Mineral Composition and Elemental Oxide Changes in Heat-Affected Soils and the Implications on Heavy Metal Immobilization by Sewage Sludge
Abstract
1. Introduction
2. Research Methods
2.1. Description of Study Area
2.2. Sewage Sludge and Soil Sample Collection and Pre-Preparation
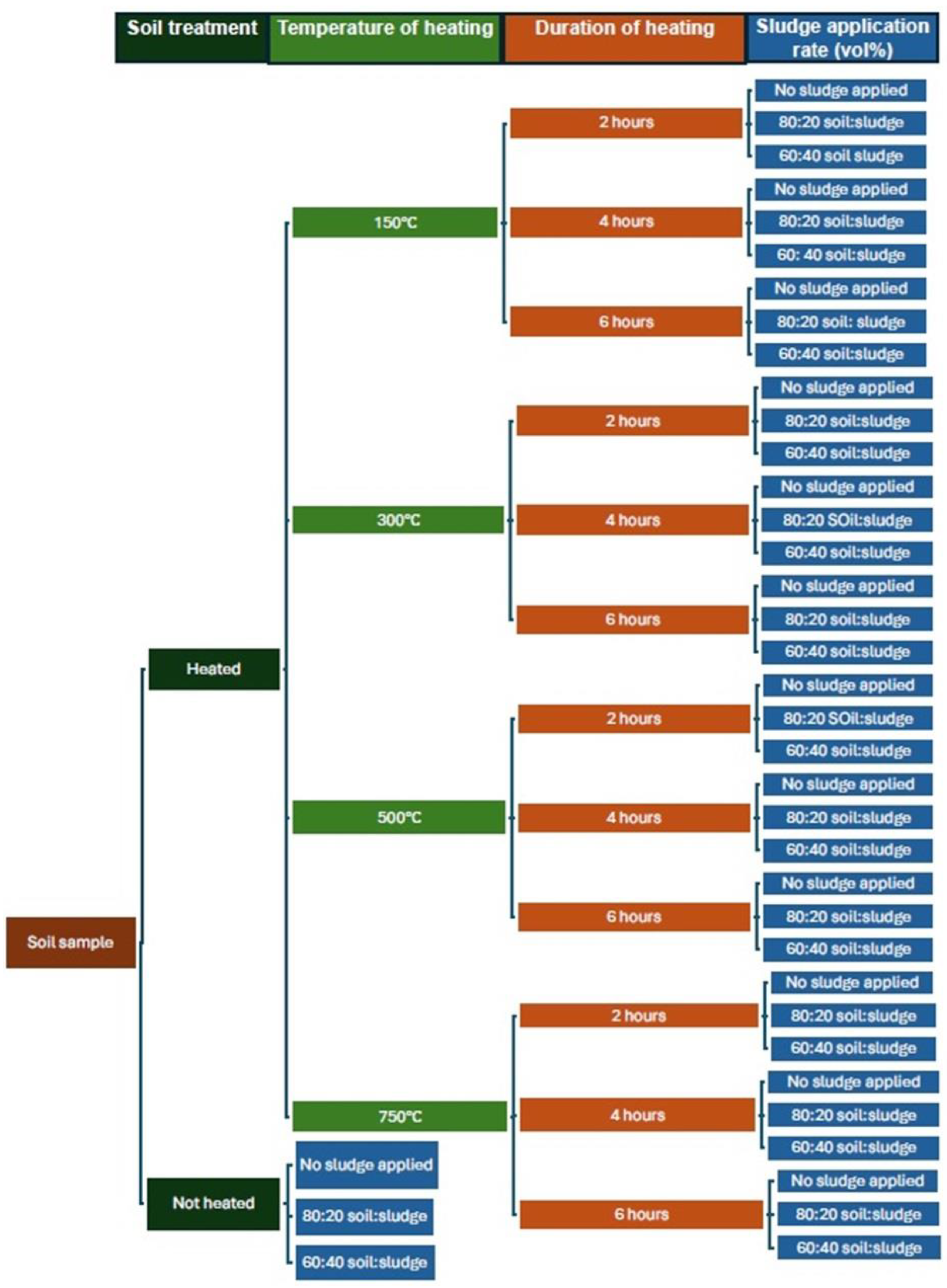
2.3. Experimental Set-Up
2.4. Characterization of Soil Samples
2.4.1. Physicochemical, Mineralogical, and Trace and Major Element Oxide Determination
2.4.2. Determination of PTE Concentrations in Different Soil Geochemical Fractions
2.4.3. Determination of the Degree of Chemical Alteration in Soil Samples
2.4.4. Determination of PTE Mobility in Sludge Amended Heated Soils
3. Results and Discussion
3.1. Physicochemical Properties of the Soil Samples
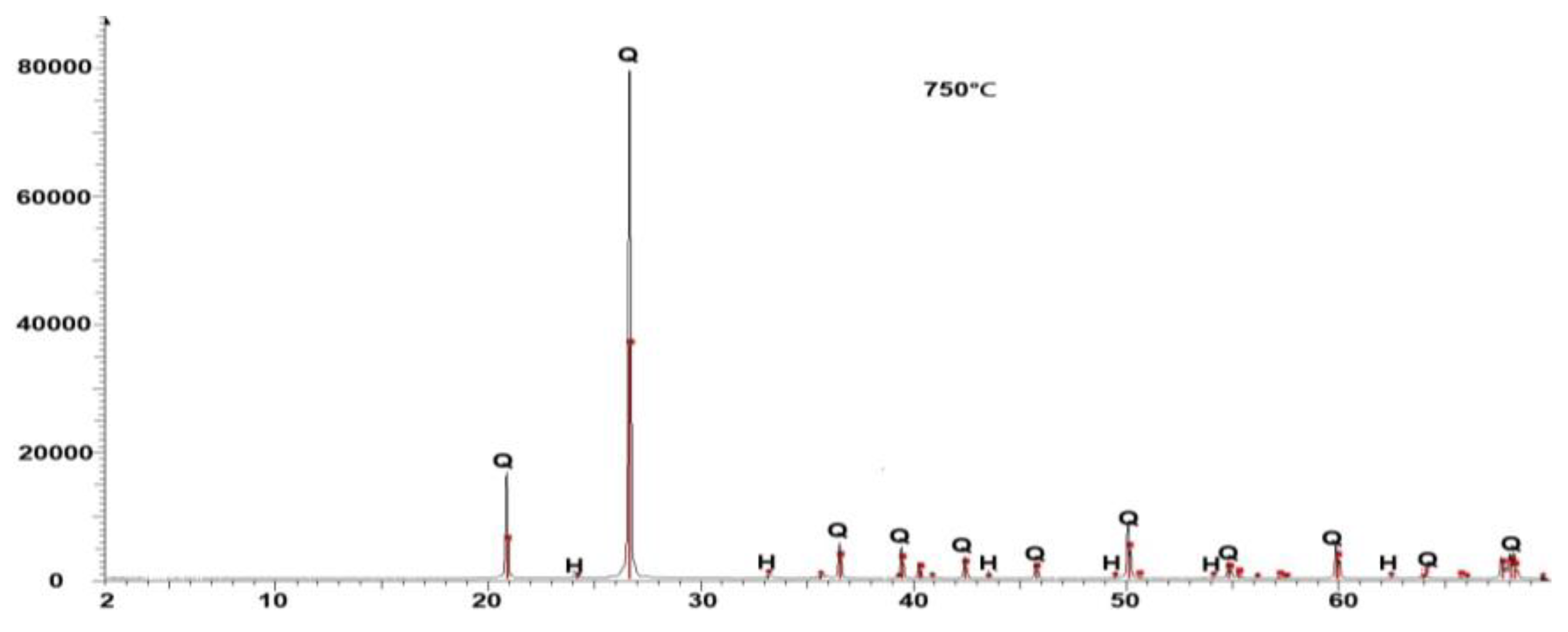
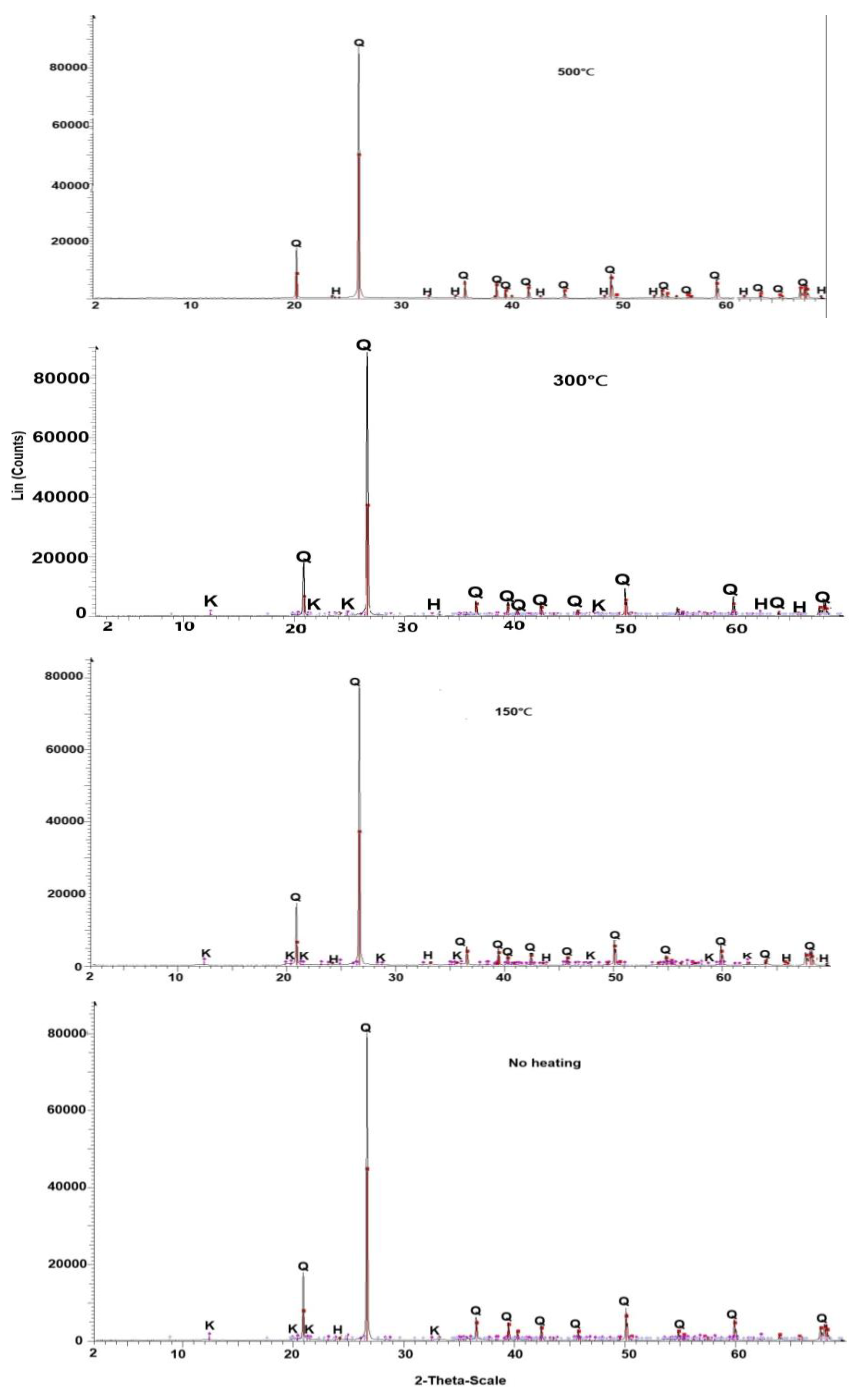
3.2. Effect of Soil Temperature Increase on Soil Mineralogy
3.3. Effect of Soil Temperature Increase on Major Element Oxides
3.4. Effect of Increased Soil Temperature on the Degree of Weathering of the Soils
3.5. Concentrations of PTEs in the Soil Samples
3.6. Segregation of PTEs into Different Geochemical Fractions in the Soils
3.7. Effect of Soil Temperature Increase on PTE Concentrations in Soils
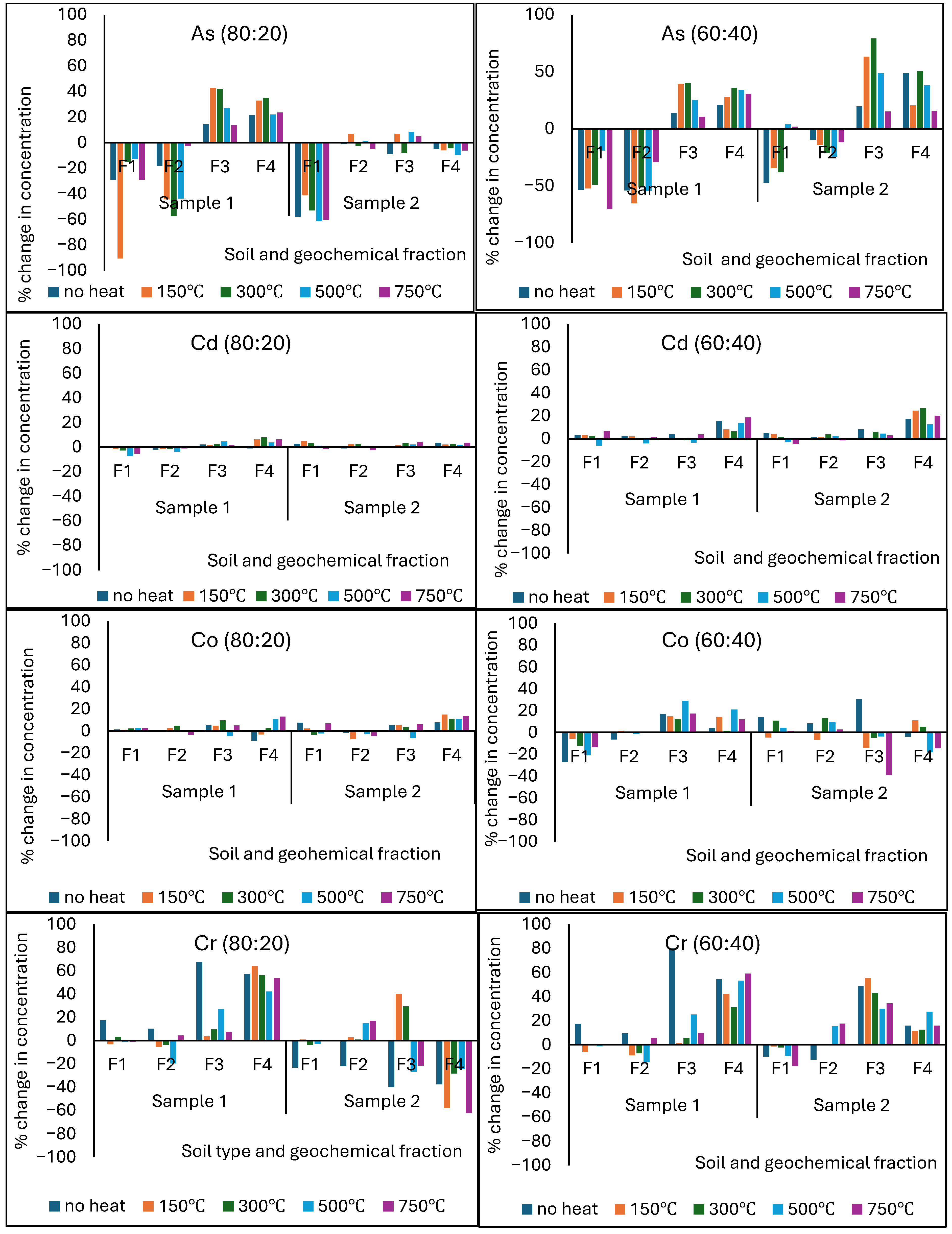
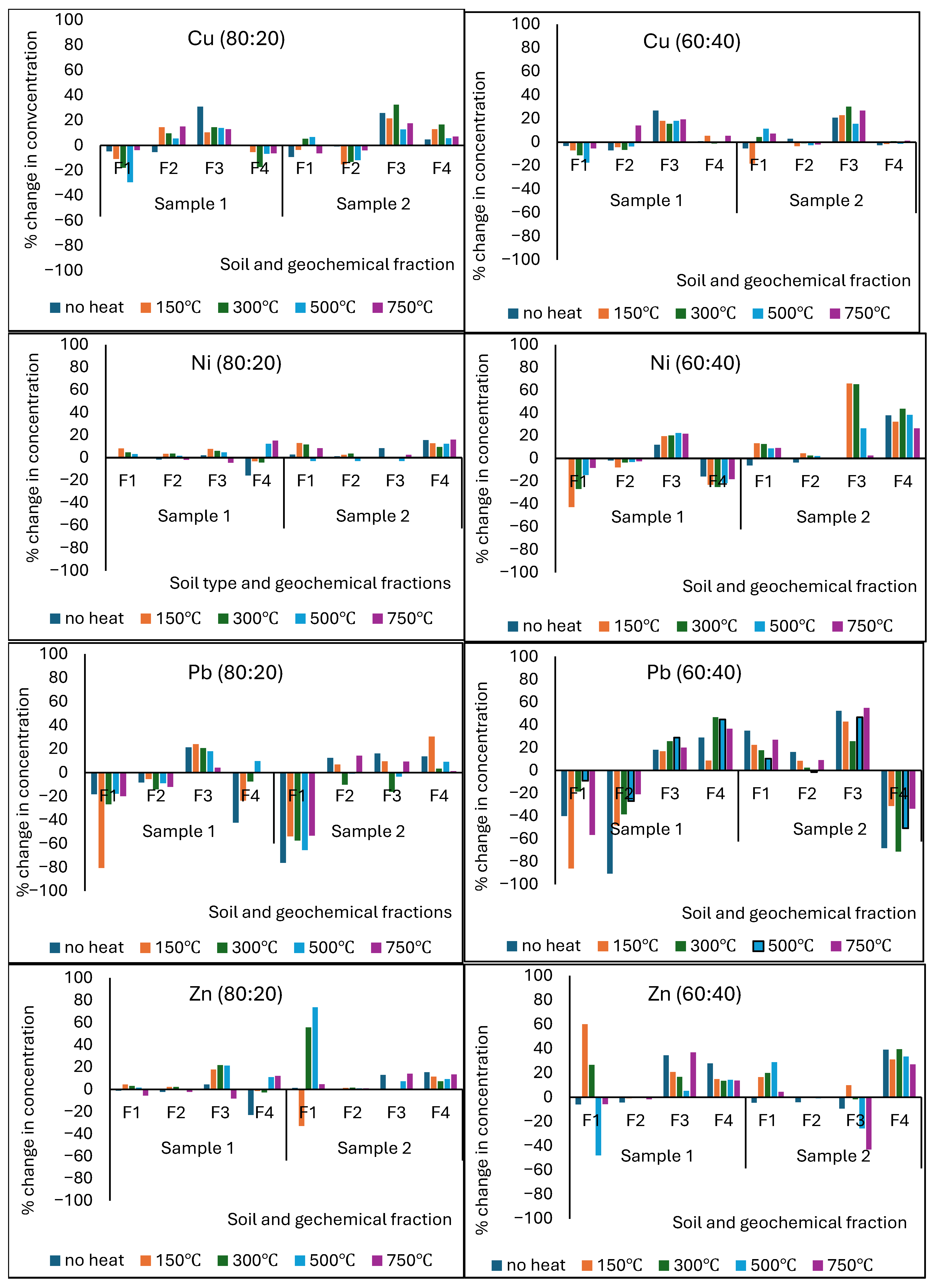
3.8. Effect of Sludge Addition on PTE Redistribution into Different Geochemical Fractions of Heat-Affected Soils
3.9. Effect of Temperature-Induced Changes in Soil Mineralogy and Elemental Oxides on PTE Segregation into Different Geochemical Fractions
3.10. Effect of Soil Temperature Increase and Sludge Application on PTE Mobility in the Soils
4. Implications on Fire-Affected Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardison, J.R. Fire and flame for plant disease control. Annu. Rev. Phytopathol. 1976, 14, 355–379. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613–614, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Cruz, G.M.; Gould, S.J.; Hollis, J.J.; McCaw, L.W. A hierarchical classification of wildland fire fuels for australian vegetation types. Fire 2018, 1, 13. [Google Scholar] [CrossRef]
