Multiple Solutions of Ore-Forming Fluids of Carbonate Rock-Related Nephrite Deposits Constrained by Hydrogen and Oxygen Isotopes
Abstract
1. Introduction
2. Methodology
3. δD and δ18O of Different Rocks in C-Type Nephrite Deposits
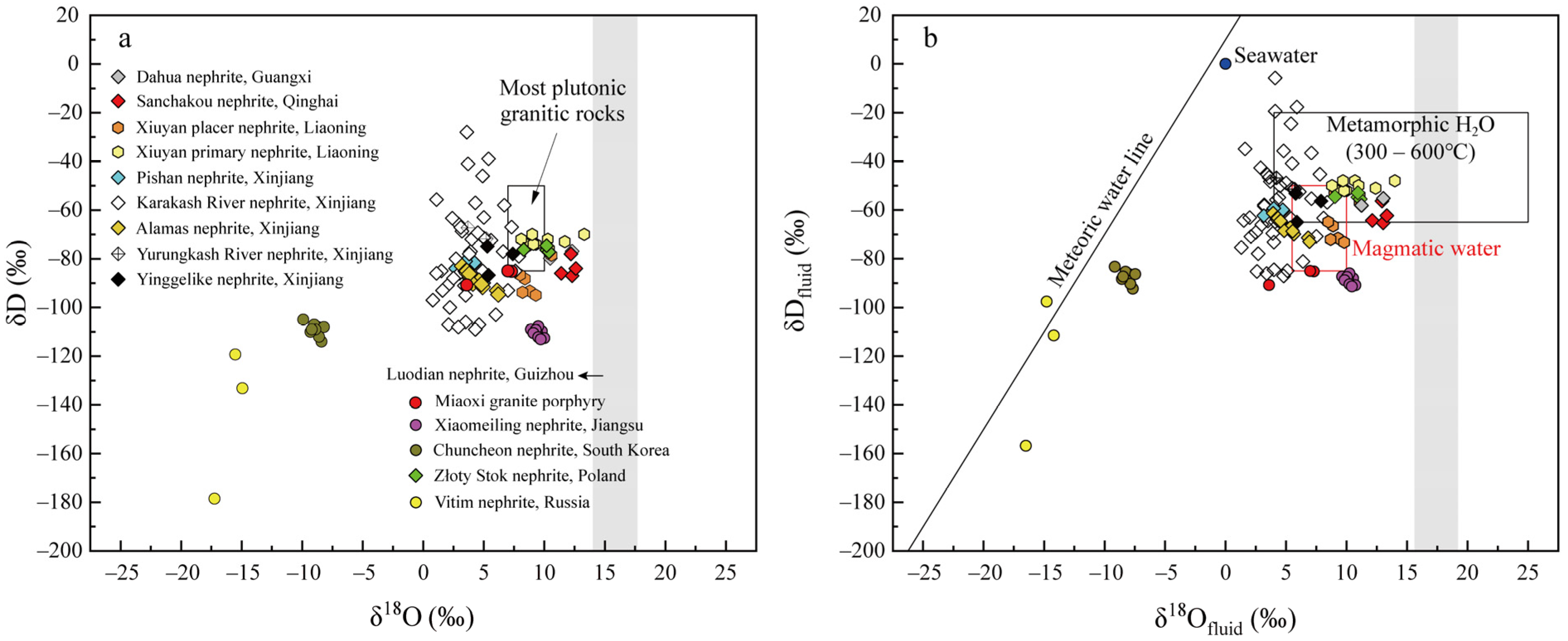
3.1. δD and δ18O of C-Type Nephrite
3.2. δD and δ18O of Intrusive Rocks and Country Rocks
4. Multi-Stage Water/Rock Interactions
4.1. Isotope Exchanges Between Water and Minerals
4.2. Taylor’s Closed Model
4.3. Fluid Mixing Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, H.P. Water/rock interactions and the origin of H2O in granitic batholiths. J. Geol. Soc. 1977, 133, 509–558. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.Q.; Abuduwayiti, M.; Wang, C.; Zhang, S.P.; Shen, C.H.; Zhang, Z.Y.; He, M.Y.; Zhang, Y.; Yang, X.D. SHRIMP U-Pb zircon ages, mineral compositions and geochemistry of placer nephrite in the Yurungkash and Karakash River deposits, West Kunlun, Xinjiang, northwest China: Implication for a magnesium skarn. Ore Geol. Rev. 2016, 72, 699–727. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Shi, G.H.; Yui, T.F.; Zhang, G.B.; Abuduwayiti, M.; Yang, L.Q.; Sun, X. Geochemistry and petrology of nephrite from Alamas, Xinjiang, NW China. J. Asian Earth Sci. 2011, 42, 440–451. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, G.H.; Xu, L.G.; Li, X.L. Mineralogy and geochemistry of nephrite jade from Yinggelike deposit, Altyn Tagh (Xinjiang, NW China). Minerals 2020, 10, 418. [Google Scholar] [CrossRef]
- Yui, T.-F.; Kwon, S.-T. Origin of a dolomite-related jade deposit at Chuncheon, Korea. Econ. Geol. 2002, 97, 593–601. [Google Scholar] [CrossRef]
- Burtseva, M.V.; Ripp, G.S.; Posokhov, V.F.; Murzintseva, A.E. Nephrites of East Siberia: Geochemical features and problems of genesis. Russ. Geol. Geophys. 2015, 56, 402–410. [Google Scholar] [CrossRef]
- Gao, K.; Fang, T.; Lu, T.J.; Lan, Y.; Zhang, Y.; Wang, Y.Y.; Chang, Y. Hydrogen and oxygen stable isotope ratios of dolomite-related nephrite: Relevance for its geographic origin and geological significance. Gems Gemol. 2020, 56, 266–280. [Google Scholar] [CrossRef]
- Nichol, D. Two contrasting nephrite jade types. J. Gemmol. 2000, 27, 193–200. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Liu, Y.; Zhang, Y.; Li, Z.; Zhang, J.; Zheng, F. Mineralogical characteristics and genesis of green nephrite from the world. Rock Miner. Anal. 2018, 37, 470–489, (In Chinese with English Abstract). [Google Scholar]
- Yui, T.F.; Yeh, H.W.; Lee, C.W. Stable isotope studies of nephrite deposits from Fengtien, Taiwan. Geochim. Cosmochim. Acta. 1988, 52, 593–602. [Google Scholar] [CrossRef]
- Li, P.; Liao, Z.T.; Zhou, Z.Y. The residual geological information in Liangzhu jades: Implications for their provenance. P. Geologist. Assoc. 2022, 133, 256–268. [Google Scholar] [CrossRef]
