Structural Regulation of Advanced Platinum-Based Core-Shell Catalysts for Fuel Cell Electrocatalysis
Abstract
1. Introduction
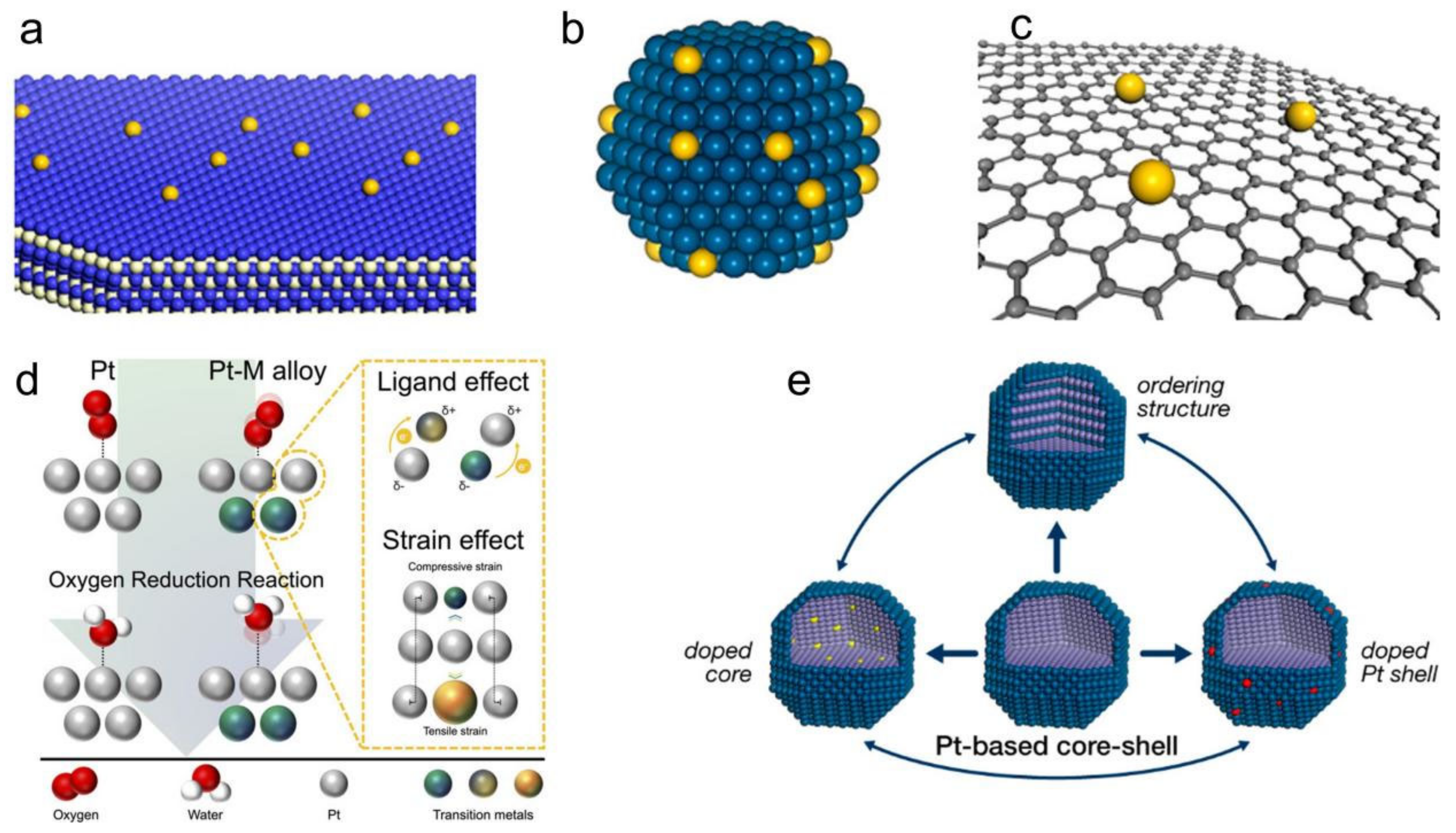
2. Characteristics of Pt-Based Core-Shell Structures
3. Regulation of Core Structure and Its Effect on Catalytic Performance
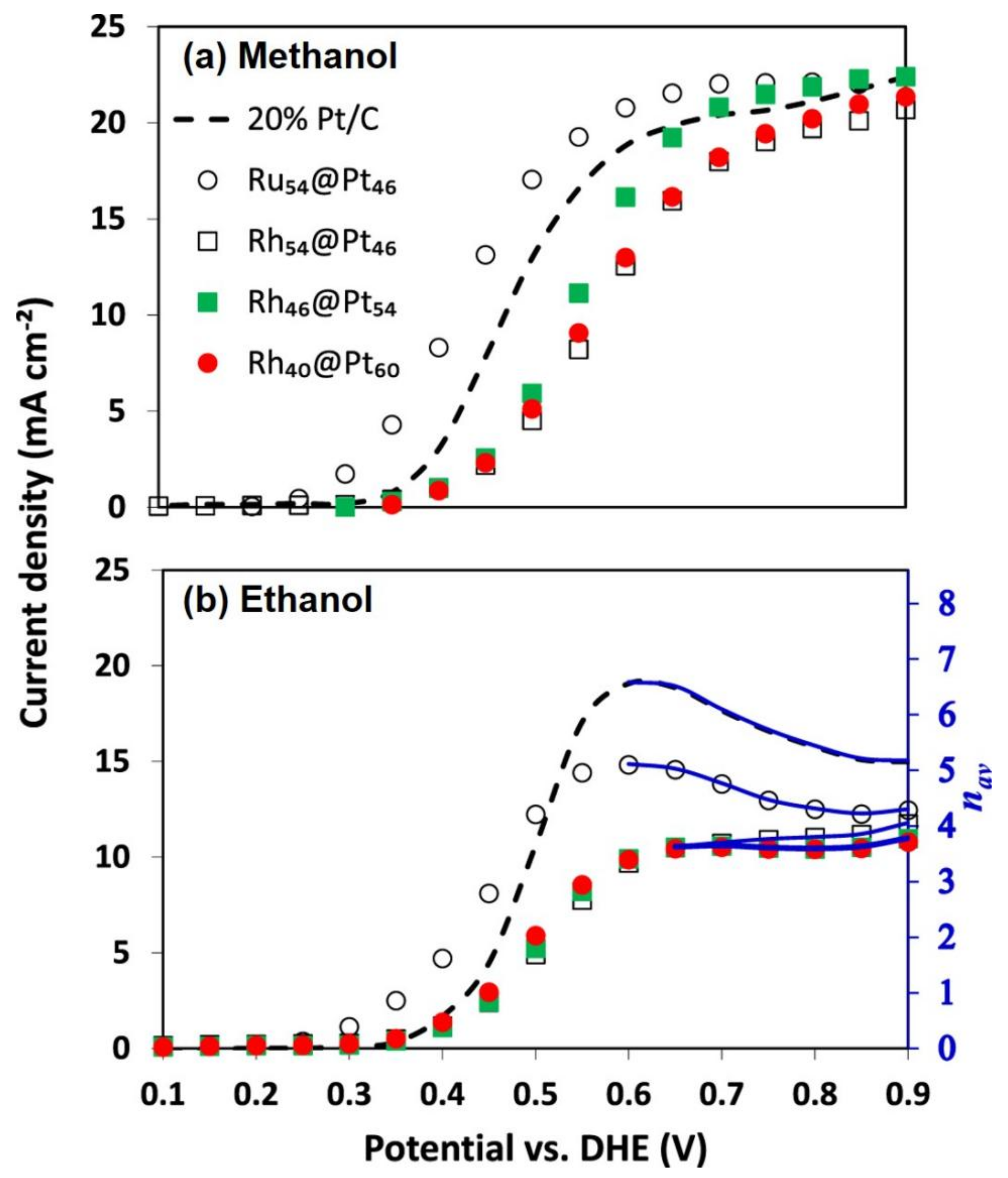
3.1. Precious Metal Core
3.2. Non-Precious Metal Core
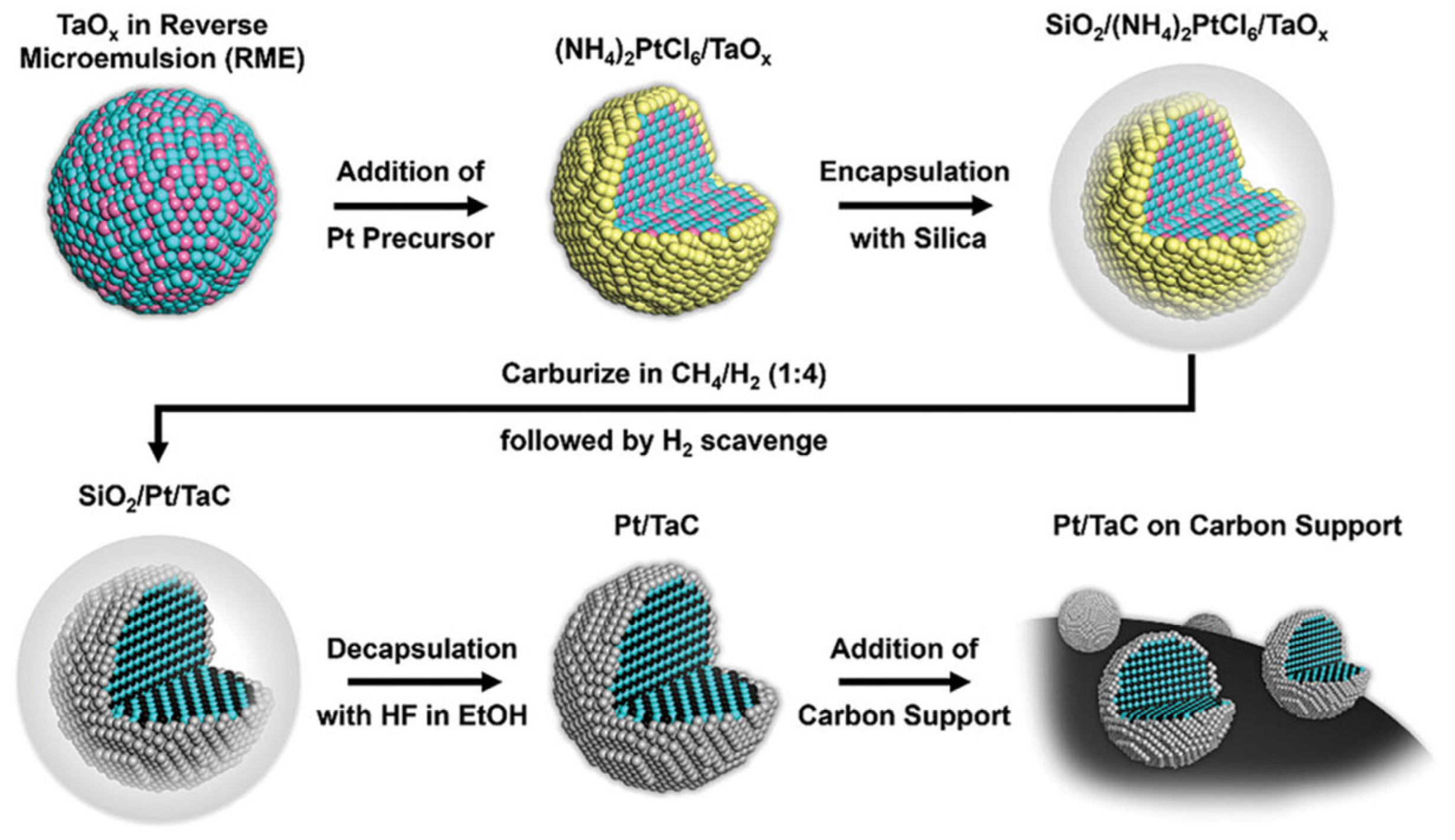
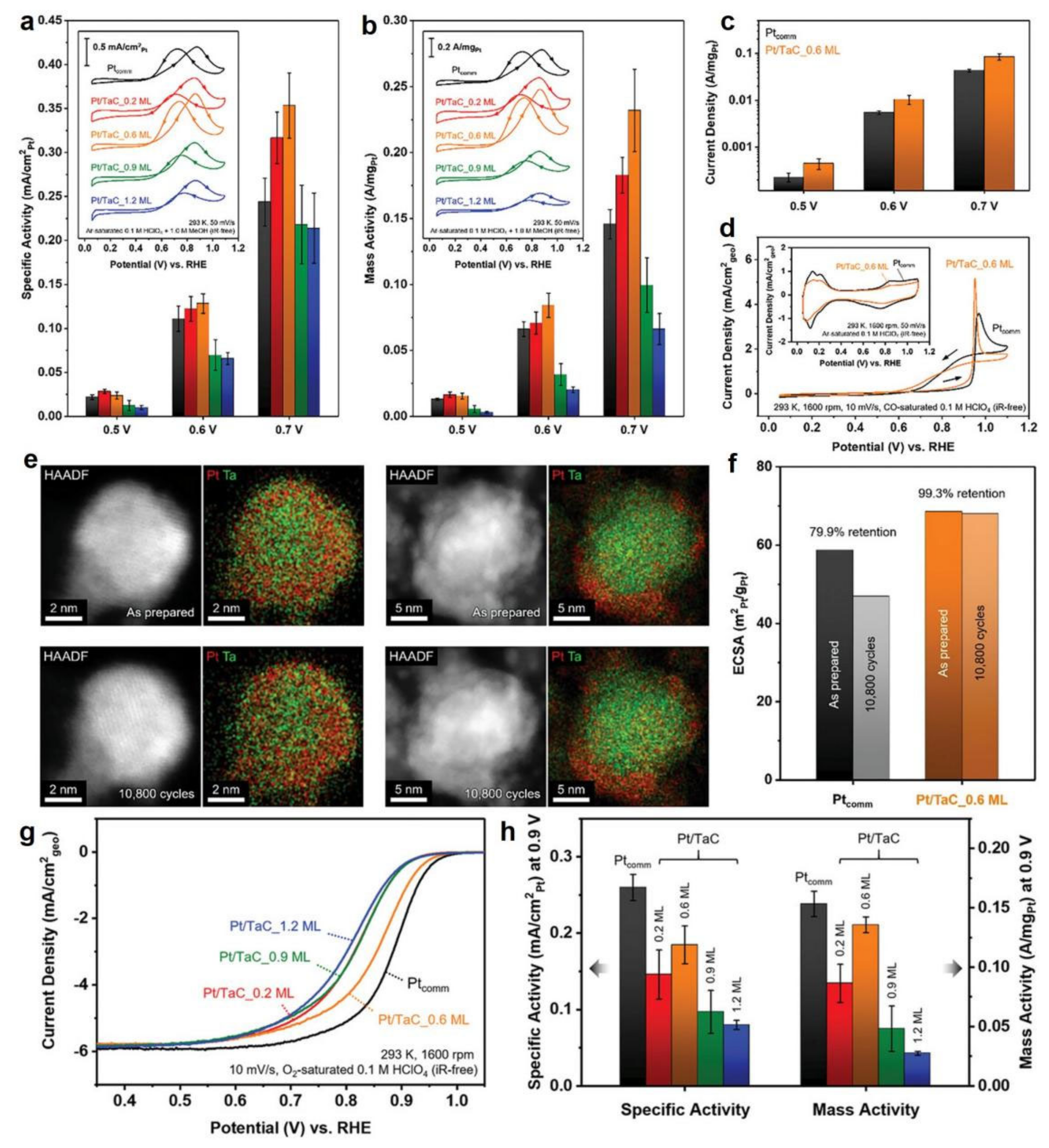
3.3. Transition Metal Carbide/Nitride Core
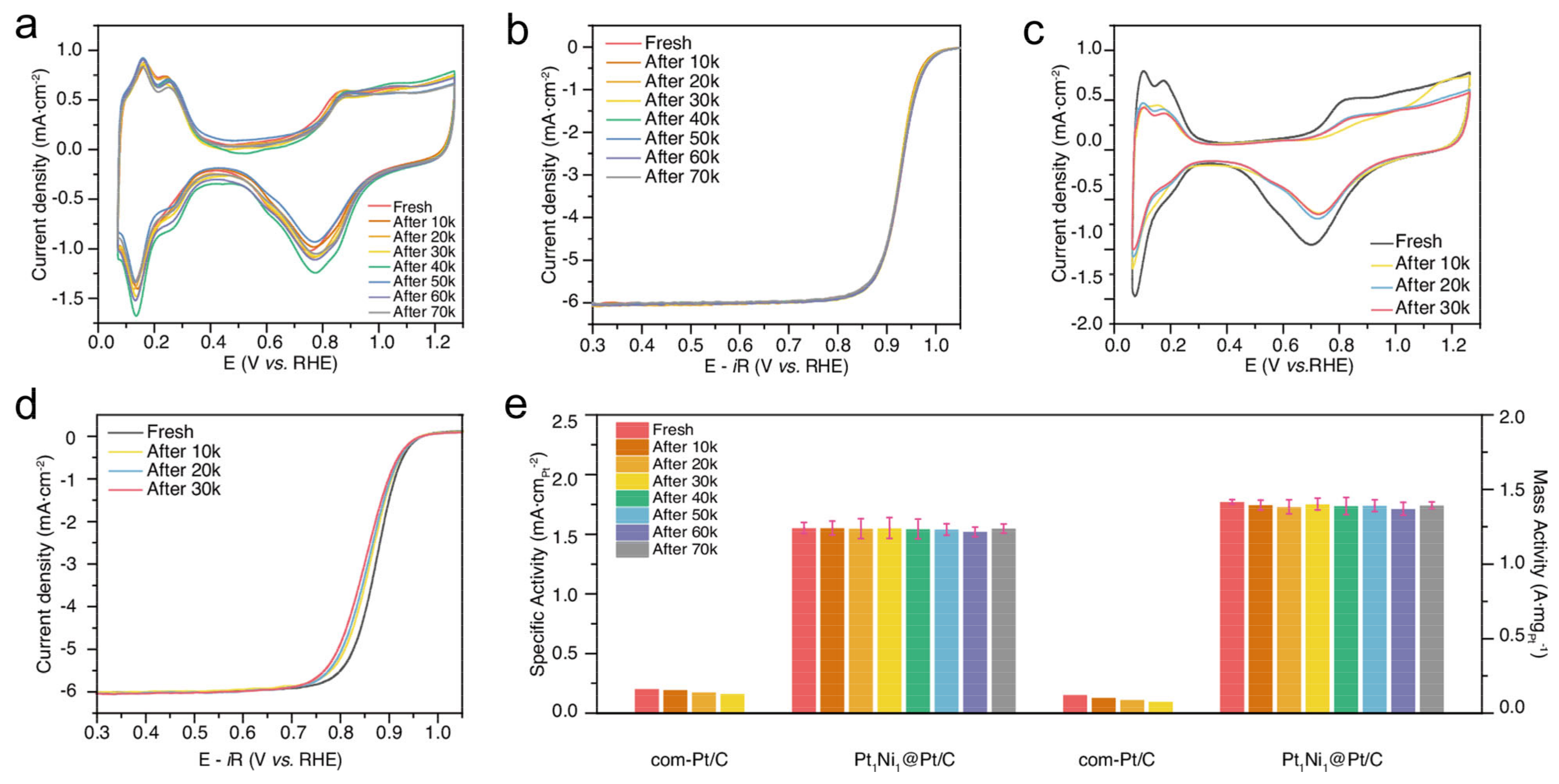
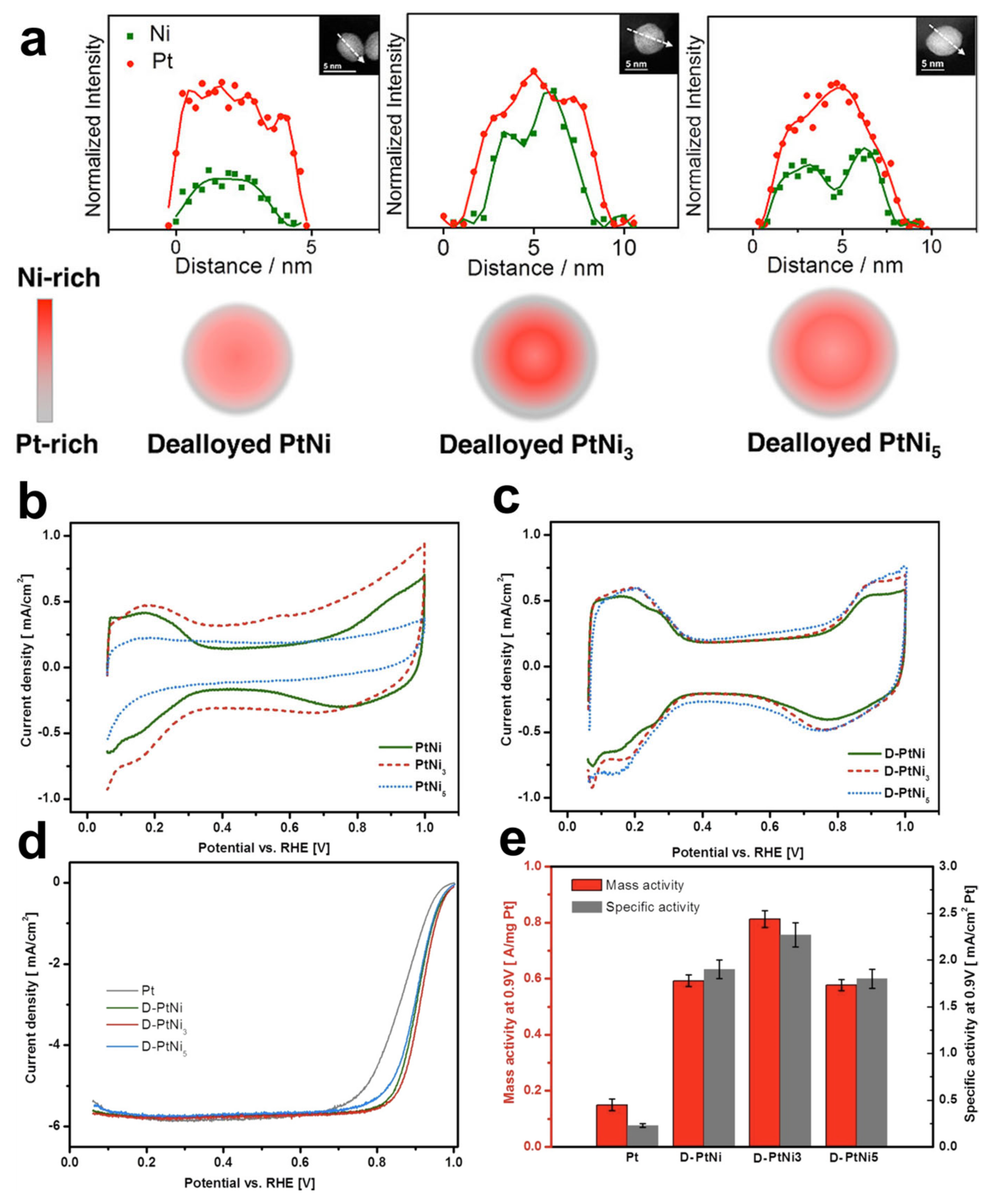
3.4. Alloy Core
3.5. Heteroelement-Doping Core
4. Regulation of Shell Structure and Its Effect on Catalytic Performance
4.1. Pt Shell Thickness
4.2. Pt Shell Alloying
4.3. Pt Shell Doping
4.4. Multi-Shell Layer

5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Gavin, M.M.; Simon, M.J.; Timothy, T.W. Global platinum group element resources, reserves and mining—A critical assessment. Sci. Total Environ. 2018, 622–623, 614–625. [Google Scholar]
- Corin, K.C.; McFadzean, B.J.; Shackleton, N.J.; O’Connor, C.T. Challenges Related to the Processing of Fines in the Recovery of Platinum Group Minerals (PGMs). Minerals 2021, 11, 533. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Zaccarini, F. Editorial for the Special Issue “Platinum-Group Minerals: New Results and Advances in PGE Mineralogy in Various Ni-Cu-Cr-PGE Ore Systems”. Minerals 2019, 9, 365. [Google Scholar] [CrossRef]
- Luo, M.C.; Zhao, Z.L.; Zhang, Y.L.; Sun, Y.J.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.Y.; Qin, Y.N.; et al. PdMo bimetallene for oxygen reduction catalysis. Nature 2019, 574, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Duanmu, K.N.; Wan, C.Z.; Cheng, T.; Zhang, L.; Dai, S.; Chen, W.X.; Zhao, Z.P.; Li, P.; Fei, H.L.; et al. Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis. Nat. Catal. 2019, 2, 495–503. [Google Scholar] [CrossRef]
- Zhang, L.; Roling, L.T.; Wang, X.; Vara, M.; Chi, M.F.; Liu, J.Y.; Choi, S.I.; Park, J.; Herron, J.A.; Xie, Z.X.; et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 2015, 349, 412–416. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Technol. 2021, 3, 100112. [Google Scholar] [CrossRef]
- Sun, S.Q.; Jin, C.X.; He, W.Z.; Li, G.M.; Zhu, H.C.; Huang, J.W. A review on management of waste three-way catalysts and strategies for recovery of platinum group metals from them. J. Environ. Manag. 2022, 305, 114383. [Google Scholar] [CrossRef]
- Tang, H.M.; Peng, Z.W.; Li, Z.Z.; Ma, Y.T.; Zhang, J.; Ye, L.; Wang, L.C.; Rao, M.J.; Li, G.H.; Jiang, T. Recovery of platinum-group metals from spent catalysts by microwave smelting. J. Clean. Prod. 2021, 318, 128266. [Google Scholar] [CrossRef]
- Chen, G.Z.; Chen, W.; Lu, R.H.; Ma, C.; Zhang, Z.D.; Huang, Z.Y.; Weng, J.N.; Wang, Z.Y.; Han, Y.H.; Huang, W. Near-Atomic-Scale Superfine Alloy Clusters for Ultrastable Acidic Hydrogen Electrocatalysis. J. Am. Chem. Soc. 2023, 145, 22069–22078. [Google Scholar] [CrossRef]
- Cai, J.L.; Chen, Y.Z.; Zhang, R.W.; Yuan, C.; Jin, Z.Y.; Chen, Y.T.; Zhang, S.M.; Zhang, J.J. Interfacial Pt-N Coordination for Promoting Oxygen Reduction Reaction. Chin. Chem. Lett. 2025, 36, 110255. [Google Scholar] [CrossRef]
- Cai, J.L.; Chen, J.X.; Chen, Y.Z.; Zhang, J.J.; Zhang, S.M. Engineering Carbon Semi-Tubes Supported Platinum Catalyst for Efficient Oxygen Reduction Electrocatalysis. iScience 2023, 26, 106730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Chen, J.; Sun, W.P.; Pan, H.G. Non-Platinum Group Metal Electrocatalysts toward Efficient Hydrogen Oxidation Reaction. Adv. Funct. Mater. 2021, 31, 2010633. [Google Scholar] [CrossRef]
- Corona, B.; Howard, M.; Zhang, L.; Henkelman, G. Computational screening of core@shell nanoparticles for the hydrogen evolution and oxygen reduction reactions. J. Chem. Phys. 2016, 145, 244708. [Google Scholar] [CrossRef]