- Rein, G.; Garcia, J.; Simeoni, A.; Tihay, V.; Ferrat, L. Smouldering natural fires: Comparison of burning dynamics in boreal peat and mediterranean humus. In International Review of Chemical Engineering, 1st ed.; WIT Press: Cambridge, MA, USA, 2008; pp. 183–192. [Google Scholar]
- Rein, G. Smouldering combustion phenomena in science and technology. Int. Rev. Chem. Eng. 2009, 1, 3–18. [Google Scholar]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Ngole-Jeme, V.M. Fire-induced changes in soil and implications on soil sorption capacity and remediation methods. Appl. Sci. 2019, 9, 3447. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Y.; Chen, X.; Sha, A.; Xiong, Z.; Luo, Y.; Peng, L.; Zou, L.; Zhao, C.; Li, Q. The easily overlooked effect of global warming: Diffusion of heavy metals. Toxics 2024, 12, 400. [Google Scholar] [CrossRef]
- Ge, L.-Q.; Cang, L.; Liu, H.; Zhou, D.-M. Effects of warming on uptake and translocation of cadmium (Cd) and copper (Cu) in a contaminated soil-rice system under free air temperature increase (FATI). Chemosphere 2016, 155, 1–8. [Google Scholar] [CrossRef]
- Sebola, T.C.; Ngole-Jeme, V.M. Temperature induced changes on heavy metal geochemical partitioning and mobility in contaminated and uncontaminated soils. Environ. Pollut. Bioavailab. 2024, 36, 2317747. [Google Scholar] [CrossRef]
- Tan, K.H.; Hajek, B.F.; Barshad, I. Thermal Analysis Techniques; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1986; pp. 151–183. [Google Scholar]
- Zihms, S.G.; Switzer, C.; Karstunen, M.; Tarantino, A. Understanding the effects of high temperature processes on the engineering properties of soils. In Proceedings of the 18th International Conference on Soil Mechanics and Geotechnical Engineering, Paris, France, 2–6 September 2013; pp. 3427–3430. [Google Scholar]
- Yusiharni, E.; Robert, J.G. Soil minerals recover after they are damaged by bushfires. In Proceedings of the 9th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; Volume 1–6, pp. 104–107. [Google Scholar]