- Jiang, B.H.; Bai, F.; Zhao, J.K. Mineralogical and geochemical characteristics of green nephrite from Kutcho, northern British Columbia, Canada. Lithos. 2021, 388–389, 106030. [Google Scholar] [CrossRef]
- Harlow, G.E.; Sorensen, S.S. Jade (nephrite and jadeitite) and serpentinite: Metasomatic connections. Int. Geol. Rev. 2005, 47, 113–146. [Google Scholar] [CrossRef]
- Burtseva, M.V.; Ripp, G.S.; Posokhov, V.F.; Zyablitsev, A.Y.; Murzintseva, A.E. The sources of fluids for the formation of nephritic rocks of the southern folded belt of the Siberian craton. Dokl. Earth Sci. Acad. Nauk. 2015, 460, 324–328. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Liao, Z.T.; Ma, T.T.; Yuan, Y. Study on ore-forming type and genetic mechanism of Sanchakou nephrite deposit in Qinghai Province. J. Tongji Univ. (Nat. Sci.). 2005, 33, 1191–1200, (In Chinese with English Abstract). [Google Scholar]
- Bai, F.; Du, J.M.; Li, J.J.; Jiang, B.H. Mineralogy, geochemistry, and petrogenesis of green nephrite from Dahua, Guangxi, Southern China. Ore Geol. Rev. 2020, 118, 103362. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.-H.; Wang, L.; Tan, J.; Wang, B. Petrochemical characteristics and genesic significance of Luodian Jade from Guizhou. Mineral. Petr. 2012, 32, 12–19, (In Chinese with English Abstract). [Google Scholar]
- Liu, X.F.; Liu, Y.; Li, Z.J.; Abuduwayiti, M.; Tian, G.Y.; Guo, D.X. The genesis and SHRIMP U-Pb zircon dating of the Pishan brown nephrite-bearing Mg-skarn deposit in Xinjiang. Acta Petrol. Mineral. 2017, 36, 259–273, (In Chinese with English Abstract). [Google Scholar]
- Liu, Y.; Deng, J.; Shi, G.H.; Sun, X.; Yang, L.Q. Geochemistry and petrogenesis of placer nephrite from Hetian, Xinjiang, Northwest China. Ore Geol. Rev. 2011, 41, 122–132. [Google Scholar] [CrossRef]
- Xu, L.G.; Wang, S.Q. Gemological characteristics and genesis of Dahua nephrite. Acta Petrol. Mineral. 2016, 35, 1–11, (In Chinese with English Abstract). [Google Scholar]
- Liao, Z.T.; Zhi, Y.X. Study on Luodian Jade from Guizhou; China University of China Press: Wuhan, China, 2017; pp. 114–118. (In Chinese) [Google Scholar]
- Yang, L. Study on Petro-Mineral Features and Genetic Mechanism of Luodian Jade, Guizhou Province. Ph.D. Thesis, Chengdu University of Technology, Chengdu, China, 2013. (In Chinese). [Google Scholar]
- Zhou, Z.Y. Research on the Ore-Forming Tectonic Background and Mechanism of Sanchakou Nephrite (Tremolite Jade) in the East Kunlun Mountains. Ph.D. Thesis, Tongji University, Shanghai, China, 2006. (In Chinese). [Google Scholar]
- Zheng, F.; Liu, Y.; Zhang, H.Q. The petrogeochemistry and zircon U-Pb age of nephrite placer deposit in Xiuyan, Liaoning. Rock Miner. Anal. 2019, 38, 438–448, (In Chinese with English Abstract). [Google Scholar]
