Abstract
Critical mineral recovery from wastewater is an enhancement of conventional mining that can help meet growing demand. This work investigates two energy wastewaters that have previously been shown to be enriched in critical minerals, oil and gas produced water in the Permian Basin and combustion residual leachate. Treatment of these two wastewaters using reverse osmosis or thermal-based methods concentrates critical minerals, which improves the economic viability of critical mineral recovery. Revenue from mineral recovery could also offset treatment costs for operators. This work evaluates the cost of treatment for each wastewater and evaluates the potential revenue from critical minerals concentrated in the brine. The levelized cost of water for combustion residual leachate ranges from USD 1.90 to USD 16.20 (USD 2023/m3 permeate) and for produced water ranges from USD 14.40 to USD 24.30 (USD 2023/m3 distillate). Recovery opportunities range from USD 0.11 to USD 1.13 (USD 2023/m3 permeate) for leachate and from USD 8.28 to USD 42.10 (USD 2023/m3 distillate) for produced water, dominated by the value of magnesium and lithium. Comparing the maximum value of critical minerals contained in produced water and the maximum treatment costs, the value of critical minerals exceeds the cost of treatment by USD 17.80/m3 distillate, which signals a potential revenue opportunity.
1. Introduction
Recovery of critical minerals from energy wastewaters presents an opportunity to supplement critical mineral [1] supplies and improve the circularity of mineral supply chains. Multiple studies have investigated the potential for mineral recovery from wastewaters [2,3,4,5,6]. In this article, coal combustion residual leachate and oil and gas produced water from the Permian Basin are selected as case studies for further evaluation because of their large volumes and critical mineral enrichment.
Coal combustion residuals (CCRs) are waste products (e.g., bottom ash, fly ash) produced when coal is fired for electricity generation. Nearly half of CCRs are stored in landfills and impoundments [7]; rainwater then percolates through these landfills and impoundments to form leachates. The annual volume estimates for these CCR leachate in landfills and impoundments were estimated in previous work to be significant, at 34.7 million cubic meters per year (MCM/year) and 311.6 MCM/year, respectively [8]. These leachates contain contaminants from the CCRs that are typically treated prior to discharge [2], leveraging advancements in adjacent fields for monitoring and control of wastewater streams [9,10]. Oil and gas produced water [11] can contain high levels of dissolved and suspended solids. Produced water quantity and composition varies significantly by region. A report for the Ground Water Protection Council estimated nearly 25.9 billion barrels of produced water were generated in the United States in 2021 [12]. A large portion of this produced water comes from Texas and New Mexico (about 9.7 billion barrels in 2021), specifically the Permian Basin. The “NEWTS [National Energy Water Treatment Speciation] Well Summary by Hydrologic Region and Subbasins in the U.S.” estimated the Hydrologic Unit Code (HUC)-8 volume as 11.3 billion barrels in 2022 [13]. Currently, produced water management is an operational risk due to the seismic and environmental implications of produced water injection. Produced water treatment has been well studied, but there are significant challenges [14].
Previous efforts have been made by the National Energy Technology Laboratory (NETL) to assess the cost of combustion residual leachate treatment and the potential recovery of critical minerals (CMs) [2,8]. These efforts were foundational in establishing the potential market for CCR leachate volumes and recovery potential. There have also been attempts to assess treatment and recovery from produced waters [3,15,16]. However, these studies for CCR leachate and produced waters do not include techno-economic assessments for the treated water costs, or the estimated quantity of critical minerals in concentrated brine following treatment.
This study aims to mitigate these gaps by assessing the cost and performance of water treatment and CM recovery from combustion residual leachate and produced waters. First, volume and composition estimates are made for each case study. Average compositions are reconciled for charge balance using total dissolved solids (TDS). The reconciled stream is used as an input for the treatment models. The treatment models estimate the treatment system requirements to achieve water quality in accordance with the respective regulations. The resultant system is costed to achieve a levelized cost of water (LCOW). Sensitivities to performance and capital and operational costs are assessed. The resultant brine is also used as a basis for estimating CM recovery potential. The mineral volumes are combined with market rates to determine the potential value of CM recovery. Finally, sensitivities to the concentration of Li and Mg are assessed.
2. Materials and Methods
The data sets and modeling techniques used to assess treatment and recovery from combustion residual leachate and produced water are described in Supplementary Section S2. Figure S1 and summarized in the following sections. The methods leverage WaterTAP 0.10.0 [17], the only available open source process optimization software tool for water treatment, to estimate the performance and cost of water treatment systems, and OLI Flowsheet: ESP (Section 2.3.2), a proprietary electrolyte simulation software tool used to estimate the resultant phases and their composition as well as validate the WaterTAP model. The data are first used to calculate the median values for leachate and produced water, along with relevant concentration profile sensitivities (Section 2.1.1 and Section 2.1.2). The WaterTAP simulation is then created with this concentration data (Section 2.3.1) using the parameters for the performance and cost simulations (Section 2.2.1 and Section 2.2.2). OLI Studio data are integrated into this WaterTAP model through reconciliation (Section 2.1.4), and a parallel OLI Flowsheet model (Section 2.3.2) is used to calculate the resultant brine for critical mineral calculations (Section 2.1.3 and Section 2.3.3) and validate the WaterTAP simulation. The WaterTAP simulation ultimately calculates the LCOW and other cost values that are shown in Section 3.
2.1. Data Collection and Processing
The following subsections describe the data used in the modeling efforts for this work.