- Ketterings, Q.M.; Bigham, J.M.; Laperche, V. Changes in soil mineralogy and texture caused by slash-and-burn fires in sumatra, indonesia. Soil Sci. Soc. Am. J. 2000, 64, 1108–1117. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Richardson, H.M. Phase changes which occur on heating kaolin clays. In The X-Ray Identification and Crystal Structures of Clay Minerals; Minerals Society Publishing: London, UK, 1972; pp. 132–142. [Google Scholar]
- Ulery, A.L.; Graham, R.C. Forest fire effects on soil color and texture. Soil Sci. Soc. Am. J. 1993, 57, 135–140. [Google Scholar] [CrossRef]
- Arocena, J.M.; Opio, C. Prescribed fire-induced changes in properties of sub-boreal forest soils. Geoderma 2003, 113, 1–16. [Google Scholar] [CrossRef]
- Frost, R.L.; Horváth, E.; Makó, É.; Kristóf, J.; Rédey, Á. Slow transformation of mechanically dehydroxylated kaolinite to kaolinite—An aged mechanochemically activated formamide-intercalated kaolinite study. Thermochim. Acta 2003, 408, 103–113. [Google Scholar] [CrossRef]
- Reynard-Callanan, J.R.; Pope, G.A.; Gorring, M.L.; Feng, H. Effects of high-intensity forest fires on soil clay mineralogy. Phys. Geogr. 2010, 31, 407–422. [Google Scholar] [CrossRef]
- Araya, S.N.; Meding, M.; Berhe, A.A. Thermal alteration of soil physico-chemical properties: A systematic study to infer response of sierra nevada climosequence soils to forest fires. Soil 2016, 2, 351–366. [Google Scholar] [CrossRef]
- Heydari, M.; Rostamy, A.; Najafi, F.; Dey, D.C. Effect of fire severity on physical and biochemical soil properties in zagros oak (Quercus brantii Lindl.) forests in Iran. J. For. Res. 2017, 28, 95–104. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 20. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Uddin, S.; Zaman, M.; Martínez-Guijarro, K.; Al-Murad, M.; Behbehani, M.; Habibi, N.; Al-Mutairi, A. Sewage sludge as soil amendment in arid soils—A trace metal, nutrient and trace organics perspective. Emerg. Contam. 2025, 11, 100420. [Google Scholar] [CrossRef]
- Kidd, P.S.; Domínguez-Rodríguez, M.J.; Díez, J.; Monterroso, C. Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere 2007, 66, 1458–1467. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of beta vulgaris plants. Chemosphere 2007, 67, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Ngole, V.M.; Ekosse, G.I.E. Copper, nickel and zinc contamination in soils within the precincts of mining and landfilling environments. Int. J. Environ. Sci. Technol. 2012, 9, 485–494. [Google Scholar] [CrossRef]