- Duan, T.Y.; Wang, S.Q. Study on stable isotopes of Xiuyan nephrite (tremolite). Acta Petrol. Mineral. 2002, 21, 115–119, (In Chinese with English Abstract). [Google Scholar]
- Gil, G.; Barnes, J.D.; Boschi, C.; Gunia, P.; Raczynski, P.; Szakmány, G.; Bendő, Z.; Péterdi, B. Nephrite from Złoty Stok (Sudetes, SW Poland): Petrological, geochemical, and isotopic evidence for a dolomite-related origin. Can. Mineral. 2015, 53, 533–556. [Google Scholar] [CrossRef]
- Kislov, E.V.; Goncharuk, I.S.; Vanteev, V.V.; Posokhov, V.F. Voimakan deposit of dolomite-type nephrite, Middle-Vitim Mountain Country: Formation conditions. Geol. Ore Depos. 2024, 66, 752–768. [Google Scholar] [CrossRef]
- Zheng, Y.F. Calculation of oxygen isotope fractionation in hydroxyl-bearing silicates. Earth Planet. Sci. Lett. 1993, 120, 247–263. [Google Scholar] [CrossRef]
- Graham, C.M.; Harmon, R.S.; Sheppard, S.M.F. Experimental hydrogen isotope studies: Hydrogen isotope exchange between amphibole and water. Am. Mineral. 1984, 69, 247–263. [Google Scholar]
- Liu, H.B.; Jin, G.S.; Li, J.J.; Han, J.; Zhang, J.F.; Zhang, J.; Zhong, F.W.; Guo, D.Q. Determination of stable isotope composition in uranium geological samples. World Nucl. Geosci. 2013, 30, 174–179, (In Chinese with English Abstract). [Google Scholar]
- Gao, K.; Shi, G.H.; Wang, M.L.; Xie, G.; Wang, J.; Zhang, X.C.; Fang, T.; Lei, W.Y.; Liu, Y. The Tashisayi nephrite deposit from South Altyn Tagh, Xinjiang, northwest China. Geosci. Front. 2019, 10, 1597–1612. [Google Scholar] [CrossRef]
- Kochnev, A.P.; Krasnov, D.A. Nephrite-bearing factors of Golyubinskoe-Ollaminskoe nephrite-bearing field (Buryat Republic). Geol. Prosp. Explor. Dev. Miner. Depos. 2017, 40, 52–65, (In Russian with English abstract). [Google Scholar]
- Wang, S.Q.; Dong, P.X. Classification, geologic characteristics and origin of the jade from Xiuyan, Liaoning Province, China. Geol. Resour. 2011, 20, 321–331, (In Chinese with English Abstract). [Google Scholar]
- Nichol, D.; Giess, H. Nephrite jade from Mastabia in Val Malenco, Italy. J. Gemmol. 2005, 29, 305–311. [Google Scholar] [CrossRef]
- Nichol, D.; Giess, H. Nephrite jade from Scortaseo, Switzerland. J. Gemmol. 2005, 29, 467–472. [Google Scholar] [CrossRef]
- Liao, Z.T.; Jing, C.; Li, P.; Shen, J.Y.; Jin, X.P. Key problems in nephrite research. J Tongji Univ. (Nat. Sci.) 2022, 50, 1073–1080. [Google Scholar]
- Wu, Z.Y.; Wang, S.Q.; Ling, X.X. Characteristics and origin of nephrite from Sangpiyu, Xiuyan County, Liaoning Province. Acta Petrol. Mineral. 2014, 33, 15–24, (In Chinese with English Abstract). [Google Scholar]
- Taylor, H.P.J. The oxygen isotope geochemistry of igneous rocks. Contrib. Mineral. Petr. 1968, 19, 1–71. [Google Scholar] [CrossRef]