2.1.1. Landfill and Impoundment Coal Combustion Residual Leachate
The composition data used for leachate is taken from the CPInfo database curated by the Electric Power Research Institute (EPRI). These data are collated for both landfill and impoundment leachate. The full data set used to develop the landfill leachate case study is contained in Supplementary Section S1.1 Table S1, and the data for impoundment leachate is contained in Supplementary Section S1.2 Table S7. The data set is filtered for values that appear in at least 20 samples. Sensitivity analyses are developed between the 25th and 75th percentile for constituents with concentrations greater than 10 mg/L including Li, Mg, and other bulk constituents. The parameters for these sensitivity analysis ranges are summarized in Table 1. These streams must be treated to match the current treatment standards for CCR leachates, outlined in Supplementary Section S1.3 Table S12.

Table 1.
Sensitivity analyses for landfill and impoundment leachate.
2.1.2. Permian Basin Produced Water
The “15,000 Most Complete” composition data for produced water from the Permian Basin are taken directly from the database collated by the USGS [18], which is curated by the NEWTS group on the Energy Data eXchange [19]. The full data sheet is contained in Supplementary Section S1.4 Table S13.
Sensitivity analyses are performed for the highest-concentration critical minerals Li and Mg between the 25th and 75th percentile, and sensitivity analyses for sodium chloride between the 25th and 60th percentile value. Composition profiles are reconciled in OLI Studio prior to use in WaterTAP and OLI Flowsheet. The CM concentrations and bulk concentration sensitivities are shown in Supplementary Section S1.4 Table S15.
2.1.3. USGS CM Market Data
The USGS CM market data [20] were collated and used in prior work on national values for landfill and impoundment leachate and the potential for recovery [2]. These market values are updated to 2023 values (Supplementary Section S2) and are combined with the mass of each CM in the brine to calculate the potential value of recovery.
2.1.4. OLI Studio Reconciliation
OLI Studio has a Stream Analyzer module that can be used to reconcile electroneutrality, pH, alkalinity, and total inorganic carbon. Raw composition data shown in Section 2.1.1 and Section 2.1.2 for leachate and produced water (full compositions in Supplementary Section S1) are input into OLI Studio. The rigorous method is used to calculate TDS. This TDS output is used in the WaterTAP modeling (Section 2.2.2). The software is used to reconcile electroneutrality by adding cations or anions (from the bulk concentration parameters) to balance the charge of the solution and to calculate the final TDS. No additional reconciliations (such as pH or alkalinity) are used. The full reconciled stream data are then used as the inlet to the OLI Flowsheet software (Section 2.3.2).
2.1.5. Constituent Data Replacement Tool (CoDaRT)
CoDaRT [21] was designed to replace missing data in energy wastewater data sets using machine learning algorithms available through Python 3.13.0. This tool can be used on its own or can integrate a user’s produced water data with the USGS data set. This tool was applied to fill in missing data using the highest-performing machine learning algorithms in both the landfill leachate (Section 2.1.1) and produced water (Section 2.1.2) data sets. The results of these runs with this tool are described in Supplementary Section S1. Tables S4 and S16 and the impact of these runs on the results are described in Section 3.4. There are insufficient data for the machine learning technique to be applied for impoundment leachate.
2.2. Design Parameters
The subsections below detail the design parameters for performance and cost analyses for the leachate and produced water treatment systems.
2.2.1. Leachate Treatment System Data
The leachate treatment system parameters are explained below and used in the subsequent modeling steps (Section 2.3.1 and Section 2.3.2). The leachate treatment system is a two-stage membrane treatment system. A brackish water reverse osmosis (BWRO) membrane is used as the first stage for lower salinities, and a seawater reverse osmosis (SWRO) membrane is used as the higher-salinity second stage for further recovery. Early attempts at modeling revealed the need for solids removal; thus, a settling tank and antiscalant are added prior to the high-pressure pump that is used to deliver the leachate to the membrane treatment system. This is illustrated in Figure 1.
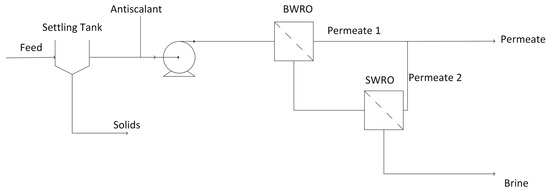
Figure 1.
Diagram of the leachate treatment system.
The parameters for the leachate treatment system are shown in Supplementary Section S2. Table S19. Justification for parameter selection is provided in Supplementary Section S2. Section S2.1 Leachate Treatment System Data. These are used for both landfill and impoundment leachate treatment (save for the differences in flow rate and feed TDS). Costs for equipment and antiscalant vary. The full range of these costs is used in the sensitivity analyses completed in Section 3.3.
2.2.2. Produced Water Treatment System Data
The produced water treatment system parameters are explained below and used in the subsequent modeling steps (Section 2.3.1). As before, WaterTAP is used to estimate the performance and cost, and OLI Flowsheet is used for detailed phase behavior as well as WaterTAP validation.
Based on a review of produced water treatment literature [22,23], the mechanical vapor compression (MVC) system was chosen as the treatment technique for produced water for volume reduction. MVC has been demonstrated at scale for produced water treatment [14]. The system consists of an evaporator/condenser and a vapor compressor. Additional heat recovery is achieved via brine and distillate heat exchangers. A diagram of this system is shown in Figure 2.