- Poggere, G.C.; Melo, V.F.; Serrat, B.M.; Mangrich, A.S.; França, A.A.; Corrêa, R.S.; Barbosa, J.Z. Clay mineralogy affects the efficiency of sewage sludge in reducing lead retention of soils. J. Environ. Sci. 2019, 80, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Mattana, S.; Petrovičová, B.; Landi, L.; Gelsomino, A.; Cortés, P.; Ortiz, O.; Renella, G. Sewage sludge processing determines its impact on soil microbial community structure and function. Appl. Soil Ecol. 2014, 75, 150–161. [Google Scholar] [CrossRef]
- Morotoba, M. Draft Scoping Report for the Proposed Township Establishment, on Ortion 36 of the Farm Varkenslaagte 119 IQ; Gauteng Merafong Local Municipality Gauteng Province: Johannesburg, South Africa, 2014; p. 68. [Google Scholar]
- Robinson, K. Overberg District Municipality Wetland Report | 2017 Local Action for Biodiversity; Overberg District Municipality; Local Action for Biodeversity (LAB): Wetlands, South Africa, 2017; p. 66. [Google Scholar]
- Drooger, S. Soil Temperatures Under a Catchment Scale Experimental Fire. Master’s Thesis, Wageningen University, Wageningen, The Netherlands, 2009; p. 72. [Google Scholar]
- Akhtar, M.; McCallister, D.L.; Eskridge, K.M. Availability and fractionation of phosphorus in sewage sludge-amended soils. Commun. Soil Sci. Plant Anal. 2002, 33, 2057–2068. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis; Technical Paper, 9; International Soil Reference and Information Centre (ISRIC): Wageningen, The Netherlands, 2002; p. 92. [Google Scholar]
- Fitton, G. X-ray fluorescence spectrometry. In Modern Analytical Geochemistry: An Introduction to Quantitative Chemical Analysis Techniques for Earth, Environmental and Material Sciences, 1st ed.; Routledge: London, UK, 1997; Volume 29, pp. 135–153. [Google Scholar]
- Council for Geosciences. Guide to the Services of the CGS Analytical Laboratory 2016. 2011. Available online: https://www.geoscience.org.za/wp-content/uploads/2022/10/CGS-Profile-2022_-08-05-2022_-v1.1.pdf (accessed on 18 March 2023).