- Rodríguez-Carrera, S.; Rodríguez-Kessler, P.L.; Ambriz-Vargas, F.; Garza-Hernández, R.; Reséndiz-Ramírez, R.; Martínez-Flores, J.S.; Benitez-Lara, A.; Martínez-Gamez, M.A.; Muñoz-Castro, A. First principles study for Ag-based core-shell nanoclusters with 3d-5d transition metal cores for the oxygen reduction reaction. Inorganica Chim. Acta 2024, 572, 122301. [Google Scholar] [CrossRef]
- Zhao, Z.H.; D’Souza, J.; Chen, F.Y.; Xia, Z.H. Rational design of efficient transition metal core–shell electrocatalysts for oxygen reduction and evolution reactions. RSC Adv. 2019, 9, 536–542. [Google Scholar] [CrossRef]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, R.W.; Sun, L.Y.; Zhang, S.M.; Zhang, J.J. Boron-alloyed porous network platinum nanospheres for efficient oxygen reduction in proton exchange membrane fuel cells. Chem. Eng. J. 2024, 485, 149998. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Dodelet, J.P.; Wu, G.; Zelenay, P. PGM-Free Cathode Catalysts for PEM Fuel Cells: A Mini-Review on Stability Challenges. Adv. Mater. 2019, 31, 1807615. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, S.M.; Zhang, J.J. Oxygen Reduction Electrocatalysis: From Conventional to Single-Atomic Platinum-based Catalysts for Proton Exchange Membrane Fuel Cells. Front. Energy 2024, 18, 206–222. [Google Scholar] [CrossRef]
- Wang, Z.C.; Chen, S.H.; Wu, W.; Chen, R.Z.; Zhu, Y.; Jiang, H.R.; Yu, L.Y.; Cheng, N.C. Tailored Lattice Compressive Strain of Pt-Skins by the L12-Pt3M Intermetallic Core for Highly Efficient Oxygen Reduction. Adv. Mater. 2023, 35, 2301310. [Google Scholar] [CrossRef]
- Wang, Z.C.; Wu, W.; Jiang, H.R.; Chen, S.H.; Chen, R.Z.; Zhu, Y.; Xiao, Y.; Lv, H.F.; Zhong, J.; Cheng, N.C. Ti Single Atom Enhancing Pt-Based Intermetallics for Efficient and Durable Oxygen Reduction. Adv. Funct. Mater. 2024, 34, 2406347. [Google Scholar] [CrossRef]
- Jiang, H.R.; Wang, Z.C.; Chen, S.H.; Xiao, Y.; Zhu, Y.; Wu, W.; Chen, R.Z.; Cheng, N.C. Atomic controlled shell thickness on Pt@Pt3Ti core-shell nanoparticles for efficient and durable oxygen reduction. J. Mater. Sci. Technol. 2025, 205, 212–220. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.Q.; Qiao, B.T.; Li, J.; Liu, J.Y.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Fairhurst, A.R.; Ransom, B.J.; Haering, D.; Stamenkovic, V.R. Role of Transition Metals in Pt Alloy Catalysts for the Oxygen Reduction Reaction. ACS Catal. 2023, 13, 14874–14893. [Google Scholar] [CrossRef]
- Zhao, X.R.; Sasaki, K. Advanced Pt-Based Core-Shell Electrocatalysts for Fuel Cell Cathodes. Acc. Chem. Res. 2022, 55, 1226–1236. [Google Scholar] [CrossRef]
- Jiang, R.Y.; Tung, S.O.; Tang, Z.; Li, L.; Ding, L.; Xi, X.G.; Liu, Y.Y.; Zhang, L.; Zhang, J.J. A review of core-shell nanostructured electrocatalysts for oxygen reduction reaction. Energy Storage Mater. 2018, 12, 260–276. [Google Scholar] [CrossRef]
- Holstein, W.L.; Rosenfeld, H.D. In-Situ X-ray Absorption Spectroscopy Study of Pt and Ru Chemistry during Methanol Electrooxidation. J. Phys. Chem. B 2005, 109, 2176–2186. [Google Scholar] [CrossRef]
- Zeng, Q.; Song, J.; Cui, P.L.; Liu, H.; Tian, L.L.; Chen, D.; Yang, J. Optimizing Lattice Strain and Electron Effect of Ultrathin Platinum Nanoshells through Core-Shell Construction toward Superior Electrocatalytic Hydrogen Evolution. Ind. Eng. Chem. Res. 2022, 61, 7529–7536. [Google Scholar] [CrossRef]
- Yim, W.L.; Klüner, T. Understanding of Adsorption and Catalytic Properties of Bimetallic Pt-Co Alloy Surfaces from First Principles: Insight from Disordered Alloy Surfaces. J. Phys. Chem. C 2010, 114, 7141–7152. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhao, X.; Yan, H.L.; Sun, L.Y.; Chen, S.L.; Zhang, S.M.; Zhang, J.J. Manipulating Pt-skin of porous network Pt-Cu alloy nanospheres toward efficient oxygen reduction. J. Colloid Interface Sci. 2023, 652, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.J.; Zhang, S.; Sun, S.H. Tuning Nanoparticle Catalysis for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.F.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef]