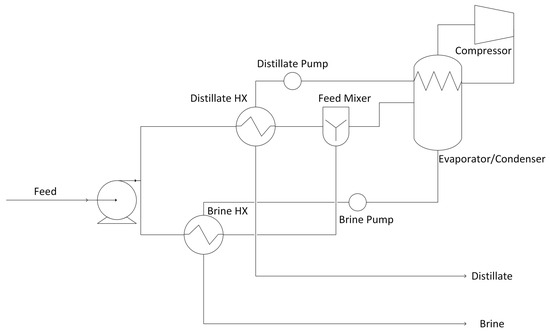
Figure 2.
Diagram of the produced water treatment system.
The data collated for the produced water treatment system are shown in Supplementary Section S2. Table S20. Explanations are provided for the selection of these parameters in Supplementary Section S2. Section S2.2 Produced Water Treatment System Data. As with leachate, additional parameters are used from Section 2.2.2. All other smaller equipment costs (such as feed mixers) are left at their default values.
2.3. Modeling Techniques
The following subsections describe the different techniques used to evaluate the data and calculate the metrics shown in the Results and Discussion Section (Section 3).
2.3.1. WaterTAP Performance and Cost Assessment
The WaterTAP package in Python [17] is an equation-oriented platform intended for both simulation and optimization (with an optimization emphasis) used to model the performance and cost of the leachate and produced water treatment systems. The leachate treatment simulation does not include further optimization as all the variables are specified, and it was elected to fix recovery to maintain similarity between the leachate simulations (for both landfill and impoundment leachate and between OLI Flowsheet and WaterTAP). However, the produced water treatment simulation optimizes LCOW by varying the size of heat exchangers and evaporators as well as the compressor ratio. The seawater property package selected takes in the inlet as water + TDS; however, there are newer property models that can input more solution diverse feed compositions.
For costing, the method created in Python by the Process Optimization and Modeling for Minerals Sustainability (PrOMMiS) [24] is adapted in the flowsheet for additional costing parameters (such as operating labor and waste disposal) beyond the WaterTAP base costing method. This method was developed for a dynamic system designed to recover REEs; there are thus significant changes to this method outlined in Supplementary Section S2.
The parameters used for overall costing are outlined in Table 2. All other parameters are left as their defaults with two exceptions: there are no land costs (assuming that treatment takes place on-site), and there are no patent costs (as this treatment system alone is insufficient for the sale of products). Solid waste disposal is also left at its default value for the solids created in the membrane treatment system. Note that the operating labor is converted from its 2022 value (24.81/h) [25] to a 2023 value using the employment cost index (ECI) for private industry workers [26] (see Supplementary Section S3).

Table 2.
WaterTAP and PrOMMiS costing parameters.
2.3.2. OLI Flowsheet Chemistry Assessment
OLI Flowsheet is used to calculate the necessary chemistry and physical parameters for determining the ultimate brine composition from the leachate and produced water treatment systems. Parameters from WaterTAP are fed into these treatment techniques (such as the optimized compression ratio); in turn, parameters from OLI Flowsheet (such as solids removal) are used in subsequent WaterTAP iterations. The full flowsheets and parameters for OLI are shown in Supplementary Section S3.
2.3.3. CM Cost Calculations
The brine composition is analyzed for the concentration of each CM available in the USGS data [2,20]. The mass (in kg/year) is multiplied by the value of each mineral (converted to USD/kg) to calculate the overall value in USD/year from each brine.
3. Results and Discussions
3.1. OLI Studio Reconciliations
The reconciliations used in the subsequent sections are generated using OLI Studio. The input data are first converted to the appropriate anions and cations (shown in detail in Supplementary Section S1).
For landfill leachate, OLI Studio reconciliation adds 64.4 mg/L of sulfate to the leachate, increasing the total sulfate from 1600 to 1664.4 mg/L. The reconciled TDS returns only 2723 mg/L (as compared to the median TDS of 4335 mg/L). This TDS is between the 20th percentile (2186 mg/L) and the 30th percentile (3181 mg/L); however, the resultant electrical conductivity is 3353.4 μmho/cm, between the 30th percentile (3080 μmho/cm) and the 40th percentile (3540 μmho/cm). TDS is typically measured as the sum of dissolved solids (regardless of whether a sample is complete or not) and may use proxy calculations (such as chloride or conductivity); thus, it is believed that the electrical conductivity may ultimately provide a more consistent result. The reconciliation predicts that calcium, barium, and aluminum will precipitate from solution (as CaCO3, BaSO4, and Al(OH)3).
For impoundment leachate, OLI Studio reconciliation adds 192.9 mg/L of sulfate, increasing this value from 109.7 mg/L to 302.6 mg/L. This is still well within the range of impoundment sulfate values, below the 70th percentile (422.9 mg/L). The reconciled TDS is 485.7 mg/L, less than half of the median TDS value (1010 mg/L) and between the 10th percentile (369.5 mg/L) and the 20th percentile (511 mg/L). However, the resultant electrical conductivity is 762.6 μmho/cm, between the median (742.5 μmho/cm) and the 60th percentile (763.8 μmho/cm). The reconciliation predicts that barium, phosphate, and aluminum will precipitate out of solution (as BaSO4, Ca5F(PO4)3 and Al(OH)3).
For produced water, the reconciliation adds 2245 mg/L of sodium to balance the charge, increasing the sodium from 31,275 mg/L to 33,519 mg/L. This is still below the 60th percentile value (39,360 mg/L). The reconciled TDS is 99,304 mg/L, still very close to the median (101,336 mg/L). The reconciliation predicts that calcium, iron, and barium will precipitate from solutions (as CaCO3, FeCO3, and BaSO4).