- Sungur, A.; Kavdir, Y.; Özcan, H.; İlay, R.; Soylak, M. Geochemical fractions of trace metals in surface and core sections of aggregates in agricultural soils. CATENA 2021, 197, 104995. [Google Scholar] [CrossRef]
- Adams, J.S.; Kraus, M.J.; Wing, S.L. Evaluating the use of weathering indices for determining mean annual precipitation in the ancient stratigraphic record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 309, 358–366. [Google Scholar] [CrossRef]
- Cullers, R.L. The geochemistry of shales, siltstones and sandstones of pennsylvanian–permian age, colorado, USA: Implications for provenance and metamorphic studies. Lithos 2000, 51, 181–203. [Google Scholar] [CrossRef]
- Goldberg, K.; Humayun, M. The applicability of the chemical index of alteration as a paleoclimatic indicator: An example from the permian of the paraná basin, brazil. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 293, 175–183. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M.. Early proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Ngole-Jeme, V.M.; Fantke, P. Ecological and human health risks associated with abandoned gold mine tailings contaminated soil. PLoS ONE 2017, 12, e0172517. [Google Scholar] [CrossRef] [PubMed]
- Schulten, H.R.; Leinweber, P. New insights into organic-mineral particles: Composition, properties and models of molecular structure. Biol. Fertil. Soils 2000, 30, 399–432. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, D.; Zeng, X.; Li, L.; Wang, A.; Liu, F.; Wang, H.; Zeng, Q.; Xiao, Z. Effect of the direct use of biomass in agricultural soil on heavy metals—Activation or immobilization? Environ. Pollut. 2021, 272, 115989. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wu, S.; Sun, L.; Tan, X.; Yu, S.; Zhang, Z. Composition of waste sludge from municipal wastewater treatment plant. Procedia Environ. Sci. 2012, 12, 964–971. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Yang, K.; Miao, G.; Wu, W.; Lin, D.; Pan, B.; Wu, F.; Xing, B. Sorption of cu2+ on humic acids sequentially extracted from a sediment. Chemosphere 2015, 138, 657–663. [Google Scholar] [CrossRef]
- Sloan, J.; Dowdy, R.; Dolan, M.; Linden, D. Long-term effects of biosolids applications on heavy metal bioavailability in agricultural soils. J. Environ. Qual. 1997, 26, 966–974. [Google Scholar] [CrossRef]
- Parkpian, P.; Klankrong, K.; DeLaune, R.; Jugsujinda, A. Metal leachability from sewage sludge-amended thai soils. J. Environ. Sci. Health Part A 2002, 37, 765–791. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Chapter one–mineral–organic associations: Formation, properties, and relevance in soil environments. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 1–140. [Google Scholar]
- Lippold, H.; Evans, N.D.M.; Warwick, P.; Kupsch, H. Competitive effect of iron(iii) on metal complexation by humic substances: Characterisation of ageing processes. Chemosphere 2007, 67, 1050–1056. [Google Scholar] [CrossRef]
- Akbarpour, F.; Gitipour, S.; Baghdadi, M.; Mehrdadi, N. Correlation between chemical fractionation of heavy metals and their toxicity in the contaminated soils. Environ. Earth Sci. 2021, 80, 726. [Google Scholar] [CrossRef]
- Gao, J.; Jansen, B.; Cerli, C.; Helmus, R.; Mikutta, R.; Dultz, S.; Guggenberger, G.; Vogel, C.; Kalbitz, K. Organic matter coatings of soil minerals affect adsorptive interactions with phenolic and amino acids. Eur. J. Soil Sci. 2018, 69, 613–624. [Google Scholar] [CrossRef]
- Stankov Jovanovic, V.P.; Ilic, M.D.; Markovic, M.S.; Mitic, V.D.; Mandic, S.D.N.; Stojanovic, G.S. Wild fire impact on copper, zinc, lead and cadmium distribution in soil and relation with abundance in selected plants of lamiaceae family from vidlic mountain (Serbia). Chemosphere 2011, 84, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.R.; Calvão, A.R.; Aranha, J. Linking wildfire effects on soil and water chemistry of the marão river watershed, portugal, and biomass changes detected from landsat imagery. Appl. Geochem. 2014, 44, 93–102. [Google Scholar] [CrossRef]
- Campos, I.; Abrantes, N.; Keizer, J.J.; Vale, C.; Pereira, P. Major and trace elements in soils and ashes of eucalypt and pine forest plantations in portugal following a wildfire. Sci. Total Environ. 2016, 572, 1363–1376. [Google Scholar] [CrossRef]
- Abraham, J.; Dowling, K.; Florentine, S. Controlled burn and immediate mobilization of potentially toxic elements in soil, from a legacy mine site in central victoria, Australia. Sci. Total Environ. 2018, 616–617, 1022–1034. [Google Scholar] [CrossRef]
- Awad, M.; Liu, Z.; Skalicky, M.; Dessoky, E.S.; Brestic, M.; Mbarki, S.; Rastogi, A.; El Sabagh, A. Fractionation of heavy metals in multi-contaminated soil treated with biochar using the sequential extraction procedure. Biomolecules 2021, 11, 448. [Google Scholar] [CrossRef]
