Modern Analogue. J. Sediment. Res. 2017, 87, 338–352. [Google Scholar] [CrossRef]
- Lawan, A.Y.; Worden, R.H.; Utley, J.E.P.; Crowley, S.F. Sedimentological and Diagenetic Controls on the Reservoir Quality of Marginal Marine Sandstones Buried to Moderate Depths and Temperatures: Brent Province, UK North Sea. Mar. Pet. Geol. 2021, 128, 104993. [Google Scholar] [CrossRef]
- Wooldridge, L.J.; Worden, R.H.; Griffiths, J.; Utley, J.E.P. How to Quantify Clay-Coat Grain Coverage in Modern and Ancient Sediments. J. Sediment. Res. 2019, 89, 135–146. [Google Scholar] [CrossRef]
- Griffiths, J.; Worden, R.H.; Wooldridge, L.J.; Utley, J.E.P.; Duller, R.A. Detrital Clay Coats, Clay Minerals, and Pyrite: A Modern Shallow-Core Analogue for Ancient and Deeply Buried Estuarine Sandstones. J. Sediment. Res. 2018, 88, 1205–1237. [Google Scholar] [CrossRef]
- Simon, N.; Worden, R.H.; Muhammed, D.D.; Utley, J.E.P.; Verhagen, I.T.E.; Griffiths, J.; Wooldridge, L.J. Sediment Textural Characteristics of the Ravenglass Estuary; Development of a Method to Predict Palaeo Sub-Depositional Environments from Estuary Core Samples. Sediment. Geol. 2021, 418, 105906. [Google Scholar] [CrossRef]
- Virolle, M.; Brigaud, B.; Féniès, H.; Bourillot, R.; Portier, E.; Patrier, P.; Derriennic, H.; Beaufort, D. Preservation and Distribution of Detrital Clay Coats in a Modern Estuarine Heterolithic Point Bar in the Gironde Estuary (Bordeaux, France). J. Sediment. Res. 2021, 91, 812–832. [Google Scholar] [CrossRef]
- Virolle, M.; Brigaud, B.; Bourillot, R.; Féniès, H.; Portier, E.; Duteil, T.; Nouet, J.; Patrier, P.; Beaufort, D. Detrital Clay Grain Coats in Estuarine Clastic Deposits: Origin and Spatial Distribution within a Modern Sedimentary System, the Gironde Estuary (South-West France). Sedimentology 2019, 66, 859–894. [Google Scholar] [CrossRef]
- Griffiths, J.; Worden, R.H.; Wooldridge, L.J.; Utley, J.E.P.; Duller, R.A.; Edge, R.L. Estuarine Clay Mineral Distribution: Modern Analogue for Ancient Sandstone Reservoir Quality Prediction. Sedimentology 2019, 66, 2011–2047. [Google Scholar] [CrossRef]
- Bello, A.M.; Usman, M.B.; Amao, A.O.; Al-Ramadan, K.; Al-Hashem, M.; Kachalla, A.; Abubakar, U.; Salisu, A.M.; Mukkafa, S.; Kwami, I.A.; et al. Diagenesis and Reservoir Quality Evolution of Estuarine Sandstones: Insights from the Cenomanian-Turonian Yolde Formation, Northern Benue Trough, NE Nigeria. Mar. Pet. Geol. 2024, 169, 107073. [Google Scholar] [CrossRef]
- Worden, R.H.; Burley, S.D. Sandstone Diagenesis: The Evolution of Sand to Stone; Wiley: Hoboken, NJ, USA, 2009; ISBN 9781444304459. [Google Scholar]
- Bello, A.M.; Charlaftis, D.; Jones, S.J.; Gluyas, J.; Acikalin, S.; Cartigny, M.; Al-Ramadan, K. Experimental Diagenesis Using Present-Day Submarine Turbidite Sands. Front. Earth Sci. 2022, 10, 952690. [Google Scholar] [CrossRef]
- Bjørlykke, K.; Ramm, M.; Saigal, G.C. Sandstone Diagenesis and Porosity Modification during Basin Evolution. Geol. Rundsch. 1989, 78, 243–268. [Google Scholar] [CrossRef]