3.2. Performance Results
The following subsections describe the performance estimates for the membrane treatment system for leachate and the MVC system for produced water. These results indicate the necessary sizing parameters used to determine the overall cost of the system.
3.2.1. Leachate Results
The inlet characteristics and preset parameters for the WaterTAP simulations are shown in Supplementary Section S2. Table S19. The OLI Flowsheet results are shown in Table 3, and the WaterTAP results are shown in Table 4. Note that a settler is placed between the BWRO and SWRO membranes in the WaterTAP simulation to better match the inlet compositions to the SWRO membrane.

Table 3.
OLI Flowsheet results.

Table 4.
WaterTAP results.
The impoundment leachate results are closer between the two simulations given the lower overall TDS levels and the lack of solids generated between the BWRO and SWRO membranes in that simulation. The higher TDS levels in the landfill leachate simulation create greater differences in the permeate; this difference is even greater without the settler in the WaterTAP simulation. Note that OLI Flowsheet does not set the A and B coefficients but rather sets the salt rejections (Section 2.1.3). These A and B coefficients are instead calculated after the simulation based on the rejections and shown in Supplementary Section S2. Table S20. The WaterTAP performance results are used for developing the cost of the process and the brine composition calculated through OLI Flowsheet are used for calculation of the potential recovery value.
3.2.2. Produced Water Results
The input values for the produced water simulation are shown in Supplementary Section S2. Table S20. Note that the brine outlet temperature and the compressor ratio are permitted to vary from these initial set points during cost optimization (see Section 2.2.2).
The OLI Flowsheet and WaterTAP results after cost optimization are shown in Table 5—initial results are shown in Supplementary Section S2. Table S22. Note that not all parameters are calculated directly; the OLI Flowsheet model does not use the area or heat transfer coefficient (U) directly but does calculate the U ×area value. Additionally, there is no “evaporator” in the OLI Flowsheet model—this area is only calculated in WaterTAP.

Table 5.
OLI Flowsheet and WaterTAP outlet parameters after cost optimization.
The cost optimization raises the compression ratio to 1.67 and the hot brine temperature to 348.15 K (from 1.4 and 345.15 K, respectively). Because of the high cost of the evaporator, it is more beneficial to have a smaller evaporator area and to raise the work performed by the compressor. This is discussed further in Section 3.3.2.
An exergy analysis was conducted using the flowsheet developed in OLI (Supplementary Section S6) found that the overall exergy efficiency was 10% with 53.3% of exergy destruction attributable to MVC.
3.3. Cost Results
Using the aforementioned PrOMMiS costing method (Section 2.2.2), the following results are presented for both leachate treatment and produced water treatment.
3.3.1. Leachate Treatment Cost Results
Using the base case values, the outlet cost parameters for the performance metrics articulated in Section 3.2.1 are presented in Table 6.

Table 6.
Cost parameters for landfill and impoundment leachate. Italics represent sub-categories of the total fixed and variable operating costs.
The cost in both cases is dominated by the fixed operating cost, the majority of which is the operating labor. This value is more than double the total as-spent capital cost for landfill leachate, and for a larger system it is still dominant (around 59 percent of the total as-spent cost for impoundment leachate). Liquid waste disposal also scales in a linear fashion with flow rate. Solid waste disposal for this system is negligible, as few solids are created in this step of treatment.
Sensitivity analyses for landfill leachate are shown in Table 7 and Figure 3. The impact of flow rate and operating labor are by far the largest on LCOW. As described in Supplementary Section S2.1, the system flow rates for this case study were determined using earlier work on national landfill and impoundment leachate volumes [8]. This study was a national aggregation, and flow rates may vary subject to local conditions. Operating labor is also subject to change dependent on the siting location and system automation. Therefore, we expect these parameters could vary significantly depending on the siting location. Using the full range of variables, the expanded LCOW range would be USD 3.00–USD 24.73 (USD 2023/m3)—this would use the minimum or maximum cost impacts from all variables (rather than single variable analyses)

Table 7.
Landfill leachate sensitivity analyses.
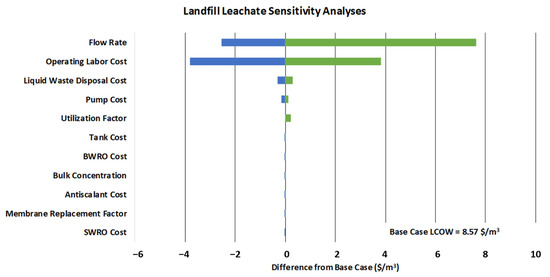
Figure 3.
Chart of sensitivity results for landfill leachate. Green represents parameter increases and blue represents parameter decreases.
For impoundment leachate, the sensitivity analyses are shown in Table 8 and Figure 4. As before, flow rate and operating labor costs are dominant; however, liquid waste disposal cost becomes a larger cost category for a larger system. Using the full range of variables, the expanded LCOW range would be USD 1.10–USD 7.63 (USD 2023/m3)—this would use the minimum or maximum cost impacts from all variables (rather than single variable analyses).

Table 8.
Impoundment leachate sensitivity analyses.
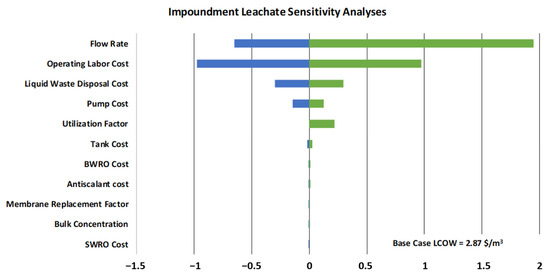
Figure 4.
