1. Introduction
Porphyry deposits are the main source of copper and molybdenum in the world, as well as an important source of gold [
1,
2,
3]. The Pulang giant porphyry Cu (–Mo–Au) deposit is located in the Zhongdian island arc, southern Yidun island arc, which is a part of the Sanjiang Tethys metallogenic domain [
4,
5]. Porphyry Cu (–Mo–Au) deposits are characterized by their typical alteration zones and minerals [
1,
6,
7]. However, the factors controlling the formation of the Pulang Cu (–Mo–Au) deposit are controversial. Most studies suggest that Cu (–Mo–Au) mineralization is closely associated with the potassic alteration zone [
8,
9,
10], whereas others have suggested that the metallogenic region of Cu (–Mo–Au) mineralization is closely related to propylitic alteration [
11,
12].
Titanite (CaTiSiO
5) is widely developed in acidic, intermediate, and alkaline igneous rocks; various types of metamorphic rocks; hydrothermally altered rocks; and some sedimentary rocks [
13]. The closure temperature of the U-Pb system of titanite is relatively high (600–700 °C). However, it is lower than that of monazite (~750 °C) and zircon (>800 °C), while being higher than the closure temperature of the
40Ar–
39Ar isotopic system of hornblende (~500 °C) and the
40Ar–
39Ar isotopic closure temperature of sericite (~300 °C to 500 °C). Titanite can be used in U(–Th)–Pb dating, which can be applied to determine or define the geochronology of the deposit [
14,
15,
16]. Furthermore, the igneous rocks contain less ancient titanites of inherited genesis, which can reveal the cooling age of initial magma. Titanite experiences complete magmatic–hydrothermal evolution and is rich in rare earth elements (REEs) and high-field-strength elements (HFSEs). The major and trace element characteristics of titanites formed by magmatic crystallization, late-stage fluid exsolution, and hydrothermal metasomatism record the geological information of continuous high- to low-temperature magmatic–hydrothermal evolution [
17,
18]. In conclusion, titanite is effective geochronometer, thermobarometer, oxybarometer, and metallogenic potential indicator mineral [
19,
20]. So, it has become an ideal research object to reveal the metal enrichment mechanism of magmatic–hydrothermal deposits.
There are three alteration zones in the Pulang porphyry deposits (potassium silicate alteration, phyllic alteration, and propylitic alteration). Given the relationship between mineralization and different alteration zones in Pulang porphyry deposits, previous studies have mainly adopted petrographic methods such as ore body morphology and mineral assemblage [
10,
11]. A recent study has reported the correlation between mineralization and biotite with different alteration characteristics (fugacity temperature, oxygen fugacity, etc.). However, no clear distinction has been defined between phyllic and propylitic alterations [
21], and a direct correlation between mineralization and the elements’ geochemical characteristics is lacking. Furthermore, the Pulang deposit exhibits a phenomenon of vertical separation of copper and molybdenum mineralization. Specifically, the intensity of copper mineralization along an exploration line remains uniform from the shallow to the deep sections, with notable high-grade mineralization in the shallow to middle depths. Conversely, Mo exhibits higher grades in the deeper sections and lower grades in the shallow sections [
10]. However, the underlying mechanism responsible for this separation and precipitation of Cu and Mo remains elusive.
In this study, detailed textural and in situ LA-ICP-MS elemental analysis of titanite grains from three alteration zones in the Pulang Cu (–Mo–Au) deposit were analyzed and compared. The results indicate the influence of elemental behavior on mineralization during magma-fluid evolution, reveal the relationship between mineralization and alteration, and throw light on the mechanism of Cu–Mo separation.
2. Geological Background
2.1. Regional Geology
The Pulang giant porphyry deposit is located in the south of the Yidun island arc, Sanjiang Tethys metallogenic domain (
Figure 1), which belongs to the eastern section of the Tethys Himalayan tectonic domain, located in the area of intense collision and compression between Gondwana and Eurasian ancient plate. The Yidun island arc was an ancient arc formed in response to the subduction of the Paleo-Tethys oceanic slab, causing the formation of multiple deposit types in Late Triassic [
22,
23,
24,
25,
26,
27]. The VMS-type Pb–Zn–Ag polymetallic deposits are developed in the northern section of the Yidun Island arc. Abundant porphyritic intrusions occurred in the southern section of the Yidun Island arc, as the causative intrusions of porphyry-(skarn) type Cu–Mo–Au deposits [
28,
29,
30].
The magmatic activities in the area are more frequent, with large areas of mainly intrusive rocks, which are divided into three stages, including Indosinian, Yanshanian, and Himalayan, among which Indosimian magmatic rocks are most widely distributed. The Upper Triassic Tumugou Formation (T
3t) and Quaternary (Q) strata are the main strata exposed in the Pulang deposit. The Tumugou Formation is composed of volcanic and clastic rocks. The volcanic rocks are mostly andesite with minor amounts of rhyolite. The clastic rocks consist of sandy slate, slate, and a small amount of limestone. The tectonic activity in the mineralized area is very complex. In addition to the major faults, secondary folds and joints are also very common, providing favorable conditions for the emplacement of magmatic rocks [
31,
32]. The mineralized area is generally hosted by a series of intermediate–felsic intrusions and controlled by the Pulang syncline paralleling to the regional NNE trending faults [
33].
2.2. Deposit Geology
The Pulang giant porphyry deposit located on the east flank of the Pulang syncline is the largest porphyry deposit developed in the Late Triassic of the study area. The main focal envelope of the ore body is the Tumgou Formation (
Figure 2).
There are different stages of magmatic activities in the Pulang mineralized area, forming the Pulang multi-stage complex. The Pulang multi-stage complex can be divided into five separate rock bodies (V is located outside the map area). The geological and geophysical data show that the five separate rock bodies are connected at depth. Quartz diorite porphyry, which formed in the early stage has the widest distribution area, followed by quartz monzonite porphyry. The quartz monzonite porphyry intruded the quartz diorite porphyry in the late stage, showing an intrusive contact relationship (
Figure 2). The main ore bodies are distributed mainly in the quartz monzonite porphyry, and the ore body shape is controlled by the morphology of the quartz monzonite porphyry. The late granite porphyry is interspersed with quartz diorite porphyry and quartz monzonite porphyry as veins. The quartz diorite porphyry is in jagged contact with the surrounding rock, and various alterations are developed. The rock bodies are more fragmented in the mineralized area, and quartz veins and broken crystal cavities are visible (
Figure 3).
2.3. Alteration Zonation and Mineral Assemblage
The Pulang complex developed typical alteration zoning. From the core of quartz monzonite porphyry to peripheral area, the alteration type changes from potassium silicate to phyllic alteration and propylitic alteration zoning [
34,
35], with extensive hornfelsic alteration zones developed in the lateral contact zone of surrounding rocks (
Figure 2). Metal sulfides (mainly pyrite and chalcopyrite) mineralization is common in the potassium silicate alteration zone and phyllic zone, and most of the ore bodies occur in the potassium silicate zone of quartz monzonite porphyry [
10].
The potassium silicate alteration zone is located in the core of the rock, mainly consisting of K-feldspar (Kfs), quartz (Qz), and biotite. Large amounts of secondary reddish K-feldspar and quartz can be found in the potassium silicate alteration, which are agglomerated and distributed in a pocket-like fashion in the rock body. Quartz variegated crystals, quartz veins, and fine-grained quartz matrix are visible, demonstrating that significant silicification has occurred. The ore body in the first mining area is confined to the potassium silicate alteration zone.
The phyllic alteration zone is mainly distributed around the potassium silicate alteration zone, and the boundary between the two alteration zones is not clear. There is a gradual transition trend from potassium silicate alteration to phyllic alteration, and some areas have overlaps. Within this alteration zone, sericitization and silicification are common, and potassic-alteration is relatively rare. The mineral composition of altered quartz monzonite porphyry mainly includes quartz and sericite, with the widespread development of fine metal sulfide veins. Near the potassium silicate alteration zone, the copper metal grade is greater than 0.2 wt.%, reaching the ore grade, while the copper metal grade is gradually lower than 0.2 wt.% toward the distal zone.