- Wan, D.F.; Wang, H.P.; Zou, T.R. Silicon and oxygen isotopic compositions of Hetian jade, Manasi green jade and Xiuyan old jade(tremolite). Acta Petrol. Mineral. 2002, 21, 11–114, (In Chinese with English Abstract). [Google Scholar]
- Degens, E.T.; Epstein, S. Relationship between O18/O16 ratios in coexisting carbonates, cherts and diatomites. J. Geol. 1962, 66, 534–542. [Google Scholar]
- Bégué, F.; Baumgartner, L.P.; Bouvier, A.-S.; Robyr, M. Reactive fluid infiltration along fractures: Textural observations coupled to in-situ isotopic analyses. Earth Planet. Sci. Lett. 2019, 519, 264–273. [Google Scholar] [CrossRef]
- Clayton, R.N.; O’Neil, J.R.; Mayeda, T.K. Oxygen isotope exchange between quartz and water. J. Geophys. Res. 1972, 77, 3057–3967. [Google Scholar] [CrossRef]
- Northrop, D.A.; Clayton, R.N. Oxygen-isotope fractionations in systems containing dolomite. J. Geol. 1966, 74, 174–196. [Google Scholar] [CrossRef]
- O’Neil, J.R.; Clayton, R.N.; Mayeda, T.K. Oxygen isotope fractionation in divalent metal carbonates. J. Chem. Phys. 1969, 51, 5547–5558. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]


| Nephrite Deposit | Sample No. | δD | δ18O | δDH2O | δ18OH2O | References | ||
|---|---|---|---|---|---|---|---|---|
| 350 °C | 400 °C | 450 °C | ||||||
| Xiaomeiling, Liyang, Jiangsu, China | LY-Q-13 | −109.0 | 8.9 | −87.3 | 9.64 | 10.10 | 10.42 | This study |
| LY-Q-14 | −109.8 | 9.8 | −88.1 | 10.54 | 11.00 | 11.32 | ||
| LY-Q-16 | −110.3 | 9.4 | −88.6 | 10.14 | 10.60 | 10.92 | ||
| LY-HL-3 | −107.7 | 9.5 | −86.0 | 10.24 | 10.70 | 11.02 | ||
| LY-HL-7 | −109.2 | 9.3 | −87.5 | 10.04 | 10.50 | 10.82 | ||
| LY-HL-10 | −110.5 | 9.1 | −88.8 | 9.84 | 10.30 | 10.62 | ||
| LY-QB-3 | −112.6 | 10.0 | −90.9 | 10.74 | 11.20 | 11.52 | ||
| LY-QB-12 | −112.2 | 9.5 | −90.5 | 10.24 | 10.70 | 11.02 | ||
| LY-QB-21 | −113.1 | 9.7 | −91.4 | 10.44 | 10.90 | 11.22 | ||
| 330 °C | 390 °C | 450 °C | ||||||
| Pishan, Xinjiang, China | 392-1 | −89.9 | 4.99 | −68.2 | 5.48 | 6.11 | 6.51 | [18] |
| 392-2 | −81.8 | 4.27 | −60.2 | 4.76 | 5.39 | 5.79 | ||
| 392-3 | −84.0 | 2.67 | −62.3 | 3.16 | 3.79 | 4.19 | ||
| 392-4 | −82.4 | 3.63 | −60.7 | 4.12 | 4.75 | 5.15 | ||
| 392-5 | −82.4 | 3.89 | −60.7 | 4.38 | 5.01 | 5.41 | ||
| 392-6 | −83.0 | 3.92 | −61.3 | 4.41 | 5.04 | 5.44 | ||
| 392-7 | −81.6 | 4.24 | −59.9 | 4.73 | 5.36 | 5.76 | ||
| 392-8 | −81.0 | 3.50 | −59.3 | 3.99 | 4.62 | 5.02 | ||
| Yingelike, Ruoqiang, Xinjiang, China | H-3 | −74.9 | 5.3 | −53.2 | 5.79 | 6.42 | 6.82 | [4] |
| H-4 | −78.0 | 7.4 | −56.3 | 7.89 | 8.52 | 8.92 | ||
| H-5 | −86.7 | 5.4 | −65.0 | 5.89 | 6.52 | 6.92 | ||
| 293 °C | 350 °C | 400 °C | ||||||
| Alamas, Xinjiang, China | ABY-1 | −86.7 | 3.8 | −65.0 | 3.7 | 4.54 | 5.00 | [3] |
| ABY-1 | −83.0 | 3.2 | −61.3 | 3.1 | 3.94 | 4.40 | ||