- Stephens, I.E.L.; Bondarenko, A.S.; Grønbjerg, U.; Rossmeisl, J.; Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012, 5, 6744–6762. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Cui, C.H.; Li, Y.N.; Gao, Y.J.; Zhu, D.M.; Gu, Y.F.; Pan, G.Y.; Zhu, Y.Q.; Zhang, T. Lattice-Strain Engineering for Heterogenous Electrocatalytic Oxygen Evolution Reaction. Adv. Mater. 2023, 35, 2209876. [Google Scholar] [CrossRef]
- Goyhenex, C.; Bulou, H.; Deville, J.P.; Tréglia, G. Compressive strain versus tensile strain: A theoretical study of Pt/Co(0001) and Co/Pt(111) heteroepitaxy. Appl. Surf. Sci. 2001, 177, 238–242. [Google Scholar] [CrossRef]
- Mathur, A.; Erlebacher, J. Effects of substrate shape, curvature and roughness on thin heteroepitaxial films of Pt on Au(111). Surf. Sci. 2008, 602, 2863–2875. [Google Scholar] [CrossRef]
- Asano, M.; Kawamura, R.; Sasakawa, R.; Todoroki, N.; Wadayama, T. Oxygen Reduction Reaction Activity for Strain-Controlled Pt-Based Model Alloy Catalysts: Surface Strains and Direct Electronic Effects Induced by Alloying Elements. ACS Catal. 2016, 6, 5285–5289. [Google Scholar] [CrossRef]
- Temmel, S.E.; Fabbri, E.; Pergolesi, D.; Lippert, T.; Schmidt, T.J. Tuning the surface electrochemistry by strained epitaxial pt thin film model electrodes prepared by pulsed laser deposition. Adv. Mater. Interfaces 2016, 3, 1600222. [Google Scholar] [CrossRef]
- Kasai, M.; Dohi, H. Growth temperature and relaxation of lattice strain in epitaxial Pt films exhibiting diffraction fringes. Surf. Sci. 2019, 689, 121461. [Google Scholar] [CrossRef]
- Pašti, I.A.; Gavrilov, N.M.; Mentus, S.V. Hydrogen adsorption on palladium and platinum overlayers: DFT study. Adv. Phys. Chem. 2011, 2011, 305634. [Google Scholar] [CrossRef]
- Bu, L.Z.; Zhang, N.; Guo, S.J.; Zhang, X.; Li, J.; Yao, J.L.; Wu, T.; Lu, G.; Ma, J.Y.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Xu, S.C.; Tsai, C.; Li, Y.Z.; Liu, C.; Zhao, J.; Liu, Y.Y.; Yuan, H.Y.; Frank, A.P.; Prinz, F.B.; et al. Direct and continuous strain control of catalysts with tunable battery electrode materials. Science 2016, 354, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.D.; Zhang, C.; Guo, R.Y.; Jiang, Y.J.; He, T.O.; Zhan, Q.; Li, R.; Zheng, Y.Z.; Li, Y.N.; Dai, S.; et al. Effect of Interfacial Interaction on Electrocatalytic Activity and Durability of Pt-Based Core-Shell Nanocatalysts. ACS Catal. 2024, 14, 11721–11732. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Wang, X.; Choi, S.I.; Roling, L.T.; Luo, M.; Ma, C.; Zhang, L.; Chi, M.F.; Liu, J.Y.; Xie, Z.X.; Herron, J.A.; et al. Palladium–platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 2015, 6, 7594. [Google Scholar] [CrossRef]
- Xie, S.F.; Choi, S.I.; Lu, N.; Roling, L.T.; Herron, J.A.; Zhang, L.; Park, J.; Wang, J.G.; Kim, M.J.; Xie, Z.X.; et al. Atomic Layer-by-Layer Deposition of Pt on Pd Nanocubes for Catalysts with Enhanced Activity and Durability toward Oxygen Reduction. Nano Lett. 2014, 14, 3570–3576. [Google Scholar] [CrossRef]
- Hunt, S.T. Engineering Carbide Nanoparticles Coated with Noble Metal Monolayers for Catalysis; Massachusetts Institute of Technology: Cambridge, MA, USA, 2016. [Google Scholar]
- Hashiguchi, Y.; Watanabe, F.; Honma, T.; Nakamura, I.; Poly, S.S.; Kawaguchi, T.; Tsuji, T.; Murayama, H.; Tokunaga, M.; Fujitani, T. Continuous-flow synthesis of Pd@Pt core-shell nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126607. [Google Scholar] [CrossRef]
- Choi, R.; Choi, S.I.; Choi, C.H.; Nam, K.M.; Woo, S.I.; Park, J.T.; Han, S.W. Designed synthesis of well-defined Pd@Pt core-shell nanoparticles with controlled shell thickness as efficient oxygen reduction electrocatalysts. Chem.-A Eur. J. 2013, 19, 8190–8198. [Google Scholar] [CrossRef]