The propylitic alteration zone is distributed mainly on the distal zone, and the distribution is more extensive. A large amount of chlorite and saussurite were developed, and carbonate veins with a small amount of pyrite are also common. The chlorite in this alteration zone mainly occurs as agglomerates or veins. The alteration zone is characterized by pyrite and chalcopyrite mineralization, but the overall mineralization is relatively weak. Alteration superposition can be seen locally, where mineralization obviously enhanced.
3. Characteristics of Titanite
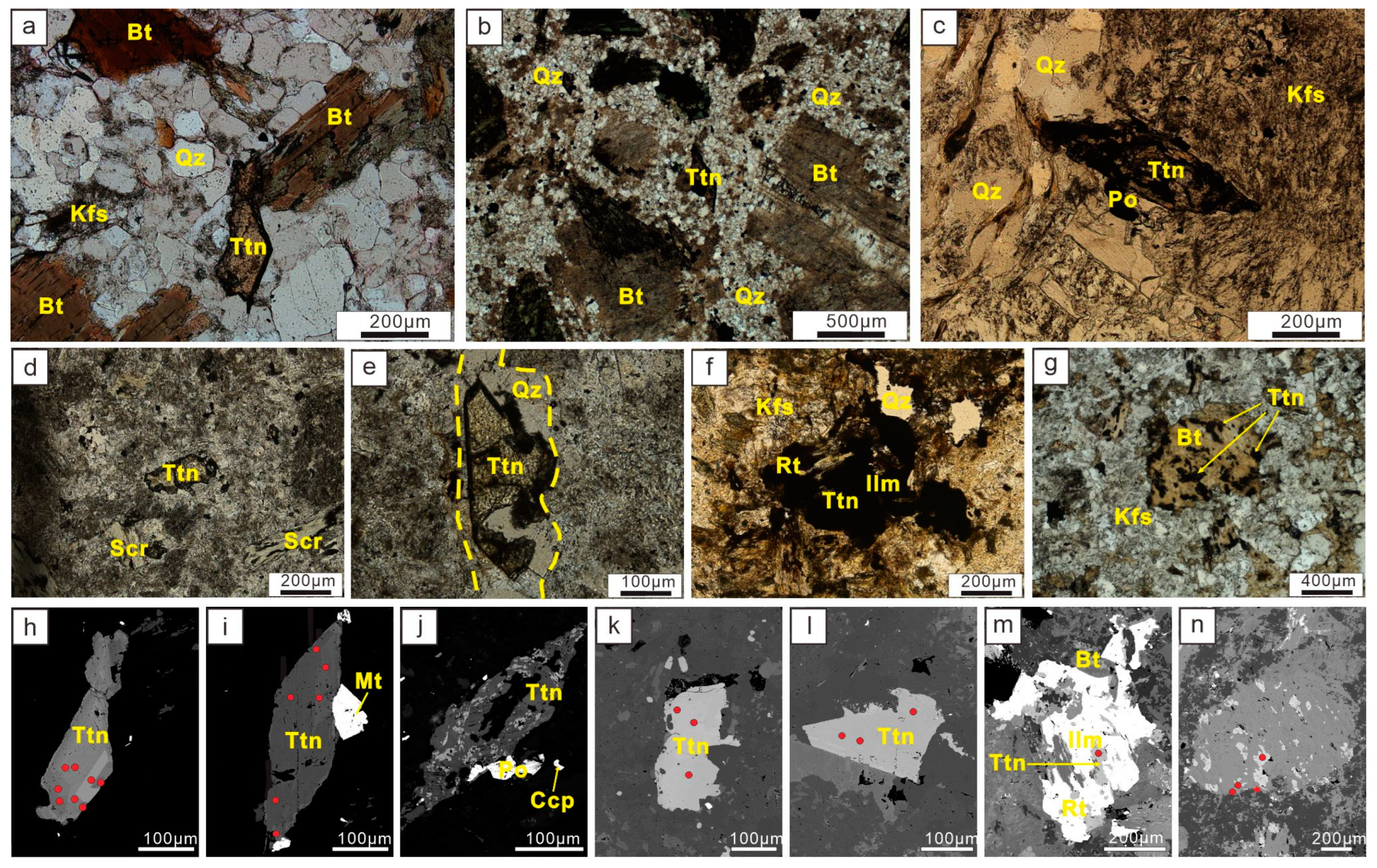
In the fresh rocks of Pulang, titanite occurs as large euhedral grains, usually larger than 200 μm. It is intergrown with quartz, primary biotite, and other typical early magmatic crystalline minerals (
Figure 4a,b). It shows characteristic internal growth zoning of typical magmatic titanite.
Titanite in the potassium silicate zone of Pulang, copper ore is usually shaped as a complete wedge or envelope, with the subhedral occurrence. The size of mineral grains ranges from 100 to 500 μm. Titanite is either paragenetic with the early crystalline minerals of magma (such as quartz) or wrapped inside the feldspar. The paragenetic association of minerals usually include apatite, magnetite, magnetic pyrite, and chalcopyrite (
Figure 4c,d). Some of the less altered titanites also have obvious zonings, and their overall characteristics are closer to those of magmatic titanites. Ilmenite is locally visible, presumably as a result of hydrothermal metasomatism of magmatic titanite.
Titanite in the phyllic alteration zone is usually paragenetically associated with typical hydrothermal alteration minerals such as ilmenite and rutile with subhedral and anhedral shapes. Some of these are visible in quartz veins, and their occurrence is strongly influenced by vein morphology (
Figure 4e). Their grains are small, usually about 50–200 μm. The titanite in this alteration zone exhibits characteristic hydrothermal titanite features [
36,
37,
38].
Titanite in the propylitic alteration zone is also often produced in biotite cleavage joints or paragenetic with ilmenite and rutile, with subhedral and anhedral (predominantly anhedral) shapes, about 50–100 μm in grain size, with obvious hydrothermal titanite characteristics (
Figure 4f,g).
4. Sampling and Analytical Methods
The analyzed samples were collected from different alteration zones of the ore body in Pulang (
Figure 2b), including quartz monzonite porphyry, quartz diorite porphyry, and granite porphyry. Quartz monzonite porphyry is altered by potassium silicate, phyllic, and propylitic alterations. Quartz diorite porphyry and granite porphyry are mainly altered by phyllic and propylitic alterations. A total of 11 rock samples were selected from the potassium silicate, phyllic, and propylitic alteration zones.
The electron probe analysis was completed at the Testing Center of Shandong Bureau of Metallurgical Geology Administration of China using a JXA-8230 electron probe microanalyzer, JEOL, Tokyo, Japan, with an operating voltage of 15 kV, operating current of 10/20 nA, analysis beam spot of 5 μm, calibration method of ZAF, and peak integration time of 20 s for main elements (content > 1%). The standard samples include the U.S. SPI mineral/metal standard and the Chinese national standard sample GSB. To ensure the stability of the standard sample, the test conditions of the standard sample are the same as the unknown sample, and each standard sample is tested at five points. Therefore, the count rate of the standard sample test is stable and reliable. A total of 154 points were analyzed.
The LA-ICP-MS trace elements measurements were performed on 7900 inductively coupled plasma mass spectrometer, Agilent Technologies Inc., State of California, United States, at Wuhan Sample Solution Analytical Technology Co., Ltd. (Wuhan, China), which corresponds to 75 points of the major elements data.