Chart of sensitivity results for impoundment leachate. Green represents parameter increases and blue represents parameter decreases.
Based on these results, it is suggested to maximize flow rate when possible (so long as operating labor is not impacted) and to minimize the cost of waste disposal as flow rate scales. This may promote a zero-liquid discharge system if waste disposal costs are too high.
3.3.2. Produced Water Cost Results
The cost results for the produced water simulations are shown in Table 9. The capital cost is more significant for the MVC system than for leachate treatment. In addition, there is a greater volume of liquid brine that requires disposal; liquid waste is far larger than the required operating labor in this case.

Table 9.
Cost parameters for produced water. Italics represent sub-categories of the total fixed and variable operating costs.
The sensitivity analyses for produced water are shown in Table 10 and Figure 5. The liquid waste disposal cost dominates; however, there are multiple large-cost categories, such as flow rate, labor, bulk concentration parameters, and evaporator parameters that have a significant impact on the LCOW as well. Note here that the bulk concentration is only increased to the 60th percentile value from the USGS data. Beyond this, 50 percent recovery by mass does not converge in the WaterTAP simulation due to solubility constraints. Using the full range of values, the LCOW varies from USD 8.60 to USD 43.60 (USD 2023/m3)—as with leachate, this would use the minimum or maximum cost impacts rather than the single variable analyses shown below.

Table 10.
Produced water sensitivity analyses.
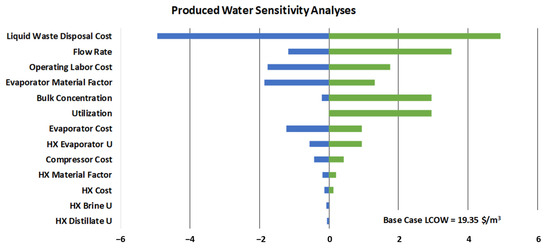
Figure 5.
Chart of sensitivity analyses for produced water. Green represents parameter increases and blue represents parameter decreases.
Additional analyses are recommended to determine how the treatment cost varies by technologies by developing assessments for alternative treatment train configurations (e.g., additional thermal technologies, membrane technologies, ion exchange, electrodialysis).
3.4. Brine Recovery Values
The value of the different brines (in terms of USD/m3 permeate or distillate) is shown in Figure 6. A summary comparing the value of the brines and the levelized cost is presented in Table 11. The landfill and impoundment leachate medians are USD 0.39/m3 permeate and USD 0.17/m3 permeate, respectively, far below the respective LCOW values for the treatment systems (USD 8.57/m3 permeate and USD 2.87/m3 permeate); thus, the cost of treatment is not likely to be offset by the value of critical minerals in these brines. The value of these brines is comprised primarily of lithium and magnesium—the sensitivity analyses range from USD 0.17 to USD 1.13/m3 permeate for landfill leachate and from USD 0.11 to USD 0.22/m3 for impoundment leachate. The CoDaRT median values for lithium and magnesium do not vary landfill leachate brine values considerably—USD 0.50/m3 permeate for Li and USD 0.39/m3 permeate for Mg. The full breakdown of the brine recovery values is featured in Supplementary Section S5.
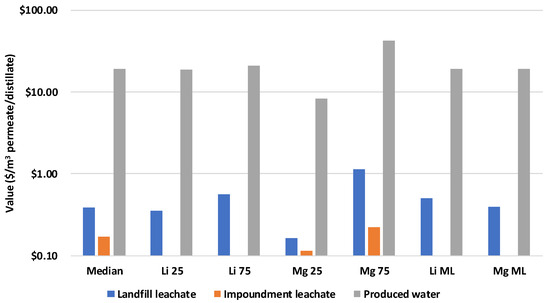
Figure 6.
Chart of brine values for different feedstocks and sensitivity analyses.

Table 11.
Comparison of the contained critical mineral value and levelized cost for each case study.
For produced water, the value of the brine for the median case is USD 19.18/m3 distillate, which is comparable to the LCOW of treatment (USD 19.40/m3 distillate). This could warrant further investigation into treatment for the sake of mineral recovery. There is significant regional variation in the composition of produced water in the Permian Basin. The value of this brine is primarily lithium and magnesium, so in order to capture the impact of some of this variation, the 25th–75th percentile values from the NEWTS USGS produced water database are used to estimate a range of values from USD 8.28 to USD 42.14/m3 distillate. The CoDaRT median values do not have a significant impact on the brine’s value—USD 19.10/m3 distillate for Li and USD 19.37/m3 distillate for Mg. As with leachate, the full breakdown of the brine recovery values is featured in Supplementary Section S5.
It is critical to define technology performance to separate Li and Mg from this concentrated brine to meet purity requirements of raw material inputs for batteries and other critical energy technologies. There are many factors that may influence the efficacy of achieving these purity levels for produced water or leachate desalination brines that have not been quantified. Future work is recommended to evaluate these factors using detailed process chemistry simulation and experimental validation.
Magnesium hydroxide (Mg(OH)2) is typically produced from brines by precipitation with an alkaline reagent. The most common reagents used to precipitate Mg are NaOH, quicklime, or ammonia [31]. In prior studies using retentate from seawater nanofiltration, Mg(OH)2 that was precipitated using NaOH has a product purity of at least 90 percent [32]. Studies examining Mg(OH)2 precipitation from sea water reverse osmosis brine using lime as a reagent found that direct lime addition yielded low product purity, but if the lime slurry was pre-screened by a 100 um screen before addition to the brine, the process yields a final product purity of 91 percent [31]. These results are summarized in Table 12 to establish a path forward for Mg recovery from produced water and leachate treatment brines.