- Beaufort, D.; Rigault, C.; Billon, S.; Billault, V.; Inoue, A.; Inoue, S.; Patrier, P. Chlorite and Chloritization Processes through Mixed-Layer Mineral Series in Low-Temperature Geological Systems—A Review. Clay Miner. Miner. 2015, 50, 497–523. [Google Scholar] [CrossRef]
- Worden, R.H.; Griffiths, J.; Wooldridge, L.J.; Utley, J.E.P.; Lawan, A.Y.; Muhammed, D.D.; Simon, N.; Armitage, P.J. Chlorite in Sandstones. Earth Sci. Rev. 2020, 204, 103105. [Google Scholar] [CrossRef]
- Beaufort, D.; Berger, G.; Lacharpagne, J.C.; Meunier, A. An Experimental Alteration of Montmorillonite to a Di + Trioctahedral Smectite Assemblage at 100 and 200 °C. Clay Miner. Miner. 2001, 36, 211–225. [Google Scholar] [CrossRef]
- Lanson, B.; Beaufort, D.; Berger, G.; Bauer, A.; Cassagnabère, A.; Meunier, A. Authigenic Kaolin and Illitic Minerals during Burial Diagenesis of Sandstones: A Review. Clay Miner. Miner. 2002, 37, 1–22. [Google Scholar] [CrossRef]
- Mosser-Ruck, R.; Cathelineau, M.; Guillaume, D.; Charpentier, D.; Rousset, D.; Barres, O.; Michau, N. Effects of Temperature, ph, and Iron/Clay and Liquid/Clay Ratios on Experimental Conversion of Dioctahedral Smectite to Berthierine, Chlorite, Vermiculite, or Saponite. Clays Clay Miner. 2010, 58, 280–291. [Google Scholar] [CrossRef]
- Charlaftis, D.; Dobson, K.J.; Jones, S.J.; Lakshtanov, D.; Crouch, J.; Cook, J. Experimental Simulation of Burial Diagenesis and Subsequent 2D-3D Characterization of Sandstone Reservoir Quality. Front. Earth Sci. 2022, 10, 766145. [Google Scholar] [CrossRef]
- Sottolichio, A.; Castaing, P.; Etcheber, H.; Maneux, E.; Schmeltz, M.; Schmidt, S. Observations of Suspended Sediment Dynamics in a Highly Turbid Macrotidal Estuary, Derived from Continuous Monitoring. J. Coast. Res. 2011, 2, 1579–1583. [Google Scholar]
- Coynel, A.; Schäfer, J.; Hurtrez, J.-E.; Dumas, J.; Etcheber, H.; Blanc, G. Sampling Frequency and Accuracy of SPM Flux Estimates in Two Contrasted Drainage Basins. Sci. Total Environ. 2004, 330, 233–247. [Google Scholar] [CrossRef]
- Castaing, P.; Allen, G.P. Mechanisms Controlling Seaward Escape of Suspended Sediment from the Gironde: A Macrotidal Estuary in France. Mar. Geol. 1981, 40, 101–118. [Google Scholar] [CrossRef]
- Allen, G.P. Sedimentary Processes and Facies in the Gironde Estuary: A Recent Model for Macrotidal Estuarine Systems. Can. Soc. Pet. Geol. 1991, 16, 29–39. [Google Scholar]
- Billy, J.; Chaumillon, E.; Féniès, H.; Poirier, C. Tidal and Fluvial Controls on the Morphological Evolution of a Lobate Estuarine Tidal Bar: The Plassac Tidal Bar in the Gironde Estuary (France). Geomorphology 2012, 169–170, 86–97. [Google Scholar] [CrossRef]
- Etcheber, H.; Schmidt, S.; Sottolichio, A.; Maneux, E.; Chabaux, G.; Escalier, J.M.; Wennekes, H.; Derriennic, H.; Schmeltz, M.; Quemener, L.; et al. Monitoring Water Quality in Estuarine Environments: Lessons from the MAGEST Monitoring Program in the Gironde Fluvial-Estuarine System. Hydrol. Earth Syst. Sci. 2011, 15, 831–840. [Google Scholar] [CrossRef]