5. Results
5.1. Major Elements
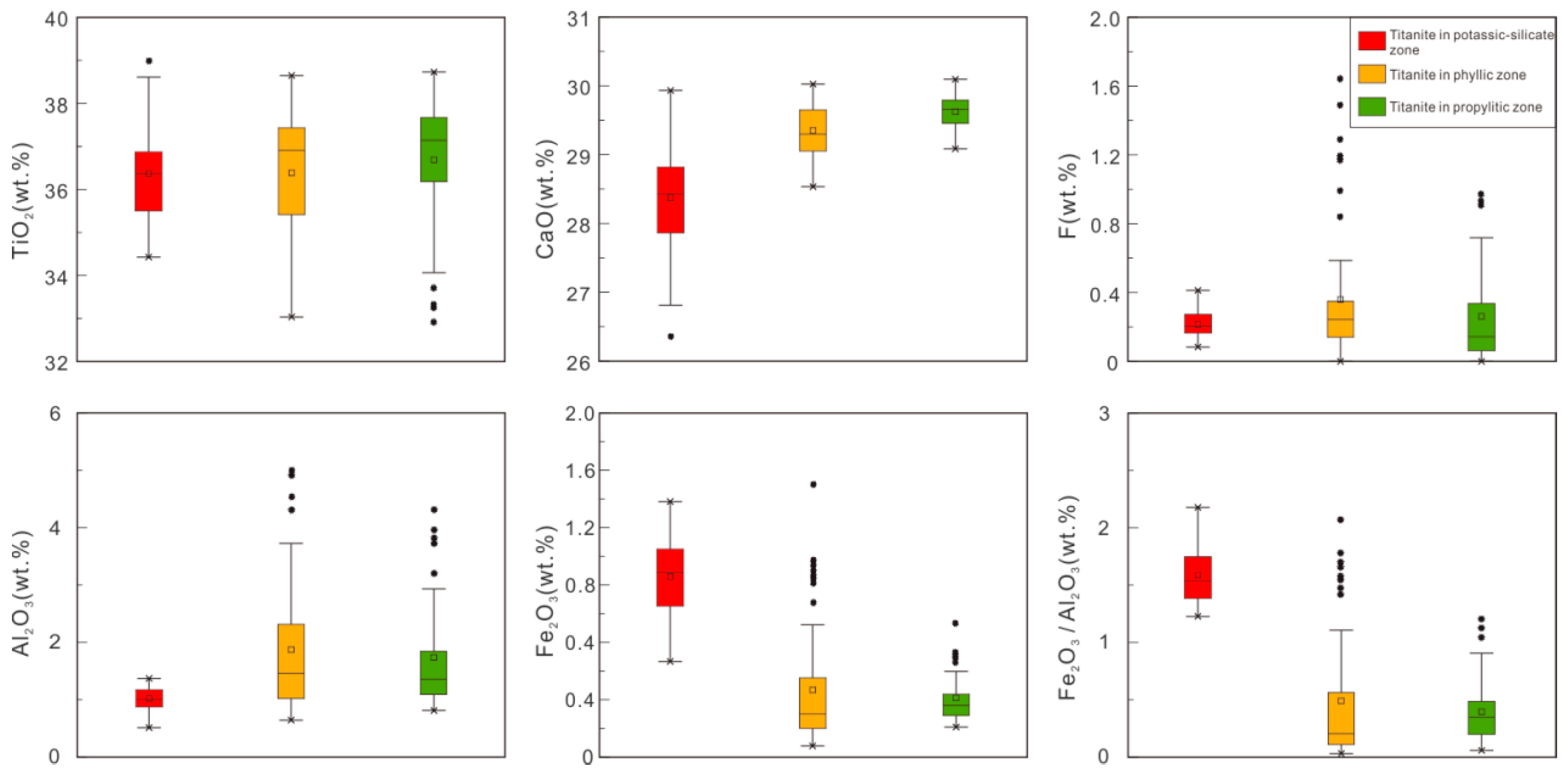
Titanite mainly contains Ti, Ca, Si, Al, Fe, and F, and their contents are correlated with their genesis [
14,
39,
40]. The obtained results of major elements in titanite are shown in
Figure 5 and
Table 1. The CaO, Fe
2O
3, Al
2O
3, and F content ranges in titanite in the potassium silicate alteration zone vary from 26.35 to 29.95 wt.%, 0.84 to 2.23 wt.%, 0.51 to 1.37 wt.%, and 0.21 wt.%, respectively, while the Al
2O
3/Fe
2O
3 ratios range from 0.46 to 0.81. The ranges of CaO, Fe
2O
3, Al
2O
3, and F content in titanite in the phyllic alteration zone vary from 28.52 to 30.02 wt.%, 0.11 to 2.41 wt.%, 0.63 to 5.00 wt.%, and 0.36 wt.%, respectively, and Al
2O
3/Fe
2O
3 ratios range from 0.48 and 26.59. The ranges of CaO, Fe
2O
3, Al
2O
3, and F content in titanite in the propylitic alteration zone vary from 29.08 to 30.10 wt.%, 0.84 to 2.23 wt.%, 0.81 to 4.31 wt.%, and 0.26 wt.%, respectively, and the Al
2O
3/Fe
2O
3 ratio ranges from 0.82 to 14.35. The titanite MnO, Na
2O, MgO, K
2O, and P
2O
5 contents in the different alteration zones are close to the detection limits. The contents of other elements such as Cl are below the detection limits of the electron probe microanalysis.
In general, the electron probe microanalysis (EMPA) data of titanite in different alteration zones of the Pulang porphyry copper deposit are similar regarding content of major elements, but some elements, such as Ca, Fe, and Al, show more significant differences. For example, the F content of titanite in the propylitic alteration zone is overall higher than titanite in the potassium silicate alteration zone and lower than titanite in the phyllic alteration zone.
5.2. Trace Elements
The trace element content in titanite is controlled by a variety of factors, including the element content in the melt, melt composition, oxygen fugacity, temperature and pressure conditions, and mineral structure. Essentially, the trace element content in titanite is controlled by the element content in melt when titanite crystallizes and the distribution coefficient of the elements between titanite and melt. The obtained results of trace elements in titanite are shown in
Table 2 and
Figure 6.
In general, the trace element content in the potassium silicate alteration zone is different from that in phyllic and propylitic alteration zones, such as Zr/Hf and Nb/Ta. The ranges of Zr/Hf of titanite in the potassium silicate, phyllic, and propylitic alteration zones vary from 2.30 to 9.44 (mean 6.17), 0.05 to 2.85 (mean 0.92), and 0.39 to 4.32 (mean 2.10), respectively. The Zr/Hf ranges of titanite in the potassium silicate, phyllic, and propylitic alteration zones vary from 7.0 to 13.1 (mean 9.0), 6.1 to 46.4 (mean 19.4), and 8.3–44.0 (mean 16.9), respectively. The ranges of Sr content of titanite in the potassium silicate, phyllic and propylitic alteration zone vary from 13 to 100 ppm (mean 58 ppm), 19 to 406 ppm (concentrated in 19–76 ppm, mean 39 ppm), and 16 to 49 ppm (mean 23 ppm), respectively, and showed obvious linear relationships.
5.3. Rare Earth Elements
The obtained results of trace elements in titanite are shown in
Table 2 and
Figure 7.
The total REE + Y content of titanite in the potassium silicate alteration zone of the Pulang copper deposit varies from 9839 to 35,764 ppm (mean 20,994 ppm), the light rare earth elements (LREEs) vary from 8116 to 25,872 ppm (mean 16,219 ppm), the LREE/HREE ratios range from 6.21 to 11.83 (mean 8.61), and the δEu range is more concentrated at 0.50–0.81 (mean 0.62). The REE + Y content in titanite in the phyllic zone varies from 417 to 7051 ppm (mean 2179 ppm), the LREE content varies from 103 to 4868 ppm (mean 1342 ppm), the LREE/HREE ratio range is concentrated at 0.95–9.20 (mean 5.22), and the δEu ranges from 0.94 to 4.18 (mean 1.79). The variation in REE + Y content of titanite in the propylitic alteration zone ranges from 403 to 8420 ppm (mean 2571 ppm), LREE content varies from 92 to 2677 ppm (mean 1368 ppm), LREE/HREE ratios range from 0.56 to 8.32 (mean 3.94), and δEu ranges from 0.45 to 3.89 (mean 1.95).
In general, all the titanite grains have a “right-leaning” chondrite-normalized REE pattern, with relative enrichment in LREEs and depletion in HREEs. Titanite in the potassium silicate alteration zone is enriched in REEs, and the chondrite-normalized REE patterns of titanite are all above the whole rock. The REE pattern of titanite in phyllic and propylitic alteration zone is dispersive, and REE content is lower than that in the potassium silicate alteration zone.