| AQB-1 | −93.1 | 6.1 | −71.4 | 6.0 | 6.84 | 7.30 | ||
| AQB-2 | −89.0 | 4.6 | −67.3 | 4.5 | 5.34 | 5.80 | ||
| AQB-3 | −85.1 | 3.5 | −63.4 | 3.4 | 4.24 | 4.70 | ||
| AQB-4 | −85.9 | 3.6 | −64.2 | 3.5 | 4.34 | 4.80 | ||
| AQB-6 | −94.7 | 6.2 | −73.0 | 6.1 | 6.94 | 7.40 | ||
| AQY-1 | −90.2 | 4.1 | −68.5 | 4.0 | 4.84 | 5.30 | ||
| AQY-2 | −85.0 | 3.6 | −63.3 | 3.5 | 4.34 | 4.80 | ||
| AQY-3 | −91.6 | 4.9 | −69.9 | 4.8 | 5.64 | 6.10 | ||
| AQY-4 | −90.4 | 4.8 | −68.7 | 4.7 | 5.54 | 6.00 | ||
| AQY-5 | −86.2 | 3.8 | −64.5 | 3.7 | 4.54 | 5.00 | ||
| 330 °C | 350 °C | 430 °C | ||||||
| Yurungkash River, Xinjiang, China | ZY2 | −71.8 | 5.2 | −51.4 | 5.7 | 6.0 | 6.6 | [19] |
| ZY3 | −67.3 | 3.7 | −46.8 | 4.2 | 4.4 | 5.1 | ||
| Karakash River, Xinjiang, China | QZY4 | −72.4 | 5.6 | −52.5 | 6.1 | 6.3 | 7.0 | |
| QZY5 | −55.7 | 1.1 | −34.9 | 1.6 | 1.8 | 2.5 | ||
| QZY6 | −71.4 | 5.0 | −51.0 | 5.5 | 5.7 | 6.4 | ||
| QZY7 | −65.7 | 2.9 | −45.2 | 3.4 | 3.6 | 4.3 | ||
| MY1 | −68.7 | 3.2 | −48.3 | 3.7 | 4.0 | 4.6 | ||
| MY2 | −63.3 | 2.4 | −42.7 | 2.9 | 3.1 | 3.8 | ||
| MY3 | −69.3 | 4.5 | −48.9 | 5.0 | 5.3 | 5.9 | ||
| MY4 | −67.1 | 3.1 | −46.6 | 3.6 | 3.8 | 4.5 | ||
| 330 °C | 390 °C | 450 °C | ||||||
| Karakash River, Xinjiang, China | MYH1 | −97 | 0.8 | −75.4 | 1.3 | 1.9 | 2.3 | [2] |
| MYH2 | −93 | 7.0 | −70.8 | 7.5 | 8.1 | 8.5 | ||
| MYH3 | −107 | 4.6 | −84.9 | 5.1 | 5.7 | 6.1 | ||
| MYH4 | −80 | 2.7 | −57.9 | 3.2 | 3.9 | 4.2 | ||
| MYH6 | −63 | 5.0 | −40.9 | 5.5 | 6.2 | 6.6 | ||
| MYH7 | −67 | 7.3 | −45.3 | 7.8 | 8.4 | 8.8 | ||
| MYH10 | −46 | 4.9 | −24.6 | 5.4 | 6.0 | 6.4 | ||
| MYH11 | −28 | 3.6 | −5.8 | 4.1 | 4.7 | 5.1 | ||
| MYH21 | −39 | 5.4 | −17.7 | 5.9 | 6.6 | 7.0 | ||
| MYH24 | −106 | 3.5 | −84.5 | 4.0 | 4.6 | 5.0 | ||
| MYH25 | −41 | 3.7 | −19.4 | 4.1 | 4.8 | 5.2 | ||
| MYH30 | −80 | 2.7 | −58.4 | 3.2 | 3.8 | 4.2 | ||
| MYH31 | −85 | 7.6 | −63.3 | 8.1 | 8.8 | 9.1 | ||
| MYH32 | −85 | 1.5 | −63.1 | 2.0 | 2.6 | 3.0 | ||
| MYH33 | −86 | 1.1 | −64.3 | 1.5 | 2.2 | 2.6 | ||
| MYH34 | −77 | 6.6 | −55.3 | 7.1 | 7.8 | 8.2 | ||
| MYH35 | −91 | 2.0 | −69.2 | 2.5 | 3.2 | 3.6 | ||
| MYH36 | −107 | 2.1 | −85.2 | 2.6 | 3.2 | 3.6 | ||
| MYH37 | −91 | 3.4 | −69.6 | 3.9 | 4.5 | 4.9 | ||
| MYH38 | −87 | 3.6 | −64.8 | 4.1 | 4.7 | 5.1 | ||
| MYH39 | −58 | 6.7 | −36.6 | 7.1 | 7.8 | 8.2 | ||
| MYH40 | −77 | 3.9 | −54.8 | 4.4 | 5.0 | 5.4 | ||
| MYH41 | −78 | 3.8 | −55.8 | 4.2 | 4.9 | 5.3 | ||
| MYH42 | −57 | 4.3 | −35.7 | 4.8 | 5.4 | 5.8 | ||
| MYH43 | −108 | 2.9 | −86.1 | 3.4 | 4.0 | 4.4 | ||
| MYH44 | −86 | 3.0 | −64.5 | 3.5 | 4.1 | 4.5 | ||
| MYH50 | −85 | 4.9 | −63.5 | 5.4 | 6.0 | 6.4 | ||
| MYH51 | −100 | 2.2 | −77.9 | 2.7 | 3.3 | 3.7 | ||
| MYH52 | −79 | 7.9 | −56.8 | 8.4 | 9.0 | 9.4 | ||
| MYH54 | −88 | 2.5 | −66.0 | 3.0 | 3.7 | 4.1 | ||
| MYH55 | −103 | 6.0 | −81.1 | 6.4 | 7.1 | 7.5 | ||