- Virolle, M.; Brigaud, B.; Luby, S.; Portier, E.; Féniès, H.; Bourillot, R.; Patrier, P.; Beaufort, D. Influence of Sedimentation and Detrital Clay Grain Coats on Chloritized Sandstone Reservoir Qualities: Insights from Comparisons between Ancient Tidal Heterolithic Sandstones and a Modern Estuarine System. Mar. Pet. Geol. 2019, 107, 163–184. [Google Scholar] [CrossRef]
- Virolle, M.; Féniès, H.; Brigaud, B.; Bourillot, R.; Portier, E.; Patrier, P.; Beaufort, D.; Jalon-Rojas, I.; Derriennic, H.; Miska, S. Facies Associations, Detrital Clay Grain Coats and Mineralogical Characterization of the Gironde Estuary Tidal Bars: A Modern Analogue for Deeply Buried Estuarine Sandstone Reservoirs. Mar. Pet. Geol. 2020, 114, 104225. [Google Scholar] [CrossRef]
- Charlaftis, D.; Jones, S.J.; Dobson, K.J.; Crouch, J.; Acikalin, S. Experimental Study of Chlorite Authigenesis and Influence on Porosity Maintenance in Sandstones. J. Sediment. Res. 2021, 91, 197–212. [Google Scholar] [CrossRef]
- Salisu, A.M.; Alhindi, H.; Hussaini, S.R.; Amao, A.O.; Al-Awah, H.; Al-ramadan, K.; Koeshidayatullah, A. 3D Microporosity Characterizations in the Heterogeneous Middle Jurassic Tuwaiq Mountain Formation: Insights into an Unconventional Sweet Spot. ACS Omega 2024, 9, 36273–36290. [Google Scholar] [CrossRef]
- Alqubalee, A.; Salisu, A.M.; Bello, A.M.; Al-Hussaini, A.; Al-Ramadan, K. Characteristics, Distribution, and Origin of Ferruginous Deposits within the Late Ordovician Glaciogenic Setting of Arabia. Sci. Rep. 2023, 13, 18430. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-Ray Diffraction and the Identification and Analysis of Clay Minerals. Geol. Mag. 1998, 135, 819–842. [Google Scholar] [CrossRef]
- Al-Ramadan, K. Illitization of Smectite in Sandstones: The Permian Unayzah Reservoir, Saudi Arabia. Arab. J. Sci. Eng. 2014, 39, 407–412. [Google Scholar] [CrossRef]
- Berger, G.; Lacharpagne, J.-C.; Velde, B.; Beaufort, D.; Lanson, B. Kinetic Constraints on Illitization Reactions and the Effects of Organic Diagenesis in Sandstone/Shale Sequences. Appl. Geochem. 1997, 12, 23–35. [Google Scholar] [CrossRef]
- Bello, A.M.; Usman, M.B.; Abubakar, U.; Al-Ramadan, K.; Babalola, L.O.; Amao, A.O.; Sarki Yandoka, B.M.; Kachalla, A.; Kwami, I.A.; Ismail, M.A.; et al. Role of Diagenetic Alterations on Porosity Evolution in the Cretaceous (Albian-Aptian) Bima Sandstone, a Case Study from the Northern Benue Trough, NE Nigeria. Mar. Pet. Geol. 2022, 145, 105851. [Google Scholar] [CrossRef]
- Taylor, T.R.; Lander, R.H.; Bonnell, L.M. Sandstone Petrography, Petrology, and Modeling; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 2022. [Google Scholar]
- Bentabol, M.; Lamarca-Irisarri, D.; Van Driessche, A.E.S.; Ryan, P.C.; Huertas, F.J. Illitization of Montmorillonite in Ammonium Solutions under Hydrothermal Conditions. Appl. Clay Sci. 2024, 258, 107478. [Google Scholar] [CrossRef]