6. Discussion
6.1. Origin of Titanite
Previous studies show that the Fe
2O
3 content in magmatic titanite and metamorphic titanite have differences (metamorphic titanite usually has a higher Al/Fe ratios). Maps of magmatic and metamorphic rock zones delineated by Kowallis in 1997 can be used based on Fe and Al content. According to the summary of previous studies, it is known that when the Al/Fe ratio is 1–2, the representative titanite is basically located within the range of granite and diorite. When the Al/Fe ratio is 3–15, the representative titanite is located within the range of metamorphic rocks [
42]. The analysis of titanite in different alteration zones of the Pulang copper deposit show that the Al/Fe ratio of titanite in the potassium silicate alteration zone is about 1, which is in the range of magmatic titanite. In contrast, all the Al/Fe ratios of titanite in the propylitic alteration zone are greater than 3, which is in the range of hydrothermal titanite (
Figure 8). Even more to the point, the Al/Fe ratios of titanite in the phyllic alteration zone combine the characteristics of magmatic and hydrothermal titanite. Part of the magmatic titanite in the potassium silicate alteration zone was metasomatized by REE-rich fluid. Al and Fe usually have low mobility in fluids. In contrast, REEs can easily form complexes in fluids rich in Cl
−, F
−, or CO₃
2−, and their migration ability is significantly enhanced. Furthermore, the Al
3+ and Fe
3+ diffusion rate in crystals is much lower than REE
3+ At low temperature hydrothermal conditions, the rebalancing of Al/Fe may be limited by dynamics, whereas REEs are more easily diffused or quickly reset through the solution–reprecipitation process. As a result, Al and Fe may not have been significantly brought in or out of the fluid, resulting in an Al/Fe value in the phyllic alteration zone similar to that of magmatic titanite in potassium silicate alteration zone, but REEs remain similar to that of titanite in the phyllic alteration zone. It probably indicates that titanite in the phyllic alteration zone partially inherited the characteristics of titanite in the potassium silicate alteration zone during the process of magmatic evolution or metasomatism with surrounding rock. This corresponds to the existence of the zone structure of titanite and the hydrothermal metasomatism characteristics in the edge of the band.
The titanite TiO
2 content is higher and F content is lower in the potassium silicate alteration zone of the Pulang copper deposit, which is closer to the magmatic titanite features overall. In contrast, the titanite TiO
2 content is lower and the F content is higher overall in the phyllic and propylitic alteration zones, which is consistent with the hydrothermal titanite features [
43,
44,
45,
46].
Since magmatic and hydrothermal titanites have differences in trace elements, the titanite type can also be judged based on the titanite trace element content characteristics. Compared to magmatic titanite, hydrothermal titanite has lower contents of high-field-strength elements (HFSEs), Zr, Hf, Nb, Ta, Th, and Mo; a higher Nb/Ta ratio; and relatively low Lu/Hf and Th/U ratios (
Figure 6). The trace elements of titanites show that phyllic and propylitic alteration zone titanites have lower Nb, Ta and Sn contents; higher Nb/Ta ratios; and lower Lu/Hf and Th/U ratios than potassium silicate titanites, which are closer to the elemental characteristics of hydrothermal titanites. The titanite in the potassium silicate zone of the Pulang copper deposit has significantly higher REE content and obvious negative Eu anomaly (
Figure 7), which are closer to the magmatic titanite characteristics, while the titanite in the phyllic and propylitic alteration zone is closer to the hydrothermal titanite characteristics.
By comparing the major, trace, and rare earth element contents in different alteration zones, it is clear that the potassium silicate to phyllic to propylitic alteration zone titanite of the Pulang copper deposit is generally consistent with the evolutionary trend of magmatic to hydrothermal titanite. The potassium silicate alteration zone titanite is closest to the unaltered magmatic titanite characteristics and can be regarded as formed at the initial stage of hydrothermal events.
6.2. Element Substitution Mechanism in Titanite
The trace element content of titanite is mainly controlled by trace element content in the melt at the time of titanite crystallization and the partition coefficient of this element between titanite and melt. The study of the elemental substitution mechanism of titanite can provide a better understanding of the compositional characteristics of magmatic–hydrothermal systems.
6.2.1. Substitution of Ti
The ideal TiO
2 content in titanite is 40.13%. However, the TiO
2 of titanite in the three alteration zones is significantly less than this value, which implies that the TiO
2 in titanite was subjected to replacement reactions, where the major elements that likely replace Ti include Al, Fe, Sn, Zr, Nb, Ta, and Mo. The main reactions include the following:
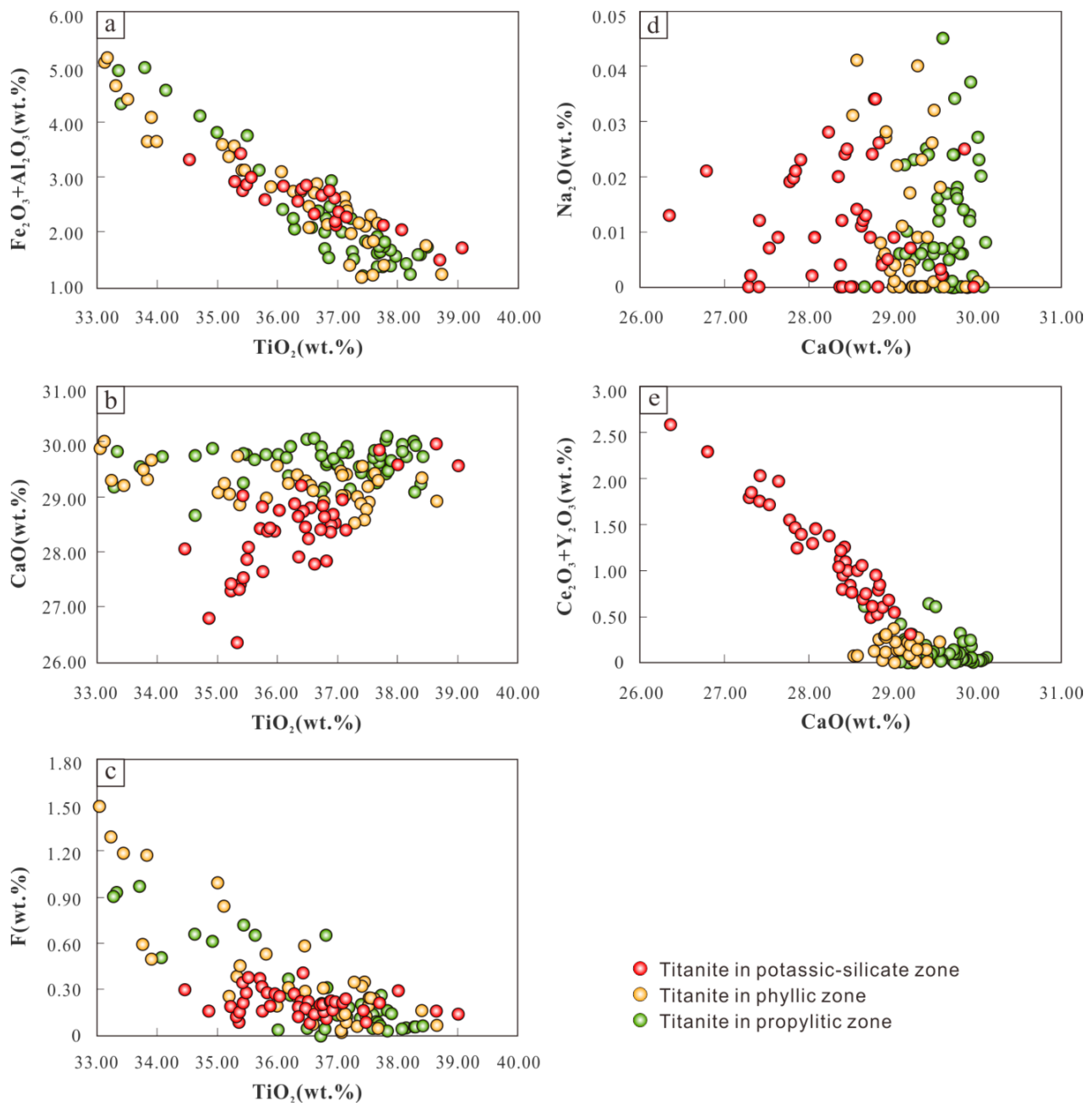
The negative correlation between TiO
2 and Al
2O
3 + Fe
2O
3 in titanite confirms the influence of the above elemental substitution (
Figure 9a).