- Zhang, J.L.; Vukmirovic, M.B.; Xu, Y.; Mavrikakis, M.; Adzic, R.R. Controlling the Catalytic Activity of Platinum-Monolayer Electrocatalysts for Oxygen Reduction with Different Substrates. Angew. Chem. Int. Ed. 2005, 44, 2132–2135. [Google Scholar] [CrossRef]
- Mahesh, I.; Sarkar, A. Scalable Production of Monolayer Shell(Pt)@Core(Pd) Nanoparticles by Electroless Cu UPD for Oxygen Reduction Reaction. Electrocatalysis 2021, 12, 127–136. [Google Scholar] [CrossRef]
- Alayoglu, S.; Zavalij, P.; Eichhorn, B.; Wang, Q.; Frenkel, A.I.; Chupas, P. Structural and architectural evaluation of bimetallic nanoparticles: A case study of Pt-Ru core-shell and alloy nanoparticles. ACS Nano 2009, 3, 3127–3137. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.S.; Wu, M.; Ning, S.L.; Huang, L.; Kang, X.W.; Chen, S.W. Ru@Pt core-shell nanoparticles: Impact of the atomic ordering of the Ru metal core on the electrocatalytic activity of the Pt shell. ACS Sustain. Chem. Eng. 2019, 7, 9007–9016. [Google Scholar] [CrossRef]
- Alayoglu, S.; Nilekar, A.U.; Mavrikakis, M.; Eichhorn, B. Ru-Pt core-shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nat. Mater. 2008, 7, 333–338. [Google Scholar] [CrossRef]
- El Sawy, E.N.; Brueckner, T.M.; Pickup, P.G. Electrochemical Oxidation of Methanol and Ethanol at Rh@Pt and Ru@Pt Catalysts. J. Electrochem. Soc. 2020, 167, 106507. [Google Scholar] [CrossRef]
- Matin, M.A.; Lee, E.; Kim, H.; Yoon, W.S.; Kwon, Y.U. Rational syntheses of core-shell Fe@(PtRu) nanoparticle electrocatalysts for the methanol oxidation reaction with complete suppression of CO-poisoning and highly enhanced activity. J. Mater. Chem. A 2015, 3, 17154–17164. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liang, Z.X.; Yang, F.; Liu, Y.W.; Chen, S.L. Ni-Pt Core-Shell Nanoparticles as Oxygen Reduction Electrocatalysts: Effect of Pt Shell Coverage. J. Phys. Chem. C 2011, 115, 24073–24079. [Google Scholar] [CrossRef]
- Jennings, P.C.; Aleksandrov, H.A.; Neyman, K.M.; Johnston, R.L. O2 Dissociation on M@Pt Core-Shell Particles for 3d, 4d, and 5d Transition Metals. J. Phys. Chem. C 2015, 119, 11031–11041. [Google Scholar] [CrossRef]
- Dhavale, V.M.; Unni, S.M.; Kagalwala, H.N.; Pillai, V.K.; Kurungot, S. Ex-situ dispersion of core-shell nanoparticles of Cu-Pt on an in situ modified carbon surface and their enhanced electrocatalytic activities. Chem. Commun. 2011, 47, 3951–3953. [Google Scholar] [CrossRef]
- Cantane, D.A.; Oliveira, F.E.R.; Santos, S.F.; Lima, F.H.B. Synthesis of Pt-based hollow nanoparticles using carbon-supported Co@Pt and Ni@Pt core-shell structures as templates: Electrocatalytic activity for the oxygen reduction reaction. Appl. Catal. B Environ. 2013, 136–137, 351–360. [Google Scholar] [CrossRef]
- Wu, H.M.; Wexler, D.; Wang, G.X.; Liu, H.K. Cocore-Ptshell nanoparticles as cathode catalyst for PEM fuel cells. J. Solid State Electrochem. 2012, 16, 1105–1110. [Google Scholar] [CrossRef]
- Yang, Z.X.; Zhang, Y.X.; Wu, R.Q. High Stability and Reactivity of Pt-Based Core-Shell Nanoparticles for Oxygen Reduction Reaction. J. Phys. Chem. C 2012, 116, 13774–13780. [Google Scholar] [CrossRef]
- Wang, R.F.; Wang, H.; Luo, F.; Liao, S.J. Core-Shell-Structured Low-Platinum Electrocatalysts for Fuel Cell Applications. Electrochem. Energy Rev. 2018, 1, 324–387. [Google Scholar] [CrossRef]
- Wang, L.; Gao, W.P.; Liu, Z.Y.; Zeng, Z.H.; Liu, Y.F.; Giroux, M.; Chi, M.F.; Wang, G.F.; Greeley, J.; Pan, X.Q.; et al. Core-Shell Nanostructured Cobalt-Platinum Electrocatalysts with Enhanced Durability. ACS Catal. 2017, 8, 35–42. [Google Scholar] [CrossRef]
- Xia, Y.N.; Gilroy, K.D.; Peng, H.C.; Xia, X.H. Seed-Mediated Growth of Colloidal Metal Nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 60–95. [Google Scholar] [CrossRef] [PubMed]
- Foucher, A.C.; Rosen, D.J.; Decker, L.K.; Macfarlane, R.J.; Murray, C.B.; Stach, E.A.; Ross, F.M. Structure and Stability of Core-Shell Cu-Pt Nanoparticles for Catalytic Applications. Chem. Mater. 2023, 35, 8758–8764. [Google Scholar] [CrossRef]