- Mosser-Ruck, R.; Pironon, J.; Cathelineau, M.; Trouiller, A. Experimental Illitization of Smectite in a K-Rich Solution. Eur. J. Mineral. 2001, 13, 829–840. [Google Scholar] [CrossRef]
- Drief, A.; Martinez-Ruiz, F.; Nieto, F.; Velilla Sanchez, N. Transmission Electron Microscopy Evidence for Experimental Illitization of Smectite in K-Enriched Seawater Solution at 50 °C and Basic PH. Clays Clay Miner. 2002, 50, 746–756. [Google Scholar] [CrossRef]
- Mosser-Ruck, R.; Cathelineau, M. Experimental Transformation of Na,Ca-Smectite under Basic Conditions at 150 °C. Appl. Clay Sci. 2004, 26, 259–273. [Google Scholar] [CrossRef]
- Ferrage, E.; Vidal, O.; Mosser-Ruck, R.; Cathelineau, M.; Cuadros, J. A Reinvestigation of Smectite Illitization in Experimental Hydrothermal Conditions: Results from X-ray Diffraction and Transmission Electron Microscopy. Am. Mineral. 2011, 96, 207–223. [Google Scholar] [CrossRef]
- Mills, M.M.; Sanchez, A.C.; Boisvert, L.; Payne, C.B.; Ho, T.A.; Wang, Y. Understanding Smectite to Illite Transformation at Elevated (>100 °C) Temperature: Effects of Liquid/Solid Ratio, Interlayer Cation, Solution Chemistry and Reaction Time. Chem. Geol. 2023, 615, 121214. [Google Scholar] [CrossRef]
- Small, J.S. Fluid Composition, Mineralogy and Morphological Changes Associated with the Smectite-to-Illite Reaction: An Experimental Investigation of the Effect of Organic Acid Anions. Clay Miner. Miner. 1994, 29, 539–554. [Google Scholar] [CrossRef]
- Du, J.; Cai, J.; Chao, Q.; Song, M.; Wang, X. Variations and Geological Significance of Solid Acidity during Smectite Illitization. Appl. Clay Sci. 2021, 204, 106035. [Google Scholar] [CrossRef]
- Guillaume, D.; Neaman, A.; Cathelineau, M.; Mosser-Ruck, R.; Peiffert, C.; Abdelmoula, M.; Dubessy, J.; Villiéras, F.; Michau, N. Experimental Study of the Transformation of Smectite at 80 and 300 °C in the Presence of Fe Oxides. Clay Miner. Miner. 2004, 39, 17–34. [Google Scholar] [CrossRef]
- Johnston, R.M.; Miller, H.G. The Effect of PH on the Stability of Smectite; Atomic Energy of Canada Ltd.: Chalk River, ON, Canada, 1984. [Google Scholar]
- Huang, W.L.; Bishop, A.M.; Brown, R.W. The Effect of Fluid/Rock Ratio on Feldspar Dissolution and Illite Formation under Reservoir Conditions. Clay Miner. Miner. 1986, 21, 585–601. [Google Scholar] [CrossRef]
- Ulmer-Scholle, D.S.; Scholle, P.A.; Schieber, J.; Raine, R.J. A Color Guide to the Petrography of Sandstones, Siltstones, Shales and Associated Rocks; American Association of Petroleum Geologists: Tulsa, OK, USA, 2015. [Google Scholar]
- Altaner, S.P.; Ylagan, R.F. Comparison of Structural Models of Mixed-Layer Illite/Smectite and Reaction Mechanisms of Smectite Illitization. Clays Clay Miner. 1997, 45, 517–533. [Google Scholar] [CrossRef]