| MYH56 | −95 | 3.5 | −73.2 | 4.0 | 4.6 | 5.0 | ||
| MYH57 | −109 | 4.3 | −87.1 | 4.8 | 5.5 | 5.9 | ||
| MYH58 | −72 | 4.0 | −50.1 | 4.5 | 5.1 | 5.5 | ||
| MYH59 | −93 | 1.6 | −70.9 | 2.1 | 2.7 | 3.1 | ||
| 350 °C | 400 °C | 450 °C | ||||||
| Dahua, Guangxi, China | D-4 | −76.9 | 12.3 | −55.2 | 13.04 | 13.50 | 13.82 | [20] |
| D-8 | −79.8 | 10.5 | −58.1 | 11.24 | 11.70 | 12.02 | ||
| Luodian, Guizhou, China | LDS | 15.8 | 16.54 | 17.00 | 17.32 | [21] | ||
| LDW1 | 16.4 | 17.14 | 17.60 | 17.92 | ||||
| LDW5 | 15.4 | 16.14 | 16.60 | 16.92 | ||||
| LDGW-1 | 17.7 | 18.44 | 18.90 | 19.22 | ||||
| LDW6 | 15.2 | 15.94 | 16.40 | 16.72 | ||||
| LDLG4 | 15.8 | 16.54 | 17.00 | 17.32 | ||||
| Luodian, Guizhou, China | LMBT-13-2 | 15.3 | 16.04 | 16.50 | 16.82 | [22] | ||
| LMTC08-7 | 14.3 | 15.04 | 15.50 | 15.82 | ||||
| 11KY080 | 15.6 | 16.34 | 16.80 | 17.12 | ||||
| 11KY181 | 16.5 | 17.24 | 17.70 | 18.02 | ||||
| ETC05-2-5 | 14.7 | 15.44 | 15.90 | 16.22 | ||||
| ETC05-2-6 | 14.5 | 15.24 | 15.70 | 16.02 | ||||
| ETC05-4 | 14.1 | 14.84 | 15.30 | 15.62 | ||||
| ETC05-6 | 14.6 | 15.34 | 15.80 | 16.12 | ||||
| ETC05-8 | 15.5 | 16.24 | 16.70 | 17.02 | ||||
| ETC05-23 | 16.3 | 17.04 | 17.50 | 17.82 | ||||
| 350 °C | 400 °C | 450 °C | ||||||
| Sanchakou, Qinghai, China | QH-176 | −86 | 11.4 | −64.3 | 12.13 | 12.60 | 13.35 | [23] |
| QH-177 | −87 | 12.3 | −65.3 | 13.03 | 13.50 | 14.25 | ||
| QH-001 | −78 | 12.2 | −56.3 | 12.93 | 13.50 | 14.15 | ||
| QHSH-001 | −84 | 12.6 | −62.3 | 13.33 | 13.80 | 14.55 | ||
| 330 °C | 390 °C | 450 °C | ||||||
| Xiuyan placer nephrite, Liaoning, China | LHM15-1 | −88.23 | 8.40 | −66.53 | 8.89 | 9.52 | 9.92 | [24] |
| LHM15-2 | −75.2 | 8.50 | −53.5 | 8.99 | 9.62 | 10.02 | ||
| LHM15-3 | −93.29 | 8.80 | −71.59 | 9.29 | 9.92 | 10.32 | ||
| LHM15-4 | −94.95 | 9.30 | −73.25 | 9.80 | 10.42 | 10.82 | ||
| LHM15-5 | −78.51 | 10.60 | −56.8 | 11.09 | 11.72 | 12.12 | ||
| LHM15-6 | −93.78 | 8.20 | −72.07 | 8.69 | 9.32 | 9.72 | ||
| LHM15-7 | −86.58 | 8.00 | −64.88 | 8.49 | 9.12 | 9.52 | ||
| 350 °C | 400 °C | 450 °C | ||||||
| Xiuyan, Liaoning, China | Y-1 | −70 | 10.0 | −48 | 10.7 | 11.20 | 11.52 | [25] |
| Y-3 | −74 | 9.3 | −52 | 10 | 10.50 | 10.82 | ||
| G-3 | −74 | 8.5 | −52 | 9.2 | 9.70 | 10.02 | ||
| G-6 | −72 | 8.1 | −50 | 8.8 | 9.30 | 9.62 | ||
| W-1 | −70 | 13.3 | −48 | 14 | 14.50 | 14.82 | ||
| W-2 | −73 | 11.7 | −51 | 12.4 | 12.90 | 13.22 | ||
| S-1a | −76 | 10.4 | −54 | 11.1 | 11.60 | 11.92 | ||
| S-1b | −72 | 10.3 | −50 | 11 | 11.50 | 11.82 | ||
| S-3a | −74 | 9.1 | −52 | 9.8 | 10.30 | 10.62 | ||
| S-3b | −70 | 9 | −48 | 9.7 | 10.20 | 10.52 | ||
| Chuncheon, South Korea | NE1 | −108 | −8.7 | −86.3 | −7.96 | −7.50 | −7.18 | [5] |
| NE2 | −114 | −8.4 | −92.3 | −7.66 | −7.20 | −6.88 | ||