Reaction (1) has less effect on the substitution of titanite Ti in the potassium silicate zone because the TiO
2 content of titanite does not have an obvious linear relationship with F content (
Figure 9c). Since the TiO
2 content of titanite in the potassium silicate zone has an obvious linear relationship with the CaO content (
Figure 9b), reaction (3) is main substitution mechanism. The linear relationship between TiO
2 and the F content of titanite is relatively obvious in the phyllic and propylitic alteration zones (
Figure 9c). However, there is no obvious correlation between TiO
2 content and CaO content (
Figure 9b), so reaction (1) is its main substitution mechanism. During the process of potassium silicate alteration to phyllic and propylitic alterations, the Nb, Ta, and Mo content in titanite decreases significantly with the increase in titanic TiO
2 content (
Figure 6), so the reaction (2) substitution mechanism in the magmatic–hydrothermal process also plays an important role.
6.2.2. Substitution of Ca
The ideal CaO content in titanite is 28.6%. However, the CaO of titanite in all three alteration zones is lower than this ideal ratio, which proves that titanite CaO underwent replacement reactions. The main elements replacing Ca include Ce, Y, Na, etc. The reactions include the following:
The Na
+ content in titanite in the three alteration zones in the Pulang copper deposit is very low and does not have an obvious linear relationship with the Ca
2+ content (
Figure 9d), so reactions (4) and (5) have a minimal effect on the substitution mechanism. The CaO and Ce
2O
3 + Y
2O
3 content in the titanite of the potassium silicate zone shows a significant negative correlation (
Figure 9e), so we speculate that reaction (6) mainly occurred in titanite in the potassium silicate alteration zone. The correlation between CaO and Ce
2O
3 + Y
2O
3 content is poor in titanite of the phyllic and propylitic zones (
Figure 9e), but Sr content keeps increasing as CaO content decreases (
Figure 6). Thus, the main substitution mechanism for CaO in these alteration zones may be reaction (7).
6.3. Magmatic–Hydrothermal Processes Revealed by Titanite
6.3.1. Oxygen Fugacity
Mineral Assemblages
Different types of titanite can correspond to different products of magmatic and hydrothermal stages, and their element characteristics can reflect the physical and chemical conditions at the time of their formation [
47]. Titanite is an important redox indicator, and it is generally recognized that the mineral assemblage of titanite, magnetite, and quartz in igneous rocks indicates the environment of high oxygen fugacity of silicate magma. The results of numerous studies show that the clinopyroxene–ilmenite assemblage reflects lower redox conditions of the magma compared to the titanite–magnetite–quartz assemblage [
48,
49,
50,
51,
52]. The titanite in the potassium silicate alteration zone of the Pulang copper deposit is usually paragenetically associated with magnetite, while the titanite in the phyllic and propylitic zones is usually paragenetic with ilmenite and rutile (
Figure 4), conjecturing a trend of lower oxygen fugacity during potassium silicate alteration → phyllic alteration → propylitic alteration.
Titanite Eu Anomaly
Different ions of the same element behave differently in the magma due to differences in their valence and radius. Magma oxygen fugacity is an important factor governing the valence and radius of element ions, which has a dramatic impact on the partitioning of elements between minerals and melts. In magmatic systems, the enrichment of Eu in minerals such as plagioclase makes the Eu depleted in the residual fluid, so that the late-stage crystallized magmatic titanite generally shows negative Eu anomalies. Since the radius of Eu2+ instead of Eu3+ is closer to the size of Ca2+, more Eu2+ is converted to Eu3+ in the oxidizing environment, converting Eu2+ into titanite so limited that it forms an obviously negative Eu anomaly.
The analysis results of titanite in different alteration zones of the Pulang copper deposit show that titanite in the potassium silicate alteration zone has an obvious negative Eu anomaly, while titanites in the phyllic and propylitic zones have an obvious positive Eu anomaly (
Figure 7). This indicates that the fluid has a high oxygen fugacity during the potassium silicate alteration stage, while the Eu value increases when entering the phyllic and propylitic alteration stages, indicating the decreasing oxygen fugacity from potassium silicate alteration to phyllic and propylitic alteration.
Titanite U/Th Ratio
Previous experiments have shown that there is a significant increase in the solubility of U when the oxygen fugacity and salinity of the fluid increase, while there is no significant change in Th. Therefore, the high U content and decreased Th/U ratio indicate that the fluid may have higher oxygen fugacity and salinity [
53]. Therefore, the U content and U/Th ratio of titanite can indicate the oxygen fugacity of the mineral formation environment.
The Th/U ratios of titanite in the potassium silicate alteration zone of the Pulang copper deposit are concentrated in the range of 2.30–9.44 (mean 6.17), the Th/U ratios of titanite in the phyllic alteration zone are concentrated in the range of 0.05–2.85 (mean 0.92), and the Th/U ratios of titanite in the propylitic zone are concentrated in the range of 0.39–4.32 (mean 2.10). Comparing the titanite compositions of the different alteration zones, it can be found that the titanite Th/U ratios are significantly higher in the potassium silicate alteration zone (
Figure 6), which proves that the formation environment of the potassium silicate alteration of the Pulang copper deposit has a higher oxygen fugacity. In the meantime, it also indicates that oxygen fugacity decreased in the process from potassium silicate alteration to phyllic and propylitic alterations.
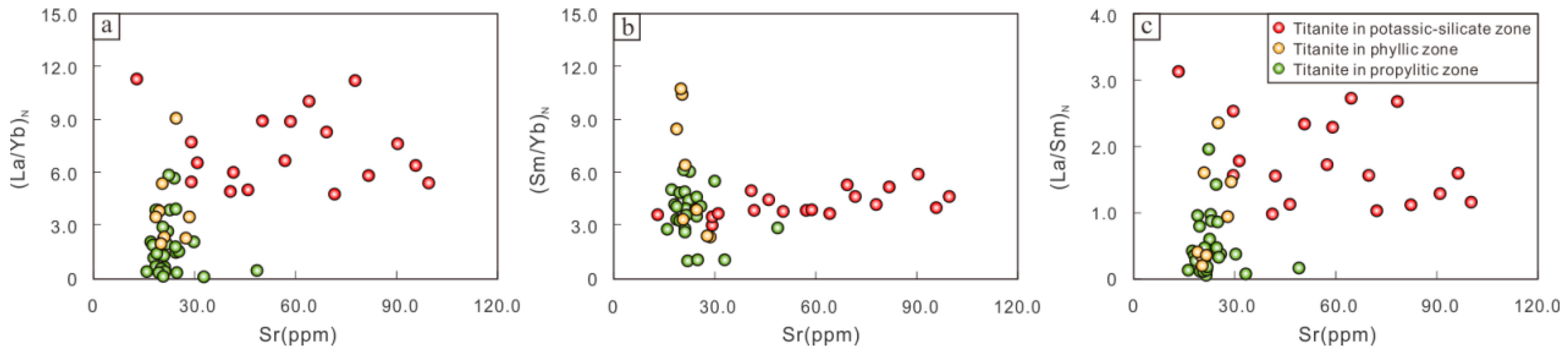
6.3.2. Indication of Magmatic Fractional Crystallization
The Sr content in titanite is mainly related to the Sr content in the melt since the Sr content in the melt decreases significantly as the feldspar crystallization continues; thus, titanite contains less Sr content. The titanite in the potassium silicate zone of the Pulang copper deposit exhibits a wide range of Sr content variation. Therefore, the Sr content in titanite can be used as an indicator to estimate the magmatic fractional crystallization. Rare earth element ratios and the Sr content diagram shows that the (La/Yb)
N, (Sm/Yb)
N, and (La/Sm)
N ratios are generally unchanged as the Sr content decreases (
Figure 10), illustrating that REE fractionation is not obvious. At this point, the titanite is more likely to be an early crystallization mineral phase of the magma, preferentially formed before other LREE-rich and MREE-rich minerals (such as orthite and apatite). The narrow variation range of the Sr content of titanite in the phyllic and propylitic zones indicates that the magmatic fractionation process had ended and was dominated by hydrothermal activity instead of magmatic fractional crystallization.