- Nadeau, P.H.; Wilson, J.; McHardy, W.J.; Tait, J.M. The Conversion of Smectite to Illite during Diagenesis: Evidence from Some Illitic Clays from Bentonites and Sandstones. Miner. Mag. 1985, 49, 393–400. [Google Scholar] [CrossRef]
- Lanson, B.; Sakharov, B.; Claret, F.; Drits, V. Diagenetic Smectite-to-Illite Transition in Clay-Rich Sediments: A Reappraisal of X-Ray Diffraction Results Using the Multi-Specimen Method. Am. J. Sci. 2009, 309, 476–516. [Google Scholar] [CrossRef]
- Bell, T.E. Microstructure in Mixed-Layer Illite/Smectite and Its Relationship to the Reaction of Smectite to Illite. Clays Clay Miner. 1986, 34, 146–154. [Google Scholar] [CrossRef]
- Roberson, H.E.; Lahann, R.W. Smectite to Illite Conversion Rates: Effects of Solution Chemistry. Clays Clay Miner. 1981, 29, 129–135. [Google Scholar] [CrossRef]
- Putnis, A. Mineral Replacement Reactions. Rev. Miner. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Whitney, G.; Northrop, H.R. Experimental Investigation of the Smectite to Illite Reaction; Dual Reaction Mechanisms and Oxygen-Isotope Systematics. Am. Mineral. 1988, 73, 77–90. [Google Scholar]
- Inoue, A.; KOHYAMA, N.; KITAGAWA, R.; WATANABE, T. Chemical and Morphological Evidence for the Conversion of Smectite to Illite. Clays Clay Miner. 1987, 35, 111–120. [Google Scholar] [CrossRef]
- Inoue, A.; Lanson, B.; Fernandes, M.; Sakharov, B.; Murakami, T.; Meunier, A.; Beaufort, D. Illite-Smectite Mixed-Layer Minerals in Hydrothermal Alteration of Volcanic Rocks: I. One-Dimensional XRD Structure Analysis and Characterisation of Component Layers. Clays Clay Miner. 2005, 53, 423–439. [Google Scholar] [CrossRef]
- Ylagan, R.F.; Altaner, S.P.; Pozzuoli, A. Reaction Mechanisms of Smectite Illitization Associated with Hydrothermal Alteration from Ponza Island, Italy. Clays Clay Miner. 2000, 48, 610–631. [Google Scholar] [CrossRef]
- Środoń, J. Nature of Mixed-Layer Clays and Mechanisms of Their Formation and Alteration. Annu. Rev. Earth Planet. Sci. 1999, 27, 19–53. [Google Scholar] [CrossRef]
- Mosser-Ruck, R.; Pignatelli, I.; Bourdelle, F.; Abdelmoula, M.; Barres, O.; Guillaume, D.; Charpentier, D.; Rousset, D.; Cathelineau, M.; Michau, N. Contribution of Long-Term Hydrothermal Experiments for Understanding the Smectite-to-Chlorite Conversion in Geological Environments. Contrib. Mineral. Petrol. 2016, 171, 97. [Google Scholar] [CrossRef]
- Robinson, D.; Schmidt, S.T.; De Zamora, A.S. Reaction Pathways and Reaction Progress for the Smectite-to-chlorite Transformation: Evidence from Hydrothermally Altered Metabasites. J. Metamorph. Geol. 2002, 20, 167–174. [Google Scholar] [CrossRef]
- Keith, T.E.C.; Bargar, K.E. Petrology and Hydrothermal Mineralogy of US Geological Survey Newberry: 2. Drill Core from Newberry Caldera, Oregon. J. Geophys. Res. Solid. Earth 1988, 93, 10174–10190. [Google Scholar] [CrossRef]
- Li, K.; Xi, K.; Cao, Y.; Niu, X.; Wu, S.; Feng, S.; You, Y. Chlorite Authigenesis and Its Impact on Reservoir Quality in Tight Sandstone Reservoirs of the Triassic Yanchang Formation, Southwestern Ordos Basin, China. J. Pet. Sci. Eng. 2021, 205, 108843. [Google Scholar] [CrossRef]