- Malinowska, E. The effect of liming and sewage sludge application on heavy metal speciation in soil. Bull Environ. Contam Toxicol 2017, 98, 105–112. [Google Scholar] [CrossRef][Green Version]
- Malinowska, E.; Jankowski, K. The effect of different doses of sewage sludge and liming on total cobalt content and its speciation in soil. Agronomy 2020, 10, 1550. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.-Q.; Wang, D.-F. Immobilization of heavy metals in sewage sludge during land application process in China: A review. Sustainability 2017, 9, 2020. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q. Effects of compost and phosphate on plant arsenic accumulation from soils near pressure-treated wood. Environ. Pollut. 2004, 132, 435–442. [Google Scholar] [CrossRef]
- Sánchez-Martín, M.; García-Delgado, M.; Lorenzo, L.F.; Rodriguez-Cruz, S.; Arienzo, M. Heavy metals in sewage sludge amended soils determined by sequential extractions as a function of incubation time of soils. Geoderma 2007, 142, 262–273. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Tripathy, S.; Hogarnal, V.; Powell, M.; Hart, B. Mobility and bioavailability of selected heavy metals in coal ash-and sewage sludge-amended acid soil. Environ. Geol. 2003, 44, 419–432. [Google Scholar] [CrossRef]
- Ngole, V.M. Using soil heavy metal enrichment and mobility factors to determine potential uptake by vegetables. Plant Soil Environ. 2011, 57, 75–80. [Google Scholar] [CrossRef]
- Rosen, V.; Chen, Y. The influence of compost addition on heavy metal distribution between operationally defined geochemical fractions and on metal accumulation in plant. J. Soils Sediments 2014, 14, 713–720. [Google Scholar] [CrossRef]
- Shrivastava, S.K.; Banerjee, D.K. Speciation of metals in sewage sludge and sludge-amended soils. Water Air Soil Pollut. 2004, 152, 219–232. [Google Scholar] [CrossRef]
- Ashworth, D.J.; Alloway, B.J. Influence of dissolved organic matter on the solubility of heavy metals in sewage-sludge-amended soils. Commun. Soil Sci. Plant Anal. 2008, 39, 538–550. [Google Scholar] [CrossRef]
- Illera, V.; Walter, I.; Souza, P.; Cala, V. Short-term effects of biosolid and municipal solid waste applications on heavy metals distribution in a degraded soil under a semi-arid environment. Sci. Total Environ. 2000, 255, 29–44. [Google Scholar] [CrossRef]
- Mendoza, J.; Garrido, T.; Castillo, G.; Martin, N.S. Metal availability and uptake by sorghum plants grown in soils amended with sludge from different treatments. Chemosphere 2006, 65, 2304–2312. [Google Scholar] [CrossRef]
- Vulkan, R.; Mingelgrin, U.; Ben-Asher, J.; Frenkel, H. Copper and zinc speciation in the solution of a soil-sludge mixture. J. Environ. Qual. 2002, 31, 193–203. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, B.; Darilek, J.L. Effect of drying on heavy metal fraction distribution in rice paddy soil. PLoS ONE 2014, 9, e97327. [Google Scholar] [CrossRef]
- Dorau, K.; Bohn, B.; Weihermüller, L.; Mansfeldt, T. Temperature-induced diurnal redox potential in soil. Environ. Sci. Process. Impacts 2021, 23, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Trehan, S.P.; Sekhon, G.S. Effect of clay, organic matter and caco3 content on zinc adsorption by soils. Plant Soil 1977, 46, 329–336. [Google Scholar] [CrossRef]
- Almås, Å.; Singh, B.R.; Salbu, B. Mobility of cadmium-109 and zinc-65 in soil influenced by equilibration time, temperature, and organic matter. J. Environ. Qual. 1999, 28, 1742–1750. [Google Scholar] [CrossRef]
- Jing, F.; Chen, X.; Yang, Z.; Guo, B. Heavy metals status, transport mechanisms, sources, and factors affecting their mobility in chinese agricultural soils. Environ. Earth Sci. 2018, 77, 104. [Google Scholar] [CrossRef]
- Latosińska, J.; Gawdzik, J. Effect of incineration temperature on the mobility of heavy metals in sewage sludge ash. Environ. Prot. Eng. 2012, 38, 31–44. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H. Fire effects on soils: The human dimension. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150171. [Google Scholar] [CrossRef]
- Badía, D.; López-García, S.; Martí, C.; Ortíz-Perpiñá, O.; Girona-García, A.; Casanova-Gascón, J. Burn effects on soil properties associated to heat transfer under contrasting moisture content. Sci. Total Environ. 2017, 601–602, 1119–1128. [Google Scholar] [CrossRef]
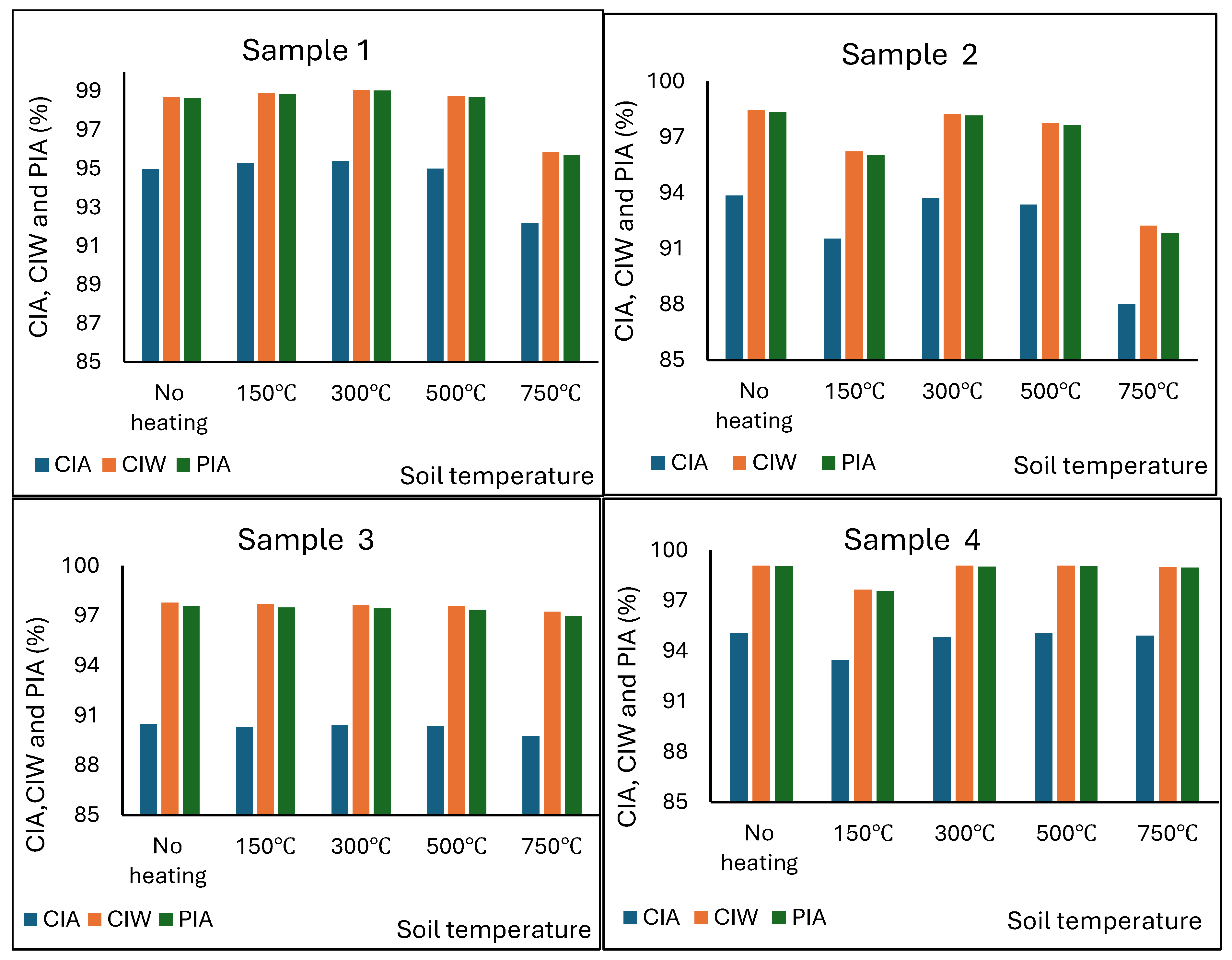

| Sample | Soil Temperature | Minerals Identified in Samples | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hematite/Goethite | K-Feldspar | Plagioclase | Quartz | Mica | Kaolinite | Chlorite | Pyrophyllite | ||
| Sample 1 | 0 | 1 | - | - | 97 | - | 2 | - | - |