- Beard, K.D.; Borrelli, D.; Cramer, A.M.; Blom, D.; Van Zee, J.W.; Monnier, J.R. Preparation and Structural Analysis of Carbon-Supported Co Core/Pt Shell Electrocatalysts Using Electroless Deposition Methods. ACS Nano 2009, 3, 2841–2853. [Google Scholar] [CrossRef]
- Chen, Y.M.; Shi, J.C.; Chen, S.L. Small-Molecule (CO, H2) Electro-Oxidation as an Electrochemical Tool for Characterization of Ni@Pt/C with Different Pt Coverages. J. Phys. Chem. C 2015, 119, 7138–7145. [Google Scholar] [CrossRef]
- Yuan, Q.B.; Duan, D.H.; Ma, Y.H.; Wei, G.Q.; Zhang, Z.L.; Hao, X.G.; Liu, S.B. Performance of nano-nickel core wrapped with Pt crystalline thin film for methanol electro-oxidation. J. Power Sources 2014, 245, 886–891. [Google Scholar] [CrossRef]
- Lim, Y.; Kim, S.K.; Lee, S.C.; Choi, J.; Nahm, K.S.; Yoo, S.J.; Kim, P. One-step synthesis of carbon-supported Pd@Pt/C core-shell nanoparticles as oxygen reduction electrocatalysts and their enhanced activity and stability. Nanoscale 2014, 6, 4038–4042. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-Like Behavior of Tungsten Carbide in Surface Catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.X.Y.; Pessemesse, Q.; Lätsch, L.; Engel, K.M.; Stark, W.J.; van Bavel, A.P.; Horton, A.D.; Payard, P.A.; Copéret, C. Role and dynamics of transition metal carbides in methane coupling. Chem. Sci. 2023, 14, 5899–5905. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.V.; Chen, J.G. Monolayer platinum supported on tungsten carbides as low-cost electrocatalysts: Opportunities and limitations. Energy Environ. Sci. 2011, 4, 3900–3912. [Google Scholar] [CrossRef]
- Yates, J.L.R.; Spikes, G.H.; Jones, G. Platinum-carbide interactions: Core–shells for catalytic use. Phys. Chem. Chem. Phys. 2015, 17, 4250–4258. [Google Scholar] [CrossRef]
- Shigeaki, O.; Takumi, K.; Yasuo, O. A high-pressure and high-temperature synthesis of platinum carbide. Solid State Commun. 2005, 133, 55–59. [Google Scholar]
- Liu, Y.; Kelly, T.G.; Chen, J.G.; Mustain, W.E. Metal Carbides as Alternative Electrocatalyst Supports. ACS Catal. 2013, 3, 1184–1194. [Google Scholar] [CrossRef]
- Hunt, S.T.; Milina, M.; Alba-Rubio, A.C.; Hendon, C.H.; Dumesic, J.A.; Roman-Leshkov, Y. Self-assembly of noble metal monolayers on transition metal carbide nanoparticle catalysts. Science 2016, 352, 974–978. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, J.S.; Göhl, D.; Paciok, P.; Gonçalves, D.S.; Lim, H.K.; Zanchet, D.; Heggen, M.; Shao-Horn, Y.; Ledendecker, M.; et al. Platinum/Tantalum Carbide Core–Shell Nanoparticles with Sub-Monolayer Shells for Methanol and Oxygen Electrocatalysis. Adv. Energy Mater. 2024, 14, 2304092. [Google Scholar] [CrossRef]
- Garg, A.; Milina, M.; Ball, M.; Zanchet, D.; Hunt, S.T.; Dumesic, J.A.; Román-Leshkov, Y. Transition-Metal Nitride Core@Noble-Metal Shell Nanoparticles as Highly CO Tolerant Catalysts. Angew. Chem. Int. Ed. 2017, 56, 8828–8833. [Google Scholar] [CrossRef]
- Weigert, E.C.; Esposito, D.V.; Chen, J.G. Cyclic voltammetry and X-ray photoelectron spectroscopy studies of electrochemical stability of clean and Pt-modified tungsten and molybdenum carbide (WC and Mo2C) electrocatalysts. J. Power Sources 2009, 193, 501–506. [Google Scholar] [CrossRef]
- Hui, Y.; Walter, V.; Claude, L.; Nicolás, A.V. Structure and Electrocatalytic Activity of Carbon-Supported Pt–Ni Alloy Nanoparticles Toward the Oxygen Reduction Reaction. J. Phys. Chem. B 2004, 108, 11024–11034. [Google Scholar]
- Hui, Y.; Nicolás, A.V.; Léger, J.M.; Claude, L. Tailoring, Structure, and Activity of Carbon-Supported Nanosized Pt-Cr Alloy Electrocatalysts for Oxygen Reduction in Pure and Methanol-Containing Electrolytes. J. Phys. Chem. B 2004, 108, 1938–1947. [Google Scholar]