- Millot, G. Geology of Clays: Weathering Sedimentology·Geochemistry; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 3662416093. [Google Scholar]
- Chamley, H. Clay Sedimentology; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 364285916X. [Google Scholar]
- Velde, B.B.; Meunier, A. The Origin of Clay Minerals in Soils and Weathered Rocks; Springer Science & Business Media: Berlin, Germany, 2008; ISBN 3540756345. [Google Scholar]
- Iijima, A. Zeolites in Petroleum and Natural Gas Reservoirs. Rev. Miner. Geochem. 2001, 45, 347–402. [Google Scholar]
- Lin, T.; Wang, W.; Ma, Q.; Kang, J.; Yang, R.; Liu, X. Formation Mechanism and Implication of Analcime in the Sandstone Reservoirs of the Permian Jingjingzigou Formation in the Jinan Sag, Southern Junggar Basin, NW China. Front. Earth Sci. 2024, 12, 1416594. [Google Scholar] [CrossRef]
- Yao, G.; Li, L.; Cai, M.; Liu, Y. Mechanisms of Salinization in a Middle Eocene Lake in the Tanggu Area of the Huanghua Depression. Mar. Pet. Geol. 2017, 86, 155–167. [Google Scholar] [CrossRef]
- Niegel, S.; Franz, M. Depositional and Diagenetic Controls on Porosity Evolution in Sandstone Reservoirs of the Stuttgart Formation (North German Basin). Mar. Pet. Geol. 2023, 151, 106157. [Google Scholar] [CrossRef]
- Zhu, S.; Cui, H.; Jia, Y.; Zhu, X.; Tong, H.; Ma, L. Occurrence, Composition, and Origin of Analcime in Sedimentary Rocks of Non-Marine Petroliferous Basins in China. Mar. Pet. Geol. 2020, 113, 104164. [Google Scholar] [CrossRef]
- Iijima, A. Effect of Pore Water to Clinoptilolite-Analcimealbite Reaction Series. J. Fac. Sci. Univ. Tokyo Sec. II 1975, 19, 133–147. [Google Scholar]
- Mallette, A.J.; Espindola, G.; Varghese, N.; Rimer, J.D. Highly Efficient Synthesis of Zeolite Chabazite Using Cooperative Hydration-Mismatched Inorganic Structure-Directing Agents. Chem. Sci. 2024, 15, 573–583. [Google Scholar] [CrossRef]
- Chipera, S.J.; Apps, J.A. Geochemical Stability of Natural Zeolites. Rev. Miner. Geochem. 2001, 45, 117–161. [Google Scholar] [CrossRef]
- Boles, J.R.; Franks, S.G. Clay diagenesis in Wilcox sandstones of Southwest Texas: Implications of smectite diagenesis on sandstone cementation. J. Sediment. Res. 1979, 49, 55–70. [Google Scholar]




| Experiment | Sample ID | Solutions | Temperature (°C) | Duration (Days) | Final Pressure (Bar) |
|---|---|---|---|---|---|
| 1 | SW@50 | Red Sea water | 50 | 14 | 46.7 |
| 2 | SW@100 | Red Sea water | 100 | 14 | 52.7 |
| 3 | SW@150 | Red Sea water | 150 | 14 | 65.1 |
| 4 | SW@200 | Red Sea water | 200 | 14 | 91.2 |
| 5 | SW@250 | Red Sea water | 250 | 14 | 116.3 |
| 6 | SF1@250 | 0.1 M KCl in Red Sea Water | 250 | 14 | 117.6 |
| 7 | SF2@100 | 0.1 M NaCl, KCl, CaCl2·2H2O, MgCl2·6H2O | 100 | 14 | 56.1 |
| 8 | SF2@150 | 0.1 M NaCl, KCl, CaCl2·2H2O, MgCl2·6H2O | 150 | 14 | 67.5 |
| 9 | SF2@200 | 0.1 M NaCl, KCl, CaCl2·2H2O, MgCl2·6H2O | 200 | 14 | 79.8 |