Table 12.
Mg recovery methods.
Precipitation of Mg(OH)2 is also often included as an impurity removal stage in Li recovery. In addition to producing saleable, high-purity Mg(OH)2, precipitation of Mg will aid downstream Li recovery.
Conventional Li processing from brine typically involves an evaporation step, Mg removal using lime followed by filtration and washing, calcium removal followed by filtration, precipitation of lithium carbonate (Li2CO3) using sodium carbonate, and filtration and washing to achieve a final Li2CO3 product [33]. Many alternative technologies have been applied to Li recovery that do not require the evaporation step, including ion exchange resins (i.e., sorbents), solvent or liquid–liquid extraction, membrane processes, electrochemical processes, selective precipitation using phosphate, and thermal-assisted methods [34,35]. Numerous literature reviews have successfully characterized these technology developments in Li separations. This study summarizes the processes that reported ending purity for Li2CO3 recovery from various synthetic and real-world brines in Table 13.

Table 13.
Li recovery methods.
4. Conclusions
The performance and cost of treatment that facilitate recovery were evaluated for produced water and combustion residual leachate. Leachate LCOW ranges from USD 1.90 to USD 16.20 (USD 2023/m3 permeate) and produced water LCOW ranges from USD 14.40 to USD 24.30 (USD 2023/m3 distillate). The potential value of the brine is also articulated—for leachate, this ranges from USD 0.11 to USD 1.13 (USD 2023/m3 permeate), and for produced water, this ranges from USD 8.28 to USD 42.10 (USD 2023/m3 distillate). Based on these values, it is unlikely that treatment would be cost effective for leachate as a precursor to mineral recovery alone; however, if treatment is required it would concentrate minerals of interest to facilitate recovery [2]. For produced water, however, the value of the brine may be sufficient to motivate recovery of Mg in the Permian Basin. Future studies should consider other basins that may have higher values of other CMs. Furthermore, Li is commonly cited by government and industry due to its prevalence in batteries [37], and future increases in demand could raise prices and improve the economics.
The lithium and magnesium recovery methods that could be employed are also articulated in this work. Purities of 91% and >99.9% have been achieved for magnesium and lithium. If these technologies are used in conjunction with treatment, a large volume of leachate or produced water can be both remediated and reused while generating critical minerals. In addition to the environmental benefit from removing pollutants from an industrial waste source, recovery of critical minerals from produced water and leachate would preclude the land use change and waste generation associated with greenfield mining. Future work of this type should consider these technologies for produced water on a national level to assess the potential for this water to assist in achieving national energy security.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min15030213/s1, Supplementary Section S1: Compositions for Leachate and Produced Water Simulations [2,21], Supplementary Section S2: Detailed Design Parameters and Costing Data [3,8,13,17,22,38,39,40,41,42,43,44,45,46,47,48,49,50,51], Supplementary Section S3: PrOMMiS Costing Method and Changes [17,24,25,26,28,29,46,52], Supplementary Section S4: OLI Flowsheets and Parameters, Supplementary Section S5: Critical Mineral Recovery Values, Supplementary Section S6: Exergy Analysis, Supplementary Data.
Author Contributions
C.A.: conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft; T.S.: data curation, writing—original draft; N.S.: conceptualization, funding acquisition, writing—review and editing, formal analysis; A.F.: conceptualization, funding acquisition, writing—review and editing, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the U.S. Department of Energy’s Office Fossil Energy and Carbon Management (FECM) through National Energy Technology Laboratory’s ongoing research under the Water Management for Power System Field Work Proposal, DE-FECM1611080, and the Critical Minerals Field Work Proposal, DE-FECM1022420.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge the review efforts of teammates Adam Atia, Susan Carr and Troy Teel. The authors would also like to thank EPRI for providing detailed constituent breakdown information and the model for their combustion residual leachate volume calculation.
Conflicts of Interest
The authors declare no conflicts of interest.
Disclaimer
This project was funded by the United States Department of Energy, National Energy Technology Laboratory, in part, through a site support contract. Neither the United States Government nor any agency thereof, nor any of their employees, nor the support contractor, nor any of their employees, make any warranty, express or implied, or assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represent that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
References
- U.S. Geological Survey. 2022 Final List of Critical Minerals; U.S. Geological Survey: Washington, DC, USA, 2022.
- Able, C.; Fritz, A.; Grol, E. Assessment of combustion residual leachate: Local treatment needs and critical mineral recovery. Resour. Conserv. Recycl. 2024, 205, 107535. [Google Scholar]
- Sener, S.E.C.; Thomas, V.M.; Hogan, D.E.; Maier, R.M.; Carbajales-Dale, M.; Barton, M.D.; Karanfil, T.; Crittenden, J.C.; Amy, G.L. Recovery of Critical Metals from Aqueous Sources. ACS Sustain. Chem. Eng. 2021, 9, 11616–11634. [Google Scholar] [CrossRef]
- Smith, K.H.; Mackey, J.E.; Wenzlick, M.; Thomas, B.; Siefert, N.S. Critical mineral source potential from oil & gas produced waters in the United States. Sci. Total Environ. 2024, 929, 172573. [Google Scholar] [PubMed]
- Gaustad, G.; Williams, E.; Leader, A. Rare earth metals from secondary sources: Review of potential supply from waste and byproducts. Resour. Conserv. Recycl. 2021, 167, 105213. [Google Scholar]
- Mackey, J.; Bain, D.J.; Lackey, G.; Gardiner, J.; Gulliver, D.; Kutchko, B. Estimates of lithium mass yields from produced water sourced from the Devonian-aged Marcellus Shale. Sci. Rep. 2024, 14, 8813. [Google Scholar]
- Texas Commission on Environmental Quality, Waste Permits Division. Topic: Coal Combustion Residuals (CCR) Surface Impoundment; Texas Commission on Environmental Quality: Austin, TX, USA, 2020.