| NE3 | −105 | −9.9 | −83.3 | −9.16 | −8.70 | −8.38 | ||
| NE4 | −107 | −9 | −85.3 | −8.26 | −7.80 | −7.48 | ||
| NE5 | −108 | −8.2 | −86.3 | −7.46 | −7.00 | −6.68 | ||
| NE6 | −112 | −8.6 | −90.3 | −7.86 | −7.40 | −7.08 | ||
| NE7 | −109 | −8.9 | −87.3 | −8.16 | −7.70 | −7.38 | ||
| NE8 | −110 | −9.3 | −88.3 | −8.56 | −8.10 | −7.78 | ||
| NE9 | −109 | −9.2 | −87.3 | −8.46 | −8.00 | −7.68 | ||
| Złoty Stok, Poland | A | −76.4 | 10.2 | −54.7 | 10.94 | 11.40 | 11.72 | [26] |
| B | −76.2 | 8.3 | −54.5 | 9.04 | 9.50 | 9.82 | ||
| C | −77.2 | 10.4 | −55.5 | 11.14 | 11.60 | 11.92 | ||
| D | −74.6 | 10.2 | −52.9 | 10.94 | 11.40 | 11.72 | ||
| Kavokta, Lower Ollomi, Golyube, Vitim, Russia | −119.3 | −15.52 | −97.6 | −14.78 | −14.32 | −14.00 | [6] | |
| −178.5 | −17.24 | −156.8 | −16.50 | −16.04 | −15.72 | |||
| −133.2 | −14.93 | −111.5 | −14.19 | −13.73 | −13.41 | |||
| Voimakan, Vitim, Russia | V1-14 | −18.5 | −17.76 | −17.30 | −16.98 | [27] | ||
| PK-1 | −18.8 | −18.06 | −17.60 | −17.28 | ||||
| PK-3 | −18.8 | −18.06 | −17.60 | −17.28 | ||||
| Nephrite Deposit | Ore Genesis | Intrusive Rock | Carbonate Rock | References |
|---|---|---|---|---|
| Xiaomeiling, Jiangsu, China | Contact metasomatism | Miaoxi granite porphyry | Qixia Formation limestone | This study |
| Pishan, Xinjiang, China | Quartz diorite | Dolomitic marble | [18] | |
| Yingelike, Ruoqiang, Xinjiang, China | Granitoids | Dolomitic marble | [4] | |
| Alamas, Xinjiang, China | Granitoids | Dolomitic marble | [3] | |
| Dahua, Guangxi, China | Diabase | Sidazhai Formation limestone | [20] | |
| Luodian, Guizhou, China | Diabase | Sidazhai Formation limestone | [21] | |
| Sanchakou, Qinghai, China | Gabbro | Qingbanshisuzhan Formation of Wanbaogou Group | [23] | |
| Chuncheon, South Korea | Chuncheon granite | Dolomitic marble | [5] | |
| Złoty Stok, Poland | Granitoids | Dolomitic marble | [26] | |
| Kavokta, Lower Ollomi, Golyube, Vitim, Russia | Granite | Dolomitic marble | [6] | |
| Voimakan, Vitim, Russia | Vitimkan intrusive complex | Dolomite marble | [27] | |
| Xiuyan, Liaoning, China | Metamorphism | Dolomite marble | [25] |
| Nephrite Deposit | Sample No. | Rock | δD | δ18O | References |
|---|---|---|---|---|---|
| Xiaomeiling, Jiangsu, China | γ-3 | Miaoxi granite porphyry | −90.8 | 3.6 | This study |
| γ-4 | −85.2 | 7.3 | |||
| γ-5 | −85.0 | 7.0 | |||
| Khaita, Vitim, Russia | Granite | 4.16 | [6] | ||
| Kavokta, Vitim, Russia | 9.93 | ||||
| Luodian, Guizhou, China | 11YK2100 | Siliceous rock | 22.4 | [22] |
| Nephrite Deposit | Sample No. | δ18O | Sample No. | δ18O* | Reference |
|---|---|---|---|---|---|
| Xiaomeiling, Jiangsu, China | P-1 | 19.5 | C-2 | 15.2 | This study |
| P-3 | 20.7 | C-3 | 14.8 | ||
| P-5 | 19.9 | C-4 | 16.4 | ||
| Chuncheon, South Korea | D-1 | 18.2 | D-18 | 2.4 | [5] |