| 10 | SF2@250 | 0.1 M NaCl, KCl, CaCl2·2H2O, MgCl2·6H2O | 250 | 14 | 108.3 |
| 11 | EW@250 | Estuarine water | 250 | 14 | 112.5 |
| 12 | SF3@250 | 0.1 M Na2CO3 | 250 | 14 | 128.7 |
| Oxides Measured in Weight Percent (wt %) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment | Sample ID | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | Na2O | K2O | ClO2 | MnO | P2O5 | SO3 | SrO | TiO2 |
| SM | SM@0 | 57.43 | 17.43 | 9.87 | 7.38 | 2.12 | 0.79 | 3.07 | 0.09 | 0.15 | 0.18 | 0.14 | 0.02 | 1.01 |
| Exp. 1 | SW@50 | 52.86 | 17.55 | 8.62 | 7.48 | 2.32 | 3.37 | 3.24 | 2.64 | 0.09 | 0.18 | 0.51 | 0.02 | 0.94 |
| Exp. 2 | SW@100 | 56.43 | 18.04 | 8.43 | 7.66 | 3.18 | 1.21 | 3.46 | 0.09 | 0.13 | 0.21 | 0.06 | 0.02 | 1.04 |
| Exp. 3 | SW@150 | 54.59 | 18.36 | 5.90 | 8.49 | 3.23 | 2.34 | 3.35 | 1.62 | 0.12 | 0.20 | 0.54 | 0.02 | 0.97 |
| Exp. 4 | SW@200 | 57.30 | 15.47 | 3.59 | 8.68 | 4.93 | 2.36 | 2.73 | 1.51 | 0.11 | 0.16 | 1.88 | 0.02 | 0.90 |
| Exp. 5 | SW@250 | 52.77 | 16.94 | 2.22 | 10.67 | 4.78 | 2.01 | 6.18 | 2.26 | 0.10 | 0.19 | 0.71 | 0.02 | 0.93 |
| Exp. 6 | SF1@250 | 54.59 | 16.12 | 6.29 | 7.19 | 2.03 | 0.73 | 9.14 | 2.39 | 0.12 | 0.18 | 0.04 | 0.01 | 0.98 |
| Exp. 7 | SF2@100 | 55.08 | 17.15 | 9.15 | 7.94 | 2.64 | 0.98 | 3.72 | 1.66 | 0.09 | 0.17 | 0.11 | 0.02 | 0.94 |
| Exp. 8 | SF2@150 | 58.00 | 16.34 | 4.04 | 9.09 | 5.25 | 0.78 | 3.44 | 1.33 | 0.08 | 0.19 | 0.06 | 0.01 | 0.99 |
| Exp. 9 | SF2@200 | 55.85 | 16.62 | 3.39 | 9.79 | 5.84 | 0.86 | 3.79 | 2.19 | 0.09 | 0.19 | 0.06 | 0.01 | 0.96 |
| Exp. 10 | SF2@250 | 58.24 | 18.08 | 1.39 | 9.58 | 5.06 | 1.94 | 3.16 | 0.77 | 0.09 | 0.22 | 0.16 | 0.01 | 1.06 |
| Exp. 11 | EW@250 | 57.06 | 16.59 | 3.26 | 8.23 | 7.62 | 0.60 | 3.59 | 1.33 | 0.12 | 0.19 | 0.05 | 0.01 | 1.35 |
| Exp. 12 | SF3@250 | 53.97 | 17.26 | 9.36 | 7.30 | 2.14 | 5.46 | 2.88 | 0.09 | 0.14 | 0.19 | 0.04 | 0.02 | 0.95 |
| Sample ID | Bulk Mineralogy (%) | Clay Mineralogy (<2 µm) (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qtz | Orth | Pla | Cal | Mus | CM | Hal | Syl | Ana | Total | Kao | Sme | Ill | Chl. | Qtz | |
| SM | 40.7 | 5.3 | 14.7 | 9.7 | 9.3 | 20.3 | - | - | - | 100.0 | 52 | 5 | 41 | - | 2 |
| Exp. 1 | 41.0 | 8.1 | 17.3 | 5.1 | 7.6 | 20.9 | - | - | - | 100.0 | 50 | 5 | 40 | - | 5 |
| Exp. 2 | 39.3 | 7.7 | 15.2 | 3.7 | 6.9 | 27.2 | - | - | - | 100.0 | 48 | 6 | 42 | - | 4 |
| Exp. 3 | 38.5 | 7.6 | 14.9 | 3.1 | 6.3 | 29.6 | - | - | - | 100.0 | 41 | 7 | 49 | - | 3 |
| Exp. 4 | 35.4 | 4.8 | 16.5 | 1.8 | 5.1 | 34.7 | 1.7 | - | - | 100.0 | 33 | 3 | 58 | - | 6 |
| Exp. 5 | 38.9 | 2.4 | 15.6 | 1.2 | 3.5 | 38.4 | - | - | - | 100.0 | 18 | 2 | 76 | - | 4 |
| Exp. 6 | 29.4 | 6.3 | 14.5 | 5.8 | 3.3 | 37.1 | - | 3.6 | - | 100.0 | 20 | TR | 80 | - | 0 |
| Exp. 7 | 38.2 | 9.1 | 18.7 | 3.0 | 8.1 | 22.9 | - | - | - | 100.0 | 44 | 2 | 48 | - | 6 |
| Exp. 8 | 36.9 | 7.4 | 14.9 | 3.9 | 7.2 | 29.7 | - | - | - | 100.0 | 40 | 1 | 52 | 4 | 3 |