| 150 | 1 | 1 | - | 96 | - | 2 | - | - | |
| 300 | 1 | - | - | 96 | - | 3 | - | - | |
| 500 | 2 | - | - | 98 | - | - | - | - | |
| 750 | 2 | 1 | - | 97 | - | - | - | - | |
| Sample 2 | 0 | 1 | - | - | 97 | - | 2 | - | - |
| 150 | 1 | 1 | 1 | 95 | - | 2 | - | - | |
| 300 | 1 | - | - | 97 | - | 2 | - | - | |
| 500 | 1 | - | - | 99 | - | 0 | - | - | |
| 750 | 1 | - | - | 99 | - | - | - | - | |
| Sample 3 | 0 | 1 | - | 1 | 87 | 2 | 4 | 4 | 1 |
| 150 | 1 | 1 | - | 88 | 2 | 3 | 4 | 1 | |
| 300 | 1 | - | 1 | 90 | 2 | 2 | 2 | 2 | |
| 500 | 1 | - | - | 93 | 2 | 2 | 2 | ||
| 750 | 1 | - | - | 98 | 1 | - | - | - | |
| Sample 4 | 0 | 1 | - | - | 96 | 1 | 2 | - | - |
| 150 | 2 | 1 | - | 96 | - | 2 | - | - | |
| 300 | 2 | - | - | 96 | - | 2 | - | 1 | |
| 500 | 1 | - | - | 99 | - | - | - | - | |
| 750 | 2 | - | - | 98 | - | - | - | - | |
| Sample | Soil Temperature (℃) | Major and Trace Element Oxide Concentrations (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | TiO2 | Al2O3 | Fe2O3(t) | MnO | MgO | CaO | Na2O | K2O | P2O5 | Cr2O3 | LOI. | Total | H2O− | ||
| Sample 1 | 0 | 82.89 | 0.51 | 6.05 | 5.90 | 0.173 | <0.01 | 0.05 | 0.03 | 0.24 | 0.069 | 0.055 | 4.15 | 100.08 | 0.95 |
| 150 | 84.24 | 0.51 | 6.27 | 5.65 | 0.175 | <0.01 | 0.05 | 0.02 | 0.24 | 0.071 | 0.050 | 2.82 | 100.06 | 0.63 | |
| 300 | 83.74 | 0.52 | 6.40 | 5.90 | 0.189 | <0.01 | 0.05 | <0.01 | 0.25 | 0.071 | 0.054 | 2.72 | 99.84 | 0.71 | |
| 500 | 84.23 | 0.52 | 6.26 | 5.87 | 0.207 | <0.01 | 0.04 | 0.04 | 0.25 | 0.074 | 0.050 | 2.59 | 100.11 | 0.72 | |
| 750 | 85.48 | 0.55 | 6.72 | 6.23 | 0.211 | <0.02 | 0.05 | 0.24 | 0.28 | 0.074 | 0.054 | 0.21 | 100.07 | 0.34 | |
| Sample 2 | 0 | 88.06 | 0.36 | 4.43 | 3.11 | 0.264 | 0.03 | 0.06 | <0.01 | 0.22 | 0.054 | 0.021 | 3.42 | 100.04 | 0.80 |
| 150 | 89.50 | 0.36 | 4.33 | 3.00 | 0.306 | 0.05 | 0.07 | 0.10 | 0.23 | 0.058 | 0.019 | 2.00 | 100.02 | 0.48 | |
| 300 | 89.39 | 0.36 | 4.49 | 3.03 | 0.283 | 0.04 | 0.07 | <0.01 | 0.22 | 0.055 | 0.021 | 1.99 | 99.95 | 0.53 | |
| 500 | 88.79 | 0.36 | 4.37 | 2.95 | 0.280 | 0.04 | 0.06 | 0.04 | 0.21 | 0.057 | 0.021 | 1.84 | 99.01 | 0.57 | |
| 750 | 90.74 | 0.38 | 4.63 | 3.20 | 0.288 | 0.04 | 0.07 | 0.32 | 0.24 | 0.059 | 0.021 | 0.18 | 100.01 | 0.28 | |
| Sample 3 | 0 | 83.61 | 0.44 | 7.02 | 4.28 | 0.208 | 0.25 | 0.11 | 0.05 | 0.58 | 0.059 | 0.028 | 3.46 | 100.09 | 0.83 |
| 150 | 84.71 | 0.43 | 6.77 | 4.16 | 0.178 | 0.24 | 0.11 | 0.05 | 0.57 | 0.057 | 0.028 | 2.42 | 99.72 | 0.62 | |
| 300 | 84.80 | 0.44 | 6.98 | 4.16 | 0.209 | 0.25 | 0.11 | 0.06 | 0.57 | 0.059 | 0.028 | 2.33 | 100.01 | 0.63 | |
| 500 | 86.59 | 0.45 | 7.19 | 4.20 | 0.212 | 0.24 | 0.11 | 0.07 | 0.59 | 0.062 | 0.027 | 0.39 | 100.13 | 0.36 | |
| 750 | 86.72 | 0.45 | 7.01 | 4.25 | 0.214 | 0.27 | 0.12 | 0.08 | 0.60 | 0.062 | 0.029 | 0.33 | 100.13 | 0.40 | |
| Sample 4 | 0 | 84.77 | 0.41 | 5.36 | 5.82 | 0.075 | <0.01 | 0.04 | <0.01 | 0.23 | 0.079 | 0.061 | 3.04 | 99.87 | 0.76 |
| 150 | 84.82 | 0.42 | 5.40 | 6.42 | 0.078 | <0.01 | 0.04 | 0.09 | 0.25 | 0.081 | 0.069 | 2.04 | 99.68 | 0.70 | |
| 300 | 85.47 | 0.42 | 5.28 | 6.33 | 0.083 | <0.01 | 0.04 | <0.01 | 0.24 | 0.084 | 0.070 | 2.06 | 100.06 | 0.62 | |
| 500 | 85.69 | 0.42 | 5.35 | 6.16 | 0.075 | <0.01 | 0.04 | <0.01 | 0.23 | 0.079 | 0.073 | 1.96 | 100.06 | 0.63 | |
| 750 | 85.64 | 0.44 | 5.95 | 7.02 | 0.084 | <0.02 | 0.04 | <0.02 | 0.26 | 0.089 | 0.082 | 0.26 | 99.83 | 0.35 | |
| Potentially Toxic Element | PTE Concentrations (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|
| Sludge | 100:0 Soil/Sludge Ratio | 80:20 Soil/Sludge Ratio | 60:40 Soil/Sludge Ratio | ||||
| Sample 1 | Sample 2 | Sample 1 | Sample 2 | Sample 1 | Sample 2 | ||
| As | 6.8 | 15 | 11.3 | 18.8 | 10.9 | 12.6 | 9.3 |
| Cd | 4.1 | 7.1 | 6.6 | 9.3 | 5.2 | 6.6 | 5.9 |
| Co | 48.9 | 67 | 51 | 71.2 | 58.4 | 52.3 | 45.3 |
| Cr | 102.3 | 185 | 105.3 | 172.6 | 102.5 | 147.1 | 107.7 |
| Cu | 157.9 | 188.3 | 176 | 203.7 | 184.8 | 152.4 | 161.5 |
| Ni | 75.6 | 87.9 | 72 | 91.1 | 77.6 | 65.5 | 63.6 |
| Pb | 87.2 | 96.3 | 103 | 98.2 | 104.4 | 86.7 | 91.9 |
| Zn | 175.1 | 187 | 189 | 186.8 | 186.9 | 182.9 | 173.5 |
| Potentially Toxic Element | Differences in PTE Concentrations (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| 80:20 Soil/Sludge | ||||||||
| Soil Sample 1 | Soil Sample 2 | |||||||
| F1 | F2 | F3 | F4 | F1 | F2 | F3 | F4 | |
| As | −0.69 | −0.34 | 0.90 | 2.31 | −0.09 | −0.08 | 2.17 | 2.02 |
| Cd | −1.60 | −1.62 | 1.19 | −0.80 | −0.79 | −0.78 | 0.61 | −0.31 |
| Co | −0.54 | −0.63 | 3.12 | 2.17 | −0.30 | −0.30 | 1.55 | 1.60 |
| Cr | −9.12 | −9.35 | 5.78 | 7.88 | −8.96 | −9.14 | 5.51 | 7.74 |
| Cu | −10.82 | −7.62 | 7.05 | 4.58 | −16.50 | −6.10 | 6.31 | 2.68 |
| Ni | −2.44 | −2.49 | 8.08 | −4.75 | −1.37 | −1.41 | 4.17 | −6.81 |