- Cui, J.L.; Zhang, D.; Liu, Z.L.; Li, C.C.; Zhang, T.T.; Yin, S.X.; Song, Y.T.; Li, H.; Li, H.H.; Li, C.Z. Carbon-anchoring synthesis of Pt1Ni1@Pt/C core-shell catalysts for stable oxygen reduction reaction. Nat. Commun. 2024, 15, 9458. [Google Scholar] [CrossRef]
- Liu, H.J.; Dou, M.L.; Wang, F.; Liu, J.J.; Ji, J.; Li, Z.L. Ordered intermetallic PtFe@Pt core-shell nanoparticles supported on carbon nanotubes with superior activity and durability as oxygen reduction reaction electrocatalysts. RSC Adv. 2015, 5, 66471–66475. [Google Scholar] [CrossRef]
- Zhu, J.B.; Xiao, M.L.; Li, K.; Liu, C.P.; Xing, W. Superior electrocatalytic activity from nanodendritic structure consisting of a PtFe bimetallic core and Pt shell. Chem. Commun. 2015, 51, 3215–3218. [Google Scholar] [CrossRef]
- Liu, Z.F.; Jackson, G.S.; Eichhorn, B.W. Tuning the CO-tolerance of Pt-Fe bimetallic nanoparticle electrocatalysts through architectural control. Energy Environ. Sci. 2011, 4, 1900. [Google Scholar] [CrossRef]
- Wang, D.L.; Xin, H.L.; Hovden, R.; Wang, H.S.; Yu, Y.C.; Muller, D.A.; DiSalvo, F.J.; Abruña, H.D. Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87. [Google Scholar] [CrossRef]
- Pan, Y.T.; Li, D.; Sharma, S.; Wang, C.; Zachman, M.J.; Wegener, E.C.; Kropf, A.J.; Kim, Y.S.; Myers, D.J.; Peterson, A.A.; et al. Ordered CoPt oxygen reduction catalyst with high performance and durability. Chem Catal. 2022, 2, 3559–3572. [Google Scholar] [CrossRef]
- Liang, J.S.; Li, N.; Zhao, Z.L.; Ma, L.; Wang, X.M.; Li, S.Z.; Liu, X.; Wang, T.Y.; Du, Y.P.; Lu, G.; et al. Tungsten-Doped L10-PtCo Ultrasmall Nanoparticles as a High-Performance Fuel Cell Cathode. Angew. Chem. Int. Ed. 2019, 58, 15471–15477. [Google Scholar] [CrossRef]
- Zhao, X.R.; Xi, C.; Zhang, R.; Song, L.; Wang, C.Y.; Spendelow, J.S.; Frenkel, A.I.; Yang, J.; Xin, H.L.; Sasaki, K. High-Performance Nitrogen-Doped Intermetallic PtNi Catalyst for the Oxygen Reduction Reaction. ACS Catal. 2020, 10, 10637–10645. [Google Scholar] [CrossRef]
- Muthuswamy, N.; de la Fuente, J.L.G.; Tran, D.T.; Walmsley, J.; Tsypkin, M.; Raaen, S.; Sunde, S.; Rønning, M.; Chen, D. Ru@Pt core-shell nanoparticles for methanol fuel cell catalyst: Control and effects of shell composition. Int. J. Hydrogen Energy 2013, 38, 16631–16641. [Google Scholar] [CrossRef]
- El Sawy, E.N.; Pickup, P.G. Formic acid oxidation at Ru@Pt core-shell nanoparticles. Electrocatalysis 2016, 7, 477–485. [Google Scholar] [CrossRef]
- Chen, T.Y.; Lin, T.L.; Luo, T.J.M.; Choi, Y.; Lee, J.F. Effects of Pt shell thicknesses on the atomic structure of Ru-Pt core-shell nanoparticles for methanol electrooxidation applications. ChemPhysChem 2010, 11, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Schwämmlein, J.N.; Stühmeier, B.M.; Wagenbauer, K.; Dietz, H.; Tileli, V.; Gasteiger, H.A.; El-Sayed, H.A. Origin of superior HOR/HER activity of bimetallic Pt-Ru catalysts in alkaline media identified via Ru@Pt core-shell nanoparticles. J. Electrochem. Soc. 2018, 165, H229–H239. [Google Scholar] [CrossRef]
- Yang, L.J.; Vukmirovic, M.B.; Su, D.; Sasaki, K.; Herron, J.A.; Mavrikakis, M.; Liao, S.J.; Adzic, R.R. Tuning the catalytic activity of Ru@Pt core-shell nanoparticles for the oxygen reduction reaction by varying the shell thickness. J. Phys. Chem. C 2013, 117, 1748–1753. [Google Scholar] [CrossRef]
- Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Core–Shell Compositional Fine Structures of Dealloyed PtxNi1−x Nanoparticles and Their Impact on Oxygen Reduction Catalysis. Nano Lett. 2012, 12, 5423–5430. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Qin, X.P.; Xiao, F.; Yang, S.L.; Xu, Y.; Tan, Z.; Li, J.D.; Yan, J.W.; Chen, Q.; Chen, M.S.; et al. The role of ruthenium in improving the kinetics of hydrogen oxidation and evolution reactions of platinum. Nat. Catal. 2021, 4, 711–718. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, P.K.; Jiang, S.P. Enhanced activity and stability of core–shell structured PtRuNi electrocatalysts for direct methanol fuel cells. Int. J. Hydrogen Energy 2016, 41, 1935–1943. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, C.X.; Guan, L.H. Chemical corrosion of PtRuCu6/C for highly efficient methanol oxidation. J. Power Sources 2016, 306, 489–494. [Google Scholar] [CrossRef]