6.4. Comparison of the Metallogenic Potentials for Different Alteration Zones Related to Porphyry
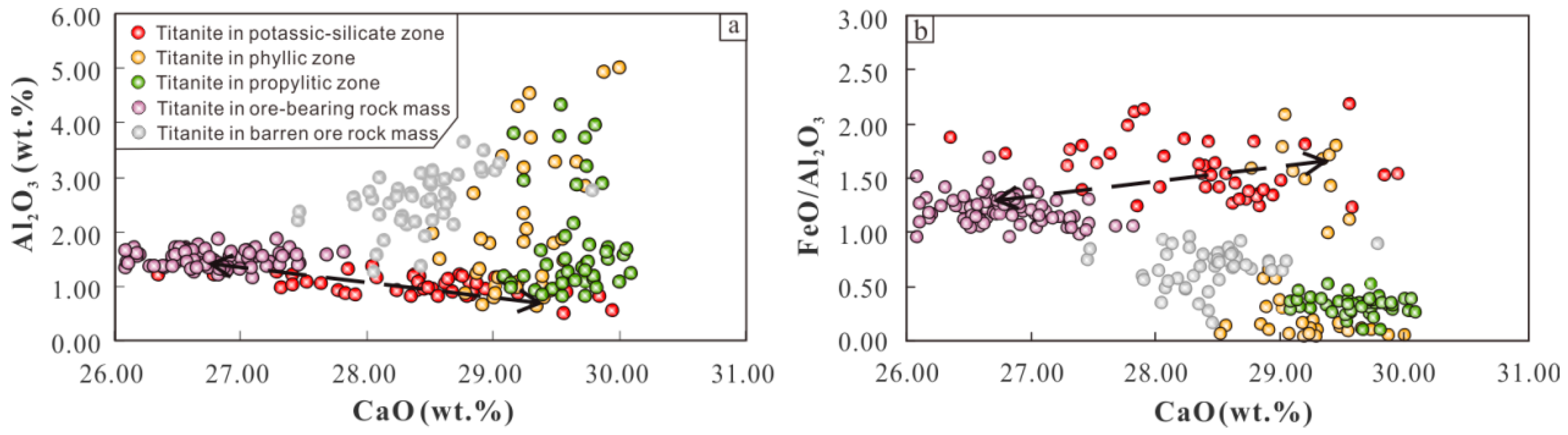
Magmatic ore-bearing potential determines the probability of the occurrence and scale of hydrothermal ore deposits associated with the magmatism. Titanite is usually enriched in Sn, Mo, and W and depleted in Cu. Therefore, the Cu content in titanite does not reflect the degree of Cu enrichment in the magma and cannot be used as an indicator for searching for copper ore deposits. However, the major elements of titanite in the potassium silicate zone of the Pulang copper deposit are characterized by low CaO and Al
2O
3 and high Fe
2O
3/Al
2O
3 (
Figure 11). The trace elements are characterized by high LREE/HREE and (Ce/Ce*)/(Eu/Eu*) ratios; high ∑REE + Y, U, Th, Ta, Nb, and Ga content; lower Sr content; and lower Zr/Hf, Nb/Ta, and Eu/Eu* ratios (
Figure 6). These are similar to the characteristics of titanite in porphyry-bearing rock bodies of the Red River–Jinshajiang alkali-rich porphyry belt [
45]. In contrast, the titanite composition of the phyllic and propylitic zones are closer to the characteristics of the ore-barren rock bodies. It is inferred that the copper mineralization of the Pulang copper deposit is more closely related to the potassium silicate alteration.
6.5. Possible Metallogenic Mechanism of Cu-Mo Separation at Pulang
Magmatic–hydrothermal evolution processes are critical in the enrichment of molybdenum. Molybdenum is an incompatible element in the crystallization of magmatic minerals, and it is generally assumed that molybdenum is enriched with an increasing degree of magmatic differentiation. However, petrological experimental studies have shown that molybdenum enrichment remains several orders of magnitude from industrial requirements after extensive magmatic fractional crystallization. Previous studies have shown that there is no linear relationship between the Mo and Sr contents in titanites (Sr content indicates the degree of magmatic differentiation) in granite bodies with different metallogenic potential, suggesting that the residual melt is not enriched in molybdenum during mineral crystallization. In the meanwhile, F-rich titanites developed in molybdenum-rich granites. The low F content in titanites from molybdenum-poor granites suggest that molybdenum enrichment may be related to the F content of the magma and that high F promotes melt/fluid separation.
The Cu content of titanite in the different alteration zones in the Pulang rock bodies associated with copper mineralization is low and does not vary significantly, while the Mo content is highly variable. Mo is mainly substituted for Ti to form titanite using the following reaction:
Mo is usually +6 valent and becomes +5 valent under the reducing conditions. The oxygen fugacity of titanite in the potassium silicate zone of the Pulang copper deposit is higher than that of titanite in the phyllic and propylitic zones, so the Mo content in titanite from the phyllic and propylitic zones will normally gradually increase as the oxygen fugacity decreases. However, the Mo content in titanite from the potassium silicate zone of the Pulang copper deposit is much higher than that in the titanite from the phyllic and propylitic zones (about 10
2–10
4 times). In contrast, the Cu content exhibits an almost constant (
Figure 12), which may suggest that molybdenum was partitioned into the fluid phase or precipitated as sulfide before medium- and low-temperature hydrothermal alteration during this process. The increased F content may have further caused the separation of copper and molybdenum and mineralization.
7. Conclusions
The composition of titanite within the potassium silicate alteration zone, phyllic alteration zone, and propylitic alteration zone of the Pulang copper deposit shows that titanite in the potassium silicate alteration zone has higher TiO2, REE, and HFSE content; higher Lu/Hf and Th/U ratios; obvious negative Eu anomalies with low Nb/Ta ratios; and greater enrichment in LREEs. This is more aligned with magmatic titanite characteristics, combined with the euhedral occurrence and mineral assemblages and the titanite characteristics from previous studies of the Pulang copper deposit. This can represent the product of the early stage of magmatic crystallization with little influence from hydrothermal alteration overall. At the late stage of phyllic and propylitic alteration, the paragenetic mineral assemblage with titanite gradually changes from titanite + magnetite to titanite + ilmenite. The Th/U ratio decreases, and the Eu content increases, suggesting the decrease in oxygen fugacity during the process of potassium silicate alteration to phyllic and propylitic alteration (in other words, during the process of magma-fluid evolution).
The formation of the porphyry copper deposit is tremendously influenced by oxygen fugacity, and high oxygen fugacity is favorable to porphyry formation. The oxygen fugacity tends to decrease during the transformation of potassium silicate alteration to phyllic and propylitic alterations. The titanite in the potassium silicate alteration zone has low CaO, Al, and Sr content; low Zr/Hf and Nb/Ta ratios; high ∑REE + Y, U, Th, Ta, Nb, and Ga content; and high Fe2O3/Al2O3, LREE/HREE ratios. These are the same as the characteristics of the ore-bearing porphyry rock bodies from previous studies, so it is assumed that the Pulang copper ore is more closely related to potassium silicate alteration. Comparing the potassium silicate alteration with the Cu elements in titanite of the propylitic zone, the Cu content is nearly unchanged during this process, and the Mo content is reduced, demonstrating that molybdenum was unloaded into the fluid phase or precipitated as sulfides before the medium- and low-temperature hydrothermal alteration during this process, which may have further led to the separation of copper and molybdenum and mineralization.