| D-2 | 18.0 | D-19 | 4.1 | ||
| D-3 | 15.0 | D-20 | 3.8 | ||
| D-4 | 14.3 | D-21 | 7.4 | ||
| D-5 | 18.2 | ||||
| D-6 | 13.7 | ||||
| D-7 | 15.1 | ||||
| D-8 | 15.9 | ||||
| D-9 | 14.7 | ||||
| D-10 | 17.6 | ||||
| D-11 | 15.5 | ||||
| D-12 | 15.5 | ||||
| D-13 | 16.9 | ||||
| D-14 | 14.0 | ||||
| D-15 | 14.4 | ||||
| D-16 | 17.8 | ||||
| D-17 | 14.2 | ||||
| Golyube, Vitim, Russia | 28.4 | 22.36 | [6] | ||
| Lower Ollomi, Vitim, Russia | 29.26 | ||||
| 26.65 | |||||
| 28.40 | |||||
| Voimakan, Vitim, Russia | KP-81-1-3 | 26.1 | [27] | ||
| Xiuyan, Liaoning, China | 9872 | 22.6 | [39] | ||
| Alamas, Xinjiang, China | S-1 | 6.1 | |||
| Luodian, Guizhou, China | EC-4B2 | 20.2 | [22] |
| Nephrite Deposit | Sample No. | Rocks | δ30Si | References |
|---|---|---|---|---|
| Luodian, Guizhou, China | 11YK2100 | Siliceous rock | 1.4 | [22] |
| LMBT-13-2 | Nephrite | 1.3 | ||
| LMTC08-7 | 1.2 | |||
| 11KY080 | 1.2 | |||
| 11KY181 | 1.1 | |||
| ETC05-2-5 | 1.4 | |||
| ETC05-2-6 | 1.7 | |||
| ETC05-4 | 1.4 | |||
| ETC05-6 | 1.3 | |||
| ETC05-8 | 1.1 | |||
| ETC05-23 | 1.2 | |||
| Luodian, Guizhou, China | LDS | Nephrite | 0.3 | [21] |
| LDW1 | 0.8 | |||
| LDW5 | 0.7 | |||
| LDGW-1 | 0.3 | |||
| LDW6 | 0.7 | |||
| LDLG4 | 0.8 | |||
| LKT001-C | Siliceous rock | 0.5 | ||
| LKT002 | 0.4 | |||
| LJGL-1 | Diabase | 0.2 | ||
| LJGL-2 | 0.0 | |||
| LJGL-4 | 0.1 |
| Stage | Mineral-Water | δ18Oi of Country Rocks | δ18O of W2 | ||
|---|---|---|---|---|---|
| 350 °C | 400 °C | 450 °C | |||
| I | Qtz-W1 | 22.4 | 17.10 | 18.34 | 19.34 |
| Dol-W1 | 20.0 | 13.76 | 14.94 | 15.88 | |
| Cal-W1 | 20.0 | 16.23 | 17.25 | 18.07 | |
| δ18O of Tr* | |||||
| II | W2-Tr | 16.36 | 17.14 | 17.83 | |
| W2-Tr | 13.02 | 13.73 | 14.36 | ||
| W2-Tr | 15.50 | 16.05 | 16.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Liao, Z.; Chen, Q.; Qi, L.; Liu, Y. Multiple Solutions of Ore-Forming Fluids of Carbonate Rock-Related Nephrite Deposits Constrained by Hydrogen and Oxygen Isotopes. Minerals 2025, 15, 272. https://doi.org/10.3390/min15030272
Li P, Liao Z, Chen Q, Qi L, Liu Y. Multiple Solutions of Ore-Forming Fluids of Carbonate Rock-Related Nephrite Deposits Constrained by Hydrogen and Oxygen Isotopes. Minerals. 2025; 15(3):272. https://doi.org/10.3390/min15030272
Chicago/Turabian StyleLi, Ping, Zongting Liao, Qi Chen, Lijian Qi, and Yungui Liu. 2025. "Multiple Solutions of Ore-Forming Fluids of Carbonate Rock-Related Nephrite Deposits Constrained by Hydrogen and Oxygen Isotopes" Minerals 15, no. 3: 272. https://doi.org/10.3390/min15030272
APA StyleLi, P., Liao, Z., Chen, Q., Qi, L., & Liu, Y. (2025). Multiple Solutions of Ore-Forming Fluids of Carbonate Rock-Related Nephrite Deposits Constrained by Hydrogen and Oxygen Isotopes. Minerals, 15(3), 272. https://doi.org/10.3390/min15030272