- Wang, R.F.; Li, H.; Feng, H.Q.; Wang, H.; Lei, Z.Q. Preparation of carbon-supported core@shell PdCu@PtRu nanoparticles for methanol oxidation. J. Power Sources 2010, 195, 1099–1102. [Google Scholar] [CrossRef]
- Zhao, H.B.; Li, L.; Yang, J.; Zhang, Y.M. Co@Pt–Ru core-shell nanoparticles supported on multiwalled carbon nanotube for methanol oxidation. Electrochem. Commun. 2008, 10, 1527–1529. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.F.; Wang, H.; Ji, S.; Key, J.L.; Li, X.Z.; Lei, Z.Q. An advantageous method for methanol oxidation: Design and fabrication of a nanoporous PtRuNi trimetallic electrocatalyst. J. Power Sources 2011, 196, 9346–9351. [Google Scholar] [CrossRef]
- Wu, Y.N.; Liao, S.J.; Guo, H.F.; Hao, X.Y. High-performance Pd@PtRu/C catalyst for the anodic oxidation of methanol prepared by decorating Pd/C with a PtRu shell. J. Power Sources 2013, 224, 66–71. [Google Scholar] [CrossRef]
- Mao, X.F.; Yang, L.C.; Yang, J.; Key, J.L.; Ji, S.; Wang, H.; Wang, R.F. A Volcano Curve: Optimizing Activity of Shell-Core PtxRuy@PdCu/C Catalysts for Methanol Oxidation by Tuning Pt/Ru Ratio. J. Electrochem. Soc. 2013, 160, H219–H223. [Google Scholar] [CrossRef]
- Zhang, J.L.; Vukmirovic, M.B.; Sasaki, K.; Nilekar, A.U.; Mavrikakis, M.; Adzic, R.R. Mixed-Metal Pt Monolayer Electrocatalysts for Enhanced Oxygen Reduction Kinetics. J. Am. Chem. Soc. 2005, 127, 12480–12481. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zeng, R.; Wang, L.G.; Jiang, L.J.; Wang, S.M.; Liu, X.P. A simple strategy to form hollow Pt3Co alloy nanosphere with ultrathin Pt shell with significant enhanced oxygen reduction reaction activity. Int. J. Hydrogen Energy 2016, 41, 21394–21403. [Google Scholar] [CrossRef]
- Wang, C.; Chi, M.F.; Li, D.G.; Strmcnik, D.; van der Vliet, D.; Wang, G.F.; Komanicky, V.; Chang, K.C.; Paulikas, A.P.; Tripkovic, D.; et al. Design and Synthesis of Bimetallic Electrocatalyst with Multilayered Pt-Skin Surfaces. J. Am. Chem. Soc. 2011, 133, 14396–14403. [Google Scholar] [CrossRef]
- Beermann, V.; Gocyla, M.; Willinger, E.; Rudi, S.; Heggen, M.; Dunin-Borkowski, R.E.; Willinger, M.G.; Strasser, P. Rh-doped Pt-Ni octahedral nanoparticles: Understanding the correlation between elemental distribution, ORR and shape stability. Nano Lett. 2016, 16, 1719–1725. [Google Scholar] [CrossRef]
- Dionigi, F.; Weber, C.C.; Primbs, M.; Gocyla, M.; Bonastre, A.M.; Spöri, C.; Schmies, H.; Hornberger, E.; Kühl, S.; Drnec, J.; et al. Controlling near-surface Ni composition in octahedral PtNi(Mo) nanoparticles by Mo doping for a highly active oxygen reduction reaction catalyst. Nano Lett. 2019, 19, 6876–6885. [Google Scholar] [CrossRef]
- Lim, J.H.; Shin, H.; Kim, M.J.; Lee, H.; Lee, K.S.; Kwon, Y.K.; Song, D.H.; Oh, S.K.; Kim, H.; Cho, E.A. Ga-doped Pt-Ni octahedral nanoparticlesas a highly active and durable electrocatalyst for oxygen reduction reaction. Nano Lett. 2018, 18, 2450–2458. [Google Scholar] [CrossRef]
- Feizabadi, A.; Chen, J.; Banis, M.N.; Yiu, Y.M.; Zhang, L.; Sun, X.; Sham, T.K. Cobalt-Doped Pd@Pt Core-Shell Nanoparticles: A Correlative Study of Electronic Structure and Catalytic Activity in ORR. J. Phys. Chem. C 2023, 127, 18843–18854. [Google Scholar] [CrossRef]
- Chen, S.M.; Chen, L.K.; Tian, N.; Hu, S.N.; Yang, S.L.; Shen, J.F.; Tang, J.X.; Wu, D.Y.; Chen, M.S.; Zhou, Z.Y.; et al. Double-Shell Confinement Strategy Enhancing Durability of PtFeTi Intermetallic Catalysts for the Oxygen Reduction Reaction. ACS Catal. 2024, 14, 16664–16672. [Google Scholar] [CrossRef]
- Patel, K.D.; Subedar, D.; Patel, F. Design and development of automotive catalytic converter using non-nobel catalyst for the reduction of exhaust emission: A review. Mater. Today Proc. 2022, 57, 2465–2472. [Google Scholar] [CrossRef]