Author Contributions
Conceptualization, X.G. and M.L.; Methodology, M.L., X.G., and G.G.; Validation, M.L., G.G., and S.G.; Formal analysis, M.L. and X.G.; Investigation, S.G. and G.G.; Resources, M.L. and X.G.; Original draft writing, M.L.; Review and editing, M.L. and X.G.; Project administration, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 41902069), the Fundamental Research Funds for the Central Universities (2-9-2022-65 and 2652023001), the National Nonprofit Fundamental Research Grant of China, Institute of Geology, China Earthquake Administration (NORSCBS22–05), and the 111 Project of the Ministry of Science and Technology, China (Grant No. BP0719021).
Data Availability Statement
Data are contained within the article.
Acknowledgments
I would like to thank Xin-Shang Bao for her generous help in data processing. Some of the testing samples were provided by Zhen Yang and Xu-Dong Liu, and the completion of the paper benefited from their hard work. Last but not least, I would like to express my sincere thanks to the reviewers for their precious comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sillitoet, R.H. Porphyry copper system. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Redmond, P.B.; Einaudi, M.T. The Bingham Canyon Porphyry Cu-Mo-Au deposit. I. Sequence of intrusions, vein formation, and sulfide deposition. Econ. Geol. 2010, 105, 43–68. [Google Scholar] [CrossRef]
- Li, W.C. The Tectonic Evolution of the Yidun Island Are and the Metallogenic Model of the Pulang Porphyry Copper Deposit, Yunnan, SW China. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2007. (In Chinese with English abstract). [Google Scholar]
- Deng, J.; Wang, Q.F.; Li, G.J.; Santosh, M. Cenozoic tectono-magmatic and metallogenic processes in the Sanjiang region, southwestern China. Earth-Sci. Rev. 2014, 138, 268–299. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Q.F.; Li, G.J. Tectonic evolution, superimposed orogeny, and composite metallogenic system in China. Gondwana Res. 2017, 50, 216–266. [Google Scholar] [CrossRef]
- Lowell, J.D.; Gumbert, J.M. Lateral and vertical alteration-mineralization zoning in porphyry ore deposits. Econ. Geol. 1970, 65, 373–408. [Google Scholar] [CrossRef]
- Cooke, D.R.; Hollings, P.; Walshe, J.L. Giant porphyry deposits: Characteristics, distribution, and tectonic controls. Econ. Geol. 2005, 100, 801–818. [Google Scholar] [CrossRef]
- Li, W.C.; Zeng, P.S.; Hou, Z.Q.; White, N.C. The Pulang Porphyry Copper Deposit and Associated Felsic Intrusions in Yunnan Province, Southwest China. Econ. Geol. 2011, 106, 79–92. [Google Scholar]
- Leng, C.B.; Cooke, D.R.; Hou, Z.Q.; Evans, N.J.; Zhang, X.C.; Chen, W.T.; Danisik, M.; McInnes, B.I.; Yang, J.H. Quantifying exhumation at the giant Pulang porphyry Cu-Au deposit using U-Pb-He dating. Econ. Geol. 2018, 113, 1077–1092. [Google Scholar] [CrossRef]
- Yang, Z. Late Triassic Mineralization of the Porphyry Copper Deposits in Yidun Arc, Southwest China. Ph.D. Thesis, University of Geosciences, Beijing, China, 2016. (In Chinese with English abstract). [Google Scholar]
- Cao, K.; Yang, Z.M.; Mavrogenes, J.; White, N.C.; Xu, J.F.; Li, Y.; Li, W.K. Geology and Genesis of the Giant Pulang Porphyry Cu-Au District, Yunnan, Southwest China. Econ. Geol. 2019, 114, 275–301. [Google Scholar] [CrossRef]
- Zhang, S.; He, W.; Gao, X.; Zhang, H.; Yuan, J. Ore-forming fluids evolution of the porphyry Cu deposits: Alteration mineralogy and thermodynamic modeling of the Pulang Cu deposit, Zhongdian district. Acta Petrol. Sin. 2020, 36, 1611–1626, (In Chinese with English abstract). [Google Scholar]
- Sun, J.F.; Yang, J.H.; Wu, F.Y.; Xie, L.W.; Yang, Y.H.; Liu, Z.C.; Li, X.H. In situ LA-ICP-MS U-Pb dating of titanite. Sci. Bull. 2012, 57, 1603–1615, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Frost, B.R.; Chamberlain, K.R.; Schumacher, J.C. Sphene (titanite): Phase relations and role as a geochronometer. Chem. Geol. 2001, 172, 131–148. [Google Scholar] [CrossRef]
- Corfu, F.; Grunsky, E.C. Igneous and Tectonic Evolution of the Batchawana Greenstone Belt, Superior Province: A U-Pb Zircon and Titanite Study. J. Geol. 1987, 95, 87–105. [Google Scholar] [CrossRef]
- Esssex, R.M.; Gromet, L.P. U-Pb dating of prograde and retrograde titanite growth during the Scandian orogeny. Geology 2000, 28, 419–422. [Google Scholar] [CrossRef]
- Cao, M.J.; Qin, K.Z.; Li, G.M.; Evans, N.J.; Jin, L. In situ LA-(MC)-ICP-MS trace element and Nd isotopic compositions and genesis of polygenetic titanite from the Baogutu reduced porphyry Cu deposit, Western Junggar, NW China. Ore Geol. Rev. 2015, 65, 940–954. [Google Scholar] [CrossRef]
- Cao, M.J. Petrogenesis and Metallogenesis of Baogutu Reduced Porphyry Copper Deposit, West Junggar, and Its Comparision with Porphyry Deposits from Balkhash. Ph.D. Thesis, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, China, 2013. (In Chinese with English abstract). [Google Scholar]
- Tiepolo, M.; Oberti, R.; Vannucci, R. Trace-element incorporation in titanite: Constraints from experimentally determined solid/liquid partition coefficients. Chem. Geol. 2002, 191, 105–119. [Google Scholar] [CrossRef]
- Deng, X.; Li, J.; Zhou, M.; Zhao, X.F.; Yan, D.R. In-situ LA-ICPMS trace elements and U-Pb analysis of titanite from the Mesozoic Ruanjiawan W-Cu-Mo skarn deposit, Daye district, China. Ore Geol. Rev. 2015, 65, 990–1004. [Google Scholar] [CrossRef]
- Jiang, J.W.; Yu, H.J.; Li, W.C. Alteration and mineralization of the giant Pulang porphyry Cu-Au deposit, southwest China: Evidence from biotite mineralogy. Ore Geol. Rev. 2024, 173, 106222. [Google Scholar] [CrossRef]
- Yang, L.Q.; Gao, X.; Shu, Q.H. Multiple Mesozoic porphyry-skarn Cu(Mo-W) systems in Yidun Terrane, east Tethys: Constraints from zircon U-Pb and molybdenite Re-Os geochronology. Ore Geol. Rev. 2017, 90, 813–826. [Google Scholar] [CrossRef]
- Deng, J.; Yang, L.Q.; Wang, C.M. Research advances of superimposed orogenesis and metallogenesis in the Sanjiang Tethys. Acta Petrol. Sin. 2011, 27, 2501–2509, (In Chinese with English abstract). [Google Scholar]
- Deng, J.; Wang, C.M.; Li, G.J. Style and process of the superimposed mineralization in the Sanjiang Tethys. Acta Petrol. Sin. 2012, 28, 1349–1361, (In Chinese with English abstract). [Google Scholar]
- Deng, J.; Ge, L.S.; Yang, L.Q. Tectonic dynamic system and compound orogeny: Additionally discussing the temporal-spatial evolution of Sanjiang orogeny, Southwest China. Acta Petrol. Sin. 2013, 29, 1099–1114, (In Chinese with English abstract). [Google Scholar]