- Able, C.; Rellergert, D.; Mazzoni, V.; Grol, E. Assessment of combustion residual leachate volume, composition, and treatment costs. J. Hazard. Mater. 2023, 457, 131731. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, S.; Ou, J.; Kouadio, K.L.; Xiong, B. Multifaceted anomaly detection framework for leachate monitoring in landfills. J. Environ. Manag. 2024, 368, 122130. [Google Scholar] [CrossRef]
- Wang, M.; Kang, J.; Liu, W.; Li, M.; Su, J.; Fang, Z.; Li, X.; Shang, L.; Zhang, F.; Guo, C. Design and study of mine silo drainage method based on fuzzy control and Avoiding Peak Filling Valley strategy. Sci. Rep. 2024, 14, 9300. [Google Scholar] [CrossRef]
- Veil, J.A.; Puder, M.G.; Elcock, D.; Redweik, R.J. A White Paper Describing Produced Water from Produce of Crude Oil, Natural Gas and Coal Bed Methane; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2004.
- ALL Consulting. U.S. Produced Water Volumes and Management Practices in 2021; Ground Water Protection Council: Oklahoma City, OK, USA, 2022. [Google Scholar]
- Romeo, L.; Siefert, N.; Wenzlick, M. NEWTS Well Summary by Hydrologic Regions and Subbasins in the U.S.; 2023. Available online: https://www.osti.gov/biblio/1986291 (accessed on 1 March 2024).
- Cooper, C.M.; McCall, J.; Stokes, S.C.; McKay, C.; Bentley, M.J.; Rosenblum, J.S.; Blewett, T.A.; Huang, Z.; Miara, A.; Talmadge, M.; et al. Oil and Gas Produced Water Reuse: Opportunities, Treatment Needs, and Challenges. ES&T Eng. 2022, 2, 347–366. [Google Scholar]
- Miranda, M.A.; Ghosh, A.; Mahmodi, G.; Xie, S.; Shaw, M.; Kim, S.; Krzmarzick, M.J.; Lampert, D.J.; Aichele, C.P. Treatment and Recovery of High-Value Elements from Produced Water. Water 2022, 14, 880. [Google Scholar] [CrossRef]
- Sanchez-Rosario, R.; Hildenbrand, Z. Produced Water Treatment and Valorization: A Techno-Economical Review. Energies 2022, 15, 4619. [Google Scholar] [CrossRef]
- WaterTAP: An Open-Source Water Treatment Model Library. National Alliance for Water Innovation, USDOE. Available online: https://github.com/watertap-org/watertap (accessed on 1 March 2024).
- Blondes, M.S.; Gans, K.D.; Engle, M.A.; Kharaka, Y.K.; Reidy, M.E.; Saraswathula, V.; Thordsen, J.J.; Rowan, E.L.; Morrissey, E.A. U.S. Geological Survey National Produced Waters Geochemical Database v2.3; United States Geological Survey: Reston, VA, USA, 22 May 2019. [CrossRef]
- Wenzlick, M.; Siefert, N.; Belarbi, Z. NEWTS USGS Produced Waters Database; National Energy Technology Laboratory EDX: Morgantown, WV, USA, 30 September 2022. [CrossRef]
- U.S. Geological Survey. Commodity Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/commodity-statistics-and-information (accessed on 1 March 2024).
- Consituent Data Replacement Tool—EDX. Available online: https://edx.netl.doe.gov/dataset/codart (accessed on 1 August 2024).
- Thiel, G.P.; Tow, E.W.; Banchik, L.D.; Chung, H.W.; Lienhard, J.H. Energy consumption in desalinating produced water from shale oil and gas extraction. Desalination 2015, 366, 94–112. [Google Scholar] [CrossRef]
- Wenzlick, M.; Siefert, N. Techno-economic analysis of converting oil & gas produced water into valuable resources. Desalination 2020, 481, 114381. [Google Scholar] [CrossRef]
- Process Optimization and Modeling for Minerals Sustainability. Available online: https://github.com/prommis/prommis/tree/main (accessed on 1 March 2024).
- U.S. Bureau of Labor Statistics. Occupational Outlook Handbook: Water and Wastewater Treatment Plant and System Operators. Bureau of Labor Statistics, 6 September 2023. Available online: https://www.bls.gov/ooh/production/water-and-wastewater-treatment-plant-and-system-operators.htm (accessed on 15 March 2024).
- Federal Reserve Bank of St. Louis. Employment Cost Index: Wages and Salaries: Private Industry Workers (ECIWAG). Available online: https://fred.stlouisfed.org/series/ECIWAG (accessed on 21 May 2024).
- U.S. Energy Information Administration. Electric Power Monthly—December 2023. U.S. Energy Information Administration, December 2023. Available online: https://www.eia.gov/electricity/monthly/archive/december2023.pdf (accessed on 1 March 2024).
- Theis, J. Quality Guidelines for Energy System Studies: Cost Estimation Methodology for NETL Assessments of Power Plant Performance; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2019.