| Exp. 9 | 37.6 | 4.0 | 15.5 | 0.5 | 3.5 | 38.9 | - | - | - | 100.0 | 34 | 0 | 55 | 8 | 3 |
| Exp. 10 | 35.2 | 3.8 | 9.7 | 0.3 | 3.3 | 46.7 | - | - | - | 100.0 | 26 | 0 | 52 | 16 | 6 |
| Exp. 11 | 35.1 | 3.5 | 14.4 | 1.9 | 5.8 | 36.9 | - | - | - | 100.0 | 25 | 1 | 48 | 24 | 3 |
| Exp. 12 | 31.2 | 5.3 | 12.1 | 9.1 | 5.2 | 13.1 | 2.4 | - | 21.7 | 100.0 | 16 | 40 | 41 | - | 3 |
| Identification | pH | Major Cations (mg/L) | TDS (ppt) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | Ca | Fe | Mg | Na | K | ||||
| SW | SW@0 | 8.12 | 57.7 | 396 | 638 | 1820 | 18,650 | 798 | 36.71 |
| Exp. 1 | SW@50 | 7.21 | 420 | 7730 | 770 | 1740 | 15,680 | 6460 | 56.19 |
| Exp. 2 | SW@100 | 6.88 | 542 | 14,260 | 1080 | 1400 | 14,900 | 7100 | 56.73 |
| Exp. 3 | SW@150 | 6.42 | 648 | 22,500 | 1690 | 939 | 12,060 | 6060 | 58.01 |
| Exp. 4 | SW@200 | 6.41 | 782 | 44,800 | 2310 | 631 | 5950 | 2470 | 58.94 |
| Exp. 5 | SW@250 | 6.34 | 1080 | 67,000 | 2350 | 431 | 5510 | 2000 | 105.2 |
| SF1 | SF1@0 | 7.78 | 70.1 | 410 | 650 | 1690 | 16,430 | 17,200 | 43.95 |
| Exp. 6 | SF1@250 | 6.86 | 477 | 58,000 | 919 | 818 | 9600 | 9060 | 104.5 |
| SF2 | SF2@0 | 5.71 | ND | 8730 | ND | 15,500 | 12,800 | 7650 | 45.33 |
| Exp. 7 | SF2@100 | 6.22 | 47.8 | 21,400 | 13.7 | 3690 | 12,500 | 9280 | 56.15 |
| Exp. 8 | SF2@150 | 6.45 | 126 | 29,900 | 16.6 | 3520 | 10,610 | 10,300 | 62.08 |
| Exp. 9 | SF2@200 | 6.28 | 520 | 42,500 | 60.1 | 600 | 6820 | 2990 | 60.69 |
| Exp. 10 | SF2@250 | 6.22 | 787 | 48,400 | 132 | 167 | 2590 | 2620 | 62.29 |
| EW | EW@0 | 7.26 | 5.2 | 12.1 | 112 | 381 | 10,600 | 70.5 | 13.02 |
| Exp. 11 | EW@250 | 6.91 | 120 | 14,200 | 5.1 | 120 | 8700 | 1250 | 26.39 |
| SF3 | SF3@0 | 11.32 | ND | ND | ND | ND | 8250 | ND | 13.79 |
| Exp. 12 | SF3@250 | 8.31 | 860 | 2910 | 1820 | 38.4 | 390 | 1140 | 7.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salisu, A.M.; Bello, A.M.; Amao, A.O.; Al-Ramadan, K. The Role of Fluid Chemistry in the Diagenetic Transformation of Detrital Clay Minerals: Experimental Insights from Modern Estuarine Sediments. Minerals 2025, 15, 317. https://doi.org/10.3390/min15030317
Salisu AM, Bello AM, Amao AO, Al-Ramadan K. The Role of Fluid Chemistry in the Diagenetic Transformation of Detrital Clay Minerals: Experimental Insights from Modern Estuarine Sediments. Minerals. 2025; 15(3):317. https://doi.org/10.3390/min15030317
Chicago/Turabian StyleSalisu, Anas Muhammad, Abdulwahab Muhammad Bello, Abduljamiu O. Amao, and Khalid Al-Ramadan. 2025. "The Role of Fluid Chemistry in the Diagenetic Transformation of Detrital Clay Minerals: Experimental Insights from Modern Estuarine Sediments" Minerals 15, no. 3: 317. https://doi.org/10.3390/min15030317
APA StyleSalisu, A. M., Bello, A. M., Amao, A. O., & Al-Ramadan, K. (2025). The Role of Fluid Chemistry in the Diagenetic Transformation of Detrital Clay Minerals: Experimental Insights from Modern Estuarine Sediments. Minerals, 15(3), 317. https://doi.org/10.3390/min15030317