- Deng, J.; Wang, Q.F.; Li, G.J. Superimposed orogeny and composite metallogenic system: Case study from the Sanjiang Tethyan belt, SW China. Acta Petrol. Sin. 2016, 32, 2225–2247, (In Chinese with English abstract). [Google Scholar]
- Yang, L.Q.; Liu, J.T.; Zhang, C. Superimposed orogenesis and metallogenesis:An example from the orogenic gold deposits in Ailaoshan gold belt, Southwest China. Acta Petrol. Sin. 2010, 26, 1723–1739, (In Chinese with English abstract). [Google Scholar]
- Deng, J.; Wang, Q.F.; Li, G.J.; Hou, Z.Q.; Jiang, C.Z.; Danyushevsky, L. Geology and genesis of the giant Beiya porphyry-skarn gold deposit, northwestern Yangtze Block, China. Ore Geol. Rev. 2016, 70, 457–485. [Google Scholar] [CrossRef]
- Chen, L. The Characteristics of Ore-Forming Magma and Tectonicsetting of the Pulang Gaint Porphyry Copper Deposit in theYunnan Province. Ph.D. Thesis, Graduate University of the Chinese Academy of Sciences (Guangzhou Institute of Geochemistry), Guangzhou, China, 2016. (In Chinese with English abstract). [Google Scholar]
- Qiu, K.F.; Yang, L.Q. Genetic feature of monazite and its U-Th-Pb dating:Critical considerations on the tectonic evolution of Sanjiang Tethys. Acta Petrol. Sin. 2011, 27, 2721–2732, (In Chinese with English abstract). [Google Scholar]
- Zeng, P.S.; Hou, Z.Q.; Li, L.H.; Qu, W.J.; Wang, H.P.; Li, W.C.; Meng, Y.F.; Yang, Z.S. Age of the Pulang porphyry copper deposit inNW Yunnan and its geological significance. Geol. Bull. China 2004, 23, 1127–1131, (In Chinese with English abstract). [Google Scholar]
- Cao, D.H.; Wang, A.J.; Li, W.C.; Wang, G.W.; Li, R.P.; Wang, Y.K. Magma Mixing in the Pulang Porphyry Copper Deposit: Evidence from Petrology and Element Geochemistry. Acta Geol. Sin. 2009, 83, 166–175, (In Chinese with English abstract). [Google Scholar]
- Li, W.; Yang, Z.; Cao, K.; Lu, Y.; Sun, M. Redox-controlled generation of the giant porphyry Cu–Au deposit at Pulang, southwest China. Contrib. Mineral. Petrol. 2019, 174, 1–34. [Google Scholar] [CrossRef]
- Guo, X.; Du, Y.S.; Pang, Z.S.; Li, S.-T.; Li, Q. Characteristics of the Ore-forming Fluids in Alteration Zones of the Pulang Porphyry Cupper Deposit in Yunnan Province and Its Metallogenic Significance. Geoscience 2009, 23, 465–471, (In Chinese with English abstract). [Google Scholar]
- Li, W.C.; Yin, G.H.; Yu, H.J.; Xue, S.R.; Wang, K.Y.; Wang, C.Y.; Wang, W.X. Characteristics of the Ore-Forming Fluid and Genesis of the Pulang Copper Deposit in Yunnan Province. J. Jilin Univ. (Earth Sci. Ed.) 2013, 43, 1436–1447, (In Chinese with English abstract). [Google Scholar]
- Harlov, D.; Tropper, P.; Seifert, W.; Nijland, T.; Förster, H.J. Formation of Al-rich titanite (CaTiSiO4O- CaAlSiO4OH) reaction rims on ilmenite in metamorphic rocks as a function of fH2O and fO2. Lithos 2006, 88, 72–84. [Google Scholar]
- Aleksandrov, S.M.; Troneva, M.A. Composition, mineral assemblages, and genesis of titanite and malayaite in skarns. Geochem. Int. 2007, 45, 1012–1024. [Google Scholar]
- Zhao, L.H.; Zeng, L.S.; Hu, M.Y.; Sun, D.Y. Rutile to titanite transformation in amphibolite and its geochemical consequences: A case study of the amphibolite from Yarlung Tsangpo suture zone. Acta Petrol. Sin. 2017, 33, 2494–2508, (In Chinese with English abstract). [Google Scholar]
- Kowallis, B.J.; Christiansen, E.H.; Griffen, D.T. Compositional variations in titanite. Geol. Soc. Am. 1997, 29, 402. [Google Scholar]
- Matthew, J.K. Titanite Petrochronology. Rev. Mineral. Geochem. 2017, 83, 419–441. [Google Scholar]
- McDonough, W.F.; Sun, S. The composition of the earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar]
- Aleinikoff, J.N.; Wintsch, R.P.; Fanning, C.M.; Dorais, M.J. U-Pb geochronology of zircon and polygenetic titanite from the Glastonbury Complex, Connecticut, USA: An integrated SEM, EMPA, TIMS, and SHRIMP study. Chem. Geol. 2002, 188, 125–147. [Google Scholar]
- Jiang, P.; Fan, H.R.; Yang, K.F.; Liu, X.; Cai, Y.C.; Yang, Y.H. Titanite-scale insights into multi-stage magma mixing in Early Cretaceous of NW Jiaodong terrane, North China Craton. Lithos 2016, 258–259, 197–214. [Google Scholar] [CrossRef]
- Pan, L.C.; Hu, R.Z.; Bi, X.W.; Li, C.; Wang, X.S.; Zhu, J.J. Titanite major and trace element compositions as petrogenetic and metallogenic indicators of Mo ore deposits: Examples from four granite plutons in the southern Yidun arc, SW China. Am. Mineral. 2018, 103, 1417–1434. [Google Scholar]
- Xu, L.L.; Bi, X.W.; Hu, R.Z.; Tang, Y.; Wang, X.; Xu, Y. LA-ICP-MS mineral chemistry of titanite and the geological implications for exploration of porphyry Cu deposits in the Jinshajiang-Red River alkaline igneous belt, SW China. Mineral. Petrol. 2015, 109, 181–200. [Google Scholar] [CrossRef]
- Che, X.D.; Linnen, R.L.; Wang, R.C.; Groat, L.A.; Brand, A.A. Distribution of trace and rare earth elements in titanite from tungsten and molybdenum deposits in Yukon and British Columbia, Canada. Can. Mineral. 2013, 51, 415–438. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Zhou, H.; Lin, H.; Yang, T. In-situ LA-ICP-MS U-Pb geochronology and trace elements analysis of polygenetic titanite from the giant Beiya gold-polymetallic deposit in Yunnan Province, Southwest China. Ore Geol. Rev. 2016, 77, 43–56. [Google Scholar] [CrossRef]
- Wones, D.R. Significance of the assemblage titanite+magnetite+quartz in granitic rocks. Am. Mineral. 1989, 74, 744–749. [Google Scholar]
- Xirouchakis, D.; Lindsley, D.H.; Frost, B.R. Assemblages with titanite (CaTiOSiO4), Ca-Mg-Fe olivine and pyroxenes, Fe-Mg-Ti oxides, and quartz. Am. Mineral. 2001, 86, 254–264. [Google Scholar] [CrossRef]
- Broska, I.; Harlov, D.; Tropper, P.; Siman, P. Formation of magmatic titanite and titanite-ilmenite phase relations during granite alteration in the Tribeč Mountains, Western Carpathians, Slovakia. Lithos 2007, 95, 58–71. [Google Scholar] [CrossRef]
- Ismail, R.; Ciobanu, C.L.; Cook, N.J.; Teale, G.S.; Giles, D.; Mumm, A.S.; Wade, B. Rare earths and other trace elements in minerals from skarn assemblages, Hillside iron oxide-copper-gold deposit, Yorke Peninsula, South Australia. Lithos 2014, 184–187, 456–477. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.C.; Chen, J.; Zhu, J.C. Mineralogical evidence for magmatic and hydrothermal processes in the Qitianling oxidized tin-bearing granite (Hunan, South China): EMP and (MC)-LA-ICPMS investigations of three types of titanite. Chem. Geol. 2010, 276, 53–68. [Google Scholar] [CrossRef]
- Bali, E.; Audétat, A.; Keppler, H. The mobility of U and Th in subduction zone fluids: An indicator of oxygen fugacity and fluid salinity. Contrib. Mineral. Petrol. 2011, 161, 597–613. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).