- Liu, L.Q.; Rao, X.B.; Zhang, S.M.; Zhang, J.J. Insight into synergy for oxygen reduction electrocatalysis of iron-nitrogen-carbon. Chem 2024, 10, 1994–2030. [Google Scholar] [CrossRef]
- Yang, Z.L.; Chen, Y.Z.; Zhang, S.M.; Zhang, J.J. Identification and Understanding of Active Sites of Non-noble Iron-Nitrogen-Carbon Catalysts for Oxygen Reduction Electrocatalysis. Adv. Funct. Mater. 2023, 33, 2215185. [Google Scholar] [CrossRef]
- Chen, M.H.; Chen, J.X.; Jia, C.G.; Luo, J.; Yang, Z.L.; Jung, J.C.Y.; Zhang, J.J.; Shengli Chen, S.L.; Zhang, S.M. Metal-free Carbon Semi-tubes for Oxygen Reduction Electrocatalysis. Cell Rep. Phys. Sci. 2023, 4, 101204. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, S.M.; Jung, J.C.Y.; Zhang, J.J. Carbons as low-platinum catalyst supports and non-noble catalysts for polymer electrolyte fuel cells. Prog. Energy Combust. Sci. 2023, 98, 101101. [Google Scholar] [CrossRef]
- Zhang, S.M.; Chen, M.H.; Zhao, X.; Cai, J.L.; Yan, W.; Yen, J.C.; Chen, S.L.; Yu, Y.; Zhang, J.J. Advanced Noncarbon Materials as Catalyst Supports and Non-Noble Electrocatalysts for Fuel Cells and Metal-Air Batteries. Electrochem. Energy Rev. 2021, 4, 336–381. [Google Scholar] [CrossRef]
- Wu, Y.N.; Liao, S.J.; Guo, H.F.; Hao, X.Y. Preparation of high-performance PdPt-Pt core-shell catalyst with shortened carbon nanotubes as support. J. Power Sources 2013, 235, 135–141. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeon, T.Y.; Kim, H.S.; Sung, Y.E.; Smyrl, W.H. Effect of Annealing PtNi Nanophases on Extended TiO2 Nanotubes for the Electrochemical Oxygen Reduction Reaction. J. Electrochem. Soc. 2008, 155, B1058. [Google Scholar] [CrossRef]
- Chinmayee, V.S.; Qin, Z.; Anthony, H.; Moylan, T.E.; Frederick, T.W.; DiSalvo, F.J. Sol-Gel Synthesis, Electrochemical Characterization, and Stability Testing of Ti0.7W0.3O2 Nanoparticles for Catalyst Support Applications in Proton-Exchange Membrane Fuel Cells. J. Am. Chem. Soc. 2010, 132, 17531–17536. [Google Scholar]
- Ho, V.T.T.; Pan, C.J.; Rick, J.; Su, W.N.; Hwang, B.J. Nanostructured Ti0.7Mo0.3O2 Support Enhances Electron Transfer to Pt: High-Performance Catalyst for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2011, 133, 11716–11724. [Google Scholar] [CrossRef] [PubMed]


| 1 | Earth-abundant and inexpensive. |
| 2 | Metallic electrical conductivity and acidic/alkaline corrosion resistance. |
| 3 | Melting point >2000 °C. |
| 4 | Shell material is insoluble in the core lattice. |
| 5 | Shell monolayer formation on the core is favorable, and interfacial bonding is strong. |
| 6 | Core has minimal or favorable modifications to shell work function and d-band |
| 7 | Readily manufactured and independently tunable core-shell architectures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Du, P.; Cheng, K.; Hua, X.; Xie, M.; Li, Y.; Zheng, Y.; Wang, Y.; Pi, C.; Zhang, S. Structural Regulation of Advanced Platinum-Based Core-Shell Catalysts for Fuel Cell Electrocatalysis. Minerals 2025, 15, 235. https://doi.org/10.3390/min15030235
Wang X, Du P, Cheng K, Hua X, Xie M, Li Y, Zheng Y, Wang Y, Pi C, Zhang S. Structural Regulation of Advanced Platinum-Based Core-Shell Catalysts for Fuel Cell Electrocatalysis. Minerals. 2025; 15(3):235. https://doi.org/10.3390/min15030235
Chicago/Turabian StyleWang, Xiaqing, Panpan Du, Kun Cheng, Xing Hua, Ming Xie, Yuyu Li, Yun Zheng, Yingying Wang, Chaoran Pi, and Shiming Zhang. 2025. "Structural Regulation of Advanced Platinum-Based Core-Shell Catalysts for Fuel Cell Electrocatalysis" Minerals 15, no. 3: 235. https://doi.org/10.3390/min15030235
APA StyleWang, X., Du, P., Cheng, K., Hua, X., Xie, M., Li, Y., Zheng, Y., Wang, Y., Pi, C., & Zhang, S. (2025). Structural Regulation of Advanced Platinum-Based Core-Shell Catalysts for Fuel Cell Electrocatalysis. Minerals, 15(3), 235. https://doi.org/10.3390/min15030235