- Fritz, A.G.; Able, C.; Mauter, M.S.; Grol, E. Aqueous Bromide Discharges from U.S. Coal-Fired Power Plants: Points of Origin, Concentration Ranges, and Effluent Treatment Costs. Energy Fuels 2023, 37, 3854–3864. [Google Scholar] [CrossRef]
- Bartholomew, T.V.; Mauter, M.S. Multiobjective Optimization Model for Minimizing Cost and Environmental Impact in Shale Gas Water and Wastewater Management. ACS Sustain. Chem. Eng. 2016, 4, 3728–3735. [Google Scholar] [CrossRef]
- Gong, M.H.; Johns, M.; Fridjonsson, E. Magnesium Recovery from Desalination Brine. Available online: https://ceed.wa.edu.au/wp-content/uploads/2018/09/9.Gong_.WaterCorp.MagnesiumRecovery.pdf (accessed on 1 March 2024).
- Morgante, C.; Vassallo, F.; Xevgenos, D.; Cipollina, A.; Micari, M.; Tamburini, A.; Micale, G. Valorisation of SWRO brines in a remote island through a circular approach: Techno-economic analysis and perspectives. Desalination 2022, 542, 116005. [Google Scholar] [CrossRef]
- Stringfellow, W.; Dobson, P. Technology for the Recovery of Lithium from Geothermal Brines. Energies 2021, 14, 6805. [Google Scholar] [CrossRef]
- Iyer, R.; Kelly, J. Lithium Production from North American Brines; Argonne National Laboratory: Lemont, IL, USA, 2022.
- Vera, M.; Torres, W.; Galli, C.; Chagnes, A.; Flexer, V. Environmental impact of direct lithium extraction from brines. Nat. Rev. Earth Environ. 2023, 4, 149–165. [Google Scholar] [CrossRef]
- Yu, H.; Naidu, G.; Zhang, C.; Wang, C.; Razmjou, A.; Han, D.S.; He, T.; Shon, H. Metal-based adsorbents for lithium recovery from aqueous resources. Desalination 2022, 539, 115951. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, P.; Tu, W.; Sun, H.; Li, S.; Zhang, Y. Lithium recovery from oil and gas produced water: Opportunities, challenges, and future outlook. J. Water Process Eng. 2023, 55, 104148. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; de la Nuez Pestana, I. Feed Spacer Geometries and Permeability Coefficients. Effect on the Performance in BWRO Spiral-Wound Membrane Modules. Water 2019, 11, 152. [Google Scholar] [CrossRef]
- Ettori, A.; Gaudichet-Maurin, E.; Aimar, P.; Causserand, C. Mass transfer properties of chlorinated aromatic polyamide reverse osmosis membranes. Sep. Purif. Technol. 2012, 101, 60–67. [Google Scholar] [CrossRef]
- Evoqua. FilmTec™ BW30XFRLE-400/34i Brackish RO Membrane (Dry). Available online: https://www.evoqua.com/en/evoqua/parts--consumables/membranes/low-fouling-membranes/filmtec-bw30xfrle-40034i-brackish-ro-membrane/ (accessed on 1 March 2024).
- Big Brand Water. Dow Filmtec BW30PRO-400/34. Available online: https://www.bigbrandwater.com/dowfilmtecbw30pro-400-34.html (accessed on 1 March 2024).
- Meratizaman, M.; Godarzi, A.A. Techno-economic feasibility of a solar-powered reverse osmosis desalination system integrated with lithium battery energy storage. Desalination Water Treat. 2019, 167, 57–74. [Google Scholar]
- Wu, Z.; Zhai, H.; Grol, E.J.; Able, C.M.; Siefert, N.S. Treatment of brackish water for fossil power plant cooling. Nat. Water 2023, 1, 471–483. [Google Scholar] [CrossRef]
- Evoqua. FilmTec™ SW30HRLE-400 Seawater RO Membrane (Wet). Available online: https://www.evoqua.com/en/evoqua/parts--consumables/membranes/seawater-membranes/8-seawater-membranes/filmtec-sw30hrle-37034-seawater-ro-membrane/ (accessed on 1 March 2024).
- Big Brand Water. Dow Filmtec SW30HRLE-400-W. Available online: https://www.bigbrandwater.com/sw30hrle-400-w.html (accessed on 1 March 2024).
- Voutchkov, N. Desalination Engineering: Planning and Design; McGraw Hill: New York, NY, USA, 2013. [Google Scholar]
- Franks, J. Typical Cost of a Lamella Plate Settler. Jim Myers & Sons, Inc. Available online: https://jmsequipment.com/typical-cost-of-a-lamella-plate-settler/ (accessed on 1 March 2024).
- Chen, L.; Xu, Q.; Gossage, J.L.; Lou, H.H. Simulation and economic evaluation of a coupled thermal vapor compression desalination process for produced water management. J. Nat. Gas Sci. Eng. 2016, 36, 442–453. [Google Scholar] [CrossRef]
- Aly, N.H.; El-Figi, A.K. Mechanical vapor compression desalination systems—A case study. Desalination 2003, 158, 143–150. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis, and Design of Chemical Processes, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2008. [Google Scholar]
- El-Sayed, Y. Designing desalination systems for higher productivity. Desalination 2001, 134, 129–158. [Google Scholar] [CrossRef]
- WaterTAP. WaterTAP Documentation. WaterTAP, [Online]. Available online: https://watertap.readthedocs.io/en/stable/index.html (accessed on 1 March 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).