| Pb | −3.90 | −3.32 | 1.65 | −2.49 | −2.06 | −3.52 | 1.52 | −2.13 |
| Zn | −2.17 | −2.19 | 2.75 | 3.58 | −1.04 | −1.04 | 1.57 | 3.87 |
| Potentially Toxic Element | 60:40 Soil/Sludge | |||||||
| Soil Sample 1 | Soil Sample 2 | |||||||
| F1 | F2 | F3 | F4 | F1 | F2 | F3 | F4 | |
| As | −2.68 | −0.34 | 0.90 | −2.55 | −1.28 | −1.58 | 2.31 | −2.18 |
| Cd | −3.50 | −1.51 | 1.15 | −0.92 | −1.88 | −0.87 | 0.65 | −0.25 |
| Co | −2.54 | −0.63 | 3.12 | −3.17 | −2.30 | −0.30 | 1.55 | −1.60 |
| Cr | −10.15 | −9.37 | 6.21 | −6.45 | −9.23 | −9.42 | 6.01 | −8.84 |
| Cu | −31.71 | −28.12 | 8.51 | −6.92 | −17.13 | −16.61 | 6.31 | −8.62 |
| Ni | −2.44 | −2.49 | 8.08 | −8.75 | −1.37 | −1.41 | 4.17 | −6.81 |
| Pb | −3.82 | −2.72 | 1.66 | −2.96 | −2.05 | −3.79 | 1.60 | −1.88 |
| Zn | −2.15 | −2.17 | 2.74 | 2.02 | −1.04 | −1.05 | 1.60 | 2.90 |
| Potentially Toxic Elements | Temperature of Heating | PTE Mobility (%) | |||||
|---|---|---|---|---|---|---|---|
| No Sludge | 80:20 | 60:40 | |||||
| Sample 1 | Sample 2 | Sample 1 | Sample 2 | Sample 1 | Sample 2 | ||
| As | no heating | 8.72 | 6.10 | 4.81 | 4.29 | 3.98 | 3.02 |
| 150 | 9.52 | 5.33 | 7.44 | 4.11 | 5.16 | 3.68 | |
| 300 | 8.61 | 6.66 | 5.80 | 4.50 | 5.30 | 2.69 | |
| 500 | 8.98 | 6.67 | 3.75 | 5.24 | 5.03 | 5.00 | |
| 750 | 8.97 | 5.52 | 4.95 | 3.64 | 5.89 | 3.24 | |
| Cd | no heating | 28.34 | 22.70 | 19.28 | 19.50 | 13.68 | 14.66 |
| 150 | 28.79 | 24.26 | 19.44 | 18.91 | 13.26 | 15.73 | |
| 300 | 29.56 | 28.57 | 19.50 | 18.84 | 18.23 | 15.51 | |
| 500 | 29.84 | 29.81 | 27.76 | 27.02 | 23.20 | 25.23 | |
| 750 | 30.88 | 24.39 | 24.32 | 20.69 | 20.40 | 17.34 | |
| Co | no heating | 5.97 | 2.10 | 4.14 | 2.35 | 6.50 | 1.08 |
| 150 | 6.11 | 5.09 | 6.07 | 5.12 | 5.17 | 4.84 | |
| 300 | 5.49 | 5.06 | 5.41 | 4.93 | 5.01 | 3.89 | |
| 500 | 5.74 | 5.07 | 4.34 | 4.87 | 6.50 | 2.63 | |
| 750 | 5.85 | 5.08 | 4.41 | 4.29 | 9.25 | 5.65 | |
| Cr | no heating | 14.14 | 16.10 | 8.98 | 19.72 | 7.46 | 15.70 |
| 150 | 19.23 | 16.91 | 17.60 | 12.06 | 15.40 | 6.90 | |
| 300 | 11.42 | 18.92 | 7.97 | 12.23 | 5.96 | 9.00 | |
| 500 | 14.31 | 19.75 | 9.04 | 11.59 | 7.55 | 7.85 | |
| 750 | 15.11 | 18.61 | 10.60 | 9.38 | 9.91 | 10.20 | |
| Cu | no heating | 26.07 | 23.01 | 19.29 | 5.47 | 15.02 | 4.90 |
| 150 | 25.78 | 24.50 | 19.56 | 5.22 | 15.64 | 5.83 | |
| 300 | 26.80 | 22.37 | 23.20 | 20.93 | 17.48 | 4.80 | |
| 500 | 25.55 | 24.16 | 18.97 | 14.73 | 15.04 | 4.39 | |
| 750 | 26.15 | 25.63 | 19.57 | 22.50 | 16.98 | 4.69 | |
| Ni | no heating | 8.39 | 7.67 | 6.47 | 3.83 | 4.47 | 2.73 |
| 150 | 9.48 | 5.38 | 7.32 | 4.45 | 5.12 | 3.14 | |
| 300 | 7.45 | 5.37 | 4.39 | 4.80 | 2.19 | 3.60 | |
| 500 | 8.58 | 5.35 | 3.63 | 4.04 | 2.01 | 2.94 | |
| 750 | 8.79 | 5.32 | 4.57 | 4.00 | 2.73 | 3.20 | |
| Pb | no heating | 26.32 | 22.33 | 12.02 | 7.58 | 18.55 | 6.91 |
| 150 | 19.80 | 29.05 | 14.83 | 7.01 | 13.51 | 7.73 | |
| 300 | 18.75 | 29.94 | 14.93 | 26.61 | 11.99 | 24.03 | |
| 500 | 23.31 | 29.15 | 17.90 | 22.31 | 13.95 | 19.56 | |
| 750 | 16.74 | 29.48 | 13.51 | 21.38 | 10.30 | 16.49 | |
| Zn | no heating | 6.79 | 12.04 | 2.07 | 4.33 | 3.26 | 1.80 |
| 150 | 6.72 | 5.19 | 2.37 | 5.77 | 4.98 | 1.40 | |
| 300 | 6.14 | 5.19 | 4.16 | 3.79 | 3.78 | 3.16 | |
| 500 | 6.76 | 6.18 | 0.51 | 4.89 | 1.57 | 0.70 | |
| 750 | 6.94 | 7.15 | 6.11 | 5.80 | 5.65 | 6.80 | |
| Potentially Toxic Elements | Temperature | Sludge Application Rate | Sludge Application Rate * Temperature |
|---|---|---|---|
| As | 0.01 | 0.01 | 0.02 |
| Cd | 0.004 | 5.07 | 0.03 |
| Co | 0.559 | 3.63 | 1.17 |
| Cr | 0.02 | 2.60 | 0.10 |
| Cu | 0.23 | 5.19 | 0.39 |
| Ni | 0.04 | 0.01 | 0.03 |
| Pb | 0.04 | 0.05 | 0.01 |
| Zn | 1.19 | 1.24 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngole-Jeme, V.M.; Sebola, C.; Ntumba, C.N. Mineral Composition and Elemental Oxide Changes in Heat-Affected Soils and the Implications on Heavy Metal Immobilization by Sewage Sludge. Minerals 2025, 15, 143. https://doi.org/10.3390/min15020143
Ngole-Jeme VM, Sebola C, Ntumba CN. Mineral Composition and Elemental Oxide Changes in Heat-Affected Soils and the Implications on Heavy Metal Immobilization by Sewage Sludge. Minerals. 2025; 15(2):143. https://doi.org/10.3390/min15020143
Chicago/Turabian StyleNgole-Jeme, Veronica Mpode, Constance Sebola, and Christophe Nsaka Ntumba. 2025. "Mineral Composition and Elemental Oxide Changes in Heat-Affected Soils and the Implications on Heavy Metal Immobilization by Sewage Sludge" Minerals 15, no. 2: 143. https://doi.org/10.3390/min15020143
APA StyleNgole-Jeme, V. M., Sebola, C., & Ntumba, C. N. (2025). Mineral Composition and Elemental Oxide Changes in Heat-Affected Soils and the Implications on Heavy Metal Immobilization by Sewage Sludge. Minerals, 15(2), 143. https://doi.org/10.3390/min15020143




